Abstract
The Hippo-YAP pathway mediates organ size control, contact inhibition, and tumorigenesis. It is a kinase cascade that inhibits the nuclear localization and transcriptional activities of YAP and TAZ. E-cadherin, cell junctions, polarity proteins, and the merlin/NF2 tumor suppressor activate the pathway to inhibit YAP/TAZ activity, while growth factor signaling inhibits the pathway to activate YAP/TAZ in the nucleus. We examined its role in the development of mouse mammary glands and tumor formation using gland reconstitution by transplantation of genetically modified mammary stem cells (MaSCs). Knockdown of YAP and TAZ with shRNA in MaSCs did not inhibit gland reconstitution. In contrast, knockdown of β-catenin blocked gland reconstitution, consistent with the known role of Wnt signaling in mammary gland development. However, we find that Hippo signaling is involved in mammary tumor formation. Expression of a constitutively active form of YAP caused rapid formation of large tumors. Moreover, knockdown of YAP/TAZ slowed the development of tumors in polyoma middle T (PyMT) transgenic mice, a well-studied mammary tumor model involving activation of several signaling pathways. YAP accumulated in nuclei of mammary glands in ErbB2/EGFR transgenic mice, suggesting that EGFR signaling affects YAP in vivo similar to cell culture. ErbB2/EGFR transgenic mice develop mammary tumors in 7–8 months, but surprisingly, MaSCs from these mice did not form tumors when transplanted into host mice. Nonetheless expression of dominant negative Lats, which inhibits hippo signaling, lead to tumor formation in ErbB2 transgenic mice, suggesting that Hippo signaling is involved EGFR induced mammary tumorigenesis.
Introduction
The Hippo signaling pathway is an important growth inhibitory pathway in organisms. It has been implicated in organ size control in Drosophila embryos as well as mammals and has been found to mediate contact inhibition of growth (Bossuyt et al., 2013; Gumbiner and Kim, 2014; Halder and Johnson, 2011; Kim et al., 2011; Tumaneng et al., 2012; Yu and Guan, 2013; Zhao et al., 2007). The pathway consists of a serine kinase cascade in association with some scaffold proteins that act to inhibit the growth promoting transcriptional activators YAP and TAZ. The Lats kinase phosphorylates YAP and TAZ, leading to their cytoplasmic retention and/or degradation. Unsurprisingly, YAP and TAZ have been implicated in cancer growth (Harvey et al., 2013), although in some cases they have been proposed to be regulated independently of Lats activity and or the Hippo pathway (Aragona et al., 2013; Halder et al., 2012).
An important question is how the inhibitory activity of the Hippo pathway is integrated with growth promoting mitogenic signaling to control proliferation and tumor growth. We and other groups found that growth factors, including EGF, and serum factors stimulate YAP nuclear localization and transcriptional activity (Fan et al., 2013; Reddy and Irvine, 2013; Yu et al., 2012). In our own studies, stimulation of YAP nuclear localization was dependent on the PI3-Kinase-PDK1 branch of the EGFR signaling pathway but independent of Akt activity (Fan et al., 2013; Kim and Gumbiner, 2015). Importantly, YAP was found to be required for the proliferative response to upstream growth signals (Fan et al., 2013; Yu et al., 2012), suggesting that it functions in parallel with other known mitogenic pathways to control growth.
Many of these findings for mammalian cells were discovered using cultured cells in vitro, since they allowed for dissection of the signaling pathway mechanisms. But to understand the significance of these mechanistic observations, it is important to test their roles in vivo. YAP has been found to mediate cell growth downstream of Gq and G11 small G-proteins in uveal melanoma (Yu et al., 2014), presumably the in vivo function of growth mediated by LPA receptors in vitro. Although the YAP homolog in Drosophila, yorkie, was found to be required for EGFR driven proliferation in glial cell (Reddy and Irvine, 2013), it is not yet known whether the Hippo pathway or YAP activity are regulated by EGFR signaling in vivo in mammals, especially in the context of cancer.
In this study we undertook to examine the roles of Hippo-YAP signaling in growth factor signaling in vivo using mouse models mammary tumorigenesis. Mammary tumorigenesis was chosen for several reasons. We chose to study tumor growth because the Hippo pathway is known to mediate contact inhibition of growth, a phenomenon most strongly implicated in cancer. EGFR signaling and PI3K signaling have both been strongly implicated in mammary tumorigenesis, both in mice and humans (Guy et al., 1992; Hardy et al., 2010; Hopkins et al., 2014; Kim and Muller, 1999; Koren and Bentires-Alj, 2013; Troyer and Lee, 2001; Wickenden and Watson, 2010). Mammary tumors also provide a very accessible and well-characterized model to facilitate these studies (Cardiff et al., 2000; Hennighausen, 2000; Lin et al., 2003). In particular, a powerful stem cell transplantation technique has been developed that allows testing of genes manipulated in cultured stem cells to be tested in in vivo reconstituted mammary glands (McCaffrey and Macara, 2009; Welm et al., 2008). We took advantage of this technique to examine the role of Hippo-YAP signaling in mammary gland growth and tumorigenesis.
Materials and Methods
MaSC transduction
MaSCs were transduced with high titer lentiviral vectors pLVTHM (Addgene Cat #12247) expressing either proteins or shRNAs to inhibit protein expression, as previously described (Wiznerowicz and Trono, 2003). The dominant negative Lats2-KR plasmid was obtained from Addgene (Plasmid #33100). The shRNAs chosen for YAP, TAZ, and β-catenin depletion were determined by screening 6 different candidates for each by their effectiveness at reducing protein expression in mouse 4T1 cells. The RNAi targeting sequences used were as follows: mouse β-catenin shRNA, 5′-GGGAGAAGCCCTTGGATAT; mouse YAP shRNA, 5′-GCACAAGAATGAAGTAGAA; mouse TAZ shRNA, 5′-TAATCACATAGAGAAAATC).
Lentiviral vectors were cotransfected with pMD2.G envelop and psPAX2 packaging plasmids (Addgene, Cat ## 12259 and 12260 respectively) into HEK293LT cells for the virus production. Viruses in the media were collected and concentrated by centrifugation through Amicon ultra centrifugal filters (Millipore), and transduced to MaSCs. Transduction of viral vectors was confirmed by checking the expression of GFP reporter gene in MaSCs. Knock-down of target gene expression in MaSCs was confirmed by immunoblotting.
MaSC transplantation
MaSC transplantation was done using an established procedure (McCaffrey and Macara, 2009; Welm et al., 2008). Briefly, Mammary glands were surgically removed from virgin female mice, which were subsequently euthanized by cervical dislocation. Mammary stem cells (MaSC) were prepared from the glands as described and transduced with lentivirus in vitro (described above). Lentivirus transduced MaSCs were orthotopically transplanted into virgin female host mice, which were manipulated under anesthesia using survival surgery. A small circular incision was made to expose the number 4 mammary gland (this gland most easily accessible and, therefore most commonly used). The number 4 nipple and blood vessel near the junction of the lymph node and the blood vessel between the number 4 and 5 fat pads was cauterized (this prevents the contamination of the fat pad with endogenous epithelial cells that fill the pad during puberty). The developing mammary gland was then removed. Transduced MaSCs from donor females were injected in 10uL volumes using a 20uL Hamilton syringe and 26 gauge needle into the remaining fat pad of the host mice. The wound was closed with wound clips.
Mice, tumor development, and histology
Virgin female wildtype FVB strain of mice were used as hosts for all experiments and as donors for many experiments. Donors also included ErbB2 transgenic mice (FVB/N-Tg(MMTVneu)202Mul/J, Jackson laboratory), PyMT transgenic mice (FVB/N-Tg(MMTV-PyVT)634Mul/J, Jackson laboratory), and constitutively active Neu transgenic mice (FVB-Tg(MMTV-Erbb2)NK1Mul/J) were obtain from W. Muller (Guy et al., 1992; Muller et al., 1988).
Transplanted mice were examined for palpable mammary tumors twice weekly. Statistical analysis was performed using Fisher’s exact test of independence, which is best for small sample size of categorical data (McDonald, 2015). Tumors were removed and examined for GFP expression and by histochemical staining. Mammary glands were dissected from all mice that appeared to lack tumors and examined by H&E staining to confirm that glands had regenerated and examined for potential smaller tumors. Dissected mammary glands or tumors were sectioned and processed for histochemical and immunohistochemical staining by the Research Histology core facility at the University of Virginia School of Medicine. YAP was immunostained using rabbit mAb to mouse YAP (Cell Signaling).
Results
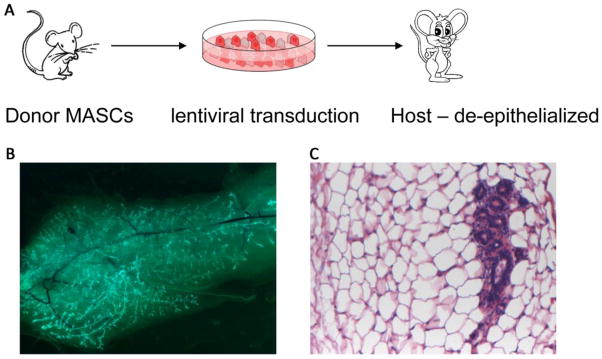
In order to investigate the role of Hippo-YAP pathway signaling in the formation of mammary tumors, we adopted a stem cell transplantation assay (figure 1). Mammary stem cells (MaSCs) obtained from a donor mouse can regenerate an entire mammary gland when transplanted into a de-epithelized mammary fat pad of a host mouse. Before transplantation, the MaSCs can be transduced using lentiviruses that either express a protein or an shRNA to inhibit the expression of a protein. An example is shown in figure 1. MaSCs transduced to express GFP regenerated branched mammary gland structures expressing GFP (B), and regenerated glandular tissue can be observed in histological sections (C).
Figure 1.
Mammary stem cell (MaSC) transplantation to evaluate gene function in mammary gland development and tumorigenesis. MaSCs are enriched from mammary glands removed from donor mice, transduced with high titer lentiviral vectors in cell culture, and transplanted into mammary fat pads of host mice from which mammary gland epithelia have been removed (A). Examples of resulting growth of mammary glands in host mice are shown; green fluorescence labeling due to EGFP expression with the lentiviral vector shows growth of transduced MaSCs (B); and histology demonstrates the presence of mammary gland tissue (B, see also figure 2).
For a positive control we examined the requirement for β-catenin in mammary gland development, which is known to depend on canonical Wnt signaling. Expression of an shRNA to β-catenin strongly inhibited the regeneration of the mammary gland; there was a complete absence of branching epithelial tissue, with only blood vessels remaining (figure 2C). Mammary gland development was previously shown to depends on β-catenin due to inhibition of β-catenin levels as a result of transgenic expression of its negative regulator axin (Hsu et al., 2001). Our result adds to those findings by directly demonstrating the requirement for β-catenin in mammary gland development. In contrast, expression of shRNAs that effectively deplete YAP and TAZ expression in cell culture in MaSCs did not inhibit mammary gland reconstitution (figure 2B), compared to control transplanted MaSCs (figure 2A), in contrast to the effects of their depletion on tumor growth (see below). Fairly normal branching epithelial ducts were regenerated, although we cannot exclude that there were more subtle defects in the branching patterns or development of fine branches. We did not examine whether there would be any effects on gland growth or differentiation during pregnancy. Thus, high levels of YAP and TAZ are not essential for mammary gland development in virgin mice. Although we cannot rule out a role for residual YAP or TAZ in such knockdown experiments, this finding is similar to findings previously reported using conditional mutant deletion of YAP/TAZ genes in mammary gland (Chen et al., 2014).
Figure 2.
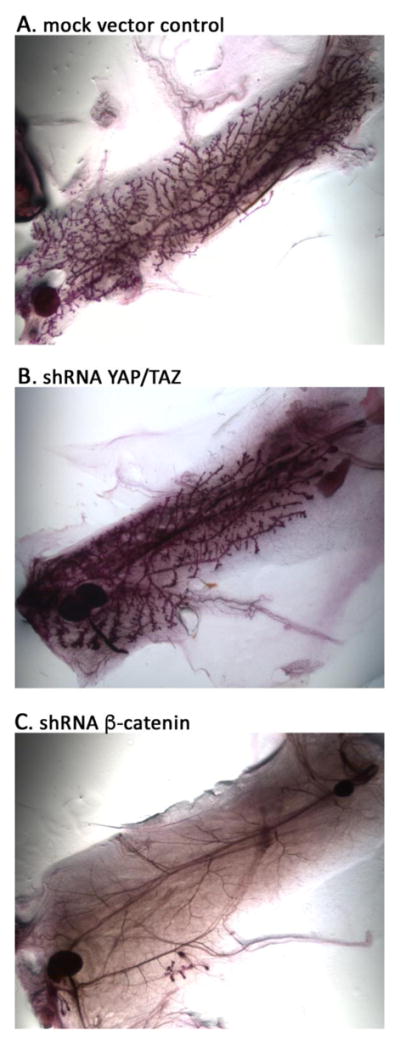
shRNA-mediated knockdown of beta-catenin, but not YAP/TAZ, inhibit mammary gland development. Photos show histology of entire dissected mammary fat pads from mice transplanted with MaSCs that were transduced with shRNAs as shown. A. Mock vector control; B. YAP/TAZ shRNA; C. β-catenin shRNA – note the lack of glandular epithelial tissue with only blood vessels remaining.
Although normal mammary gland development may not depend on YAP or TAZ, limiting their activities could be important to prevent overgrowth or tumor development. We therefore asked whether expression of a constitutively active form of YAP could affect mammary gland development or tumor formation. We expressed a mutant form of YAP with 5 serine residues mutated to alanine (5S>A) to prevent their phosphorylation in MaSCs. One of these residues, S127, is a well established target of Lats, and the S127A mutation inhibits its cytoplasmic retention, but several other nearby phospho serine residues have also been implicated in Lats dependent regulation (Hao et al., 2008; Zhao et al., 2007) (see discussion). Mammary glands regenerated from 5S>A YAP expressing MaSCs, but caused rapid formation of large palpable mammary tumors (figure 3). Co-expression of GFP shows that the histologically detected tumors arose from the MaSCs. Tumors developed within 3 months in 4 out of 9 animals transplanted with 5S>A YAP expressing MaSCs; 2/4 from MaSCs obtained from WT mice and 2/5 from ErbB2 tg mice (Table 1). Compared to the control mock treated MaSCs, which formed no tumors when obtained from either WT or ErbB2 transgenic mice, these differences were statistically significant (p = 0.013 and p = 0.026, respectively) by criteria of Fischer’s Exact Test. Thus, constitutively active YAP can behave as a fairly potent oncogene for mammary tumorigenesis.
Figure 3.
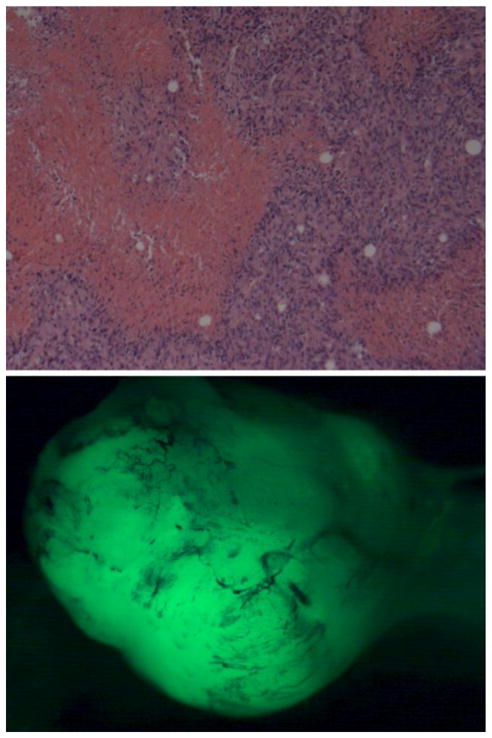
Large mammary tumors formed by expression of constitutively activated YAP in transplanted MaSCs. Palpable tumors were dissected out and stained. Top panel – H&E staining shows tumor tissue; bottom – shows that mass of tumor cells was derived from transplanted MaSCs which also express EGFP.
Table 1.
Summary of frequency of tumor formation caused by transplanted MaSCs obtained from WT or transgenic (tg) mice with various genetic manipulations prior to transplantation. Row 4 is just the tg mice alone without transplantation. Row 1 differs significantly from row 2, p = 0.013; row 3 differs significantly from row 5, p = 0.026; row 7 differs significantly from row 5, p = 0.012; row 7 vs row 8, p = 0.2, not statistically significant.
| Type transplanted MaSC | Numbers of tumors | Time until palpable tumors |
|---|---|---|
| 1. YAP-5S>A | 2/4 mice | ~ 3 months |
| 2. WT control | 0/27 | >13 months |
| 3. YAP-5S>A in ErbB2 tg | 2/5 mice | ~ 3 months |
| 4. ErbB2 tg mice (not transplants) | most mice | > 7 months |
| 5. ErbB2 tg MaSC grafts | 0/23 mice | >13 months |
| 6. ErbB2 tg MaSC grafts +YAP/TAZ shRNA | 0/15 mice | >13 months |
| 7. ErbB2 tg MaSC grafts + dom neg Lats | 3/8 mice | ~ 6 months |
| 8. WT MaSC grafts + dom neg Lats | 0/5 mice | 15 months |
We therefore asked whether YAP or TAZ play a role in mammary tumors induced by known signaling oncogenes. A very well studied model of mammary tumorigenesis is induced by polyoma middle T (PyMT) (Lin et al., 2003). PyMT binds to and activates numerous signaling pathways downstream of growth factor receptors, including Src family kinases, PI3kinase, ShcA and PLC γ; (Campbell et al., 1994; Courtneidge and Smith, 1983; Su et al., 1995; Whitman et al., 1985). We obtained MaSCs from donor animals with transgenic expression of PyMT in mammary gland under the control of the MMTV promoter; these mice normally develop aggressive mammary tumors at high frequency. These PyMT MaSCs were transduced with the lentivirus expressing shRNA to YAP and TAZ (same as used in figure 2) and transplanted into host mammary fat pads. Compared to mock transduced controls, expression of YAP and TAZ shRNAs substantially delayed the development of mammary tumors (figure 4). Given the tumorigenic potency of PyMT and the fact that it activates so many signaling proteins, it is not surprising that YAP/TAZ depletion did not completely block tumor formation. Nonetheless, these data show that YAP and/or TAZ play a role in mediating the oncogenesis in this model.
Figure 4.
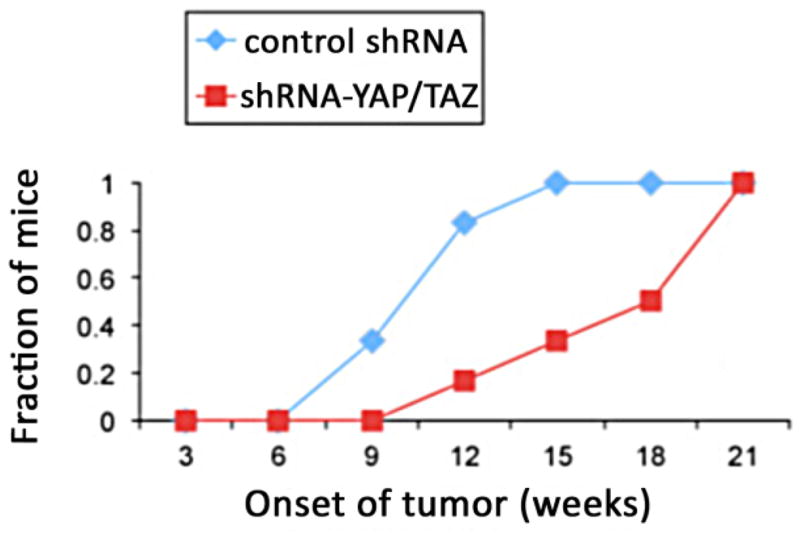
shRNA- mediated depletion of YAP and TAZ slow the growth of PyMT induced mammary tumors. MaSCs were derived from PyMT-transgenic mouse (driven by mammary MMTV promoter) and transduced with shRNA lentiviral vectors prior to transplantation into host mice. The number of mice with palpable mammary tumors was counted over time for treated and control groups.
We then undertook experiments to investigate whether Hippo signaling plays a role in tumorigenesis caused more specifically by oncogenic EGFR signaling. To do so we used a well established and commonly studied model of mammary tumorigenesis induced by overexpression of ErbB2 in mammary gland under control of the MMTV promoter (Guy et al., 1992). As reported in the literature, these transgenic mice developed mammary tumors in ~ 7–8 months (not shown). These mice exhibited more nuclear YAP staining in their mammary glands, even at early times before the development of tumors, compared to WT mice, which had mostly cytoplasmic YAP (figure 5). This suggests that EGFR overexpression inhibits Hippo pathway signaling in the mammary gland in vivo, similar to EGFR activation in mammary cell lines in vitro (Fan et al., 2013).
Figure 5.
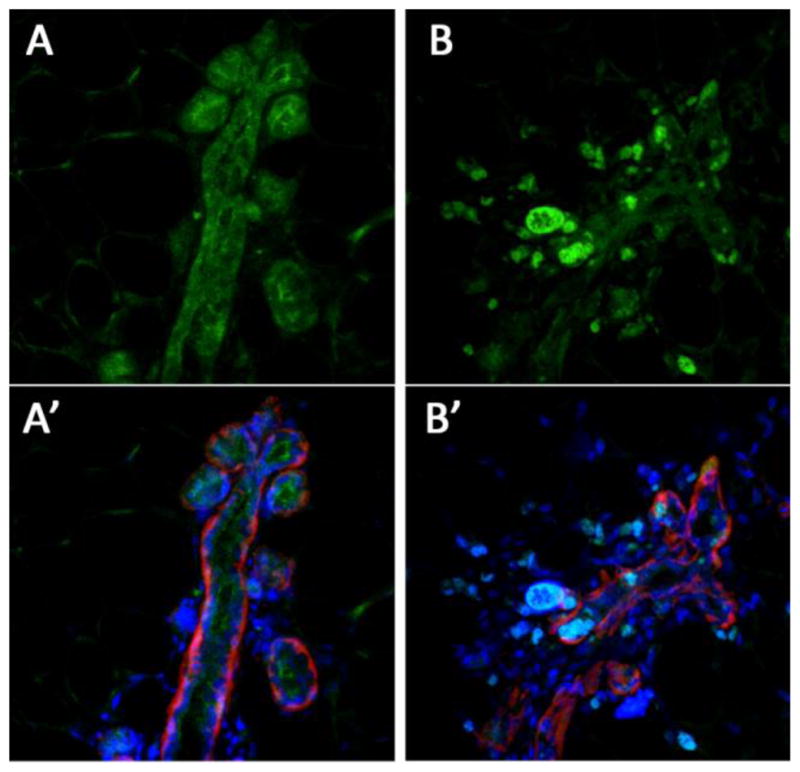
Nuclear localization of YAP in mammary glands of transgenic mice expressing the ErbB2 growth factor receptor. A, A′ – control normal mouse mammary gland. B, B′ – mammary gland from ErbB2 transgenic animal. Green – IF staining for YAP, Red – IF staining for laminin to show basement membranes, blue – nuclear stain.
To try to evaluate whether Hippo signaling plays a role in EGFR induced tumorigenesis, we carried out MaSC transplantation experiments utilizing MaSCs from these ErbB2 transgenic mouse. Prior to transplantation we genetically perturbed the pathway in these ErbB2 overexpressing MaSCs via lentiviral transduction expressing: mock shRNA for the control, YAP and TAZ shRNA as in figure 4, or expression of a dominant negative kinase dead form of Lats1 (Zhao et al., 2007) as a way to inhibit Hippo signaling and enhance YAP nuclear activity. The number of mice with palpable mammary tumors was determined over a period of 13 months and the results are shown in table 1. Importantly, expression of the dominant negative Lats1 construct increased the number of tumors formed in a rapid time frame, 3 out of 8 mice in 6 – 7 months compared to 0 out of 23 control ErbB2 transgenic mice after 13 months, which is statistically significant (p = 0.012, Fischer’s exact test). Dominant negative Lats expression by itself in WT MaSCs did not cause tumors (0 out of 5 mice after 15 months), suggesting that tumor formation resulting from Lats inhibition occurs only in the presence of the ErbB2 oncogene, although the small sample size resulted in a low statistical significance (p = 0.2, Fischer’s exact test).
A surprising and interesting finding was that transplantation of control mock transfected MaSCs from ErbB2 transgenic mice failed to give rise to any tumors, even after long time periods, 13 months or more (table 1), in contrast to 7–8 months for transgenic animals themselves that we used for donors. We also tested a more aggressive and faster model of EGFR induced mammary tumors, with transgenic donor mice expressing a constitutively activated Neu EGFR (Guy et al., 1992; Muller et al., 1988), but the reconstituted glands still failed to form any tumors after 8 months, compared to 4–6 months for the transgenic donor mice themselves (not shown). Although tumors did not form, the transplanted cells did reconstitute mammary glands in all cases. Unfortunately this lack of tumors in reconstituted mammary glands complicated our attempts to directly test whether YAP is required for ErbB2 induced mammary tumorigenesis. Nonetheless, this surprising result is revealing about the mechanism of EGFR induced mammary tumorigenesis, because it suggests that EGFR activation in the mammary epithelial stem cell compartment derived from developed donor glands alone is insufficient to drive tumor formation. It seems unlikely that this was due to the small number of cells transplanted initially, since these transplants grew into complete branching mammary glands of normal size without causing tumors. Although it is possible that the ErbB2 activity is lower as a result of cell transplantation, ErbB2 expression is driven by the strong MMTV promoter in these animals, and the fact that the constitutively active Neu receptor oncogene also failed to cause tumors makes this less likely. Also, the accumulation of nuclear YAP in these mammary glands compared to controls as well as the formation of tumors by ErbB2 transgenic MaSCs when dominant negative Lats is expressed suggest that the ErbB2 was active in these cells. An interesting possibility is that ErbB2 activity is also active in a non-epithelial compartment of the mammary gland; such as vascular cells or immune cells, which are not transplanted along with MaSCs. The MMTV promoter is thought to act primarily in the epithelial compartment, but it is only a partially specific promoter expressed in other tissues as well. Another possibility is that the ErbB2 needs to be overexpressed at an earlier critical time in development, and expression in the formed stem cell compartment is insufficient to cause tumor growth. This finding raises important questions about EGFR oncogenesis that may be interesting to investigate in future studies.
Discussion
Several findings in this manuscript demonstrate that Hippo-YAP signaling has a role in growth factor induced mammary tumorigenesis in vivo. Constitutive activation of YAP in transplanted MaSCs induces rapid development of mammary tumors, and loss of YAP and TAZ expression reduces the rate of mammary tumorigenesis induced by the PyMT oncogene, which activates many pathways downstream of signaling receptors. Oncogenic ErbB2 expression in mammary glands stimulated nuclear accumulation of YAP in the mammary gland, even before tumors develop. Interference with Lats activity in ErbB2 expressing mammary glands using expression of a dominant negative kinase dead mutant of Lats also induces tumors. Although the surprising lack of tumors resulting from transplanted ErBb2-transgenic MaSCs limited our ability to directly test the requirement for YAP/TAZ in ErbB2-induced tumors, these findings together support a role for Hippo signaling in growth factor induced mammary tumorigenesis in vivo.
Expression of YAP with constitutively activating mutations harboring 5 S>A mutations induced tumors in about half of the transplanted animals. This result differs from an earlier report using a YAP with only an S127A mutation, which did not induce hyperplasia but led to defects in terminal differentiation during pregnancy (Chen et al., 2014). We did not examine the effects of 5S>A YAP on gland development or tumorigenesis during pregnancy. Although the S127 residue has been the most well characterized target of Lats, it is clear from phostag gel experiments that numerous phosphorylation sites are involved in Hippo-Lats regulation of YAP (Hao et al., 2008; Zhao et al., 2007); moreover, the 5S>A mutation has been used in previous in vitro experiments to strongly activated it’s activity. The roles of all the serines is not certain, but our findings demonstrate that in the context of mammary tumorigenesis in vivo they play a role in regulation of YAP activity above and beyond the role of S127 alone. Cell culture experiments have shown that the S381 and S384 residues of YAP are involved in the regulation of its stability (Zhao et al., 2010) and the combinatorial mutation of S127 and S381 residues, or mutations of 5 S>A were sufficient to transform NIH3T3 cells (Zhao et al., 2009; Zhao et al., 2010). The S61 residue of YAP is also phosphorylated by AMPK during glucose starvation, which inactivates the transcriptional activity of YAP (Wang et al., 2015).
There are some reports claiming that nuclear localization of YAP can be regulated independently of the Hippo pathway/action of the Lats kinase. Our results suggest that Lats is involved in mammary tumor growth, because expression of dominant negative Lats stimulates tumor formation in ErbB2 transgenic animals. Dominant negative Lats has been shown to interfere with YAP phosphorylation and stimulate its nuclear accumulation (Zhao et al., 2007) and since Lats (or Warts in Drosophila) is probably the kinase that best defines the Hippo pathway (Bossuyt et al., 2013; Gumbiner and Kim, 2014; Yu and Guan, 2013), our finding suggests that Hippo signaling is involved in the regulation of YAP during tumorigenesis.
We found that YAP/TAZ is important in a well-characterized model of mammary tumorigenesis, one driven by expression of the PyMT oncogene. A similar finding was reported for this model of tumorigenesis using conditional ablation of the YAP gene in mammary gland (Chen et al., 2014). Our finding confirms this result and also demonstrates clearly that YAP/TAZ act in cells derived from the epithelial stem cell compartment, since we studied mammary glands reconstituted from transplantation of MaSCs. The PyMT oncogene activates a number of pathways that act downstream of growth factors, including PI3K and Src (Campbell et al., 1994; Courtneidge and Smith, 1983; Su et al., 1995; Whitman et al., 1985), which we and others have implicated as stimulators of YAP/TAZ nuclear accumulation and activity (Fan et al., 2013; Kim and Gumbiner, 2015; Li et al., 2016; Taniguchi et al., 2015). The direct activation of many different signaling pathways makes it difficult to know which pathway(s) depend(s) on YAP/TAZ for their effects on tumor growth, and it is possible that tumor growth gradually overcomes loss of YAP/TAZ due to this broad stimulation of signaling pathways.
Induction of tumors by members of the EGFR family provide a more well defined signaling pathway involved in mammary tumorigenesis, both in humans and in mice (Guy et al., 1992; Kim and Muller, 1999; Muller et al., 1988). Our previous in vitro cultured mammary cell experiments suggested that the Hippo pathway mediates part of the growth effects of EGF signaling, and therefore we addressed whether this is the case for mammary tumor growth in vivo. Our finding that ErbB2 expression caused YAP to accumulate in nuclei in mammary glands, even prior to overt tumorigenesis, is consistent with our cell culture findings and provides evidence that EGFR signaling in vivo can also inhibit hippo signaling and lead to nuclear YAP. Moreover our finding that dominant negative Lats stimulates ErbB2 transgenic MaSCs to grow tumors provides additional evidence that the Hippo pathway interacts with EGFR signaling in vivo in the mammary gland, at least in the context of tumorigenesis.
Our findings provide support for the overall hypothesis that deregulation of the Hippo pathway and activation of YAP play a role in tumorigenesis and human cancer. The role of the Hippo pathway and YAP in human cancer have been difficult to prove because very few cancer causing mutations are found in Hippo pathway genes (Pan, 2015), although a recent study found an infrequent but highly penetrant activating mutation in YAP associated with lung cancer (Chen et al., 2015). Amplification or overexpression of YAP may be a more prevalent mechanism. YAP is known to transform mammary cells in culture (Chan et al., 2008). Over-expression of YAP/TAZ is commonly observed in subtypes of breast cancer including luminal B, Her2 type and triple negative breast cancers (Min Kim et al., 2015; Vici et al., 2014). Hyper-activation of YAP/TAZ is associated with poor clinical outcome in human breast cancer patients(Bartucci et al., 2015; Kim et al., 2015). YAP/TAZ play oncogenic roles in the breast cancer by promoting invasion and metastasis (Chan et al., 2008; Lamar et al., 2012), increasing chemoresistance (Bartucci et al., 2015; Lai et al., 2011) as well as sustaining self-renewal and tumor-initiation potentials of breast cancer stem cells (Cordenonsi et al., 2011). Our findings of the oncogenic activity of YAP in mammary stem cells in vivo bridge the findings of its transforming activity in cultured mammary cells to the role of YAP in human breast cancer.
Acknowledgments
We wish to thank Yongliang Huo for teaching us the MaSC transplantation method while at UVA. We also thank Nam Gyun Kim and Alisha Mendonsa for reading drafts of the manuscript and providing feedback. The work in this manuscript was supported by NIH Grant R01 GM098615 to BMG.
References
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, Amabile MI, Pilozzi E, Patrizii M, Biffoni M, Maugeri-Sacca M, Piccolo S, De Maria R. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- Bossuyt W, Chen CL, Chen Q, Sudol M, McNeill H, Pan D, Kopp A, Halder G. An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene. 2013 doi: 10.1038/onc.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KS, Ogris E, Burke B, Su W, Auger KR, Druker BJ, Schaffhausen BS, Roberts TM, Pallas DC. Polyoma middle tumor antigen interacts with SHC protein via the NPTY (Asn-Pro-Thr-Tyr) motif in middle tumor antigen. Proc Natl Acad Sci U S A. 1994;91:6344–6348. doi: 10.1073/pnas.91.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- Chen HY, Yu SL, Ho BC, Su KY, Hsu YC, Chang CS, Li YC, Yang SY, Hsu PY, Ho H, Chang YH, Chen CY, Yang HI, Hsu CP, Yang TY, Chen KC, Hsu KH, Tseng JS, Hsia JY, Chuang CY, Yuan S, Lee MH, Liu CH, Wu GI, Hsiung CA, Chen YM, Wang CL, Huang MS, Yu CJ, Chen KY, Tsai YH, Su WC, Chen HW, Chen JJ, Chen CJ, Chang GC, Yang PC, Li KC. R331W Missense Mutation of Oncogene YAP1 Is a Germline Risk Allele for Lung Adenocarcinoma With Medical Actionability. J Clin Oncol. 2015;33:2303–2310. doi: 10.1200/JCO.2014.59.3590. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes & development. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Courtneidge SA, Smith AE. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983;303:435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–2574. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM, Kim NG. The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci. 2014;127:709–717. doi: 10.1242/jcs.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- Hardy KM, Booth BW, Hendrix MJ, Salomon DS, Strizzi L. ErbB/EGF signaling and EMT in mammary development and breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:191–199. doi: 10.1007/s10911-010-9172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. Mouse models for breast cancer. Breast Cancer Res. 2000;2:2–7. doi: 10.1186/bcr20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins BD, Hodakoski C, Barrows D, Mense SM, Parsons RE. PTEN function: the long and the short of it. Trends Biochem Sci. 2014;39:183–190. doi: 10.1016/j.tibs.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W, Shakya R, Costantini F. Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. J Cell Biol. 2001;155:1055–1064. doi: 10.1083/jcb.200107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Muller WJ. The role of the epidermal growth factor receptor family in mammary tumorigenesis and metastasis. Exp Cell Res. 1999;253:78–87. doi: 10.1006/excr.1999.4706. [DOI] [PubMed] [Google Scholar]
- Kim HM, Jung WH, Koo JS. Expression of Yes-associated protein (YAP) in metastatic breast cancer. Int J Clin Exp Pathol. 2015;8:11248–11257. [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210:503–515. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren S, Bentires-Alj M. Mouse models of PIK3CA mutations: one mutation initiates heterogeneous mammary tumors. FEBS J. 2013;280:2758–2765. doi: 10.1111/febs.12175. [DOI] [PubMed] [Google Scholar]
- Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. alphaE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev. 2016;30:798–811. doi: 10.1101/gad.274951.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. The American journal of pathology. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, I, Macara G. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009;23:1450–1460. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JH. Handbook of Biological Statistics. Sparky House Publishing; Baltimore, MD: 2015. [Google Scholar]
- Min Kim H, Kim SK, Jung WH, Koo JS. Metaplastic carcinoma show different expression pattern of YAP compared to triple-negative breast cancer. Tumour Biol. 2015;36:1207–1212. doi: 10.1007/s13277-014-2735-x. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Pan D. YAPing Hippo Forecasts a New Target for Lung Cancer Prevention and Treatment. J Clin Oncol. 2015;33:2311–2313. doi: 10.1200/JCO.2015.61.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Liu W, Schaffhausen BS, Roberts TM. Association of Polyomavirus middle tumor antigen with phospholipase C-gamma 1. J Biol Chem. 1995;270:12331–12334. doi: 10.1074/jbc.270.21.12331. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, Sandborn WJ, Hardiman G, Raz E, Maehara Y, Yoshimura A, Zucman-Rossi J, Guan KL, Karin M. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer KL, Lee DC. Regulation of mouse mammary gland development and tumorigenesis by the ERBB signaling network. J Mammary Gland Biol Neoplasia. 2001;6:7–21. doi: 10.1023/a:1009560330359. [DOI] [PubMed] [Google Scholar]
- Tumaneng K, Russell RC, Guan KL. Organ size control by Hippo and TOR pathways. Curr Biol. 2012;22:R368–379. doi: 10.1016/j.cub.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vici P, Mottolese M, Pizzuti L, Barba M, Sperati F, Terrenato I, Di Benedetto A, Natoli C, Gamucci T, Angelucci D, Ramieri MT, Di Lauro L, Sergi D, Bartucci M, Dattilo R, Pagliuca A, De Maria R, Maugeri-Sacca M. The Hippo transducer TAZ as a biomarker of pathological complete response in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Oncotarget. 2014;5:9619–9625. doi: 10.18632/oncotarget.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welm BE, Dijkgraaf GJ, Bledau AS, Welm AL, Werb Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell stem cell. 2008;2:90–102. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Wickenden JA, Watson CJ. Key signalling nodes in mammary gland development and cancer. Signalling downstream of PI3 kinase in mammary epithelium: a play in 3 Akts. Breast Cancer Res. 2010;12:202. doi: 10.1186/bcr2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77:8957–8961. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, Zhao J, Chen X, Zhang L, Wang CY, Bastian BC, Zhang K, Guan KL. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–1098. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]