Abstract
Disease and other health hazards pose serious threats to the persistence of wild ape populations. The total chimpanzee population at Gombe National Park, Tanzania, has declined from an estimated 120 to 150 individuals in the 1960's to around 100 individuals by the end of 2013, with death associated with observable signs of disease as the leading cause of mortality. In 2004, we began a non-invasive health-monitoring program in the two habituated communities in the park (Kasekela and Mitumba) with the aim of understanding the prevalence of health issues in the population, and identifying the presence and impacts of various pathogens. Here we present prospectively collected data on clinical signs (observable changes in health) in the chimpanzees of the Kasekela (n=81) and Mitumba (n=32) communities over an 8-year period (2005–2012). First, we take a population approach and analyze prevalence of clinical signs in five different categories: gastrointestinal system (diarrhea), body condition (estimated weight loss), respiratory system (coughing, sneezing etc.), wounds/lameness, and dermatologic issues by year, month, and community membership. Mean monthly prevalence of each clinical sign per community varied, but typically affected <10% of observed individuals. Secondly, we analyze the presence of clinical signs in these categories as they relate to individual demographic and social factors (age, sex, and dominance rank) and simian immunodeficiency virus (SIVcpz) infection status. Adults have higher odds of being observed with diarrhea, loss of body condition, and wounds or lameness when compared to immatures, while males have a higher probability of being observed with wounds or lameness than females. In contrast, signs of respiratory illness appear not to be related to chimpanzee-specific factors and skin abnormalities are very rare. For a subset of known-rank individuals, dominance rank predicts the probability of wounding/lameness in adult males, but does not predict any adverse clinical signs in adult females. Instead, adult females with SIVcpz infection are more likely to be observed with diarrhea, a finding that warrants further investigation. Comparable data are needed from other sites to determine whether the prevalence of clinical signs we observe are relatively high or low, as well as to more fully understand the factors influencing health of wild apes at both the population and individual level.
Keywords: chimpanzees, disease, clinical signs, health-monitoring
INTRODUCTION
Infectious diseases are widely recognized as a direct threat to the conservation of many wildlife populations [Altizer et al., 2003; Daszak et al., 2001]. Among African great apes, numerous disease outbreaks have been associated with significant morbidity and mortality [Leendertz et al., 2006]. Examples include Ebola virus in Taï chimpanzees (Pan troglodytes verus) [Formenty et al., 1999] and western lowland gorillas (Gorilla gorilla gorilla) [Bermejo et al., 2006; Walsh et al., 2003], anthrax in Taï chimpanzees [Leendertz et al., 2004], an “AIDS-like” disease in Mahale chimpanzees (Pan troglodytes schweinfurthii) [Nishida et al., 2003], and human metapneumovirus in chimpanzees at Mahale and Taï [Kaur et al., 2008; Köndgen et al., 2010] and mountain gorillas (Gorilla beringei beringei) [Palacios et al., 2011]. Given that all wild great apes are endangered, infectious disease may both directly and indirectly exacerbate population declines. Thus, researchers and managers at several field sites have instituted health monitoring programs for animals in their study areas. In principle, identifying patterns of illness in wild populations provides a foundation for understanding transmission dynamics and helps inform the design and implementation of conservation management strategies [Travis et al., 2008].
For diagnosis of specific pathogens, direct sampling of blood and/or tissues is ideal. However, obtaining the requisite invasive samples from wild non-human primates is complicated due to anesthesia-associated risks and issues related to disrupting habituation and/or behavioral research [Gillespie et al., 2008; Lukasik, 2002; Travis et al., 2008]. As such, managers in protected areas typically prefer non-invasive methods. Such methods include a form of syndromic surveillance, which entails the collection of pre-diagnosis health data on disease “syndromes” such as respiratory, dermatologic, gastrointestinal, etc., that can signal a case or outbreak [Henning, 2004]. This surveillance is often combined with non-invasive fecal and urine sampling, and collection of tissue samples from deceased individuals [Gillespie, 2006; Gillespie et al., 2008; Leendertz et al., 2006]. A combination of one or all of these health-monitoring methods have been in place for varying periods of time at several African great ape sites (e.g., chimpanzees: Kanyawara, Uganda [Ashford et al., 2000; Krief et al., 2005; Masi et al., 2012], Budongo, Uganda [Zommers et al., 2013], Taï Forest, Cote d'Ivoire [Kaiser et al., 2010; Köndgen et al., 2010; Leendertz et al., 2004; Metzger et al., this volume], Fongoli, Senegal [Howells et al., 2011], Bossou, Guinea [Fujita, 2011], Mahale, Tanzania [Hanamura et al., 2008; Kaur et al., 2008], Gombe, Tanzania [Gillespie et al., 2010; Keele et al., 2009; Lonsdorf et al., 2006; Lukasik 2002; Parsons et al., 2014; Terio et al., 2011], Nyungwe, Rwanda [Martz et al., this volume], western lowland gorillas: Bai Hokou, Central African Republic [Masi et al., 2012; Morton et al., 2013], Goualougo, Republic of Congo [Gillespie et al., 2009], mountain gorillas: Rwanda, Uganda, Democratic Republic of Congo [Ashford et al., 1990; Decision Tree Writing Group, 2006; Rwego et al., 2008]). An ongoing issue with non-invasive sample collection for pathogen identification is that the necessary diagnostic tests have simply not been developed or validated for many diseases of concern in these species (although this has improved greatly over the past decade, e.g., see Calvignac-Spencer et al. [2012]) and/or the spectrum of possible pathogens in a population is unknown.
While regular, non-invasive, population-wide screening for a variety of pathogens would constitute the ideal health-monitoring program in wild apes, it is virtually impossible to implement due to diagnostic, financial, and other logistical constraints. As such, syndromic health surveillance on individuals already undergoing observation for behavioral research or tourism purposes is a more practical and feasible option for systematic and ongoing health-monitoring. Clinical signs are observed indicators of potential medical conditions, and are detected by both human and veterinary doctors to assist with diagnosis [Stedman, 2005]. In wild apes, clinical signs have been collected and reported from a variety of protected areas. The Gorilla Doctors (formerly the Mountain Gorilla Veterinary Project) began monitoring the health of wild mountain gorillas in the late 1980s and subsequently pioneered the use of syndromic surveillance to trigger a clinical decision-making process for when to conduct veterinary interventions [Decision Tree Writing Group, 2006]. Mountain gorillas remain the only African great ape in which illnesses and injuries are routinely treated via veterinary intervention. Information on clinical signs was collected by Krief et al. [2005], in conjunction with fecal and urine analysis as part of a 5 month study in the early 2000s on the health of chimpanzees in Kanyawara, Uganda, but no specific population-level or temporal patterns were reported. A later study by the same team [Masi et al., 2012] compared clinical signs, and fecal and urine data from chimpanzees in Kanyawara (collected from July to December 2006) and western lowland gorillas in Bai Hokou, Central African Republic (collected from May to July 2008 and December 2008 to February 2009). Respiratory clinical signs were most commonly observed in both species and no species differences were found in the proportion of days with respiratory symptoms. Chimpanzees had a higher occurrence of both diarrhea and wounds than gorillas. In contrast, gorillas had a higher occurrence of eye lesions, and only gorillas were found to have skin lesions. In this study, fecal and urine parameters showed significant seasonal changes but no analyses of seasonal effects on clinical signs were reported. Also at Bai Hokou, Morton et al. [2013] reported that respiratory events accounted for over 80% of all health issues observed in gorillas during a 3-year period, while wounds, diarrhea, eye and skin issues were observed at much lower levels. In addition, an increase in respiratory clinical signs was significantly associated with habituation and tourist presence but whether this was due to increased transmission or improved detection is unknown. Lonsdorf et al. [2011] used an 8-year (1979–1987) retrospective dataset on respiratory clinical signs to investigate baseline (i.e., non-outbreak) patterns of respiratory illness in a subset of Gombe chimpanzees. The authors found that baseline rates of respiratory illness in individual chimpanzees were positively correlated with provisioning of bananas by humans (a practice that has since been discontinued) during the dry season. Importantly, none of these previous studies report the prevalence of clinical signs of ill health.
The prevalence of health issues in a population is a measure calculated from data on individual animals, in which the probability of particular types of health issues occurring is expected to vary over the lifespan and by sex. In higher vertebrates, the immune system undergoes substantial changes from the prenatal period through infancy and juvenility, as young individuals are exposed to new pathogens [Brinkworth & Thorn, 2013; Ygberg & Nilsson, 2012]. In addition, sex differences in health and longevity are found not only in humans [e.g., Wang et al., 2012] but are common across taxa. In general, males tend to show reduced immunocompetence [Folstad & Karter, 1992; Nunn et al., 2009] and have been reported to have higher rates of parasitism [Moore & Wilson, 2002; Skorping & Jensen, 2004], likely due to the common male life history strategy of investing in competitive ability at the expense of maintenance [Muehlenbein & Bribiescas, 2005].
In group-living animals, an individual's health status may also be influenced by social factors. The size of social groups [Nunn & Heymann, 2005], subgroup structuring [Nunn et al., 2015], and position in a social network [Rimbach et al., 2015; Rushmore et al., 2013] have all been proposed to affect population-level disease transmission dynamics. Understanding social factors affecting primate health and longevity at the individual level is more challenging due to the necessity of sufficient long-term datasets, but more are becoming available. Many primate species exhibit dominance hierarchies and as such, dominance rank has been a social metric of particular interest and one that may affect the sexes differently. For example, in chacma baboons [Silk et al., 2010] and rhesus macaques [Blomquist et al., 2011] high-ranking females live longer than low-ranking individuals. In contrast, the health benefits of high rank in males have been debated due to correlations between high rank and increased testosterone, and testosterone's putative role as an immunosuppressant [reviewed in Muehlenbein & Bribiescas, 2005]. Several reviews have summarized the equivocal relationship between rank and health in males that may depend upon the stability of the hierarchy and whether high rank correlates to higher or lower physiological stress [e.g., Habig & Archie, 2015; Sapolsky, 2005]. For example, in male chimpanzees of the Ngogo community (Kibale National Park, Uganda), both high dominance rank and fecal testosterone levels were positively correlated with an individual's parasite richness [Muehlenbein, 2006]. High-ranking males also had higher rates of infection with Oeosophagostomum sp. in the Kanyawara community of Kibale [Krief et al., 2010]. However, in baboons, high-ranking males were less likely to become ill and recovered more quickly than low-ranking males and mid-ranking males had the highest incidence of injuries [Archie et al., 2012].
Among the great apes, very little has been documented regarding sex, age, and social status differences in health indicators. The primary focus thus far has been on parasitism with studies highlighting the variation between sites. As mentioned above, Muehlenbein [2006] reported a positive relationship between high rank and parasitism in male chimpanzees in the Ngogo community of Kibale National Park, Uganda. Gillespie et al. [2010] found no differences between male and female chimpanzees at Gombe National Park, Tanzania in the prevalence of six out of seven pathogenic species. However, adults had higher parasite prevalence and richness than immature individuals. In contrast, Masi et al. [2012] found no age/sex class differences in prevalence and mean parasite load in either chimpanzees (at Kanyawara, Uganda) or western lowland gorillas (at Bai Hokou, Central African Republic). Sex differences in urinalysis have also been conducted at Kanyawara and Bai Hokou, with chimpanzee and gorilla females showing higher concentrations of erythrocytes than males [Masi et al., 2012].
Here we utilize prospectively collected data from habituated chimpanzees at Gombe National Park, Tanzania to examine clinical signs over an 8-year period (2005–2012). We had two main objectives: (i) to analyze the prevalence of clinical signs at the community level and (ii) to investigate individual-level social and demographic correlates of ill health. Chimpanzee societies have fission–fusion dynamics in which members of a community form temporary subgroups or “parties” [Goodall, 1986]. These subgroups form as a result of a combination of factors which may include food availability, sexual state of females, and social relationships with other individuals [Anderson et al., 2002; Goodall, 1986; Matsumoto-Oda et al., 1998; Mitani et al., 2002]. Furthermore, adult male and female chimpanzees show distinct sex differences in behavior. These include differences in feeding and ranging patterns, such that females typically range and feed in small overlapping core while males range more broadly throughout the territory [Murray et al., 2007; Williams et al., 2002; Wrangham & Smuts, 1980]. Male chimpanzees also participate in more direct physical aggression than females, both within communities during sexual coercion and competition for dominance status and mates, and between communities during cooperative territorial defense [Wilson et al., 2014]. However, female–female aggression can be severe in the contexts of infanticide and immigration; residents (older, higher-ranking females) often attack new immigrants [Pusey et al., 2008a]. Eastern chimpanzees (P.t. schweinfurthii), also exhibit distinct sex differences in sociality, such that adult females are significantly less gregarious than adult males, spending much of their time accompanied only by their dependent offspring (Gombe: [Murray et al., 2007], Kanyawara: [Emery Thompson et al., 2007], Mahale: [Hasegawa, 1990]). The chimpanzee mating system is promiscuous, and direct parental care is provided solely by mothers. Offspring are nutritionally dependent on their mother through infancy until weaning between the ages of 3 and 5 years, but remain behaviorally dependent (i.e., continually traveling and socializing with) through the juvenile years, many until the age of 10 [Goodall, 1968; Pusey, 1990].
The total chimpanzee population at Gombe appears to have declined from as many as 120–150 in the 1960s [Pusey et al., 2007] to 96–100 at the beginning of 2013 [Jane Goodall Institute Research Center, Duke University, unpublished data] with death associated with observable signs of disease as the leading cause of mortality [Williams et al., 2008]. Major epidemics at Gombe included suspected polio in 1966, sarcoptic mange in 1997, and respiratory disease outbreaks throughout the study [Goodall, 1983, 1986; Mlengeya, 2000; Nutter, 1996; Walton et al., 2004; Williams et al., 2008]. In addition, individual chimpanzees have been screened for infection with simian immunodeficiency virus of chimpanzees (SIVcpz) using non-invasive methods since late 2000 [Santiago et al., 2003]. SIVcpz infection has been found to result in increased mortality [Keele et al., 2009], and has been identified to be a major contributing factor in the decline of the Kalande community [Rudicell et al., 2010]. To address this, a comprehensive non-invasive health-monitoring program, the Gombe Ecosystem Health Program, was initiated in these communities in mid-2004 with the aim of understanding the prevalence of health issues in the population and identifying the presence and impacts of various pathogens [Gillespie et al., 2010; Keele et al., 2009; Lonsdorf et al., 2006; Parsons et al., 2014, 2015; Terio et al., 2011; Travis et al., 2008].
For this contribution, we first analyze the prevalence of clinical signs in six categories: gastrointestinal, body condition, respiratory, wounds, lameness, and skin issues as they relate to community membership, year, and season (month). The climate in Gombe is highly seasonal based on rainfall, with a wet season from (on average) November to April and a dry season from May to October. Several studies have reported seasonal differences in chimpanzee behavior (e.g., party size [Murray et al., 2006; Wrangham, 1977], activity budgets [Lodwick et al., 2004]). Specifically, we predicted that diarrhea and weight loss would show monthly and community differences given that these factors correlate to the occurrence of gastrointestinal parasites [Gillespie et al., 2010] and seasonal influence on body mass [Pusey et al., 2005]. We also predicted annual differences, given that outbreaks of gastrointestinal disease may occur in some years, but not others. Similarly, we predicted annual, seasonal, and community differences in respiratory clinical signs, given the known seasonal variation in baseline respiratory illness [Lonsdorf et al., 2011] and that outbreaks have affected the communities at different times of the year and in different years [Williams et al., 2008]. We did not make any a priori predictions regarding patterns for wounding or lameness as these are not presumed to be infectious and are more likely to be related to chimpanzee agonistic interactions. Similarly, we did not make a priori predictions regarding dermatologic problems as we were unsure whether/how frequently this clinical sign is observed in chimpanzees [following Masi et al., 2012] outside of one reported outbreak of sarcoptic mange in this population [Walton et al., 2004].
We also examined whether age, sex, dominance rank, and SIVcpz status predict the odds of a given individual being observed with particular clinical signs. We made separate predictions for each clinical sign category according to age class, sex, and rank where justified by previous research. We also analyzed whether SIVcpz status was correlated with each of the signs; however, we did not make specific predictions regarding infection status given that SIV-associated clinical signs may not occur until just prior to death. We predicted that diarrhea would be significantly related to age class (adults more than immatures), but not sex, given the previous findings on parasitism in this population [Gillespie et al., 2010]. In contrast, we did not make any a priori predictions for age class, sex, or rank regarding poor body condition (estimated weight loss) as this clinical sign may represent a variety of underlying conditions. We predicted that respiratory illness would be seen more frequently in males, given their increased sociality and the above-mentioned relationship between testosterone and altered immune responses. However, we did not make any a priori predictions regarding rank or age class. We predicted an increased frequency of wounds and lameness in males and adults, given that most contact aggression in chimpanzees occurs in adult males. For the subset of individuals for whom we have information on dominance rank, we predicted that wounding and lameness would be negatively correlated with high rank status in males. Finally, we did not make any a priori predictions regarding skin issues.
METHODS
Study Site and Long-Term Data Collection
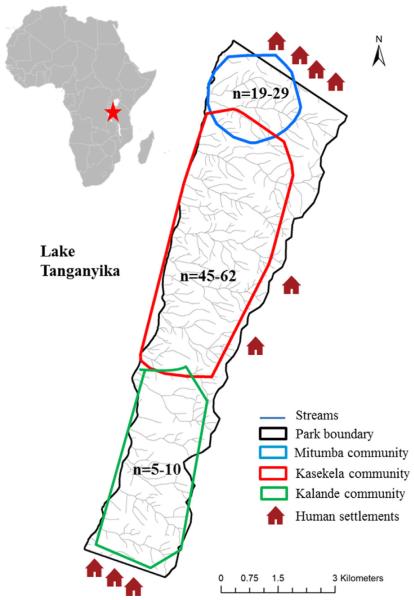
Gombe is a small (land area=35 km2) national park located on the western border of Tanzania and is currently home to three chimpanzee communities. Our study focused on the two habituated communities in the park, Kasekela and Mitumba. The Kasekela community range is in the center of the park (approximately 1802 ha), while the Mitumba community range is between the Kasekela community to the south and Mwamgongo village, which borders the northern boundary of the park (approximately 468 ha). Members of the Kalande community in the south of the park have been monitored regularly since 1999 [Rudicell et al., 2010], but remain largely unhabituated and thus have been excluded from this study. Figure 1 shows a map of Gombe, including approximate chimpanzee ranges and the location of human settlements around the park.
Fig. 1.
Map of Gombe National Park, showing the relative community size and ranges of the three chimpanzee communities in Gombe National Park, as well as the locations of human settlements on the park's boundaries.
The Kasekela community was habituated in the early 1960s and full-day focal follows on individual chimpanzees commenced in 1973. Habituation of the Mitumba community began in the mid-1980s and full-day focal follows commenced in the mid-1990s [Pusey et al., 2008b]. During these follows, a team of two researchers records group composition and location every 15 min, continuous data on feeding behavior, and ad libitum data social interactions. Researchers attempt to follow the majority of adult chimpanzees in each community at least once per month as part of this behavioral record. Additionally, in the larger Kasekela community, follows on family groups (mother, infant, and next oldest offspring) are conducted with the aim of targeting each family once per month (see [Lonsdorf et al., 2014] for a detailed description).
Health Data Collection
In mid-2004, we initiated a prospective health-monitoring system for surveillance of clinical signs of ill health which was linked to the daily focal follows. As part of the daily focal follows on both adults and family groups, a Daily Health Record (hereafter “DHR”—see Fig. 2 for an English translation the form) is filled out by experienced Tanzanian field research staff who have, on average, more than 15 years of experience observing chimpanzees. In the Mitumba community, seven individuals collected health data, all of whom were present for every year of the 8-year study. In the larger Kasekela community, 17 different individuals contributed data and were present for an average of 5.4 years of the 8-year study (range 2–8). All of the researchers work in pairs and all DHRs were reviewed and confirmed by the veterinary project manager to maintain consistency and reliability. A DHR was completed on the focal individual whether it appeared healthy or unhealthy. If an individual other than the primary subject of the follow was observed ill, an additional health record was generated for the ill individual. The DHR is a form modified from the data collection system that was piloted by Lukasik [2002] and was in use at the time by the Mountain Gorilla Veterinary Project [Decision Tree Writing Group, 2006]; the data definitions we used for our clinical signs of interest are comparable (see Table I).
Fig. 2.
English translation of the Daily Health Record datasheet used to collect data on individual chimpanzee clinical signs in Gombe National Park (Field staff use a version translated to Kiswahili).
TABLE I.
Definitions of Normal and Abnormal Clinical Sign Categories Employed in the Gombe Chimpanzee Health-Monitoring Project
| Clinical sign category | Normal | Abnormal |
|---|---|---|
| Gastrointestinal | Feces with expected consistency and distinct shape. | Watery: stools no longer retain shape or consistency. |
| Body condition | Normal weight for individual. | Thin: estimated <10% loss of body weight. |
| Very thin: >10% loss of body weight | ||
| Respiratory | No or very rare coughing or sneezing over course of observation. No difficulty breathing. No mucus running from nose. | Presence of mucus and/or presence of cough one or more times per hour of observation. |
| Wound | None present. Skin intact and no gashes, punctures or swelling observed. | An injury to any tissues of body caused by trauma or disease. Skin may be ruptured or area affected may be swollen. |
| Lame | Normal movement of whole body. | Abnormal movement of one or more limbs leading to limping. |
| Skin | Skin and hair as expected for species. | Rash: Redness of skin with or without pustules, or flakey, whitish looking pieces of epidermis. Loss of hair: reduced density of hair. |
Data definitions have been adapted from the clinical decision tree of the Mountain Gorilla Veterinary Project [Decision Tree Writing Group, 2006].
All paper data collected were entered into IMPACT™ (Internet-Supported Management Program to Assist Conservation Technology). IMPACT™ was originally created for mountain gorillas by the Mountain Gorilla Veterinary project (now, Gorilla Doctors: http://www.gorilladoctors.org/saving-lives/gorilla-health-monitoring-and-interventions), who collaborated with us to modify it for chimpanzees. For this study, we analyzed data for the complete years of 2005–2012. During this time, the Kasekela community size ranged from 45 to 62 individuals, comprised of 9–14 adult (12 years of age or older) males, 8–25 adult females, and 18–26 immatures. The Mitumba community size ranged from 19 to 29 individuals, comprised of 2–5 adult males, 7–10 adult females, and 10–15 immatures [Jane Goodall Institute Research Center, Duke University, unpublished data]. Over this 8-year period, we collected 4804 DHRs from 81 individuals in Kasekela and 2620 DHRs from 32 individuals in Mitumba. For the community-level analyses contained here, the average monthly number of individuals in Kasekela with a DHR was 33 (SD±9) and in Mitumba was 13 (SD±4). For the individual-level analyses, the average number of DHRs collected per individual in Kasekela was 40 (SD±25) and in Mitumba was 44 (SD±30).
All data collection was observational and all research was approved by the required Tanzanian governing bodies, including the Tanzanian Commission for Science and Technology, the Tanzanian Wildlife Research Institute, and Tanzania National Parks. The research in this study adhered to the American Society of Primatologists' Principles for Ethical Treatment of Non-Human Primates.
Data Collation and Analyses
Preliminary analyses revealed that 61% of DHR observations of lameness co-occurred with wounds, so we combined these clinical signs to indicate the presence of wounds and/or lameness for both the community prevalence and individual analyses. No other pairs of signs co-occurred on more than 25% of observations.
Community prevalence
Prevalence refers to the percentage of cases of disease in a population at a particular point in time [Porta et al., 2014]. Both new and existing cases contribute to this summary measure, and thus it is an important measure for evaluating population-level disease impacts and risks. The ability to measure true prevalence is nearly impossible in wild animals, as consistent sampling of the entire population is not feasible. However, Gombe is fairly unique in that we are able to observe a majority of the members of each habituated community once per month. Hereafter, we use the term prevalence to refer to the estimated prevalence based on the number of observed individuals per month in each community.
Outcome variable
To analyze annual, seasonal, and community patterns of health, we generated a monthly community prevalence of abnormal clinical signs in each of five categories: gastrointestinal (diarrhea), body condition (estimated weight loss), respiratory, wounds/lameness, and skin. Therefore, each clinical sign category had 192 months of data (12 months×8 years×2 communities) in which prevalence was calculated as the number of individuals observed with that sign/the number of total individuals observed. For the gastrointestinal category specifically, we analyzed only observations of watery feces, as an indicator of diarrhea, and therefore focused on more obvious and extreme signs of gastrointestinal abnormality. While this is a more conservative approach, these data may underestimate the prevalence of gastrointestinal-related illness/disorders. For all other categories, we analyzed any abnormal indicator. The unit of time used for this study was calendar month given that the focal follows that these data were paired with were conducted on a monthly basis. Thus, if an individual was observed more than once in a given month, and any of their observations were abnormal, they were classified as abnormal for that particular clinical sign for the month. It is possible that observations that occur on the last and first days of the month may over-represent the same event. However, we examined last day/first day observations and these happened only twice in Kasekela (out of a possible 96 month/year combinations×81 different individuals) and three times in Mitumba (out of a possible 96 month/year combinations×32 individuals), so are unlikely to substantially affect our results.
Predictor variables
For the community-level analyses, we had three predictor variables of interest: (i) calendar year—to investigate inter-annual variation in health signs; (ii) calendar month—to investigate seasonality, which has been documented previously for both respiratory signs [Lonsdorf et al., 2011] and gastrointestinal parasites [Gillespie et al., 2010]; and (iii) community residence—to investigate whether the two communities show differing patterns of clinical signs. While a direct measure of rainfall per month would be the ideal measure of seasonal influences, these data are not available for most of the study period. As such, we used calendar month as a proxy. We analyzed monthly prevalence patterns using general linear models (GLMs) using Proc GLM in SAS version 9.2. We first fit a full model with all single variables and all two-way interactions and subsequently excluded all non-significant model terms to arrive at the final model.
Individual factors
Outcome variable
To analyze social and demographic correlates of clinical signs in individual chimpanzees, we generated a monthly presence/absence of abnormal observations for each individual and each clinical sign. For example, if a particular individual had two DHRs for the month and one of them indicated coughing, and no other abnormal signs were reported on either DHR, that individual was assigned a “1” for respiratory, and a “0” for all other clinical sign categories. We summarized presence or absence of signs over the month so that we could include a seasonal variable in statistical analyses and so that multiple observations of the same health issue in a month did not artificially overinflate our clinical sign count.
Predictor variables
Age and sex
We investigated the relationships between sex (M, F) and age and probability of having an observation of ill health. Age was assigned according to age at the start of the calendar month, and was divided into two categories—adult (12 years of age and older) and immature (<12 years of age) following Foerster et al. [2015]. This age cutoff was chosen because 12 years of age is the earliest recorded age that a male at Gombe has fathered offspring [Wroblewski et al., 2009] and several females at Gombe have given birth between 11 and 12 years of age [Jane Goodall Institute Research Center, Duke University, unpublished data].
SIV status
Chimpanzees in Gombe are screened quarterly for the presence of SIVcpz antibodies in their fecal samples. All DHR observations were assigned positive SIVcpz status if the date of the DHR followed the date of a fecal sample that was confirmed Western immunoblot blot positive according to standardized methods [Santiago et al., 2003]. DHRs were assigned a negative SIVcpz status if the date of observation occurred at least 30 days prior to a fecal sample found negative for SIVcpz specific antibodies [Fiebig et al., 2003]. To be as conservative as possible, observations that occurred within the 30-day window prior to the most recent antibody negative sample, and could therefore come from chimpanzees who were acutely infected but whose anti-SIVcpz antibody levels were still negative, were assigned an “unknown” status and excluded from the individual-level analyses. Similarly, DHR observations that occurred in the time period between a last confirmed negative and first confirmed positive sample for an individual were assigned an “unknown” status and excluded.
Categorical dominance rank
We analyzed a subset of individuals to investigate the relationship between categorical dominance rank and each clinical sign. Dominance rankings were only available for adults of the KK community. For both males and females, we calculated dominance ranks based on pant-grunts given and received by same sex individuals over 2 year periods from 2005 to 2011. Pant-grunts are formal indicators of subordination in chimpanzees [Bygott, 1979]. In each 2-year period, we calculated the Modified David's Scores (MDS) [de Vries et al., 2006] separately for males and females and assigned each individual to a categorical rank based on their MDS. Chimpanzees with a MDS >0.5SD above the mean are considered “high,” those within 0.5SD of the mean are “middle” and all those <0.5 below the mean are “low.” Rank category assignment was the same for each year in the 2-year window. Using a categorical measure of rank follows the precedence of other studies in both male [Gilby et al., 2013] and female [Foerster et al., 2015] chimpanzees.
Annual, seasonal, and community patterns
We also included year, calendar month, and community membership (Kasekela or Mitumba), in our analyses to control for any annual, seasonal, or community-specific effects.
Statistical Analyses
For the full dataset, we constructed separate statistical models for each category of clinical sign and modeled the presence of each sign in an individual in a given month as a binomial dependent variable using Generalized Estimating Equations (GEEs). GEEs can control for repeated sampling of subjects and/or time periods and are particularly useful for analyzing correlated binary response data [Diggle et al., 2002]. We fit all models using a logit link function and an independent correlation structure to account for repeated and uneven sampling on individual chimpanzees. Each predictor variable was examined as a fixed effect along with two interaction terms: age×sex and SIV status×sex. Fixed effects were first examined in univariate models and significant variables were added to a multivariate model. The best model was selected using the lowest quasilikelihood under the independence model criterion (QIC) [Cui, 2007; Hanley, 2003]. For all chimpanzee-specific variables (age, sex, rank, and SIV status) that were significant, we conducted post hoc pairwise comparisons to generate odds ratios and 95% confidence intervals for the effect of interest. We used Proc GENMOD in SAS version 9.2 (Cary, NC) for all analyses.
To investigate the effects of rank, we conducted separate within-sex analyses on adults in Kasekela, given the known differences in socioecological pressures on male and female chimpanzees [Wrangham & Smuts, 1980], and that male dominance interactions involve substantially more direct contact aggression than female dominance interactions [Pusey et al., 1997]. The presence/absence of a clinical sign in each category was then modeled as described above for the following variables: year, month, categorical rank, and SIV status. (The variables community, age, and sex were no longer relevant to these analyses).
RESULTS
Community Prevalence
Figure 3 through 7 show the prevalence of each clinical sign by calendar month and community. Monthly means±SE are displayed on the same Y-axis scale (−0.02 to 0.12) for all clinical sign categories for comparison purposes, except for the figure for skin abnormalities (Fig. 7) given that the prevalence of this clinical sign was much less frequent. Table II shows, by year and by community, the maximum monthly prevalence of each clinical sign category observed in that year. The minimum monthly prevalence for all categories was zero.
Fig. 3.
Mean monthly prevalence of diarrhea for the Kasekela (KK) and Mitumba (MT) chimpanzee communities in Gombe National Park from 2005 to 2012. Months 5–10 represent the typical dry season, while months 11–4 are relatively wetter. Errors bar represent±SE for the 8-year period.
Fig. 7.
Mean monthly prevalence of abnormal skin observations for the Kasekela (KK) and Mitumba (MT) chimpanzee communities in Gombe National Park from 2005 to 2012. Months 5–10 represent the typical dry season, while months 11–4 are relatively wetter. Errors bar represent±SE for the 8-year period.
TABLE II.
Annual and Overall Prevalence Maximums (Highest Monthly Prevalence in That Year) by Community (KK, Kasekela; MT, Mitumba) of Abnormal Observations in Five Different Clinical Sign Categories for Chimpanzees in Gombe National Park
| Diarrhea |
Weight |
Respiratory |
Wound/lame |
Skin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | KK | MT | KK | MT | KK | MT | KK | MT | KK | MT |
| 2005 | 0.20 | 0.33 | 0.04 | 0.33 | 0.12 | 0.33 | 0.17 | 0.20 | 0.08 | 0.00 |
| 2006 | 0.08 | 0.07 | 0.13 | 0.10 | 0.36 | 0.27 | 0.15 | 0.25 | 0.05 | 0.00 |
| 2007 | 0.13 | 0.15 | 0.06 | 0.09 | 0.28 | 0.09 | 0.11 | 0.10 | 0.04 | 0.00 |
| 2008 | 0.10 | 0.00 | 0.03 | 0.09 | 0.05 | 0.42 | 0.07 | 0.27 | 0.00 | 0.00 |
| 2009 | 0.03 | 0.05 | 0.00 | 0.20 | 0.09 | 0.46 | 0.05 | 0.15 | 0.00 | 0.00 |
| 2010 | 0.07 | 0.00 | 0.02 | 0.05 | 0.02 | 0.00 | 0.06 | 0.22 | 0.00 | 0.00 |
| 2011 | 0.10 | 0.05 | 0.05 | 0.07 | 0.28 | 0.10 | 0.14 | 0.25 | 0.03 | 0.00 |
| 2012 | 0.07 | 0.08 | 0.10 | 0.07 | 0.33 | 0.12 | 0.10 | 0.09 | 0.05 | 0.00 |
| All | 0.20 | 0.33 | 0.13 | 0.33 | 0.36 | 0.46 | 0.17 | 0.27 | 0.08 | 0.00 |
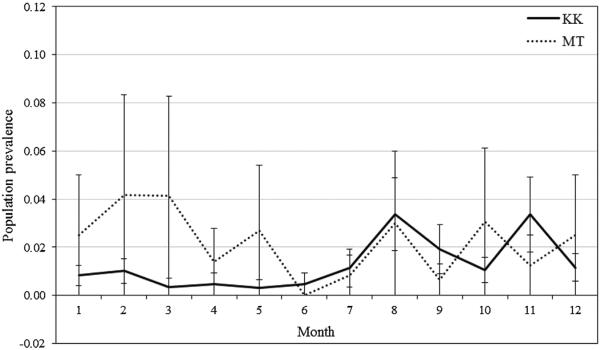
The mean monthly prevalence of diarrhea ranged from 0.3% to 4.8% in Kasekela, and 0% to 5.8% in Mitumba (Fig. 3). As we hypothesized, the prevalence of observed diarrhea was significantly affected by all three predictors of interest. The final model (R2=77.6) included terms for year (F=2.88, df=7, P=0.0093), month (F=4.19, df=11, P<0.0001), community (F=7.51, df=1, P=0.0074), and an interaction term for year×month (F=2.79, df=77, P<0.0001) and year×community (F=2.50, df=8, P=0.0218). Figure 3 shows the mean monthly prevalence for the 8 years of the study and illustrates both the monthly differences and that the Kasekela community had significantly higher prevalence of diarrhea than the Mitumba community. In terms of annual differences, Table II shows that 2005 had a much higher maximum prevalence for both communities than other years. We conducted post hoc pairwise comparisons using the Tukey–Kramer method for year and found that 2005 had significantly higher prevalence of diarrhea than both 2009 (P=0.0213) and 2010 (P=0.0073).
Observations of abnormal body condition/weight loss resulted in a mean monthly prevalence range of 0.3–3.4% for Kasekela and 0–4.3% for Mitumba (Fig. 4). Prevalence of weight loss was predicted by the interaction of year×community (F=2.33, df=8, P=0.0209, R2=15.2). Figure 4 illustrates the variability of these abnormal observations across calendar months. Furthermore, the Kasekela and Mitumba communities often had large differences in the maximum monthly prevalence (e.g., in 2005 and 2009) of this clinical sign (Table II). We conducted post hoc pairwise comparisons using the Tukey–Kramer method for year and found that 2005 had significantly more observations of estimated weight loss when compared to 2010 (P=0.0335).
Fig. 4.
Mean monthly prevalence of abnormal weight observations for the Kasekela (KK) and Mitumba (MT) chimpanzee communities in Gombe National Park from 2005 to 2012. Months 5–10 represent the typical dry season, while months 11–4 are relatively wetter. Errors bar represent±SE for the 8-year period.
The mean monthly prevalence of respiratory disease ranged from 0.6% to 9% in Kasekela and 0% to 8.7% in Mitumba (Fig. 5) and was significantly affected by all three predictors of interest. The final model (R2=63.8) included terms for year×month (F=1.44, df=88, P=0.0446), and year×community (F=2.12, df=8, P=0.0420). Figure 5 illustrates the monthly and community differences. Table II shows that in nearly every year, the Kasekela and Mitumba communities had large differences in the maximum monthly prevalence.
Fig. 5.
Mean monthly prevalence of abnormal respiratory observations for the Kasekela (KK) and Mitumba (MT) chimpanzee communities in Gombe National Park from 2005 to 2012. Months 5–10 represent the typical dry season, while months 11–4 are relatively wetter. Errors bar represent±SE for the 8-year period.
Monthly prevalence of wounds/lameness ranged from 1.6% to 6% in Kasekela and 1.1% to 9% in Mitumba (Fig. 6). The observed prevalence of wounds/lameness was not significantly predicted by any of our model terms. The interaction term for community×month was significant only at the P<0.10 level (F=1.47, df=23, P=0.0857), and Table II shows that in 6 out of 8 years (excluding 2007 and 2012), there was a higher maximum prevalence in Mitumba than in Kasekela. Figure 6 illustrates the monthly and community differences and shows that the Mitumba community tended to have a higher prevalence than the Kasekela community, especially in the latter half of the calendar year. It is important to note that we did not attempt to measure the age of wounds, so this is not necessarily a measure of “new” wounds each month, but a measure of presence/absence in the month.
Fig. 6.
Mean monthly prevalence of wounds/lameness observations for the Kasekela (KK) and Mitumba (MT) chimpanzee communities in Gombe National Park from 2005 to 2012. Months 5–10 represent the typical dry season, while months 11–4 are relatively wetter. Errors bar represent±SE for the 8-year period.
The prevalence of dermatologic issues was much lower than that of the other clinical sign categories. Kasekela monthly prevalence ranged from 0% to 1.4% and no skin problems were observed in Mitumba (Fig. 7). The final model (R2=22.2) included terms for community (F=16.52, df=1, P<0.0001), year (F=2.41, df=7, P=0.0223), and an interaction term for community×year (F=2.41, df=7, P=0.0223). The absence of observed skin abnormalities in the Mitumba community resulted in our significant community difference. Figure 7 illustrates a lack of pattern of skin abnormalities by calendar month in Kasekela, but Table II illustrates annual differences in Kasekela. However, we conducted post hoc pairwise comparisons using the Tukey–Kramer adjustment and did not find any specific pairs of years that were significantly different from each other.
Individual Factors
We removed individual DHRs that had unknown SIVcpz status, which resulted in a final dataset of 4376 individual chimpanzee months (n=103 individuals, 42 males, 61 females) for the full dataset analyses. The number of adults and immatures in the dataset was 64 and 54, respectively (some individuals aged into the adult category during the study and their data appear in both categories). The number of SIVcpz-positive and negative individuals was 16 and 89, respectively (two individuals changed status over the course of the study and appear in both categories). Table III summarizes the number of individuals, total number of records and number of chimpanzee months with clinical signs of ill health present for each category in the full dataset (all individuals, both communities). The subset of data for which we had individuals of both known SIVcpz status and categorical dominance ranks was comprised of 785 individual chimpanzee months for males (n=14 individuals, 3/14 SIVcpz+) and 1139 individual chimpanzee months for females (n=28 individuals, 6/28 SIVcpz+). Table IV provides the number of individuals, total number of records and number of chimpanzee months with clinical signs of ill health present for each category for Kasekela adults, separately by sex. Table V summarizes the statistical results for each dataset.
TABLE III.
Number of Individuals, Records, and Abnormal Records According to Community, Sex, Age Class, and SIVcpz Status for Chimpanzees in Gombe National Park
| Community | Sex | Age class | SIVcpz status | Individuals | Total records | Diarrhea | Weight | Respiratory | Wound/lame | Skin |
|---|---|---|---|---|---|---|---|---|---|---|
| KK | Female | Adult | − | 27 | 1104 | 19 | 5 | 25 | 38 | 1 |
| + | 8 | 198 | 13 | 5 | 5 | 13 | 3 | |||
| Immature | − | 17 | 455 | 6 | 0 | 9 | 8 | 5 | ||
| + | 1 | 23 | 0 | 0 | 0 | 0 | 0 | |||
| Male | Adult | − | 14 | 693 | 17 | 16 | 21 | 52 | 6 | |
| + | 3 | 140 | 3 | 4 | 2 | 8 | 1 | |||
| Immature | − | 18 | 474 | 8 | 0 | 16 | 15 | 2 | ||
| + | 3 | 25 | 0 | 1 | 0 | 0 | 0 | |||
| MT | Female | Adult | − | 11 | 592 | 4 | 14 | 34 | 36 | 0 |
| + | 1 | 80 | 0 | 1 | 4 | 4 | 0 | |||
| Immature | − | 7 | 126 | 0 | 1 | 2 | 0 | 0 | ||
| + | 0 | NA | NA | NA | NA | NA | NA | |||
| Male | Adult | − | 3 | 144 | 6 | 4 | 5 | 16 | 0 | |
| + | 1 | 83 | 2 | 4 | 0 | 13 | 0 | |||
| Immature | − | 11 | 239 | 1 | 0 | 3 | 10 | 0 | ||
| + | 0 | NA | NA | NA | NA | NA | NA |
TABLE IV.
Number of Individuals, Records, and Abnormal Records According to Sex, Dominance Rank, and SIVcpz Status for Chimpanzees in Gombe National Park
| Sex | Rank | SIVcpz status | Individuals | Total records | Diarrhea | Weight | Respiratory | Wound/lame | Skin |
|---|---|---|---|---|---|---|---|---|---|
| Female | L | − | 8 | 266 | 5 | 0 | 4 | 9 | 0 |
| + | 1 | 21 | 4 | 0 | 0 | 0 | 0 | ||
| M | − | 17 | 387 | 8 | 0 | 12 | 6 | 0 | |
| + | 5 | 151 | 9 | 1 | 5 | 7 | 0 | ||
| H | − | 10 | 314 | 5 | 5 | 6 | 9 | 1 | |
| + | 0 | NA | NA | NA | NA | NA | NA | ||
| Male | L | − | 7 | 141 | 4 | 0 | 3 | 4 | 0 |
| + | 1 | 57 | 0 | 0 | 1 | 1 | 0 | ||
| M | − | 10 | 399 | 10 | 14 | 10 | 35 | 5 | |
| + | 2 | 37 | 0 | 3 | 1 | 3 | 1 | ||
| H | − | 5 | 114 | 1 | 0 | 4 | 3 | 0 | |
| + | 1 | 37 | 0 | 0 | 0 | 0 | 0 |
TABLE V.
Summary of Variables Tested and Their Statistical Significance for Each Clinical Sign in Three Different Datasets for Chimpanzees in Gombe National Park: Full dataset = All Individuals, Both communities; KK Males = Known-Rank Adult Males of the Kasekela Community; KK Females = Known-Rank Adult Females
| Full dataset | Diarrhea | Weight | Respiratory | Wound/lame | Skin |
|---|---|---|---|---|---|
| Sex | NS | NS | NS | 0.0015 | NS |
| Age class | 0.0392 | 0.0010 | NS | <0.0001 | NS |
| SIVcpz status | NS | NS | NS | NS | NS |
| Community | 0.0775 | NS | NS | NS | NS |
| Month | 0.0001 | 0.0052 | <0.0001 | NS | NS |
| Year | 0.0232 | NS | 0.0025 | NS | NS |
| Age × sex | NS | NS | NS | NS | NS |
| SIVcpz status × sex | NS | NS | NS | NS | NS |
|
| |||||
| KK males | |||||
|
| |||||
| Rank | NS | NS | NS | 0.0033 | NS |
| SIVcpz status | NS | NS | NS | NS | NS |
| Month | NS | NS | NS | NS | NS |
| Year | NS | NS | NS | NS | NS |
|
| |||||
| KK females | |||||
|
| |||||
| Rank | NS | NS | NS | NS | NS |
| SIVcpz status | 0.0032 | NS | NS | NS | NS |
| Month | NS | NS | NS | NS | NS |
| Year | NS | NS | NS | NS | NS |
Full dataset (all individuals, both communities)
As predicted, the odds of an individual chimpanzee displaying the clinical sign of diarrhea was significantly related to age class (GEE: Wald χ21=4.25, P=0.0392). Adults had a higher of being observed with diarrhea than immatures (OR: 1.83, 95%CI 1.03–3.23). However, neither sex nor SIVcpz status significantly predicted diarrhea. Corresponding to the community-level analyses, month (GEE: Wald χ211=37.93, P=0.0001) and year (GEE: Wald χ27=16.22, P=0.0232) were also significant. In addition, membership in the KK community marginally predicted the probability of diarrhea (GEE: Wald χ21=3.12, P=0.0775, OR: 2.12, 95%CI 0.92–4.87).
The odds of an individual being observed in poor body condition (weight loss) was also predicted by age category (GEE: Wald χ21=10.75, P=0.0010), with adults having a higher odds of poor condition than immature individuals (OR: 12.06, 95%CI 2.72–53.39). Calendar month (GEE: Wald χ211 = 26:63, P=0.0052) was also retained in the final model, with August showing the highest odds of estimated weight loss. No other predictor variables were significant.
Counter to our predictions, no individual-specific variables predicted the odds of being observed with respiratory clinical signs. The best fit model for this clinical sign included month (GEE: Wald χ211=54.25, P<0.0001) and year (GEE: Wald χ27=22.08, P=0.0025).
In contrast, only individual-specific variables predicted the odds of being observed with wounds and/or lameness, with adults being observed with this sign significantly more than immatures (GEE: Wald χ21=16.22, P<0.0001, OR: 2.96, 95%CI 1.76–5.11) and females significantly less than males (GEE: Wald χ21=10.12, P=0.0015, OR: 0.53, 95%CI 0.35–0.78).
The odds of an individual being observed with skin abnormalities was not predicted by any of our proposed variables of interest.
Reduced dataset (within sex, known-ranked adults, kasekela only)
Among adult males, categorical rank significantly predicted the odds of being observed with wounds and/or lameness (GEE: Wald χ22=11.43, P=0.0033). We conducted pairwise comparisons of rank categories and found that the odds of being observed with wounds and/or lameness was significantly lower in both high-ranking males (OR=0.21, 95%CI 0.06–0.70, P=0.0110) and low-ranking males (OR=0.27, 95%CI 0.11–0.70, P=0.0069) when compared to medium-ranking males. No other clinical sign was predicted by our variables of interest.
Among adult females, categorical rank did not significantly predict the odds of being observed with any clinical sign. However, negative SIVcpz status significantly decreased the odds of being observed with diarrhea (GEE: Wald χ21=8.66, P=0.0032, OR=0.23, 95%CI 0.08–0.61). No other clinical sign was predicted by our variables of interest.
DISCUSSION
Given that disease is a major concern for endangered wildlife, and apes in particular, several programs have been initiated over the past decade to better understand the frequency of and factors influencing ape health. However, data on the population prevalence of different illnesses or injuries is largely unavailable, making it difficult to determine whether particular areas/study sites are at “more” or “less” risk of ill health than others. Here we present the first description of community-level and individual-level patterns of clinical signs in a wild chimpanzee population collected over an 8-year period.
Community Prevalence
The prevalence of gastrointestinal issues as measured by diarrhea was significantly related to all three of our predictors: year, month, and community. Annual differences were present, particularly in 2005, which had a significantly higher prevalence than other years. Unfortunately, we cannot determine the cause of the high prevalence in 2005 since our comprehensive screening for gastrointestinal pathogens did not begin until 2006. We also found monthly differences in diarrheal prevalence that largely correspond to the patterns of gastrointestinal parasitism reported previously for this community [Gillespie et al., 2010]. High prevalence was generally found for many parasite species at the beginning of the calendar year when rainfall is high and chimpanzee party size is relatively large. However, Gillespie et al. [2010] also reported higher prevalence of many parasites species in the Mitumba community, perhaps reflecting their status as an edge community. In this analysis, we see higher prevalence of diarrhea in Kasekela, particularly during the early dry season (months 5–7). The recent discovery of a pig-associated pathogenic protozoa (Cryptosporidium suis) exclusively in the Kasekela community may contribute to the observed community differences, but we do not yet have enough data to determine whether this pathogen shows a seasonal pattern [Parsons et al., 2015]. Overall, this suggests that diarrhea is likely a clinical sign for a variety of underlying health issues and/or seasonal changes in diet and requires more detailed attention. At the only other chimpanzee site to report data on gastrointestinal clinical signs (Kanyawara), diarrhea was observed in at least one individual on approximately 20% of observation days over a 6-month period [Masi et al., 2012]. However, prevalence was not reported, making inter-site comparisons difficult.
Observed prevalence of poor body condition (estimated by weight loss) differed by year and the effect of year differed in the two communities. This potential “emaciation” may indicate a response to food availability or an infectious process that impacted the communities differently in different years—or both. However, both diarrhea and estimated weight loss were higher in the Mitumba community in 2005, and as mentioned above, we cannot determine a specific cause. It is interesting that calendar month did not significantly predict prevalence of abnormally low weight given earlier findings for the Kasekela community that chimpanzees are on average significantly lighter (as measured by a hanging scale) in dry season months and that weight tracks mean rainfall in the previous calendar month [Pusey et al., 2005]. There are a several potential explanations for this difference that may be acting in combination, but we examine two here. One important difference is that we focused on estimated loss of weight in our health data collection; whereas, observations of weight gain were not collected. Additionally, we considered “thin” and “very thin” together as an abnormal clinical sign, since we did not rely upon a standardized numeric body condition scoring system. As such, our weight observations do not have comparable resolution to the body mass data analyzed by Pusey et al. [2005]. Secondly, it is also possible that the food availability in the park has changed over the years such that there is now more food in the dry season and less overall variation in weight. Satellite imagery taken of the park over the years shows an increase of vegetation overall since the 1970s [Pusey et al., 2007]. In support of this possibility, Pusey et al. [2005] showed higher masses from the 1990s onwards compared to the 1970s and 1980s, which also corresponded to an expansion of the Kasekela chimpanzee range. To our knowledge, systematic data on observations of poor body condition/weight loss have not been reported from other sites, so inter-site comparisons based upon standardized body condition scoring systems should be a future goal.
Respiratory disease is the single largest health concern for habituated wild apes. Outbreaks of respiratory disease have accounted for significant morbidity and mortality in chimpanzees at Gombe [Goodall, 1986; Williams et al., 2008], Mahale [Hanamura et al., 2008; Kaur et al., 2008], Taï forest [Köndgen et al., 2010], and Bossou [Fujita, 2011]. The average monthly means range up to 9% in Kasekela and up to 8.7% in Mitumba. However, maximum prevalence ranged up to 36% in Kasekela and 46% in Mitumba (Table II). These large differences between average and maximum prevalence indicate the presence of outbreaks of respiratory disease. It is important to note that outbreaks are included in our calculations here, and thus, the baseline (non-outbreak) prevalence of respiratory disease is lower. Our analyses here show significant interactions of year with month, and year with community, suggesting that outbreaks are driving these results, given that outbreaks have occurred in different years and months in the two communities. The population prevalence of respiratory signs during outbreaks has been reported for many of the abovementioned studies from other chimpanzees study sites, but no data have been reported outside of outbreaks. In the Kanyawara chimpanzees, the respiratory-related clinical signs of coughing and sneezing were observed on approximately 50–55% and 40% of observation days, respectively [Masi et al., 2012]. However, here again, we are unable to make direct comparisons without comparative estimates of prevalence.
We did not make a priori predictions regarding the effects of year, month, or community on prevalence of wounds/lameness, as we assumed that wounds were more likely to be related to chimpanzee social behavior (e.g., aggression, sexual coercion) than an infectious process. Indeed, we found that none of our predictors were significantly related to wounding, but there was a non-significant tendency for a higher prevalence of wounds in Mitumba. This may relate to the fact that during the study period, there was more intense intracommunity aggression in Mitumba, as well as several intercommunity interactions during which Kasekela chimpanzees initiated aggressive interactions against Mitumba [Wilson, unpublished data]. Wounds were also observed quite regularly at Kanyawara (between 40% and 45% of observations days), but population prevalence was not estimated [Masi et al., 2012].
The prevalence of observed dermatological conditions was much lower than for all other clinical signs. This corresponds to our assumption that outside of a single outbreak of sarcoptic mange [Walton et al., 2004], skin abnormalities are relatively rare in Gombe. Skin lesions were also absent in the Kanyawara community [Masi et al., 2012]. However, other study sites report seeing dermatologic issues much more regularly (e.g., chimpanzees in the Goualougo Triangle, Republic of Congo [Sanz, personal communication]) so more comparative data from multiple sites are necessary. One limitation of these data is that full examination of this system requires a much closer view of the animal, which is often precluded by research and tourism viewing-distance rules in the park. For instance, most of the dermatologic clinical signs recorded by Gorilla Doctors program are the result of close examination during veterinary interventions [Travis, personal communication]. Interestingly, at Gombe, we have observed skin abnormalities only in the Kasekela community over the 8-year study period. While observational data are imperfect, this finding is certainly interesting and warrants further investigation. We have not sampled or identified the cause of the skin issues observed in Kasekela, but many of them appear similar in description to the dermatophytosis (white, scaly patches) described in the Mahale chimpanzees, which from 2001 to 2002 appeared in almost 31% of the population [Nishida et al., 2007].
Individual Factors
We present here the first report of the effects of age, sex, SIVcpz status, and rank differences on observable clinical signs of ill health in wild chimpanzees. Such data are not only important from a conservation perspective, but also for understanding the different pressures that individuals undergo as a result of their sex and life stage. By monitoring clinical signs of ill health in known individuals with substantial longterm data, we can begin to tease apart the complex interplay of health, behavior, and ecology.
In the full dataset, we found age class differences in the odds of being observed with diarrhea, as well as monthly, annual and community-specific differences that correspond to the abovementioned community-level analyses. We predicted age class differences given that Gillespie et al. [2010] found higher prevalence of most pathogenic parasites in adults as compared to immatures. This is not to suggest that diarrhea is directly correlated with parasitism and as discussed above, this clinical sign warrants more detailed investigation to determine the full scope of gastrointestinal pathogens that may be involved.
We also found age class differences that suggest that adults have higher odds of being observed with poor body condition. This finding corresponds to the above finding on diarrhea, although diarrhea and weight loss co-occur in only 4 out of 4,376 chimpanzee months. Moreover, increased parasitism can result in weight loss in the absence of diarrhea. Alternatively, it is possible that the smaller body sizes and/or variation in growth rates of younger chimpanzees make it less likely for field staff to observe poor body condition. That is, for immature individuals, “lack of weight gain/growth” may be a better indicator of a health issue than poor body condition. Employment of photographic documentation coupled with a quantitative body condition scoring scheme would be an important next step to further investigate this finding. In addition, month significantly predicted abnormally low weight in an individual, corresponding to previously published data from this population showing that chimpanzees tend to be lighter in the dry season [Pusey et al., 2005]. However, month did not predict the prevalence of this clinical sign in the population as a whole, perhaps indicating that some members of the population are more affected by seasonal changes in food availability than others. For example, we know that high-ranking females have larger core areas in the dry season [Murray et al., 2007], which may buffer them against severe weight loss.
Counter to our predictions, the probability of being observed with respiratory disease was not predicted by any of our chimpanzee-specific variables and was only significantly related to seasonal and annual variables. We originally predicted that males would have higher odds of being observed with this sign given that their increased gregariousness may increase risk of exposure to respiratory pathogens. While we did not find such an effect, males appear to play a significant transmission role when it comes to the onset of respiratory outbreaks [Wolf et al, unpublished data]. Thus, it may be that males are key to respiratory disease transmission, as has been found for parasite transmission [Skorping & Jensen, 2004], but that once exposed, individuals of all age/sex classes are equally likely to become ill. Differing effects on age/sex classes may also depend on the particular pathogen in question. Therefore, as diagnostic technologies improve and we are better able to identify specific pathogens, we will improve our capability to understand respiratory disease correlates.
In contrast, the odds of being observed with wounds and/or lameness was only predicted by the individual-specific variables of age and sex, with adults and males showing increased odds of this clinical sign. This corresponds well with what we know about chimpanzee social behavior, in that most physical contact aggression occurs between males during either territorial defense or conflicts associated with dominance status [Muller & Mitani, 2005; Wilson & Wrangham, 2003]. In the subset of males for whom we have categorical dominance rank data, we predicted that wounding/lameness would vary directly and inversely with rank categories. That is, low-ranking males would show the highest probability of being observed with this clinical sign, high-ranking males would show the lowest, and medium-ranking males would fall in the middle. However, we found that medium-ranking males had higher rates of wounding than either high- or low-ranking males. This corresponds to findings in male baboons [Archie et al., 2012] and suggests that perhaps mid-ranking males sustain higher rates of wounding because they are more likely to be engaged in aggressive interactions associated with moving up or down the hierarchy. In this reduced dataset, none of the other clinical signs were predicted by our variables of interest (year, month, rank, SIVcpz status), which likely reflects the very low number of observations in this subset of data (see Table II).
For adult females, categorical dominance rank did not predict the odds of being observed with any of the five clinical signs. This is in contrast to the findings regarding differences in wounding/lameness according to dominance rank in males. However, dominance interactions between female chimpanzees typically involve less physical contact aggression [Pusey et al., 1997] and are manifested in access to better foraging areas for higher-ranking individuals [Murray et al., 2006]. In fact, female–female aggression is quite infrequent [Miller et al., 2014; Murray et al., 2007] though in rare circumstances it can be severe [Pusey et al., 2008a]. Male to female aggression is more common and occurs in the context of mating and/or sexual coercion [Feldblum et al., 2014; Muller et al., 2007]. At Gombe, low-ranking females receive more aggression from males than high-ranking females [Markham et al., 2014], but this may not be severe enough to cause detectable differences in wounding and lameness.
SIVcpz infection status did not predict the probability of being observed with any of the five clinical signs in the full dataset. Previous research in Gombe demonstrated that SIVcpz infection is associated with increased mortality and reduced reproductive success, as well as histopathological findings consistent with end-stage AIDS [Keele et al., 2009; Terio et al., 2011]. At first glance, the lack of clinical signs of illness in SIVcpz infected chimpanzees may thus seem surprising. However, HIV-1 positive humans, who are not on highly active antiretroviral therapy (HAART), do not typically exhibit signs of clinical illness until the late stages of their infection, generally 1 or 2 years prior to death [Collaborative Group on AIDS Incubation and HIV Survival, 2000]. This symptomatic period may be even shorter in chimpanzees given that they do not receive any human-like primary care such as antibiotics, etc. Indeed, two chimpanzees with documented CD4 T cell loss and AIDS-like pathology upon necropsy did not show weight loss until the final 2.5 and 5 months prior to death, respectively. The absence of clinical signs during most of the infection is also consistent with studies of the chimpanzee gut microbiome, which revealed compositional differences between SIVcpz positive and negative individuals only during the end-stage of infection within a few months before death [Barbian et al., this volume]. Nonetheless, among adult females, where 6 of 28 were SIVcpz positive, infection conferred a higher probability of being observed with diarrhea. Although SIVcpz infected adult males did not demonstrate a similar association, only three such individuals were included in the dataset, suggesting that available data are not sufficient to draw definitive conclusions. Furthermore, we have recently confirmed the presence of multiple species of Cryptosporidium in Gombe, including a human-associated zoonotic strain of C. hominis [Parsons et al., 2015]. Cryptosporidium has been linked to outbreaks of diarrheal disease in both humans and livestock and co-infections with Cryptosporidium contribute substantially to mortality in HIV-1 infected individuals [e.g., Colford et al., 1996]. Additional work will be necessary to investigate SIVcpz infection status and co-infection with other pathogens.
SUMMARY/CONCLUSIONS
At Gombe, the clinical sign with the highest mean monthly prevalence was respiratory illness, followed by wounding and lameness, diarrhea and poor body condition/weight loss, and dermatologic issues. Given that time and resources are limited, these data are useful to inform prioritization of sample collection as well as investigation of potential management options. For example, in the case of respiratory clinical signs, a decision could be made to do intensive “outbreak” sampling at a pre-defined threshold. Our findings regarding diarrhea and weight loss suggests that more extensive investigation of gastrointestinal pathogens and their interaction with seasonality are warranted and these are ongoing [Gillespie et al., 2010; Parsons et al., 2015; Terio et al., this volume]. In contrast, if wounding and lameness are largely predicted by chimpanzee behavior rather than by infectious processes, sampling and/or management interventions are less likely to be relevant. Furthermore, skin abnormalities appear to be quite rare, but an increase in prevalence in the future would be a signal that something has changed and may trigger more intensive sampling and investigation. The existing community differences are also intriguing and warrant further investigation. Finally, while there are pairs of years that are significantly different from each other for some clinical signs, there is no overall increase in prevalence of ill health for any sign over the course of the study.
In addition, we have shown that the odds of an individual chimpanzee being observed with particular clinical signs are predicted by demographic and social factors. Adults have a higher probability of being observed with diarrhea, weight loss and wounds and/or lameness, while males have a higher probability than females of being observed with wounds and/or lameness. In contrast, respiratory illness appears not to be related to individual-specific factors. For a subset of known-rank individuals, dominance rank predicts the probability of wounding/lameness in adult males, but does not predict any clinical signs in adult females. Instead, adult females with SIVcpz infection are more likely to be observed with diarrhea, a finding that warrants further investigation.
While collection of clinical sign data is an informative, relatively low cost, and fairly easily implemented method to monitor the health of wild populations already under observation, limitations and challenges remain. At the most basic level, we cannot monitor what we cannot see, so perfect detection is unattainable in wild animals that live in a dense forest and are not observable every day. Gombe and other study sites with habituated populations are our best option for implementing such a technique and the collection of long-term clinical sign data by other sites will hopefully allow for future comparative analyses. While not all signs imply the existence of disease, understanding the average prevalence and ranges of given signs aids in decision-making for follow up observations and/or more extensive biological sampling to definitively diagnose disease. However, as described above, diagnostic testing greatly increases the financial and logistical challenges. Finally, the management philosophy should determine the options for intervention (or not), which in turn drives the design of the system and data collection needs. Defining an acceptable level of risk (since zero risk does not occur) for the prevalence and consequences of adverse health outcomes is the key to designing a system to monitor and respond to these threats. This is something that each management authority and its partners have to address individually, and we encourage those discussions prior to any investment in this area [Travis et al., 2008]. Nevertheless, collection of clinical sign data is a valuable component of a multi-faceted health-monitoring system in wild primates, and is often more logistically and financially feasible than systematic population-wide sample collection for pathogen screening. Such data can provide important insights for behavioral studies, management strategies and conservation planning.
ACKNOWLEDGMENTS
The authors thank the Jane Goodall Institute and Tanzania National Parks (TANAPA) for initiating and supporting the 50+ year research tradition at Gombe, including the current health-monitoring project. In addition, we thank the Gombe Stream Research Centre field staff, especially Juma Baranyikwa, Gabo Paulo, Matendo Msafiri, Iddi Issa, and Baraka Gilagiza. Special thanks are due to the Honorable Dr. Titus Mlengeya Kamani for key support of this research and to the Gorilla Doctors for valuable database support. Permission to carry out research at Gombe was granted by the Government of Tanzania, Tanzania National Parks, Tanzania Commission for Science and Technology, and the Tanzania Wildlife Research Institute. We thank Emma Finestone, Emma Lantz and Anna Sjodin for data management assistance. Additionally, monetary support and invaluable time and effort were provided by staff and volunteers at Lincoln Park Zoo's Davee Center for Epidemiology and Endocrinology and Lester E. Fisher for the Study and Conservation of Apes.
Contract grant sponsor: US Fish and Wildlife Great Ape Conservation Fund; contract grant sponsor: Arcus Foundation; contract grant sponsor: Leo S. Guthman Foundation; contract grant sponsor: National Institutes of Health; contract grant numbers: R01 AI58715, R00 HD057992.
REFERENCES
- Altizer S, Harvell D, Friedle E. Rapidevolutionary dynamics and disease threats to biodiversity. Trends in Ecology and Evolution. 2003;18:589–596. [Google Scholar]
- Anderson DP, Nordheim EV, Boesch C, Moermond TC. Factors influencing fission-fusion grouping in chimpanzees in the Tai National Park, Cote d'Ivoire. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioral diversity in chimpanzees and bonobos. Cambridge University Press; Cambridge: 2002. pp. 90–101. [Google Scholar]
- Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proceedings of the National Academy of Sciences. 2012;109:9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford RW, Reid GDF, Butynski TM. The intestinal faunas of man and mountain gorillas in a shared habitat. Annals of Tropical Medicine and Parasitology. 1990;84:337–340. doi: 10.1080/00034983.1990.11812477. [DOI] [PubMed] [Google Scholar]
- Ashford RW, Reid GDF, Wrangham RW. Intestinal parasites of the chimpanzee Pan troglodytes, in Kibale Forest, Uganda. Annals of Tropical Medicine and Parasitology. 2000;94:173–179. doi: 10.1080/00034980057518. [DOI] [PubMed] [Google Scholar]
- Bermejo M, Rodríguez-Teijeiro JD, Illera G, et al. Ebola outbreak killed 5000 gorillas. Science. 2006;314:1564. doi: 10.1126/science.1133105. [DOI] [PubMed] [Google Scholar]
- Blomquist GE, Sade DS, Berard JD. Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta) International Journal of Primatology. 2011;32:193–208. [Google Scholar]
- Brinkworth JF, Thorn M. Vertebrate immune system evolution and comparative primate immunity. In: Brinkworth JG, Pechenkina K, editors. Primates, pathogens, and evolution, developments in primatology: progress and prospects 38. Springer; New York: 2013. pp. 17–64. [Google Scholar]
- Bygott JD. Agonistic behaviour, dominance and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg DA, McCown ER, editors. The great apes. Benjamin/Cummings; Menlo Park: 1979. pp. 405–427. [Google Scholar]
- Calvignac-Spencer S, Leendertz S, Gillespie TR, Leendertz FH. Wild great apes as sentinels and sources of infectious disease. Clinical Microbiology and Infection. 2012;18:521–527. doi: 10.1111/j.1469-0691.2012.03816.x. [DOI] [PubMed] [Google Scholar]
- Colford JM, Tager IB, Hirozawa AM, et al. Cryptosporidiosis among patients infected with human immunodeficiency virus. Factors related to symptomatic infection and survival. American Journal of Epidemiology. 1996;144:807–816. doi: 10.1093/oxfordjournals.aje.a009015. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on AIDS Incubation and HIV Survival Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- Cui J. QIC program and model selection in GEE analyses. The Stata Journal. 2007;7:209–220. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica. 2001;78:103–116. doi: 10.1016/s0001-706x(00)00179-0. [DOI] [PubMed] [Google Scholar]
- Decision Tree Writing Group Clinical response decision tree for the mountain gorilla (Gorilla beringei) as a model for great apes. American Journal of Primatology. 2006;68:909–927. doi: 10.1002/ajp.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Animal Behaviour. 2006;71:585–592. [Google Scholar]
- Diggle PJ, Heagerty PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. 2nd edition Oxford University Press; New York, NY: 2002. [Google Scholar]
- Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW. Core area quality is associated with variance in reproductive success in chimpanzees at Kanyawara, Kibale National Park. Animal Behaviour. 2007;73:501–512. [Google Scholar]
- Feldblum JT, Wroblewski EE, Rudicell RS, et al. Sexually coercive male chimpanzees sire more offspring. Current Biology. 2014;24:2855–2860. doi: 10.1016/j.cub.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- Foerster S, McLellan A, Schroepfer-Walker K, et al. Social bonds in the dispersing sex: partner preferences among adult female chimpanzees. Animal Behaviour. 2015;105:139–152. doi: 10.1016/j.anbehav.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. The American Naturalist. 1992;139:603–622. [Google Scholar]
- Formenty P, Boesch C, Wyers M, et al. Ebola virus outbreak among wild chimpanzees living in a rain forest of Cote d'Ivoire. Journal of Infectious Diseases. 1999;179:S120–S126. doi: 10.1086/514296. [DOI] [PubMed] [Google Scholar]
- Fujita S. Health monitoring. In: Matsuzawa T, Humle T, Sugiyama Y, editors. The chimpanzees of bossou and nimba. Springer; Tokyo: 2011. pp. 353–359. [Google Scholar]
- Gilby IC, Brent LJN, Wroblewski EE, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology. 2013;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie TR. Non-invasive assessment of gastrointestinal parasite infections in free-ranging primates. International Journal of Primatology. 2006;27:1129–1143. [Google Scholar]
- Gillespie TR, Nunn CL, Leendertz FH. Integrative approaches to the study of primate infectious disease: implications for biodiversity conservation and global health. Yearbook of Physical Anthropology. 2008;51:53–69. doi: 10.1002/ajpa.20949. [DOI] [PubMed] [Google Scholar]
- Gillespie TR, Morgan D, Deutsch JC, et al. A legacy of low impact logging does not elevate prevalence of potentially pathogenic protozoa in free-ranging chimpanzees and lowland gorillas in the Republic of Congo. Ecohealth. 2009;6:557–564. doi: 10.1007/s10393-010-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie TR, Lonsdorf EV, Canfield EP, et al. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. American Journal of Physical Anthropology. 2010;143:534–544. doi: 10.1002/ajpa.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The behavior of free-living chimpanzees in the Gombe Stream Reserve. Animal Behavior Monographs. 1968;1:161–311. [Google Scholar]
- Goodall J. Population dynamics during a 15 year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Zeitshcrift Fur Tierpsychologie. 1983;61:1–60. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: patterns of behavior. Belknap Press of Harvard University Press; Cambridge, Massachusetts: 1986. p. 673. [Google Scholar]
- Habig B, Archie EA. Social status, immune response and parasitism in males: a meta-analysis. Philosophical Transactions of the Royal Society Part B. 2015;370 doi: 10.1098/rstb.2014.0109. 20140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamura S, Kiyono M, Lukasik-Braum M, et al. Chimpanzee deaths at Mahale caused by a flu-like disease. Primates. 2008;49:77–80. doi: 10.1007/s10329-007-0054-1. [DOI] [PubMed] [Google Scholar]
- Hanley JA. Statistical analysis of correlated data using generalized estimating equations: an orientation. American Journal of Epidemiology. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Hasegawa T. Sex differences in ranging patterns. In: Nishida T, editor. The chimpanzees of Mahale. University of Tokyo Press; Tokyo: 1990. pp. 100–114. [Google Scholar]
- Henning KJ. Morbidity and Mortality Weekly Report. 2004. What is syndromic surveillance? pp. 7–11. [Google Scholar]
- Howells ME, Pruetz J, Gillespie TR. Patterns of gastrointestinal commensals and parasites as an index of population and ecosystem health: the case of sympatric western chimpanzees (Pan troglodytes verus) and guinea baboons (Papio hamadryas papio) at Fongoli, Senegal. American Journal of Primatology. 2011;73:173–179. doi: 10.1002/ajp.20884. [DOI] [PubMed] [Google Scholar]
- Kaiser M, Löwa A, Ulrich M, et al. Wild chimpanzees are infected with five plasmodium species. Emerging Infectious Diseases. 2010;6:1956–1969. doi: 10.3201/eid1612.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Singh J, Tong S, et al. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. American Journal of Primatology. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Jones JH, Terio KA, et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köndgen S, Schenk S, Pauli G, Hoesch C, Leendertz F. Noninvasive monitoring of respiratory viruses in wild chimpanzees. Ecohealth. 2010;7:332–341. doi: 10.1007/s10393-010-0340-z. [DOI] [PubMed] [Google Scholar]
- Krief S, Huffman MA, Sevenet T, et al. Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. International Journal of Primatology. 2005;26:467–490. [Google Scholar]
- Krief S, Vermeulen B, Lafosse S, et al. Nodular worm infection in wild chimpanzees in western Uganda: a risk for human health? PLoS Neglected Tropical Diseases. 2010;4:e630. doi: 10.1371/journal.pntd.0000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leendertz FH, Ellerbrok H, Boesch C, et al. Anthrax kills wild chimpanzees in a tropical rainforest. Nature. 2004;30:451–452. doi: 10.1038/nature02722. [DOI] [PubMed] [Google Scholar]
- Leendertz FH, Pauli G, Maetz-Rensing K, et al. Pathogens as drivers of population declines: the importance of systematic monitoring in great apes and other threatened mammals. Biological Conservation. 2006;131:325–337. [Google Scholar]
- Lodwick JL, Borries C, Pusey AE, Goodall J, McGrew WC. From nest to nest: influence of ecology and reproduction on the active period of adult Gombe chimpanzees. American Journal of Primatology. 2004;64:249–260. doi: 10.1002/ajp.20076. [DOI] [PubMed] [Google Scholar]
- Lonsdorf E, Travis D, Pusey A, Goodall J. Using retrospective health data from the Gombe chimpanzee study to inform future monitoring efforts. American Journal of Primatology. 2006;908:897–908. doi: 10.1002/ajp.20296. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Murray CM, Lonsdorf EV, et al. A retrospective analysis of factors correlated to chimpanzee (Pan troglodytes schweinfurthii) respiratory health at Gombe National Park, Tanzania. Ecohealth. 2011;8:26–35. doi: 10.1007/s10393-011-0683-0. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Markham AC, Heintz MR, et al. Sex differences in wild chimpanzee behavior emerge during infancy. PLoS ONE. 2014;9:e99099. doi: 10.1371/journal.pone.0099099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasik M. Establishing a long term veterinary project for free-ranging chimpanzees in western Tanzania. Pan African News. 2002;9:13–17. [Google Scholar]
- Markham AC, Santymire RM, Lonsdorf EV, et al. Rank effects on social stress in lactating chimpanzees. Animal Behaviour. 2014;87:195–202. doi: 10.1016/j.anbehav.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi S, Chauffour S, Bain O, et al. The influence of seasonality on great ape health: a case study of chimpanzees and western gorillas. PLoS ONE. 2012;7:e49805. doi: 10.1371/journal.pone.0049805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Oda A, Hosaka K, Huffman MA, Kawanaka K. Factors affecting party size in chimpanzees of the Mahale mountains. International Journal of Primatology. 1998;19:999–1011. [Google Scholar]
- Miller JA, Pusey AE, Gilby IC, et al. Competing for space: female chimpanzees are more aggressive inside than outside their core areas. Animal Behavior. 2014;87:147–152. doi: 10.1016/j.anbehav.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani JC, Watts DP, Lwanga JS. Ecological and social correlates of chimpanzee party size and composition. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioral diversity in chimpanzees and bonobos. Cambridge University Press; Cambridge: 2002. pp. 102–111. [Google Scholar]
- Mlengeya T. TANAPA Veterinary Department Annual Report. 2000. Respiratory disease outbreak in the chimpanzee population of Gombe National Park. 2000/2001. [Google Scholar]
- Moore SL, Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. [DOI] [PubMed] [Google Scholar]
- Morton FB, Todd A, Lee P, Masi S. Observational monitoring of clinical signs during the last stage of habituation in a wild western gorilla group in Bai Hokou, Central African Republic. Folia Primatologica. 2013;84:118–133. doi: 10.1159/000350916. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP. Intestinal parasite infections and fecal steroid levels in wild chimpanzees. America Journal of Physical Anthropology. 2006;130:546–550. doi: 10.1002/ajpa.20391. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. American Journal of Human Biology. 2005;17:527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW. Male coercion and the costs of promiscuous mating for female chimpanzees. Proceedings of the Royal Society, Series B. 2007;274:1009–1014. doi: 10.1098/rspb.2006.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Mitani JC. Conflict and cooperation in wild chimpanzees. Advances in the Study of Behavior. 2005;35:275–331. [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and dominance rank among wild female chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees: towards an ideal despotic distribution. Animal Behaviour. 2007;74:1795–1804. [Google Scholar]
- Nishida T, Corp N, Hamai M, et al. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. American Journal of Primatology. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- Nishida T, Fujita S, Matsusaka T, Shimada M, Kitopeni R. Dermatophytosis of M group chimpanzees, Mahale mountains, Tanzania. Pan Africa News. 2007;14:5–6. [Google Scholar]
- Nunn CL, Heymann EW. Malaria infection and host behaviour: a comparative study of Neotropical primates. Behavioral Ecology and Sociobiology. 2005;59:30–37. [Google Scholar]
- Nunn CL, Lindenfors P, Pursall R, Rolff J. On sexual dimorphism in immune function. Philosophical Transactions of the Royal Society Series B. 2009;364:61–69. doi: 10.1098/rstb.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn CL, Jordán F, McCabe CM, Verdolin JL, Fewell JH. Infectious disease and group size: more than just a numbers game. Philosophical Transactions of the Royal Society Part B. 2015;370 doi: 10.1098/rstb.2014.0111. 20140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter FB. Respiratory disease claims the lives of at least seven Gombe chimps. Pan African News. 1996;31:3. [Google Scholar]
- Palacios G, Lowenstine L, Cranfield MR, et al. Human metapneumovirus infection in wild mountain gorillas, Rwanda. Emerging Infectious Disease. 2011;17:711–713. doi: 10.3201/eid1704.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MB, Gillespie TR, Lonsdorf EV, et al. Global positioning system data-loggers: a tool to quantify fine-scale movement of domestic animals to evaluate potential for zoonotic transmission to an endangered wildlife population. PLoS ONE. 2014;9:e110984. doi: 10.1371/journal.pone.0110984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MB, Travis DA, Lonsdorf EV, et al. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Neglected Tropical Diseases. 2015;9:e0003650. doi: 10.1371/journal.pntd.0003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta MS, Greenland S, Hernán M, et al. A dictionary of epidemiology. 6th edition Oxford University Press; Oxford: Oxford: 2014. [Google Scholar]
- Pusey AE. Behavioral changes at adolescence in chimpanzees. Behaviour. 1990;115:203–246. [Google Scholar]
- Pusey AE, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. International Journal of Primatology. 2005;26:3–31. [Google Scholar]
- Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conservation Biology. 2007;21:623–634. doi: 10.1111/j.1523-1739.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Murray C, Wallauer W, et al. Severe aggression among female pan troglodytes schweinfurthii at gombe national park, Tanzania. International Journal of Primatology. 2008a;29:949–973. [Google Scholar]
- Pusey AE, Wilson ML, Collins DA. Human impacts, disease risk, and population dynamics in the chimpanzees of Gombe National Park, Tanzania. American Journal of Primatology. 2008b;70:738–744. doi: 10.1002/ajp.20567. [DOI] [PubMed] [Google Scholar]
- Rimbach R, Bisanzio D, Galvis N, et al. Brown spider monkeys (Ateles hybridus): a model for differentiating the role of social networks and physical contact on parasite transmission dynamics. Philosophical Transactions of the Royal Society B. 2015;370 doi: 10.1098/rstb.2014.0110. 20140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudicell RS, Holland Jones J, Wroblewski EE, et al. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathogens. 2010;6:e1001116. doi: 10.1371/journal.ppat.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore J, Caillaud D, Matamba L, et al. Social network analysis of wild chimpanzees provides insights for predicting infectious disease risk. Journal of Animal Ecology. 2013;82:976–986. doi: 10.1111/1365-2656.12088. [DOI] [PubMed] [Google Scholar]
- Rwego IB, Isabirye-Basuta G, Gillespie TR, Goldberg TL. Gastrointestinal bacterial transmission among humans, mountain gorillas, and domestic livestock in Bwindi Impenetrable National Park, Uganda. Conservation Biology. 2008;22:1600–1607. doi: 10.1111/j.1523-1739.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- Santiago M, Lukasik M, Kamenya S, et al. Foci of endemic simian immunodeficiency virus infection in wild-living eastern chimpanzees (Pan troglodytes schweinfurthii) Journal of Virology. 2003;77:7545–7562. doi: 10.1128/JVI.77.13.7545-7562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Berman TJ, et al. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Skorping A, Jensen K. Disease dynamics: all caused by males? Trends in Ecology and Evolution. 2004;19:219–220. doi: 10.1016/j.tree.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Stedman TL. Stedman's medical dictionary for the health professions and nursing. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- Terio KA, Kinsel MJ, Raphael J, et al. Pathologic lesions in chimpanzees (Pan trogylodytes schweinfurthii) from gombe national park, Tanzania, 2004–2010. Journal of Zoo and Wildlife Medicine. 2011;42:597–607. doi: 10.1638/2010-0237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis DA, Lonsdorf EV, Mlengeya T, Raphael J. A science-based approach to managing disease risks for ape conservation. American Journal of Primatology. 2008;70:745–750. doi: 10.1002/ajp.20566. [DOI] [PubMed] [Google Scholar]
- Walsh PD, Abernethy KA, Bermejo M, et al. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422:611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- Walton SF, Dougall A, Pizzutto S, et al. Genetic epidemiology of Sarcoptes scabiei (Acari: sarcoptidae) in northern Australia. International Journal of Parasitology. 2004;34:839–849. doi: 10.1016/j.ijpara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wang H, Dwyer-Lindgren L, Lofgren KT, et al. Agespecific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- Williams J, Liu HY, Pusey AE. Costs and benefits of grouping for female chimpanzees at Gombe. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge University Press; Cambridge, U.K.: 2002. pp. 192–203. [Google Scholar]
- Williams J, Lonsdorf E, Wilson M, et al. Causes of death in the Kasekela chimpanzees of Gombe National Park. Tanzania. American Journal of Primatology. 2008;70:766–777. doi: 10.1002/ajp.20573. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Boesch C, Fruth B, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513:414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- Wilson M, Wrangham R. Intergroup relations in chimpanzees. Annual Review of Anthropology. 2003;32:363–392. [Google Scholar]
- Wrangham RW. Feeding behavior of chimpanzees in gombe national park Tanzania. In: Clutton-Brock TH, editor. Primate ecology. Academic Press; London, U.K.: 1977. pp. 504–538. [Google Scholar]
- Wrangham RW, Smuts BB. Sex differences in the behavioral ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproductive Fertility Supplement. 1980;28:13–31. [PubMed] [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, et al. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Animal Behavior. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ygberg S, Nilsson A. The developing immune system—from foetus to toddler. Acta Pædiatrica. 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- Zommers Z, Macdonald DW, Johnson PJ, Gillespie TR. Impact of human activities on chimpanzee ground use and parasitism (Pan troglodytes) Conservation Letters. 2013;6:264–273. [Google Scholar]