Abstract
Dendritic cells (DCs) initiate immunity and also antigen-specific tolerance mediated by extrathymic regulatory T (Treg) cells. Yet it remains unclear how DCs regulate induction of such tolerance. Here we report that efficient induction of Treg cells was instructed by BTLA+DEC205+CD8+CD11c+ DCs and the immunomodulatory functions of BTLA. In contrast, T cell activation in steady state by total CD11c+ DCs that include a majority of DCs that do not express BTLA did not induce Treg cells and had no lasting impact on subsequent immune responses. Engagement of HVEM, a receptor of BTLA, promoted Foxp3 expression in T cells through upregulation of CD5. In contrast, T cells activated in the absence of BTLA and HVEM-mediated functions remained CD5lo and therefore failed to resist the inhibition of Foxp3 expression in response to effector cell-differentiating cytokines. Thus DCs require BTLA and CD5-dependent mechanisms to actively adjust tolerizing T cell responses under steady state conditions.
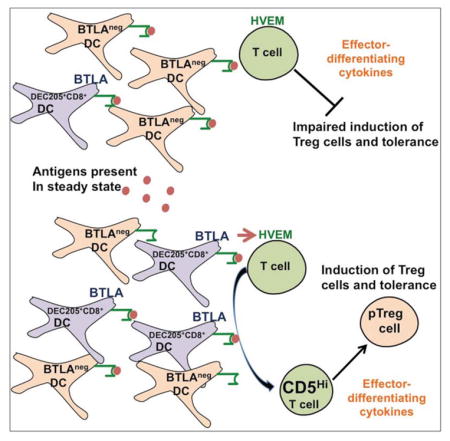
Graphical abstract
INTRODUCTION
Dendritic cells (DCs) prime and also regulate immune responses (Steinman, 2012). In the steady state, defined by the absence of pro-inflammatory stimuli, the outcome of T cell activation by DCs results in T cell tolerance (Hawiger et al., 2001; Ohnmacht et al., 2009; Probst et al., 2003; Steinman et al., 2003). Further, specialized types of DCs have tolerogenic functions (Belz et al., 2002; Coombes et al., 2007; Gottschalk et al., 2013; Idoyaga et al., 2013). However, potentially tolerogenic CD8+, CD103+ or DEC205+ DCs constitute only relatively minor subpopulations among all CD11c+ DCs in the lymphoid organs. Therefore the relevance of tolerogenic functions by such DCs remains unclear in the context of T cell responses to specific antigens that are also being presented by other DCs.
Foxp3-expressing (Foxp3+) peripheral (p) regulatory T (Treg) cells converted by DCs in steady state from extrathymic T cells which induce de novo Foxp3 expression, prevent specific subsequent autoimmune responses (Coombes et al., 2007; Hadeiba et al., 2008; Jones et al., 2015; Josefowicz et al., 2012b; Kretschmer et al., 2005; Sun et al., 2007). Tolerogenic DCs that induce Treg cells are characterized by production of various immunomodulatory metabolites and cytokines (Coombes et al., 2007; Li and Flavell, 2008; Manicassamy et al., 2009; Mascanfroni et al., 2013; Mucida et al., 2007; Munn et al., 2002). Further, specific immunomodulatory molecules such as CTLA-4 and PD-L1 function in tolerance and Treg cell induction by DCs (Fife et al., 2009; Francisco et al., 2009; Probst et al., 2005; Wang et al., 2008; Wing et al., 2008). B and T lymphocyte associated (BTLA), an immunoglobulin domain superfamily protein, is expressed in T cells and antigen presenting cells including CD8+ DCs, where it acts as a ligand for the herpesvirus entry mediator (HVEM), a tumor necrosis factor receptor superfamily member, expressed in resting and activated T cells (Cheung et al., 2005; Murphy and Murphy, 2010; Steinberg et al., 2013; Watanabe et al., 2003). The functions of HVEM and BTLA can govern T cell responses including their memory and regulatory functions (Flynn et al., 2013; Sharma et al., 2014; Soroosh et al., 2011).
In addition to extrinsic signals, a conversion of pTreg cells is mediated by the intrinsic specificity to self and tolerizing antigens. Self-reactive T cells are characterized by increased expression of CD5 that promotes conversion of such CD5hi cells into Foxp3+ pTreg cells by blocking mTOR activated in response to effector cell-differentiating cytokines (Henderson et al., 2015). Therefore CD5 selectively regulates induction of Treg cells without compromising an overall high plasticity of immune responses among a total T cell repertoire. The expression of CD5 in T cells increases in response to either self-peptide(p)MHC in the thymus or tolerizing antigens presented by DCs in the periphery (Azzam et al., 1998; Hawiger et al., 2004). The expression of CD5 in T cells exiting the thymus represents a spectrum rather than discrete amounts and therefore specific upregulation of CD5 expression in T cells by peripheral DCs may be crucial for instructing the conversion of extrathymic Treg cells (Azzam et al., 2001; Hawiger et al., 2004; Henderson et al., 2015). This raises a question of how such a tolerogenic upregulation of CD5 expression in T cells is governed to ensure a specific induction of Treg cells in response to tolerizing antigens presented by DCs.
Here we show that efficient induction of Treg cells relies on a specific T cell stimulation by DCs that use BTLA and CD5-dependent mechanisms to actively adjust tolerizing T cell responses under steady state conditions. In contrast, T cells activated by DCs in the absence of BTLA and HVEM-mediated signals do not upregulate CD5 and are therefore susceptible to inhibition of Foxp3 expression by effector cell-differentiating cytokines. We propose a model whereby antigens available to most CD11c+ DCs that do not express BTLA (BTLAneg), fail to provide a BTLA-mediated signal to upregulate T cell expression of CD5 that promotes induction of pTreg cells. In contrast, only specific T cell stimulation by tolerogenic BTLA+ DCs induces pTreg cells and tolerance.
RESULTS
CD11c+ DCs fail to alter a long-term antigen-specific autoimmune response
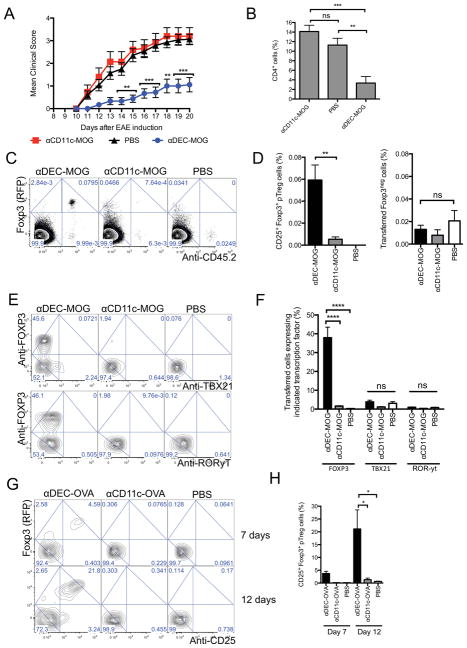
Presentation of myelin oligodendrocyte glycoprotein (MOG) to T cells by peripheral DCs prevents subsequent experimental acute encephalomyelitis (EAE) (Hawiger et al., 2004; Idoyaga et al., 2013; Jones et al., 2015; Yogev et al., 2012). To examine the impact on such tolerance by antigens that are acquired and presented by the entire population of CD11c+ DCs, we developed a recombinant chimeric antibody (denoted here as αCD11c-MOG) to deliver MOG35-55 peptide to all CD11c+ DCs in vivo. CD11c mediates efficient antigen uptake for presentation to T cells (Castro et al., 2008). To help exclude a non-specific targeting by αCD11c-MOG, we used the same mutated mouse heavy and light constant regions that we previously designed for the highly specific αDEC-MOG that delivers MOG to DEC205+ DCs (Hawiger et al., 2001; Hawiger et al., 2004) (Figure S1A) and Experimental Procedures. We confirmed targeting in vivo of αCD11c-MOG to subsets of CD11c+DCs that either expressed or not expressed both DEC205 and CD8α (DEC205+CD8+ and DEC205negCD8neg DC) and verified the specific activation and proliferation of T cells (Figure S1B–D). We then pre-treated multiple groups of B6 mice with αCD11c-MOG and αDEC-MOG 6 weeks before we immunized such mice with MOG35-55 in adjuvant to induce EAE. In agreement with previously published results (Hawiger et al., 2004; Jones et al., 2015), MOG delivered to DEC205+ DCs, prevented EAE and spinal cord T cell infiltrations. In contrast, a treatment with MOG delivered to all CD11c+ DCs had no impact on subsequently induced EAE (Figure 1A and B). We conclude that antigens available to all CD11c+ DCs fail to provide long-lasting specific tolerance.
Figure 1. Activation of T cells by CD11c+ DCs in steady state has no lasting impact on autoimmune responses (A and B).
Targeting of MOG to all CD11c+ DCs fails to prevent subsequently induced EAE. Mice were treated with chimeric antibodies as indicated 6 weeks before induction of EAE. (A) Graphs show mean EAE disease scores (n=10–20). (B) Results show mean percentages of CD4+ T cells in spinal cords 19 days after EAE induction (n=3–4). (C and D) CD11c+ DCs fail to induce pTreg cells in steady state. Sorted 2D2 Foxp3negCD25neg T cells were adoptively transferred and analyzed by flow cytometry 6 weeks after treatment of recipients with chimeric antibodies as indicated. (C) Plots show Foxp3 (RFP) expression and anti-CD45.2 staining intensity among total CD4+ T cells from splenocytes. (D) Graphs show percentages of remaining Foxp3negCD25neg T cells and converted Foxp3+CD25+ pTreg cells among total CD4+ splenocytes (n=5–7). (E and F) Expression of transcription factors. Mice treated as in (C and D) and analyzed after 2 weeks. (E) Plots show anti-FOXP3 and anti-TBX21 (upper panel) or anti-RORyt (lower panel) intracellular staining intensity in transferred CD4+ T cells among splenocytes. (F) Graphs show percentages of cells that were positively stained for FOXP3, TBX21 and RORyt (n=4). (G and H) CD11c+ DCs fail to induce pTreg cells from T cells of various TCR-specificity. Sorted OTII Foxp3negCD25neg T cells were transferred into recipient mice and analyzed by flow cytometry at indicated days after indicated treatments. (G) Plots show Foxp3 (RFP) expression and anti-CD25 staining intensity in transferred CD4+ T cells from splenocytes. (H) Graphs show percentages of pTreg cells (n=3). (C, E and G) Numbers in quadrants indicate corresponding percentages. Results represent one of two to four similar experiments. (A, B, D, F and H) Graphs show mean +/− standard error of mean (SEM), * P< 0.05, ** P< 0.01 ***, P< 0.001 and **** P< 0.0001 determined by one-way or two-way ANOVA, n = number of mice per group from two to four independent experiments. Please see also Figure S1.
The pTreg cells induced de novo by DCs are required for long-lasting tolerance to block responses by newly enlisted encephalitogenic T cells (Jones et al., 2015). Therefore we examined the de novo conversion of Foxp3+ Treg cells from MOG-specific (2D2) Foxp3-neagtive (Foxp3neg) T cells responding to MOG delivered to either all CD11c+ DCs or only DEC205+ DCs. As reported previously (Jones et al., 2015), we observed pTreg cells persisted in animals treated with αDEC-MOG. In contrast, and consistent with the absence of tolerance from EAE in mice 6 weeks after the treatment with αCD11c-MOG, we found negligible numbers of pTreg cells but similar small numbers of Foxp3neg T cells in all groups (Figure 1C and D). Further, we observed no transient induction of Foxp3 expression in such T cells at multiple earlier time points after a treatment with αCD11c-MOG (Figure S1E). Therefore T cell activation by all CD11c+ DCs does not efficiently induce pTreg cells.
CD11c+ DCs can induce specific effector cell responses (Iwasaki and Medzhitov, 2015; Palucka and Banchereau, 2013). Although effector T cells undergo skewing typically under pro-inflammatory conditions, CD11c+ DCs could induce opposing tolerogenic and encephalitogenic T helper-1 (Th1) and Th17 effector T cell responses in steady state. However, we found only similar low expression of the corresponding transcription factors TBX21 and RORyt (Figure 1E and F). The failure of CD11c+ DCs to differentiate T cells into either pTreg cells or effector T cells indicated anergic phenotype of such T cells consistent with a failure to expand to a re-challenge with MOG peptide (Figure S1F and G). Also, mice pre-treated with αCD11c-MOG became resistant to EAE induced 1 week later (Figure S1H). However, in the absence of the de novo conversion of pTreg cells, this CD11c+ DC-induced tolerance was only transient and mice became susceptible to EAE within 6 weeks (Figure 1A and B). Overall, we conclude that T cell activation by all CD11c+ DCs in steady state leads to a transient unresponsiveness of T cells that do not persist and fail to differentiate into pTreg cells to prevent a subsequent antigenic challenge.
To further extend our studies, we developed αCD11c-OVA (Experimental Procedures). We then examined cognate OVA-specific (OTII) T cell responses to OVA peptide delivered to either all CD11c+ DCs or only DEC205+ DCs using αDEC-OVA. As expected, we found about 21% of pTreg cells within 12 days after treatment with αDEC-OVA. In contrast, we found only negligible numbers of pTreg cells after treatment with αCD11c-OVA (Figure 1G and H), despite a similar activation of T cells in αDEC-OVA and αCD11c-OVA-treated groups (Figure S1I). We conclude that CD11c+ DCs fail to induce pTreg cells from T cells of various TCR-specificity.
DEC205+CD8+ DCs promote induction of pTreg cells
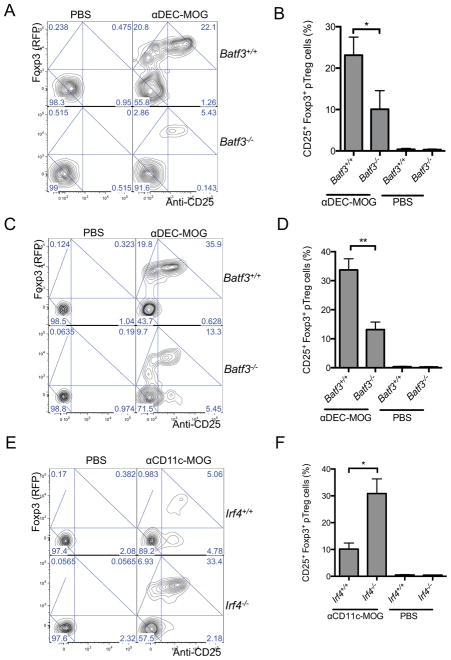
DEC205+CD8negDCs have been proposed to induce Treg cells (Idoyaga et al., 2013). However, other studies suggested a preferential induction of iTreg cells by DEC205+CD8+ DCs (Wang et al., 2008; Yamazaki et al., 2008). To determine such roles of DEC205+CD8+ DCs in vivo, we used Batf3−/− mice characterized by reduced numbers of these DCs (Hildner et al., 2008). 12 and 21 days after a treatment with αDEC-MOG we observed, correspondingly, only about 5–10% and 13% conversion of specific T cells into pTreg cells in the Batf3−/− mice, whereas the pTreg cell induction in Batf3+/+ mice remained at about 22% and 36%, respectively (Figure 2A–D). Therefore similar to the functions in thymus (Perry et al., 2014), peripheral Batf3-dependent DEC205+CD8+ DCs induce pTreg cells in vivo when antigen is delivered specifically to these DCs.
Figure 2. DEC205+CD8+ DCs mediate induction of pTreg cells.
(A–D) Batf3-dependent DCs are required for pTreg cell induction. Sorted 2D2 Foxp3negCD25neg T cells were adoptively transferred into Batf3+/+ and Batf3−/− recipient mice that were treated as indicated. Plots show Foxp3 (RFP) expression and anti-CD25 staining intensity in transferred CD4+ T cells from splenocytes analyzed by flow cytometry after 12 days (A) or 21 days (C). Graphs show percentages of pTreg cells (n=4–5) after 12 days (B) or 21 days (D). (E and F) Conversion of pTreg cells depends on the proportion of the DEC205+CD8+ DCs among CD11c+ DCs. Cells as in (A) were transferred into Irf4−/− and Irf4+/+ recipient mice treated as indicated. (E) Plots show Foxp3 (RFP) expression and anti-CD25 staining intensity in transferred CD4+ T cells from splenocytes analyzed by flow cytometry after 21 days. (F) Graphs show percentages of pTreg cells (n=9–11). (A, C and E) Numbers in quadrants indicate corresponding percentages. Results represent one of three to four similar experiments. (B, D and F) graphs show mean +/− SEM, * P< 0.05 and ** P< 0.01 determined by one-way ANOVA, n = number of mice per group from three to four independent experiments. Please see also Figure S2.
The failure to induce pTreg cells by CD11c+ DCs that include DEC205+CD8+ DCs (Figure 1) could result from targeting through the CD11c integrin receptor. Alternatively, induction of pTreg cells may be inefficient in the presence of the majority of non-DEC205+CD8+ DCs presenting the same antigen. Therefore we used a ItgaxcreIrf4fl/fl (Irf4−/−) genetic mouse model characterized by a proportional increase of DEC205+CD8+ DCs (Figure S2A) due to a reduction of numbers of DEC205negCD8neg DCs (Persson et al., 2013; Schlitzer et al., 2013). We found that in Irf4−/− mice treated with αCD11c-MOG the pTreg cell conversion rate increased about 3–6 fold compared to the corresponding pTreg cell induction in Irf4+/+ mice, despite similar T cell activation in both types of recipients (Figure 2E and F and Figure S2B). This indicates that pTreg cell conversion depends on the proportion of the DEC205+CD8+ DCs among all CD11c+ DCs that can present the same antigen. We collectively conclude that T cells stimulated predominantly by DEC205+CD8+ DCs are converted to pTreg cells in vivo.
Tolerogenic induction of pTreg cells depends on BTLA
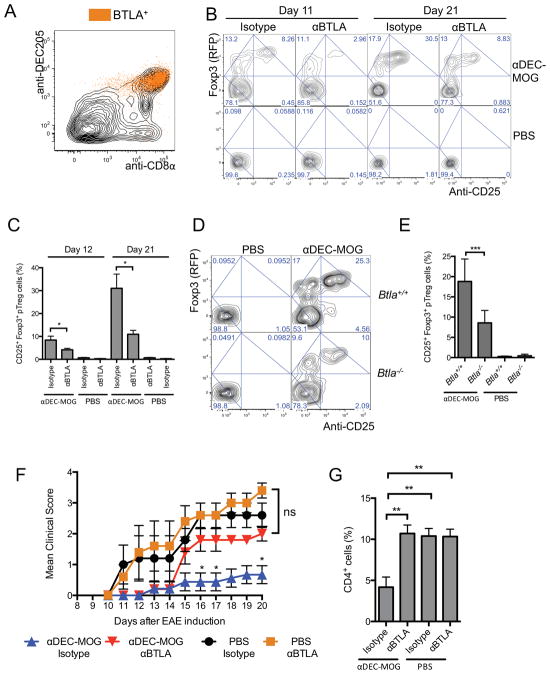
The immunemodulatory molecules such as PD-L1 expressed by CD8+ DCs help mediate the induction of Treg cells by these DCs (Shortman and Heath, 2010; Wang et al., 2008). However, it remains unclear how a conversion of pTreg cells is specifically determined by tolerogenic DCs in vivo. CD8+ DCs express BTLA (Flynn et al., 2013) and we confirmed BTLA to be specifically expressed in DEC205+CD8+ DCs from multiple lymphoid organs (Figure 3A and S3A). We therefore hypothesized that these DCs may require BTLA to induce pTreg cells.
Figure 3. BTLA is required for induction of tolerogenic pTreg cells.
(A) BTLA is specifically expressed in DEC205+CD8+ DCs. The plot shows anti-DEC205 and anti-CD8α staining intensities analyzed by flow cytometry in splenic CD11c+MHCII+ DCs with superimposed distribution of BTLA+ events. (B and C) Blocking of BTLA reduces DC-mediated pTreg cell conversion. 2D2 Foxp3negCD25neg T cells were transferred into recipient mice treated with chimeric antibodies and αBTLA or isotype control as indicated. (B) Plots show Foxp3 (RFP) expression and anti-CD25 staining intensity in transferred CD4+ T cells from splenocytes analyzed by flow cytometry after 11 and 21 days. (C) Graphs show percentages Foxp3+CD25+ pTreg cells (n=4–5). (D and E) BTLA is required for DC-mediated pTreg cell induction. 2D2 Foxp3negCD25neg T cells were adoptively transferred into Btla+/+ and Btla−/− recipient mice that were treated as indicated. (D) Plots show Foxp3 (RFP) expression and anti-CD25 staining intensity in transferred CD4+ T cells from splenocytes analyzed by flow cytometry after 9 days. (E) Graphs show percentages of pTreg cells (n=4–6). (F and G) Blocking of BTLA abolishes DC-induced tolerance against EAE. Mice were treated as indicated and subsequently treated with αBTLA or isotype control 6 weeks before induction of EAE. (F) Graphs show mean EAE disease scores (n=10–20). (G) Results show mean percentages of CD4+ T cells in spinal cords 19 days after EAE induction (n=3–4). (B and D) Numbers in quadrants indicate corresponding percentages. (A, B and D) Results represent one of three similar experiments. (C, E, F and G) Graphs show mean +/− SEM, * P< 0.05, ** P< 0.01 and *** P< 0.001 determined by one-way or two-way ANOVA, n = number of mice per group from two to three independent experiments. Please see also Figure S3.
To test such a role for BTLA, we tested an impact on pTreg cell conversion of blocking BTLA by αBTLA (6A6) (Hurchla et al., 2005) that prevents BTLA binding to its ligand HVEM. In mice treated with αBTLA we found a consistent about 70% decrease in conversion of Foxp3+ pTreg cells (Figure 3B and C). In contrast, blocking of BTLA did not alter expression of effector transcription factors TBX21 and RORyt in the remaining T cells or initial T cell activation following αDEC-MOG treatment (Figure S3B and C).
A treatment with αBTLA could also impact functions of BTLA expressed in T cells (Albring et al., 2010). Therefore we used Btla−/− and Btla+/+ mice as 2D2 T cell recipients. We found that the specific pTreg cell conversion in Btla−/− recipient mice treated with αDEC-MOG decreased by about 50–60% compared to Btla+/+ recipients (Figure 3D and E) despite similar specific T cell activation and similar numbers of DEC205+CD8+ DCs in Btla−/− and Btla+/+ recipient mice (Figure S3D and E). We also observed an increased induction of iTreg cells by Btla+/+ DEC205+CD8+ DCs as compared to DEC205negCD8neg DCs and Btla−/− DEC205+CD8+ DCs (Figure S3F and G). This was accompanied in cultured DCs by a partial, non-specific down-regulation of BTLA expression consistent with its moderate impact on in vitro iTreg cell induction (Figure S3H). In contrast to specific functions of BTLA in DCs, we observed a similar conversion of pTreg cells from Btla−/− or Btla+/+ T cells in vivo (Figure S3I and J). We collectively conclude that BTLA promotes an efficient de novo conversion of Treg cells by DCs.
A genetic deletion of BTLA increases severity of EAE (Watanabe et al., 2003). Therefore to examine the role of BTLA in specific anti-EAE tolerance, we pre-treated B6 mice with αDEC-MOG followed by a treatment with αBTLA. After 6 weeks we then immunized such mice to induce EAE. We observed a similar EAE induction in groups pre-treated with either 6A6 αBTLA antibody or an isotype control in the absence of MOG targeting to DCs, consistent with a lack of an impact of a transient blockade of BTLA on EAE induced 6 weeks later. In contrast, in mice treated with αDEC-MOG, despite an initial resistance to EAE, a concomitant blocking of BTLA abolished the DC-induced anti-EAE tolerance in agreement with the role of BTLA in induction by DCs of pTreg cells to efficiently inhibit EAE (Figure 3F and G).
BTLA and HVEM function to upregulate CD5 expression in peripheral T cells
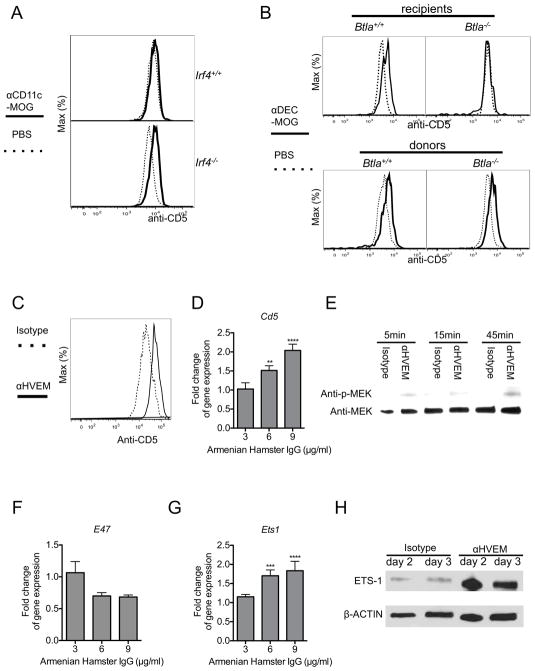
2D2 T cells in mice treated with αDEC-MOG respond with an increased expression of CD5 that precedes a de novo induction of Foxp3 expression (Henderson et al., 2015). In contrast, we found an unaltered expression of CD5 in T cells from mice treated with either PBS or αCD11c-MOG (Figure S4A). Therefore consistent with the inefficient conversion of pTreg cells in response to stimulation by all CD11c+ DCs, these DCs also fail to increase expression of CD5 in T cells. However, T cells upregulated CD5 expression in response to MOG delivered by αCD11c-MOG in Irf4−/− mice that have an increased proportion of BTLA+DEC205+CD8+ DC, consistent with the role of these DCs in mediating pTreg cell induction (Figure 4A). To determine if BTLA functions are required for this specific increase of CD5 expression, we examined adoptively transferred 2D2 T cells responding to MOG delivered to DEC205+CD8+ DCs in Btla−/− and Btla+/+ recipient mice. As expected in Btla+/+ mice Foxp3neg T cells increased expression of CD5. However, expression of CD5 remained low in T cells recovered from Btla−/− mice (Figure 4B-upper panel). Further, T cells stimulated in vitro by Btla+/+ DEC205+CD8+ DCs had higher expression of CD5 than T cells responding to DEC205negCD8neg DCs or Btla−/− DEC205+CD8+ DCs, further consistent with the specific functions of BTLA to mediate CD5 induction (Figure S4B). In contrast, we observed a similar activation and CD5 expression in Btla+/+ and Btla−/− T cells in vivo (Figure S4C and Figure 4B-lower panel). We collectively conclude that BTLA is required for the DEC205+CD8+ DC-mediated upregulation of CD5 expression in T cells.
Figure 4. Upregulation of CD5 in T cells depends on BTLA and HVEM functions.
(A) BTLA+DEC205+CD8+CD11c+ DCs increase CD5 expression in T cells in vivo. 2D2 Foxp3negCD25neg T cells were adoptively transferred into Irf4+/+ and Irf4−/− recipient mice treated as indicated. Overlaid histograms show staining intensity with anti-CD5 in transferred Foxp3neg T cells among splenocytes analyzed by flow cytometry after 9 days. (B) BTLA is required for DC-mediated upregulation of CD5 in T cells. Upper panel - 2D2 Foxp3negCD25neg T cells were transferred into Btla+/+ and Btla−/− recipient mice that were treated as indicated. Lower panel - Btla+/+ and Btla−/− 2D2 Foxp3negCD25neg T cells were transferred into recipient mice that were treated as indicated. Overlaid histograms show staining intensity with anti-CD5 in transferred Foxp3neg CD4+ T cells among splenocytes analyzed by flow cytometry after 9 days. (C) Engagement of HVEM in T cells induces CD5 upregulation. Naïve 2D2 Foxp3negCD25neg CD4+ T cells were stimulated in vitro for 3 days with αCD3 and αCD28 and in the presence of either αHVEM or isotype control followed by cross-linking with a secondary reagent. Overlaid histograms show CD5 expression in Foxp3neg CD4+ T cells as indicated. (D) Engagement of HVEM in T cells induces Cd5 gene expression. Naïve 2D2 CD4+Foxp3negCD25neg T cells were stimulated for 2 days in vitro in the presence of either αHVEM or isotype control at indicated concentrations followed by cross-linking with a secondary reagent. Gene expression was analyzed by quantitative real-time RT-PCR, normalized for expression of HPRT and calculated using ΔΔCT method. Graph shows a fold difference in αHVEM over isotype treated with an arbitrary value indicating no change set at 1. (E) Engagement of HVEM in T cells activates MEK phosphorylation. Immunoblot analysis in lysates of T cells stimulated as in (D) and for the indicated times. (F and G) Engagement of HVEM in T cells reduces E47 gene expression (F) and induces Ets1 gene expression (G). T cells were stimulated and gene expression analyzed as in (D). (H) Engagement of HVEM in T cells increases ETS1 protein expression. Immunoblot analysis in lysates of T cells stimulated as in (D) and for the indicated times. (A, B, C, E, and H) Results represent one of two to three similar experiments. (D, F, and G) Results (n=4) from two independent experiments represent mean +/− SEM. ** p ≤0.01 *** p ≤0.001 **** p ≤0.0001, analyzed by two-way ANOVA. Please see also Figure S4.
To investigate if engagement of HVEM, a receptor for BTLA, upregulates expression of CD5, we cross-linked HVEM in naïve sorted Foxp3negCD25neg 2D2 and polyclonal CD4+ T cells also activated through TCR stimulation. We found that an additional engagement of HVEM upregulated surface expression of CD5 and increased Cd5 gene expression (Figure S4D and Figure 4C and D). HVEM can govern multiple signaling pathways in immune and epithelial cells (Shui et al., 2012; Shui et al., 2011; Steinberg et al., 2011). In developing CD5hi T cells a specific engagement of HVEM had no impact on STAT3 phosphorylation and a minimal impact on TCR-induced NFkB activation (Figure S4E-G). In contrast, HVEM engagement increased phosphorylation of mitogen-activated protein kinase (MAPK) kinase (MEK) (Figure 4E). MEK activation can increase expression of E26 avian leukemia oncogene 1 (ETS1) as well as decrease an expression of transcription factor 3 (TCF-3 also known as E2A or E47), correspondingly, positive and negative regulators of Cd5 expression (Khanna et al., 2011; Page et al., 2004; Paumelle et al., 2002; Tung et al., 2001; Yang et al., 2004). We found a decreased expression of E2A (E47) and an increased expression and abundance of ETS1 in developing CD5hi T cells, consistent with their specific functions in transcriptional regulation of Cd5 expression (Figure 4F–H). We conclude that HVEM governs in developing CD5hi T cells pathways regulating expression of CD5.
HVEM-mediated upregulation of CD5 relieves inhibition of Treg cell induction by effector cell-differentiating cytokines
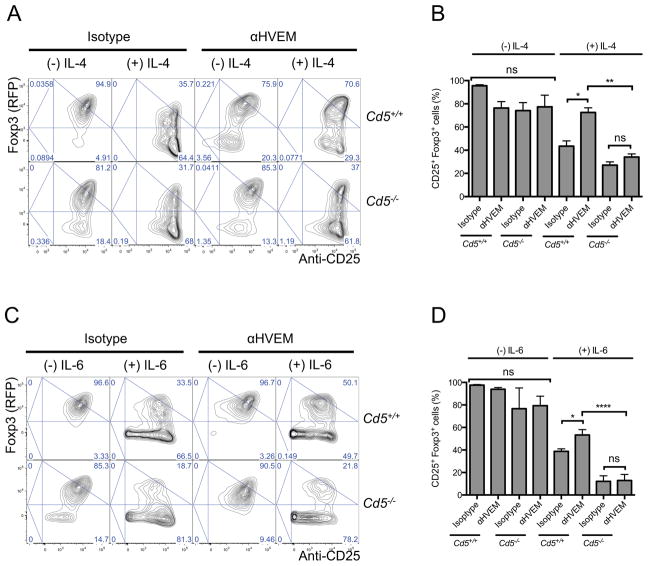
The CD5hi T cells, but not CD5lo or Cd5−/− T cells, can efficiently convert into Treg cells in the presence of effector cell-differentiating cytokines (Henderson et al., 2015). We engaged HVEM in naïve Cd5+/+ and Cd5−/− 2D2 CD4+ T cells cultured under Treg cell-differentiating conditions and in the absence or presence of IL-4 or IL-6 (Figure 5A–D). We found a similar high rate of Treg cell induction in Cd5+/+ and Cd5−/− T cells in the absence of these cytokines. Without an engagement of HVEM, IL-4 or IL-6 decreased such Treg cell induction by about 60–80% both in Cd5+/+ and Cd5−/− T cells. However, consistent with its role to increase expression of CD5, an engagement of HVEM overcame an IL-4 and IL-6-mediated inhibition of Treg cell induction and over 70% and 50% of Cd5+/+ T cells converted to Treg cells despite the presence of IL-4 and IL-6, respectively (Figure 5A–D). In contrast, a conversion of Cd5−/− T cells into Treg cells remained low at about 37% and 21% in the presence of IL-4 and IL-6, respectively, despite an engagement of HVEM (Figure 5A–D), In contrast, an engagement of HVEM had no effect on the induction of iTreg cells in the absence of IL-4 or IL-6. We also observed a similar CD5-dependent promotion of Treg cell induction upon engagement of HVEM in polyclonal T cells (Figure S5A and B). Thus an engagement of HVEM in T cells leads to the upregulation of CD5 expression to promote induction of Treg cells.
Figure 5. Engagement of HVEM in T cells induces CD5-dependent Treg cell induction.
(A and B) HVEM-mediated upregulation of CD5 relieves inhibition of Treg cell induction by IL-4. (A) Naïve Cd5+/+ and Cd5−/− 2D2 Foxp3negCD25neg CD4+ T cells were stimulated for 5 days in vitro with αCD3 and αCD28 in the presence of TGF-β and αHVEM or isotype control followed by cross-linking with a secondary reagent and also in the presence or absence of IL-4. Plots show Foxp3 (RFP) expression and anti-CD25 staining intensity. (B) Graphs show percentages of Foxp3+CD25+ cells (n=3–4). (C and D) HVEM-mediated upregulation of CD5 relieves inhibition of Treg cell induction by IL-6. (C) T cells were cultured as in (A) but in the presence or absence of IL-6. Plots show Foxp3 (RFP) expression and anti-CD25 staining intensity. (D) Graphs show percentages of Foxp3+CD25+ cells in the indicated groups (n=3–5). (A and C) Numbers in quadrants indicate corresponding percentages. Results represent on of two to three similar experiments. (B and D) Results represent mean +/− SEM, * P< 0.05, ** p ≤0.01 and **** p ≤0.0001 determined by one-way ANOVA, Results in each group are from two to three independent experiments. Please see also Figure S5.
DISCUSSION
In this study we have identified a crucial mechanism governing induction of extrathymic Treg cells and tolerance by DCs. BTLA and HVEM-mediated functions increase in T cells the expression of CD5 to instruct Treg cell differentiation. Thus, only T cells specifically activated by BTLA+DEC205+CD8+ DCs efficiently convert into tolerogenic pTreg cells. In contrast, Treg cell conversion is impaired in T cells that respond to antigens presented by the majority of CD11c+ DCs that are BTLAneg.
CD11c+ DCs in lymphoid tissues constitute phenotypically heterogeneous populations whose development is dependent on distinct transcription factors (Satpathy et al., 2012). This diversity matches the versatile T cell responses induced by such DCs (Guilliams et al., 2014; Mellman, 2013; Steinman, 2012). In the pro-inflammatory environment, priming of T cells by DCs leads to immune responses. In contrast, the presentation of antigens by DCs in steady state can result in multiple mechanisms of T cell tolerance (Hawiger et al., 2001; Ohnmacht et al., 2009; Probst et al., 2003; Steinman et al., 2003). However, a broad tolerogenic outcome of antigenic presentation by DCs in steady state could hamper subsequent protective immune responses against cross-reactive antigens. Therefore induction of tolerance by DCs may rather be mediated by specialized “tolerogenic” DCs to enhance the specificity of such immune regulation (Coquerelle and Moser, 2010; Merad et al., 2013). Yet, despite the proposed tolerance-inducing functions of some DCs, a precise functional definition of tolerogenic DCs remains elusive. The proposed populations of tolerogenic DCs expressing CD8, DEC205 or CD103, include the “lymphoid resident” DCs and migratory DCs that transfer peripheral antigens to the lymphoid tissues (Coquerelle and Moser, 2010; Lukacs-Kornek and Turley, 2011; Merad et al., 2013; Randolph et al., 2008; Shortman and Heath, 2010). However, it remains unknown if the induction of tolerance requires T cell activation by antigens whose presentation is restricted to tolerogenic DCs. Our findings that only antigens presented specifically by BTLA+DEC205+CD8+ DCs induce T cell tolerance, support a model of tolerance that relies on a compartmentalization of antigenic presentation by various DC types. Our results further indicate that by governing the crucial tolerogenic functions of DCs, BTLA helps to further shape a plasticity of DC-mediated T cell responses determined by various pathways (Iwasaki and Medzhitov, 2015; Kumamoto et al., 2013; Sancho and Reis e Sousa, 2012; Segura and Amigorena, 2013; Steinman, 2012). In contrast, the roles in specific immune responses of BTLA expressed solely in various types of immune cells require further investigation.
A conversion of Foxp3+ pTreg cells by DCs mediates tolerance and induction of pTreg cells by DCs is independent of other potential antigen presenting cells (Esterhazy et al., 2016; Hawiger et al., 2010; Jones et al., 2015; Kretschmer et al., 2005). Several mechanisms proposed to mediate the induction of Treg cells by DCs rely on specific cytokines and metabolites as well as signaling by PD-1 and CTLA-4 (Coombes et al., 2007; Francisco et al., 2009; Li and Flavell, 2008; Manicassamy et al., 2009; Mascanfroni et al., 2013; Mucida et al., 2007; Probst et al., 2005; Sun et al., 2007; Wang et al., 2008; Yogev et al., 2012). Although the relevant mechanisms by which these immunomodulatory pathways induce Foxp3+ Treg cells remain incompletely understood, such pathways may directly affect the cell-intrinsic mechanisms that induce Foxp3 expression (Benoist and Mathis, 2012; Josefowicz et al., 2012a).
However, the regulation of immune tolerance also depends on the specificity of T cells to self and tolerizing antigens as reflected by expression of CD5 (Azzam et al., 1998; Klein et al., 2014). CD5 is a surface protein that recruits multiple positive and negative regulators of T cell signaling (Soldevila et al., 2011). CD5 expression parallels T cell receptor (TCR) signal strength during thymic T cell development leading to an increased CD5 expression in developing CD4+ T cells bearing high affinity TCR for self-antigens (Azzam et al., 1998). Despite the functions of CD5 as a negative regulator of TCR signaling in thymus, the peripheral CD5hi T cells remain responsive to antigenic stimulation and can form cross-reactive effector T cells thereby risking the development of autoimmune responses (Klein et al., 2014; Mandl et al., 2013; Persaud et al., 2014). Recently, we established that CD5 instructs the conversion of such self-reactive and potentially autoagressive extrathymic CD5hi T cells into pTreg cells by modulating their responsiveness to effector cell-differentiating cytokines (Henderson et al., 2015). In addition to thymic development, CD5 expression can also increase in response to peripheral tolerogenic signals (Hawiger et al., 2004). Therefore an upregulation of CD5 expression is a common mechanism for both thymic and peripheral processes to instruct subsequent induction of pTreg cells (Henderson et al., 2015).
However, mechanisms governing in peripheral T cells the expression of CD5 and the impact of such mechanisms on tolerance remained unknown. Our results now indicate that only BTLA+DEC205+CD8+ DCs specifically mediate a tolerizing upregulation of CD5 expression in T cells. Therefore, the antigens acquired and presented predominantly by these tolerogenic DCs may be perceived as “tolerizing” by T cells. In contrast, antigens that are available to all CD11c+ DCs and presented by a majority of non-tolerogenic BTLAneg DCs fail to provide a BTLA-mediated signal to upregulate CD5 expression in T cells and do not alter the subsequent immune responses in steady state. Our results also reveal that HVEM, a receptor of BTLA, expressed in T cells, governs expression of transcription factors ETS1 and E2A (E47) that regulate expression of CD5. Therefore functions of BTLA and HVEM enable DCs to link the T cell responsiveness to specific tolerogenic antigens with a CD5-dependent process governing pTreg cell induction to maintain the de novo-induced peripheral T cell tolerance. In the absence of these specific functions of BTLA and CD5, the steady state conditions alone may not facilitate a sufficient induction of tolerance by DCs. Therefore, we propose that through the combined functions of BTLA and CD5 tolerogenic DCs can actively adjust T cell responses by favoring a differentiation of CD5hi T cells that can more readily become tolerizing pTreg cells.
The differences in cytokine milieu between in vivo and ex vivo environments as well as phenotypic alterations of DCs in vitro may contribute to the specific variations of especially iTreg cell induction, also possibly regulated by multiple mechanisms (Wang et al., 2008; Yamazaki et al., 2008). However, the functions of BTLA and HVEM are both necessary and sufficient to promote a differentiation of Treg cells that are crucial to govern T cell tolerance and prevention of autoimmunity by DCs in vivo.
In conclusion, by showing that induction of pTreg cells relies on specific DCs that use BTLA and CD5-dependent mechanisms to actively adjust T cell responses, our findings help to further define and extend a concept of tolerogenic DCs. In contrast, a majority of CD11c+ DCs are BTLAneg and therefore are not tolerogenic even in the steady state. Thus a selective induction of pTreg cells by specialized tolerogenic functions of BTLA+ DCs may promote tolerance while helping to maintain an overall high plasticity of immune responses among the total repertoire of T cells that respond to antigens presented by all DCs.
EXPERIMENTAL PROCEDURES
For complete experimental procedures see supplemental information.
Mice
Cd5−/− (Tarakhovsky et al., 1994), Foxp3RFP (Wan and Flavell, 2005), anti-MOG TCR transgenic (2D2) (Bettelli et al., 2003), OTII TCR Tg (Barnden et al., 1998), Batf3−/− (Hildner et al., 2008), Irf4fl/fl (Klein et al., 2006), Cd11c-Cre (Caton et al., 2007) and Btla−/− (Watanabe et al., 2003) mice were previously described and are available from the Jackson Laboratory. All mice were bred on a CD45.1 or CD45.2 congenic C57BL/6 background. 6–8 week old sex and age-matched littermates were used for all experiments. All mice were maintained in our facility under specific pathogen free conditions and used in accordance with guidelines of the Saint Louis University Institutional Animal Care and Use Committee.
Chimeric antibodies
Chimeric antibodies were produced as previously described (Hawiger et al., 2001; Hawiger et al., 2004). Chimeric antibodies were injected in PBS intraperitoneally at 15μg/mouse (MOG-delivering antibodies) or 125 ng/mouse (OVA-delivering antibodies) as established previously (Hawiger et al., 2004; Hawiger et al., 2010).
Flow cytometry and antibodies used for staining
Adoptive transfers
Lymph nodes and spleen cells were pooled and CD4+ T cells were enriched by depleting CD8+, B220+, CD11c+, CD11b+ and NK1.1+ cells with magnetic microbeads (Miltenyi) and then cell sorted on ARIA III (BD). 5x106 cells/mouse were injected intravenously in tail veins. In all experiments transferred T cells and recipients were congenically labeled based on CD45.1 and CD45.2 expression.
Antibody Injections
αBTLA (6A6) or isotype control (Armenian Hamster IgG) antibodies (BioXCell) in PBS were injected intraperitoneally 150μg/mouse 4 hours and 26 hours after chimeric antibodies injection.
EAE Model
To induce EAE mice were injected with 100μg synthetic Myelin Oligodendrocyte Glycoprotein peptide (MOG35-55, Yale Keck Protein Synthesis Facility) in Complete Freund’s Adjuvant subcutaneously in each flank. Pertussis Toxin (List Biological Laboratories Inc.) was injected 200ng per mouse in PBS intraperitoneally on days 0 and 2 after MOG35-55 injections. Clinical score of EAE was graded on a scale of 1–4.
Cell cultures
Lymph node and spleen cells were pooled and CD4+ T cells were enriched using magnetic microbeads (Miltenyi) and then sorted on ARIA III (BD). 0.25x106/well T cells were cultured with anti-CD3 (145-2C11)(1 μg/ml) and anti-CD28 (37.51)(1.5 μg/ml) in RPMI-1640 media containing 10% FBS, Penicillin-Streptomycin, L-Glutamine, β-Mercaptoethanol, Sodium pyruvate (Gibco), recombinant IL-2 (200 units/ml), and in some experiments TGF-β (1–4 ng/ml) (BioLegend). Anti-CD3 was cross-linked by addition of anti-Armenian Hamster IgG (15 μg/ml) after 20 min incubation at 37°C. In some experiments recombinant IL-4 (2ng/ml) or IL-6 (8ng/ml) were additionally added. Additionally, anti-HVEM (HMHV-1B18) (BioLegend) or Isotype control (Armenian Hamster IgG) (BioXCell) were added to some cultures at 6 μg/ml or as indicated and were cross-linked by addition of anti-Armenian Hamster IgG (15 μg/ml) after 20 min incubation at 37°C. Alternatively LNs and spleens were dissociated in 5% FCS RPMI and incubated in the presence of collagenase D (Roche) and EDTA as described before (Hawiger et al., 2001; Hawiger et al., 2004), CD11C+ DCs were enriched using microbeads (Miltenyi) and then sorted on ARIA III (BD). 0.35–0.7x105/well DCs and CD11cneg cells were cultured with 0.2x106/well T cells in RPMI-1640 media containing 10% FBS, Penicillin-Streptomycin, L-Glutamine, β-Mercaptoethanol (Gibco), and Sodium pyruvate (Gibco). In some experiments synthetic MOG35-55 (60 μg/ml), recombinant IL-2 (200 units/ml), and TGF-β (2 ng/ml) were also added or T cells were labeled with 3 μM CFSE (Sigma) in 5% FCS RPMI at 37°C for 20 min.
Immunoblot and Real-time RT- PCR analysis
Statistical analysis
In all experiments data was pooled from two to four independent experiments and individual P values were calculated using Student’s t-test with Welch’s correction, one-way ANOVA or two-way ANOVA.
Supplementary Material
Acknowledgments
The authors would like to thank Joy Eslick and Sherri L. Koehm (Saint Louis University) for expert help with flow cytometry and Ziva Misulovin (Saint Louis University) for help with immunoblots. This work was supported in part by grants from the National Multiple Sclerosis Society (RG5019A) and National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI113903) (both to D.H.). This publication is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors declare no financial conflict of interests.
Footnotes
Author Contributions
A.J. designed and performed experiments, interpreted data and wrote the manuscript. J.B. performed some of the in vitro experiments and interpreted data, L. K. performed RT-PCR analysis and interpreted data, A.O. performed some of the chimeric antibody production, R.M.T. participated in preparation of manuscript, C.G. maintained experimental animals and participated in preparation of experiments and manuscript. D.H. conceived, designed and oversaw experiments, interpreted data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albring JC, Sandau MM, Rapaport AS, Edelson BT, Satpathy A, Mashayekhi M, Lathrop SK, Hsieh CS, Stelljes M, Colonna M, et al. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. The Journal of experimental medicine. 2010;207:2551–2559. doi: 10.1084/jem.20102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. Journal of immunology. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. The Journal of experimental medicine. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. The Journal of experimental medicine. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harbor perspectives in biology. 2012;4:a007021. doi: 10.1101/cshperspect.a007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. The Journal of experimental medicine. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur J Immunol. 2008;38:2263–2273. doi: 10.1002/eji.200838302. [DOI] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. The Journal of experimental medicine. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear PG, et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunological reviews. 2010;234:317–334. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- Esterhazy D, Loschko J, London M, Jove V, Oliveira TY, Mucida D. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nature immunology. 2016;17:545–555. doi: 10.1038/ni.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nature immunology. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn R, Hutchinson T, Murphy KM, Ware CF, Croft M, Salek-Ardakani S. CD8 T cell memory to a viral pathogen requires trans cosignaling between HVEM and BTLA. PloS one. 2013;8:e77991. doi: 10.1371/journal.pone.0077991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk C, Damuzzo V, Gotot J, Kroczek RA, Yagita H, Murphy KM, Knolle PA, Ludwig-Portugall I, Kurts C. Batf3-dependent dendritic cells in the renal lymph node induce tolerance against circulating antigens. J Am Soc Nephrol. 2013;24:543–549. doi: 10.1681/ASN.2012101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nature reviews Immunology. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nature immunology. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. The Journal of experimental medicine. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Wan YY, Eynon EE, Flavell RA. The transcription cofactor Hopx is required for regulatory T cell function in dendritic cell-mediated peripheral T cell unresponsiveness. Nature immunology. 2010;11:962–968. doi: 10.1038/ni.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JG, Opejin A, Jones A, Gross C, Hawiger D. CD5 Instructs Extrathymic Regulatory T Cell Development in Response to Self and Tolerizing Antigens. Immunity. 2015;42:471–483. doi: 10.1016/j.immuni.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. Journal of immunology. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. The Journal of clinical investigation. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nature immunology. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Opejin A, Henderson JG, Gross C, Jain R, Epstein JA, Flavell RA, Hawiger D. Peripherally Induced Tolerance Depends on Peripheral Regulatory T Cells That Require Hopx To Inhibit Intrinsic IL-2 Expression. Journal of immunology. 2015;195:1489–1497. doi: 10.4049/jimmunol.1500174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012a;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012b;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Okkeri J, Bilgen T, Tiirikka T, Vihinen M, Visakorpi T, Westermarck J. ETS1 mediates MEK1/2-dependent overexpression of cancerous inhibitor of protein phosphatase 2A (CIP2A) in human cancer cells. PloS one. 2011;6:e17979. doi: 10.1371/journal.pone.0017979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nature reviews Immunology. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nature immunology. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature immunology. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Lukacs-Kornek V, Turley SJ. Self-antigen presentation by dendritic cells and lymphoid stroma and its implications for autoimmunity. Current opinion in immunology. 2011;23:138–145. doi: 10.1016/j.coi.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2- dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nature immunology. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Dendritic cells: master regulators of the immune response. Cancer immunology research. 2013;1:145–149. doi: 10.1158/2326-6066.CIR-13-0102. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annual review of immunology. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. The Journal of experimental medicine. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annual review of immunology. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. The Journal of experimental medicine. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page JL, Wang X, Sordillo LM, Johnson SE. MEKK1 signaling through p38 leads to transcriptional inactivation of E47 and repression of skeletal myogenesis. The Journal of biological chemistry. 2004;279:30966–30972. doi: 10.1074/jbc.M402224200. [DOI] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Human dendritic cell subsets in vaccination. Current opinion in immunology. 2013;25:396–402. doi: 10.1016/j.coi.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumelle R, Tulasne D, Kherrouche Z, Plaza S, Leroy C, Reveneau S, Vandenbunder B, Fafeur V. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene. 2002;21:2309–2319. doi: 10.1038/sj.onc.1205297. [DOI] [PubMed] [Google Scholar]
- Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nature immunology. 2014;15:266–274. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, Gudjonsson S, Hakansson U, Reizis B, Kotarsky K, Agace WW. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–720. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nature immunology. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annual review of immunology. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annual review of immunology. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nature immunology. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends in immunology. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Sharma S, Rajasagi NK, Veiga-Parga T, Rouse BT. Herpes virus entry mediator (HVEM) modulates proliferation and activation of regulatory T cells following HSV-1 infection. Microbes Infect. 2014;16:648–660. doi: 10.1016/j.micinf.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunological reviews. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Shui JW, Larange A, Kim G, Vela JL, Zahner S, Cheroutre H, Kronenberg M. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012;488:222–225. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui JW, Steinberg MW, Kronenberg M. Regulation of inflammation, autoimmunity, and infection immunity by HVEM-BTLA signaling. Journal of leukocyte biology. 2011;89:517–523. doi: 10.1189/jlb.0910528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldevila G, Raman C, Lozano F. The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Current opinion in immunology. 2011;23:310–318. doi: 10.1016/j.coi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P, Doherty TA, So T, Mehta AK, Khorram N, Norris PS, Scheu S, Pfeffer K, Ware C, Croft M. Herpesvirus entry mediator (TNFRSF14) regulates the persistence of T helper memory cell populations. The Journal of experimental medicine. 2011;208:797–809. doi: 10.1084/jem.20101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunological reviews. 2011;244:169–187. doi: 10.1111/j.1600-065X.2011.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MW, Huang Y, Wang-Zhu Y, Ware CF, Cheroutre H, Kronenberg M. BTLA interaction with HVEM expressed on CD8(+) T cells promotes survival and memory generation in response to a bacterial infection. PloS one. 2013;8:e77992. doi: 10.1371/journal.pone.0077992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annual review of immunology. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annual review of immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakhovsky A, Muller W, Rajewsky K. Lymphocyte populations and immune responses in CD5-deficient mice. Eur J Immunol. 1994;24:1678–1684. doi: 10.1002/eji.1830240733. [DOI] [PubMed] [Google Scholar]
- Tung JW, Kunnavatana SS, Herzenberg LA, Herzenberg LA. The regulation of CD5 expression in murine T cells. BMC Mol Biol. 2001;2:5. doi: 10.1186/1471-2199-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nature immunology. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. Journal of immunology. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Contag CH, Felsher D, Shachaf CM, Cao Y, Herzenberg LA, Herzenberg LA, Tung JW. The E47 transcription factor negatively regulates CD5 expression during thymocyte development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3898–3902. doi: 10.1073/pnas.0308764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, von Stebut E, Probst HC, van den Broek M, Riethmacher D, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.