Abstract
Idiopathic pulmonary fibrosis (IPF) is a lethal human disease with short survival time and few treatment options. Herein, we demonstrated that discoidin domain receptor 2 (DDR2), a receptor tyrosine kinase that predominantly transduces signals from fibrillar collagens, plays a critical role in the induction of fibrosis and angiogenesis in the lung. In vitro cell studies showed that DDR2 can synergize the actions of both transforming growth factor (TGF)-β and fibrillar collagen to stimulate lung fibroblasts to undergo myofibroblastic changes and vascular endothelial growth factor (VEGF) expression. In addition, we confirmed that late treatment of the injured mice with specific siRNA against DDR2 or its kinase inhibitor exhibited therapeutic efficacy against lung fibrosis. Thus, this study not only elucidated novel mechanisms by which DDR2 controls the development of pulmonary fibrosis, but also provided candidate target for the intervention of this stubborn disease.
Introduction
Idiopathic pulmonary fibrosis (IPF), the most common fibrotic conditions in the lung, is a chronic and lethal human disease with unknown etiology. IPF patients have a median survival of ~2–3 years after diagnosis because of an irreversible loss of lung function and respiratory failure.1 Although a variety of inflammatory insults are associated with the development of IPF, an undeniable fact is that this type fibrotic disorder is clinically recalcitrant to treatment with immunosuppressive agents,2 leading to the recent shift of concept for IPF treatment away from anti-inflammation toward antifibrosis.3 However, although there has been a huge rise in clinical trials with antifibrotic drugs during the past decade, it was until recently that two pharmacological agents were approved for the treatment of moderate IPF.4
The pathogenesis of pulmonary fibrosis involves alveolar epithelial cell injury, inflammatory cell infiltration, as well as fibroblast recruitment and activation.5 It has been long accepted that the activated fibroblasts (so-called myofibroblasts), characterized by de novo expression of α-smooth muscle actin (α-SMA), are the key effector cells in various fibrotic diseases in which they have stronger contractile activity and are responsible for the secretion of exaggerated amounts of extracellular matrix (ECM) molecules with the subsequent scaring and destruction of the tissue architecture. The formation of myofibroblasts can be driven by cytokines, matrix proteins, and biomechanical tension.6 Among these myofibroblast-modulating factors, transforming growth factor (TGF)-β is considered to be the most powerful driver of fibroblast ECM production and tissue fibrosis characterized to date and agents blocking its activation have been under clinical trial for IPF.3 TGF-β regulates myofibroblast gene expression through canonical Smad pathway and non-Smad pathways such as mitogen-activated protein kinase family and PI3K/Akt.7
Recently, the dynamically altered ECM microenvironment was suggested to act as positive feedback stimuli for lung fibroblast behaviors and the progression of lung fibrosis.8,9 Because fibrillar collagens are major components of fibrotic lung matrices,10 understanding how fibroblasts or myofibroblasts receive and transmit signals from fibrillar collagens will definitely favor the development of novel drugs to intervene in the influence from abnormal ECM.
Discoidin domain receptors (DDRs), including DDR1 and DDR2, are unique receptor tyrosine kinases because they signal in response to nondiffusible collagens rather than diffusible cytokines. Unlike the quick-on and quick-off activation pattern of growth factor receptors, DDRs display a slow but sustained kinetic of phosphorylation upon collagen binding, which induces cell differentiation, migration, and invasion.11 In contrast to DDR1 that is primarily expressed in epithelial cells and activated by multiple types of collagens, DDR2 is abundantly expressed in fibroblasts or cells of mesenchymal origin and activated by fibrillar collagens and type X collagen.12,13 Accumulating evidences indicate that DDR2 acts as a marker as well as a key regulator of epithelial mesenchymal transition (EMT).14,15,16,17 The deficient expression of DDR2 in vivo can not only cause some developmental defects such as dwarfing and infertility,18,19,20 but also lead to several pathological changes, such as tumor progression, arthritis, and choroidal neovascularization.21,22,23,24 Previous development or search of DDR-targeting drugs has yielded several candidate compounds,25,26,27,28 most of which do not distinguish DDR1 from DDR2. Dasatinib, a Food and Drug Administration (FDA)-approved drug used to treat chronic myelocytic leukemia, was reported to potently inhibit the kinase activity of DDRs.25 Two recent clinical studies demonstrated that the lung cancer patients who carry oncogenic mutations of DDR2 showed response to dasatinib treatment.29,30
The only in vivo evidence for a role of DDR2 in fibrotic disease showed that DDR2 knockout mice exhibited exaggerated severity of chronic hepatic fibrosis.31 Although a previous in vitro study has demonstrated that DDR2 promotes lung fibroblast proliferation and migration,32 till now it remains unclear whether and how DDR2 contributes to the pathogenesis of pulmonary fibrosis. In this study, we initially found that DDR2 mutant mice were refractory to induction of experimental lung fibrosis. Furthermore in vitro studies showed that DDR2 can synergize the actions of both TGF-β and fibrillar collagen to stimulate lung fibroblasts to undergo myofibroblastic changes and vascular endothelial growth factor (VEGF) expression. We also confirmed that DDR2-targeting strategies potently inhibited the further progression of established lung fibrosis. Thus, our data suggest the potential of DDR2 as a therapeutic target for treatment of pulmonary fibrosis.
Results
A deficiency or downregulation of DDR2 prevents lung fibrosis
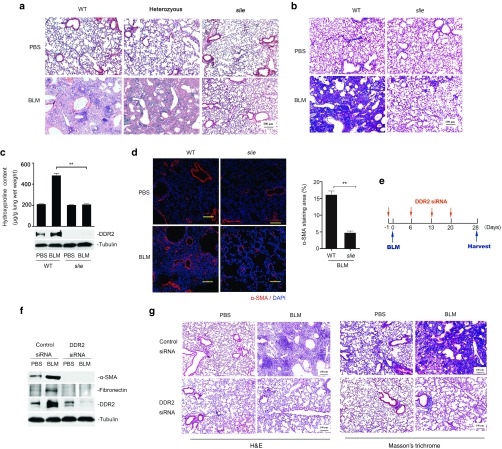
To determine the exact role of DDR2 in the development of pulmonary fibrosis, a well-established DDR2 mutant mouse colony (slie),19 was firstly subjected to bleomycin-induced lung injury for 4 weeks. The results showed that bleomycin insult caused a dramatic reduction of alveolar space in both wild-type and heterozygous slie mutant mice, but exhibited no effect on the homozygous slie animals (Figure 1a). The bleomycin-challenged lungs from slie mice also had much less accumulation in collagens than those from control group, as measured by Masson staining, hydroxyproline content and quantitative polymerase chain reaction (qPCR) analysis of collagen I (col1α1) mRNA expression (Figure 1b,c and Supplementary Figure S1a). Consistent with the differences in collagen deposition, there were much fewer α-SMA-positive myofibroblasts as well as downregulated level of active form of TGF-β1 in slie lung tissues when compared with wild-type controls upon bleomycin treatment (Figure 1d and Supplementary Figure S1b). Given that massive lung fibrosis usually leads to respiratory failure and eventual death, we measured the lifespan of mice under exposure to high dose of bleomycin (25 mg/kg). All wild-type animals died 21 days after bleomycin administration, whereas, five in six slie mice still survive until day 28 (see Supplementary Figure S2). The protective effect of DDR2 deficiency on lung injury was also observed in a fluorescein isothiocyanate (FITC)-driven model (see Supplementary Figure S3). In the following experiments, the bleomycin model is mostly used because it is relatively well characterized and mimics many of the features of human IPF patients.33
Figure 1.
Discoidin domain receptor 2 (DDR2) facilitates experimental lung fibrosis. (a–d) Ddr2 deficient mice were resistant to bleomycin-induced fibrosis in the lung. Wild-type or slie mutant mice were administered bleomycin and the lung tissues were harvested 4 weeks later. Representative images of H&E a and Masson's trichrome staining b are shown. Scale bars, 100 µm. The whole lung homogenates from three mice were used for hydroxyproline content assay and immunoblot analysis, respectively c. Immunofluorescent staining of myofibroblast marker α-smooth muscle actin (α-SMA) (red) was demonstrated in d. Right histogram shows the percentage of α-SMA staining area with regard to the total picture area (10 fields/group). **P < 0.01. (e–g) DDR2 knockdown prevents pulmonary fibrosis. One day before bleomycin challenge, C57BL/6 mice were instilled with small interfering RNA (siRNA) solutions and this treatment was repeated once a week until the lung tissues were collected on day 28 (e). The protein extracts from three mice were pooled and subjected to immunoblot (f). The images in g show H&E and Masson's trichrome staining, respectively. Scale bars, 100 µm. H&E, Hematoxylin and Eosin.
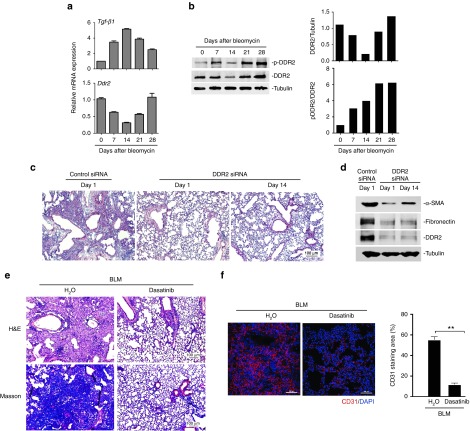
Next, we tested the effects of lung-specific downregulation of DDR2 expression through RNA interference approach on the fibrotic outcome. qPCR analysis indicated that the silencing effect of the intranasally applied DDR2 siRNA could last around 1 week (see Supplementary Figure S4). Thus, in the animal experiment, siRNA treatment was repeated once a week for a total of 4 weeks (Figure 1e). Compared with control (scramble) siRNA, specific siRNA against DDR2 that led to a prominent reduction of DDR2 protein expression prevented the bleomycin-induced abnormality of lung structure and accumulation of ECM proteins, such as fibronectin and collagens (Figure 1f,g). The above data collectively indicate that a deficiency or reduction in DDR2 expression attenuates lung fibrosis in animal models.
DDR2 controls lung myofibroblast activation induced by either TGF-β or collagen I
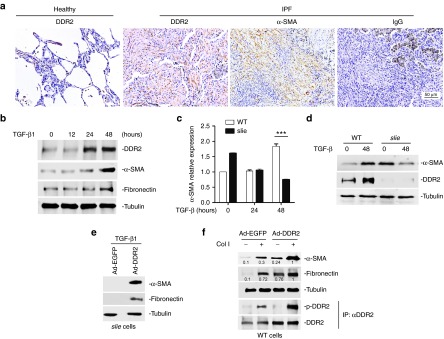
To establish the potential links of DDR2 with human fibrotic disease, we measured its expression in IPF patients by immunohistochemical staining. The protein level of DDR2 was much higher in IPF lungs than in healthy ones, with most of the DDR2 positive cells presenting a fibroblastic spindle shape and resembling those α-SMA-expressing myofibroblasts (Figure 2a). To provide in vitro evidence supporting this result, we analyzed DDR2 expression profile during the course of lung myofibroblast activation. The results demonstrated that in response to TGF-β1 stimulation, DDR2 protein level in primary mouse lung fibroblasts started to increase from the time point of 24 hours and then reached a high level at 48 hours, which preceded the upregulation of myofibroblastic genes α-SMA and fibronectin (Figure 2b).
Figure 2.
Discoidin domain receptor 2 (DDR2) controls lung myofibroblast activation. (a) DDR2 is highly expressed in human idiopathic pulmonary fibrosis (IPF) patients. Represent images of immunohistochemistry. (b) DDR2 expression is increased during the differentiation of lung myofibroblasts. Primary mouse lung fibroblasts were treated transforming growth factor (TGF)-β1 for various durations. (c–d) The absent expression of DDR2 impedes the TGF-β1-induced differentiation of lung fibroblasts. Primary lung fibroblasts from control or slie mice were stimulated with TGF-β1 and then subjected to quantitative polymerase chain reaction (qPCR) c and immunoblot analysis d, respectively. ***P < 0.001. (e–f) DDR2 enhances both TGF-β1 and collagen I-driven myofibroblast activation. Lung fibroblasts from slie mice were infected with either EGFP- or DDR2-expressing adenovirus for 24 hours and then stimulated with TGF-β1 for 48 hours e. Lung fibroblasts from wild-type mice were treated with recombinant adenovirus for 24 hours and then cultured on collagen I-coated surface for another 24 hours. For detection of phosphorylated DDR2, the cell lysates were immunoprecipitated with anti-DDR2 and then subjected to immunoblot with antiphosphotyrosine f.
To explore whether DDR2 is directly involved in the acquisition of myofibroblastic characteristics, mouse lung fibroblasts from wild-type or slie mice were incubated with TGF-β1 for various durations and the mRNA and protein expression of α-SMA was measured by qPCR and immunoblot, respectively. Although the basal level of α-SMA was higher in slie cells than in wild-type ones, the TGF-β1-induced α-SMA expression was markedly hampered by the complete loss of DDR2 expression (Figure 2c,d and Supplementary Figure S5). The inability of TGF-β1 to drive the expression of myofibroblast markers in slie cells could be rescued by the introduction of DDR2-expressing adenovirus (Figure 2e). These data collectively indicate a dependence of TGF-β-elicited myofibroblast differentiation on DDR2 expression.
As collagen-induced activation of DDR2 can regulate multiple cell behaviors, we were wondering whether this signaling cascade similarly had the potential to modulate lung myofibroblast formation. As expected, the addition of collagen I caused increase in the tyrosine phosphorylation of DDR2 in mouse lung fibroblasts, which was more prominent in DDR2-transduced cells than in green fluorescent protein (EGFP) control cells. Coinciding with this phenomenon, the highest expression densities of α-SMA and fibronectin were also observed in collagen I-treated DDR2-overexpressing cells (Figure 2f). Overall, it appears that DDR2 participates in both TGF-β- and collagen I-triggered lung fibroblast conversion into myofibroblasts.
The protein kinases involved in DDR2 control of lung myofibroblast differentiation
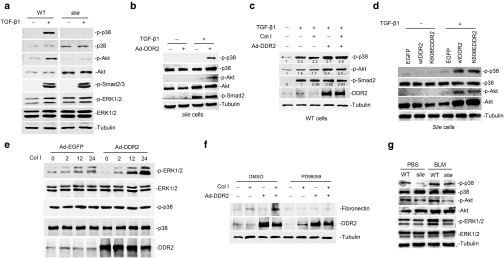
We then tried to explore the molecular mechanisms underlying DDR2 regulation of lung myofibroblast biology. To this end, the phosphorylation levels of major downstream mediators of TGF-β were initially analyzed. Compared with wild-type lung fibroblasts, DDR2-null cells were almost completely resistant to TGF-β1-induced activation of p38 and Akt. In sharp contrast, under TGF-β1 treatment, the two cells did not show discernable difference in the phosphorylation levels of ERK1/2 and Smad2/3 (Figure 3a). The unresponsiveness of slie cells to TGF-β1 induction of p38 and Akt kinase activities could be restored by the re-expression of DDR2 (Figure 3b). We further examined the effects of DDR2 activity on TGF-β1 signaling. In wild-type lung fibroblasts, the capabilities of TGF-β1 to activate p38 and Akt were not affected by collagen I treatment (Figure 3c, lane 3), but enhanced by the transduction of DDR2-expressing adenovirus (Figure 3c, lane 4). This promoting action of DDR2 failed to be amplified by simultaneous addition of collagen I (Figure 3c, lane 5). To provide additional evidence supporting this result, slie lung fibroblasts were infected with lentivirus expressing either wild type DDR2 or the kinase-dead mutant form of DDR2 (K608E),34 and the transduced cells were then incubated with TGF-β1 in the presence of collagen I. We found that K608EDDR2, although resistant to collagen I-induced tyrosine phosphorylation (see Supplementary Figure S6), displayed an equal ability with the wild-type protein in synergizing with TGF-β1 to drive the activation of p38 and Akt (Figure 3d). These data imply that the influence of DDR2 on TGF-β pathway might be independent of its activation conferred by ligand binding.
Figure 3.
The protein kinases involved in mediating discoidin domain receptor 2 (DDR2) control of lung myofibroblast activation. (a) DDR2 deficiency in lung fibroblasts impairs the transforming growth factor (TGF)-β1-induced activation of p38 and Akt. Wild-type or slie lung fibroblasts were treated with TGF-β1 for 30 minutes and the cell lysates were then subjected to immunoblot analysis of the indicated proteins. (b,c) The influence of DDR2 overexpression and collagen treatment on TGF-β1 signaling. The indicated lung fibroblasts were infected with recombinant adenovirus for 24 hours and then were treated with either TGF-β1 or the combination of TGF-β1 and collagen I. For the combination treatment, the cells were firstly incubated with or without 10 μg/ml collagen solutions and then stimulated with TGF-β1 for another 30 minutes. (d) DDR2 affects TGF-β1 pathway independent of its activation status. Slie lung fibroblasts were infected lentivirus expressing either wild-type DDR2 or a kinase dead mutant form of DDR2 (K608EDDR2). Fortyeight hours after virus infection, the cells were treated with collagen I for 2 hours and then stimulated with TGF-β1 for another 30 minutes. (e) DDR2 strengthens the collagen I-triggered activation of ERK1/2. Mouse lung fibroblasts expressing EGFP or DDR2 were treated with collagen I (10 μg/ml) for various durations. (f) ERK1/2 cascade is responsible for the collagen I-induced lung myofibroblast activation. Twenty four hours after adenovirus infection, mouse lung fibroblasts were cultured on collagen-coated surface for another 24 hours in the presence of dimethyl sulfoxide or 20 μmol/l PD98059. (g) The kinase activities in bleomycin-injured mouse lung. ERK, extracellular signal-regulated kinase; EGFP, green fluorescent protein.
Our previous studies have established that the activation of DDR2 signaling in response to ligand stimulation could elicit cellular effects through ERK1/2 pathway in osteoblasts and synoviocytes.34,35 Here, we found that in mouse lung fibroblasts, collagen I treatment also generated a gradual and persistent increase in the phosphorylation of ERK1/2. This effect could be strengthened by the ectopic expression of DDR2. Nevertheless, the activity of p38 appeared to be unresponsive to either collagen treatment or DDR2 overexpression (Figure 3e). This finding promoted us to determine whether the activities of ERK1/2 contributed to collagen I/DDR2-induced myofibroblast features. It was found that an inhibitor of ERK1/2 pathway PD98059 totally blocked collagen I induction of fibronectin with or without exogenous expression of DDR2 (Figure 3f).
Evaluation of the pathological significance of the above molecular links indicated that the lungs from bleomycin-damaged slie mice had markedly lower phosphorylation levels of p38, Akt, and ERK1/2 than those from wild-type group (Figure 3g). Altogether, these in vitro and in vivo data overwhelmingly suggest that DDR2 regulation of myofibroblast program during lung fibrosis might be achieved through synergizing the TGF-β-induced activation of p38 and Akt as well as the collagen I-induced activity of ERK1/2.
DDR2 modulates the angiogenic process during pulmonary fibrosis
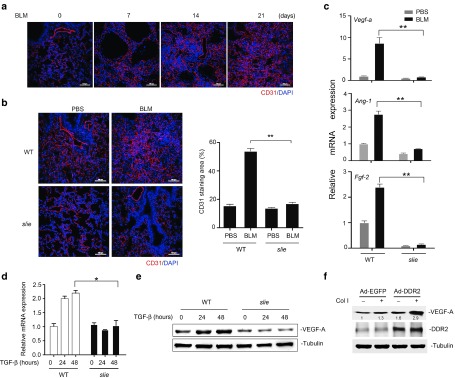
The contribution of neovascularization to the progression of lung fibrosis, although controversial in human studies,36 has been highlighted by growing animal evidences.37,38,39 In light of our recent identification of the critical role of DDR2 in the angiogenic activity of vascular endothelial cells as well as in tumor angiogenesis,22 we were attracted to test whether DDR2 had any effects on the vascular remodeling in the bleomycin-induced lung fibrosis model. Endothelial marker CD31 staining indicated that bleomycin treatment induced gradual increases in the lung microvessel densities (Figure 4a). At day 21 postbleomycin, DDR2 mutant mice displayed much less numbers of lung microvessels than their corresponding controls (Figure 4b). In addition, the lung tissues from slie mice had downregulated mRNA expression of major proangiogenic genes, with the greatest difference being observed for Vegf-a whose protein product is thought to be the most potent angiogenic factor (Figure 4c).
Figure 4.
Discoidin domain receptor 2 (DDR2) promotes lung fibrosis-related angiogenesis in vivo and myofibroblast expression of vascular endothelial growth factor (VEGF) in vitro. (a) C57BL/6 mice were challenged with bleomycin for various durations and the lung tissues were subjected to immunofluorescent staining of endothelial marker CD31. Scale bars, 100 µm. (b,c) DDR2 deficiency inhibits the bleomycin-induced vascular remodeling in the lung. Wild-type or slie mutant mice were treated with bleomycin for 21 days and the lung tissues were immunofluorescent stained with antibody recognizing mouse CD31. Right histogram shows the percentage of CD31 staining area with regard to the total picture area (10 fields/group). **P < 0.01 (b). The lung tissues were subjected to quantitative polymerase chain reaction (qPCR) analysis of the mRNA expression of the indicated proangiogenic cytokines. **P < 0.001 (c). (d,e) DDR2 is required for transforming growth factor (TGF) -β1 induction of VEGF-A in lung fibroblasts. Primary lung fibroblasts from control or slie mice were stimulated with TGF-β and the relative expression level of VEGF-A was determined by qPCR (d) and western blot (e), respectively. *P < 0.05. (f) DDR2 up-regulates the collagen I-induced VEGF-A expression. The green fluorescent protein (EGFP)- or DDR2-overexpressing mouse lung fibroblasts were treated with collagen I for 24 hours. The protein levels of VEGF-A and DDR2 were analyzed by immunoblot.
Because VEGF-A has been documented to be released by TGF-β-treated lung fibroblasts,40 we then performed in vitro experiments to study the possibility of DDR2 modulation of its expression in this type of cells. The results showed that the TGF-β1 induction of Vegf-a transcription was observed only in wild-type but not in DDR2-deficient mouse lung fibroblasts (Figure 4d,e), demonstrating an indispensible role of DDR2 in TGF-β1-induced expression of VEGF-A. Unlike TGF-β1, collagen I was not an effective inducer for VEGF-A expression in normal lung fibroblasts (Figure 4f, lane 2). However, once exogenous DDR2 was introduced into the cells, the ability of collagen I to induce VEGF-A expression was dramatically enhanced (Figure 4f, lane 4). Combining these results we can conclude that DDR2 might favor the angiogenic sprouting of blood vessels in the fibrotic lung tissues partially through regulation of myofibroblast expression of VEGF.
DDR2-targeting strategies demonstrate therapeutic efficacy against lung fibrosis
It is generally considered that the bleomycin-provoked pathological progression in the lung at least includes three phases: epithelial injury, inflammatory infiltration, and tissue remodeling. We first profiled the expression and activation changes of DDR2 after bleomycin challenge. It was demonstrated that the highest transcription of TGF-β1 occurred at day 14 (Figure 5a, upper panel), implying maximal lung damage at this time point. In contrast, upon bleomycin exposure both the mRNA and protein expression of DDR2 started to decrease and reached its lowest level at day 14, followed by expression recovery thereafter (Figure 5a, bottom panel and Figure 5b). Unlike the profile of total DDR2 protein, DDR2 activation measured as the ratio of phosphorylated form of DDR2 to total DDR2 exhibited a trend of gradual increase (Figure 5b). These results suggest that during the process of experimental lung fibrosis DDR2 might serve a primary function at the stage of tissue remodeling which started postday 14. To confirm this speculation, we evaluated the consequence of delayed intervention of DDR2 against lung fibrosis. The beneficial effects of administering DDR2 siRNA from day 14, although slightly reduced, were largely preserved, when compared with treatment schedule beginning from the onset of injury (day 1) (Figure 5c,d).
Figure 5.
Discoidin domain receptor 2 (DDR2) -targeting strategies have therapeutic efficacy against lung fibrosis. (a,b) The expression and activation pattern of DDR2 during bleomycin-induced lung fibrosis. Lung tissues from bleomycin-treated C57BL/6 mice were collected at various time points for analysis of the mRNA (a) and protein (b) expression of the indicated genes. The histograms in b depict results quantified using densitometry scanning. (c,d) Late treatment with DDR2 small interfering RNA (siRNA) still potently inhibits the development of lung fibrosis. 1 day after bleomycin instillation, C57BL/6 mice were treated with siRNA immediately or from 14 days later. At day 28, the mouse lungs were subjected to Hematoxylin and Eosin (H&E) staining c and immunoblot analysis d. (e,f) DDR2 kinase inhibitor dasatinib attenuates pulmonary fibrosis. Fourteen days after bleomycin treatment, the C57BL/6 mice received daily oral gavage of dasatinib. Two weeks later, the mouse lung tissues were collected for analysis of the lung structure e and vascular density f. The histogram in f represents the percentage of CD31 staining. **P < 0.001.
Because DDR2 can modulate the activation of lung myofibroblasts in an activation-dependent manner, we tried to assess whether lung fibrosis-related events could be prevented by pharmacological inhibitors of DDR2 kinase. For this aim, dasatinib that is an FDA-approved drug as well as a potent DDR2 inhibitor,25 was exploited in our animal experiments. Fourteen days after bleomycin challenge, the mice were subjected to daily administration of dasatinib for 2 weeks. As demonstrated in Figure 5e, dasatinib yielded strong inhibitory effect on the development of lung fibrosis. In addition, this drug also sharply suppressed the bleomycin-induced increases in angiogenesis (Figure 5f). These results collectively indicate that even when applied after maximal lung injury has been established, DDR2-targeting strategies were still highly effective in preventing fibrotic tissue formation.
Targeting of DDR2 limits the progression of established lung fibrosis
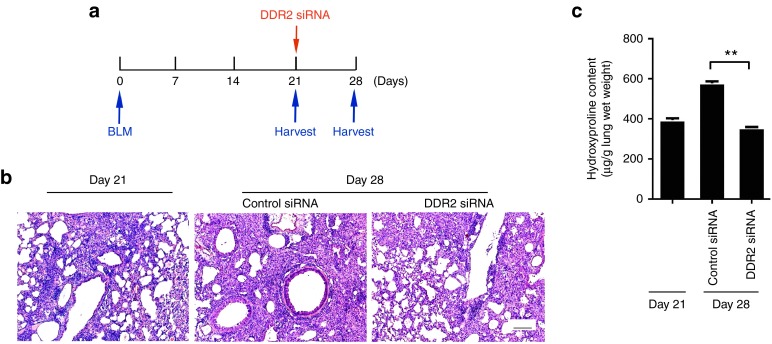
The important role of DDR2 in mediating collagen I-induced myofibroblast activation signifies that targeting DDR2 may have the potential to restrain the deterioration of established scar through interrupting the fibrillar collagen-driven positive feedback events. In order to examine this possibility in vivo, the fibrotic status of animals harvested at day 21 after bleomycin, a time point with some extent of fibrosis, was compared with that of those receiving inhibition of DDR2 from day 21 onwards until day 28 (Figure 6a). It was shown that the administration of DDR2 siRNA resulted in almost no further destruction of lung structure and accumulation of collagen matrix (Figure 6b,c), indicating that DDR2 blockade limits the progression of established fibrosis.
Figure 6.
Discoidin domain receptor 2 (DDR2) knockdown limits the progression of established lung fibrosis. (a) Schematic of late treatment with DDR2 small interfering RNA (siRNA). (b, c) 21 days after bleomycin challenge, the mice were either sacrificed or continuously received treatment with siRNA. On day 28, the mouse lung tissues were subjected to histological analysis b and hydroxyproline content assay c, respectively. Data are representative of three independent experiments. **P < 0.01.
Discussion
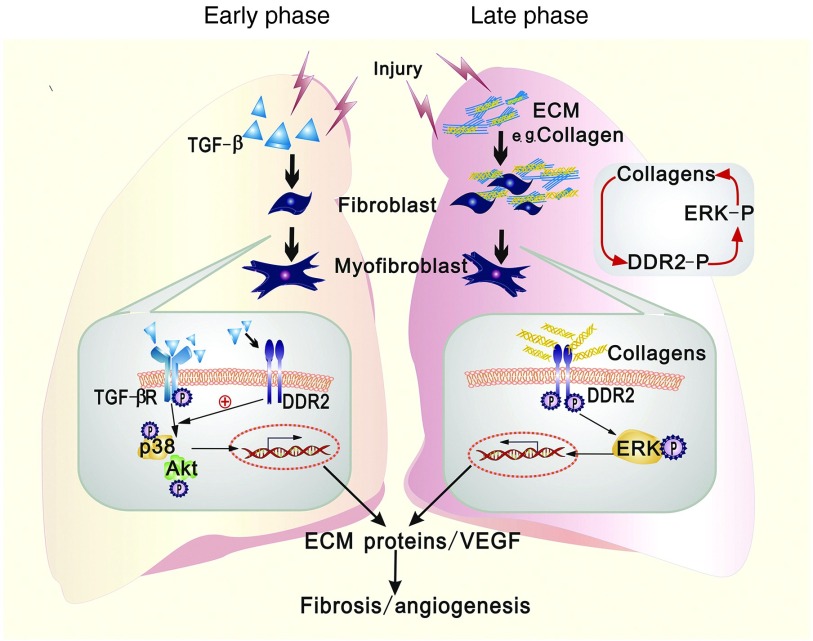
Based on the data from this study, we arrive at the following hypotheses regarding the role of DDR2 in lung fibrillogenesis (Figure 7)1. In the early phase of fibrotic reaction, the induced expression of DDR2 in lung fibroblasts by TGF-β can synergize with TGF-β-initiated signals to specifically trigger the activation of p38 and Akt in an activation-independent manner, which accelerates the cell phenotypic transition toward myofibroblasts.2 When the fibrotic process is already established, the abundant fibrillar collagens within fibrotic foci may gradually augment the activation of myofibroblasts via DDR2/ERK axis in a positive feedback manner.3 In both phases, DDR2 promotes the oversynthesis of ECM components as well as the secretion of VEGF, resulting in massive fibrosis and angiogenesis.
Figure 7.
Schematic depicting the role of discoidin domain receptor 2 (DDR2) in mediating the activation of myofibroblasts during lung fibrosis. In the early phase of fibrotic reaction, transforming growth factor (TGF)-β stimulation of lung fibroblasts induces up-regulated expression of DDR2, which may enable DDR2 to cooperate with TGF-β receptor to activate p38 and Akt, consequently leading to the cell phenotypic transition toward myofibroblasts. This action of DDR2 is independent of the presence of its ligands, fibrillar collagens (left). When the fibrotic process is already established, the abundant fibrillar collagens within fibrotic foci may gradually augment the activation of myofibroblasts via the activation of DDR2/ extracellular signal-regulated kinase (ERK) axis in a positive feedback manner (right). In both phases, DDR2 promotes the oversynthesis of extracellular matrix (ECM) components as well as the secretion of vascular endothelial growth factor (VEGF), which eventually causes massive fibrosis and angiogenesis.
The persistence of myofibroblasts in injured tissues can trigger a relentless scarring process and finally cause fibrosis. Although the in vivo correlation of DDR2 with myofibroblast abundance has been previously documented in other models,31,41 it remains poorly understood how DDR2 modulates the activation of this type of cells. A major finding of this study is that DDR2 expression deficiency hampers TGF-β1 induction of lung myofibroblast phenotypes. However, an intriguing phenomenon is that under resting conditions, DDR2-null lung fibroblasts express a higher level of α-SMA than wild-type ones, which was not observed in DDR2 mutant mice. In the light of the recent report that DDR2 silencing retards fibroblast migration,42 we infer that the upregulation of basal α-SMA expression caused by DDR2 deficiency may reflect a cellular mechanism to compensate for the complete loss of key migration molecules in vitro.
Although the unresponsiveness of Smad activity to DDR2 has been documented in some types of cells,17,43 its impact on non-Smad pathways under TGF-β stimulation has not been explored. We revealed that DDR2 could specifically strengthen the TGF-β-induced activation of p38 and Akt in lung fibroblasts. Because the cascades of both p38 and Akt were well established as key intermediates of lung fibroblast–myofibroblast differentiation as well of experimental lung fibrosis,44,45,46,47,48 our results suggest that DDR2 downregulation might not only suppress TGF-β-induced initiation of fibrotic reaction in the lung but also theoretically avoid undesirable potential side effects caused by pan-TGF-β blockade (autoimmunity and carcinogenesis). In addition, our disclosure of their crosstalk will also shed light on investigation of DDR2 function under other physiological or pathological situations where TGF-β plays a central role. Notably, the promoting effect of DDR2 on TGF-β signaling was shown to have nothing to do with the activation status of DDR2. This complies with the fact that DDR2 constitutively interacts with its coreceptor in other type of cells.49 Our ongoing study is to find the molecules that are responsible for determining the selectivity of DDR2 on TGF-β non-Smad pathway.
It was recently shown that the IPF patient-derived ECM could stimulate lung fibroblasts to express genes encoding ECM proteins detected in IPF tissue,9 strongly supporting the concept that the abnormal ECM components accumulated within fibrotic lung might act as positive-feedback stimuli for the development of lung fibrosis.50 Compared with normal lung matrices, IPF lung was enriched in several types of collagens,10 with at least collagen types I and III being reported to activate DDR2.12 Our identification of the inducible effects of collagen I/DDR2 cascades on lung myofibroblast formation implies that DDR2 might be an attractive molecular target for the interruption of the fibrillar collagen-induced positive feedback loop events. This conjecture can be partially supported by our results that DDR2 knockdown from day 21, a time point with obvious established scar, can almost completely inhibit the further disorder of lung structure. Even collagen I was reported to induce DDR1 expression in human lung fibroblasts, this process was demonstrated to rely on the activation of DDR2 signaling.51
In addition to through direct regulation of cell differentiation, DDR2 may raise the abundance of myofibroblast subsets through advancing the recruitment of its precursor cells. One such evidence is that DDR2 can regulate lung fibroblast proliferation and migration.32 Another possibility rests on the established concepts that alveolar epithelial cells can serve as an important source of lung fibroblasts via EMT,52,53 and that DDR2 is a critical regulator of EMT.15,16,54 A most recent study reported that DDR2 can regulate neutrophil chemotaxis.55 Given neutrophil's well-characterized contribution to IPF,56 we do not exclude the possibility that such a regulatory link may also exist in the bleomycin-treated model, although we did not investigate this point. However, even with this possibility, our results showing that starting DDR2 siRNA treatment from day 14, a time point of maximal injury as well as the onset of tissue remodeling, had similar protective effect as prevention schedule, give a clue that DDR2 control of postinflammation events might determine its influence on the final outcome of fibrosis.
We recently demonstrated that DDR2 promotes the angiogenic activity of endothelial cells in vitro.22 However, according to the in vivo data from our lab and others,21,24 DDR2-deficient stroma can also accelerate angiogenesis under some pathological conditions. We reasoned that the positive regulation of DDR2 on endothelial function may be overbalanced out by its negative modulation on surrounding cell secretion of proangiogenic cytokines, finally making DDR2 gives rise to an overall inhibitory outcome in angiogenesis.24 In this study, we found that DDR2 enhances both TGF-β1- and collagen I-driven expression of VEGF-A in lung fibroblasts. This in vitro evidence coincides with the in vivo observation that a genetic deficiency of Ddr2 impedes lung neovascularization in bleomycin-induced fibrosis model. Accumulating evidences indicate that antiangiogenesis therapy may be a promising treatment option for pulmonary fibrosis.38,39,57 Notably, nintedanib, a triple angiokinase inhibitor (VEGFR, platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor (FGFR)), recently became the first approved drug for IPF treatment.58 Therefore, our current data collectively indicate that DDR2 control of lung myofibroblast behaviors could produce impacts on both angiogenic and fibrotic processes.
Although dasatinib does not distinguish DDR2 from DDR1 whose knockout mice were similarly refractory to bleomycin-induced lung fibrosis,25,59 it is unlikely that the therapeutic effects of dasatinib treatment starting from 14 days after bleomycin in our model could equally benefit from its inhibition of the two members because DDR1 was considered to primarily regulate the function of epithelial cells and macrophages.59,60 The expression of DDR1 in lung fibroblasts, although positive, was reported to rely on DDR2 signaling.51 Whatever, multitarget effect is a common characteristic for most kinase inhibitors, which sometimes is conducive to enhancing their efficacy in inhibiting disease progression. For instance, dasatinib suppression of Src activity may additionally contribute to the action of this drug on TGF-β-induced myofibroblast events because Src kinase was reported to serve as a key mediator of TGF-β–p38 pathway.61,62 The results derived by the authors, together with the most recently published data,63 provide important preclinical evidence for the use of dasatinib in the treatment of IPF.
In summary, the data from this study strongly suggest that during pulmonary fibrosis, DDR2 not only participates in both the initiation and maintenance of fibrotic reaction, but also affects both ECM production and angiogenesis. These features make DDR2 may represent a versatile therapeutic target in this stubborn disease.
Materials and Methods
Animal experimental protocols. The heterozygous slie mutant mice, carrying deletion mutation spanning Ddr2 exon 1–17,19 were provided by Jackson Lab (Bar Harbor, Maine) and intercrossed to produce homozygotes for use. All animal studies were performed according to the protocols and guidelines of the institutional care and use committee. To induce pulmonary damage, 6- to 8-week-old sex- and age-matched wild-type or slie mice were intranasally dropped with bleomycin (Nippon Kayaku, Tokyo, Japan) at 5 mg/kg body weight (BW) or FITC (Sigma, St Louis, MO) at 6 mg/kg BW. For in vivo silencing of DDR2 in mouse lung, a nonspecific control siRNA or DDR2-specific siRNA was modified with 2′-methoxy (2′-OMe) by Genepharma Company (Shanghai, China) to improve stability. The sense sequences of siRNAs are as following: DDR2, 5′-TTGAGATGAATACTAGCTTAG-3′; control, 5′-TTCTCCGAACGTGTCACGTT-3′. The silencing effect of DDR2 siRNA has been validated by our previous in vitro study.35 One OD siRNA was dissolved in 40 μl D5W solution (5% D-Glucose) and used to treat one mouse per time through nasal instillation as described earlier.64 For each group, the siRNA treatment was conducted once a week before the harvest. Dasatinib (Sprycel, Bristol-Myers Squibb, New York, NY) was dissolved in 80 mmol/l citric acid (pH 2.1) to make a stock solution of 10 mg/ml. For animal use, dasatinib were given to the mice orally (10 mg/kg BW) by gavage once a day. At least five animals were used in each treatment group and three individual experiments were performed.
Isolation of mouse lung fibroblasts and treatment protocols. The lungs dissected from 4–5-week mice were cut into small pieces and then immersed in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing collagenase II (2 mg/ml), trypsin (2.5 mg/ml), Dnase I (2 mg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml) for 12 hours at 37 °C after phosphate-buffered saline wash. The clumps of tissues were removed through filtering and the cells were collected from the supernatant by centrifuge, followed by their maintenance in Dulbecco's modified Eagle's medium supplemented with heat-inactivated 10% fetal bovine serum (FBS). The phenotypes of fibroblasts after passage 3 were characterized by immunostaining of the epithelial marker pan-cytokeratin and the fibroblastic marker vimentin.
For functional studies, the cells were used between passages 4 and 6. To induce the cell differentiation, the cells were stimulated with 10 ng/ml TGF-β1 (PeproTech, Rocky Hill, NJ) or cultured on collagen I (Roche Diagnostics, Mannheim, Germany)-coated surface (8 µg/cm2) for 48 hours. Recombinant adenovirus expressing EGFP or full length DDR2 was packaged and purified by Vector Gene Technology Company (Beijing, China), and used to infect the cells with 10 viral particles/cell. For inhibition of ERK pathway, the cells were pretreated with 20 µmol/l PD98059 (Calbiochem, San Diego, CA) or dimethyl sulfoxide.
Histological analysis, immunohistochemistry, and immunofluorescence staining. Human healthy (20 subjects) and IPF (4 males and 2 females) lung tissue sections were obtained from Biomax (Rockville, MD). The study protocol was approved by the ethics committee of the Fourth Military Medical University. Paraffin sections of mouse lungs (4 μm) were prepared for H&E and Masson's trichrome staining or immunohistochemistry. Masson's trichrome staining was performed according to the manufacturer's instructions (Baso, Zhuhai, China).
For immunohistochemical detection of DDR2 and α-SMA, the sections were dewaxed and rehydrated through graded alcohol and then subject to antigen retrieval by high pressure cooking for 2 minutes in 10 mmol/l citrate acid (pH 6.0). The antibodies against human DDR2 (R&D Systems, Minneapolis, MN) and human α-SMA (Abcam, Cambridge, UK) were used at a dilution of 1: 100 and 1 : 200, respectively. Rabbit or mouse immunoglobulin G (IgG) was used as a negative control. Antibody binding was visualized by a horseradish peroxidase-conjugated secondary antibody system (Sigma-Aldrich, St. Louis, MO).
For immunofluorescence staining of CD31, the specific primary antibody (BD Biosciences, San Jose, CA) was used at 1: 50. The fluorescent signal was excited and captured by confocal laser-scanning microscopy (Nikon-A1 Tokyo, Japan). Quantification of the vascular density was done by analyzing at least five sections and five fields/section.
Lentivirus package. The cDNAs of wild-type human DDR2 or a kinase-dead mutant DDR2 (K608EDDR2) that have been described earlier34 were inserted into lentiviral pCDH vector (Provided by Professor Jian Zhang, The Fourth Military Medical University, Xi'an, China). For the package of virus, human embryo kidney 293T-cells from the American Type Culture Collection were transiently transfected with pCDH, PMD2.G, and PSPAX2 at a ratio of 4 : 1 : 3 with Lipofectamin2000 (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, the supernatant were collected and then 1 ml of them was used to infect lung fibroblasts seeded on six-well plate.
Determination of hydroxyproline content. A commercial hydroxyproline kit from Jiancheng Institute of Biotechnology (Nanjing, China) was used following the provider's instructions. Briefly, fresh lung tissues were weighted and hydrolyzed to release hydroxyproline. After a series of chemical reactions, a pink color solution was formed and then subjected to measurement of absorbance at 560 nm. The hydroxyproline content of each sample was calculated by comparing with the standards. Results were expressed as micrograms of hydroxyproline per gram wet lung weight.
Reverse transcription-qPCR. Total RNA was extracted using the RNeasy kit (QIAGEN Hilden, Germany). cDNA was synthesized using the Super-Script II First-Strand Synthesis System (Invitrogen). qPCR was performed using a Prism 7500 real-time thermocycler (Applied BioSystems San Diego, CA). The primer sequences for mouse Ddr2, Gapdh, Vegf-a, Ang-1, and Fgf-2 have been described earlier.22,35 Mouse α-SMA: GACGCTGAAGTATCCGATAGAACACG (forward); CACCATCTCCAGAGTCCAGCACAAT (reverse); mouse Tgf-β1: AGCGGACTACTATGCTAAAGAGGTCACCC (forward); CCAAGGTAACGCCAGGAATTGTTGCTATA (reverse); mouse col1α1: CATGTTCAGCTTTGTGGACC (forward); TTCTGTACGCAGGTGATTGG (reverse). qPCR data indicate the relative mRNA expression level of target gene. Gapdh was used as an internal reference control. Bar graphs are the mean ± SD of three separate experiments.
Immunoblot analysis. Cultured cells or mouse lung tissues were harvested and lysed in Radio-Immunoprecipitation Assay (RIPA) buffer (0.05 mol/l Tris–HCl pH 7.4, 0.15 mol/l NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mmol/l ethylenediamine tetraacetic acid (EDTA), 1 mmol/l phenylmethylsulfonyl fuoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin and Phospho-STOP). Protein concentration was determined by BCA protein assay kit (Pierce Biotech, Rockford, IL) and equal amounts of protein were resolved by 10% sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes (Amersham Biosciences, Buckinghamshire, UK). After blocking in Tris-buffered saline containing 5% skim milk and 0.1% Tween-20, membranes were incubated with primary antibodies, followed by incubation with horseradish peroxidase (HRP)-labeled secondary antibodies. The dilution of primary antibodies for immunoblot was as follows: DDR2 (1 : 1000, R&D); β-actin (1 : 500, Booster); Tublin (1 : 1000, Booster); α-SMA (1 : 500, abcam), phosphotyrosine specific monoclonal antibody 4G10 (1 : 1000, Millipore).
Statistical analysis. Statistical analyses were performed using the Statistical Product and Service Solutions (SPSS) software program. One-way analyses of variance (ANOVAs) followed by least significant difference (LSD)-t tests were used to make comparisons between pairs of groups. Student's t-test was used to examine the differences between the two groups of data. A two-tailed P-value of <0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. The expression levels of fibrotic markers in the lung of DDR2 mutant mice. Figure S2. DDR2 deficiency confers the mice survival advantage in response to high dose of bleomycin. Figure S3. DDR2 mutant mice are protected from FITC-driven lung fibrosis. Figure S4. The duration of in vivo knockdown of DDR2. Figure S5. DDR2-deficient lung fibroblasts are refractory to TGF-β1-induced expression of α-SMA. Figure S6. DDR2 kinase-dead mutant is resistant to collagen-induced activation.
Acknowledgments
This work was supported by the Chinese National Key Basic Research and Development Program (2010CB529705), grants from National Natural Science Foundation of China (81100044) and the Natural Science Foundation of Ningxia (NZ14056). The authors have no conflicts of interest to disclose. The authors greatly thank Wei Zhang (The Fourth Military Medical University, Xi'an, China) for providing us the DDR2-expressing adenovirus stock and Qingguo Yan (The Fourth Military Medical University, Xi'an, China) for technical assistance of immunohistochemistry analysis. H.Z., H.B., X.B., and S.Z. contributed equally to this article.
Supplementary Material
References
- Raghu, G, Collard, HR, Egan, JJ, Martinez, FJ, Behr, J, Brown, KK et al.; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. (2011). An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard, HR and King, TE Jr (2001). Treatment of idiopathic pulmonary fibrosis: the rise and fall of corticosteroids. Am J Med 110: 326–328. [DOI] [PubMed] [Google Scholar]
- Rafii, R, Juarez, MM, Albertson, TE and Chan, AL (2013). A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis 5: 48–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil, K (2015). Pharmaceutical approval update. P T 40: 33–35. [PMC free article] [PubMed] [Google Scholar]
- Wolters, PJ, Collard, HR and Jones, KD (2014). Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B, Phan, SH, Thannickal, VJ, Prunotto, M, Desmoulière, A, Varga, J et al. (2012). Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, AM, Shen, Z, Ritzenthaler, JD and Roman, J (2006). Myofibroblast transdifferentiation in obliterative bronchiolitis: tgf-beta signaling through smad3-dependent and -independent pathways. Am J Transplant 6: 2080–2088. [DOI] [PubMed] [Google Scholar]
- Shimbori, C, Gauldie, J and Kolb, M (2013). Extracellular matrix microenvironment contributes actively to pulmonary fibrosis. Curr Opin Pulm Med 19: 446–452. [DOI] [PubMed] [Google Scholar]
- Parker, MW, Rossi, D, Peterson, M, Smith, K, Sikström, K, White, ES et al. (2014). Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, AJ, Hadley, R, Cornett, AM, Dreffs, AA, Matthes, SA, Tsui, JL et al. (2012). Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186: 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiathan, RR, Marco, M, Leitinger, B, Kleer, CG and Fridman, R (2012). Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 31: 295–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, W, Gish, GD, Alves, F and Pawson, T (1997). The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1: 13–23. [DOI] [PubMed] [Google Scholar]
- Leitinger, B and Kwan, AP (2006). The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol 25: 355–364. [DOI] [PubMed] [Google Scholar]
- Goldsmith, EC, Hoffman, A, Morales, MO, Potts, JD, Price, RL, McFadden, A et al. (2004). Organization of fibroblasts in the heart. Dev Dyn 230: 787–794. [DOI] [PubMed] [Google Scholar]
- Maeyama, M, Koga, H, Selvendiran, K, Yanagimoto, C, Hanada, S, Taniguchi, E et al. (2008). Switching in discoid domain receptor expressions in SLUG-induced epithelial-mesenchymal transition. Cancer 113: 2823–2831. [DOI] [PubMed] [Google Scholar]
- Walsh, LA, Nawshad, A and Medici, D (2011). Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix Biol 30: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K, Corsa, CA, Ponik, SM, Prior, JL, Piwnica-Worms, D, Eliceiri, KW et al. (2013). The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol 15: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador, JP, Azcoitia, V, Tuckermann, J, Lin, C, Olaso, E, Mañes, S et al. (2001). The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep 2: 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano, K, Marín de Evsikova, C, Young, J, Wnek, C, Maddatu, TP, Nishina, PM et al. (2008). A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol Endocrinol 22: 1866–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargal, R, Cormier-Daire, V, Ben-Neriah, Z, Le Merrer, M, Sosna, J, Melki, J et al. (2009). Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am J Hum Genet 84: 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiola, I, Olaso, E, Crende, O, Friedman, SL and Vidal-Vanaclocha, F (2012). Discoidin domain receptor 2 deficiency predisposes hepatic tissue to colon carcinoma metastasis. Gut 61: 1465–1472. [DOI] [PubMed] [Google Scholar]
- Zhang, S, Bu, X, Zhao, H, Yu, J, Wang, Y, Li, D et al. (2014). A host deficiency of discoidin domain receptor 2 (DDR2) inhibits both tumour angiogenesis and metastasis. J Pathol 232: 436–448. [DOI] [PubMed] [Google Scholar]
- Xu, L, Servais, J, Polur, I, Kim, D, Lee, PL, Chung, K et al. (2010). Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum 62: 2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, T, Zhu, J, Bu, X, Zhao, H, Zhang, S, Chang, Y et al. (2015). The anti-angiogenic role of discoidin domain receptor 2 (DDR2) in laser-induced choroidal neovascularization. J Mol Med (Berl) 93: 187–198. [DOI] [PubMed] [Google Scholar]
- Day, E, Waters, B, Spiegel, K, Alnadaf, T, Manley, PW, Buchdunger, E et al. (2008). Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol 599: 44–53. [DOI] [PubMed] [Google Scholar]
- Sun, X, Phan, TN, Jung, SH, Kim, SY, Cho, JU, Lee, H et al. (2012). LCB 03-0110, a novel pan-discoidin domain receptor/c-Src family tyrosine kinase inhibitor, suppresses scar formation by inhibiting fibroblast and macrophage activation. J Pharmacol Exp Ther 340: 510–519. [DOI] [PubMed] [Google Scholar]
- Colzani, M, Noberini, R, Romanenghi, M, Colella, G, Pasi, M, Fancelli, D et al. (2014). Quantitative chemical proteomics identifies novel targets of the anti-cancer multi-kinase inhibitor E-3810. Mol Cell Proteomics 13: 1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, M, Duan, L, Luo, J, Zhang, L, Lu, X, Zhang, Y et al. (2013). Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem 56: 3281–3295. [DOI] [PubMed] [Google Scholar]
- Hammerman, PS, Sos, ML, Ramos, AH, Xu, C, Dutt, A, Zhou, W et al. (2011). Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov 1: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitini, V, Arrigo, C, Di Mirto, C, Mondello, P and Altavilla, G (2013). Response to dasatinib in a patient with SQCC of the lung harboring a discoid-receptor-2 and synchronous chronic myelogenous leukemia. Lung Cancer 82: 171–172. [DOI] [PubMed] [Google Scholar]
- Olaso, E, Arteta, B, Benedicto, A, Crende, O and Friedman, SL (2011). Loss of discoidin domain receptor 2 promotes hepatic fibrosis after chronic carbon tetrachloride through altered paracrine interactions between hepatic stellate cells and liver-associated macrophages. Am J Pathol 179: 2894–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, PA and Jarai, G (2012). Discoidin domain receptors regulate the migration of primary human lung fibroblasts through collagen matrices. Fibrogenesis Tissue Repair 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, BB and Hogaboam, CM (2008). Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L152–L160. [DOI] [PubMed] [Google Scholar]
- Su, J, Yu, J, Ren, T, Zhang, W, Zhang, Y, Liu, X et al. (2009). Discoidin domain receptor 2 is associated with the increased expression of matrix metalloproteinase-13 in synovial fibroblasts of rheumatoid arthritis. Mol Cell Biochem 330: 141–152. [DOI] [PubMed] [Google Scholar]
- Zhang, Y, Su, J, Yu, J, Bu, X, Ren, T, Liu, X et al. (2011). An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation. J Bone Miner Res 26: 604–617. [DOI] [PubMed] [Google Scholar]
- Hanumegowda, C, Farkas, L and Kolb, M (2012). Angiogenesis in pulmonary fibrosis: too much or not enough? Chest 142: 200–207. [DOI] [PubMed] [Google Scholar]
- Burdick, MD, Murray, LA, Keane, MP, Xue, YY, Zisman, DA, Belperio, JA et al. (2005). CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med 171: 261–268. [DOI] [PubMed] [Google Scholar]
- Wan, YY, Tian, GY, Guo, HS, Kang, YM, Yao, ZH, Li, XL et al. (2013). Endostatin, an angiogenesis inhibitor, ameliorates bleomycin-induced pulmonary fibrosis in rats. Respir Res 14: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, XM, Li, WC, Liu, DS, Li, YP, Wen, FQ, Feng, YL et al. (2009). VEGFR-2 antagonist SU5416 attenuates bleomycin-induced pulmonary fibrosis in mice. Int Immunopharmacol 9: 70–79. [DOI] [PubMed] [Google Scholar]
- Tabata, C, Tabata, R, Kadokawa, Y, Hisamori, S, Takahashi, M, Mishima, M et al. (2007). Thalidomide prevents bleomycin-induced pulmonary fibrosis in mice. J Immunol 179: 708–714. [DOI] [PubMed] [Google Scholar]
- Olaso, E, Lin, HC, Wang, LH and Friedman, SL (2011). Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Herrera, ML and Quezada-Calvillo, R (2012). DDR2 plays a role in fibroblast migration independent of adhesion ligand and collagen activated DDR2 tyrosine kinase. Biochem Biophys Res Commun 429: 39–44. [DOI] [PubMed] [Google Scholar]
- Makino, K, Jinnin, M, Aoi, J, Hirano, A, Kajihara, I, Makino, T et al. (2013). Discoidin domain receptor 2-microRNA 196a-mediated negative feedback against excess type I collagen expression is impaired in scleroderma dermal fibroblasts. J Invest Dermatol 133: 110–119. [DOI] [PubMed] [Google Scholar]
- Hu, Y, Peng, J, Feng, D, Chu, L, Li, X, Jin, Z et al. (2006). Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-beta1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung 184: 33–42. [DOI] [PubMed] [Google Scholar]
- Matsuoka, H, Arai, T, Mori, M, Goya, S, Kida, H, Morishita, H et al. (2002). A p38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 283: L103–L112. [DOI] [PubMed] [Google Scholar]
- Moran, N (2011). p38 kinase inhibitor approved for idiopathic pulmonary fibrosis. Nat Biotechnol 29: 301. [DOI] [PubMed] [Google Scholar]
- Conte, E, Fruciano, M, Fagone, E, Gili, E, Caraci, F, Iemmolo, M et al. (2011). Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One 6: e24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X, Han, J, Chen, ZZ, Qi, BW, Wang, GC, Ma, YH et al. (2010). A phosphoinositide 3-kinase-gamma inhibitor, AS605240 prevents bleomycin-induced pulmonary fibrosis in rats. Biochem Biophys Res Commun 397: 311–317. [DOI] [PubMed] [Google Scholar]
- Ishigame, H, Zenewicz, LA, Sanjabi, S, Licona-Limón, P, Nakayama, M, Leonard, WJ et al. (2013). Excessive Th1 responses due to the absence of TGF-β signaling cause autoimmune diabetes and dysregulated Treg cell homeostasis. Proc Natl Acad Sci USA 110: 6961–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauboer, ME, Boeijen, FR, Emson, CL, Turner, SM, Zandieh-Doulabi, B, Hanemaaijer, R et al. (2014). Extracellular matrix proteins: a positive feedback loop in lung fibrosis? Matrix Biol 34: 170–178. [DOI] [PubMed] [Google Scholar]
- Ruiz, PA and Jarai, G (2011). Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J Biol Chem 286: 12912–12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby, LM and Waters, CM (2010). Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, BC, Liebler, JM, Luby-Phelps, K, Nicholson, AG, Crandall, ED, du Bois, RM et al. (2005). Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg, M and Neilson, EG (2009). Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119: 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota, SA, Ng, J, Lueng, A, Khajah, M, Parhar, K, Li, Y et al. (2011). NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 17: 1359–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi, Y, Yamadori, I, Fujita, J, Yoshinouchi, T, Ueda, N and Táchira, J (1997). The role of neutrophils in the pathogenesis of idiopathic pulmonary fibrosis. Chest 112: 1338–1343. [DOI] [PubMed] [Google Scholar]
- Wang, X, Zhu, H, Yang, X, Bi, Y and Cui, S (2010). Vasohibin attenuates bleomycin induced pulmonary fibrosis via inhibition of angiogenesis in mice. Pathology 42: 457–462. [DOI] [PubMed] [Google Scholar]
- McCormack, PL (2015). Nintedanib: first global approval. Drugs 75: 129–139. [DOI] [PubMed] [Google Scholar]
- Avivi-Green, C, Singal, M and Vogel, WF (2006). Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 174: 420–427. [DOI] [PubMed] [Google Scholar]
- Matsuyama, W, Wang, L, Farrar, WL, Faure, M and Yoshimura, T (2004). Activation of discoidin domain receptor 1 isoform b with collagen up-regulates chemokine production in human macrophages: role of p38 mitogen-activated protein kinase and NF-kappa B. J Immunol 172: 2332–2340. [DOI] [PubMed] [Google Scholar]
- Nam, S, Kim, D, Cheng, JQ, Zhang, S, Lee, JH, Buettner, R et al. (2005). Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res 65: 9185–9189. [DOI] [PubMed] [Google Scholar]
- Galliher, AJ and Schiemann, WP (2007). Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res 67: 3752–3758. [DOI] [PubMed] [Google Scholar]
- Yilmaz, O, Oztay, F and Kayalar, O (2015). Dasatinib attenuated bleomycin-induced pulmonary fibrosis in mice. Growth Factors 33: 366–375. [DOI] [PubMed] [Google Scholar]
- Bitko, V, Musiyenko, A, Shulyayeva, O and Barik, S (2005). Inhibition of respiratory viruses by nasally administered siRNA. Nat Med 11: 50–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.