Abstract
Mechanical stress plays a key role in the development of cartilage degradation in osteoarthritis (OA). Nevertheless, the role of long noncoding RNAs in mechanical stress-induced regulation of chondrocytes remains unclear. The aim of this study was to explore the function of mechanical stress-related long noncoding RNAs in cartilage. Tissue samples were collected from 50 patients and chondrocytes were exposed to cyclic tensile strain (CTS). A total of 107 lncRNAs were differentially expressed in damaged cartilage versus intact cartilage. Of these lncRNAs, 51 were upregulated and 56 were downregulated in the damaged tissue. The TMSB4 pseudogene, lncRNA-MSR, was upregulated in the damaged cartilage and was activated in chondrocytes in response to mechanical stress. Furthermore, lncRNA-MSR regulated the expression of TMSB4 by competing with miRNA-152 in chondrocytes. Our results demonstrated that upregulation of lncRNA-MSR initiates pathological changes that lead to cartilage degradation, and the inhibition of lncRNA-MSR could represent a potential therapeutic target for OA.
Introduction
Osteoarthritis (OA) is a degenerative joint disease characterized by articular cartilage degradation and is the leading cause of physical disability.1 In OA, the medial compartment of the articular cartilage is the most susceptible to degeneration, whereas the lateral compartment remains relatively unaffected.2,3 Differences in mechanical factors underlie the disparity in disease susceptibility between the medial and lateral compartment; the damaged cartilage regions are usually subjected to mechanical loading, whereas intact regions are not. In particular, chondrocytes in the damaged cartilage are susceptible to mechanical stress and the tensile properties of the damaged cartilage are lost due to the destruction of the collagen network.4,5
During normal movements in vivo, articular chondrocytes are exposed to compression loads of 15%, which leads to 5% tensile strain in chondrocytes.6 In vitro, chondrocytes with a tensile strength at a magnitude of 10% or higher have proinflammatory functions, whereas a magnitude of 5% exerts protective and anti-inflammatory effects.7,8 In our study, we applied cyclic tensile strain (CTS) with 10% elongation for 24 hours and CTS with 5% as a control. Thymosin β-4 (TMSB4) is the principal intracellular G-actin sequestering protein involved in the binding of monomeric actin. The function of TMSB4 is to prevent actin filament polymerization while maintaining a pool of actin monomers to use when the cell requires filament assembly.9 Previous studies have shown that TMSB4 expression is upregulated at all time points during mechanical load in cartilage10 and TMSB4 can activate the induction of metalloproteinase in chondrocytes.11
Pseudogenes, a subclass of long noncoding RNAs (lncRNAs) that developed from protein-coding genes but have lost the ability to produce proteins, have long been viewed as nonfunctional genomic relics of evolution.12 LncRNAs are a diverse class of transcribed RNA molecules with a length of more than 200 nucleotides and limited protein coding potential.13 Many lncRNAs are differentially expressed in different tissues and under various developmental and pathological conditions.14,15 We have previously shown that a number of lncRNAs are differentially expressed in OA and normal cartilage, and identified a vimentin pseudogene, lncRNA-CIR, in OA.16
In the present study, we investigated the mechanism of lncRNAs on mechanical stress-induced degeneration of articular cartilage during OA progression. We identified a small number of new lncRNAs that are upregulated or downregulated in different cartilage regions. Specifically, we identified a new TMSB4 pseudogene lncRNA related to mechanical stress (lncRNA-MSR). We showed lncRNA-MSR controls TMSB4 expression by functioning as a competing endogenous RNA (ceRNA) for microRNAs (miRNAs), which leads to the disruption of the cytoskeleton and the increased expression of matrix metalloproteinases (MMPs). We also showed that lncRNA-MSRs participate in chondrocyte ECM degradation and in the occurrence and development of OA.
Results
LncRNA expression profiles in the different regions of cartilage
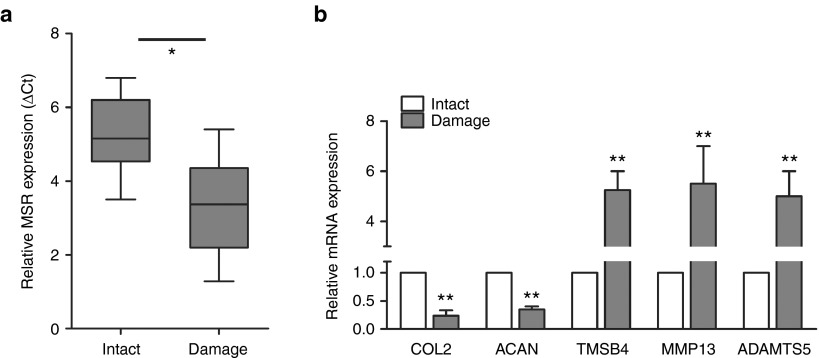
Cartilage fragments of intact or damaged tissue that satisfied the criteria for inclusion were selected for the experiments. A hierarchical clustering showed the expression of lncRNAs and protein-coding RNAs in the cartilage (Supplementary Figure S1). We identified 107 lncRNAs that were differentially expressed in the damaged cartilage compared to the intact cartilage. Of these lncRNAs, 51 were upregulated and 56 were downregulated in the damaged tissue samples (Supplementary Table S1). The differentially expressed mRNAs were selected for gene ontology (GO) analysis. We selected significant GO terms including “response to stress” and “cytoskeleton organization” according to the GO enrichment analyses (Supplementary Figure S4). To validate the microarray data, we performed real-time PCR on the selected genes using 50 pairs of cartilage samples. We identified a new TMSB4 pseudogene lncRNA related to mechanical stress, which we called lncRNA-MSR. LncRNA-MSR is located at 20q13.13, and its primary source is HGNC: 11888. Gene ID: 7120 in NCBI. The parent gene TMSB4 is at Xq21.3-q2. HGNC: 11881. Gene ID: 7114 in NCBI. There are 37 base differences between lncRNA-MSR and the parent gene (Supplementary Table S2). In addition, we used RNAFOLD to predict the secondary structure of lncRNA-MSR, and bioinformatics results indicated that lncRNA-MSR is a long noncoding RNA and lack of protein-coding potential (Supplementary Figure S2). The data confirmed that the TMSB4 pseudogene lncRNA (lncRNA-MSR) was significantly upregulated in the damaged region compared with the intact region of the cartilage in OA (Figure 1a). Furthermore, the expression of COL2A1 and aggrecan (ACAN), which are regulated in OA, were significantly decreased, whereas the expression of TMSB4, MMP13, and ADAMTS5 were increased in the damaged region of the cartilage compared to the intact region (Figure 1b).
Figure 1.
The differentially expressed genes in the different cartilage regions. (a) The expression of lncRNA-MSR was analyzed using quantitative polymerase chain reaction (qPCR). The ΔCt values were used to measure gene expression, which was normalized to the expression level of GAPDH. (Intact region group = 50, damaged region group = 50, *P < 0.05, **P < 0.01). (b) The expression of TMSB4, COL2A1, ACAN, MMP13, and ADAMTS5 were analyzed using qPCR. The results are shown as the mean ± standard error of the mean of at least three independent experiments (*P < 0.05, **P < 0.01).
The newly identified lncRNA-MSR is upregulated by mechanical stress
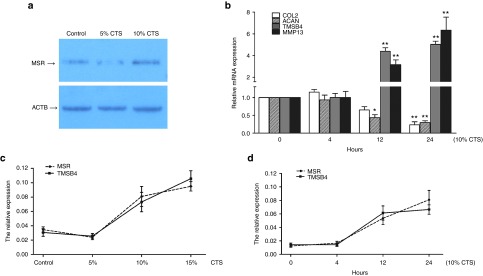
To investigate the effect of biomechanics on chondrocytes, the cells were exposed to preoptimized magnitudes of CTS using Flexcell-5000. Northern blotting analysis showed that the expression of lncRNA-MSR in chondrocytes was increased by CTS with a magnitude of 10% compared to 5% (Figure 2a). ECM-related genes (COL2A1 and ACAN) were not induced by CTS over a period of 4 hours; however, the expression of MMP13 and TMSB4 increased with the duration of CTS, and the expression of COL2A1 and ACAN were significantly downregulated over 24 hours (Figure 2b). We also confirmed that the expression of lncRNA-MSR and TMSB4 increased after chondrocytes were exposed to CTS at a magnitude of 10% and higher (Figure 2c). Furthermore, the expression of lncRNA-MSR and TMSB4 were regulated in parallel with the duration of CTS (Figure 2d). Chondrocytes were exposed to optimized CTS with a magnitude of 10% (0.5 Hz) for 24 hours throughout the study.
Figure 2.
The newly identified lncRNA-MSR in chondrocytes is stimulated by mechanical stress. (a) The expression of lncRNA-MSR in chondrocytes by cyclic tensile strain (CTS) at a magnitude of 5% and 10% was examined using Northern blotting. ACTB was used as an internal control. (b) The chondrocytes were stimulated with mechanical stress for the indicated times. The expression of COL2A1, ACAN, TMSB4, and MMP13 were analyzed using qPCR at 0, 4, 12, and 24 hours (the 0 hour timepoint was used for comparisons). (c) The expression of TMSB4 and lncRNA-MSR in chondrocytes were analyzed using qPCR at the various magnitudes of CTS. (d) The expression of TMSB4 and lncRNA-MSR in chondrocytes were analyzed using qPCR for the various durations of CTS. The results are shown as the mean ± SEM of at least three independent experiments (*P < 0.05, **P < 0.01).
The effects of lncRNA-MSR on the expression of TMSB4 and the degradation of the ECM in chondrocytes
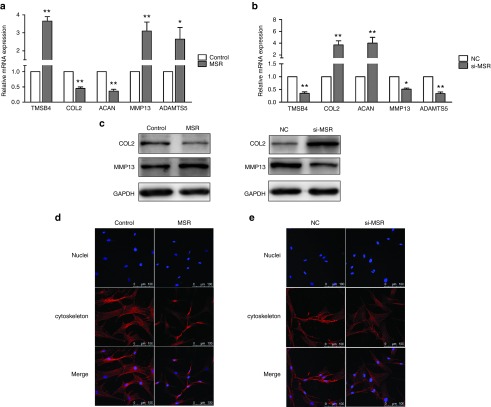
To analyze the effects of lncRNA-MSR on the expression of TMSB4, we transfected chondrocytes with p-lncMSR and si-MSR (Supplementary Figure S3). The results showed that the overexpression of lncRNA-MSR increased TMSB4 expression (Figure 3a). In addition, lncRNA-MSR knockdown induced the repression of TMSB4 expression at both the transcriptional and protein level (Figure 3b). Studies have reported that upregulation of TMSB4 prevents the exchange of the G-actin-bound nucleotide, resulting in the inhibition of actin polymerization.11,17 Therefore, we investigated the effects of lncRNA-MSR on ECM degradation and cytoskeleton structure in the chondrocytes (Supplementary Figure S5). The overexpression of lncRNA-MSR significantly decreased the expression of COL2A1 and ACAN, and promoted the expression of MMP13 and ADAMTS5 in chondrocytes (Figure 3a,c). LncRNA-MSR knockdown had the opposite effects on the expression of these markers (Figure 3b,c). Furthermore, the overexpression of lncRNA-MSR induced cytoskeleton remodeling, disorganization of F-actin and a concomitant cell retraction (Figure 3d); however, lncRNA-MSR knockdown reversed the mechanical stress-induced disorganization of F-actin (Figure 3e).
Figure 3.
The effects of lncRNA-MSR on TMSB4 expression and ECM in chondrocytes. (a, b) The mRNA expression of TMSB4, COL2A1, ACAN, MMP13, and ADAMTS5 were determined after overexpression of lncRNA-MSR or siRNA knockdown of lncRNA-MSR. (c) The chondrocytes were transfected with p-lncMSR or si-MSR, and the protein expression of COL2A1 and MMP-13 was analyzed using Western blotting. GAPDH was used as loading control. (d, e) The chondrocytes were transfected with p-lncMSR or si-MSR, and the cells were fixed and stained with rhodamine phalloidin and Hoechst 33342. Bar = 100 µm. The results are shown as the mean ± SEM of at least three independent experiments (*P < 0.05, **P < 0.01 p-lncMSR versus control (treated with empty plasmid) and si-MSR versus the negative control (NC) siR-Ribo).
The effects of lncRNA-MSR on TMSB4 expression as a ceRNA in chondrocytes
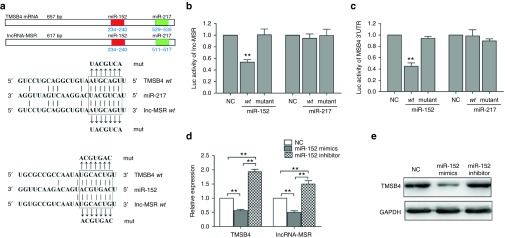
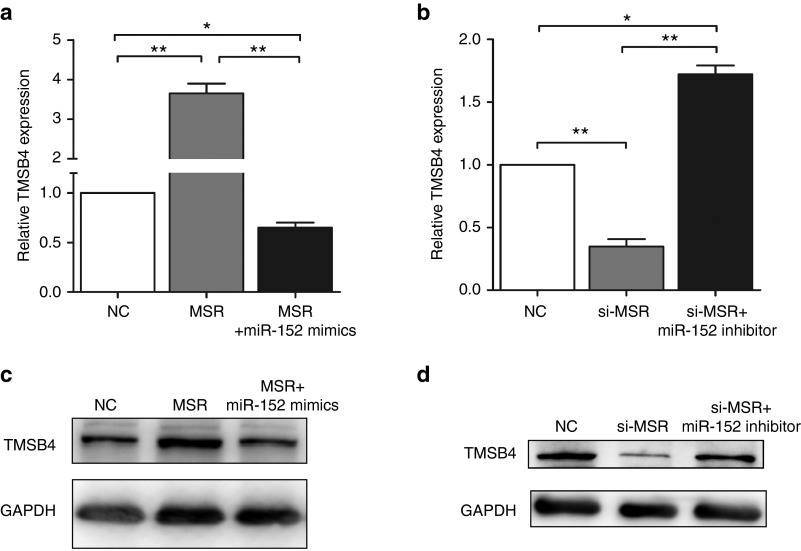
Many pseudogene transcripts exert regulatory control of their ancestral gene's expression and function as competing endogenous RNA (ceRNA), competing for miRNAs that target the common sequences.18,19,20 We predicted miRNAs using the TargetScan, microRNA.org, and miRanda prediction algorithms.21,22,23 We found that two microRNAs (miR-152 and miR-217) directly targeted the conserved sequences of both lncRNA-MSR and TMSB4 (Figure 4a). The miR-REPORT luciferase reporter was constructed to determine whether miRNA could directly target the 3'-UTR of TMSB4 and lncRNA-MSR. The luciferase signal of the wild-type lncRNA-MSR reporter and wild-type TMSB4 3'-UTR reporter was suppressed by miR-152, whereas the luciferase signal of the mutant reporters was not affected (Figure 4b,c). In addition, the luciferase activity was not influenced by miR-217. Furthermore, the overexpression of miR-152 repressed TMSB4 and lncRNA-MSR expression, and the inhibition of miR-152 increased the expression of TMSB4 and lncRNA-MSR at both the transcriptional and protein level in the chondrocytes (Figure 4d,e). These results demonstrated that both TMSB4 and lncRNA-MSR expression was directly downregulated by miR-152. Furthermore, we found that high expression of TMSB4 induced by overexpression of lncRNA-MSR was downregulated by miR-152 (Figure 5a,c), and the repression of TMSB4 through inhibiting lncRNA-MSR was upregulated by the miR-152 inhibitor (Figure 5b,d). Therefore, we concluded that lncRNA-MSR functioned as a ceRNA by competing for binding with miR-152 to regulate TMSB4 expression.
Figure 4.
LncRNA-MSR is targeted by TMSB4-targeting miR-152. (a) TMSB4 and lncRNA-MSR 3'-UTRs contain a highly conserved domain. Targeted miRNA seed matches within the high homology region are conserved between TMSB4 and lncRNA-MSR. The miRNAs binding to TMSB4 and lncRNA-MSR are matched by solid lines. (b, c) The luciferase reporter analysis of either the wild-type or mutant TMSB4 and lncRNA-MSR 3'-UTR activity. The miRNAs were cotransfected with the wild-type or mutant vector, and scrambled miRNA was used as a negative control. (d) The primary chondrocytes were transfected with the miR-152 mimic or inhibitor. Scrambled miRNA was used as a negative control. The expression of lncRNA-MSR and TMSB4 was analyzed using qPCR. (e) The protein expression of TMSB4 was analyzed using western blotting with GAPDH as loading control. The results are shown as the mean ± SEM of at least three independent experiments (*P < 0.05, **P < 0.01).
Figure 5.
The effects of lncRNA-MSR on TMSB4 expression as a ceRNA in chondrocytes. (a) The chondrocytes were transfected with p-lncMSR or cotransfected with p-lncMSR and the miR-152 mimics. The expression of TMSB4 was determined using qPCR. (b) The chondrocytes were transfected with si-MSR or cotransfected with si-MSR and the miR-152 inhibitor. The expression of TMSB4 was determined using qPCR. (c, d) The protein expression of TMSB4 was analyzed using western blotting. GAPDH was used as loading control. The results are shown as the mean ± SEM of at least three independent experiments (*P < 0.05, **P < 0.01).
Verification of a schematic model for the ceRNA function of lncRNA-MSR in chondrocytes
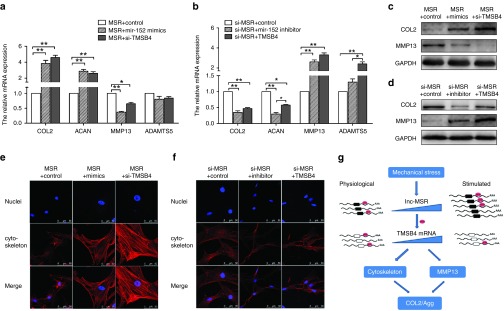
To validate the pathway of mechanical stress in chondrocytes, we cotransfected miR-152 mimics and p-lncMSR into chondrocytes. The effects of lncRNA-MSR were rescued by miR-152 and the expression of COL2A1 and ACAN increased. In addition, the expression of MMP13 significantly decreased at the transcriptional and protein levels (Figure 6a,c). The effects of lncRNA-MSR on ECM degradation were also rescued by TMSB4 knockdown at the transcriptional and protein level (Figure 6a,c). Furthermore, we inhibited miR-152 in the lncRNA-MSR knockdown chondrocytes. The inhibition of miR-152 reversed the increased expression of the ECM-related genes that were induced by the depletion of lncRNA-MSR (Figure 6b,d). The overexpression of TMSB4 also reversed the protection of ECM by lncRNA-MSR knockdown (Figure 6b,d). Moreover, the overexpression of lncRNA-MSR disrupted the actin cytoskeleton, but ectopic expression of miR-152 eliminated this effect. Similarly, TMSB4 knockdown inhibited cytoskeleton disassembly (Figure 6e). LncRNA-MSR knockdown maintained the integrity of the cytoskeleton organization, but the miR-152 inhibitor induced cytoskeleton reorganization; the overexpression of TMSB4 also similarly induced cytoskeleton reorganization (Figure 6f). Finally, we proposed a schematic model in which lncRNA-MSR is activated by mechanical stress and functions as a ceRNA to promote the expression of TMSB4 by competitively binding with miR-152. This induces cytoskeleton disorganization and MMP expression, leading to ECM degradation (Figure 6g).
Figure 6.
The mechanism of lncRNA-MSR regulation of ECM expression by functioning as a ceRNA. (a) The chondrocytes were cotransfected with the p-lncMSR and miR-152 mimics or cotransfected with p-lncMSR and si-TMSB4. The expression of ECM was analyzed using qPCR. (b) The protein expression of COL2A1 and MMP-13 was analyzed using Western blotting. (c) The chondrocytes were cotransfected with p-lncMSR and miR-152 inhibitor or cotransfected with si-MSR and p-TMSB4. The expression of ECM was analyzed using qPCR. (d) The protein expression of COL2A1 and MMP-13 was analyzed using western blotting. The findings in B and D were quantitatively analyzed using western blot images. The results are shown as the mean ± SEM of at least three independent experiments (*P < 0.05, **P < 0.01). (e) The chondrocytes were cotransfected with the p-lncMSR and miR-152 mimics or cotransfected with p-lncMSR and si-TMSB4, and the cells were stained with rhodamine phalloidin. Bar = 50 µm. (f) The chondrocytes were co-transfected with si-MSR and miR-152 inhibitor or cotransfected with si-MSR and p-TMSB4, and cells were stained with rhodamine phalloidin. Bar = 50 µm. (g) A schematic model showing that lncRNA-MSR was stimulated by mechanical stress and regulated TMSB4 expression through the binding of miR-152, which induced cytoskeleton disassembly and ECM degradation.
Discussion
In our study, we found that lncRNA-MSR, which is activated by mechanical stress, induced cytoskeleton remolding and MMP production, thereby promoting cartilage degradation. And we considered that the inhibition of lncRNA-MSR by siRNA could represent a potential therapeutic target for OA. OA is a degenerative joint disease, thus, local treatment could achieve good therapeutic effect and the side effect is less. So far, numerous previous studies, both in the laboratory and clinic, have demonstrated the potential of nucleic acids as therapeutic agents, particularly with their promise of specificity and functional diversity. But there are also challenges need to overcome, such as therapeutic agents' delivery, pharmacokinetics, immunogenicity, and toxicity.24
Pseudogenes are genomic loci that resemble real genes, but are considered biologically inconsequential because they harbor premature stop codons, deletions or insertions, and frameshift mutations that abrogate their translation into functional proteins.20 The number of pseudogenes is similar to the number of coding genes and they represent a significant proportion of the transcriptome.25 Despite lacking true promoters, processed pseudogenes utilize proximal regulatory elements to mediate their transcription.26 Many pseudogene transcripts belonging to lncRNAs exhibit tissue-specificity and are involved in various biological process.27 However, to date, very few pseudogenes have been functionally characterized. Processed pseudogenes in mouse oocytes have been previously reported to generate endogenous small interfering RNAs (endo-siRNAs) that downregulate the expression of cognate genes through conventional RNA interference.28 In our previous study, we hypothesized that lncRNA-CIR may either act as an siRNA itself to suppress the expression of genes or it may induce mRNA to form endo-siRNAs to downregulate vimentin.16 According to recent studies, only a few pseudogenes undergo antisense transcription. However, the transcripts of pseudogenes competing with cognate genes for miRNA binding have been extensively investigated. We suggest that lncRNA-MSR is the decoy for the ancestral gene TMSB4. Lnc-MSR acts similarly to a “sponge” and can affect the distribution of miRNA-152 on its targets. In this decoy manner, ectopic miR-152 expression is closely related to the expression of lncRNA-MSR and TMSB4. Given that no homolog of lncRNA-MSR has been found in other species, no in vivo models are currently available to study this mechanism in detail.
TMSB4 has numerous biological activities, including in wound healing, apoptosis, inflammatory responses, and angiogenesis, which fundamentally depend upon cell migration.9 TMSB4 may indirectly affect MMP expression by inducing changes in the cytoskeleton. Alternatively, TMSB4 may induce MMP expression in response to a mechanical stimulus either directly or as part of a second messenger signaling cascade.10 We also found lncRNA-CIR was differentially expressed in our study and its target gene was vimentin, which is another vital component of the cytoskeleton. Vimentin is a determinant of cell stiffness and is important in signal transduction and the relay of mechanical signals to downstream biochemical responses.29 Therefore, the cytoskeleton plays a vital role in chondrocytes subjected to mechanical stress. Furthermore, lncRNAs are involved in the mechanical response of chondrocytes and have a regulatory effect on the process. Although the function of many lncRNAs has yet to be elucidated, determining the precise molecular mechanisms of lncRNAs in mechanical stress is critical to understanding the pathogenesis of OA and exploring novel potential targets for therapy.
Materials and Methods
Patients and specimens. OA cartilage was isolated from the knee joints of 50 patients undergoing total knee arthroplasty and processed for histological examination. Informed consent was obtained from all tissue donors included in the current study. The study was approved by the Human Ethics Committee of Peking University Third Hospital (China). Methods were carried out in “accordance” with the approved guidelines.
Histological examination. The cartilage specimens were dehydrated in graded alcohols and xylene, embedded in paraffin, and serially sliced into 5 mm sagittal sections. The sections were stained with Toluidine Blue, Safranin-O, and Hematoxylin and Eosin according to standard protocols. Histological changes were graded according to a modified Mankin scale.30 A score of <4 points was considered intact cartilage and a score of >6 represented damaged cartilage.31
Microarray and quantitative real-time PCR. Arraystar lncRNA labeling, array hybridization and data processing were performed as previously described.16 For miRNA quantitative PCR analysis, reverse transcription of specific miRNAs was performed using Bulge-Loop miRNA primer sets (RiboBio, Guangzhou, China.), according to the manufacturer's instructions. For mRNA analysis, total RNA was reverse transcribed using target-specific primers. The expression of constituently active genes GAPDH and U6 were used to calculate the mRNA and miRNA expression, respectively, using the 2-ΔΔCT method.34 The primers used in the present study were as follows:
COL2A1 forward: 5′-TGGACGATCACGAAACC-3′, reverse: 5′-GCTGCGGATGCTCTCAATCT-3′;
ACAN forward: 5′-ACTCTGGGTTTTCGTGACTCT-3′, reverse: 5′-ACACTCAGCGAGTTGTCATGG-3′;
MMP13 forward: 5′-ACTGAGAGGCTCCGAGAAATG-3′, reverse: 5′-GAACCCCGCATCTTGGCTT-3′;
ADAMTS5 forward: 5′-GAACATCGACCAACTCTACTCCG-3′, reverse: 5′-CAATGCCCACCGAACCATCT-3′;
TMSB4 forward: 5′-CCAGACTTCGCTCGTACTCG-3′, reverse: 5′-ATTTCACTGTCGTCCCACCC-3′;
lncRNA-MSR forward: 5′-ACTACTGACAACGAAGGCCG-3′, reverse: 5′- TTTCACTGTTGTCCCACCCC-3′; and lncRNA-CIR forward: 5′-ACACTTGCAAGCCTGGGTAG-3′, reverse: 5′-CCATTTTCCTGTTGGTGCGG-3′. The PCR products were confirmed by sequencing.
Northern blotting. Northern blotting was performed as previously described.33,34,35 For all the groups, 20 µg of total RNA was analyzed on an agarose gel and transferred to a Hybond N+ nylon membrane (Pharmacia). The membranes were cross-linked using ultraviolet light at 5,000 uJ/cm2.
Primary culture of chondrocytes and exposure to mechanical stress. Donor chondrocytes were isolated as previously described. Chondrocytes (5 × 105/well) were grown on ProNectin F-coated Bioflex 6-well culture plates (Flexcell International, Hillsborough, NC) to 80% confluence. CTS experiments were performed using an FX-5000 Flexercell system (Flexcell International, McKeesport, PA). CTS was enforced at 5, 10, and 15% elongation (0.5 Hz) for various periods of time.6,36
Luciferase assay. LncRNA-MSR containing the putative binding site for miR-152 and its identical sequence with a mutation of the miR-152 seed sequence were inserted between the restrictive sites Xho I and Not I of the pmiR-RB-Report and validated using sequencing. The pmiR-RB-Report luciferase reporter vectors of the TMSB4 3'-UTR were constructed as above. HeLa cells were transfected with wild type or mutated reporter vectors, miRNA mimics or negative control. Lysates were harvested 24 hours after transfection. Renilla luciferase activities were consecutively measured according to the dual-luciferase assay manual (Promega).
Plasmid construction, RNA interference, and transfection. The full-length lncRNA-MSR was introduced into the pEGFP-C1 vector, and the correct constructs were verified using DNA sequencing and designated as p-lncMSR. Similarly, full-length TMSB4 was cloned into the vector and designated as p-TMSB4. Small interfering RNAs (siRNAs) against lncRNA-MSR (named si-MSR) and TMSB4 (named si-TMSB4) were designed and synthesized by RiboBio. The sequence of the functional si-MSR is AUGAGGCGUGUGCCGUCAAdTdT and the sequence of the functional si-TMSB4 is CCCAUCUGUCUAUCUAUCUdTdT. For miRNA transfection, miR-152 mimics, miR-152 inhibitors and negative control (RiboBio) were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Western blot. Total protein was extracted using lysis buffer (50 mmol/l of Tris HCl, pH 7.4, 150 of mmol/l NaCl, 1% NonidetP40, and 0.1% sodium dodecyl sulfate) and the concentration was determined using a bicinchoninic acid protein assay kit (Pierce). The blots were probed overnight at 4 °C with antibodies against COL2A1 (1:1,000 dilution; Abcam), MMP-13 (1:3,000 dilution; Abcam) and TMSB4 (1:2,000 dilution; Abcam), followed by incubation for 1 hour at room temperature with horseradish peroxidase-conjugated secondary antibody (1:4,000 dilution; Santa Cruz Biotechnology).
Cytoskeleton staining. Cells were rinsed in PBS and fixed with 4% paraformaldehyde for 15 minutes at room temperature. Chondrocytes probed with rhodamine phalloidin were incubated at room temperature in the dark for 30 minutes. Subsequently, the samples were washed in PBS and incubated with Hoechst 33342 for 5 minutes. The cells were observed under a confocal microscope (FV 1000 Olympus IX-81, Olympus, Tokyo, Japan).
Statistics. Statistically significant differences between multiple groups were calculated using an analysis of variance. The results from two groups were evaluated using t-tests. The results are reported as the mean ± standard error of the mean. A P < 0.05 was considered statistically significant. All experiments were performed and analyzed in triplicate. The data analysis was performed using SPSS software (version 15.0, SPSS, Chicago, IL).
SUPPLEMENTARY MATERIAL Figure S1. The differential expression of lncRNAs in the different regions. Figure S2. The secondary structure of lncRNA-MSR was predicted using RNAFOLD. Figure S3. The effect of 3 different small interfering RNAs (siRNAs) against TMSB4 (si-TMSB4) was analyzed using qPCR. Figure S4. Gene ontology (GO) analysis of differentially expressed mRNAs. Figure S5. The effects of lncRNA-MSR on COL2 degradation. Table S1. Differentially Expressed LncRNAs in damage versus intact Cartilage. Table S2. The sequence between lncRNA-MSR and the parent gene.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 90919022, 81101390, 81330040), Specialized Research Fund for the Doctoral Program of Higher Education (20110001130001). Author contributions: Study conception and design by Q.L., X.H., X.Z., L.D., C.Z., Y.A. Acquisition of data by Q.L., C.Z., Y.A. Analysis and interpretation of data by Q.L., C.Z., Y.A. Competing interests: The authors declare that they have no competing interests.
Supplementary Material
References
- Wang, Q, Rozelle, AL, Lepus, CM, Scanzello, CR, Song, JJ, Larsen, DM et al. (2011). Identification of a central role for complement in osteoarthritis. Nat Med 17: 1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer, M, Grässel, S, Straub, RH, Schett, G, Dinser, R, Grifka, J et al. (2009). Differential transcriptome analysis of intraarticular lesional vs intact cartilage reveals new candidate genes in osteoarthritis pathophysiology. Osteoarthritis Cartilage 17: 328–335. [DOI] [PubMed] [Google Scholar]
- Meng, J, Ma, X, Ma, D and Xu, C (2005). Microarray analysis of differential gene expression in temporomandibular joint condylar cartilage after experimentally induced osteoarthritis. Osteoarthritis Cartilage 13: 1115–1125. [DOI] [PubMed] [Google Scholar]
- Kawakita, K, Nishiyama, T, Fujishiro, T, Hayashi, S, Kanzaki, N, Hashimoto, S et al. (2012). Akt phosphorylation in human chondrocytes is regulated by p53R2 in response to mechanical stress. Osteoarthritis Cartilage 20: 1603–1609. [DOI] [PubMed] [Google Scholar]
- Thomas, RS, Clarke, AR, Duance, VC and Blain, EJ (2011). Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis. Arthritis Res Ther 13: R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, S, Deschner, J, Long, P, Verma, A, Hofman, C, Evans, CH et al. (2004). Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis Rheum 50: 3541–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassner, R, Buckley, MJ, Georgescu, H, Studer, R, Stefanovich-Racic, M, Piesco, NP et al. (1999). Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol 163: 2187–2192. [PMC free article] [PubMed] [Google Scholar]
- Patwari, P, Cook, MN, DiMicco, MA, Blake, SM, James, IE, Kumar, S et al. (2003). Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum 48: 1292–1301. [DOI] [PubMed] [Google Scholar]
- Huff, T, Müller, CS, Otto, AM, Netzker, R and Hannappel, E (2001). beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol 33: 205–220. [DOI] [PubMed] [Google Scholar]
- Blain, EJ, Mason, DJ and Duance, VC (2003). The effect of cyclical compressive loading on gene expression in articular cartilage. Biorheology 40: 111–117. [PubMed] [Google Scholar]
- Blain, EJ, Mason, DJ and Duance, VC (2002). The effect of thymosin beta4 on articular cartilage chondrocyte matrix metalloproteinase expression. Biochem Soc Trans 30(Pt 6): 879–882. [DOI] [PubMed] [Google Scholar]
- Poliseno, L (2012). Pseudogenes: newly discovered players in human cancer. Sci Signal 5: re5. [DOI] [PubMed] [Google Scholar]
- Wilusz, JE, Sunwoo, H and Spector, DL (2009). Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23: 1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer, TR, Dinger, ME and Mattick, JS (2009). Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159. [DOI] [PubMed] [Google Scholar]
- Ørom, UA, Derrien, T, Beringer, M, Gumireddy, K, Gardini, A, Bussotti, G et al. (2010). Long noncoding RNAs with enhancer-like function in human cells. Cell 143: 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q, Zhang, X, Dai, L, Hu, X, Zhu, J, Li, L et al. (2014). Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol 66: 969–978. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont, PJ, Furman, MI, Wachsstock, D, Safer, D, Nachmias, VT and Pollard, TD (1992). The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell 3: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalla, D, Yario, T, Steitz, JA and Steitz, J (2010). Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328: 1563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan, Z, Deng, Z, Shatseva, T, Fang, L, He, C and Yang, BB (2011). Expression of CD44 3'-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Nucleic Acids Res 39: 3026–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliseno, L, Salmena, L, Zhang, J, Carver, B, Haveman, WJ and Pandolfi, PP (2010). A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465: 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betel, D, Wilson, M, Gabow, A, Marks, DS and Sander, C (2008). The microRNA.org resource: targets and expression. Nucleic Acids Res 36(Database issue): D149–D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, B, Enright, AJ, Aravin, A, Tuschl, T, Sander, C and Marks, DS (2004). Human MicroRNA targets. PLoS Biol 2: e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, BP, Burge, CB and Bartel, DP (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20. [DOI] [PubMed] [Google Scholar]
- Gogtay, NJ and Sridharan, K (2016). Therapeutic nucleic acids: current clinical status. Br J Clin Pharmacol 82: 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, PM, Zheng, D, Zhang, Z, Carriero, N and Gerstein, M (2005). Transcribed processed pseudogenes in the human genome: an intermediate form of expressed retrosequence lacking protein-coding ability. Nucleic Acids Res 33: 2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney, E, Stamatoyannopoulos, JA, Dutta, A, Guigó, R, Gingeras, TR, Margulies, EH et al.; ENCODE Project Consortium; NISC Comparative Sequencing Program; Baylor College of Medicine Human Genome Sequencing Center; Washington University Genome Sequencing Center; Broad Institute; Children's Hospital Oakland Research Institute. (2007). Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447: 799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer, TR, Dinger, ME and Mattick, JS (2009). Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159. [DOI] [PubMed] [Google Scholar]
- Tam, OH, Aravin, AA, Stein, P, Girard, A, Murchison, EP, Cheloufi, S et al. (2008). Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub, P (1995). Intermediate filaments and gene regulation. Physiol Chem Phys Med NMR 27: 377–400. [PubMed] [Google Scholar]
- Mankin, HJ, Dorfman, H, Lippiello, L and Zarins, A (1971). Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 53: 523–537. [PubMed] [Google Scholar]
- Sato, T, Konomi, K, Yamasaki, S, Aratani, S, Tsuchimochi, K, Yokouchi, M et al. (2006). Comparative analysis of gene expression profiles in intact and damaged regions of human osteoarthritic cartilage. Arthritis Rheum 54: 808–817. [DOI] [PubMed] [Google Scholar]
- Livak, KJ and Schmittgen, TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Liu, T, Shen, D, Xing, S, Chen, J, Yu, Z, Wang, J et al. (2013). Attenuation of exogenous angiotensin II stress-induced damage and apoptosis in human vascular endothelial cells via microRNA-155 expression. Int J Mol Med 31: 188–196. [DOI] [PubMed] [Google Scholar]
- Cheng, W, Liu, T, Jiang, F, Liu, C, Zhao, X, Gao, Y et al. (2011). microRNA-155 regulates angiotensin II type 1 receptor expression in umbilical vein endothelial cells from severely pre-eclamptic pregnant women. Int J Mol Med 27: 393–399. [DOI] [PubMed] [Google Scholar]
- Liu, T, Huang, Y, Chen, J, Chi, H, Yu, Z, Wang, J et al. (2014). Attenuated ability of BACE1 to cleave the amyloid precursor protein via silencing long noncoding RNA BACE1AS expression. Mol Med Rep 10: 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, P, Gassner, R and Agarwal, S (2001). Tumor necrosis factor alpha-dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro. Arthritis Rheum 44: 2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.