Abstract
Background
The emergence of multiple-drug resistance bacteria has become a major threat and thus calls for an urgent need to search for new effective and safe anti-bacterial agents.
Objectives
This study aims to evaluate the anticancer and antibacterial activities of secondary metabolites from Penicillium sp., an endophytic fungus associated with leaves of Garcinia nobilis.
Methods
The culture filtrate from the fermentation of Penicillium sp. was extracted and analyzed by liquid chromatography-mass spectrometry, and the major metabolites were isolated and identified by spectroscopic analyses and by comparison with published data. The antibacterial activity of the compounds was assessed by broth microdilution method while the anticancer activity was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Results
The fractionation of the crude extract afforded penialidin A-C (1–3), citromycetin (4), p-hydroxyphenylglyoxalaldoxime (5) and brefelfin A (6). All of the compounds tested here showed antibacterial activity (MIC = 0.50 — 128 µg/mL) against Gramnegative multi-drug resistance bacteria, Vibrio cholerae (causative agent of dreadful disease cholera) and Shigella flexneri (causative agent of shigellosis), as well as the significant anticancer activity (LC50 = 0.88 – 9.21 µg/mL) against HeLa cells.
Conclusion
The results obtained indicate that compounds 1–6 showed good antibacterial and anticancer activities with no toxicity to human red blood cells and normal Vero cells.
Keywords: Garcinia nobilis, endophytic fungi, secondary metabolites, antibacterial activity, anticancer activity
Introduction
Resistance to antibiotics remains a global challenge to the healthcare sector in a large part of the world in both developing and developed countries. The spread of multidrug resistant (MDR) bacteria in hospital and community settings remains a widely unresolved problem and a heavy burden to health services. Despite advances in antibiotic therapy, infectious complications remain an important cause of mortality and morbidity among hospitalized patients.1
Natural products are organic compounds formed by living organisms in response to external stimuli such as nutritional changes, infection and competition. Biologically active natural products produced by plants, animals, insects, fungi, bacteria and protozoans have been isolated to be used in pharmaceutical drug discovery and design.2,3 Endophytes are microbes which reside in living plant tissues without causing injury or diseases to the hosts.4,5 Most of endophytes are capable of producing active metabolites and some of these compounds are proven to have medical values. For example, terpenoids, alkaloids, phenylpropanoids, aliphatic compounds, polyketides and peptides isolated from endophytic fungi have been reported to possess antimicrobial and cytotoxic activities.6
In our continuous search of antibacterial and anticancer drugs from natural source, we targeted one endophytic fungus Penicillium sp, harbored in leaves of a Cameroonian medicinal plant Garcinia nobilis Engl. (Clusiaceae). Garcinia, one of the biggest genera of the family Guttiferae, has been found to be a rich source of xanthones,7 biflavonoids, benzophenones8 as well as triterpenoids.9 Phenolic constituents from Garcinia species have been reported to possess various biological activities, including antibacterial, 10,11,12 cytotoxic13,14 and pro-oxidant15 activities. They also displayed inhibitory activity against α-glucosidase, glycation16 and human immunodeficiency virus (HIV).17
The present study was therefore designed to evaluate the cytotoxic and antibacterial activities of secondary metabolites from the culture media of Penicillium sp. isolated from the healthy leaf of Gacinia nobilis. Details of their antibacterial properties against Gram-negative multi-drug resistance bacteria such as Vibrio cholerae and Shigella flexneri, as well as their cytotoxic activities against HeLa (cancer cell lines), Vero and human red blood (normal) cells are reported herein.
Materials and methods
General experimental procedures
The high resolution mass spectra were obtained with an LTQ-Orbitrap Spectrometer (Thermo Fisher, USA) equipped with a HESI-II source. The spectrometer was operated in positive mode (1 spectrum s−1; mass range: 100–1000) with nominal mass resolving power of 60 000 at m/z 400 with a scan rate of 1 Hz). It was equipped with automatic gain control to provide high-accuracy mass measurements within 2 ppm deviation using an internal standard; Bis(2-ethylhexyl)phthalate : m/z = 391.28428. The spectrometer was attached with an Agilent (Santa Clara, USA) 1200 high performance liquid chromatography (HPLC) system consisting of LC-pump, photodiode array (PDA) detector (λ = 260 nm), auto sampler (injection volume 5 µ L) and column oven (30 °C). The following parameters were used for experiments: spray voltage 5 kV, capillary temperature 260 °C, tube lens 70 V. Nitrogen was used as sheath gas (50 arbitrary units) and auxiliary gas (5 arbitrary units). Helium served as the collision gas. The separations were performed by using a Nucleodur C18 Gravity column (50 × 2 mm, 1.8 µm particle size) with a H2O (+ 0.1% HCOOH) (A) / acetonitrile (+ 0.1% HCOOH) (B) gradient (flow rate 300 µL/min). Samples were analyzed using a gradient program as follows: 95% A isocratic for 10 min, linear gradient to 100% B over 14 min, after 100% B isocratic for 4 min, the system returned to its initial condition (80% A) within 0.5 min, and was equilibrated for 4.5 min. The separation was carried out by preparative HPLC run for 20 min on a Gilson apparatus with ultra-violet (UV) detection at 220 nm using a Nucleodur C18 Isis column (Macherey-Nagel, Düren, Germany), 5 µm (250 × 16 mm) with a H2O (A) / CH3OH (B) gradient (flow rate 4 mL/min). Samples were separated by using a gradient program as follows: 60% A isocratic for 2 min, linear gradient to 100% B over 18 min, after 100% B isocratic for 5 min, the system returned to its initial condition (60% A) within 0.50 min, and was equilibrated for 4.50 min. The Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker DRX-500 MHz spectrometer
Sampling of Garcinia nobilis
Leaves of G. nobilis were collected in Mount Etinde, Southwest region Cameroon. The plant material was identified at the Cameroon National Herbarium, Yaoundé, where the voucher specimen (50779/HNC/Cam/Mt Zamangoue) is deposited.
Isolation and identification of the endophytic fungus
The healthy leaf was firstly cleaned by washing several times under running tap water and then cut into small slices, followed by successive surface sterilization in 70% ethanol and NaOCl (6–14% active chlorine) for 2 min and finally with sterile distilled water for three times. Plant material was then dried in between the folds of sterile filter papers and deposited on a Petri dish containing potato dextrose agar (PDA) medium (200 g potato, 20 g dextrose, and 15 g agar in 1 L of H2O, supplemented with 100 mg/L of chloramphenicol to suppress bacterial growth) and incubated at 25 °C until the outgrowth of endophytic fungi was discerned. Hyphal tips originating from plant segments were transferred to potato dextrose agar (PDA). Each fungal isolate was checked for purity and transferred to the new medium by the hyphal tip method. A total of eight fungi were morphologically distinguishable. One of these isolates, CAM64 was selected for further studies based on it morphotype and the LC-MS profile of its crude extract from small scale fermentation. The fungus was identified as Penicillium sp. according to morphologic traits and a molecular biological protocol by DNA amplification and sequencing as described by Douanla et al (2013).18
Fermentation, extraction and isolation of secondary metabolites
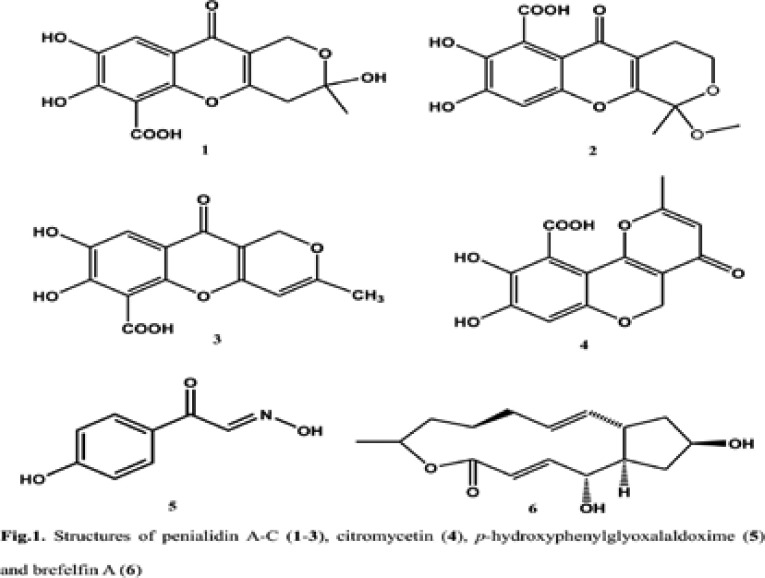
Fermentation was done on a shaker for 10 days at 25°C in 500 mL × 10 Erlenmeyer flasks each containing 300 mL of sterile potato dextrose broth (PDB) medium.19 Its solid state fermentation was done in 10 flasks (1 L each), each flask containing 100 g rice and 100 mL water enriched with 0.3% peptone and extracted after 40 days growing at 25°C with ethyl acetate to yield 3.2 g of crude extract. Isolation of penialidins (A-C) and citromycetin (1–4) was carried out as previously described.19 Isolation of hydroxyphenylglyoxalaldoxime (5) and brefelfin A (6) was done as follow. From solid state fermentation of Penicillium sp., the ethyl acetate crude extract (3.2g) was firstly partitioned with cyclohexane to remove fatty material. The resulted polar fraction (1.8 g) was then subjected to column chromatography using sephadex LH-20 to afford four main fractions (Fr1–Fr4) after thin layer chromatography (TLC) monitoring. Fractions Fr2 (96 mg) and Fr3 (105 mg) were purified by preparative HPLC eluting with a gradient of methanol-H2O+ 0.1% trifluoroacetic acid (TFA) to yield hydroxyphenylglyoxalaldoxime (5) C8H8O3N (4.6 mg, tR = 6.73 min, high-resolution electron impact-mass spectrometry (HRESIMS) at 166.05022 [M+H]+: (calcd. 166.05042) and brefelfin A (6) C16H24O4 (3.5 mg, tR = 12.06 min, HRESIMS at 281.17957 [M+H]+: (calcd. 281.17528). Their structures (Fig. 1) were easily determined from their high resolution mass spectrometry combined to proton and carbon 13 nuclear magnetic resonance (1H and 13C-NMR), by introducing these data into antibase20 and finally confirmed by two-dimension nuclear magnetic resonance (2D NMR) spectra. The chemical shifts were in agreement with those reported in the literature.21
Fig. 1.
Structures of penialidin A–C (1–3), citromycetin (4), p-hydroxyphenylglyoxalaldoxime (5) and brefelfin A (6)
Antibacterial assays
Microbial growth conditions
A total of five bacterial strains were tested for their susceptibility to compounds, and these strains were taken from our laboratory collection (kindly provided by Dr. T. Ramamurthy, NICED, Kolkata). Among the clinical strains of Vibrio cholerae used in this study, strains NB2 and SG24(1) belonged to O1 and O139 serotypes, respectively. These strains were able to produce cholera toxin and hemolysin. The other strains used in this study were V. cholerae non-O1, non-O139 (strains CO6 and PC2); and Shigella flexneri SDINT. The V. cholerae non-O1 and non-O139 strains, were positive for hemolysin production but negative for cholera toxin production. The bacterial strains were maintained on agar slant at 4 °C and subcultured on a fresh appropriate agar plates 24 h prior to any antibacterial test. The Mueller Hinton Agar (MHA) was used for the activation of bacteria. The Mueller Hinton Broth (MHB) and nutrient agar (Hi-Media) were used for the MIC and MBC determinations respectively.
Inocula preparation
Suspensions of bacteria were prepared in MHB from cells arrested during their logarithmic phase growth (4h) on MHB at 37 °C. The turbidity of the microbial suspension was read spectrophotometrically at 600 nm and adjusted to an OD of 1.0 with MHB, which is equivalent to 2×108 CFU/mL. From this prepared solution, other dilutions were made with MHB to yield 1x106 CFU/mL.22
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC and MBC of compounds 1–6 were assessed using the broth microdilution method recommended by the National Committee for Clinical Laboratory Standards23,24 with slight modifications. Each test sample was dissolved in dimethylsulfoxide (DMSO) to give a stock solution. The 96-well round bottom sterile plates were prepared by dispensing 180 µl of the inoculated broth (1×106CFU/mL) into each well. A 20 µL aliquot of the compounds was added. The concentrations of sample tested were 0.125, 0.25, 0.50, 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024, and 2048 µg/mL. The final concentration of DMSO in each well was < 1% [preliminary analyses with 1% (v/v) DMSO did not inhibit the growth of the test organisms]. Dilutions of ampicillin and chloramphenicol served as positive controls, while broth with 20 µL of DMSO was used as negative control. Plates were covered and incubated for 24 h at 37°C. After incubation, minimum inhibitory concentrations (MIC) were read visually; all wells were plated to nutrient agar (Hi-Media) and incubated for 24 h at 37 °C. The lowest concentrations that yielded no growth after this subculturing were taken as the minimum bactericidal concentration (MBC) values.
Anticancer assays
HeLa (Human cervical cancer cell line, ATCC No. CCL-2) and monkey Vero cells (normal non-cancer cells, ATCC No. CCL-81), obtained from the American Type Culture Collection (ATCC) were used in this study. Anticancer activity was determined using the 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, USA) assay reported by Mosmann25 for the human cervical cancer cell lines (HeLa cells) and monkey Vero cells. This cell viability assay is based on living cells' property to transform the MTT dye tetrazolium ring into a purple-colored formazan structure due to the action of mitochondrial and other dehydrogenases inside the cell. The color intensity yielded by the cell population is directly proportional to the number of viable cells, and one can quantify the absorbance measurements using mathematical parameters. Each test sample was dissolved in dimethylsulfoxide (DMSO) to give a stock solution. Compounds 1–6 were prepared from the stock solutions by serial dilution in RPMI 1640 to give a volume of 100 µL in each well of a microtiter plate (96-well). Each well was filled with 100 µL of cells at 2 × 105 cells/mL. The assay for each concentration of compound was performed in triplicates and the culture plates were kept at 37°C with 5% (v/v) CO2 for 24 hours. After removing the supernatant of each well and washing twice by PBS, 20 µL of MTT solution (5 mg/ mL in PBS) and 100 µL of medium were then introduced. After 4 h of incubation, 100 µL of DMSO was added to each well to dissolve the formazan crystals and the absorbance values at 490 nm were measured with a microplate reader (Bio-RAD 680, USA). The relative cell viability (%) was expressed as a relative percentage of treated cells to the untreated control cells (TC/UC × 100). The rate of cell inhibition was calculated using the following formula: inhibition rate = [1− (ODtest/ODnegative control)] ×100%. The LC50 values were calculated as the concentration of test sample resulting in a 50% reduction of absorbance compared to untreated cells. Cells treated with paclitaxel + RPMI 1640 served as positive control whereas cells left untreated + 1% (v/v) DMSO + RPMI 1640 were used as negative control.
Hemolytic assay
Whole blood (10 mL) from a healthy individual was collected into a conical tube containing heparin as an anticoagulant (blood group O was used). Authorization for the collection of blood was obtained from the Medical and Ethical Committee. The written informed consent for participation in the study was obtained from a parent of 40 years old. Erythrocytes were harvested by centrifugation for 10 min at 1,000 × g at room temperature and washed three times in PBS solution. The top layer (plasma) and the next, milky layer (buffy coat with a layer of platelets on top of it) were then carefully aspirated and discarded. The cell pellet was resuspended in 10 mL of PBS solution and mixed by gentle aspiration with a Pasteur pipette. This cell suspension was used immediately.
Hemolysis test was performed to determine cellular toxicity of the compounds as previously described.26 Compounds 1–6, at concentrations ranging from 0.25 to 512 µg/mL, were incubated with an equal volume of 1% human red blood cells in phosphate buffered saline (10 mM PBS, pH 7.4) at 37°C for 1 h. 1% human red blood cells in buffer was used as a non-hemolytic control whereas buffer containing 1% Triton X-100 and 1% human red blood cells served as a 100% hemolytic control. Cell lysis was monitored by measuring the release of hemoglobin at 595 nm with a spectrophotometer (Thermo Scientific, USA). Percent hemolysis was calculated as follows: [(A595 of sample treated with compound - A595 of sample treated with buffer) / (A595 of sample treated with Triton X-100 − A595 of sample treated with buffer)] × 100.
Statistical analysis
Statistical analysis was carried out using Statistical Package for Social Science (SPSS, version 12.0). The experimental results were expressed as the mean ± Standard Deviation (SD). Group comparisons were performed using One Way ANOVA followed by Waller-Duncan Post Hoc test. A p value of 0.05 was considered statistically significant.
Results
Antibacterial activity
Compounds 1–6 were examined in vitro against bacterial species and the results are depicted in Table 1. All compounds showed different degrees of antibacterial activities against the tested bacterial pathogens. Vibrio cholerae SG24 (1) was the most sensitive bacteria while Vibrio cholerae CO6 was the most resistant. Compound 3 (MIC = 0.50–16 µg/mL) was the most active among the isolated compounds following in decreasing order by compound 2 (MIC = 4–32 µg/mL), compound 1 (MIC = 8–32 µg/ mL), compound 5 (MIC = 32–64 µg/mL), compounds 4 and 6 (MIC = 64–128 µg/mL).
Table 1.
Inhibition parameters (MIC, MBC) of compounds 1–6 and reference antibacterial drugs (µg/mL)
| Antibacterial activity (MIC and MBC in µg/mL) | ||||||
| Compounds | Inhibition parameters |
Vibrio cholerae |
Vibrio cholerae |
Vibrio cholerae |
Vibrio cholerae |
Shigella flexneri |
| SG24 (1) | CO6 | NB2 | PC2 | SDINT | ||
| 1 | MIC | 8 | 16 | 32 | 32 | 16 |
| MBC | 16 | 32 | 64 | 64 | 32 | |
| MBC/MIC | 2 | 2 | 2 | 2 | 2 | |
| 2 | MIC | 4 | 32 | 16 | 8 | 16 |
| MBC | 4 | 64 | 16 | 8 | 32 | |
| MBC/MIC | 1 | 2 | 1 | 1 | 2 | |
| 3 | MIC | 0.50 | 16 | 8 | 0.50 | 8 |
| MBC | 1 | 32 | 16 | 2 | 16 | |
| MBC/MIC | 2 | 2 | 2 | 4 | 2 | |
| 4 | MIC | 64 | 128 | 128 | 128 | 64 |
| MBC | 64 | 128 | 128 | 128 | 64 | |
| MBC/MIC | 1 | 1 | 1 | 1 | 1 | |
| 5 | MIC | 64 | 64 | 32 | 32 | 32 |
| MBC | 128 | 128 | 64 | 64 | 64 | |
| MBC/MIC | 2 | 2 | 2 | 2 | 2 | |
| 6 | MIC | 64 | 128 | 64 | 128 | 128 |
| MBC | 4 | 256 | 64 | 256 | 128 | |
| MBC/MIC | 1 | 2 | 1 | 2 | 1 | |
| Ampicillin | MIC | 16 | 16 | >512 | >512 | >512 |
| MBC | 16 | 16 | >512 | >512 | >512 | |
| MBC/MIC MIC MBC | 1 | 1 | / | / | / | |
| Chloramphenicol | MIC | 4 | 16 | 8 | 1 | 64 |
| MBC | 8 | 64 | 32 | 4 | >512 | |
| MBC/MIC | 2 | 4 | 4 | 4 | / | |
/: not determined; MIC: minimum inhibitory concentration; MBC: minimum bactericidal concentration
The antibacterial activities of compounds 1–6 (MIC = 8 – 32 µg/mL) were in some cases equal to or more important than those of ampicillin and chloramphenicol; highlighting their good antibacterial potency. Collectively, these findings showed that compounds 1–6 have broadspectrum antibacterial activity and are effective against ampicillin-resistant and chloramphenicol-resistant bacteria. The lowest MIC value of 0.50 µg/mL was recorded on Vibrio cholera SG24 (1) and Shigella flexneri with compound 3 while the lowest MBC value of 1µg/mL was obtained on Vibrio cholerae SG24 (1) with compound 3. However, the highest MIC value of 128 µg/mL was recorded on Vibriocholerae CO6, Vibrio cholerae NB2 and Vibrio cholerae PC2 with compound 3 and on Vibrio cholerae CO6, Vibrio cholerae NB2, Shigella flexneri with compound 6 while the highest MBC value of 256 µg/mL was obtained on Vibrio cholerae CO6 and Vibrio cholerae PC2 with compound 6. A lower MBC/MIC (≤4) value signifies that a minimum amount of compound is used to kill the microbial species, whereas, a higher value signifies the use of comparatively more amount of compound for the control of any microorganism. No activity was noted for ampicillin against Vibrio cholerae NB2, Vibrio cholerae PC2, Shigella flexneri SDINT at concentrations up to 512 µg/mL. However, these multi-drug resistant bacterial strains were sensitive to compounds 1–6. This finding suggests the antibacterial potency of these compoundsin particular for the treatment of ampicillin-resistant and chloramphenicol- resistant bacteria.
Hemolytic activity
In this study, none of the tested compounds showed hemolytic activities against human red blood cells at concentrations up to 512 µg/mL (results not shown) indicating that they are non-toxic to normal cells.
Anticancer activity
Compounds 1–6 were evaluated for their cytotoxicity against human cancer cells (HeLa cells) and normal noncancer cells (Vero cells) and the results are summarized in Table 2. The isolated compounds exhibit an inhibitory effect against tumor cell growth, with varying efficiencies (LC50 = 0.88 — 9.21 µg/mL) and selectivities (SI = 19.70– 315.47). The lowest LC50 value (corresponding to the most anticancer compound) was found with compound 6 followed in decreasing order by compound 5 > compound 3, compound 4 > compound 1, compound 2. The tested compounds also showed significant anticancer activity to HeLa cells (LC50 = 0.88 — 9.21 µg/mL) when compared with Vero cells (LC50 = 124.72 – 277.62 µg/ mL) indicating that they are less toxic to normal cells. The positive control paclitaxel showed LC50 values of 0.57 and 68.51 µg/mL against HeLa and Vero cells, respectively.
Table 2.
Anticancer (LC50 in µg/mL) of compounds from 1–6 and their selectivity index (SI).
| Compounds | Cytotoxicty (LC50 in µg/mL) | Selectivity Index* | ||||||
| HeLa cells | Vero cells | HeLa cells |
V. cholerae SG24 (1) |
V. cholerae CO6 |
V. cholerae NB2 |
V. cholerae PC2 |
S. flexneri SDINT |
|
| 1 | 6.33 ± 0.66a | 124.72 ± 1.27a | 19.70 | 15.59 | 7.79 | 3.89 | 3.89 | 7.79 |
| 2 | 6.74 ± 0.27a | 158.24 ± 2.86b | 23.47 | 39.56 | 4.94 | 9.89 | 19.78 | 9.89 |
| 3 | 8.13 ± 0.18b | 233.26 ± 3.73c | 28.69 | 466.52 | 14.57 | 29.15 | 466.52 | 29.15 |
| 4 | 9.21 ± 0.79b | 261.14 ± 2.87d | 28.35 | 4.08 | 2.04 | 2.04 | 2.04 | 4.08 |
| 5 | 2.06 ± 0.23c | 195.41 ± 1.79e | 94.85 | 3.05 | 3.05 | 6.10 | 6.10 | 6.10 |
| 6 | 0.88 ± 0.12d | 277.62 ± 1.38f | 315.47 | 4.33 | 2.16 | 4.33 | 2.16 | 2.16 |
| Paclitaxel | 0.57 ± 0.03e | 68.51 ± 0.19g | 120.19 | / | / | / | / | / |
/: not determined, SI = LC50 on Vero cells /MIC or LC50 on HeLa cells;
SI obtained from average MIC. Each LC50 value represents the mean ± SD (n = 3). In the same column, LC50 values marked with different superscript letters (a-g) are significantly different (p < 0.05).
Discussion
The findings of the present study showed that the antibacterial activities varied with the bacterial strains. These variations may be due to genetic differences between the bacteria. The results of MIC and MBC showed that the MIC values obtained were four times equal to or lesser than the MBCs on the corresponding (sensitive) bacteria, suggesting the bactericidal effects of the concerned samples.27,28,29 This is interesting in view of the perspective of developing new antibacterial drugs from endophytic fungus. The present study showed antibacterial activity of penialidin A-C (1–3), citromycetin (4), p-hydroxyphenylglyoxalaldoxime (5) and brefelfin A (6) from Penicillium sp. against the bacterial species and this is the first report on the activity of these compounds against these types of pathogenic strains. However, the results of compounds 1 and 2 are in agreement with those of the literature against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Acinetobacter sp.19
Although no definite structure-activity relationship could be determined, some structural features that might have influenced the antibacterial activity of polyketide compounds (1–4) can be drawn from the comparison of the chemical structures of compounds with different activities. Compound 3 was the most active polyketide compound, followed by 2, 1 and 4. Together, it appears that, 2-hydroxyl, 2-methoxy and 10-carboxyl groups play a greater role in increasing the antimicrobial activity based on the substitution patterns of the aromatics rings. The overall results of this study can be considered as very promising in the perspective of new drugs discovery from endophytic fungus sources, especially when the medical importance of tested bacteria is considered. Acute watery diarrhea accounts for 80% of the cases (death account for 50%) in the developing world.30
Among the diarrheal diseases, cholera is a serious epidemic disease caused by the gram-negative bacterium Vibrio cholerae.31 Vibrio cholerae, serotypes O1 and O139 has ability to produce an enterotoxin, cholera toxin that is a major determinant of virulence for cholera. Among the other virulence factors, ElT or hemolysin produced by Vibrio cholerae is also reportedly a potent toxin with both enterotoxic and cytotoxic activities.32,33 Emergence of multiply drug-resistant Vibrio cholerae is a serious clinical problem in the treatment and containment of the disease, as reflected by the increase in the fatality rate from 1% to 5.3% after the emergence of drug-resistant strains in Guinea-Bissau during the 1996–1997 epidemic of cholera.34 Such findings trace the importance of discovering new substances against which these organisms are sensitive. Generally, these bacterial strains were sensitive to the isolated compounds.
MTT assay measures the cell viability based on the reduction of yellow tetrazolium MTT to purple formazon dye by mitochondrial dehydrogenase enzyme. The amount of formazon reflects the number of metabolically active viable cells.35 Compound 3 was the most antibacterial compound while compounds 5 and 6 were the most anticancer samples. Interestingly, the anticancer of compounds 5 and 6 can be considered more important when taking into consideration the criterion of the American National Cancer Institute (NCI) regarding the anticancer of pure compounds (LC50 < 4 µg/mL).36 The results of the anticancer activity also showed that the isolated compounds exhibit an inhibitory effect against tumor cell growth, with varying efficiencies (LC50 = 0.88 — 9.21 µg/mL) and selectivities (SI = 19.70– 315.47). The anticancer mechanisms associated to each compound may explain the cytotoxic potency of these compounds. Selectivity is important because most anticancer drugs currently in use induce serious adverse effects. In this study, Selectivity Index (SI) of active compounds was determined in order to investigate whether the cytotoxic activity was specific to cancer cells/bacterial strains. The SI of the samples is defined as the ratio of cytotoxicity (LC50 values) on normal non-cancer cells (Vero cells) to cancer cells (HeLa cells) or bacterial cells: SI = LC50 on Vero cells / LC50 on HeLa cells or MIC. Test agents with SI higher than three were considered to have good selectivity towards cancer cells.37 The SI values of the tested compounds against the HeLa cells ranged from 19.70 to 315.47 and could be considered as good. Apart from compounds 4 on V. cholerae CO6, V. cholerae NB2 and V. cholerae PC2 and compound 6 on V. cholerae CO6, V. cholerae PC2 and S. flexneri, the SI values of the tested compounds against the bacterial strains ranged from 3.05 to 466.52 and could be considered as high.
Conclusion
The chemical analysis of the ethyl acetate extract of Penicillium sp., an endophytic fungus associated with leaves of Garcinia nobilis afforded six known compounds including penialidin A-C (1–3), citromycetin (4), p-hydroxyphenylglyoxalaldoxime (5) and brefelfin A (6). Compounds 1–6 showed good antibacterial and anticancer activities with no toxicity to human red blood cells and normal Vero cells. These compounds could be explored in more details in the future to develop novel antibacterial/anticancer drugs.
Acknowledgements
This work was supported by grants of the German Academic Exchange Service (DAAD), grant A/12/90548 to Jouda Jean-Bosco for his Ph.D studies. Also, Jean-de-Dieu Tamokou acknowledges funding from the Indian Ministry of Education and Research through their CV Raman fellowship grant.
Conflict of interest
We declare no conflict of interest.
References
- 1.Valle DLJ, Andrade JI, Puzon JJM, Cabrera EC, Rivera WL. Antibacterial activities of ethanol extracts of Philippine medicinal plants against multidrug-resistant bacteria. Asian Pac J Trop Biomed. 2015;5(7):532–540. doi: 10.1016/j.apjtb.2015.04.005. (25):3077-85.doi:10.1016/j.lfs.2004.07.009. [DOI] [Google Scholar]
- 2.Willian RS. The role of natural products in a modern drug discovery program. Drug Discov Today. 2000;5(2):39–41. doi: 10.1016/S1359-6446(99)01443-9. [DOI] [PubMed] [Google Scholar]
- 3.Rollinger JM, Langer T, Stuppner H. Strategies for efficient lead structure discovery from natural products. Curr Med Chem. 2006;13(13):1491–1507. doi: 10.2174/092986706777442075. [DOI] [PubMed] [Google Scholar]
- 4.Petrini O, Sieber TN, Toti L, Viret O. Ecology, metabolite production, and substrate utilization in endophytic fungi. Nat Toxins. 1992;1(3):185–196. doi: 10.1002/nt.2620010306. [DOI] [PubMed] [Google Scholar]
- 5.Strobel GA. Endophytes as sources of bioactive products. Microbes Infect. 2003;5(6):535–544. doi: 10.1016/S1286-4579(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 6.Astuti P, Wahyono, Nababan OA. Antimicrobial and cytotoxic activities of endophytic fungi isolated from Piper crocatum Ruiz & Pav. Asian Pac J Trop Biomed. 2014;4(2):S592–S596. doi: 10.12980/APJTB.4.2014APJTB-2014-0073. [DOI] [Google Scholar]
- 7.Bennett GJ, Lee H-H. Xanthones from the Guttiferae. Phytochemistry. 1989;28(4):967–998. doi: 10.1016/0031-9422(89)80170-0. [DOI] [Google Scholar]
- 8.Waterman PG, Hussain RA. Systematic significance of xanthones, benzophenones and biflavonoids in Garcinia. Biochem Syst Ecol. 1983;11(1):21–28. doi: 10.1016/0305-1978(83)90025-X. [DOI] [Google Scholar]
- 9.Nguyen DLH, Harrison LJ. Xanthones and triterpenoids from the bark ofGarcinia vilersiana. Phytochemistry. 2000;53(1):111–114. doi: 10.1016/S0031-9422(99)00391-X. [DOI] [PubMed] [Google Scholar]
- 10.Permana D, Lajis NH, Mackeen MM, Ali AM, Aimi N, Kitajima M, Takayama H. Isolation and bioactivities of constituents of the roots of Garcinia atroviridis. J Nat Prod. 2001;64(7):976–979. doi: 10.1021/np000563o. [DOI] [PubMed] [Google Scholar]
- 11.Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, Suksamrarn A. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem Pharm Bull. 2003;51(7):857–859. doi: 10.1248/cpb.51.857. http://doi.org/10.1248/cpb.51.857. [DOI] [PubMed] [Google Scholar]
- 12.Hay AE, Helesbeux JJH, Duval O, Labaïed M, Grellier P, Richomme P. Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci. 2004;75(25):3077–3085. doi: 10.1016/j.lfs.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Shadid KA, Shaari K, Abas F, Israf DA, Hamzah AS, Syakroni N, Saha K, Lajis NH. Cytotoxic cagedpolyprenylated xanthonoids and a xanthone from Garcinia cantleyana. Phytochemistry. 2007;68(20):2537–3244. doi: 10.1016/j.phytochem.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Zhao M, Yang B, Bai W. Immunomodulatory and anticancer activities of phenolics from Garcinia mangostana fruit pericarp. Food Chem. 2009;116(4):969–973. doi: 10.1016/j.foodchem.2009.03.064. [DOI] [Google Scholar]
- 15.Wu CC, Lu YH, Wei BL, Yang SC, Won SJ, Lin CN. Phloroglucinols with prooxidant activity from Garcinia subelliptica. J Nat Prod. 2008;71(2):246–250. doi: 10.1021/np070507o. [DOI] [PubMed] [Google Scholar]
- 16.Fouotsa H, Lannang AM, Mbazoa CD, Rasheed S, Bishnu PM, Zulfiqar A, Krishna PD, NKengfack AE, Farzana S, Muhammad IC, Sewald N. Xanthones inhibitors of a-glucosidase and glycation from Garcinia nobilis. Phytochem Lett. 2012;5(2):236–239. doi: 10.1016/j.phytol.2012.01.002. [DOI] [Google Scholar]
- 17.Gustafson KR, Blunt JW, Munro MHG, Fuller RW, McKee TC, Cardellina JH, II, McMahon JB, Cragg GM, Boyd MR. The Guttiferones, HIV-inhibitory benzophenones from Symphonia globulifera, Garcinia livingstonei, Garcinia ovalifolia and Clusia rosea. Tetrahedron. 1992;48(46):10093–10102. [Google Scholar]
- 18.Douanla MC, Langer E, Talontsi MF. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecol. 2013;6(3):212–222. doi: 10.1016/j.funeco.2013.01.004. [DOI] [Google Scholar]
- 19.Jouda J-B, Kusari S, Lamshöft M, Talontsi MF, Douala MC, Wandji J, Spiteller M. Penialidins A-C with strong antibacterial activities from Penicilliumsp., an endophytic fungus harboring leaves of Garcinia nobilis. Fitoterapia. 2014;98:209–214. doi: 10.1016/j.fitote.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Laatsch H. AntiBase, a data base for rapid dereplication and structure determination of microbial natural products. Weinheim, Germany: Wiley VCH; 2013. [Google Scholar]
- 21.Betina V. Biological effects of the antibiotic brefeldin A (Decumbin, Cyanein, Ascotoxin, Synergisidin): a retrospective. Folia Microbiol. 1992;37(1):3–11. doi: 10.1007/BF02814572. [DOI] [PubMed] [Google Scholar]
- 22.Tamokou J-de-D, Tsemeugne J, Fondjo SE, Sarkar P, Kuiate JR, Djintchui NA, Sondengam LB, Bag KP. Antibacterial and cytotoxic activities and SAR of some azo compounds containing thiophene backbone. Pharmacologia. 2016;7(4):182–192. doi: 10.5567/pharmacologia.2016.182.192. [DOI] [Google Scholar]
- 23.NCCLS, author. Approved guideline, M26-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. Methods for determining bactericidal activity of antimicrobial agents. [Google Scholar]
- 24.NCCLS, author. Approved Standards, M7-A4. Wayne, PA: National Committee for Clinical Laboratory Standards; 1997. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Situ H, Bobek LA. In vitro assessment of antifungal therapeutic potential of salivary histatin-5, two variants of histatin-5, and salivary mucin (MUC7) domain 1. Antimicrob Agents Chemother. 2000;44(6):1485–1493. doi: 10.1128/AAC.44.6.1485-1493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamokou JD, Mpetga SDJ, Lunga PK, Tene M, Tane P, Kuiate JR. Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from the stem bark of Albizia adianthifolia (Mimosoideae) BMC Complement Altern Med. 2012;12:99. doi: 10.1186/1472-6882-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Djouossi MG, Tamokou JD, Ngnokam D, Kuiate JR, Tapondjou AL, Harakat D, Laurence-Nazabadioko LV. Antimicrobial and antioxidant flavonoids from the leaves of Oncoba spinosa Forssk. (Salicaceae) BMC Complement Altern Med. 2015;15:134. doi: 10.1186/s12906-015-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamokou JD, Tala FM, Wabo KH, Kuiate JR, Tane P. Antimicrobial activities ofmethanol extract and compounds from stem Bark of Vismia rubescens. J Ethnopharmacol. 2009;124(3):571–575. doi: 10.1016/j.jep.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 30.Tullock J, Richards L. Childhood diarrhea and acute respiratory infections in developing countries. The Med J Australia. 1993;159(1):46–51. doi: 10.5694/j.1326-5377.1993.tb137705.x. [DOI] [PubMed] [Google Scholar]
- 31.Nair GB, Ramamurthy T, Bhattacharya SK, Mukhopadhyay AK, Garg S, Bhattacharya MK, Takeda T, Takeda Y, Deb BC. Spread of Vibrio choleraeO139 Bengal in India. J Infect Dis. 1994;169(5):1029–1034. doi: 10.1093/infdis/169.5.1029. [DOI] [PubMed] [Google Scholar]
- 32.Ichinose Y, Yamamoto K, Nakasone N, Tanabe MJ, Takeda T, Miwatani T, Iwanaga M. Enterotoxicity of El-Tor-like haemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987;55(5):1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramamurthy T, Bag PK, Pal A, Bhattacharya SK, Bhattacharya MK, Sen D, Shimada T, Takeda T, Nair GB. Virulence patterns of V. cholerae non-O1 isolated from hospitalized patients with acute diarrhoea in Calcutta, India. J Med Microbiol. 1993;39(4):310–317. doi: 10.1099/00222615-39-4-310. [DOI] [PubMed] [Google Scholar]
- 34.Dalsgaard A, Forslund A, Bodhidatta D, Serichantalergs C, Pitarangsi L, Pang T, Shimada T, Echeverria P. A high proportion of V. cholerae isolated from children with diarrhoea in Bangkok, Thailand are multiple antibiotic resistant and belong to heterogeneous non-O1, non-O139 O-serotypes. Epidemiol Infect. 1999;122(2):217–226. doi: 10.1017/S0950268899002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Loosdrecht AA, Beelan RHJ, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MMAC. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods. 1994;174(1–2):311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 36.Tanamatayarat P, Limtrakul P, Chunsakaow S, Duangrat C. Screening of some rubiaceous plants for cytotoxic activity against Cervix Carcinoma (KB-3-1) Cell Line. Thai J Pharm Sci. 2003;27:167–172. [Google Scholar]
- 37.Mahavorasirikul W, Viyanant V, Chaijoroenkul W, Itharat A, Na-Bangchang K. Cytotoxicity activity of Thaimedicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern Med. 2010;10:5. doi: 10.1186/1472-6882-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]