Abstract
In addiction, notably, Alcohol Use Disorder (AUD), patients often have a tendency to fail to acknowledge the reality of the disease and to minimize the physical, psychological, and social difficulties attendant to chronic alcohol consumption. This lack of awareness can reduce the chances of initiating and maintaining sobriety. Presented here is a model focusing on compromised awareness in individuals with AUD of mild to moderate cognitive deficits, in particular, for episodic memory impairment—the ability to learn new information, such as recent personal experiences. Early in abstinence, alcoholics can be unaware of their memory deficits and overestimate their mnemonic capacities, which can be investigated with metamemory paradigms. Relevant neuropsychological and neuroimaging results considered suggest that the alcoholics’ impairment of awareness of their attenuated memory function can be a clinical manifestation explained mechanistically by neurobiological factors, including compromise of brain systems that result in a mild form of mnemonic anosognosia. Specifically, unawareness of memory impairment in AUD may result from a lack of personal knowledge updating attributable to damage in brain regions or connections supporting conscious recollection in episodic memory. Likely candidates are posterior parietal and medial frontal regions known to be integral part of the Default Mode Network (DMN) and the insula leading to an impaired switching mechanism between the DMN and the Central-Executive Control (i.e., Lateral Prefronto-Parietal) Network. The cognitive concepts and neural substrates noted for addictive disorders may also be relevant for problems in self-identification of functional impairment resulting from injury following war-related blast, sport-related concussion, and insidiously occurring dementia.
Keywords: alcoholism, anosognosia, brain, memory, metamemory
INTRODUCTION: THE CONCEPT, HISTORY, AND RELEVANCE
Alcohol use disorder (AUD) is a highly prevalent worldwide public health problem (Esser et al. 2014; Grant et al. 2015; Rehm et al. 2015). In the United States, 14 percent of adults (i.e., 32.6 million Americans with drinking problems during a 12-month period) currently suffer from an AUD, but only about 20 percent of those with an alcohol-related problem had ever sought treatment (Grant et al. 2015). In 2011, the American Society of Addiction Medicine (ASAM 2011) released a new definition of addiction, including alcohol dependence, labeling it as “a primary, chronic disease of brain reward, motivation, memory and related circuitry. Dysfunction in these circuits leads to characteristic biological, psychological, social and spiritual manifestations” (http://www.asam.org/quality-practice/definition-of-addiction).
Mild to moderate mnemonic and nonmnemonic deficits have been observed in individuals with AUD, including episodic memory impairment—the ability to learn new information, such as recent personal experiences. Episodic memory relies on the encoding, storage, and retrieval of personally-experienced events that are associated with specific spatial and temporal encoding contexts. This function enables conscious recollection of events from one’s personal past and the mental projection of anticipated events into one’s subjective future (Wheeler et al. 1997). Furthermore, recollection of episodic events includes autonoetic awareness, which is the impression of re-experiencing or reliving the past and mentally traveling back in subjective time (Tulving 2001).
In the case of alcohol addiction, once the alcohol withdrawal step is completed, some patients benefit from cognitive-behavioral treatment, during which they are taught to anticipate high-risk situations to relapse (Berglund et al. 2003; Assanangkornchai and Srisurapanont 2007; Clay et al. 2008). These methods require sufficient learning capabilities for acquisition of new knowledge about the risks and sequelae of alcoholism and for engagement in effective strategies for avoiding future high-risk situations. Nevertheless, not all alcoholic patients are cognitively able to acquire such complex novel knowledge as memory impairment, a shortcoming that could hamper the acquisition of novel semantic information and cognitive procedures (Pitel et al. 2007b). Therefore, memory compromise has the potential to impede alcoholics from benefiting fully from cognitive and behavioral treatment approaches for alcohol dependence. Furthermore, episodic memory abilities may be essential for AUD patients to improve the level of motivation to change their alcoholic behavior, especially in contributing to ‘consciousness raising’ of alcohol problem reality (i.e., gaining knowledge about the problem behavior and the advantages of changing) (Blume et al. 2005; Le Berre et al. 2012). Successful alcohol treatment and subsequent abstinence may require high-level cognitive processes affected in alcoholism and unachievable by sober alcoholics or alcoholics with Korsakoff’s Syndrome (KS), who by definition exhibit extensive impaired episodic learning. This assumption is supported by links observed between cognitive dysfunctions and treatment outcome in alcoholic subjects (Tapert et al. 2004).
Early in abstinence, patients with AUD are often unaware of their memory deficits and overestimate their memory capacities. This disability has been considered as an impairment in metamemory, which refers to personal knowledge about one’s own memory ability with implications for cognitive processes relevant for monitoring and controlling memory. If patients overestimate their memory abilities, clinical treatment might be only partially beneficial, thereby placing patients at risk of laboring under the illusion that they have sufficiently consolidated essential information to assure maintenance of their abstinence or reduced alcohol consumption in their daily life. Further, poor awareness of memory compromise in AUD can jeopardize decision-making judgments about addiction management in everyday life. These problems also indirectly contribute to difficulties in maintaining sobriety and are analogous to the need to seek help by patients with Alzheimer’s disease in treatment seeking, healthcare decision-making, and medication management (Cosentino et al. 2011; Karlawish et al. 2005). In this review, we assume that even alcoholic patients without demonstrable neurological complication could suffer a mild form of anosognosia for memory deficits observed in alcoholic amnesic patients with Korsakoff’s syndrome.
The term anosognosia was introduced by Babinski to describe unawareness, or literally ‘lack of knowledge,’ of left hemiplegia in stroke patients (Babinski 1914, 1918). To explain anosognosia following brain disease, Weinstein and Kahn promoted a motivational theory where explicit denial of disabilities was considered a psychological defense mechanism (Weinstein and Kahn 1953, 1955). In particular, these authors postulated that anosognosia was associated with specific premorbid personality attitudes, such that patients with explicit verbal denial of their illness - even hemiplegia - had always perceived illness as an imperfection, sought for prestige and the esteem of others, and denied inadequacies despite implicit knowledge of them (Weinstein and Kahn 1953). This theory has been challenged, however, by clinical and neuroimaging studies reporting that anosognosia was preferentially associated with lateralized brain damage in the right rather than left hemisphere, and typically invoking prefrontal and parietal cortices known to support features of self-awareness (Bisiach et al. 1986; Heilman 1991; Marcel et al. 2004; Rainville et al. 2003; Venneri and Shanks 2004; Vuilleumier 2004). The initial use of the term anosognosia to describe unawareness of hemiplegia (Babinski 1914) has since been extended to include unawareness of cognitive impairment in a broad spectrum of neurological disorders including aphasia (Rubens and Garrett 1991), amnesic syndromes (McGlynn and Schacter 1989), dementia (Barrett et al. 2005; Reed et al. 1993), and cognitive deficits following brain injury (Prigatano 1991). Here, we explore the cognitive neuropsychological aspects of anosognosia exclusive of its motivational component when discussing unawareness of memory impairment in alcoholism. We use the terms anosognosia, impaired awareness, and unawareness interchangeably. To date, a wide variety of cognitive deficits have been identified in AUD, notably in episodic memory, working memory, and executive functions (Beatty et al. 1995; Brokate et al. 2003; Fama et al. 2004; Goldstein et al. 2004; Moselhy et al. 2001; Nixon et al. 1998; Noel et al. 2001; Pitel et al. 2007a; Sullivan et al. 2000; Zinn et al. 2004; Bates et al. 2002). These functional difficulties have been related to structural and functional brain changes in the fronto-cerebellar and limbic circuitries (Beresford et al. 2006; Cardenas et al. 2007; Chanraud et al. 2007; Laakso et al. 2000; Le Berre et al. 2014a; Makris et al. 2008; Pfefferbaum et al. 1997; Pitel et al. 2012; Rosenbloom et al. 2003; Sullivan 2003; Sullivan and Pfefferbaum 2005; Sullivan et al. 1995; Chanraud et al. 2010). Our aim is to address the question of whether unawareness of memory impairment in AUD is related to damage of specific brain systems and whether evidence indicates that resulting cognitive deficits militate against individuals with AUD from gaining and maintaining awareness of their cognitive functioning, indicative of mild anosognosia. We start by describing an existing cognitive model and component processes relevant to anosognosia for memory disorders. Then, we consider specific studies, which investigated the pattern of impaired metamemory in alcoholic patients, notably using monitoring measures such as the Feeling-Of-Knowing (FOK) and Retrospective Confidence Judgments (RCJ) assessed during an episodic memory task. Lastly considered are cognitive correlates and brain substrates of metamemory impairment relevant to understanding of the lack of awareness of memory impairment observed in some AUD patients early in abstinence.
PSYCHOLOGICAL MODEL OF AWARENESS: THE COGNITIVE AWARENESS MODEL (CAM)
Relevant psychological constructs for understanding anosognosia for memory impairment in addiction are described in the Cognitive Awareness Model (CAM, Figure 1) developed to account for the heterogeneity of anosognosia in Alzheimer’s disease (AD) (Agnew and Morris 1998; Hannesdottir and Morris 2007; Morris and Hannesdottir 2004; Morris and Mograbi 2013). Although this model was built in the context of dementia, it offers a useful theoretical framework to explain the variance of anosognosia observed in other pathologies such as focal and diffuse traumatic brain injury in addition to AUD.
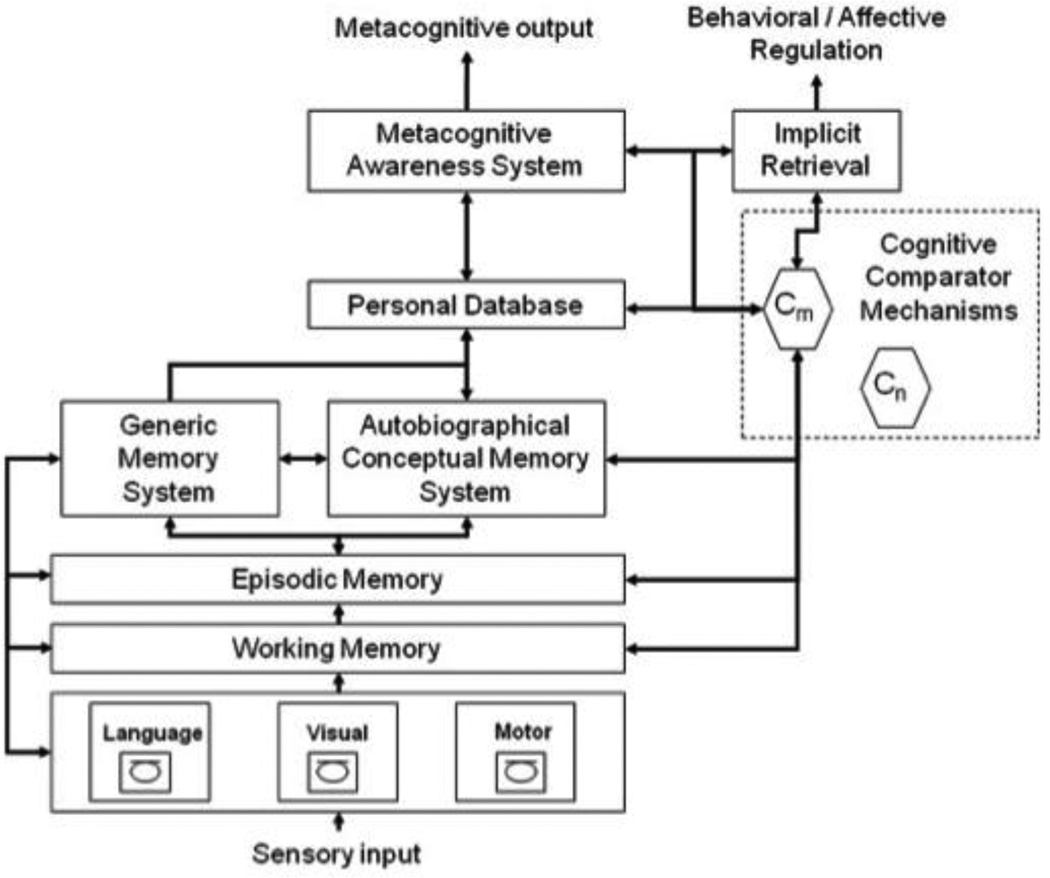
Figure 1.
The Cognitive Awareness Model (CAM, Morris and Mograbi, 2013)
The CAM model involves a separate awareness system, the Metacognitive Awareness System, which supports conscious awareness of ability or error. This model also postulates two systems that process awareness: the personal knowledge base and the Mnemonic Comparator System. The personal knowledge base is the semanticized (that is, non-episodic) representation of one’s own memory ability contributing to one’s self-structure and supported by autobiographical memory. Integrated in a central executive system, The Mnemonic Comparator System refers to monitoring processes under executive control, enabling the comparisons between incoming information actually supported by the current memory function and personal knowledge about one’s own memory functioning stored in the Personal Database. An updating of the Personal Database would occur if the Mnemonic Comparator System is able to detect discrepancies between information about the current memory state and information stored in the Personal Database. The resulting new knowledge would then be released through the Metacognitive Awareness System, which enables conscious self-awareness of one’s mnemonic ability (Morris and Mograbi 2013).
Depending on which component is damaged, this model postulates that different types of anosognosic disorders will be manifest. This model suggests different forms of unawareness, where primary anosognosia results from a failure in the Metacognitive Awareness System and secondary anosognosia results from disruptions in lower metacognitive mechanisms contributing to processing awareness (Hannesdottir and Morris 2007). In primary anosognosia, the information signaling a mnemonic error and emerging from the comparator mechanism is not registered in awareness. The secondary awareness category can be divided in two types of anosognosia: mnemonic and executive. Mnemonic anosognosia refers to a failure in updating the personal knowledge database due to memory deficits, which impede long-term consolidation of newly updated information in semantic personal knowledge. Consequently, the affected persons do not perceive changes in their memory functioning and believe that their memory skills are as good as before the disease onset. Executive anosognosia reflects impairment on a higher-order executive level involving comparator mechanisms (i.e., breakdown in the ‘Mnemonic Comparator System’) and leads to difficulties in conducting comparisons with information stored in the personal knowledge base. Thus, the CAM model affords the opportunity to determine which specific cognitive factor explains anosognosia for memory deficits, in our case for AUD, and therefore could help to orient and adapt cognitive rehabilitation to compensate for or remedy the lack of awareness of cognitive impairment.
METAMEMORY: DEFINITION AND ASSESSMENT
Metamemory refers to personal knowledge and subjective beliefs about one’s general memory function, memory capacities, and strategies contributing to optimal learning (Flavell 1971; Flavell and Wellman 1977). This knowledge set is frequently assessed with subjective measures collected independently of ongoing memory activities, such as questionnaires that compare a patient’s report with a clinician’s or caregiver’s evaluation. Before performing a memory task, this metacognitive and introspective knowledge allows set up of appropriate strategies and anticipation and planning of successive memory processes required to cognitive task success.
Metamemory also designates the monitoring and control processes operating during the acquisition, retention, and retrieval of information in memory (Nelson and Narens 1990). The control component refers to regulation actions applied during a memory activity such as selection and use of strategies or decisions on allocation of time and cognitive resources to meet task demands. The monitoring component assesses the progress and success of memory functioning; new information is emergent from this monitoring and consequently modifies the metacognitive knowledge database.
Self-monitoring mechanisms have been assessed with global or item-by-item predictions collected during semantic or episodic memory tasks. For global predictions, participants were asked to predict how many words they could recall before and after learning, for example, 20 word pairs. For item-by-item predictions, two main monitoring judgments have been used in chronic alcoholism: the prospective Feeling-Of-Knowing (FOK) measure and the retrospective confidence judgment (RCJ) collected during an episodic memory task. The prospective FOK judgment is usually collected using the recall-judgment-recognition paradigm (Hart 1965), in which participants learn word pairs, rate their future ability to recognize previously learned information not directly accessible in memory (i.e., information that participants failed to recall), followed by a recognition task. Prediction of future recognition performance is thereafter compared with actual recognition performance to assess the FOK accuracy. In contrast with the prospective judgment task, retrospective confidence judgments (RCJ) are made following a recall or recognition task, when participants rate their confidence about the level of success of information retrieval. These retrospective judgments are then compared with actual recognition performance to assess RCJ accuracy.
METAMEMORY IN ALCOHOLISM: SUBJECTIVE REPRESENTATION AND MONITORING PROCESSES
Subjective representation of memory impairment in nonamnesic alcoholics
In studies of chronic alcoholism, metacognitive knowledge has been assessed with questionnaires, such as the Metamemory In Adulthood (MIA) questionnaire (Dixon and Hultsch 1983) and the Memory Functioning Questionnaire (MFQ) (Gilewski et al. 1990). Assessing one’s beliefs on general memory functioning and on one’s own memory functioning, these questionnaires were completed by alcoholics and were then compared with ratings by healthy control participants. While episodic memory deficits are commonly reported in nonamnesic alcoholics, memory awareness assessed by subjective measures is seldom considered and, when examined, is variable.
In AUD studies, some patients believed they were as capable as controls on everyday life memory activities, thus reflecting a tendency toward overestimation of memory abilities (Le Berre et al. 2010). Others felt that their memory failure was more serious and pervasive than controls (Le Berre et al. 2016). Nevertheless, even when the latter group of alcoholics reported subjective memory complaint, at the same time, these patients did not acknowledge more pronounced memory decline with age than the controls nor with the evolution of their disease. One possible explanation is that subjective representation of their actual memory functioning, i.e., their personal knowledge database, may not have been completely updated and would only reflect their memory skills at some time earlier in life, perhaps before or at the beginning of their alcoholism onset. Alcoholics also expressed a higher level of anxiety during memory tasks than controls. This enhanced anxiety was hypothesized as 1) resulting from a more anxious personality in alcoholic patients, 2) indirectly reflecting emotional reactions related to memory failures even in patients without overt awareness of their memory decline, a sign of an implicit form of awareness in chronic alcoholism (Mograbi and Morris 2013), or 3) both.
Finally, alcoholics in general did not report using additional internal or external memory strategies than controls (i.e., write notes as a reminder, plan daily schedule in advance, concentrate more on information they want to retain) to adapt to and compensate for their memory problems (Le Berre et al. 2010, 2016). Therefore, even when some patients explicitly acknowledged their memory difficulties or at least manifested implicit awareness through emotional reactions, alcoholics failed to adjust their behavioral strategies to overcome their everyday life memory problems. This observation raises the question of potential impairment in the control processes of metamemory in chronic alcoholism, currently untested objectively.
Cognitive profile of impaired FOK and intact RCJ in nonamnesic alcoholics
As observed in alcoholic KS patients, a cognitive profile of impaired FOK and intact RCJ was also observed in nonamnesic alcoholics. In two different studies -- one from France and one from the U.S. -- collecting FOK judgments for newly learned information (i.e., episodic), alcoholic patients early in abstinence showed episodic memory deficits compared with controls and generated inaccurate FOK predictions, especially with a tendency to overestimate their memory skills (Le Berre et al. 2010, 2016). This alcohol-related metamemory deficit, however, did not extend to the retrospective confidence measure (RCJ) on recognition performance, wherein alcoholics accurately judged their success in recognizing episodic information (Le Berre et al. 2016).
The cognitive profile of impaired FOK with intact RCJ for recognition during an episodic memory paradigm observed in uncomplicated chronic alcoholics can be interpreted in the framework of the CAM model (Agnew and Morris 1998; Morris and Hannesdottir 2004; Morris and Mograbi 2013). The alcoholic pattern of impaired metamemory runs counter to either a primary or secondary executive anosognosia, in that alcoholics were still able to perceive a memory failure consciously when it occurred during recognition performance. In this context, the pattern of metamemory performance would be more consistent with secondary, mnemonic anosognosia. Alcoholic patients would be still capable of monitoring their memory performance once they experienced the memory task, indicative of relatively intact integrity of the Mnemonic Comparator System and of the Metacognitive Awareness System proposed in the CAM model. However, the alcoholics apparently failed to consolidate updated information into their personal long-term database due to specific memory dysfunction (Hannesdottir and Morris 2007). With this scenario, the alcoholics compared their current memory functioning with outdated self-beliefs, thereby preventing accurate assessment of future memory performance.
In a recent review investigating the relation between mood and anosognosia in Alzheimer’s disease (Mograbi and Morris 2014), depression and anxiety were associated with awareness assessed by self-report or clinician ratings. Specifically, higher depressed mood correlated with more apparent awareness in dementia. In the framework of the CAM model, Mograbi and Morris (2014) postulated that “depressed mood may lead to negative bias or depressive realism when evaluating self-ability, influencing self appraisals (i.e., Metacognitive Awareness system) or biasing retrieval of personal information (personal data base).” Anxiety and depression disorders are highly prevalent in AUD (Grant et al. 1995, 2004) and could potentially influence the metamemory profile observed in alcoholic patients. To date, however, relations between mood and awareness of memory impairment using the FOK and RCJ measures have not been reported in chronic alcoholism. Even when alcoholics demonstrated higher anxious personality revealed by “trait anxiety” form of the State-Trait Anxiety Inventory (STAI) questionnaire (Spielberger et al. 1983), this emotional component did not account for the lack of FOK accuracy in alcoholics patients early in abstinence (Le Berre et al. 2010). Further to this point, significant correlations were not observed between the FOK and RCJ measures and depression symptom scores assessed by the Beck Depression Inventory (BDI) questionnaire in sober alcoholics (Le Berre et al. 2016).
COGNITIVE CORRELATES OF METAMEMORY
Both executive dysfunction and episodic memory deficits occur in alcoholism (Beatty et al. 1995; Brokate et al. 2003; Fama et al. 2004; Goldstein et al. 2004; Moselhy et al. 2001; Nixon et al. 1998; Noel et al. 2001; Pitel et al. 2007a; Sullivan et al. 2000; Zinn et al. 2004). The exploration of cognitive correlates of metamemory using an episodic FOK paradigm extends our understanding of unawareness of memory deficits in chronic alcoholic patients. Specifically, the co-occurrence and association of episodic memory deficits and executive dysfunction contribute to metamemory impairment in chronic alcoholics (Le Berre et al. 2010).
Using a “Remember-Know” paradigm (Gardiner et al. 2002), impairment in conscious recollection was demonstrated in uncomplicated alcoholics early in abstinence (Le Berre et al. 2010; Pitel et al. 2007a). For this paradigm, during a recognition task, patients were asked to specify their state of consciousness for each correctly recognized word, using one of three options: “Remember answers” when they remembered the learning episode and were able to provide details about it (i.e., conscious recollection), “Know answers” when they knew that they had learned the information but were unable to give details about the learning episode (i.e., familiarity), and “Guess answers” when they were supposed to have learned the word but were not at all sure about it. Alcoholic patients generated fewer Remember answers than controls (Le Berre et al. 2010; Pitel et al. 2007a). Episodic FOK accuracy has been predicted by the percentage of ‘Remember’ answers, suggesting that episodic memory deficits, especially conscious recollection compromise, may contribute to the metamemory impairment observed in alcoholics (Le Berre et al. 2010). Further, alcoholics fail to use the contextual information related to the target to make their FOK predictions. A growing number of studies converged on the idea that recollection, reflecting the retrieval of qualitative information such as the context of a prior episode (Yonelinas 2002), may be a fundamental cognitive process underlying FOK judgments (Brewer et al. 2010; Hicks and Marsh 2002; Sacher et al. 2009; Souchay et al. 2007; Thomas et al. 2011). Supporting this hypothesis are studies using the Remember-Know paradigm and showing that episodic FOK accuracy is linked to recollection (Boduroglu et al. 2014; Hicks and Marsh 2002; Souchay et al. 2007).
The metamemory compromise observed in AUD patients early in abstinence was also associated with executive dysfunction, notably, decline in verbal fluency performance (Le Berre et al. 2010). This result is consistent with the accessibility hypothesis (Koriat 1993), which postulates that FOK judgments are based on the partial accessibility of semantic, perceptive, or emotional features linked to the target information. Indeed, fluency performance involves organizational ability and strategies to search information in memory. Focused attentional capacities, as used in fluency performance, enable selective retrieval of pertinent information linked to a target while ignoring irrelevant information. Such ability allows for the generation of accurate FOK judgments. Moreover, performance on fluency tasks was significantly predictive of episodic memory disorders, including recollection impairment, in chronic alcoholic patients (Pitel et al. 2007a). Thus, fluency impairment could contribute to recollection difficulties and indirectly promote metamemory compromise.
In summary, in AUD patients early in abstinence, we posit that compromise of conscious recollection and strategic mnemonic search abilities are the principal cognitive mechanisms of metamemory decline (Le Berre et al. 2010). This possibility suggests that alcoholic-related unawareness of memory deficits can be attributed to compromise of a constellation of cognitive mechanisms. Cognitive correlates of alcoholics’ metamemory impairment are consistent with the hypothesis of secondary mnemonic anosognosia following from a specific memory dysfunction. Due to conscious recollection deficits, alcoholics experience difficulties in retrieving specific episodic information rich with contextual details. This impairment is further obstructive to updating knowledge about self-functioning. Consequently, affected alcoholics are likely to generate predictions about their cognitive abilities based on semanticized (implicit) and remote memories of self-ability and poor self-reflection (autonoetic), and thus maintain a petrified concept of self (Mograbi et al. 2009).
BRAIN SUBSTRATES OF METAMEMORY
Brain lesion studies have demonstrated that prefrontal cortex damage plays a critical role in episodic FOK and also in RCJ accuracy (Modirrousta and Fellows 2008; Schnyer et al. 2004; Pannu and Kaszniak 2005). Conflicting results regarding the pattern of metamemory deficits were observed in patients with frontal brain damage: Pannu et al. observed impaired RCJ accuracy but accurate FOK (Pannu et al. 2005), whereas Schnyer et al. reported FOK inaccuracy but intact RCJ (Schnyer et al. 2004). These discrepancies can be explained by neuroanatomical differences with more lateral prefrontal cortex damage occurred in a majority of patients observed by Pannu et al., whereas an overlapping region of damage was found in the right ventromedial prefrontal cortex in frontal patients studied by Schnyer et al. The cognitive profile of impaired FOK with intact RCJ was also highlighted in patients with brain damage in a more dorsal region of the medial prefrontal cortex and in the left hemisphere, suggesting that medial prefrontal cortex (mPFC) damage in either hemisphere or damage to dorsal mPFC alone is sufficient to disrupt FOK (Modirrousta and Fellows 2008).
Amnesic patients with temporal lobe damage could accurately predict their memory performance using episodic FOK, thereby excluding temporal lobe damage as a neural substrate of metamemory impairment (Janowsky et al. 1989; Modirrousta and Fellows 2008). Contrarily, functional neuroimaging studies provide evidence that accuracy in episodic FOK predictions in healthy subjects do implicate temporal lobe activation (Kikyo and Miyashita 2004; Schnyer et al. 2005). Specifically, FOK predictions involved a left fronto-temporal brain network, including the inferior prefrontal cortex for the creation of cues activating information in memory, the hippocampus and temporal cortex for storage and retrieval memory contents, and the ventromedial prefrontal cortex for ensuring the ‘monitoring’ of these retrieval processes in memory (Schnyer et al. 2005).
Recently, relations between memory awareness assessed using a modified episodic FOK task and structural integrity of brain regions implicated in self-awareness processes (i.e., prefrontal, temporal, parietal, anterior and posterior cingulate cortices, hippocampus and insula) were investigated in a mixed group of patients with mild Alzheimer’s disease and cognitively healthy older adults (Cosentino et al. 2015). Lower FOK accuracy was associated only with smaller right insular volume in this group of cognitively diverse older adults. Cosentino et al. (2015) discussed the role of the insula in memory awareness in the context of its contribution in conscious detection of errors assuming that it could be consistent with a form of primary or secondary executive anosognosia proposed in the CAM model (Hannesdottir and Morris 2007).
Inaccuracy in assessing future recognition performance for episodic information (i.e., FOK), but accuracy in confidence ratings of actual recognition performance (i.e., RCJ), has been demonstrated in abstinent alcoholic participants (Le Berre et al. 2016). In the same group of uncomplicated alcoholics, correlation analyses between FOK and RCJ measures and bilateral gray matter volume of hypothesis-driven regions of interest (ROI), i.e., the frontal, temporal, parietal, cingulate cortices plus the insular cortex and bilateral volumes of the hippocampi showed that (1) lower FOK accuracy was correlated with smaller insular volumes and (2) lower RCJ accuracy was associated with smaller volumes of the bilateral frontal cortex, cingulate cortex, and hippocampus. Further, the bilateral insula volume endured as a significant unique predictor of the FOK accuracy after accounting for variance from bilateral frontal, cingulate, and hippocampal volumes. By contrast, bilateral frontal, cingulate and hippocampal volumes each endured as significant selective predictors of the RCJ accuracy controlling for bilateral insula volume in separate multiple regressions. Therefore, examination of brain structure-function relations revealed a double dissociation (Fama and Sullivan 2014), where FOK accuracy was selectively related to insular volume but not frontolimbic structures, whereas retrospective confidence accuracy was selectively related to a constellation of fronto-limbic structural volumes but not insula volumes in sober alcoholics (Le Berre et al. 2016). Given that the insula is hypothesized as a key neural substrate supporting components of self-awareness (Craig 2009; Schmitz and Johnson 2007), this alcohol-related impaired FOK/intact RCJs profile could be explained by the idea that prospective FOK and retrospective RCJs both implicate online memory monitoring mechanisms but differ in that FOK judgments require additional self-inferential processes to generate accurate future estimations.
CONCLUSION
The neuropsychological and neuroimaging findings on mnemonic functioning suggest that the lack of awareness of memory deficits exhibited by some uncomplicated alcoholic patients at alcohol treatment entry can be explained mechanistically by neurobiological substrates.
The cognitive performance profile of impaired prospective FOK judgments with intact RCJ indicates that chronic alcoholism does not necessarily lead to a global compromise in metamemory skills. A similar pattern of inaccuracy in FOK for recently learned information (Shimamura and Squire 1986) with preserved RCJs for recall (Shimamura and Squire 1988) emerged in studies exploring the metamemory profile in amnesic alcoholics, that is, those with KS. Alcoholics who have sustained severe thiamine deficiency or depletion are at risk for developing Wernicke’s encephalopathy and, if untreated or undertreated, go on to express KS (Sechi and Serra 2007; Thomson et al. 2012), which is marked by a disproportionate impairment of episodic memory compared with other components of cognitive functioning (Kopelman 1995, 2002). One of the core clinical manifestations in KS is a severe difficulty in acknowledging their profound amnesia, namely, anosognosia for their memory impairment. Episodic memory deficits observed in KS appeared to fall along an impairment continuum from mild to moderate in uncomplicated alcoholism to severe deficits in alcoholism with KS (Butters and Brandt 1985; Pitel et al. 2008; Sullivan and Pfefferbaum 2009). This neuropsychological continuum could reflect a neuroanatomical continuity in alcoholics without neurological complications to alcoholics with such complications, specifically, KS (Le Berre et al. 2014a; Pitel et al. 2012; Sullivan and Pfefferbaum 2009). A continuum of mnemonic impairment raises the possibility that even nonamnesic alcoholics could suffer a mild form of anosognosia for their memory deficits.
The cognitive profile of impaired FOK and intact RCJ is consistent with a secondary, mnemonic anosognosia attendant to specific memory impairments (Hannesdottir and Morris 2007). Due to conscious recollection compromise, alcoholics are at risk of failing to update information in their personal long-term knowledge database. Thus, predictions regarding their own cognitive capacities may be based on biased comparisons between their current memory functioning and semanticized and outdated memories of their self-ability (Mograbi et al. 2009). Damage in or functional disconnection between brain regions known to be involved in a core recollection network could affect successful recollection and create failure in metamemory functioning (Johnson and Rugg 2007; Hayama et al. 2012; King et al. 2015; see Kim 2010; Rugg and Vilberg 2013, for reviews). This brain network encompasses the hippocampus and parahippocampal cortex, medial prefrontal cortex, and posterior midline and lateral parietal cortex (angular gyrus). These brain regions are an integral part of the Default Mode Network (DMN) particularly implicated in internally focused tasks such as autobiographical memory retrieval, self-reference activities, and imaging the future (Buckner et al. 2008), which could be essential for generating accurate prospective FOK judgments (Figure 2, blue bracket = the Default Mode Network (DMN) supporting recollection and internally driven processes contributing to update knowledge about self functioning in the Personal Data Base). Neuroimaging studies in alcohol dependence have shown volume deficits in brain structures involved in the DMN (Agartz et al. 1999; Beresford et al. 2006; Cardenas et al. 2007, 2011; Fein et al. 2009; Jang et al. 2007; Le Berre et al. 2014a, 2014b, 2016; Makris et al. 2008; Moselhy et al. 2001; Pfefferbaum et al. 1997; Sullivan et al. 1995). In addition to evidence for structural damage in the DMN, activation synchrony among nodes of this resting state brain network are disrupted in chronic alcoholics (Chanraud et al. 2011). During resting-state functional MRI, connectivity analyses identified weaker within-default mode network connectivity and expanded connectivity to regions outside the DMN in alcoholics relative to the controls (Müller-Oehring et al. 2015).
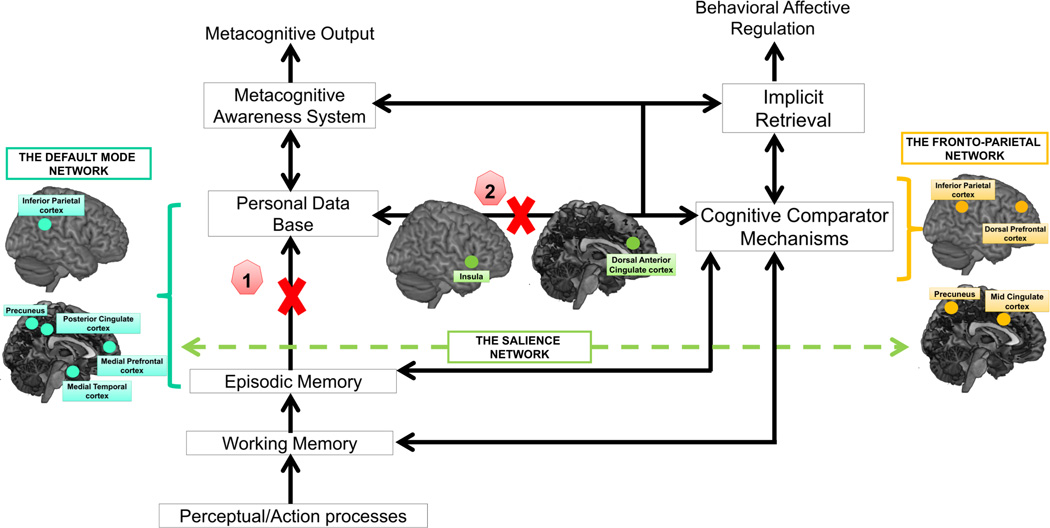
Figure 2.
An anosognosia model based on a version of the CAM (Morris and Mograbi, 2013) and the network model of insula function (Menon and Uddin, 2010) describing cognitive and brain network mechanisms of unawareness of memory impairment. Metamemory deficit in AUDs may result from a lack of personal knowledge updating due to 1) damage in brain regions or connections supporting conscious recollection in episodic memory, likely candidates are posterior parietal and medial frontal regions known to be integral part of the Default Mode Network (DMN) and 2) damage in the insula leading to an impaired switching mechanism between the Default Mode Network and the Central-Executive Control (i.e. Lateral Prefronto-Parietal) Network.
Personal Data Base = semantic memories regarding one’s past cognitive performance; Cognitive Comparator Mechanisms = monitoring processes under executive control enabling the comparisons between information regarding the current memory function and information stored in the Personal Data Base; Metacognitive Awareness System = system enabling conscious self-awareness of one’s mnemonic abilities; Implicit Retrieval = implicit processes supporting implicit awareness of cognitive failure.
Blue bracket = the Default Mode Network (DMN) supporting recollection and internally driven processes contributing to update knowledge about self functioning in the Personal Data Base; orange bracket = the Central-Executive Control (i.e. Lateral Prefronto-Parietal) network supporting the cognitive comparator mechanisms; green dashed line = switching mechanisms operating by the salience network between the Default Mode Network and the Central-Executive Control Network.
The assumption of a direct disconnection between the Personal Data Base and the Mnemonic Comparator System can also explain the lack of personal knowledge updating, according evidence that the insula may be a specific brain substrate of FOK accuracy in AUD. The insula is a brain region structurally affected by chronic alcohol exposure with altered shape (Jung et al. 2007), decreased cortical thickness (Momenan et al. 2012), volume deficits (Demirakca et al. 2011; Makris et al. 2008; Senatorov et al. 2015), and cerebral blood perfusion deficits (Sullivan et al. 2013). The insula has been proposed as a central node in the salience network initiating and enabling a dynamic switching between other large-scale networks, especially between the DMN supporting self-referential mental activity and the central executive (CE) network including the dorsolateral prefrontal and posterior parietal cortices (Menon and Uddin 2010) (Figure 2, orange bracket = the Central-Executive Control (i.e. Lateral Prefronto-Parietal) network supporting the cognitive comparator mechanisms, green dashed line = switching mechanisms operating by the salience network between the Default Mode Network and the Central-Executive Control Network).
The studies considered have implications for adapting clinical treatment to address unawareness of memory deficits not only in alcoholism but also in other conditions marked by poor insight into acquired cognitive deficits, such as war-related blast injury (Betthauser et al. 2012; Sullivan 2012; Sayer 2012) and repeated closed-head injury in contact sports (Cournoyer and Tripp 2014). After alcohol withdrawal, it would seem essential to conduct a neuropsychological screening aimed at detection of cognitive impairment, potentially contributing to poor awareness and reduced efficiency and efficacy of cognitive-behavioral therapy. Especially in chronic alcoholism, a conscious recollection deficit is associated with difficulties in acknowledging memory impairments. Therefore, for patients with lack of awareness, it may be beneficial to deploy a cognitive rehabilitation program focused on memory, especially recollection and consolidation of personal knowledge.
It has been demonstrated that FOK judgment accuracy may be influenced by initial learning, i.e., the quality of the original encoding. FOK judgment accuracy improved when stimuli were repeatedly presented at encoding (Hertzog et al. 2010) and when participants were engaged in deep rather than shallow encoding (Lupker et al. 1991). Conversely, divided attention at encoding impaired FOK accuracy (Sacher et al. 2009). With our postulated mnemonic anosognosia in AUD, we assume that reinforcing the initial learning process by giving, for example, additional time to study new information and enabling repetitive exposure to the new material would facilitate the consolidation of new information in strengthening the memory trace and consequently would improve AUD patients’ abilities to generate accurate prospective FOK judgments. In studies conducted in alcoholics early in abstinence (Le Berre et al. 2010, 2016), patients were exposed to new words during a single, limited time. Further studies are needed to investigate the influences of degree of learning on the FOK in AUD given that, to our knowledge, no study addressed this question for FOK failures specifically in alcoholism.
We conclude that it is essential to keep in mind that to the extent that self-awareness has neurobiological basis, this phenomenon remains under the influence of social and interpersonal factors as well as psychological processes. Clare et al. (2012) emphasized the relevance of a biopsychosocial approach to understanding the factors that influence unawareness of impairment in the context of dementia, which could also apply in AUD. Further to this point, Hebb (1959) wrote: “I have emphasized the significance of neurological ideas for psychology, and it must be conceded that psychological thought has sometimes been abortive because not keeping abreast with neurological developments. But this is a two-way street; the neurologist must likewise keep in touch with the development of psychological knowledge, for some of the most important evidence concerning the nature of brain function can only be obtained from the study of behaviour” (page 273).
Acknowledgments
This research was supported by grants from AA010723, AA017168, and AA017923.
REFERENCES
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of general psychiatry. 1999;56(4):356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Agnew SK, Morris RG. The heterogeneity of anosognosia for memory impairment in Alzheimer’s disease: a review of the literature and a proposed model. Aging & Mental Health. 1998;2:7–19. [Google Scholar]
- Assanangkornchai S, Srisurapanont M. The treatment of alcohol dependence. Current opinion in psychiatry. 2007;20(3):222–227. doi: 10.1097/YCO.0b013e3280fa837d. [DOI] [PubMed] [Google Scholar]
- Babinski J. Contribution à l’ étude des troubles mentaux dans l’hémiplegie organique cérébrale (anosognosie) Revue Neurologique. 1914;27:845–847. [Google Scholar]
- Babinski J. Anosognosie. Revue Neurologique. 1918;31:365–367. [Google Scholar]
- Barrett AM, Eslinger PJ, Ballentine NH, Heilman KM. Unawareness of cognitive deficit (cognitive anosognosia) in probable AD and control subjects. Neurology. 2005;64(4):693–699. doi: 10.1212/01.WNL.0000151959.64379.1B. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Experimental and clinical psychopharmacology. 2002;10(3):193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug and alcohol dependence. 1995;37(3):247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, et al. Hippocampus volume loss due to chronic heavy drinking. Alcoholism, clinical and experimental research. 2006;30(11):1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Berglund M, Thelander S, Salaspuro M, Franck J, Andreasson S, Ojehagen A. Treatment of alcohol abuse: an evidence-based review. Alcoholism, clinical and experimental research. 2003;27(10):1645–1656. doi: 10.1097/01.ALC.0000090144.99832.19. [DOI] [PubMed] [Google Scholar]
- Betthauser LM, Bahraini N, Krengel MH, Brenner LA. Self-report measures to identify post traumatic stress disorder and/or mild traumatic brain injury and associated symptoms in military veterans of Operation Enduring Freedom (OEF)/Operation Iraqi Freedom (OIF) Neuropsychology review. 2012;22(1):35–53. doi: 10.1007/s11065-012-9191-4. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Vallar G, Perani D, Papagno C, Berti A. Unawareness of disease following lesions of the right hemisphere: anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia. 1986;24(4):471–482. doi: 10.1016/0028-3932(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA. Memory, executive cognitive function, and readiness to change drinking behavior. Addictive behaviors. 2005;30(2):301–314. doi: 10.1016/j.addbeh.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Boduroglu A, Pehlivanoglu D, Tekcan AI, Kapucu A. Effects of self-referencing on feeling-of-knowing accuracy and recollective experience. Memory (Hove, England) 2014:1–12. doi: 10.1080/09658211.2014.925927. [DOI] [PubMed] [Google Scholar]
- Brewer GA, Marsh RL, Clark-Foos A, Meeks JT. Noncriterial recollection influences metacognitive monitoring and control processes. Quarterly journal of experimental psychology (2006) 2010;63(10):1936–1942. doi: 10.1080/17470210903551638. [DOI] [PubMed] [Google Scholar]
- Brokate B, Hildebrandt H, Eling P, Fichtner H, Runge K, Timm C. Frontal lobe dysfunctions in Korsakoff’s syndrome and chronic alcoholism: continuity or discontinuity? Neuropsychology. 2003;17(3):420–428. doi: 10.1037/0894-4105.17.3.420. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Butters N, Brandt J. The continuity hypothesis: the relationship of long-term alcoholism to the Wernicke-Korsakoff syndrome. Recent developments in alcoholism. 1985;3:207–226. doi: 10.1007/978-1-4615-7715-7_17. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biological psychiatry. 2011;70(6):561–567. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. NeuroImage. 2007;34(3):879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cerebral cortex (New York, NY : 1991) 2011;21(10):2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, Pfefferbaum A, Sullivan EV. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35(9):1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L, Nelis SM, Martyr A, Roberts J, Whitaker CJ, Markova IS, et al. The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: testing a biopsychosocial model. International journal of geriatric psychiatry. 2012;27(2):167–177. doi: 10.1002/gps.2705. [DOI] [PubMed] [Google Scholar]
- Clay SW, Allen J, Parran T. A review of addiction. Postgraduate medicine. 2008;120(2):E01–E07. doi: 10.3810/pgm.2008.07.1802. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Brickman AM, Griffith E, Habeck C, Cines S, Farrell M, et al. The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia. 2015;75:163–169. doi: 10.1016/j.neuropsychologia.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Metcalfe J, Cary MS, De Leon J, Karlawish J. Memory Awareness Influences Everyday Decision Making Capacity about Medication Management in Alzheimer’s Disease. International journal of Alzheimer’s disease. 2011;2011:483897. doi: 10.4061/2011/483897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournoyer J, Tripp BL. Concussion knowledge in high school football players. Journal of athletic training. 2014;49(5):654–658. doi: 10.4085/1062-6050-49.3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Hultsch DF. Structure and development of metamemory in adulthood. Journal of gerontology. 1983;38(6):682–688. doi: 10.1093/geronj/38.6.682. [DOI] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Preventing chronic disease. 2014;11:E206. doi: 10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcoholism, clinical and experimental research. 2004;28(11):1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fama R, Sullivan EV. Methods of association and dissociation for establishing selective brain-behavior relations. Handbook of clinical neurology. 2014;125:175–181. doi: 10.1016/B978-0-444-62619-6.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Chu R, Barakos J. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcoholism, clinical and experimental research. 2009;33(10):1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell JH. First Discussant’s Comments: What is Memory Development the Development of? Human Development. 1971;14:272–278. [Google Scholar]
- Flavell JH, Wellman HM. Metamemory. In: Kail RV, Hagen JW, editors. Perspectives on the development of memory and cognition. Hillsdale, NJ: Laurence Erlbaum Associates; 1977. pp. 3–33. [Google Scholar]
- Freud S. Abrégé de psychanalyse. Paris: PUF; 1949. [Google Scholar]
- Gardiner JM, Ramponi C, Richardson-Klavehn A. Recognition memory and decision processes: a meta-analysis of remember, know, and guess responses. Memory (Hove, England) 2002;10(2):83–98. doi: 10.1080/09658210143000281. [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychology and aging. 1990;5(4):482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug and alcohol dependence. 1995;39(3):197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hannesdottir K, Morris RG. Primary and secondary anosognosia for memory impairment in patients with Alzheimer’s disease. Cortex. 2007;43(7):1020–1030. doi: 10.1016/s0010-9452(08)70698-1. [DOI] [PubMed] [Google Scholar]
- Hart JT. Memory and the feeling-of-knowing experience. Journal of educational psychology. 1965;56(4):208–216. doi: 10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Hayama HR, Vilberg KL, Rugg MD. Overlap between the neural correlates of cued recall and source memory: evidence for a generic recollection network? Journal of cognitive neuroscience. 2012;24(5):1127–1137. doi: 10.1162/jocn_a_00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. Intelligence, brain function and the theory of mind. Brain. 1959;82(2):260–275. doi: 10.1093/brain/82.2.260. [DOI] [PubMed] [Google Scholar]
- Heilman KM. Anosognosia: possible neuropsychological mechanisms. In: Prigatano GP, Schacter DL, editors. Awareness of deficit after brain injury. New York: Oxford University Press; 1991. pp. 53–62. [Google Scholar]
- Hertzog C, Dunlosky J, Sinclair SM. Episodic feeling-of-knowing resolution derives from the quality of original encoding. Memory & cognition. 2010;38(6):771–784. doi: 10.3758/MC.38.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks JL, Marsh RL. On predicting the future states of awareness for recognition of unrecallable items. Memory & cognition. 2002;30(1):60–66. doi: 10.3758/bf03195265. [DOI] [PubMed] [Google Scholar]
- Jang DP, Namkoong K, Kim JJ, Park S, Kim IY, Kim SI, et al. The relationship between brain morphometry and neuropsychological performance in alcohol dependence. Neuroscience letters. 2007;428(1):21–26. doi: 10.1016/j.neulet.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Memory and metamemory: comparisons between patients with frontal lobe lesions and amnesic patients. Psychobiology. 1989;17(1):3–11. [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cerebral cortex. 2007;17(11):2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Jung YC, Jang DP, Namkoong K, Ku J, Kim JJ, Park S, et al. Shape deformation of the insula in alcoholics: reduction of left-right asymmetry. Neuroreport. 2007;18(17):1787–1791. doi: 10.1097/WNR.0b013e3282f193b4. [DOI] [PubMed] [Google Scholar]
- Karlawish JH, Casarett DJ, James BD, Xie SX, Kim SY. The ability of persons with Alzheimer disease (AD) to make a decision about taking an AD treatment. Neurology. 2005;64(9):1514–1519. doi: 10.1212/01.WNL.0000160000.01742.9D. [DOI] [PubMed] [Google Scholar]
- Kikyo H, Miyashita Y. Temporal lobe activations of “feeling-of-knowing” induced by face-name associations. NeuroImage. 2004;23(4):1348–1357. doi: 10.1016/j.neuroimage.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- King DR, de Chastelaine M, Elward RL, Wang TH, Rugg MD. Recollection-related increases in functional connectivity predict individual differences in memory accuracy. The Journal of neuroscience. 2015;35(4):1763–1772. doi: 10.1523/JNEUROSCI.3219-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff syndrome. The British journal of psychiatry : the journal of mental science. 1995;166(2):154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Disorders of memory. Brain : a journal of neurology. 2002;125(Pt 10):2152–2190. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Koriat A. How do we know that we know? The accessibility model of the feeling of knowing. Psychological review. 1993;100(4):609–639. doi: 10.1037/0033-295x.100.4.609. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behavioural brain research. 2000;109(2):177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Pinon K, Vabret F, Pitel AL, Allain P, Eustache F, et al. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcoholism, clinical and experimental research. 2010;34(11):1888–1898. doi: 10.1111/j.1530-0277.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Pitel AL, Chanraud S, Beaunieux H, Eustache F, Martinot JL, et al. Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: Comparison of effects in France and the United States. Human brain mapping. 2014a doi: 10.1002/hbm.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Müller-Oehring EM, Kwon D, Serventi M, Pfefferbaum A, Sullivan EV. Differential compromise of prospective and retrospective metamemory monitoring and their dissociable structural brain correlates, Cortex. 2016 doi: 10.1016/j.cortex.2016.05.002. http://dx.doi.org/10.1016/j.cortex.2016.05.002. [DOI] [PMC free article] [PubMed]
- Le Berre AP, Rauchs G, La Joie R, Mezenge F, Boudehent C, Vabret F, et al. Impaired decision-making and brain shrinkage in alcoholism. European psychiatry. 2014b;29(3):125–133. doi: 10.1016/j.eurpsy.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Vabret F, Cauvin C, Pinon K, Allain P, Pitel AL, et al. Cognitive barriers to readiness to change in alcohol-dependent patients. Alcoholism, clinical and experimental research. 2012;36(9):1542–1549. doi: 10.1111/j.1530-0277.2012.01760.x. [DOI] [PubMed] [Google Scholar]
- Lupker SJ, Harbluk JL, Patrick AS. Memory for things forgotten. Journal of experimental psychology Learning, memory, and cognition. 1991;17(5):897–907. doi: 10.1037//0278-7393.17.5.897. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biological psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel AJ, Tegner R, Nimmo-Smith I. Anosognosia for plegia: specificity, extension, partiality and disunity of bodily unawareness. Cortex. 2004;40(1):19–40. doi: 10.1016/s0010-9452(08)70919-5. [DOI] [PubMed] [Google Scholar]
- McGlynn SM, Schacter DL. Unawareness of deficits in neuropsychological syndromes. Journal of clinical and experimental neuropsychology. 1989;11(2):143–205. doi: 10.1080/01688638908400882. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Medial prefrontal cortex plays a critical and selective role in ‘feeling of knowing’ meta-memory judgments. Neuropsychologia. 2008;46(12):2958–2965. doi: 10.1016/j.neuropsychologia.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Brown RG, Morris RG. Anosognosia in Alzheimer’s disease--the petrified self. Consciousness and cognition. 2009;18(4):989–1003. doi: 10.1016/j.concog.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Morris RG. Implicit awareness in anosognosia: clinical observations, experimental evidence, and theoretical implications. Cognitive neuroscience. 2013;4(3–4):181–197. doi: 10.1080/17588928.2013.833899. [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Morris RG. On the relation among mood, apathy, and anosognosia in Alzheimer’s disease. Journal of the International Neuropsychological Society : JINS. 2014;20(1):2–7. doi: 10.1017/S1355617713001276. [DOI] [PubMed] [Google Scholar]
- Momenan R, Steckler LE, Saad ZS, van Rafelghem S, Kerich MJ, Hommer DW. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry research. 2012;204(2–3):101–111. doi: 10.1016/j.pscychresns.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Morris RG, Hannesdottir K. Loss of ‘awareness’ in Alzheimer’s disease. In: MRG, BJT, editors. The cognitive neuropsychology of Alzheimer’s disease. Oxford: Oxford University Press; 2004. pp. 275–296. [Google Scholar]
- Morris RG, Mograbi DC. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex. 2013;49(6):1553–1565. doi: 10.1016/j.cortex.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol and alcoholism. 2001;36(5):357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. The Resting Brain of Alcoholics. Cerebral cortex (New York, NY : 1991) 2015;25(11):4155–4168. doi: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: a theoretical framework and new finding. In: GH B, editor. The Psychology of Learning and Motivation. New York: Academic Press; 1990. pp. 125–141. [Google Scholar]
- Nixon SJ, Tivis RD, Jenkins MR, Parsons OA. Effects of cues on memory in alcoholics and controls. Alcoholism, clinical and experimental research. 1998;22(5):1065–1069. [PubMed] [Google Scholar]
- Noel X, Van der Linden M, Schmidt N, Sferrazza R, Hanak C, Le Bon O, et al. Supervisory attentional system in nonamnesic alcoholic men. Archives of general psychiatry. 2001;58(12):1152–1158. doi: 10.1001/archpsyc.58.12.1152. [DOI] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: a review. Neuropsychology review. 2005;15(3):105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW, Rapcsak SZ. Metamemory for faces following frontal lobe damage. Journal of the International Neuropsychological Society : JINS. 2005;11(6):668–676. doi: 10.1017/S1355617705050873. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism, clinical and experimental research. 1997;21(3):521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, de la Sayette V, Viader F, et al. Episodic and working memory deficits in alcoholic Korsakoff patients: the continuity theory revisited. Alcoholism, clinical and experimental research. 2008;32(7):1229–1241. doi: 10.1111/j.1530-0277.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, et al. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcoholism, clinical and experimental research. 2007a;31(7):1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Chételat G, Le Berre AP, Desgranges B, Eustache F, Beaunieux H. Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology. 2012;78(17):1330–1333. doi: 10.1212/WNL.0b013e318251834e. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Witkowski T, Vabret F, Guillery-Girard B, Desgranges B, Eustache F, et al. Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcoholism, clinical and experimental research. 2007b;31(2):238–248. doi: 10.1111/j.1530-0277.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Prigatano GP. Disturbances of self-awareness of deficit after traumatic brain injury. In: Prigatano GP, Schacter DL, editors. Awareness of deficit after brain injury: Clinical and theoretical issues. New York: Oxford University Press; 1991. pp. 111–126. [Google Scholar]
- Rainville C, Giroire JM, Periot M, Cuny E, Mazaux JM. The impact of right subcortical lesions on executive functions and spatio-cognitive abilities: a case study. Neurocase. 2003;9(4):356–367. doi: 10.1076/neur.9.4.356.15548. [DOI] [PubMed] [Google Scholar]
- Reed BR, Jagust WJ, Coulter L. Anosognosia in Alzheimer’s disease: relationships to depression, cognitive function, and cerebral perfusion. Journal of clinical and experimental neuropsychology. 1993;15(2):231–244. doi: 10.1080/01688639308402560. [DOI] [PubMed] [Google Scholar]
- Rehm J, Anderson P, Barry J, Dimitrov P, Elekes Z, Feijao F, et al. Prevalence of and potential influencing factors for alcohol dependence in Europe. European addiction research. 2015;21(1):6–18. doi: 10.1159/000365284. [DOI] [PubMed] [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol research & health. 2003;27(2):146–152. [PMC free article] [PubMed] [Google Scholar]
- Rubens AB, Garrett MF. Anosognosia of linguistic deficits in patients with neurological deficits. In: Prigatano GP, Schacter DL, editors. Awareness of deficit after brain injury: Clinical and theoretical issues. New York: Oxford University Press; 1991. pp. 40–52. [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Current opinion in neurobiology. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Taconnat L, Souchay C, Isingrini M. Divided attention at encoding: effect on feeling-of-knowing. Consciousness and cognition. 2009;18(3):754–761. doi: 10.1016/j.concog.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Sayer NA. Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annual review of medicine. 2012;63:405–419. doi: 10.1146/annurev-med-061610-154046. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Toward a cognitive neuropsychology of awareness: implicit knowledge and anosognosia. Journal of clinical and experimental neuropsychology. 1990;12(1):155–178. doi: 10.1080/01688639008400962. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience and biobehavioral reviews. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Nicholls L, Verfaellie M. The role of VMPC in metamemorial judgments of content retrievability. Journal of cognitive neuroscience. 2005;17(5):832–846. doi: 10.1162/0898929053747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, LaFleche G, Nicholls L, Kaszniak AW. A role for right medial prefontal cortex in accurate feeling-of-knowing judgements: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42(7):957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet neurology. 2007;6(5):442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Damadzic R, Mann CL, Schwandt ML, George DT, Hommer DW, et al. Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain : a journal of neurology. 2015;138(Pt 1):69–79. doi: 10.1093/brain/awu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Memory and metamemory: a study of the feeling-of-knowing phenomenon in amnesic patients. Journal of experimental psychology Learning, memory, and cognition. 1986;12(3):452–460. doi: 10.1037//0278-7393.12.3.452. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Long-term memory in amnesia: cued recall, recognition memory, and confidence ratings. Journal of experimental psychology Learning, memory, and cognition. 1988;14(4):763–770. doi: 10.1037//0278-7393.14.4.763. [DOI] [PubMed] [Google Scholar]
- Souchay C, Moulin CJ, Clarys D, Taconnat L, Isingrini M. Diminished episodic memory awareness in older adults: evidence from feeling-of-knowing and recollection. Consciousness and cognition. 2007;16(4):769–784. doi: 10.1016/j.concog.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism, clinical and experimental research. 2003;27(9):1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. War-related PTSD, blast injury, and anosognosia. Neuropsychology review. 2012;22(1):1–2. doi: 10.1007/s11065-012-9188-z. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism, clinical and experimental research. 1995;19(1):110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Muller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, et al. A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biological psychiatry. 2013;74(7):547–555. doi: 10.1016/j.biopsych.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180(4):583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol and alcoholism. 2009;44(2):155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism, clinical and experimental research. 2000;24(5):611–621. [PubMed] [Google Scholar]
- Tapert SF, Ozyurt SS, Myers MG, Brown SA. Neurocognitive ability in adults coping with alcohol and drug relapse temptations. The American journal of drug and alcohol abuse. 2004;30(2):445–460. doi: 10.1081/ada-120037387. [DOI] [PubMed] [Google Scholar]
- Thomas AK, Bulevich JB, Dubois SJ. Context affects feeling-of-knowing accuracy in younger and older adults. Journal of experimental psychology Learning, memory, and cognition. 2011;37(1):96–108. doi: 10.1037/a0021612. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Guerrini I, Marshall EJ. The evolution and treatment of Korsakoff’s syndrome: out of sight, out of mind? Neuropsychology review. 2012;22(2):81–92. doi: 10.1007/s11065-012-9196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and common sense: how far apart? Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2001;356(1413):1505–1515. doi: 10.1098/rstb.2001.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneri A, Shanks MF. Belief and awareness: reflections on a case of persistent anosognosia. Neuropsychologia. 2004;42(2):230–238. doi: 10.1016/s0028-3932(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Anosognosia: the neurology of beliefs and uncertainties. Cortex. 2004;40(1):9–17. doi: 10.1016/s0010-9452(08)70918-3. [DOI] [PubMed] [Google Scholar]
- Weinstein EA, Kahn RL. Personality factors in denial of illness. AMA archives of neurology and psychiatry. 1953;69(3):355–367. doi: 10.1001/archneurpsyc.1953.02320270076008. [DOI] [PubMed] [Google Scholar]
- Weinstein EA, Kahn RL. Denial of illness. Symbolic and physiological aspects. Springfield, IL: Charles C. Thomas; 1955. [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychological bulletin. 1997;121(3):331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Zinn S, Stein R, Swartzwelder HS. Executive functioning early in abstinence from alcohol. Alcoholism, clinical and experimental research. 2004;28(9):1338–1346. doi: 10.1097/01.alc.0000139814.81811.62. [DOI] [PubMed] [Google Scholar]