INTRODUCTION
Metabolic Syndrome (MetS) is a complex disorder that includes abdominal obesity, glucose intolerance and/or insulin resistance, high blood pressure and dyslipidemia [1]. MetS, as well as its individual components have been identified as important modifiable risk factors for cognitive decline and are associated with cognitive underperformance, mild cognitive impairment and dementia [2–4]. T2DM, an important cardiovascular risk factor, is linked with cognitive decline, abnormal brain metabolism and atrophy [5–8]. Diffusion Tensor Imaging (DTI) has been used to quantify microstructural alterations in white mater that may impact cognitive performance [9–11]. DTI-derived metrics of white matter integrity are considered a more sensitive tool compared to structural MRI and might reveal subtle changes before those are detectable by conventional structural MRI metrics[12].
The association among individual cardiovascular risk factors and cognitive function has been widely studied [9], but the effect of MetS as a whole on brain structure and cognitive performance has not been well studied [13]. Prior studies that evaluated the impact of MetS on white matter (WM) microstructure have focused on analyses of the main WM tracts [13, 14] and did not analyzed specific brain regions. In addition, a limited number of studies attempting to link the impact of MetS on regional white matter microstructure to worse cognitive performance [15], reported that a Fractional Anisotropy (FA) decrease is associated to worse processing speed function. In the current study, we aimed to determine the impact of MetS on white matter microstructure and cognitive performance in older adults, a population group consistently linked to the presence of cognitive decline secondary to a higher presence of risk factors (T2DM, hypertension, obesity, dyslipidemia) [16–19]. We hypothesized that 1) MetS is associated with microstructural white matter abnormalities in the brain, and 2) these abnormalities would be associated with worse cognitive performance in subjects with MetS compared to controls.
METHODS
Subjects
This study was a case-control study of cognitive performance coupled with neuroimaging in T2DM vs non-diabetic controls conducted at the Syncope and Falls in the Elderly Laboratory, General Clinical Research Center and at the Center for Advanced Magnetic Resonance Imaging, at the Beth Israel Deaconess Medical Center (BIDMC), between August 2009 and July 2013. Patients underwent an initial visit, followed by a 2-year follow up visit. The current report is a cross-sectional post-hoc analysis of data collected in the baseline visit. Inclusion criteria for the original study were: age 50–85, diagnosis of T2DM and treated for more than 5 years, nondiabetic controls, hypertensive (BP >140/90 mmHg and/or treated for hypertension) and normotensive (BP <140/90 mmHg and no medical history of hypertension).
Subjects were originally recruited via local advertisement, and screened by medical history. Subjects that met inclusion/exclusion criteria signed an informed consent form (ICF) as approved by the Institutional Review Board (IRB) at BIDMC.
Exclusion criteria were: type 1 diabetes, acute medical conditions, myocardial infarction or major surgeries in last six months, history of stroke, dementia or Mini-Mental State Examination (MMSE) less than 24, carotid stenosis, heart or valvular disease, liver or renal failure or transplant, severe hypertension (systolic BP >200mmHg and/or diastolic BP >110mmHg, or treated with three or more antihypertensive medications), seizures, malignant tumors, alcohol abuse, recreational drug use, and morbid obesity (body mass index (BMI) >40). Magnetic resonance imaging (MRI) exclusion criteria were: metallic bioimplants (e.g., pacemakers and valve replacements not compatible with 3 Tesla MRI guidelines), claustrophobia or inability to cooperate.
A total of 131 subjects were enrolled, and 75 subjects completed the study. Subjects were excluded for the following reasons: 29 were ineligible after signing ICF for violation of one or more inclusion/exclusion criteria, 10 withdrew consent, 12 were terminated (cancer, inappropriate conduct, hyperglycemia, stroke, arrhythmia, etc.), and 5 were lost to follow up. Of the 75 patients completing the study, another 20 patients were excluded from the present analysis due to incomplete MRI datasets. We divided the remaining 55 patients of our cohort into two groups, those meeting criteria for MetS and those who were MetS-free (controls). MetS was defined according to the Consensus statement from the International Diabetes Federation [20], which includes the following criterias: increased triglycerides (≥150mg/dl or in treatment), reduced HDL cholesterol (<40mg/dl in men, <50mg/dl in women, or in treatment), increased BP (SBP≥130 and/or DBP≥85mgHg, or in treatment), increased fasting glucose (>100mg/dl or in treatment) and increased waist circumference (≥102cm in men, ≥88cm in women).
The MetS group included a total of 32 subjects (age 64.8 ± 7.8, 56.25% female, 28 T2DM [duration 14.6 ± 8.5 years]) and 23 age-, gender-, and education-matched non-diabetic normotensive controls. Subjects with T2DM were treated for more than five years and were either normotensive (blood pressure (BP) <140/90 mmHg and no medical history of hypertension), or hypertensive (BP >140/90 mmHg and/or treated for hypertension). The control group had normal fasting glucose and glycated hemoglobin (HbA1c).
Protocol
All subjects completed a medical history questionnaire, physical and neurological examinations. Fasting laboratory chemistries were obtained to measure blood glucose, HbA1c and lipid profile. Twenty-four hour BP was obtained every 20 minutes using a wearable 24-hour home monitoring device (Dynapulse, Inc. Vista, CA).
Neuropsychological measures
The neuropsychological assessment included executive function (Trial Making Test (TMT); Verbal fluency (VF), including phonemic and semantic fluency tasks), verbal learning and memory function (Hopkins Verbal Learning Test-Revised (HVLT), including a Total Recall, Delayed, Retention, and Recognition Discrimination index), a measure of visual-spatial ability and visual memory function (Rey-Osterreith Complex Figure Test (ROCF), including Immediate Recall (I.R.) and Delayed Recall (D.R.) tests), a measure of attention (Digit Span (DS)), and MMSE. The test results were analyzed using age-, gender-, race- and education-adjusted standardized T-scores.
Magnetic resonance imaging
MRI data were acquired using a 3-Tesla, GE GHX MRI scanner with a quadrature and eight channel phase array head coils (GE Medical Systems, Milwaukee, WI). Studies used a 3-D magnetization prepared rapid acquisition with gradient echo (MP-RAGE) and fluid attenuated inversion recovery (FLAIR) sequences to obtain anatomical images. Image analysis was developed using interactive data language (IDL, Research Systems, Boulder, Colorado, USA) and MATLAB (MathWorks, Natick, Massachusetts, USA). T1- and T2-weighted anatomical images (MP-RAGE and FLAIR) were co-registered non-linearly to the MNI152 standard template and segmented to calculate regional gray matter (GM) and white matter (WM) in main anatomical lobes and their subregions (SPM, University College London, UK). MP-RAGE and FLAIR images were co-registered to a standard template and segmented to calculate regional GM, WM, and white matter hyperintensities (WMHs) volume in the frontal, temporal, parietal and occipital regions using statistical parametric mapping software package (SPM, University College London, UK). WMHs were identified by thresholding of hyperintense pixels on FLAIR images with > 30% increase in signal intensity compared with the global WM average for each patient, and normalized for total brain volume[21].
Diffusion tensor imaging
DTI was acquired using a diffusion-weighted (DW)-EPI sequence [TR = 10,000 ms; TE = 80 ms; slice thickness = 5 mm; no gap; in-plane resolution = 1 mm, independent diffusion gradient directions using b= s/mm2, and one with b=0 s/mm2].
Data were preprocessed for head motion, and distortions due to eddy currents. Non-brain tissue was removed using the brain extraction tool (BET) implemented in the FSL package. DTI data was calculated for each voxel after fitting the diffusion tensor model to each voxel using FMRIB’s Diffusion Toolbox (FDT) from the FSL package. We used LONI probabilistic brain atlas, which has been thresholded 50% of their probability leading to shrinkage of the included ROI and restricted it to white matter boundary. Those ROIs are binarized and transformed to subject’s native space using inverse transformation matrix. In each ROI we extracted fraction anisotropy (FA), mean diffusivity (MD), axial diffusivity (LD), and radial diffusivity (RD).
These diffusion parameters were used as a measure to quantify the integrity of the microstructural WM, since it’s been proven that changes in the water diffusion within the tissues is highly predictive of alterations in its microstructural integrity[22].
Statistical analysis
JMP Pro 10.0.0 (SAS Institute, Cary, NC) was used to perform all statistical analysis. Descriptive analyses were used for demographic characteristics. Demographic data, cognitive function, brain volumes and DTI metrics were compared between the groups using one-way ANOVA and non-parametric tests. Least square (LS) models were used to assess the association between DTI metrics, brain volumes and changes in cognitive function. The models were adjusted for age, sex, HbA1c, 24-hour mean BP, BMI, fasting glucose, and the presence of hyperlipidemia and WMHs. Age was included in all the analyses as a covariate to account for differences in WM volume due to age-related decline[23]. Global and regional DTI metrics were used as independent variables. The dependent variables were the cognitive results. We present r2adjusted for model covariates and selected r2adjusted >0.1, and p<0.05 for a more conservative approach. To minimize repeated measures effect, each brain region was modeled separately.
RESULTS
Demographic and clinical results
Demographic and clinical characteristics were compared between group using one-way ANOVA and non-parametric tests and are shown in Table 1. The MetS group and the controls did not differ in age, sex, or education years. As expected, subjects with MetS had a significantly greater BMI, 24-hour systolic and mean BP, HbA1c, triglycerides and the presence of hyperlipidemia by medical history, as compared to controls. Subjects with MetS had worse performance on the verbal fluency, learning and delayed recall tasks (VF: T score (p=0.01), VF: animals T-score (p=<0.0001), HVLT: Total Recall T-score (p=<0.0001), and HVLT: Delayed Recall T-score (p=0.003)), as compared to the controls.
Table 1.
Characteristics of the study group
| MetS group (n=32) | Control group (n=23) | P value | |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age | 64.8±7.8 | 66.9±10.9 | NS |
|
| |||
| Gender, %female | 56.25% | 52.17% | NS |
|
| |||
| Body mass index, kg/m2 | 29.8±5.8 | 25±3.1 | 0.0003 |
|
| |||
| Education, y | 15±3.1 | 16±3 | NS |
|
| |||
| Cardiovascular and Metabolic Outcomes | |||
|
| |||
| Hemoglobin A1c, % | 7.1±1.3 | 5.7±0.3 | <0.0001 |
|
| |||
| Diabetes mellitus type 2, % | 87.5% | 0% | |
|
| |||
| Hypertension, % | 100% | 0% | |
|
| |||
| Hyperlipidemia medical history, y | 21±65.63% | 7±30.43% | 0.01 |
|
| |||
| Triglycerides, mg/dL | 142.8±95.6 | 94.5±42 | 0.01 |
|
| |||
| HDL, mg/dL | 48.7±16.2 | 55.1±16.9 | NS |
|
| |||
| 24hr systolic blood pressure, mmHg | 133.2±7.3 | 126.7±8.1 | 0.008 |
|
| |||
| 24hr diastolic blood pressure, mmHg | 71.1±7.1 | 67.7±8.4 | NS |
|
| |||
| 24hr mean blood pressure, mmHg | 91.7±6.5 | 86.7±7.7 | 0.01 |
|
| |||
| Imaging characteristics | |||
|
| |||
| Whole brain FA | 0.3±0.01 | 0.3±0.01 | NS |
|
| |||
| Whole brain MD | 0.9±0.06 | 0.9±0.06 | NS |
|
| |||
| Whole brain RD | 1.2±0.07 | 1.1±0.06 | NS |
|
| |||
| Whole brain LD | 0.7±0.06 | 0.7±0.06 | NS |
|
| |||
| Global GM/ICV | 0.37±0.05 | 0.39±0.04 | NS |
|
| |||
| Global WM/ICV | 0.25±0.03 | 0.27±0.02 | NS |
|
| |||
| Global CSF/ICV | 0.36±0.07 | 0.32±0.06 | NS |
|
| |||
| Hippocampus | 0.007±0.0009 | 0.07±0.0009 | NS |
|
| |||
| WMHs | 15.6±6.4 | 12.6±3.9 | 0.03 |
|
| |||
| L angular gyrus-FA | 0.2±0.02 | 0.3±0.03 | 0.02 |
|
| |||
| R angular gyrus-FA | 0.2±0.03 | 0.3±0.02 | 0.03 |
|
| |||
| L postcentral gyrus-LD | 1.15±0.06 | 1.11±0.06 | 0.05 |
|
| |||
| R angular gyrus-RD | 0.76±0.08 | 0.71±0.07 | 0.04 |
|
| |||
| Cognitive Outcomes | |||
|
| |||
| Hopkins Verbal Learning T Score | |||
| - Total Recall | 45.8±12.7 | 59±8.3 | <0.0001 |
| - Delayed Recall | 44.1±12.9 | 54±10.1 | 0.002 |
| - Retention % | 81.5±18.6 | 89.4±15.2 | NS |
| - Recognition Discrimination index | 47.1±11 | 52.1±7.3 | 0.05 |
|
| |||
| Rey-Osterrieth Complex Figure Test T Score | |||
| - Immediate recall | 42.2±17.2 | 51.5±16.6 | 0.05 |
| - Delayed recall | 43.3±17.1 | 49.5±16.4 | 0.1 |
|
| |||
| Verbal Fluency T Score | 46.3±10.4 | 52.3±7.9 | 0.01 |
|
| |||
| Verbal Fluency: animals T Score) | 31.6±12.3 | 48.3±11.3 | <0.0001 |
|
| |||
| Trail Making T Score | 48.7±12.4 | 51.3±9.8 | NS |
|
| |||
| Mini-Mental State Examination | 28.8±1.3 | 28.8±1.6 | NS |
Groups did not differ in normalized global GM, WM volumes and whole brain DTI metrics. MetS group had greater global WMHs volume (p=0.03). We observed differences in regional DTI metrics in FA, MD, LD and RD values. The MetS group showed several abnormalities in the angular gyrus in both hemispheres, e.g. lower FA values in L angular gyrus (0.02) and R angular gyrus (0.03), and higher RD values in R angular gyrus (0.02) and higher LD values in the L postcentral gyrus (0.05) (Table 1). No differences between regional GM and WM volumes were observed between the groups.
Associations between microstructural measures and cognition
The relationship between global and regional DTI metrics and cognitive performance was examined using LS models adjusting for age, mean systolic BP, HbA1c, BMI, presence of hyperlipidemia and global WMHs volume.
a. Global DTI metrics and cognitive performance
In the entire cohort, a better performance on the semantic category of the verbal fluency task was positively associated with the whole brain FA, a measure of infrastructural integrity (R2adj=0.1, p=0.007), and negatively with the whole brain MD, an inverse measure of membrane and cellular density [24], (R2adj=0.1, p=0.01), LD (R2adj=0.09, p=0.02), and negatively with RD, a measure of WM de- or dysmyelination (R2adj=0.1, p=0.006). Subjects with MetS had a positive association between the verbal fluency task and whole brain FA (R2adj=0.2, p=0.005), and a negative correlation with MD (R2adj=0.3, p=0.001), LD (R2adj=0.2, p=0.004) and RD (R2adj=0.3, p=0.0007). No similar association was seen in the control group, which might indicate that the deleterious effect of MetS on cognitive performance is mediated through WM microstructure alterations.
b. Regional DTI metrics and cognitive performance
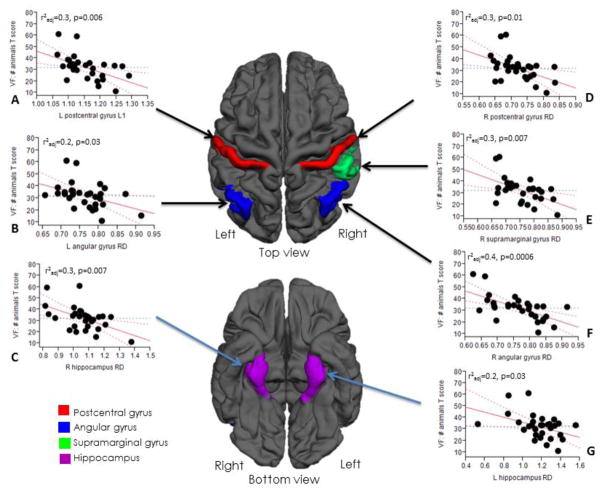
Table 2 shows the relationships between regional DTI metrics and cognitive performance in the MetS group using four LS models (1–4) adjusting for age, sex, HbA1c, 24-hour mean BP, BMI, presence of hyperlipidemia and global WMHs volume. Significant negative associations were found between the semantic fluency task and WM diffusivity measures, in contrast to the control group in which no significant associations were observed. The strongest associations were found with the WM microstructure of the postcentral, supramarginal, angular and parahippocampal gyri, and the hippocampal region. In model 2, adjusted for age, HbA1c, 24-hour mean BP and global WMHs volume, a lower score in cognitive performance was correlated with higher diffusivity metrics in the L postcentral gyrus (LD [R2adj =0.3, p=0.006]), L angular gyrus (RD [R2adj =0.2, p=0.03]), R hippocampus (RD [R2adj =0.3, p=0.007]), R postcentral (RD [R2adj 0.3, p=0.01]), R supramarginal gyrus (RD[R2adj =0.3, p=0.007]), R angular gyrus (RD[R2adj =0.4, p=0.0006]) and L hippocampus (RD[R2adj =0.2, p=0.03]) (Figure 1). We also found similarly significant associations in models 1, 3 and 4. A negative HbA1c effect was observed on the VF: animals T-score and a less intense association between HbA1c and DTI parameters was seen. Similar interaction was observed between a lower HVLT: Total Recall T-score and a higher diffusivity metric in the R angular gyrus (RD [R2adj =0.2, p=0.01]) in the MetS group. In controls, no significant associations between microstructural WM abnormalities and cognitive performance were observed. We also investigated whether there was an association between normalized regional GM in the aforementioned areas and the VF: animals T-score, using it as a predictive variable in the same 4 models. In models 1, 3, 4 and for most regions in model 2, there was no association between regional GM and cognitive performance. Only for R angular gyrus RD and L supramarginal gyrus RD in model 2 the association was statistically significant.
Table 2.
Relationship between cognitive performance and DTI metrics (MetS group)
| VF: animals (T- score) | L postcentral gyrus – LD | L postcentral gyrus – RD | R postcentral gyrus - RD | L supramarginal gyrus – LD | L supramarginal gyrus - RD | L angular gyrus - RD | R angular gyrus - MD | R angular gyrus - RD | |
|---|---|---|---|---|---|---|---|---|---|
| LS Model–1 |
r2adj | 0.2 | 0.3 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.3 |
| P | 0.003 | 0.001 | 0.01 | 0.005 | 0.009 | 0.04 | 0.02 | 0.0008 | |
| HbA1c | 0.04 | 0.01 | 0.03 | 0.05 | 0.02 | NS | NS | NS | |
| LS Model–2 |
r2adj | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.4 |
| P | 0.006 | 0.001 | 0.01 | 0.004 | 0.01 | 0.03 | 0.02 | 0.0006 | |
| HbA1c | NS | NS | NS | NS | NS | NS | NS | NS | |
| LS Model–3 |
r2adj | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | NS | 0.1 | 0.3 |
| P | 0.003 | 0.001 | 0.01 | 0.007 | 0.009 | NS | 0.02 | 0.001 | |
| HbA1c | 0.04 | 0.01 | 0.03 | 0.03 | 0.01 | 0.05 | NS | 0.03 | |
| LS Model-4 |
r2adj | 0.2 | 0.3 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.3 |
| P | 0.003 | 0.001 | 0.01 | 0.007 | 0.009 | 0.04 | 0.02 | 0.001 | |
| HbA1c | NS | 0.02 | 0.04 | 0.03 | 0.02 | NS | NS | 0.04 | |
|
|
|||||||||
Model 1= adjusted for age, sex, HbA1c, and mean BP; Model 2= adjusted for age, HbA1c, mean BP and the global WMHs volume; Model 3= adjusted for age, HbA1c, mean BP, and the presence of hyperlipidemia; Model 4= adjusted for age, HbA1c, mean BP and BMI. Abbreviations: Right (R); Left (L); Verbal fluency (VF); Fractional anisotropy (FA), Mean diffusivity (MD), Axial diffusivity (LD), Radial diffusivity (RD); Least squares (LS).
Fig 1.
The associations between white matter microstructural integrity and cognitive performance adjusting for age, HbA1c, mean BP and the presence of WMHs in the MetS group. (A), (B), (C), (D), (E), (F), (G): Higher values in L postcentral L1 (A), L angular gyrus RD (B), R hippocampus RD (C), R postcentral gyrus RD (D), R supramarginal gyrus RD (E), R angular gyrus RD (F) and L hippocampus RD (G) are associated with lower performance in cognitive function. No significant associations were observed in the control group. Abbreviations: LD – Axial Diffusivity, RD – Radial Diffusivity, VF – Verbal fluency
c. The critical role of hyperglycemia
In our study, poor glycemic control (HbA1c) was the only metabolic component associated with worse cognitive performance in univariate analyses (Figure 2). This suggests that chronic hyperglycemia may be one of the main pathophysiologic mechanisms underlying cognitive decline associated with MetS and is further highlighted in the multiple regression models of Table 2: HbA1c was the only variable that retained a robust independent negative association with cognitive performance, whereas the rest of MetS components (hyperlipidemia, obesity, hypertension) were not independently associated with either cognitive performance or DTI metrics. The duration of T2DM did not have an impact on our findings, either in univariate or multivariate regression analyses (results not shown). No relation between WMHs and cognition or DTI metrics was observed in our analysis.
Fig 2.
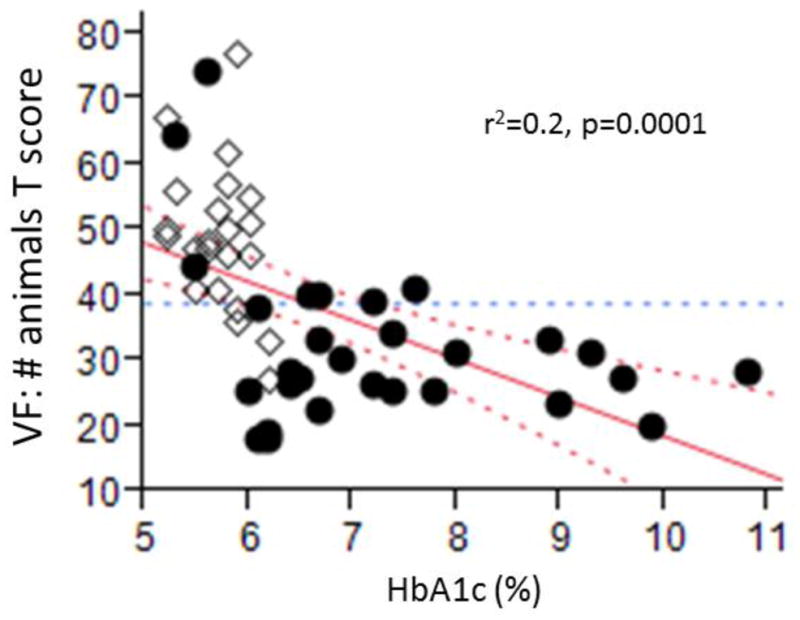
The inverse association between HbA1c and VF: animals T score. Between the groups, a higher level of HbA1c at baseline is associated with a lower VF: animals T score. This supports the notion the glucose control in one of the factors that influence the most the cognitive performance in patients with MetS. Circles – participants with MetS; Diamond – control participants. Abbreviations: VF – Verbal fluency
We also examined possible associations between global vasoreactivity and gray and white matter volume, as well as global DTI metrics and did not find one. Additionally, we explored the relationship between a panel of inflammatory and other serum biomarkers and cognitive outcomes and imaging metrics and found no significant associations in either group. The panel included C-reactive protein, leptin, insulin-like growth factor-1, interleukin-6, tumor necrosis factor-α, intercellular adhesion molecule-1, vascular cell adhesion molecule -1.
DISCUSSION
This study evaluated the impact of MetS on brain structure and cognitive performance in older adults and the main findings are: 1) MetS is associated with impaired WM microstructural integrity in the angular, postcentral, supramarginal, parahippocampal gyri and the hippocampus all of which are key areas of neural networks implicated in verbal learning and memory processing [21–23]. 2) Subjects in the MetS group performed worse in the cognitive domains of verbal fluency and verbal recall compared to controls. 3) This cognitive underperformance was mediated by the microstructural WM abnormalities in specific brain regions. 4) Hyperglycemia was the only MetS component that was associated with abnormal cerebral microstructure integrity as well as worse cognitive performance.
MetS subjects performed markedly worse in specific cognitive domains of verbal fluency and visuospatial memory, as compared to healthy controls. However, their overall results on MMSE (mean 28.8) were similar to controls which would label them as “cognitively intact” [28]. Therefore, it is plausible that older people with MetS may underperform semantic VF and verbal recall, both of which that are considered surrogate markers of executive function and learning and memory[29] and this dysfunction might not be detected using routine clinical bedside testing. Cognitive testing was adjusted for age and education level, thus minimizing the effects of these variables, but the association could be even stronger in the elderly or people with poor control of MetS components (e.g. HbA1c, blood pressure or morbid obesity).
T2DM, HTN and other cardiovascular risk factors that are components of MetS have been individually associated with worse cognitive performance [30, 31] and subsequent cognitive decline [32, 33] in prior studies. In our cohort, chronic hyperglycemia (HbA1c), among all MetS components, was the only one that was robustly associated with worse score in the VF test both in univariate and multivariate analyses in our sample. Our MetS group had well controlled blood pressure (average SBP of 133mmHg) and relatively good glycemic control (average HbA1c 7.1). Our models were adjusted for the effects of blood pressure, BMI and hyperlipidemia but their effects were not significant. It is possible that the impact of hyperglycemia or HTN could be even stronger in a general population sample with less well controlled vascular risk factors.
Besides cognitive performance, hyperglycemia was also associated with microstructural changes in WM affecting specific brain regions rather than with a global load of WMHs (Table 2). DTI measures evaluated in our study, are markers of tissue diffusion, axonal integrity and myelin sheath integrity [22], and therefore may be more sensitive to early demyelinating processes. It follows that the most likely underlying pathologic change in the WM would be demyelinating rather than axonal in nature. Indeed, in our study the association with worse cognitive performance was driven primarily by worse RD, which is considered a better marker of myelin disruption [24] as opposed to LD which is more likely to represent axonal damage [22, 24, 34] and was not markedly different between controls and MetS subjects.
Our results are in accordance with prior studies that have revealed an association between T2DM, WM microstructure disruption and cognitive deficits [31–36]. MetS group had a higher global load of WMHs, which is considered a classic hallmark imaging sign of the cumulative effect of cardiovascular risk factors on the subcortical WM [41, 42], but neither global nor regional GM volumes were different between the two groups in our study, a difference which we might have been able to detect in a larger patient sample. The reasons for predilection of WM microstructural abnormalities are likely to stem from multiple mechanisms exerting their effects on WM structure and integrity. T2DM has been associated with large and small vessel disease, altered regional vasoreactivity [43], worse vasodilatation capacity [44] and reduced regional gray matter perfusion [45]. Given the fact that WM is more susceptible to ischemia compared to GM [46, 47], it is be possible that a vascular mechanism plays a central WM pathology which is further supported by several studies demonstrating reduced perfusion and vasoreactivity in areas of leukoaraiosis [44–46]. Glucotoxicity associated with chronic hyperglycemia and glycemic variability may be another mechanism underlying WM and T2DM abnormalities [51].
The associations between regional DTI metrics and cognitive performance we report are statistically robust, they account for ~20–40% of the variability in the cognitive performance after accounting for imbalances in cardiometabolic risk factors (Table 2). Given that our groups were otherwise well balanced with regards to age, education level, gray matter volume and that we excluded a prior conditions such as stroke, seizures, malignant tumors, alcohol abuse, recreational drug use, major surgery there do not seem to be other obvious biologic factors that could explain these relationships. However, it should be noted that there are other aspects of cerebral WM network performance that our imaging methods might not have captured and which, if added, could shed more light into the pathophysiology of the process. Specifically, although WM structural alterations lead to impaired structural connectivity [9, 52], we have not accounted for this aspect of white matter network pathology. Accounting for functional connectivity could add another layer towards more elaborate characterization of WM network performance [53, 54].
Our study has some weaknesses, such as a relatively small sample, relatively well controlled T2DM, hypertension and other vascular risk factors that that might have underestimated their biologic effect. We did not have enough power to stratify patients into different degrees of MetS severity. Tractographic analysis, which could have potentially shed additional light on the effect of DM in specific WM tracts, was not performed. Also, the cross-sectional nature of our study allows assumptions regarding associations but prohibits causal inferences to be made. It also prohibits us from making any assumptions regarding the trajectory of cognitive performance and potential for developing dementia in the future on individual patients.
Strengths of our study include prospective data collection, use of detailed and standardized cognitive test battery, high-quality 3T imaging with both global and regional metrics, which allowed detection of differences in small regions which would have otherwise been diluted if only global measurements were taken. Excluding subjects with strokes and dementia, and the use of a relatively healthy control group allowed us to “isolate” the cognitive and anatomical effect of MetS and associated vascular risk factors as much as possible. In conclusion, our study provides evidence of an association between the presence of MetS and the development of abnormalities in the microstructural WM integrity.
In summary, we observed that subjects with MetS had impaired global and regional diffusivity parameters, which translated in a worse cognitive performance. These associations suggest that the cumulative effect of the cardiometabolic factors present in MetS may negatively affect the microstructural WM integrity early in the disease process, before macrostructural MRI changes become apparent. However, WMHs were not associated with worse learning parameters. It is important to point out that previous studies have mostly found a link between these cardiometabolic factors and a decline in executive function [55], but our results add new evidence to the presence of underperformance in domains of verbal fluency and visuospatial memory as well. In our patient group, hyperglycemia seems to exert the most deleterious effect of all the components of MetS. DTI measures may provide a sensitive tool to detect abnormalities in the WM, before they manifest on anatomical MR scans as brain atrophy. Neuropsychological assessment of verbal fluency may be more sensitive to MetS related cognitive disturbance than routine bedside clinical evaluation. A larger prospective study may be useful to further elucidate the role of DTI metrics as predictor of mid-life risk of MetS for subsequent gross anatomical MRI changes and cognitive decline.
Acknowledgments
The study was supported by NIH-NIA 1R01- AG0287601A2, NIH-NIDDK 5R21 DK084463, 1R01DK13902-01A1, American Diabetes Association, Clinical 1-03-CR-23 and 1-06-CR-25 to Dr. Vera Novak. The project described was supported by Grant Number UL1 RR025758- Harvard Clinical and Translational Science Center and M01-RR-01032, from the National Center for Research Resources.
Footnotes
Authors Contributions
Freddy Alfaro MD falfarom@bidmc.harvard.edu - contributed to study conduct, performed DTI data and statistical analysis, and wrote the first version of this manuscript.
Vasileios-Arsenios Lioutas MD vlioutas@bidmc.harvard.edu – performed DTI data and statistical analysis, and wrote the first version of this manuscript.
Daniela A. Pimentel MD danita17@gmail.com - contributed to the study conduct and to manuscript preparation.
Chen-Chih Chung MD dizzierblue@yahoo.com.tw – contributed to study conduct and manuscript preparation.
Francisco Bedoya MD francisco.bedoya@upch.pe - contributed to study conduct and manuscript preparation.
Woo-Kyoung Yoo MD, PhD mdwooky@gmail.com - processed DTI data and contributed to manuscript preparation
Vera Novak MD, PhD vnovak@bidmc.harvard.edu - designed the study and protocol, overseen all aspects of the study conduct and experiments and manuscript preparation.
Dr. Freddy Alfaro reports no disclosures.
Dr. Vasileios-Arsenios Lioutas reports no disclosures.
Dr. Daniela Pimentel reports no disclosures.
Dr. Chen-Chih Chung reports no disclosures.
Dr. Francisco Bedoya reports no disclosures.
Dr. Woo-Kyoung Woo PhD reports no disclosures.
Dr. Vera Novak MD PhD was funded by NIH-NIA 1R01- AG0287601A2, NIH-NIDDK 5R21 DK084463, NIH-NIDDK 1R01DK13902-01A1, American Diabetes Association, Clinical 1-03-CR-23 and 1-06-CR-25.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 3.Kaffashian S, Dugravot A, Elbaz A, et al. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology. 2013;80:1300–1306. doi: 10.1212/WNL.0b013e31828ab370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raffaitin C, Féart C, Le Goff M, et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt R, Launer LJ, Nilsson L-G, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53:687–692. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 6.Kreis R, Ross BD. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology. 1992;184:123–130. doi: 10.1148/radiology.184.1.1319074. [DOI] [PubMed] [Google Scholar]
- 7.Manschot SM, Brands AMA, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 8.Sprandel MCO, Hueb WA, Segre A, et al. Alterations in lipid transfers to HDL associated with the presence of coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14:107. doi: 10.1186/s12933-015-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reijmer YD, Brundel M, de Bresser J, et al. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care. 2013;36:137–144. doi: 10.2337/dc12-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 11.Del Bene A, Ciolli L, Borgheresi L, et al. Is type 2 diabetes related to leukoaraiosis? an updated review. Acta Neurol Scand. 2015;132:147–155. doi: 10.1111/ane.12398. [DOI] [PubMed] [Google Scholar]
- 12.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yates KF, Sweat V, Yau PL, et al. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segura B, Jurado MA, Freixenet N, et al. Microstructural white matter changes in metabolic syndrome: a diffusion tensor imaging study. Neurology. 2009;73:438–444. doi: 10.1212/WNL.0b013e3181b163cd. [DOI] [PubMed] [Google Scholar]
- 15.Segura B, Jurado MA, Freixenet N, et al. White matter fractional anisotropy is related to processing speed in metabolic syndrome patients: a case-control study. BMC Neurol. 2010;10:64. doi: 10.1186/1471-2377-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopman DS, Mosley TH, Catellier DJ, et al. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65:876–881. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- 17.Tamashiro-Duran JH, Squarzoni P, de Duran FLS, et al. Cardiovascular risk in cognitively preserved elderlies is associated with glucose hypometabolism in the posterior cingulate cortex and precuneus regardless of brain atrophy and apolipoprotein gene variations. AGE. 2012;35:777–792. doi: 10.1007/s11357-012-9413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke K, Gaser C, Manor B, Novak V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci. 2013;5:90. doi: 10.3389/fnagi.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasa RN, Rossetti HC, Gupta MK, et al. Cardiovascular Risk Factors Associated with Smaller Brain Volumes in Regions Identified as Early Predictors of Cognitive Decline. Radiology. 2016;278:198–204. doi: 10.1148/radiol.2015142488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckel RH, Alberti KGMM, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet Lond Engl. 2010;375:181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 21.Novak V, Last D, Alsop DC, et al. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29:1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurother J Am Soc Exp Neurother. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Alexander AL, Hurley SA, Samsonov AA, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011;1:423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackert VH, den Heijer T, Oudkerk M, et al. Hippocampal head size associated with verbal memory performance in nondemented elderly. NeuroImage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- 27.Chen KHM, Chuah LYM, Sim SKY, Chee MWL. Hippocampal region-specific contributions to memory performance in normal elderly. Brain Cogn. 2010;72:400–407. doi: 10.1016/j.bandc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.O’Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65:963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duff K, Schoenberg MR, Scott JG, Adams RL. The relationship between executive functioning and verbal and visual learning and memory. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2005;20:111–122. doi: 10.1016/j.acn.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 32.Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 33.Hughes TM, Sink KM. Hypertension and Its Role in Cognitive Function: Current Evidence and Challenges for the Future. Am J Hypertens. 2015 doi: 10.1093/ajh/hpv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu J-L, Chen Y-L, Leu J-G, et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. NeuroImage. 2012;59:1098–1105. doi: 10.1016/j.neuroimage.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wang Y, Wang J, et al. White matter integrity disruptions associated with cognitive impairments in type 2 diabetic patients. Diabetes. 2014;63:3596–3605. doi: 10.2337/db14-0342. [DOI] [PubMed] [Google Scholar]
- 36.Qiu C, Sigurdsson S, Zhang Q, et al. Diabetes, markers of brain pathology and cognitive function: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol. 2014;75:138–146. doi: 10.1002/ana.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yau PL, Kluger A, Borod JC, Convit A. Neural substrates of verbal memory impairments in adults with type 2 diabetes mellitus. J Clin Exp Neuropsychol. 2014;36:74–87. doi: 10.1080/13803395.2013.869310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kodl CT, Franc DT, Rao JP, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–3089. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kullmann S, Schweizer F, Veit R, et al. Compromised white matter integrity in obesity. Obes Rev Off J Int Assoc Study Obes. 2015;16:273–281. doi: 10.1111/obr.12248. [DOI] [PubMed] [Google Scholar]
- 40.Kullmann S, Callaghan MF, Heni M, et al. Specific white matter tissue microstructure changes associated with obesity. NeuroImage. 2015;125:36–44. doi: 10.1016/j.neuroimage.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 43.Chung C-C, Pimentel D, Jor’dan AJ, et al. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015;85:450–458. doi: 10.1212/WNL.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Last D, Alsop DC, Abduljalil AM, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiehuis AM, Vincken KL, van den Berg E, et al. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51:1321–1326. doi: 10.1007/s00125-008-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arakawa S, Wright PM, Koga M, et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke J Cereb Circ. 2006;37:1211–1216. doi: 10.1161/01.STR.0000217258.63925.6b. [DOI] [PubMed] [Google Scholar]
- 47.Purkayastha S, Fadar O, Mehregan A, et al. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2014;34:228–234. doi: 10.1038/jcbfm.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marstrand JR, Garde E, Rostrup E, et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke J Cereb Circ. 2002;33:972–976. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- 50.Markus HS, Lythgoe DJ, Ostegaard L, et al. Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J Neurol Neurosurg Psychiatry. 2000;69:48–53. doi: 10.1136/jnnp.69.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui X, Abduljalil A, Manor BD, et al. Multi-scale glycemic variability: a link to gray matter atrophy and cognitive decline in type 2 diabetes. PloS One. 2014;9:e86284. doi: 10.1371/journal.pone.0086284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reijmer YD, Fotiadis P, Martinez-Ramirez S, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain J Neurol. 2015;138:179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saggio ML, Ritter P, Jirsa VK. Analytical Operations Relate Structural and Functional Connectivity in the Brain. PloS One. 2016;11:e0157292. doi: 10.1371/journal.pone.0157292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scaccianoce E, Laganà MM, Baglio F, et al. Combined DTI-fMRI Analysis for a Quantitative Assessment of Connections Between WM Bundles and Their Peripheral Cortical Fields in Verbal Fluency. Brain Topogr. 2016 doi: 10.1007/s10548-016-0516-0. [DOI] [PubMed] [Google Scholar]
- 55.Kesse-Guyot E, Julia C, Andreeva V, et al. Evidence of a cumulative effect of cardiometabolic disorders at midlife and subsequent cognitive function. Age Ageing. 2015;44:648–654. doi: 10.1093/ageing/afv053. [DOI] [PubMed] [Google Scholar]