Abstract
IMPORTANCE
Bariatric surgery induces significant weight loss for severely obese patients, but there is limited evidence of the durability of weight loss compared with nonsurgical matches and across bariatric procedures.
OBJECTIVES
To examine 10-year weight change in a large, multisite, clinical cohort of veterans who underwent Roux-en-Y gastric bypass (RYGB) compared with nonsurgical matches and the 4-year weight change in veterans who underwent RYGB, adjustable gastric banding (AGB), or sleeve gastrectomy (SG).
DESIGN, SETTING, AND PARTICIPANTS
In this cohort study, differences in weight change up to 10 years after surgery were estimated in retrospective cohorts of 1787 veterans who underwent RYGB from January 1, 2000, through September 30, 2011 (573 of 700 eligible [81.9%] with 10-year follow-up), and 5305 nonsurgical matches (1274 of 1889 eligible [67.4%] with 10-year follow-up) in mixed-effects models. Differences in weight change up to 4 years were compared among veterans undergoing RYGB (n = 1785), SG (n = 379), and AGB (n = 246). Data analysis was performed from September 9, 2014, to February 12, 2016.
EXPOSURES
Bariatric surgical procedures and usual care.
MAIN OUTCOMES AND MEASURES
Weight change up to 10 years after surgery through December 31, 2014.
RESULTS
The 1787 patients undergoing RYGB had a mean (SD) age of 52.1 (8.5) years and 5305 nonsurgical matches had a mean (SD) age of 52.2 (8.4) years. Patients undergoing RYGB and nonsurgical matches had a mean body mass index of 47.7 and 47.1, respectively, and were predominantly male (1306 [73.1%] and 3911 [73.7%], respectively). Patients undergoing RYGB lost 21% (95% CI, 11%-31%) more of their baseline weight at 10 years than nonsurgical matches. A total of 405 of 564 patients undergoing RYGB (71.8%) had more than 20% estimated weight loss, and 224 of 564 (39.7%) had more than 30% estimated weight loss at 10 years compared with 134 of 1247 (10.8%) and 48 of 1247 (3.9%), respectively, of nonsurgical matches. Only 19 of 564 patients undergoing RYGB (3.4%) regained weight back to within an estimated 5% of their baseline weight by 10 years. At 4 years, patients undergoing RYGB lost 27.5% (95% CI, 23.8%-31.2%) of their baseline weight, patients undergoing AGB lost 10.6% (95% CI, 0.6%-20.6%), and patients undergoing SG lost 17.8% (95% CI, 9.7%-25.9%). Patients undergoing RYGB lost 16.9% (95% CI, 6.2%-27.6%) more of their baseline weight than patients undergoing AGB and 9.7% (95% CI, 0.8%-18.6%) more than patients undergoing SG.
CONCLUSIONS AND RELEVANCE
Patients in the Veterans Administration health care system lost substantially more weight than nonsurgical matches and sustained most of this weight loss in the long term. Roux-en-Y gastric bypass induced significantly greater weight loss among veterans than SG or AGB at 4 years. These results provide further evidence of the beneficial association between surgery and long-term weight loss that has been demonstrated in shorter-term studies of younger, predominantly female populations.
Obesity is a chronic disease, and prior research has clearly demonstrated that bariatric surgery is the most effective intervention for inducing weight loss among obese patients.1-4 Much of this evidence is based on relatively short 1- to 3-year follow-up from randomized clinical trials3 that suggest that bariatric procedures are superior to nonsurgical interventions, Roux-en-Y gastric bypass (RYGB) induces greater weight loss than sleeve gastrectomy (SG), and RYGB and SG are superior to adjustable gastric banding (AGB) for weight loss.3,5
Much less is known about the durability of weight loss after bariatric surgery because few studies6-13 have reported outcomes from patient cohorts with sufficient long-term follow-up. Results from previous published studies are limited by having small sample sizes,9,12,14,15 being from single institutions, not having nonsurgical comparators,13,16-18 or reporting outcomes from obsolete bariatric procedures.19 In a systematic review20 of 7371 bariatric studies, only 29 studies (0.4%) associated with 7971 patients had at least 80% retention and 2 years of follow-up. Only 4 studies had 5 or more years of follow-up. In those 4 studies, weight regain began 3 years after surgery for patients undergoing RYGB and AGB, implying that the long-term effects of these procedures might not be durable. It is unclear whether weight loss after bariatric surgery is superior to nonsurgical care in the long term.19,21,22 There is a need for outcomes studies from large, controlled, multisite cohorts that have a high degree of long-term follow-up.20 There is also a need to understand the long-term weight loss outcomes of men and older patients who undergo bariatric surgery because most prior studies6-13 have included predominantly younger women and obesity-related complications tend to be more severe in men. Thus, the benefits of bariatric surgery may be greater in men. Knowing long-term outcomes from bariatric surgery is important because obesity is a chronic disease, and the results of its treatments are most meaningful for long-term not short-term outcomes.
To address persistent questions about the long-term durability of bariatric surgery, we compared 10-year weight change between patients undergoing RYGB and nonsurgical matches from a large, multisite, clinical cohort.23 Finally, to better understand the comparative effectiveness of the 3 major surgical procedures (RYGB, SG, and AGB),24,25 we compared weight change up to 4 years after surgery, a period for which we had complete follow-up information for patients undergoing SG, which is a more recently developed procedure.
Methods
Study Design and Study Population
A retrospective cohort study was performed of Veterans Affairs (VA) bariatric surgery patients and a matched cohort of severely obese veterans who had not undergone bariatric surgery. This study was approved by the VA Surgical Improvement Program of the VA Office of Patient Care Services, the institutional review boards (including waiver of informed consent) of the Durham and North Texas VA medical centers, and the Group Health Research Institute. Veterans who underwent any bariatric surgical procedure in VA bariatric centers or community hospitals reimbursed by the VA from January 1, 2000, through September 30, 2011, were studied (eFigure 1 in the Supplement). Data analysis was performed from September 9, 2014, to February 12, 2016. After excluding veterans with missing preoperative body mass index (BMI) (calculated as the weight in kilograms divided by height in meters squared) or a BMI less than 35, no valid bariatric procedure code, a baseline diagnosis considered a medical exclusion for surgery, a presurgical stay longer than 5 days, or no recorded inpatient stay at date of surgery, the surgical cohort included 1844 veterans undergoing RYGB, 381 undergoing SG, 249 undergoing AGB, and 26 undergoing other procedures.
Potential matches for patients undergoing RYGB were identified from VA electronic health records using sequential stratification matching.26-28 Sequential stratification resembles a series of n = 1 trials in which each trial start date was each surgical patient's surgery date and allows matching of treated to untreated patients in longitudinal studies where controls have multiple potential index dates and evolving comorbid health condition incidence. Potential matches were identified based on sex, diabetes diagnosis, race, VA region, age, Diagnostic Cost Group score, and BMI measured within 6 months before surgery. Up to 3 matches were selected for each surgical case based on the smallest caliper29 that preserved covariate balance while minimizing the loss of patients undergoing RYGB for lack of comparable matches. Potential matches often had many BMI measurements during the study period so they could match to more than one surgical patient. Controls who underwent bariatric surgery at a later date contributed person-time to the control group until their surgery date. From the original sample of 1844 patients undergoing RYGB,23 we excluded patients who died within 1 year of surgery (n = 52 patients), who lacked postsurgical weights (n = 4), and who had inpatient weights only after surgery (n = 1). The analysis of patients undergoing RYGB and nonsurgical matches included 1787 patients undergoing RYGB and 5305 nonsurgical matches, representing 5052 unique individuals because controls were allowed to match to multiple surgical patients.
For an unmatched comparison of postsurgical weight changes across surgical procedures, we excluded patients who underwent vertical banded gastroplasty (n = 8) or biliary pancreatic diversion (n = 12), who died within 1 year of surgery (n = 61), who only had inpatient weights after surgery (n = 1), who lacked postsurgical weights (n = 6), or who did not have a weight measurement within 5 years after surgery (n = 2 patients undergoing RYGB). This resulted in a final sample of 1785 patients undergoing RYGB, 246 undergoing AGB, and 379 undergoing SG (eFigure 2 in the Supplement).
Definition and Cleaning of Weight Outcome
Follow-up weight data were obtained from measurements recorded in the electronic health records during outpatient visits from January 1, 2000, through December 31, 2014. The primary outcome was percentage change in weight at follow-up compared with baseline, which is less confounded by baseline BMI than other commonly reported measures (eg, percentage of excess weight loss).30 Baseline weight data on the surgery day was available for 78.6% of patients undergoing RYGB but only 2.2% of nonsurgical matches, and the mean (SD) number of days for the baseline weight measurement before surgery was 7.3 (24.0) for patients undergoing RYGB and 59.8 (48.6) for nonsurgical matches.
Postsurgical weight measurements are highly variable and have a nonlinear trend, so we developed a multistep outlier-detection algorithm that examined the SD of consecutively measured weights (eAppendix in the Supplement). An algorithm to identify multiple weight measures on the same day, or weights that deviated from clinically plausible trends over time, excluded a small proportion (4201 of 89 757 [4.7%]) of weight measurements.
Statistical Analysis
The statistical analysis was developed to estimate mean trajectories of the percentage of weight change and estimates at specific postsurgical years. Penalized spline mixed-effects models were used, with piecewise linear functions with pre-specified knots included as fixed and random effects at the population-average and subject-specific levels.31,32 This approach allowed us to estimate overall mean trajectories and individual trajectories with enough flexibility to reflect weight change fluctuations during an individual's entire follow-up (eFigure 3 in the Supplement illustrates 75 cases).
Because of the extent of weight change in the 2 years after surgery, our primary model specification for the overall mean included a linear term for time and knots at 3 months, 6 months, and 1, 2, 3, 5, 7, and 9 years. All time terms were interacted with a treatment indicator to allow differential estimates for patients undergoing RYGB and nonsurgical matches using HPMIXED in SAS statistical software, version 9.4 (SAS Institute Inc). The model also included individual-level random effects with knots at 6 months and 1, 2, 3, 5, 7, and 9 years; a subject-level random slope to account for correlation among patients’ repeated measures; and a VA geographic area–level random slope to account for correlation between each pair matched within VA geographic area and patients matching more than once. Estimate statements were used to generate the predicted percentage of weight change at 1, 3, 5, 7 and 10 years of follow-up for the patients undergoing RYGB compared with the nonsurgical matches. The model's empirical Bayes estimates were used to calculate patients’ predicted weights at 1, 3, 5, 7, and 10 years only for those with a weight measurement within a 12-month interval of (6 months before to 6 months after) and at least 1 measurement after each follow-up year, which were used to classify each patient as having lost less than 5%, 5% or more, 10% or more, 20% or more, and 30% or more of their baseline weight. The 12-month interval requirement modestly reduced the number of patients for whom individual weights could be predicted. For example, 573 surgical patients were followed up for at least 10 years, but only 564 of these 573 had a weight measurement between 9.5 and 10.5 years. Patients’ predicted weights at 10 years of follow-up were used to calculate mean weight loss and excess body weight loss from baseline.
A similar model with knots specified at 3 months, 6 months, and 1, 2, and 3 years was used to compare weight change through 4 years of follow-up across surgical procedures, which had shorter follow-up because of the recent introduction of the SG procedure and study end date of December 31, 2014 (eFigure 2 in the Supplement). To understand the effect of baseline differences in patients who underwent each surgical procedure, patients’ predicted percentage of weight loss at 4 years was regressed on surgery type while adjusting for baseline BMI, comorbidity score, demographics, and diabetes status (eTable in the Supplement).
Results
Characteristics of Patients Undergoing RYGB and Nonsurgical Matches
Patients undergoing RYGB had a mean (SD) age of 52.1 (8.5) years and nonsurgical matches had a mean (SD) age of 52.2 (8.4) years. Patients undergoing RYGB and nonsurgical matches were predominantly white (1503 [84.1%] and 4452 [83.9%], respectively); 981 (54.9%) and 2927 (55.2%) had diagnosed diabetes and 595 (33.3%) and 1742 (32.8%) were superobese (BMI ≥50) at baseline, respectively. Patients undergoing RYGB had a mean (SD) BMI of 47.7 (7.8) and 1306 (73.1%) were male, whereas nonsurgical matches had a mean (SD) BMI of 47.1 (7.2) and 3911 (73.7%) were male. Hypertension, dyslipidemia, and depression were common in both groups (Table). Follow-up rates were high (eFigure 1 in the Supplement): 95.6% up to 3 years and 81.9% up to 10 years for surgical patients and 91.1% up to 3 years and 67.4% up to 10 years for nonsurgical matches.
Table.
Baseline Characteristics of Bariatric Surgical Patients by Surgical Procedure Type and Nonsurgical Matchesa
| Characteristic | AGB Group (n = 246) | SG Group (n = 379) | RYGB Group (n = 1787) | Nonsurgical Matches (n = 5305) |
|---|---|---|---|---|
| Variables included in match | ||||
| Male sex | 181 (73.6) | 291 (76.8) | 1306 (73.1) | 3911 (73.7) |
| Diagnosed diabetes | 123 (50.0) | 215 (56.7) | 981 (54.9) | 2927 (55.2) |
| White race | 177 (72.0) | 284 (74.9) | 1503 (84.1) | 4452 (83.9) |
| Nonwhite or unknown race | 69 (28.0) | 95 (25.1) | 284 (15.9) | 853 (16.1) |
| Age, mean (SD), y | 52.6 (9.7) | 53.5 (9.6) | 52.1 (8.5) | 52.2 (8.4) |
| BMI at baseline, mean (SD) | 42.7 (6.6) | 43.8 (6.6) | 47.7 (7.8) | 47.1 (7.2) |
| Superobese (BMI ≥50) | 32 (13.0) | 58 (15.3) | 595 (33.3) | 1742 (32.8) |
| Diagnostic Cost Group score, mean (SD) | 0.74 (0.69) | 0.81 (0.72) | 0.91 (0.75) | 0.84 (0.69) |
| Married | 136 (55.3) | 195 (51.5) | 962 (53.8) | 2609 (49.2) |
| Required to pay VA copayments | 32 (13.0) | 42 (11.1) | 195 (10.9) | 485 (9.1) |
| Exempt from VA copayments because of disability | 141 (57.3) | 233 (61.5) | 963 (53.9) | 2727 (51.4) |
| Exempt from VA copayments because of low income | 58 (23.6) | 79 (20.8) | 488 (27.3) | 1597 (30.1) |
| Geographic region (VISN) | ||||
| New England (VISN 1) | 1 (0.4) | 2 (0.5) | 15 (0.8) | 44 (0.8) |
| Upstate New York (VISN 2) | 1 (0.4) | 0 | 8 (0.5) | 23 (0.4) |
| New York or New Jersey (VISN 3) | 4 (1.6) | 45 (11.9) | 5 (0.3) | 17 (0.3) |
| VISN4 Network (VISN 4) | 7 (2.9) | 43 (11.4) | 196 (11.0) | 571 (10.8) |
| Capitol (VISN 5) | 0 | 1 (0.3) | 14 (0.8) | 40 (0.8) |
| Middle Atlantic (VISN 6) | 3 (1.2) | 21 (5.5) | 21 (1.2) | 63 (1.2) |
| Southeast (VISN 7) | 1 (0.4) | 6 (1.6) | 40 (2.2) | 118 (2.2) |
| Sunshine (VISN 8) | 7 (2.9) | 2 (0.5) | 135 (7.6) | 400 (7.5) |
| Middle South (VISN 9) | 10 (4.1) | 63 (16.6) | 209 (11.7) | 633 (11.9) |
| Ohio (VISN 10) | 1 (0.4) | 14 (3.7) | 60 (3.4) | 175 (3.3) |
| Veterans in Partnership (VISN 11) | 0 | 0 | 8 (0.5) | 27 (0.5) |
| Great Lakes (VISN 12) | 1 (0.4) | 8 (2.1) | 56 (3.1) | 165 (3.1) |
| Heartland (VISN 15) | 0 | 0 | 31 (1.7) | 101 (1.9) |
| South Central (VISN 16) | 60 (24.4) | 0 | 89 (5.0) | 270 (5.1) |
| Texas (VISN 17) | 58 (23.6) | 66 (17.4) | 153 (8.6) | 453 (8.5) |
| Southwest (VISN 18) | 2 (0.8) | 1 (0.3) | 26 (1.5) | 82 (1.6) |
| Rocky Mountains (VISN 19) | 1 (0.4) | 1 (0.3) | 15 (0.8) | 45 (0.9) |
| Northwest (VISN 20) | 2 (0.8) | 10 (2.6) | 153 (8.6) | 451 (8.5) |
| Sierra Pacific (VISN 21) | 20 (8.1) | 48 (12.7) | 248 (13.9) | 721 (13.6) |
| Desert Pacific (VISN 22) | 55 (22.4) | 46 (12.1) | 215 (12.0) | 644 (12.1) |
| Midwest (VISN 23) | 12 (4.9) | 2 (0.5) | 90 (5.0) | 262 (4.9) |
| Diagnosesb | ||||
| Hypertension | 183 (74.4) | 308 (81.3) | 1446 (80.9) | 3762 (70.9) |
| Dyslipidemia | 145 (58.9) | 270 (71.2) | 1049 (58.7) | 2738 (51.6) |
| Arthritis | 66 (26.8) | 105 (27.7) | 496 (27.8) | 812 (15.3) |
| Depression | 108 (43.9) | 171 (45.1) | 790 (44.2) | 1739 (32.8) |
| Coronary artery disease | 36 (14.6) | 68 (17.9) | 364 (20.4) | 1006 (19.0) |
| GERD | 83 (33.7) | 138 (36.4) | 621 (34.8) | 1027 (19.4) |
| Asthma | 21 (8.5) | 39 (10.3) | 219 (12.3) | 520 (9.8) |
| Fatty liver disease | 15 (6.1) | 23 (6.1) | 122 (6.8) | 31 (0.6) |
| PTSD | 41 (16.7) | 84 (22.2) | 309 (17.3) | 830 (15.6) |
| Alcohol abuse | 12 (4.9) | 12 (3.2) | 70 (3.9) | 340 (6.4) |
| Other substance abuse | 6 (2.4) | 9 (2.4) | 71 (4.0) | 234 (4.4) |
| Schizophrenia | 6 (2.4) | 3 (0.8) | 32 (1.8) | 285 (5.4) |
Abbreviations: AGB, adjustable gastric banding; BMI, body mass index (calculated as the weight in kilograms divided by height in meters squared); GERD, gastrointestinal reflux disease; PTSD, posttraumatic stress disorder; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; VA, Department of Veterans Affairs; VISN, Veterans Integrated Service Network.
Data are presented as number (percentage) of study participants unless otherwise indicated. Sample sizes reflect surgical patients and nonsurgical matches who are still alive at 1 year of follow-up. In addition, matches who underwent bariatric surgery within 1 year of follow-up are excluded. A total of 1787 patients underwent RYGB in the case vs match analysis, but only 1785 patients underwent RYGB in the surgical procedures analysis because 2 of these patients only had observed weights at more than 5 years after surgery.
All diagnoses were identified from inpatient and outpatient visit records using International Classification of Diseases, Ninth Revision codes.
Ten-Year Weight Change in Patients Undergoing RYGB and Nonsurgical Matches
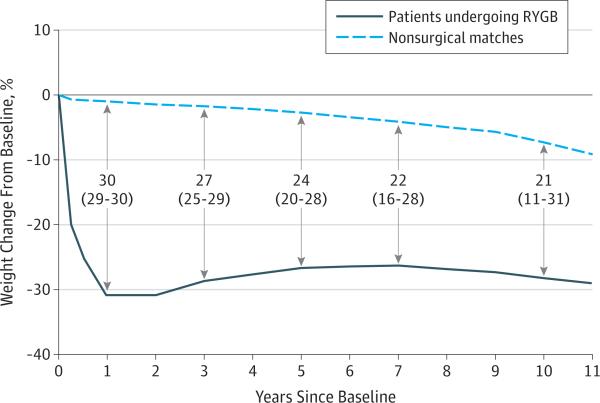
Patients undergoing RYGB lost an estimated 31.0% (95% CI, 30.4%-31.6%) of their baseline weight at 1 year (Figure 1), whereas nonsurgical matches lost 1.1% (95% CI, 0.7%-1.6%) of their baseline weight at 1 year. Thus, patients undergoing RYGB lost 29.9% (95% CI, 29.3%-30.5%) more of their baseline weight at 1 year than nonsurgical matches, and this difference remained clinically significant at 26.9% (95% CI, 24.7%-29.1%) at year 3, 24.0% (95% CI, 19.9%-28.1%) at year 5, and 22.2% (95% CI, 16.0%-28.5%) in year 7. At year 10, patients undergoing RYGB had maintained significantly greater weight loss (21.3%; 95% CI, 11.4%-31.1%) than nonsurgical matches. Patients undergoing RYGB lost 28.6% (95% CI, 19.5%-37.6%) of their baseline weight at 10 years, whereas nonsurgical matches lost 7.3% (95% CI, 1.4%-13.3%) of their baseline weight at 10 years.
Figure 1. Differences in Estimated Weight Changes Among Patients Undergoing Roux-en-Y Gastric Bypass (RYGB) and Nonsurgical Matches.
Estimated values, differences, and 95% CIs (shown in parentheses) were generated from a penalized spline mixed-effects model (7092 patients: 5305 nonsurgical matches and 1787 patients undergoing RYGB). Numbers and arrows in the center of the figure represent the differences and 95% CIs of the differences between nonsurgical matches and patients undergoing RYGB at years 1, 3, 5, 7, and 10. The sample for whom there was follow-up weight data for each year are as follows: year 1, n = 6894 patients (5131 nonsurgical matches and 1763 patients undergoing RYGB); year 3, n = 6301 (4629 nonsurgical matches and 1672 patients undergoing RYGB); year 5, n = 5172 (3748 nonsurgical matches and 1424 patients undergoing RYGB); year 7, n = 3942 (2806 nonsurgical matches and 1136 patients undergoing RYGB); and year 10, n = 1847 (1274 nonsurgical matches and 573 patients undergoing RYGB).
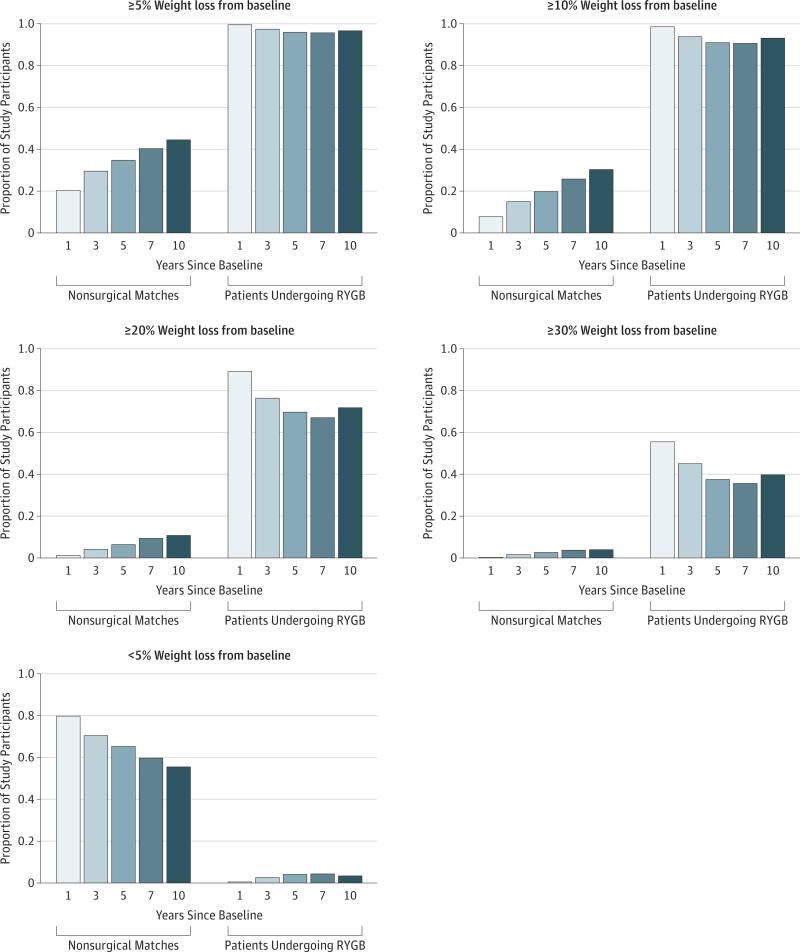
Among patients undergoing RYGB with a 10-year weight (n = 564), mean weight loss from baseline was 41.3 kg, and mean excess body weight loss from baseline was 56.4%. Mean weight loss for nonsurgical matches with a 10-year weight (n = 1247) was 6.3 kg, and mean excess body weight loss was 7.7%. At 10 years, 405 of 564 patients undergoing RYGB (71.8%) maintained weight loss of more than an estimated 20% of their baseline weight, and 224 of 564 (39.7%) maintained more than an estimated 30% weight loss (Figure 2). Only 19 of 564 patients undergoing RYGB (3.4%) were within 5% of their original baseline weight at 10 years compared with 692 of 1247 nonsurgical matches (55.5%).
Figure 2. Proportion of Patients Undergoing Roux-en-Y Gastric Bypass (RYGB) and Nonsurgical Matches With Weight Loss of Less Than 5%, 5% or More, 10% or More, 20% or More, or 30% or More at 1, 3, 5, 7, and 10 Years.
Predicted weights at 1, 3, 5, 7, and 10 years after baseline were estimated from a penalized spline mixed-effects model. Only individuals who had a weight measurement within 12 months of (6 months before to 6 months after) and a measurement after the year after surgery were included in this figure. The sample sizes for the proportions at each year are as follows: year 1, n = 6615 (4883 nonsurgical matches and 1732 patients undergoing RYGB); year 3, n = 5900 (4307 nonsurgical matches and 1593 patients undergoing RYGB); year 5, n = 4867 (3519 nonsurgical matches and 1348 patients undergoing RYGB); year 7, n = 3736 (2652 nonsurgical matches and 1084 patients undergoing RYGB); and year 10, n = 1811 (1247 nonsurgical matches and 564 patients undergoing RYGB).
Differences in Patient Characteristics Across Procedures
The RYGB, SG, and AGB subgroups of surgical patients were different in several respects (Table). Patients undergoing SG were older, less likely to be married, and more likely to be male or have diagnosed diabetes than the patients undergoing RYGB or AGB. Patients undergoing RYGB were more likely to be superobese or white than the patients undergoing SG or AGB. There was also significant regional variation in surgical procedures being performed, with 173 of 246 AGBs (70.3%) performed in 3 regions compared with only 112 of 379 SG cases (29.5%) and 475 of 1787 RYGB cases (25.6%) in those regions.
Trends in Postsurgical Body Weight by Procedure
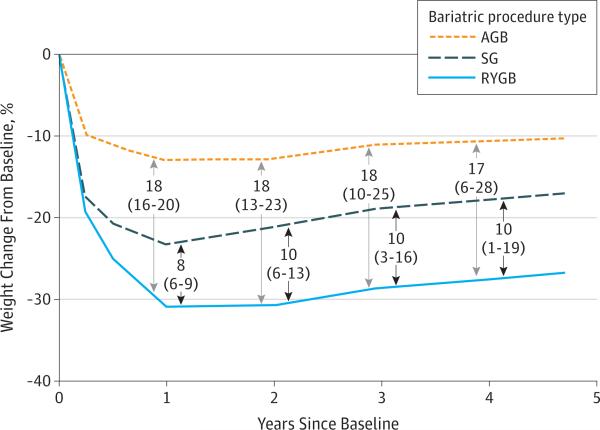
Patients undergoing RYGB experienced the greatest weight loss in each year of the 4-year follow-up. Patients undergoing SG experienced less weight loss, and patients undergoing AGB lost the least weight (Figure 3). At 1 year, patients undergoing RYGB lost 30.9% of their baseline weight (95% CI, 30.2%-31.6%), patients undergoing AGB lost 13.0% (95% CI, 11.1%-14.9%), and patients undergoing SG lost 23.4% (95% CI, 21.8%-24.7%). Patients undergoing RYGB lost 17.9% (95% CI, 15.9%-19.9%) more of their baseline weight at 1 year than patients undergoing AGB and 7.6% (95% CI, 5.9%-9.2%) more than patients undergoing SG. At 4 years, patients undergoing RYGB lost 27.5% of their baseline weight (95% CI, 23.8%-31.2%), patients undergoing AGB lost 10.6% (95% CI, 0.6%-20.6%), and patients undergoing SG lost 17.8% (95% CI, 9.7%-25.9%). Patients undergoing RYGB had lost 16.9% (95% CI, 6.2%-27.6%) more of their baseline weight at 4 years than patients undergoing AGB and 9.7% (95% CI, 0.8%-18.6%) more than patients undergoing SG.
Figure 3. Differences in Estimated Percentage of Weight Change From Baseline by Surgical Procedure Type.
Estimated values, differences, and 95% CIs (shown in parentheses) were generated from a penalized spline mixed-effects model (2410 patients: 246 in the adjustable gastric banding [AGB] group, 379 in the sleeve gastrectomy [SG] group, and 1785 in the Roux-en-Y gastric bypass [RYGB] group). Numbers and arrows in the center of the figure represent the differences and 95% CIs of the differences between the AGB and RYGB groups (top) and the SG and RYGB groups (bottom) at years 1, 2, 3, and 4. The sample for whom there was follow-up weight data for each year and procedure are as follows: year 1, n = 2373 patients (244 patients undergoing AGB, 374 patients undergoing SG, and 1755 patients undergoing RYGB); year 2, n = 2300 (237 patients undergoing AGB, 363 patients undergoing SG, and 1700 patients undergoing RYGB); year 3, n = 2183 (230 patients undergoing AGB, 325 patients undergoing SG, and 1628 patients undergoing RYGB); and year 4, n = 1845 (202 patients undergoing AGB, 181 patients undergoing SG, and 1462 patients undergoing RYGB).
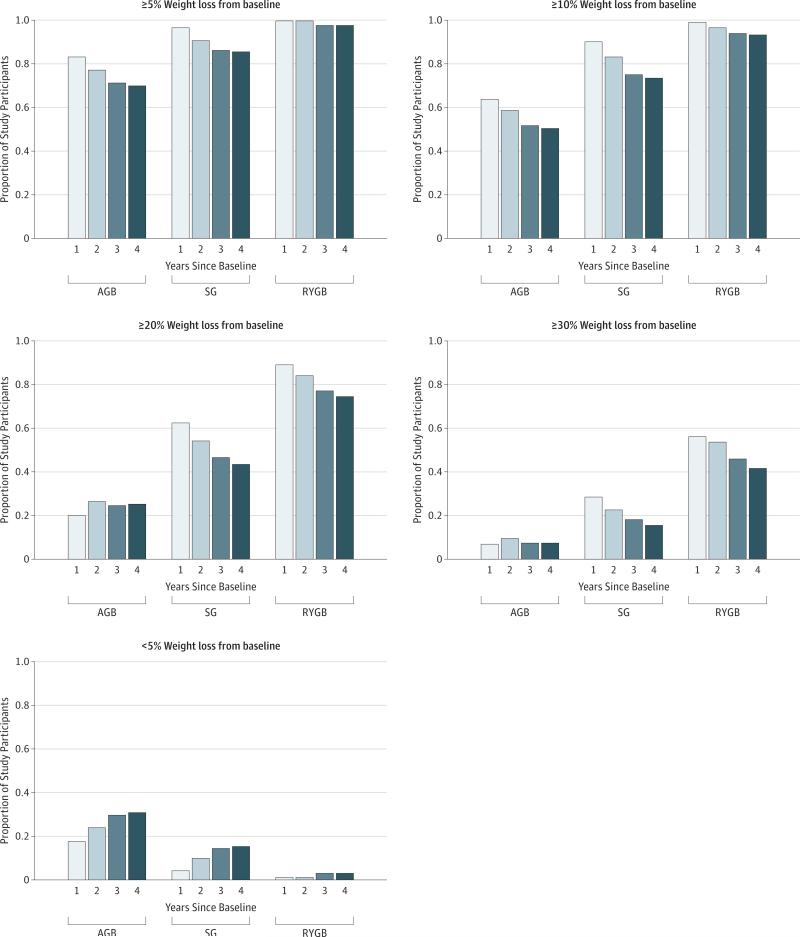
Among patients with a 4-year weight, mean weight loss from baseline was 41.0 kg for patients undergoing RYGB (n = 1431), 15.4 kg for patients undergoing AGB (n = 200), and 25.3 kg for patients undergoing SG (n = 178). The mean (SD) excess body weight loss at 4 years from baseline was 28.6% (30.5%) for patients undergoing AGB, 43.0% (28.1%) for patients undergoing SG, and 60.0% (26.1%) for patients undergoing RYGB. A greater proportion of patients undergoing RYGB lost an estimated 20% or more and 30% or more of their baseline weight in years 1 to 4 than patients undergoing SG or AGB (Figure 4). A greater proportion of patients undergoing AGB lost less than 5% of their baseline weight at 4 years than patients undergoing SG and RYGB (61 of 200 [30.5%] vs 26 of 178 [14.6%] vs 35 of 1431 [2.5%]).
Figure 4. Proportion of Patients Undergoing Adjustable Gastric Banding (AGB), Sleeve Gastrectomy (SG), and Roux-en-Y Gastric Bypass (RYGB) With Weight Loss of Less Than 5%, 5% or More, 10% or More, 20% or More, or 30% or More at 1, 2, 3, and 4 Years by Procedure.
Individual predicted weights at 1, 2, 3, and 4 years after surgery were estimated from a penalized spline mixed-effects model. Only patients who had a weight measurement within 12 months (6 months before to 6 months after) of the year after surgery were included in this figure. The sample sizes for the proportions at each year are as follows: year 1, n = 2336 patients (243 patients undergoing AGB, 369 patients undergoing SG, and 1724 patients undergoing RYGB); year 2, n = 2231 (233 patients undergoing AGB, 358 patients undergoing SG, and 1640 patients undergoing RYGB); year 3, n = 2116 (225 patients undergoing AGB, 315 patients undergoing SG, and 1576 patients undergoing RYGB); and year 4, n = 1809 (200 patients undergoing AGB, 178 patients undergoing SG, and 1431 patients undergoing RYGB).
Discussion
In this study, we address an evidence gap about long-term weight change associated with current bariatric procedures using a large multisite cohort of veterans in the United States with excellent follow-up. We found that patients undergoing RYGB were able to sustain significantly greater weight loss than nonsurgical matches up to 10 years after surgery. The nonsurgical matches experienced modest weight loss, most likely because of age-related changes. A total of 405 patients undergoing RYGB (71.8%) maintained 20% or greater weight loss at 10 years, which is similar to the 34 patients undergoing RYGB (73%) in the Swedish Obese Subjects study.19 In contrast to the Swedish Obese Subjects study, our follow-up rate for 10-year weight measures was substantially higher (81.9% vs 66.0% of those eligible).
In the only other long-term controlled study of patients undergoing RYGB in the United States with a high follow-up rate (96%), Adams and colleagues21 reported that 76% of patients undergoing RYGB sustained 20% or greater weight loss at 6 years, which is consistent with our results. We also found that 224 of 564 patients undergoing RYGB (39.7%) maintained a 30% or greater weight loss at 10 years, which is comparable to the 41% of patients in the study by Adams et al21 who maintained greater than 30% weight loss at 6 years. Furthermore, we found that only 19 of 564 patients undergoing RYGB (3.4%) had regained weight back to within 5% of their original baseline weight at 10 years, demonstrating that the long-term failure rate of RYGB is very low. These findings provide additional strong evidence for durable long-term weight loss among patients undergoing RYGB.
Sleeve gastrectomy is a relatively new procedure, so we were not able to evaluate outcomes for SG for the full 10-year study duration because there was only a high degree of follow-up for 4 years. For the 4 years after the initial operation, patients undergoing RYGB had the largest weight loss with somewhat smaller weight loss observed for patients undergoing SG and even less for patients undergoing AGB. These observations are consistent with previous studies1,17,18,20 of shorter-term (1-3 years) bariatric surgery weight loss. Significant weight regain was rare for patients undergoing RYGB. Nearly 1 in 3 patients undergoing AGB (61 of 200 [30.5%) regained all their lost weight (within 5% of baseline) by 4 years after surgery, whereas 26 of 178 patients undergoing SG (14.6%) and only 35 of 1431 patients undergoing RYGB (2.5%) experienced this degree of weight regain. Patients who regain weight may still have better long-term health outcomes than patients who never lost significant weight, but weight regain is one reason why the AGB procedure has fallen out of favor in recent years.25
Head-to-head comparisons of surgical procedures with long-term follow-up are important to inform procedure selection by patients and surgeons because, to our knowledge, only 3 large, multisite, US-based studies13,16,21 have been published comparing weight change with more than 2 years of follow-up. Because of the recent introduction of SG, long-term outcome data for the safety and durability of SG procedures are lacking. This is a critical evidence gap, given that SG is now the most commonly performed procedure in the United States (51.7% of all procedures in 2014).25
More evidence is needed on postsurgical complications, disease resolution, and long-term mental health outcomes to help surgical candidates choose the procedure that is best for them. Engaging patients in a high-quality shared decision-making33 conversation about their weight loss treatment options (including no treatment) is critical because prior studies34-36 have found that patients have unrealistic expectations of the weight loss that bariatric surgery will help them achieve. Untreated severely obese patients are unlikely to achieve significant weight loss, although the nonsurgical matches in our study experienced modest weight loss, most likely because of age-related changes.
This study has several limitations. First, patients were not randomized, risking unobserved confounding that persisted after matched comparisons. Second, nonsurgical matches did not receive formal weight loss treatment, but it is possible that a small subset participated in the VA's MOVE! program. However, national MOVE! participation and mean weight loss among participants have been low,37 so possible inclusion of MOVE! participants in our study is unlikely to have biased our outcomes estimates. Third, comorbid health conditions of the Diagnostic Cost Group score were identified using International Classification of Diseases, Ninth Revision diagnosis codes, which can be inaccurate and do not account for disease severity. Fourth, there may be bias attributable to loss to follow-up. Fifth, weight data were not systematically and routinely collected as part of a prospective data collection effort as in the Longitudinal Assessment of Bariatric Surgery,12 so weights at specific time points were model-estimated predictions.
This study also has several important strengths, including having a high 10-year follow-up rate (81.9% for surgical patients and 67.4% for controls), a high degree of generalizability because we included outcomes from many different surgeons across multiple sites, inclusion of carefully matched nonsurgical controls, and direct comparison of the 3 most common surgical procedures currently performed. Our results are consistent with the only other long-term observational study with a control population (Swedish Obese Subjects study) and with shorter-term comparative studies of weight change after RYGB, AGB, and SG procedures.17,38 Finally, this study provides evidence of long-term weight loss after bariatric surgery for older men for whom evidence is sparse because all prior studies6-13 were predominantly composed of women in their 40s.
Conclusions
Among obese patients receiving care in the VA health care system, veterans who underwent RYGB lost much more weight than nonsurgical matches and were able to sustain most of this weight loss in the long term. We found that RYGB induced significantly more weight loss at 4 years than SG or AGB. These results provide further evidence for the beneficial association between surgery and long-term weight loss that has been demonstrated in shorter-term studies of younger, predominantly female populations.
Supplementary Material
Key Points.
Questions How much weight loss can be expected on average 10 years after undergoing Roux-en-Y gastric bypass (RYGB) and how does weight loss 4 years after bariatric surgery compare among patients undergoing RYGB, adjustable gastric banding, and sleeve gastrectomy?
Findings In this cohort study, patients undergoing RYGB lost 21% more of their baseline weight than matched nonsurgical patients at 10 years, and RYGB induced significantly greater weight loss than sleeve gastrectomy or adjustable gastric banding at 4 years.
Meaning Bariatric surgery, especially RYGB, is effective at promoting long-term weight loss among obese patients.
Acknowledgments
Funding/Support: This research was funded by grant IIR 10-159 from the Office of Research and Development, Health Services Research and Development Service, Department of Veterans Affairs (principal investigator, Dr Maciejewski). Dr Maciejewski was also supported by Research Career Scientist award RCS 10-391 from the Department of Veterans Affairs.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. This study was reviewed by the Surgical Quality Data Use Group of the Veterans Affairs National Surgery Office for adherence to the data use agreement.
Footnotes
Author Contributions: Drs Maciejewski and Olsen had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Maciejewski, Arterburn, Smith, Yancy, Livingston, Olsen.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Maciejewski, Arterburn, Van Scoyoc, Livingston, Olsen.
Critical revision of the manuscript for important intellectual content: Maciejewski, Arterburn, Smith, Yancy, Weidenbacher, Livingston, Olsen.
Statistical analysis: Arterburn, Van Scoyoc, Smith, Olsen.
Obtaining funding: Maciejewski, Arterburn.
Administrative, technical, or material support: Maciejewski, Arterburn, Weidenbacher, Livingston.
Study supervision: Maciejewski, Arterburn, Livingston.
Conflict of Interest Disclosures: Dr Maciejewski reported receiving institutional grants from the Department of Veterans of Affairs and the Agency for Healthcare Research and Quality, receiving an institutional contract from the Centers for Medicare & Medicaid Services, and owning Amgen stock because of his spouse's employment. Dr Arterburn reported receiving institutional grants from the National Institutes of Health, the Patient Centered Outcomes Research Institute, the Department of Veterans of Affairs, and the Informed Medical Decisions Foundation and receiving payment for travel expenses from the Informed Medical Decisions Foundation. Dr Olsen reported receiving institutional grants from the National Institutes of Health, the Patient Centered Outcomes Research Institute, and the Department of Veterans of Affairs. Drs Smith and Yancy reported receiving institutional grants from the National Institutes of Health and the Department of Veterans of Affairs. No other disclosures were reported.
Disclaimer: Dr Livingston is a full-time editor at JAMA but was not involved in the editorial review of the paper at the journal. The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs, the US government, Duke University, the University of Texas Southwestern Medical Center, the Group Health Research Institute, or the University of Washington.
Additional Contributions: Theodore Berkowitz, MS, Center for Health Services Research in Primary Care, Durham Veterans Affairs Medical Center, Durham, North Carolina, generated all the figures. No financial compensation was given for this work.
TB
REFERENCES
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(41):1–190, 215-357, iii-iv. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- 3.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. doi: 10.1136/bmj.g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trastulli S, Desiderio J, Guarino S, et al. Laparoscopic sleeve gastrectomy compared with other bariatric surgical procedures: a systematic review of randomized trials. Surg Obes Relat Dis. 2013;9(5):816–829. doi: 10.1016/j.soard.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312(9):915–922. doi: 10.1001/jama.2014.10540. [DOI] [PubMed] [Google Scholar]
- 7.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer PR, Bhatt DL, Kirwan JP, et al. STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes: 3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716–726. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149(7):707–715. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150(10):931–940. doi: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courcoulas AP, Christian NJ, Belle SH, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 15.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–2249. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arterburn D, Powers JD, Toh S, et al. Comparative effectiveness of laparoscopic adjustable gastric banding vs laparoscopic gastric bypass. JAMA Surg. 2014;149(12):1279–1287. doi: 10.1001/jamasurg.2014.1674. [DOI] [PubMed] [Google Scholar]
- 17.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–420. doi: 10.1097/SLA.0b013e31822c9dac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlin AM, Zeni TM, English WJ, et al. Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257(5):791–797. doi: 10.1097/SLA.0b013e3182879ded. [DOI] [PubMed] [Google Scholar]
- 19.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 20.Puzziferri N, Roshek, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien PE, Brennan L, Laurie C, Brown W. Intensive medical weight loss or laparoscopic adjustable gastric banding in the treatment of mild to moderate obesity: long-term follow-up of a prospective randomised trial. Obes Surg. 2013;23(9):1345–1353. doi: 10.1007/s11695-013-0990-3. [DOI] [PubMed] [Google Scholar]
- 23.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 24.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25(10):1822–1832. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 25.Ponce J, Nguyen NT, Hutter M, Sudan R, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in the United States, 2011-2014. Surg Obes Relat Dis. 2015;11(6):1199–1200. doi: 10.1016/j.soard.2015.08.496. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy EH, Taylor JM, Schaubel DE, Williams S. The effect of salvage therapy on survival in a longitudinal study with treatment by indication. Stat Med. 2010;29(25):2569–2580. doi: 10.1002/sim.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YP, Propert KJ, Rosenbaum PR. Balanced risk set matching. J Am Stat Assoc. 2001;96(455):870–882. [Google Scholar]
- 28.Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005;61(3):721–728. doi: 10.1111/j.1541-0420.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 29.Gu XS, Rosenbaum PR. Comparison of multivariate matching methods: structures, distances and algorithms. J Comput Graph Stat. 1993;2(4):405–420. [Google Scholar]
- 30.Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring) 2013;21(8):1519–1525. doi: 10.1002/oby.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzmaurice GL, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; New York, NY: 2004. [Google Scholar]
- 32.Durbán M, Harezlak J, Wand MP, Carroll RJ. Simple fitting of subject-specific curves for longitudinal data. Stat Med. 2005;24(8):1153–1167. doi: 10.1002/sim.1991. [DOI] [PubMed] [Google Scholar]
- 33.Hsu C, Liss DT, Westbrook EO, Arterburn D. Incorporating patient decision aids into standard clinical practice in an integrated delivery system. Med Decis Making. 2013;33(1):85–97. doi: 10.1177/0272989X12468615. [DOI] [PubMed] [Google Scholar]
- 34.Wee CC, Jones DB, Davis RB, Bourland AC, Hamel MB. Understanding patients’ value of weight loss and expectations for bariatric surgery. Obes Surg. 2006;16(4):496–500. doi: 10.1381/096089206776327260. [DOI] [PubMed] [Google Scholar]
- 35.Kaly P, Orellana S, Torrella T, Takagishi C, Saff-Koche L, Murr MM. Unrealistic weight loss expectations in candidates for bariatric surgery. Surg Obes Relat Dis. 2008;4(1):6–10. doi: 10.1016/j.soard.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Heinberg LJ, Keating K, Simonelli L. Discrepancy between ideal and realistic goal weights in three bariatric procedures: who is likely to be unrealistic? Obes Surg. 2010;20(2):148–153. doi: 10.1007/s11695-009-9982-8. [DOI] [PubMed] [Google Scholar]
- 37.Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration's MOVE! Weight Management Program. Transl Behav Med. 2011;1(4):551–560. doi: 10.1007/s13142-011-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis. 2014;10(3):396–403. doi: 10.1016/j.soard.2014.02.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.