Abstract
Radiation therapy is a staple approach for cancer treatment, whereas radioresistance of cancer cells remains a substantial clinical problem. In response to ionizing radiation (IR) induced DNA-damage, cancer cells can sustain/activate pro-survival signaling pathways, leading to apoptotic resistance and induction of cell cycle checkpoint/DNA repair. Previous studies show that Rac1 GTPase is overexpressed/hyperactivated in breast cancer cells and is associated with poor prognosis. Studies from our laboratory reveal that Rac1 activity is necessary for G2/M checkpoint activation and cell survival in response to IR exposure of breast and pancreatic cancer cells. In the present study, we investigated the effect of Rac1 on the survival of breast cancer cells treated with hyper-fractionated radiation (HFR), which is used clinically for cancer treatment. Results in this report indicate that Rac1 protein expression is increased in the breast cancer cells that survived HFR compared to parental cells. Furthermore, this increase of Rac1 is associated with enhanced activities of ERK1/2 and NF-κB signaling pathways and increased levels of anti-apoptotic protein Bcl-xL and Mcl-1, which are downstream targets of ERK1/2 and NF-κB signaling pathways. Using Rac1 specific inhibitor and dominant negative mutant N17Rac1, here we demonstrate that Rac1 inhibition decreases the phosphorylation of ERK1/2 and IκBα, as well as the levels of Bcl-xL and Mcl-1 protein in the HFR-selected breast cancer cells. Moreover, inhibition of Rac1 using either small molecule inhibitor or dominant negative N17Rac1 abrogates clonogenic survival of HFR-selected breast cancer cells and decreases the level of intact PARP, which is indicative of apoptosis induction. Collectively, results in this report suggest that Rac1 signaling is essential for the survival of breast cancer cells subjected to HFR and implicate Rac1 in radioresistance of breast cancer cells. These studies also provide the basis to explore Rac1 as a therapeutic target for radioresistant breast cancer cells.
Keywords: hyper-fractionated radiation, breast cancer, Rac1, ERK1/2, AKT, IκBα, survival
INTRODUCTION
Radiation therapy (RT) is routinely used for breast cancer treatment.1 While ionizing radiation (IR) delivered by RT causes DNA-damage in cancer cells that can lead to cell death, radioresistance (primary or acquired) remains a major problem in clinic.2 Thus, there is a need to improve our understanding of the mechanisms that protect cancer cells from RT-induced cytotoxicity.
In response to IR, cancer cells activate several mechanisms that promote DNA repair and survival.3 Among these, activation of ATM/ATR, PI3K/AKT and MEK/ERK signaling pathways are commonly observed following IR treatment of cancer cells.3,4 While the ATM/ATR signaling pathway plays an essential role in cell cycle checkpoint activation that leads to cell cycle arrest and DNA repair,5 PI3K/AKT and MEK/ERK signaling pathways promote survival through up-regulation of anti-apoptotic factors (e.g. Bcl2/Bcl-xL/Mcl-1) and inhibition of pro-apoptotic factors (e.g. Bid/Bad).3,4
The NFκB signaling pathway plays an important role in cell proliferation and survival in the inflammatory response.6 When inactive, NFκB is sequestered by the inhibitory κB protein (IκB) in the cytoplasm.6 Upon stimulation by inducers including radiation, IκB becomes phosphorylated by IκK kinases and subjected to proteasomal degradation.6 This releases the sequestered NFκB, allowing it to translocate into the nucleus and induce targeted gene expressions.6 Additionally, IR-induced ATM and reactive oxygen species (ROS) can further enhance the activation of NFκB pathway.7 The best validated NFκB gene targets include Bcl-2, Bcl-xL and Mcl-1, which are members of the anti-apoptotic Bcl-2 family.8
Ras-related C3 botulinum toxin substrate 1 (Rac1), a member of Rho family GTPases, plays important roles in cell migration and survival.9 Rac1 exists in either an active GTP-bound state or inactive GDP-bound state.10 Rac1 is activated by its GEFs (Guanine nucleotide Exchange Factors), which accelerate GDP to GTP exchange, and inhibited by its GAPs (GTPase-Activating Proteins), which stimulate GTP hydrolysis.10 In its active state, Rac1 interacts with downstream effectors to activate numerous signaling pathways.11,12 Rac1 has been reported to activate ERK1/2 signaling via PAK1/2 kinases, which phosphorylate Raf1 and MEK1 to facilitate the formation of the Raf/MEK/ERK complex.13–15 Rac1 also interacts with PI3K to activate PI3K/AKT signaling16,17 and plays an essential role in AKT activation following UV or sphingosine 1-phosphate treament.18,19 Both AKT and ERK1/2 signaling pathways have been shown to promote survival after IR.3,20–25 In addition, Rac1 is required for IR-induced ROS production and ATM activation,3,26,27 which activates the NFκB signaling pathway.28
Rac1 and its modulators (GEFs/GAPS) are implicated in cancer development, invasion and metastasis.10 Overexpression/hyperactivity of Rac1 has been associated with cancer therapy resistance.29–31 For instance, aberrant Rac1 amplification/activation is linked to chemo/radio resistance of head and neck squamous cell carcinomas (HNSCC) and glioblastoma cells, and the HNSCC cells resistant to cisplatin or radiation displayed an increased Rac1 expression, activity and translocation to the nuclei.31–34 Further, inhibition of Rac1 using either pharmacological inhibitor or siRNA restores the chemo/radio sensitivity of these cancer cells.31,34 Rac1 is also shown to play an essential role in the resistance of breast cancer cells to trastuzumab (anti-HER2 therapy) and this involves PTEN inactivation and overexpression of insulin-like growth factor-1 receptor.35 Consistently, high-throughput RNAi screens identify Rac1 amplification as one of the most biologically relevant mechanisms of anti-HER2 therapy resistance in breast cancer.30
We recently reported a new Rac1 function in the regulation of the IR response of breast and pancreatic cancer cells.26,27 We show that Rac1 is rapidly activated by IR and is required for ATM/ATR activation and cell survival following IR. Similarly, other studies reported that Rac1 deficiency reduces DNA damage checkpoint response, DNA repair and survival after exposure to IR and UV.36 In this study, we investigated the role of Rac1 in the response of human breast cancer cells to hyper-fractionated radiation (HFR), a protocol currently used for cancer therapy. Results in this report demonstrate that Rac1 signaling is required for the survival of breast cancer cells following HFR, suggesting a clinical potential of targeting Rac1 for radiosensitization of breast cancer cells.
RESULTS
Rac1 is overexpressed in human breast cancer cells
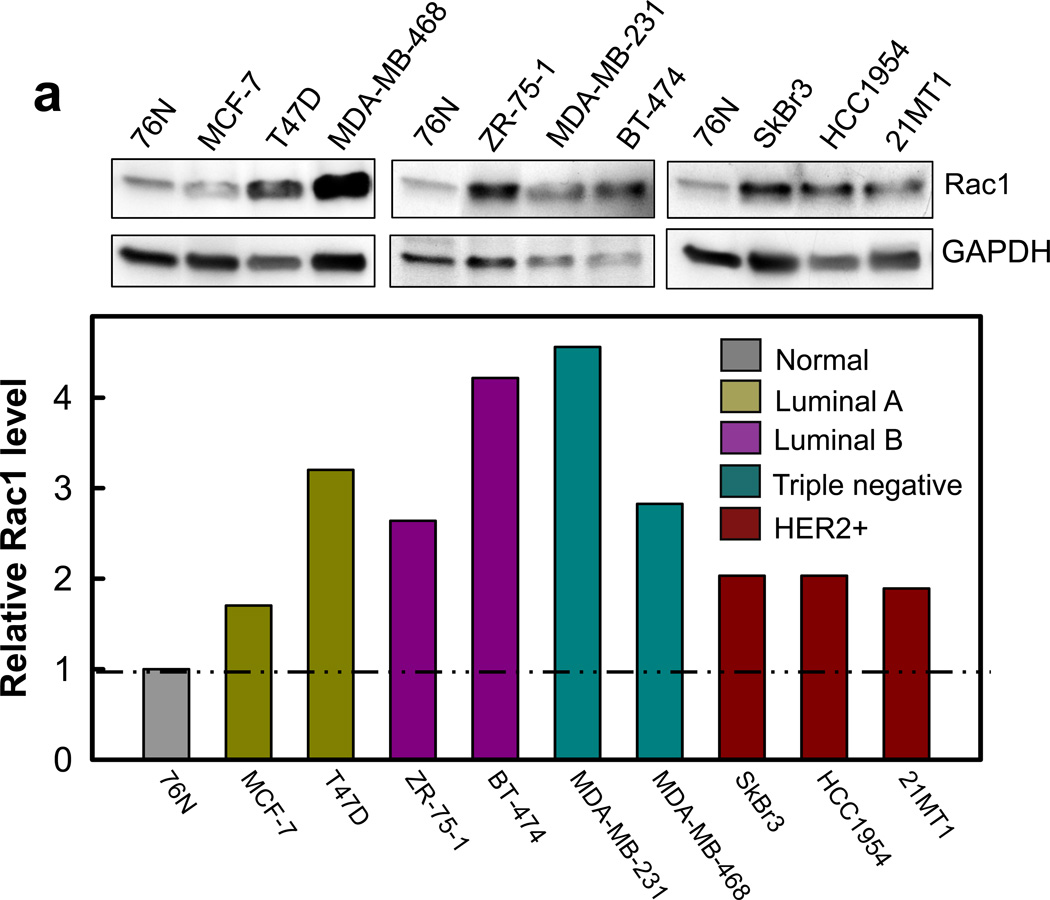
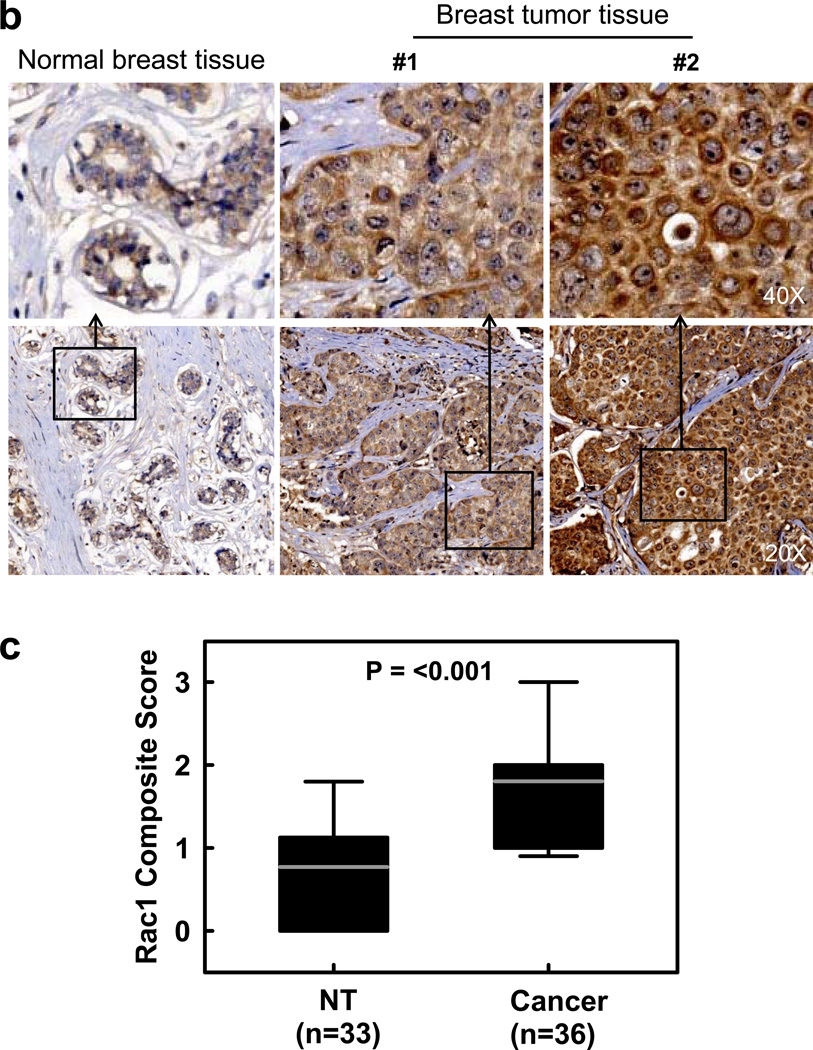
We analyzed Rac1 protein expression in a panel of normal and malignant breast cell lines. The genetic characteristics of these cell lines were previously reported and are summarized in Supplementary Table S1.37–39 As shown in Figure 1a, immunoblotting detected an average of 2–5 fold higher levels of Rac1 in the breast cancer cell lines compared to 76N normal human mammary epithelial (HME) cells. To assess whether Rac1 overexpression observed in the breast cancer cell lines translates to clinical samples, we assessed Rac1 expression by immunohistochemistry (IHC) in a TMA, which consists of 33 cases of human normal breast/benign tumor tissues (NT) and 36 cases of human malignant breast tumor tissues. As shown in Figure 1b–c, IHC detected specific immunostaining for Rac1 with moderate to strong levels in the majority of breast tumor tissues, whereas the NT samples were negative or weakly positive for Rac1 staining. Among the TMA samples, 64% (23/36) of breast tumors had strong to moderately high levels of Rac1 expression, whereas weak to no Rac1 expression was observed in 58% (19/33) of NT samples. The analysis also showed that 18% (6/33) of NT samples had strong to moderately high levels of Rac1 expression. Overall, IHC analysis indicates a significantly higher level of Rac1 expression in breast tumor samples compared to NT samples (p<0.001).
Figure 1.
Rac1 is overexpressed in breast cancer cells. (a) Upper panel: normal mammary epithelial cells (76N) and breast cancer cells of subtype luminal A (MCF-7 and T47D), and luminal B (BT-474 and ZR-75-1), triple-negative (MDA-MB-231 and MDA-MB-468) and HER2 positive (SkBr3, HCC1954, 21MT-1) were analyzed for Rac1 protein expression by Western blot analysis. As a protein loading control, the level of GAPDH in cell lysates was assessed. Lower panel: immunoblot densities of Rac1 and GAPDH were quantified using ImageJ analytical program (NIH) and relative Rac1 expression versus GAPDH determined. (b) Representative IHC analysis shows a distinct increase in immunostaining of Rac1 in malignant breast tumor tissues compared to normal breast tissues. (c) Box plot shows composite score of Rac1 expression in normal breast/benign tumor tissues (NT) and cancerous breast tissue (Cancer), analyzed by IHC.
Rac1 is activated in breast cancer cells responding to IR
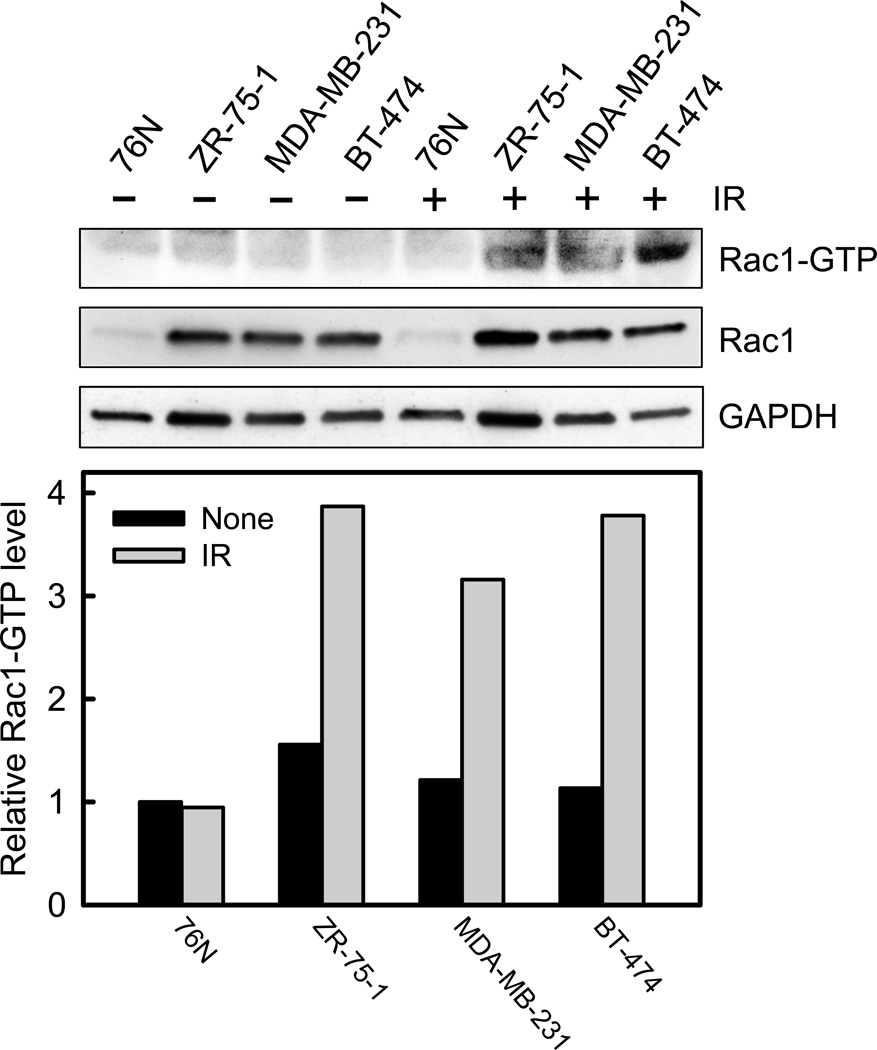
We have previously shown that IR activates Rac1 in MCF-7 cells.26 We therefore tested whether IR causes a similar effect on other normal and cancerous mammary cell lines. As shown in Figure 2, Rac1 activity (Rac1-GTP) increased approximately 4-fold in ZR-75-1, MDA-MB-231 and BT-474 breast cancer cells within 15 min following IR exposure. However, no increase in Rac1 activity was detected in 76N HME cells after IR.
Figure 2.
Rac1 activity is increased following irradiation in breast cancer cells compared to normal breast cells. Upper panel: indicated cells were harvested before and 15 min after IR (10-Gy) and analyzed for Rac1 activity (Rac1-GTP), as described in Materials and Methods. As controls, protein levels of Rac1 and GAPDH in cell lysates were assessed. Lower panel: immunoblot densities of Rac1-GTP and Rac1 total protein were quantified using ImageJ software and relative Rac1-GTP level versus Rac1 total protein level determined.
Rac1 expression is increased in the breast cancer cells that survived HFR
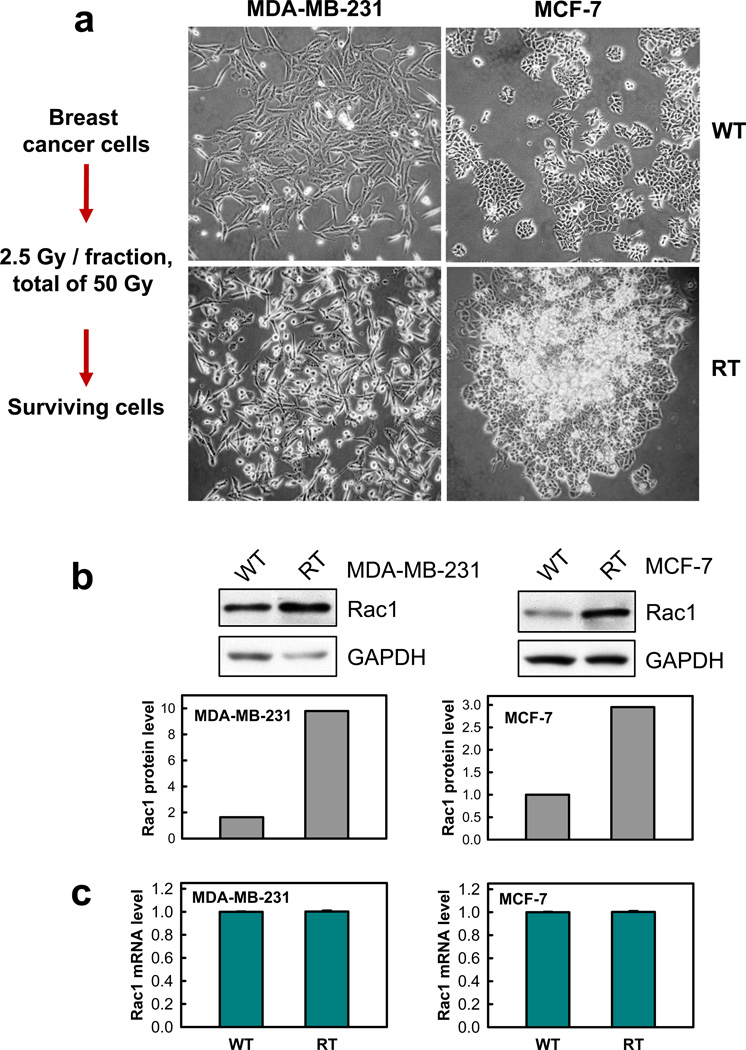
Since aberrant amplification/activation of Rac1 is associated with poor prognosis of breast cancer,10,40 we assessed the effect of HFR on Rac1 and its downstream pro-survival signaling pathways in breast cancer cells. For this study, MDA-MB-231 and MCF-7 cells were subjected to a clinical protocol of HFR (50-Gy in 2.5-Gy daily) and the surviving cells were selected following treatment (Figure 3a).
Figure 3.
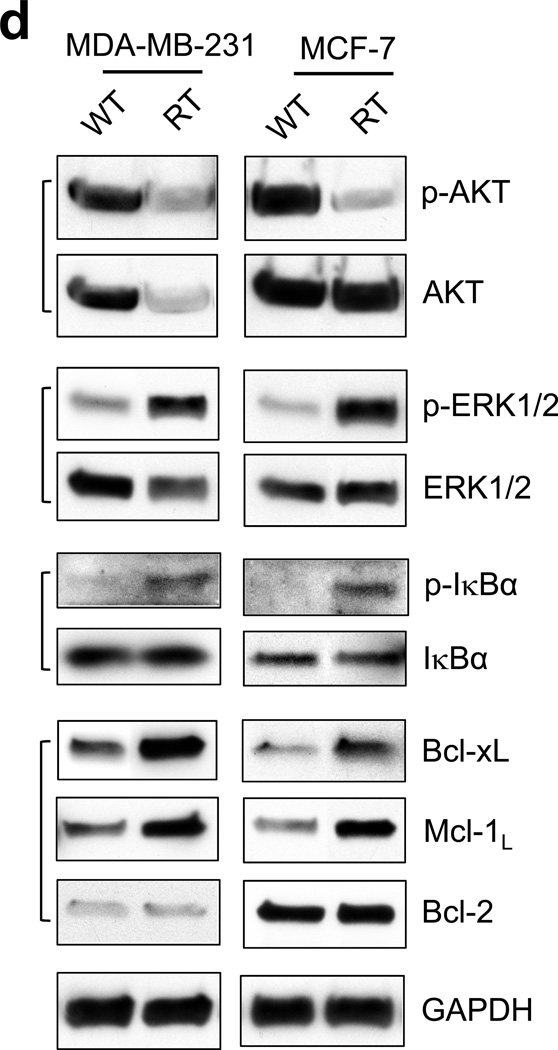
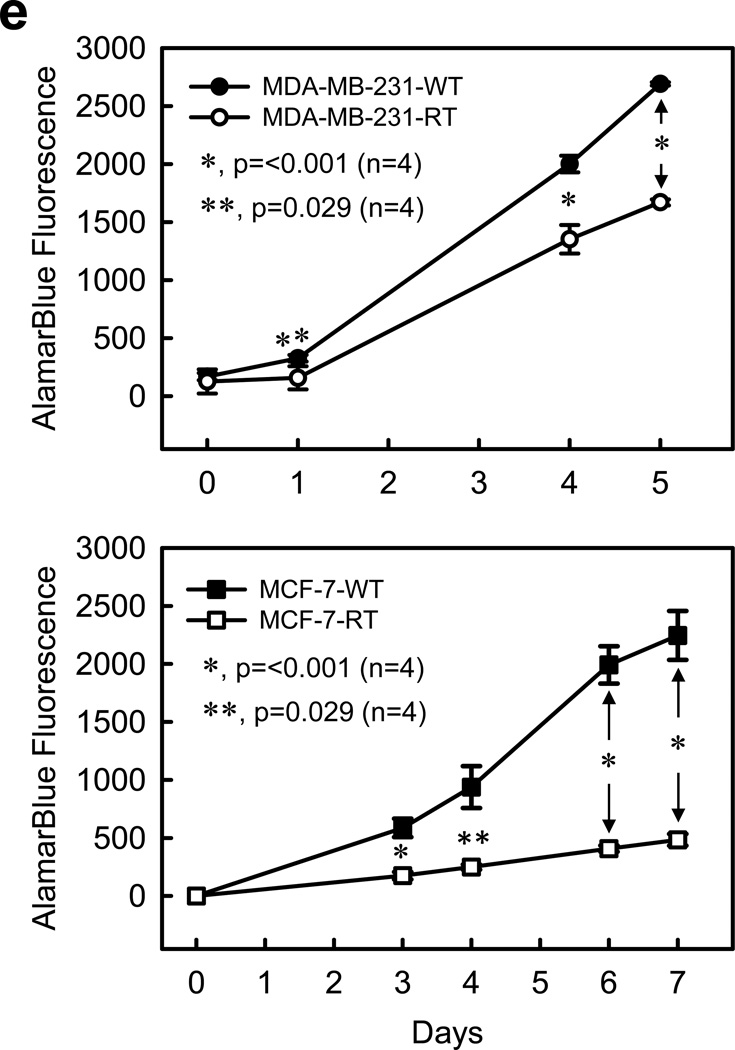
Breast cancer cells that survived a clinical dose of HFR exhibit different cell morphology and increased Rac1 protein expression compared to parental cells. (a) Left panel: procedure for selecting the breast cancer cells that survived clinical dose of HFR. Right panel: representative images of MCF-7 and MDA-MB-231 cells were obtained before (WT) and after (RT) HFR treatment. (b) Upper panel: indicated breast cancer cells were analyzed for protein levels of Rac1 and GAPDH by immunoblotting. Bottom panel: immunoblot densities of Rac1 and GAPDH proteins were quantified using ImageJ software and relative Rac1 expression versus GAPDH determined. (c) mRNA levels of Rac1 and GAPDH in the indicated cells were analyzed by RT-PCR and relative Rac1 mRNA level versus GAPDH mRNA determined. The results are shown as mean±s.d. of RT-PCR analyses in tetraplicate samples (n=4). (d) Log-phase growing MCF-7 and MDA-MB-231 cells treated without (WT) or with (RT) HFR were analyzed for phosphorylation and/or level of AKT, ERK1/2, IκBα, Bcl-xL, Mcl-1L and Bcl-2 by immunoblotting. GAPDH was assessed as a protein loading control. (e) The indicated cells were analyzed for growth kinetics using AlamarBlue assay, as described previously.59 At indicated time points, 10% of AlamarBlue reagent was added to the cells, incubated for 130 min and measured for fluorescence intensity using a SpectraMax M5 plate reader (Molecular Devices, Inc.) at ex/em 544/590 nm.
As shown in Figure 3a, MDA-MB-231 and MCF-7 cells that survived the HFR (RT) were morphologically different from their corresponding wild-type (WT) parental cells. These cells appeared rounded-up and less adherent to substratum compared to parental cells. We next analyzed the Rac1 protein level in the HFR-treated cells. As shown in Figure 3b, when compared to their parental cells, approximate 10- and 3-fold increases in Rac1 protein level were detected in MDA-MB-231-RT and MCF-7-RT cells, respectively.
To investigate the mechanism by which Rac1 expression is increased in the breast cancer cells treated with HFR, Rac1 mRNA levels in WT and RT cells were examined by RT-PCR. As shown in Figure 3c, both MDA-MB-231-RT and MCF-7-RT cells expressed the same level of Rac1 mRNA as their respective parental cells. Thus, the increase in Rac1 protein expression in the HFR-treated cells apparently involves a post-transcriptional mechanism.
Breast cancer cells that survived HFR exhibit altered pro-survival signaling properties compared to their parental cells
Since the breast cancer cells treated with HFR survived 50-Gy fractionated radiation delivered over 4 weeks, it is expected that substantial changes in biology occurred in these cells compared to their parental cells. Therefore, we compared activities and/or levels of several pro-survival signaling pathways in the HFR-treated cells with the corresponding parental cells. As shown in Figure 3d, AKT phosphorylation was unexpectedly decreased in both MDA-MB-231-RT and MCF-7-RT cells. Furthermore, AKT protein level was also markedly decreased in MDA-MB-231-RT cells compared to MDA-MB-231-WT cells, whereas it remained unchanged in MCF-7-RT cells relative to MCF-7-WT cells. In contrast, phosphorylation of ERK1/2 and IκB, indicative of activation of the ERK1/2 and NF-κB signaling pathways, were markedly increased in both MDA-MB-231-RT and MCF-7-RT cells relative to their respective parental cells (Figure 3d).
To determine the biological impact of the up-regulation in ERK1/2 and NF-κB signaling pathways in the HFR-treated breast cancer cells, we analyzed the downstream targets of these signaling pathways. As shown in Figure 3d, immunoblotting indicates notable increases in anti-apoptotic proteins Bcl-xL and Mcl-1L in both MDA-MB-231-RT and MCF-7-RT cells compared to their parental cells. In contrast, no difference in Bcl-2 protein levels were observed between the HFR-treated cells and untreated cells. These results indicate augmentation of pro-survival signaling activities in the breast cancer cells that survived HFR.
Since both ERK1/2 and NF-κB signalings can promote survival and proliferation, we compared growth kinetics of HFR-treated cells with their parental cells. Results in Figure 3e show that both MDA-MB-231-RT and MCF-7-RT cells grew significantly slower than their parental cells.
We next compared the HFR-treated cells and untreated cells for their cell cycle response to IR. As shown in Supplementary Figure S1, both MDA-MB-231-RT and MCF-7-RT cells, either with or without IR exposure, displayed different cell cycle profiles when compared to the corresponding parental cells. It is noticeable that MDA-MB-231-RT cells contained a larger proportion of 2N-DNA content cell population, indicative of G1 phase cells, than MDA-MB-231-WT cells, either with or without IR exposure (Supplementary Figure S1, upper panel). On the other hand, MCF-7-RT cells appeared to contain a larger proportion of 4N-DNA content cell population, indicative of cells in G2/M phase, compared to MCF-7-WT cells, with/without IR exposure (Supplementary Figure S1, lower panel). In response to IR exposure, both untreated and HFR-treated MDA-MB-231 and MCF-7 cells underwent dose-dependent G2/M cell cycle arrest, indicating the presence of a functional G2/M checkpoint in these cells.
Collectively, these results indicate that the HFR-selected cells adopted many changes in biological characteristics in order to survive the cytotoxicity induced by HFR.
Inhibition of Rac1 abrogates up-regulation of the pro-survival signaling pathways in the HFR-selected breast cancer cells
As shown in Figure 3b–d, HFR treatment of breast cancer cells resulted in an up-regulation of Rac1 expression and a concomitant increase in activities of ERK1/2 and NF-κB pro-survival signaling pathways. We therefore assessed the effect of Rac1 on these signalings.
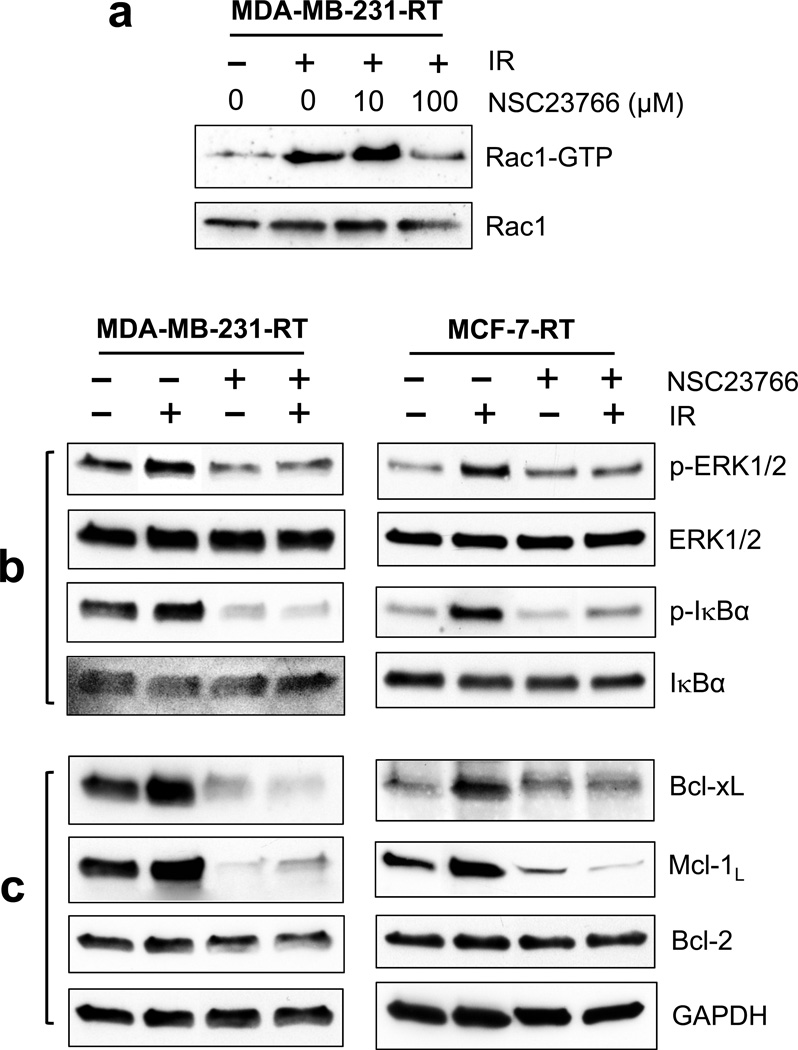
We first tested the effect of Rac1 inhibition by NSC23766 on these signaling pathways and their downstream targets, the Bcl-2 family members. We previously demonstrated that incubation with NSC23766 abrogates IR-induced Rac1 activation in MCF-7 cells.26 Similarly, incubation with NSC23766 abolished Rac1 activation after IR in MDA-MB-231-RT cells (Figure 4a).
Figure 4.
Effect of Rac1 inhibition on the key components of pro-survival signaling pathways in HFR-selected breast cancer cells treated with/without radiation. (a) MDA-MB-231-RT cells were incubated with NSC23766 at the indicated doses for 1 h and exposed to IR (10-Gy). The cells were then incubated for 15 min at 37°C and analyzed for Rac1 activity (Rac1-GTP) and protein level (Rac1). (b) and (c) cells were incubated for 1 h in the presence or absence of NSC23766 (100 µM) and treated with/without 10-Gy IR. After 2 h incubation following IR, the cells were analyzed for phosphorylation and level of ERK1/2 and IκBα (b). After 48 h post IR, the cells were analyzed for levels of Bcl-xL, Mcl-1L, Bcl-2 and GAPDH by immunoblotting (c).
As shown in Figure 4b, IR exposure induced noticeable increases in phosphorylation of ERK1/2 and IκBα in both MDA-MB-231-RT and MCF-7-RT cells, whereas the increases were completely abrogated by incubation with Rac1 inhibitor NSC23766. Rac1 inhibition by NSC23766 also diminished the basal phosphorylations of ERK1/2 and IκBα in un-irradiated MDA-MB-231-RT cells (Figure 4b).
We next examined the effect of Rac1 on the expression of Bcl-xL, Mcl-1L and Bcl-2, which are downstream targets of ERK1/2 and NF-κB pathways. As shown in Figure 4c, IR exposure resulted in increased protein expression of Bcl-xL and Mcl-1L in both MDA-MB-231-RT and MCF-7-RT cells. However, these IR effects were abolished in both cell lines by the presence of Rac1 inhibition. Rac1 inhibition also decreased the basal protein expression of Bcl-xL and Mcl-1L in un-irradiated MDA-MB-231-RT cells. In contrast, neither IR nor Rac1 inhibition had any detectable effect on the steady-state level of Bcl-2 protein in both MDA-MB-231-RT and MCF-7-RT cells.
In summary, these results suggest that Rac1 is required for the maintenance of hyperactive pro-survival pathways in the breast cancer cells selected by HFR.
Ectopic expression of dominant negative N17Rac1 mutant diminishes pro-survival signaling pathways in the HFR-selected breast cancer cells
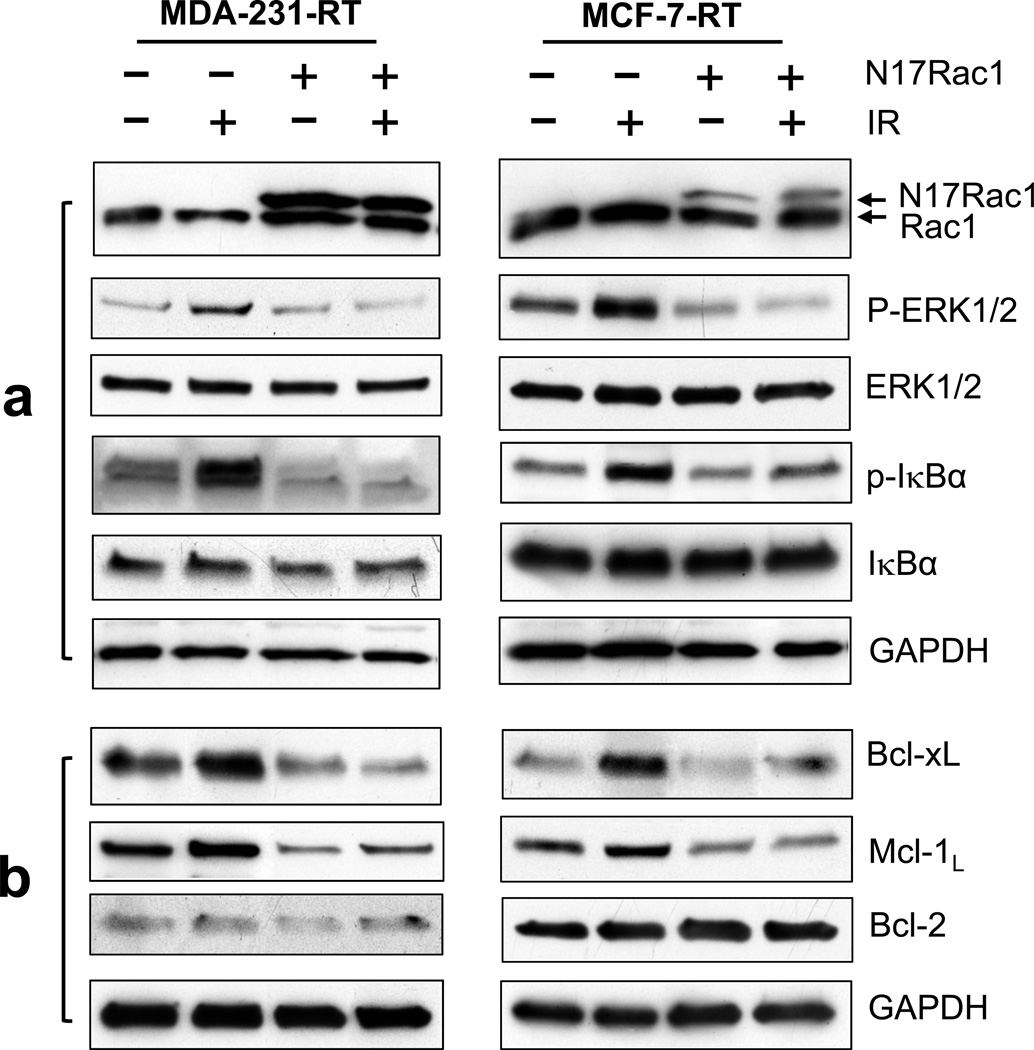
Using an adenoviral vector expressing N17Rac1, a dominant negative Rac1 mutant,41 we tested the effect of Rac1 on the pro-survival signaling pathways in MDA-MB-231-RT and MCF-7-RT cells in the presence or absence of IR. As shown in Figure 5a, immunoblotting detected the ectopically expressed N17Rac1, which migrates slightly slower than the endogenous wild-type Rac1.26,27 Results in Figure 5a showed that the IR-induced increases in ERK1/2 and IκBα phosphorylation in MDA-MB-231-RT and MCF-7-RT cells were completely blocked by the expression of N17Rac1 mutant.
Figure 5.
Effect of N17Rac1 dominant negative mutant on the key components of pro-survival signaling pathways in HFR-selected breast cancer cells treated with/without radiation. (a) Indicated cells were transduced for 24 h with adenoviral vector expressing N17Rac1 or control adenoviral vector (10 pfu/cell), treated with/without 10-Gy IR, incubated for 2 h and analyzed for phosphorylation and/or level of N17Rac1, ERK1/2 and IκB by immunoblotting. (b) At 48 h post IR, the cells were assessed for levels of Bcl-xL, Mcl-1L, Bcl-2 and GAPDH.
We next examined the effect of N17Rac1 mutant on downstream targets of ERK1/2 and NF-κB signaling pathways. As shown in Figure 5b, while IR exposure resulted in an increase in protein levels of Bcl-xL and Mcl-1L in the control-transduced MDA-MB-231-RT and MCF-7-RT cells, this effect was abrogated by the expression of N17Rac1. In addition, in the absence of IR, N17Rac1 expression also resulted in decreases in protein levels of Bcl-xL and Mcl-1L in MDA-MB-231-RT and MCF-7-RT cells (Figure 5b). Consistent with the effect observed with the Rac1 inhibitor NSC23766, N17Rac1 expression also had little effect on the steady-state level of Bcl-2.
Inhibition of Rac1 blocks survival of the HFR-selected breast cancer cells but not normal mammary epithelial cells
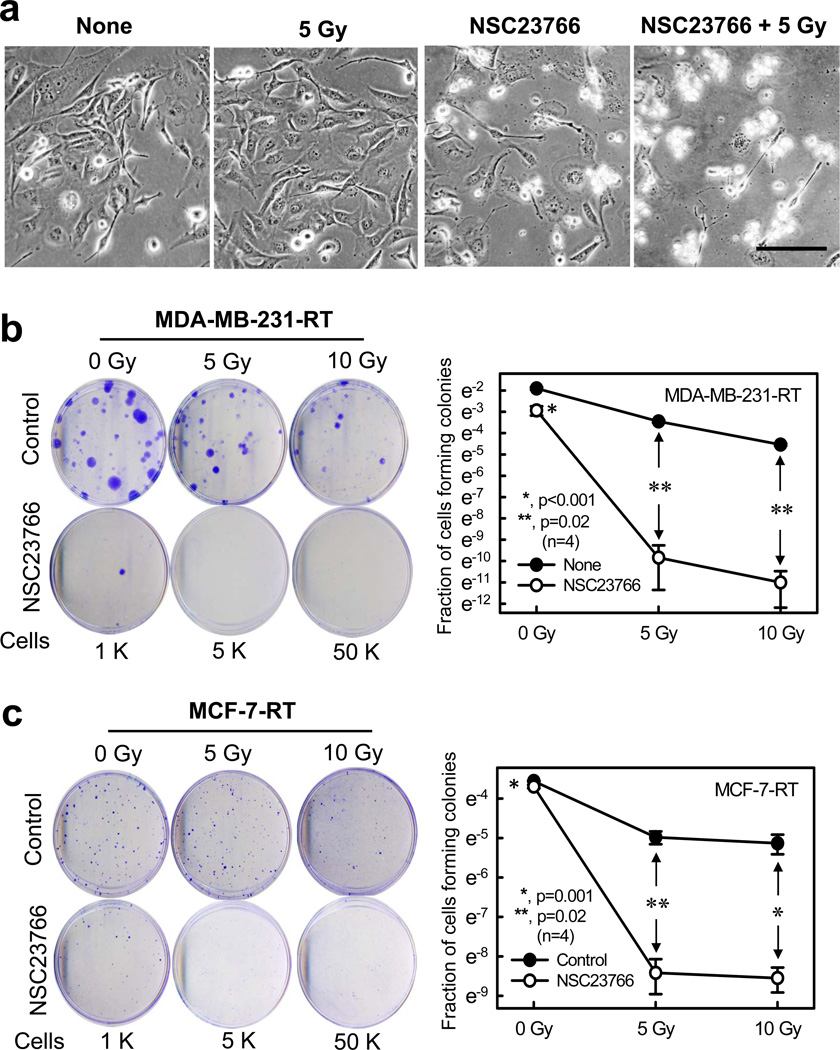
Since inhibition of Rac1 either by NSC23766 or by N17Rac1 mutant resulted in suppression of pro-survival signaling activities (Figure 4–5), we examined the effect of Rac1 inhibition on cell survival, in the presence or absence of IR. As shown in Figure 6a, while IR itself had little effect on the morphology of MDA-MB-231-RT cells, inhibition of Rac1 by NSC23766 caused >50% of cells to round-up and shrink, which is indicative of cytotoxicity.42 IR exposure in the presence of NSC23766 resulted in a further increase in cytotoxicity compared to the cells treated with NSC23766 alone, as >90% of the cells treated with both IR and NSC23766 rounded-up and detached from substratum (Figure 6a), indicating a synergistic cytotoxic effect.
Figure 6.
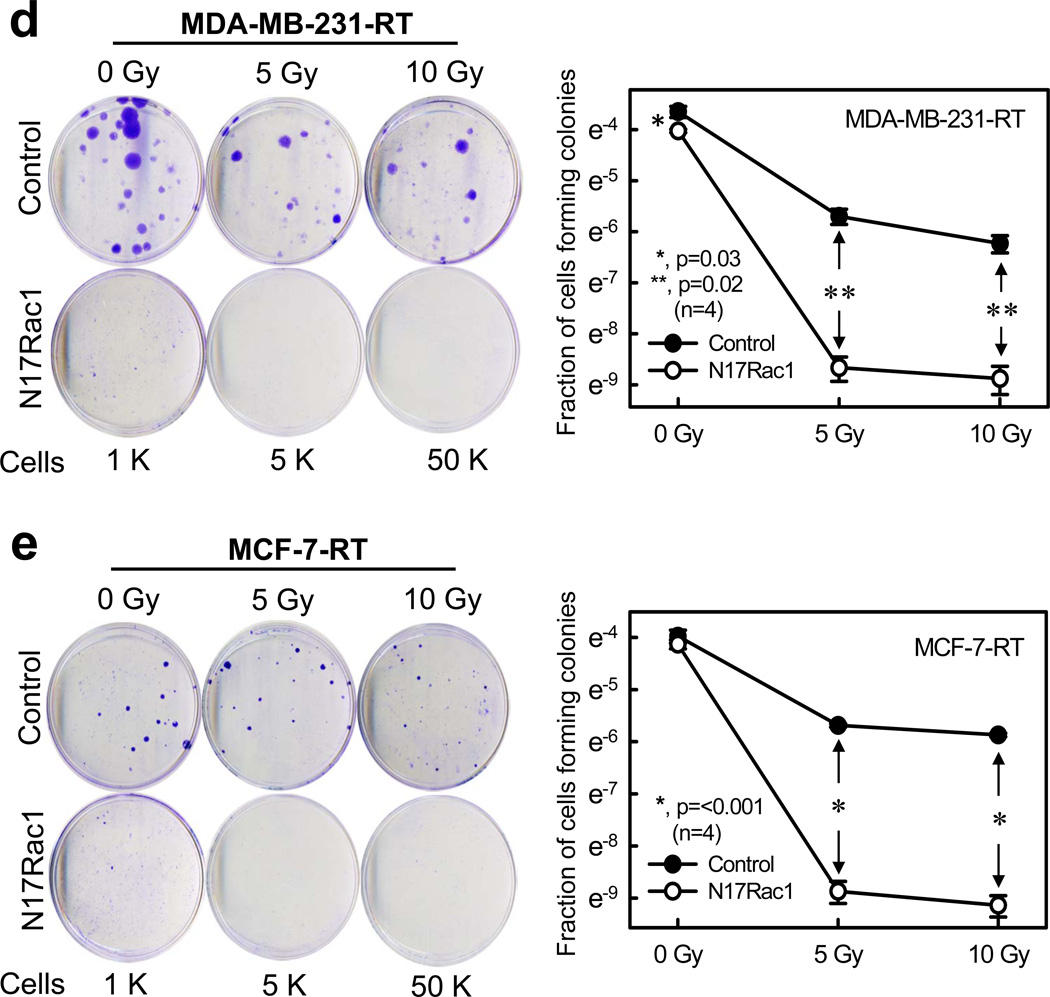
Inhibition of Rac1 abrogates clonogenic survival of the HFR-selected breast cancer cells. (a) MDA-MB-231-RT cells were incubated for 1 h in the presence or absence of NSC23766 (100 µM) and exposed to IR (5-Gy). The cells were incubated for 3 h post IR, washed, incubated for an additional 2 days and photographed using phase-contrast optics. The scale bar represents 100 µm. (b) MDA-MB-231-RT cells were incubated for 1 h with/without NSC23766 and exposed to increasing doses of IR. After 3 h incubation post IR, the cells were washed and incubated in growth medium for 2 weeks. Left panel: representative sample dishes from the clonogenic assay are shown. Right panel: number of colonies in the resulting samples were quantified using the ImageJ analytical program and the results are shown as mean±s.d. of two set of experiments in duplicate samples. (c) MCF-7-RT cells were treated as described above. Left panel: representative sample dishes from the clonogenic assay are shown. Right panel: numbers of colonies in the samples were quantified using the ImageJ analytical program and the results shown as mean±s.d. of two set of experiments in duplicate samples. (d) MDA-MB-231-RT cells were infected with Ad.N17Rac1 or Ad.Control (10 pfu/cell) for 24 h and exposed to IR (5 or 10-Gy) or left non-irradiated. The cells were incubated in growth medium for 14 days and assessed for amount of colonies. Left panel: representative sample dishes from the clonogenic assay. Right panel: number of colonies in the samples were quantified and the results shown as mean±s.d. of two set of experiments in duplicate samples. (e) MCF-7-RT cells were treated as described above. Left panel: representative sample dishes from the clonogenic assay. Right panel: numbers of colonies in the samples was quantified and the results shown as mean±s.d. of two set of experiments in duplicate samples.
We verified the cytotoxic effect of Rac1 inhibition using a clonogenic assay. As shown in Figure 6b–c, while IR exposure alone resulted in a modest dose-dependent decrease in clonogenic survival of MDA-MB-231-RT and MCF-7-RT cells, IR exposure in the presence of NSC23766 resulted in the striking eradication of clonogenic survival of these cells. In the presence of NSC23766, viability of MDA-MB-231-RT cells treated with 5- and 10-Gy of IR was respectively decreased by >6 orders of magnitude compared to their corresponding irradiated controls (Figure 6b, p=0.02, n=4). Similarly, MCF-7-RT cells treated with 5- and 10-Gy of IR in the presence of NSC23766 showed a decrease of clonogenic viability by >3 orders of magnitude compared to their corresponding irradiated controls (Figure 6c: 5-Gy, p=0.02, n=4; 10-Gy, p=0.001, n=4). Treatment with NSC23766 alone also resulted in a significant reduction in clonogenic survival of both MDA-MB-231-RT and MCF-7-RT cells relative to their respective untreated control cells (Figure 6b–c, 0-Gy, p<0.001, n=4).
We have previously shown that Rac1 inhibition alone has little effect on the survival of wild-type MCF-7 cells, while it synergized with IR, abrogating clonogenic survival of MCF-7-WT cells after IR.26 We therefore tested whether Rac1 inhibition affects clonogenic survival of MDA-MB-231-WT cells. As shown in Supplementary Figure S2, Rac1 inhibition by NSC23766 resulted in a noticeable but statistically insignificant reduction in the number of colonies formed by these cells. Consistent with the result obtained from MCF-7-WT cells,26 inhibition of Rac1 by NSC23766 also abrogated clonogenic survival of MDA-MB-231-WT cells after IR (Supplementary Figure S2). For a comparison, we also tested the effect of Rac1 inhibition on survival of 76N human normal mammary epithelial cells, which expressed lower Rac1 levels compared to MCF-7-WT and MDA-MB-231-WT cells (Figure 1a). Results in Supplementary Figure S3 showed that, while IR resulted in a dose-dependent decrease in survival of 76N cells, inhibition of Rac1 had no additional effect on the survival of these cells following IR.
To verify the effect of Rac1 inhibition on 76N cell survival after IR, we analyzed the phosphorylation of ERK1/2 and IκB in these cells. As shown in Supplementary Figure S4a, relative to positive control, 76N cell lysate incubated with GTPγs prior to Rac1 activity assay, 76N cells express very low Rac1 activity and this activity was inhibited by incubation with NSC23766. As shown in Supplementary Figure S4b, while IR exposure induced a subtle, if any, increase in phosphorylation of ERK1/2 and IκB in 76N cells, presence of NSC23766 had little effect on these phosphorylations. Incubation with NSC23766 might result in a slight increase in ERK1/2 phosphorylation in 76N cells (Supplementary Figure S4b, p-ERK1/2). We next assessed the effect of Rac1 inhibition on the expression of Bcl-xL, Mcl-1L and Bcl-2 proteins in the 76N cells treated with/without IR. Supplementary Figure S4c showed that, while Rac1 inhibition did not affect the protein expression of Bcl-xL and Bcl-2, it reduced Mcl-1L protein level in both irradiated and non-irradiated 76N cells.
Ectopic expression of N17Rac1 mutant inhibits clonogenic survival of the HFR-selected breast cancer cells
Using an adenoviral vector expressing N17Rac1 dominant negative mutant,41 we verified the cytotoxic effect of Rac1 inhibition on MDA-MB-231-RT and MCF-7-RT cells. As shown in Figure 6d–e, while Ad.Control-transduced cells showed a dose dependent decrease in clonogenic survival following IR exposure, transduction with Ad.N17Rac1 abolished clonogenic survival after IR in both HFR selected cell lines. As shown in Figure 6d, N17Rac1 expressing MDA-MB-231-RT cells exposed to 5- and 10-Gy of IR showed >3 orders of magnitude decrease in clonogenic survival compared to the corresponding irradiated controls (p=0.02, n=4). A similar result was also obtained using MCF-7-RT cells transduced with Ad.N17Rac1 (Figure 6e, p=<0.001, n=4). Additionally, ectopic N17Rac1 expression itself resulted in a reduction in clonogenicity in both lines of HFR-selected cells in the absence of IR. However, while this effect of N17Rac1 on un-irradiated MDA-MB-231-RT cells was statistically significant (Figure 6d, 0-Gy, p=0.029, n=4), its effect on MCF-7-RT cells was insignificant (Figure 6e, 0-Gy, p=0.343, n=4). It should be noted that the size of colonies formed by the N17Rac1 expressing cells, in both MDA-MB-231-RT or MCF-7-RT cells, were smaller than their corresponding control cells (Figure 6d–e)
Collectively, results of these studies suggest that Rac1-mediated pro-survival signalings are essential for the survival of breast cancer cells in response to HFR treatment. Additionally, the HFR-selected breast cancer cells, which express a higher level of Rac1 than their parental cells, are more sensitive to Rac1 inhibition than their parental controls, suggesting an addiction of the HFR-treated cells to Rac1 signaling for survival.
Rac1 inhibition induces apoptosis in the HFR-selected breast cancer cells
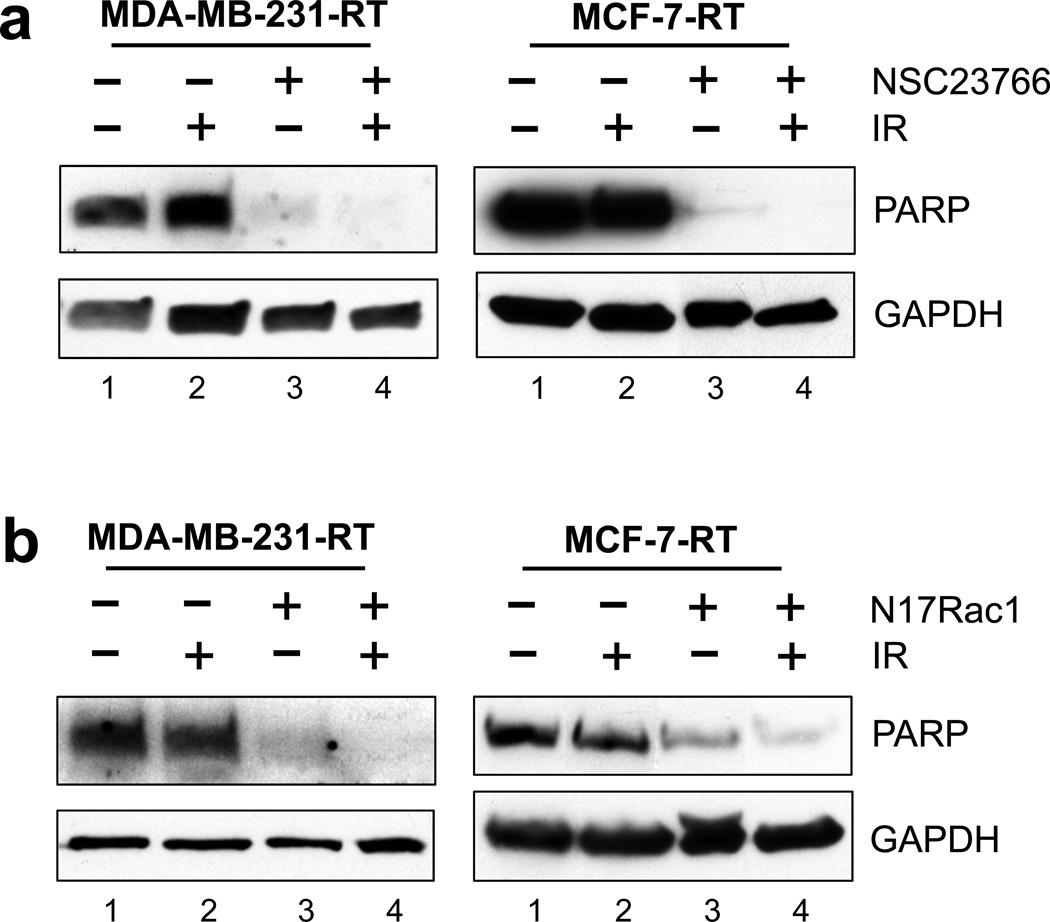
To investigate the mechanisms involved in the decrease in survival of the HFR-selected breast cancer cells by Rac1 inhibition, we assessed the integrity of PARP in these cells in the presence or absence of Rac1 inhibition. Cleavage of PARP is a hallmark of apoptosis and it occurs during the execution phase of programmed cell death.43
As shown in Figure 7a, in the absence of NSC23766, IR exposure had no detectable effect on the levels of intact PARP in both MDA-MB-231-RT and MCF-7-RT cells, determined at 48 h post IR. In contrast, inhibition of Rac1 by NSC23766 alone resulted in a marked decrease in the level of intact PARP in both MDA-MB-231-RT and MCF-7-RT cells. Additionally, IR exposure in the presence of Rac1 inhibition eliminated the residual intact PARP that was present in the cells treated with NSC23766 alone (Figure 7a, lane 4 vs. 3).
Figure 7.
Inhibition of Rac1 induces apoptosis in the HFR-survived breast cancer cells. (a) Indicated cells were treated with/without IR (10-Gy) in the presence or absence of NSC23766 (100 µM), incubated for 2 days and analyzed for PARP and GAPDH protein expression by immunoblotting. (b) Cells were infected with Ad.Rac1N17 or Ad.Control (10 pfu/cell) for 24 h and exposed to IR (10-Gy) or left untreated. After 48 h incubation, cells were analyzed for PARP and GAPDH protein expression by immunoblotting.
To verify the effect of Rac1 inhibition on PARP, cells were transduced with Ad.N17Rac1 or Ad.Control and exposed to IR. As shown in Figure 7b, transduction with Ad.N17Rac1 resulted in a marked decrease in levels of intact PARP in both MDA-MB-231-RT and MCF-7-RT cells compared to the control-transduced cells (lanes 3 vs. lane 1). Furthermore, IR exposure of N17Rac1 expressing cells resulted in a further decrease of intact PARP relative to the un-irradiated N17Rac1-tranduced cells (Figure 7b, lane 4 vs. 3).
Collectively, these studies indicate that inhibition of Rac1 in the HFR-selected breast cancer cells results in apoptosis induction.
DISCUSSION
Hyperactive Rac1 signaling has been implicated in cancer development and associated with poor prognosis.40,44,45 In this study, we observe a significant up-regulation of Rac1 protein expression in cancerous versus normal HME cells and tissues (see Figure 1). Furthermore, IR induces a rapid Rac1 activation in breast cancer cells and the breast cancer cells that survived the clinical HFR protocol reveal an increase in Rac1 protein expression, which is associated with an overall increase in pro-survival signaling activities (Figure 2–3). Additionally, the HFR-selected cells display very different cell cycle distribution profiles compared to their parental cells, with or without IR, and the changes are apparently cell type specific (see Supplementary Figure S1). Moreover, the HFR-selected cells grow significantly slower than parental cells (Figure 3e). These results indicate that the HFR-selected cells have gone through adaptive changes to resist the cytotoxic pressure generated by HFR and suggest an involvement of Rac1 in the survival of the breast cancer cells treated with HFR.
Activation of AKT, ERK1/2 and NF-κB signaling pathways following IR has been implicated in survival and radioresistance.28,46,47 Rac1 is required for PI3K/AKT activation by lipopolysaccharide and MEK/ERK activation by 12-O-tetradecanoylphorbol-13-acetate.48,49 In addition, Rac1 is essential for activation of the NF-κB signaling pathway during inflammation and in the initiation of colorectal cancer that carries mutations in APC.50,51 These studies suggest that AKT, ERK1/2 and NF-κB pro-survival signalings are downstream targets of Rac1. Results in this report demonstrate that HFR results in increased Rac1 expression and increased phosphorylation of ERK1/2 and IκBα in the HFR-selected cells (see Figure 3d). Furthermore, Rac1 inhibition abrogates the HFR-induced phosphorylation of ERK1/2 and IκBα, and the expression of downstream anti-apoptotic proteins Bcl-xL and Mcl-1L (see Figure 4–5). These results suggest an involvement of Rac1-regulated ERK1/2 and NF-κB signaling pathways in the survival of breast cancer cells following HFR.
Our previous studies indicate that inhibition of Rac1 in wild-type breast and pancreatic cancer cells blocks survival of irradiated cells, whereas it has little effect on the survival of non-irradiated cells.26,27 However, results of the current study indicate that the HFR-selected breast cancer cells exhibit sensitivity to Rac1 inhibition in the absence of IR (see Figure 6–7). Biochemical analyses indicate that Rac1 inhibition alone reduces phosphorylation of ERK1/2 and IκBα, as well as levels of anti-apoptotic proteins Bcl-xL and Mcl-1L in the non-irradiated HFR-selected cells (see Figure 4–5). In contrast, Rac1 inhibition by NSC23766 does not suppress the survival of normal 76N HME cells that express very little Rac1, whether with/without IR (Supplementary Figure S3). Consistently, inhibition of Rac1 also does not decrease phosphorylation of ERK1/2 or IκBα in 76N cells treated with/without IR. These results suggest a sequential increase in dependency on Rac1 for survival from normal HME cells → primary breast cancer cells → HFR-selected cells.
Both Bcl-2 and Bcl-xL have been shown to play critical roles in anticancer therapeutic resistance.52,53 While the two proteins share 45% sequence identity,54 studies demonstrate some differences in their anti-apoptotic functions responding to stimuli. For instance, Fiebig et al. show that Bcl-2 overexpression blocks the apoptosis induced by ceramide or thapsigargin, but has no effect on doxorubicin- or TNFα-induced apoptosis.54 On the other hand, Bcl-xL overexpression can block the apoptosis induced by all four stimuli.54 In the present study, we show that Bcl-xL expression is up-regulated following HFR, whereas Bcl-2 level is unaffected by HFR (Figure 3d). Consistently, Rac1 inhibition in the HFR-treated cells abolishes the up-regulation of Bcl-xL but had little effect on Bcl-2 protein level (see Figure 4–5). Another Bcl-2 family member Mcl-1L is also upregulated following HFR and this up-regulation is abrogated by Rac1 inhibition (see Figure 4–5). These results suggest a role for Rac1 in the regulation of Bcl-xL and Mcl-1L in response to HFR and implicate Bcl-xL and Mcl-1L in the survival of breast cancer cells after HFR.
It is noticed that IR induces an increase in Mcl-1L protein in both normal 76N and breast cancer cells, but only causes an increase in Bcl-xL protein in breast cancer cells (see Figure 4–5 and Supplementary Figure S4). These results suggest that different mechanisms are involved in the regulation of Mcl-1L and Bcl-xL expression in response to IR and additional genetic alterations may be required for the upregulation of Bcl-xL following IR. Furthermore, since Rac1 inhibition abolishes HFR or IR-induced Mcl-1L and Bcl-xL, Rac1 is apparently required for the upregulation of these proteins after HFR or IR. Future studies are needed to elucidate the molecular pathways that upregulate these anti-apoptotic molecules in response to HFR.
RT is a staple cancer treatment approach, whereas its efficacy is still limited by radioresistance. While RT induces cytotoxicity in cancer cells, it concurrently activates multiple pro-survival signaling pathways,3,4 which can act conjointly to reduce the magnitude of radiation-induced cytotoxicity and promote radioresistance. Results in this report provide evidence supporting a key role for Rac1 in the survival of breast cancer cells following HFR. Studies to explore the clinical potential of targeting Rac1 signaling for radiosensitization of cancer cells are currently underway and will be reported in due course.
MATERIALS AND METHODS
Cell culture and treatment
Human breast cancer cell lines 21MT-1, BT-474, HCC1954, MCF-7, MDA-MB-231, MDA-MB-468, SkBr3, T47D and ZR75-1 were recently obtained from ATCC. 76N is a line of human primary mammary epithelial cells immortalized using human telomerase.37 The 76N cell line is a kind gift from Dr. Vimla Band (University of Nebraska Medical Center). Cell culture and treatment are detailed in Supplementary Materials.
Rac1 inhibitor NSC2376655 was obtained from Tocris Bioscience and dissolved in DMSO.
Antibodies and immunoblotting
Antibodies are listed in Supplementary Materials. Immunoblotting was performed as described previously.22,56
Tissue microarray (TMA) and immunohistochemistry
The clinical specimen for IHC was a commercial TMA (BR723) (US Biomax). The TMA included 33 cases of human breast normal or benign tumor tissues (NT) and 36 cases of human malignant breast tumor tissues at various stages. The TMA was analyzed for Rac1 expression by IHC, as described previously,57,58 using a Rac1 specific antibody (PA1–091) at 1:400 dilution. The Rac1 immunostaining intensity was evaluated by a UNMC pathologist who was blinded to the clinical information.
Rac1 activity assay
Rac1 activity was assayed using a Rac1 assay kit (Upstate Biotechnology), as described in our previous studies.26,27
RT-PCR analysis
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) and analyzed for human Rac1 and GAPDH mRNA by real-time RT-PCR using the RT2 Real-Time™ Syber Green RT-PCR system (SuperArray Bioscience). The relative Rac1 mRNA expression was adjusted with GAPDH mRNA levels. The PCR primer sequences are included in Supplementary Materials.
Growth kinetics
Growth kinetics was determined using AlamarBlue assay (Life Technologies) as described previously.59
Cell cycle analysis
Fluorescence-activated cell sorting (FACS) was performed as described previously.22
Adenoviral vectors and adenoviral infections
Recombinant adenovirus N17Rac1 (Ad.N17Rac1) and dl312 (Ad.Control) were kindly provided by Dr. Toren Finkel (NIH, Bethesda, MD). In Ad.N17Rac1, the Rac1 cDNA contains a Ser to Asp substitution at position 17 and functions as a dominant negative mutant.60
Adenoviral infection was performed as described previously.61
Clonogenic survival assay
Clonogenic assay was performed and quantified as described previously.26,27,62
Statistical analysis
Composite-score was evaluated by multiplying the values of the IHC-staining intensity and the percent of immunoreactive cells.57,58 The data are shown as mean±s.d. P-values were determined using Student’s t-test and P≤0.05 was considered as significant. All statistical analyses were done using SigmaPlot.
Supplementary Material
Acknowledgments
We thank Dr. Vimla Band for 76N cells and Dr. Toren Finkel for Ad.N17Rac1 and Ad.dl312 adenoviral vectors. This work was supported, in parts, by Pilot Project Funding from 5P30GM106397 to YY and AN, and P50CA127297 to SKB, MO and YY.
Footnotes
CONFLICT OF INTERST
The authors declare no conflict of interest.
REFERENCES
- 1.DeSantis C, Siegel R, Jemal A. Cancer treatment and survivorship: facts and figures 2014–2015. American Cancer Society. 2014–2015:3–6. [Google Scholar]
- 2.Skvortsova I, Debbage P, Kumar V, Skvortsov S. Radiation resistance: Cancer stem cells (CSCs) and their enigmatic pro-survival signaling. Semin Cancer Biol. 2015;35:39–44. doi: 10.1016/j.semcancer.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 4.Hein AL, Ouellette MM, Yan Y. Radiation-induced signaling pathways that promote cancer cell survival (review) Int J Oncol. 2014;45:1813–1819. doi: 10.3892/ijo.2014.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Nag SA, Zhang R. Targeting the NFkappaB signaling pathways for breast cancer prevention and therapy. Curr Med Chem. 2015;22:264–289. doi: 10.2174/0929867321666141106124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen BPC, Li M, Asaithamby A. New insights into the roles of ATM and DNA-PKcs in the cellular response to oxidative stress. Cancer Letters. 2012;327:103–110. doi: 10.1016/j.canlet.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin AS. Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol Rev. 2012;246:327–345. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 9.Bosco E, Mulloy J, Zheng Y. Rac1 GTPase: A “Rac” of All Trades. Cell Mol Life Sci. 2009;66:370. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. Rac signaling in breast cancer: A tale of GEFs and GAPs. Cell Signal. 2012;24:353–362. doi: 10.1016/j.cellsig.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 12.Brown JH, Del Re DP, Sussman MA. The Rac and Rho Hall of Fame: A Decade of Hypertrophic Signaling Hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 13.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK Signaling Stimulates Extracellular Signal-Regulated Kinase (ERK) Activation by Regulating Formation of MEK1-ERK Complexes. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, et al. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 15.Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol. 2003;162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315(Pt 3):775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolias KF, Cantley LC, Carpenter CL. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 18.Murga C, Zohar M, Teramoto H, Gutkind JS. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene. 2002;21:207–216. doi: 10.1038/sj.onc.1205036. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–3216. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 20.Abbott DW, Holt JT. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274:2732–2742. doi: 10.1074/jbc.274.5.2732. [DOI] [PubMed] [Google Scholar]
- 21.Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, et al. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 22.Yan Y, Black CP, Cowan KH. Irradiation-induced G2/M checkpoint response requires ERK1/2 activation. Oncogene. 2007;26:4689–4698. doi: 10.1038/sj.onc.1210268. [DOI] [PubMed] [Google Scholar]
- 23.Toulany M, Lee K-J, Fattah KR, Lin Y-F, Fehrenbacher B, Schaller M, et al. Akt Promotes Post-Irradiation Survival of Human Tumor Cells through Initiation, Progression, and Termination of DNA-PKcs-Dependent DNA Double-Strand Break Repair. Mol Cancer Res. 2012;10:945–957. doi: 10.1158/1541-7786.MCR-11-0592. [DOI] [PubMed] [Google Scholar]
- 24.Sahlberg SH, Gustafsson AS, Pendekanti PN, Glimelius B, Stenerlow B. The influence of AKT isoforms on radiation sensitivity and DNA repair in colon cancer cell lines. Tumour Biol. 2014;35:3525–3534. doi: 10.1007/s13277-013-1465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimura T, Kakuda S, Ochiai Y, Kuwahara Y, Takai Y, Fukumoto M. Targeting the AKT/GSK3β/Cyclin D1/Cdk4 Survival Signaling Pathway for Eradication of Tumor Radioresistance Acquired by Fractionated Radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:540–548. doi: 10.1016/j.ijrobp.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 26.Yan Y, Greer PM, Cao PT, Kolb RH, Cowan KH. RAC1 GTPase plays an important role in gamma-irradiation induced G2/M checkpoint activation. Breast Cancer Res. 2012;14:R60. doi: 10.1186/bcr3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan Y, Hein AL, Etekpo A, Burchett KM, Lin C, Enke CA, et al. Inhibition of RAC1 GTPase sensitizes pancreatic cancer cells to gamma-irradiation. Oncotarget. 2014;5:10251–10270. doi: 10.18632/oncotarget.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magné N, Toillon R-A, Bottero V, Didelot C, Houtte PV, Gérard J-P, et al. NF-κB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Letters. 2006;231:158–168. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Bid HK, Roberts RD, Manchanda PK, Houghton PJ. RAC1: An Emerging Therapeutic Option for Targeting Cancer Angiogenesis and Metastasis. Mol Cancer Ther. 2013;12:1925–1934. doi: 10.1158/1535-7163.MCT-13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wetterskog D, Shiu KK, Chong I, Meijer T, Mackay A, Lambros M, et al. Identification of novel determinants of resistance to lapatinib in ERBB2-amplified cancers. Oncogene. 2014;33:966–976. doi: 10.1038/onc.2013.41. [DOI] [PubMed] [Google Scholar]
- 31.Skvortsov S, Dudás J, Eichberger P, Witsch-Baumgartner M, Loeffler-Ragg J, Pritz C, et al. Rac1 as a potential therapeutic target for chemo-radioresistant head and neck squamous cell carcinomas (HNSCC) Br J Cancer. 2014;110:2677–2687. doi: 10.1038/bjc.2014.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold CR, Abdelmoez A, Thurner G, Debbage P, Lukas P, Skvortsov S, et al. Rac1 as a multifunctional therapeutic target to prevent and combat cancer metastasis. Oncoscience. 2014;1:513–521. doi: 10.18632/oncoscience.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dokmanovic M, Hirsch DS, Shen Y, Wu WJ. Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther. 2009;8:1557–1569. doi: 10.1158/1535-7163.MCT-09-0140. [DOI] [PubMed] [Google Scholar]
- 34.Sooman L, Ekman S, Andersson C, Kultima H, Isaksson A, Johansson F, et al. Synergistic interactions between camptothecin and EGFR or RAC1 inhibitors and between imatinib and Notch signaling or RAC1 inhibitors in glioblastoma cell lines. Cancer Chemother Pharmacol. 2013;72:329–340. doi: 10.1007/s00280-013-2197-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Wang Z, Jiang Y, Yang C. Inactivation of Rac1 reduces Trastuzumab resistance in PTEN deficient and insulin-like growth factor I receptor overexpressing human breast cancer SKBR3 cells. Cancer Letters. 2011;313:54–63. doi: 10.1016/j.canlet.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Espinha G, Osaki J, Magalhaes Y, Forti F. Rac1 GTPase-deficient HeLa cells present reduced DNA repair, proliferation, and survival under UV or gamma irradiation. Mol Cell Biochem. 2015;404:281–297. doi: 10.1007/s11010-015-2388-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhao X, Malhotra GK, Lele SM, Lele MS, West WW, Eudy JD, et al. Telomerase-immortalized human mammary stem/progenitor cells with ability to self-renew and differentiate. Proc Natl Acad Sci U S A. 2010;107:14146–14151. doi: 10.1073/pnas.1009030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subik K, Lee JF, Baxter L, Strzepek T, Costello D, Crowley P, et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer (Auckl) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 40.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, et al. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 41.Moore KA, Sethi R, Doanes AM, Johnson TM, Pracyk JB, Kirby M, et al. Rac1 is required for cell proliferation and G2/M progression. Biochem J. 1997;326(Pt 1):17–20. doi: 10.1042/bj3260017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 44.Leve F, Morgado-Díaz JA. Rho GTPase signaling in the development of colorectal cancer. J Cell Biochem. 2012;113:2549–2559. doi: 10.1002/jcb.24153. [DOI] [PubMed] [Google Scholar]
- 45.Kunz M. Oncogenes in melanoma: An update. Eur J Cell Biol. 2014;93:1–10. doi: 10.1016/j.ejcb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Bussink J, van der Kogel AJ, Kaanders JHAM. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 47.Munshi A, Ramesh R. Mitogen-Activated Protein Kinases and Their Role in Radiation Response. Genes Cancer. 2013;4:401–408. doi: 10.1177/1947601913485414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang D, Li D, Cao L, Wang L, Zhu S, Xu T, et al. Positive Feedback Regulation of Proliferation in Vascular Smooth Muscle Cells Stimulated by Lipopolysaccharide Is Mediated through the TLR 4/Rac1/Akt Pathway. PLoS One. 2014;9:e92398. doi: 10.1371/journal.pone.0092398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Pedersen E, Basse A, Lefever T, Peyrollier K, Kapoor S, et al. Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo. Oncogene. 2010;29:3362–3373. doi: 10.1038/onc.2010.95. [DOI] [PubMed] [Google Scholar]
- 50.Cuadrado A, Martín-Moldes Z, Ye J, Lastres-Becker I. Transcription Factors NRF2 and NF-κB Are Coordinated Effectors of the Rho Family, GTP-binding Protein RAC1 during Inflammation. J Biol Chem. 2014;289:15244–15258. doi: 10.1074/jbc.M113.540633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myant Kevin B, Cammareri P, McGhee Ewan J, Ridgway Rachel A, Huels David J, Cordero Julia B, et al. ROS Production and NF-κB Activation Triggered by RAC1 Facilitate WNT-Driven Intestinal Stem Cell Proliferation and Colorectal Cancer Initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opferman JT. Attacking cancer’s Achilles heel: antagonism of anti-apoptotic BCL-2 family members. FEBS J. 2015 doi: 10.1111/febs.13472. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hata AN, Engelman JA, Faber AC. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6:213. doi: 10.1186/1471-2407-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan Y, Spieker RS, Kim M, Stoeger SM, Cowan KH. BRCA1-mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene. 2005;24:3285–3296. doi: 10.1038/sj.onc.1208492. [DOI] [PubMed] [Google Scholar]
- 57.Shiao YH, Palli D, Caporaso NE, Alvord WG, Amorosi A, Nesi G, et al. Genetic and immunohistochemical analyses of p53 independently predict regional metastasis of gastric cancers. Cancer Epidemiol Biomarkers Prev. 2000;9:631–633. [PubMed] [Google Scholar]
- 58.Charafe-Jauffret E, Tarpin C, Bardou VJ, Bertucci F, Ginestier C, Braud AC, et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an ‘inflammatory signature’. J Pathol. 2004;202:265–273. doi: 10.1002/path.1515. [DOI] [PubMed] [Google Scholar]
- 59.Pessetto ZY, Yan Y, Bessho T, Natarajan A. Inhibition of BRCT(BRCA1)-phosphoprotein interaction enhances the cytotoxic effect of olaparib in breast cancer cells: a proof of concept study for synthetic lethal therapeutic option. Breast Cancer Res Treat. 2012;134:511–517. doi: 10.1007/s10549-012-2079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 61.Yan Y, Haas JP, Kim M, Sgagias MK, Cowan KH. BRCA1-induced apoptosis involves inactivation of ERK1/2 activities. J Biol Chem. 2002;277:33422–33430. doi: 10.1074/jbc.M201147200. [DOI] [PubMed] [Google Scholar]
- 62.Cai Z, Chattopadhyay N, Liu WJ, Chan C, Pignol J-P, Reilly RM. Optimized digital counting colonies of clonogenic assays using ImageJ software and customized macros: Comparison with manual counting. Int J Radiat Biol. 2011;87:1135–1146. doi: 10.3109/09553002.2011.622033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.