Abstract
We previously found that body mass index (BMI) strongly predicted response to ketamine. Adipokines have a key role in metabolism (including BMI). They directly regulate inflammation and neuroplasticity pathways and also influence insulin sensitivity, bone metabolism and sympathetic outflow; all of these have been implicated in mood disorders. Here, we sought to examine the role of three key adipokines—adiponectin, resistin and leptin—as potential predictors of response to ketamine or as possible transducers of its therapeutic effects. Eighty treatment-resistant subjects who met DSM-IV criteria for either major depressive disorder (MDD) or bipolar disorder I/II and who were currently experiencing a major depressive episode received a single ketamine infusion (0.5 mg kg −1 for 40 min). Plasma adipokine levels were measured at three time points (pre-infusion baseline, 230 min post infusion and day 1 post infusion). Overall improvement and response were assessed using percent change from baseline on the Montgomery–Asberg Depression Rating Scale and the Hamilton Depression Rating Scale. Lower baseline levels of adiponectin significantly predicted ketamine’s antidepressant efficacy, suggesting an adverse metabolic state. Because adiponectin significantly improves insulin sensitivity and has potent anti-inflammatory effects, this finding suggests that specific systemic abnormalities might predict positive response to ketamine. A ketamine-induced decrease in resistin was also observed; because resistin is a potent pro-inflammatory compound, this decrease suggests that ketamine’s anti-inflammatory effects may be transduced, in part, by its impact on resistin. Overall, the findings suggest that adipokines may either predict response to ketamine or have a role in its possible therapeutic effects.
INTRODUCTION
Mood disorders are associated with a pro-inflammatory state, insulin resistance, increased sympathetic outflow and diminished neuroplasticity in the central nervous system (CNS).1 Interestingly, adipokines influence all these processes, having important roles in inflammation, insulin sensitivity and sympathetic outflow via their actions at central loci.2,3 Their plasma and cerebrospinal fluid levels are significantly correlated, so that their presence in plasma may transduce CNS effects.4,5 It should be noted that adipokines are also expressed in the CNS.6 Our group recently demonstrated that patients with the highest body mass index (BMI) responded best to the N-methyl-D-aspartate antagonist ketamine.7 Ketamine is rapidly distributed to peripheral tissues and crosses the blood–brain barrier (BBB). Ketamine also displays high lipid solubility, and its low plasma protein binding facilitates its rapid transfer across the BBB, which directly affects brain lipids.8,9
Here, we focus on three key adipokines: adiponectin, resistin and leptin. Adiponectin significantly promotes insulin sensitivity, counters inflammation and atherosclerosis, inhibits bone resorption and bone loss, gains access to the brain and promotes adaptive neuroplasticity in the CNS.3,10–15 In the periphery, adiponectin is secreted entirely by adipocytes and potently promotes healthy insulin sensitivity. Animals and humans with a congenital lipodystrophy or fat deficiency are still insulin resistant even in the absence of fat, because insulin resistance is pronounced in the absence of fat-derived adiponectin;16 adiponectin administration in the context of lipodystrophy reverses insulin resistance.17 Adiponectin in the periphery is active at many sites, notably the pancreas, where it promotes insulin sensitivity,3,18 and it has anti-inflammatory effects at many sites as well.19 Low adiponectin levels within the normal range are associated with an increased incidence of coronary artery disease and type II diabetes. There is a continuous gradient, so that low–normal adiponectin levels are associated with increased coronary and metabolic disease, whereas their incidences decrease with progressively higher levels of adiponectin.13,15
Because cerebrospinal fluid and plasma levels of adiponectin are significantly correlated, adiponectin is likely to gain access to the CNS.4 BBB adiponectin receptors presumably facilitate this transport. Adiponectin also exerts insulin-sensitizing effects in the CNS and, as noted above, adiponectin in the CNS promotes adaptive neuroplasticity.20 We now know that adiponectin originating in plasma and transported to the cerebrospinal fluid exerts CNS effects,21 and that adiponectin is also produced locally in the CNS.6,22 A previous study found that adiponectin levels were reduced around the clock in patients with major depressive disorder (MDD),23 and pre-clinical data also positively link adiponectin levels to depression. In experimental animals, reduced adiponectin levels caused by haploinsufficiency led to increased social aversion, anhedonia and learned helplessness, and caused impaired feedback on the hypothalamic–pituitary–adrenal axis.24 In addition, intracerebroventricular injection of an adiponectin neutralizing antibody was found to precipitate depressive-like behaviors.25 On the other hand, central adiponectin infusion produced antidepressant-like behavioral effects in normal-weight mice as well as in diet-induced obese mice.26–28
The adipokine resistin is among the most potent pro-inflammatory compounds.29,30 Higher levels are associated with multiple inflammatory diseases including rheumatoid arthritis, systemic lupus erythematosis, autoimmune thyroid disease, inflammatory bowel disease and others.29,31 A related phenomenon is the fact that resistin potently stimulates tumor necrosis factor-α (TNF-α),32 and ketamine has anti-inflammatory properties, particularly with regard to TNF-α-induced inflammation.33 Moreover, TNF-α is consistently elevated in a subgroup of patients with depressive illness.34 Studies in patients with depressive illness found that patients with atypical depression had depressive features that correlated positively with resistin, and that resistin levels fell significantly after antidepressant treatment. In addition, resistin was originally thought to promote insulin resistance in humans. This effect, however, was found to be pronounced in mice but minimal in human beings.2 Like adiponectin, resistin is produced and released by visceral fat. Its levels correlate positively with BMI. This correlation with BMI is likely to reflect higher levels of resistin.
The adipokine leptin is synthesized by adipocytes and transported across the BBB to regulate appetite, thermogenesis and energy homeostasis, and to promote adaptive neuroplasticity.2,35 Leptin’s effects seem salutary in some circumstances and damaging in others. For instance, it promotes healthy neuroplasticity36 and exerts antidepressant responses in animal models;28,37 conversely, it also activates the sympathetic nervous system,38 promotes bone resorption by a CNS mechanism,39 and results in leptin resistance in key tissues such as the β-cells of the pancreas, thus leading to insulin resistance. Leptin has multiple other effects depending on the prevailing metabolic and neuronal milieu. Recent data indicate that leptin acts in the CNS as a neural growth factor in the hypothalamus, rapidly inducing neuronal arborizations35,36,40 that occurred well before the feeding effects of the administered leptin.40 In addition, pre-clinical data suggest that leptin interacts with the glutamatergic system in a manner relevant to depressive illness and, hence, to ketamine’s therapeutic effects. For instance, conditional knock-out mice with deletion of the leptin receptor in glutamatergic neurons in forebrain structures including the hippocampus and medial prefrontal cortex displayed depressive-like behaviors.41 These included anhedonia, behavioral despair, enhanced learned helplessness and social withdrawal,41 and were blocked by an N-methyl-D-aspartate antagonist.41 These findings suggest that leptin receptor signaling has an important role in forebrain glutamatergic neurons that regulate depression-related behaviors and underscores the possible connection between leptin receptor signaling and N-methyl-D-aspartate receptors in modulating depression-related behaviors.41 Interestingly, experimental animals whose response to severe stressors consisted of depressive-like behaviors remitted after leptin administration.37 In addition, Eikelis and colleagues38 demonstrated that CNS leptin secretion in patients with depressive illness was reduced compared with levels in healthy controls. Thus, a pharmacological intervention that promotes leptin secretion could potentially be effective in treating depression.
It should also be noted that specific changes have been observed in lipid profiles associated with specific diagnoses and mood state in individuals with mood disorders. A large (n = 2305) cross-sectional study showed that patients with bipolar depression had higher triglycerides and low-density lipoprotein levels, as well as lower high-density lipoprotein levels, than individuals with MDD,42 supporting the role of diagnostic- and state-specific changes in lipids in individuals with mood disorders.
As noted above, higher leptin levels activate the sympathetic nervous system, have an adverse effect on bone mineral density, and can result in leptin resistance in the pancreas, exacerbating the degree of insulin resistance. In light of the above observations, this study attempted to address the following questions. First, do baseline levels of adipokines predict a positive response to the rapid antidepressant effects of ketamine? Second, if so, does a baseline index indicating a more adverse metabolic state (for example, high resistin levels) predict response to ketamine? This would be analogous to the observation that antidepressants are most effective in the most severely depressed patients.43 Third, does ketamine rapidly alter plasma adipokine levels? Fourth, do these effects differ in patients with bipolar disorder (BD) versus those with MDD? Finally, what are the potential clinical implications of these findings?
MATERIALS AND METHODS
Patient selection, study design and outcome measures
Eighty inpatients (males and females, ages 18–65 years) with either treatment-resistant MDD (n = 49) or BD-I/II (n = 31) who were currently experiencing a major depressive episode lasting at least 4 weeks were included in this study, which combined data from three different ketamine trials. The clinical trials identifier NCT00088699 includes these three substudies investigating: (1) ketamine in bipolar depression, (2) ketamine and riluzole and (3) ketamine’s mechanism of action. The design was similar to that of the original studies from which these data were obtained.44,45 Each study was a double-blind, randomized, placebo-controlled, cross-over trial assessing the antidepressant efficacy of ketamine for treatment-resistant depression. One study had an initial open-label phase (up to 230 min post infusion).44 Treatment resistance was defined as a current or past history of lack of response to at least two adequate antidepressant or neuromodulatory (including electroconvulsive therapy) trials as defined by our modified version of the Antidepressant Treatment History Form.46 All patients had no diagnosis of alcohol or substance abuse or dependence in the past 90 days as determined by the Structured Clinical Interview for DSM-IV-TR. All participants had a Montgomery–Asberg Depression Rating Scale (MADRS) score of at least 20 at baseline, were unmedicated for at least 2 weeks (5 weeks for fluoxetine) before their first infusion (except for 15 individuals with BD-I who were receiving lithium or valproate), and were in good medical health, as determined by medical history, physical examination, and routine blood and urine laboratory tests. All patients had similar diets during the studies. The studies were approved by the NIH Combined Neuroscience institutional review board and written informed consent was provided by all participants before study entry.
Patients received a single infusion of ketamine hydrochloride (0.5 mg kg −1) over 40 min. Here we report results from 60 min prior to infusion (baseline), 230 min post infusion and day 1 post infusion, as >88% of all those who respond to ketamine do so within 230 min post infusion.47 Ratings included the MADRS and the 17-item Hamilton Depression Rating Scale (HAM-D), both of which were administered at the same time points as those used for peripheral blood collection. Adipokine levels were examined using samples only through day 1, given that maximum antidepressant response to ketamine generally reaches its maximum by that point. Overall response to ketamine was defined by percent change in MADRS and HAM-D score compared with baseline; negative values reflect a reduction in depressive symptoms. Response was defined as a 50% or greater decrease in depression rating scale scores from baseline, and remission was defined as scoring lower than an 8 on the MADRS.
An additional model examined the role of depression subtype on change in adipokine levels following ketamine. Patients were classified as having either ‘melancholic depression’, ‘atypical depression’ or ‘neither’ (based on the clinician’s checklist).
Adipokine measurements
Whole-blood samples were collected using the Vacutainer system. Baseline samples were obtained at 0800 hours for all patients. Samples were centrifuged at 3000 r.p.m. at 4 °C for 10 min and stored at − 80 °C until assay performance. Circulating plasma levels of adiponectin, leptin and resistin were measured using the high-sensitivity multiplex Luminex immunoassay (xMAP Technology, Austin, TX, USA) and fluorescently color-coded magnetic microsphere beads (R&D Systems; Minneapolis, MN, USA) according to the manufacturer’s instructions. Samples were diluted 1:10, measured in duplicate and blinded to clinical information. The standard cocktail was created as a fourfold dilution series to concentrations ranging from 117.44 to 481 020 pg ml−1 for adiponectin, 25 to 103 080 pg ml −1 for leptin and 2.47 to 10 100 pg ml −1 for resistin. After the addition of a biotinylated antibody cocktail and streptavidin-PE, levels of all analytes were determined with a Bio-Plex Magpix Multiplex Reader (Bio-Rad; Hercules, CA, USA). Concentration values were calculated automatically with Bio-Plex Manager MP Software (Philadelphia, PA, USA) by generating a five parameter logistic curve-fit standard curve for each analyte.
Statistics
Kolmogorov–Smirnov tests indicated that peripheral adiponectin was normally distributed. Leptin and resistin were not normally distributed, so they were transformed using a natural log.
Linear mixed models were used to examine changes in adipokines. Time was a fixed factor with maximum likelihood estimates and a compound symmetry covariance structure for resistin and leptin but a scaled identity covariance structure for adiponectin. Post hoc t-tests compared values using all pairwise comparisons with a Bonferroni correction.
Bivariate associations were assessed with Pearson correlation. Correlations examined baseline demographic factors, baseline adipokine levels and antidepressant response as well as changes in adipokines and depression rating scale scores at 230 min and day 1 post infusion.
All tests were two-tailed with significance set at P<0.05. A Bonferroni correction was applied for the number of adipokines examined (three) with each type of analysis, so a cutoff of P<0.0167 was used. Data are presented as mean ± standard deviation. All statistical analyses were completed using IBM SPSS Version 21 (Armonk, NY, USA) or GraphPad 6 Software (San Diego, CA, USA).
RESULTS
Clinical and demographic characteristics for all 80 subjects are summarized in Table 1. Baseline adiponectin levels were related to age (r = 0.29, P = 0.003), baseline leptin levels were related to BMI (r = 0.52, P<0.001) and gender (r = 0.42, P<0.001), and baseline resistin levels were not related to any variables of interest.
Table 1.
Demographic and clinical information (n =80)
| Total (n =80)
|
Bipolar (n =31)
|
MDD (n = 49)
|
P-value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender (female) | 41 | 51 | 20 | 65 | 21 | 43 | 0.07 |
| Family history of mood disoders | 69 | 86 | 28 | 90 | 41 | 84 | 0.52 |
| Mean | S.d. | Mean | S.d. | Mean | S.d. | P-value | |
|
| |||||||
| Age | 44.3 | 12.1 | 46.1 | 11.0 | 43.1 | 12.8 | 0.28 |
| Age of onset (years) | 18.5 | 10.2 | 17.2 | 7.3 | 19.4 | 11.6 | 0.34 |
| Current episode (months) | 44.9 | 90.5 | 18.4 | 21.0 | 61.8 | 111.9 | 0.04 |
| Duration of Illness (years) | 25.8 | 12.4 | 28.9 | 10.6 | 23.9 | 13.2 | 0.08 |
| Previous episodes | 32.7 | 40.1 | 43.2 | 37.7 | 25.8 | 40.5 | 0.07 |
| BMI | 29.2 | 5.8 | 30.1 | 6.3 | 28.7 | 5.4 | 0.33 |
| HAM-D (baseline) | 21.3 | 4.0 | 21.2 | 3.9 | 21.3 | 4.1 | 0.87 |
| MADRS (baseline score) | 33.3 | 4.7 | 32.8 | 4.4 | 33.5 | 4.9 | 0.54 |
| MADRS (percent change from baseline) | |||||||
| 230 min | − 35.7 | 32.8 | − 40.3 | 32.6 | − 32.8 | 32.9 | 0.32 |
| Day 1 | − 34.2 | 35.4 | − 41.1 | 34.4 | −29.9 | 35.6 | 0.17 |
Abbreviations: BMI, body mass index; HAM-D, Hamilton Depression Rating Scale; MADRS, Montgomery–Asberg Depression Rating Scale; MDD, major depressive disorder.
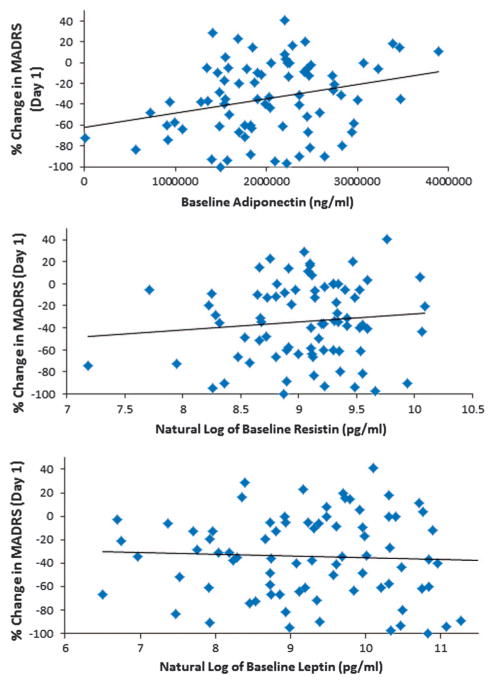
Lower baseline adiponectin levels correlated with superior antidepressant response to ketamine (percent change from baseline) at 230 min post infusion (MADRS: r = 0.25, P = 0.03; HAM-D: r = 0.22, P = 0.051) and at day 1 (MADRS: r = 0.28, P = 0.01; HAM-D: r = 0.34, P = 0.002; Figure 1). Controlling for age did not affect the relationship. Baseline leptin and resistin levels were not related to antidepressant response (MADRS, leptin: 230 min (r = − 0.06, P = 0.63), day 1 (r = − 0.05, P = 0.67); resistin: 230 min (r = 0.05, P = 0.66), day 1 (r = 0.11, P = 0.35)). After Bonferroni correction, the correlation between adiponectin levels at day 1 remained significant. None of the correlations between change in adipokine levels and change in depression rating scale scores were significant.
Figure 1.
Baseline plasma adiponectin levels were strongly associated with improvement in depressive symptoms one day after a single ketamine infusion. No such effects were seen for leptin or resistin.
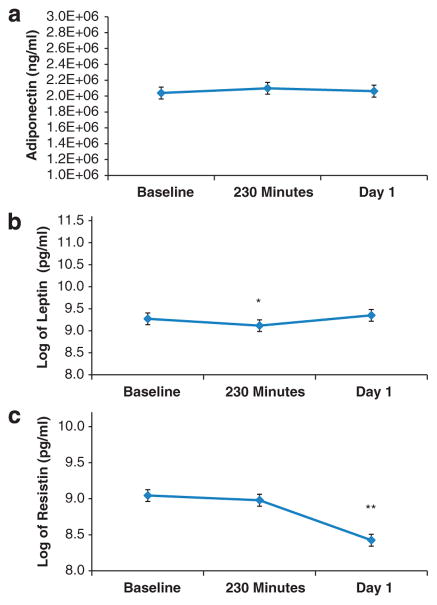
Plasma adiponectin levels did not change significantly from baseline to post-ketamine infusion (F2,236 = 0.16, P = 0.85; Figure 2, Table 2). Natural log-transformed plasma leptin levels differed significantly over time (F2,157 = 9.82, P <0.001); values were lower than baseline at 230 min post infusion (P = 0.008), but not at day 1 (P = 0.29; Figure 2, Table 2). Leptin levels were lower at 230 min than at day 1 (P<0.001). Natural log-transformed plasma resistin levels also differed significantly over time (F2,157 = 40.06, P <0.001); values were significantly lower than baseline at day 1 post infusion (P<0.001), but not at 230 min (P = 0.79). Resistin levels were higher at 230 min than at day 1 (P<0.001; Figure 2, Table 2).
Figure 2.
(a) Adiponectin, (b) leptin and (c) resistin plasma levels at baseline (pre-treatment), 230 min post-ketamine infusion and day 1 post-ketamine infusion. *P<0.001, **P<0.001.
Table 2.
Adipokine levels at baseline and post-ketamine infusion (230 min and day 1; n =80)
| Estimated marginal means | Total (n = 80)
|
Bipolar (n = 31)
|
MDD (n = 49)
|
P-value | |||
|---|---|---|---|---|---|---|---|
| Mean | S.e.m. | Mean | S.e.m. | Mean | S.e.m. | ||
| Adiponectin | 0.85 | ||||||
| Baseline | 2 038 940 | 74 709 | 1 918 493 | 118 570 | 2 115 141 | 95 306 | |
| 230 min | 2 098 711 | 74 709 | 2 009 068 | 118 570 | 2 155 424 | 95 306 | |
| Day 1 | 2 062 758 | 75 180 | 1 960 504 | 118 570 | 2 128 797 | 96 294 | |
| Leptin (natural log) | <0.001 | ||||||
| Baseline | 9.272 | 0.133 | 9.57 | 0.21 | 9.081 | 0.168 | |
| 230 min | 9.117 | 0.133 | 9.42 | 0.21 | 8.923 | 0.168 | |
| Day 1 | 9.350 | 0.133 | 9.54 | 0.21 | 9.228 | 0.169 | |
| Resistin (natural log) | <0.001 | ||||||
| Baseline | 9.044 | 0.082 | 8.71 | 0.07 | 9.259 | 0.054 | |
| 230 min | 8.980 | 0.082 | 8.60 | 0.07 | 9.221 | 0.054 | |
| Day 1 | 8.425 | 0.083 | 7.16 | 0.07 | 9.239 | 0.054 | |
Abbreviation: MDD, major depressive disorder.
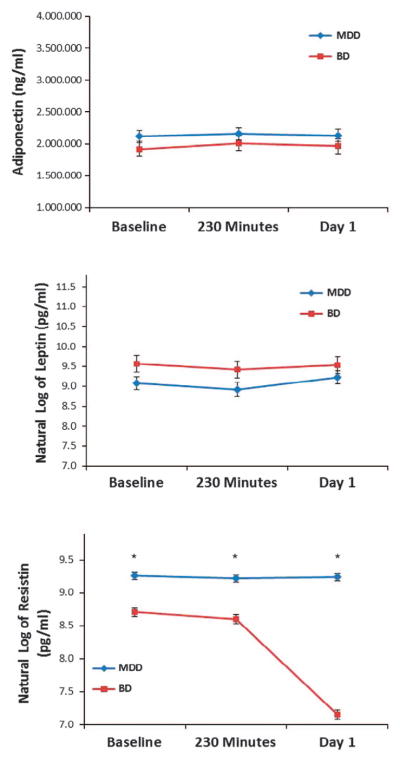
Diagnosis (MDD versus BD) was examined using models of change over time as an additional factor of interest; the interaction between diagnosis and time was also examined (Figure 3). Resistin levels were significantly lower in BD patients throughout the full course of the ketamine trial (diagnosis: F1,78 = 203.72, P<0.001; diagnosis × time: F2,155 = 271.36, P<0.001), but the largest difference between groups was at day 1. Diagnosis did not appear to have a role with adiponectin (diagnosis: F1,233 = 3.71, P = 0.06; diagnosis × time: F2,233 = 0.03, P = 0.97) or leptin levels (diagnosis: F1,78 = 2.74, P = 0.10; diagnosis × time: 2,155 = 1.82, P = 0.17). After controlling for baseline levels, no difference was observed between diagnostic groups with regard to either adiponectin or leptin levels. However, a significant interaction was seen between diagnosis and time for resistin (P<0.001), suggesting a larger difference in resistin levels between diagnostic groups at day 1 (Figure 3).
Figure 3.
The potential role of diagnosis in adipokine levels. Resistin was significantly lower in patients with bipolar disorder (BD) versus those with major depressive disorder (MDD) throughout the full course of the ketamine trial. Diagnosis did not significantly influence leptin or adiponectin levels. *P<0.001.
As a follow-up to these diagnostic differences, a separate model compared BD patients receiving lithium with those receiving valproate. Controlling for baseline levels, no difference between mood stabilizers was observed for either adiponectin (P = 0.53) or leptin (P = 0.51). There was a significant interaction between mood stabilizer and time (P = 0.04) for resistin, but the post hoc tests were not significant.
For leptin, the main effect of depression subtype (‘melancholic’, ‘atypical’ or ‘neither’) was significant (P = 0.003). Specifically, post hoc tests indicated significantly higher levels of leptin in the ‘atypical’ group than in the ‘neither’ group (P<0.001); the ‘atypical’ and ‘melancholic’ groups were not significantly different (P = 0.87), and the interaction with time was not significant (P = 0.98). Depression subtype was also a significant factor for resistin (P = 0.02). The ‘atypical’ group had significantly higher resistin levels than the ‘melancholic’ group. No significant group differences or group by time interactions were observed for adiponectin (group: P = 0.20; group × time: P = 0.80). Controlling for BMI eliminated the subtype difference with leptin but not resistin.
Finally, we looked at BMI and found that it was significantly correlated with leptin levels at baseline (r = 0.57, P<0.001), but not with adiponectin (r = − 0.08, P = 0.47) or resistin (r = 0.01, P = 0.95) levels. Although BMI was independently related to antidepressant response at 230 min (r = − 0.30, P = 0.006) and at day 1 (r = − 0.27, P = 0.02), it did not alter the lack of association between leptin and response to ketamine in a regression model at either 230 min post infusion (standardized β = 0.13, P = 0.30) or at day 1 (standardized β = 0.11, P = 0.38).
DISCUSSION
In this study of three key adipokines—adiponectin, resistin and leptin—we found that low baseline plasma adiponectin levels predicted rapid clinical response to ketamine. Individuals with an antidepressant response to ketamine had subtle changes in leptin levels and a significant decrease in plasma levels of resistin compared to baseline levels (pre-ketamine infusion).
In addition, we found that the lower the baseline plasma adiponectin levels, the greater the response to ketamine. Adiponectin has many positive roles in promoting good health; it helps combat insulin resistance and exerts significant anti-inflammatory effects. We also found that subjects with the lowest levels of plasma adiponectin had an individual course of illness characterized by more affective episodes. Thus, it appears that lower plasma adiponectin levels may imply decreased insulin sensitivity, increased inflammation and a larger number of prior, clinically significant affective episodes.
Studies investigating responsiveness to antidepressants based on severity of illness have obtained mixed results. A large meta-analysis of 45 antidepressant drug trials that included 4782 patients with mild depression and 911 patients with severe depression reported that the response rate in severely depressed patients was significantly greater in than in mildly ill patients.43 A similarly large study that examined response rates to electroconvulsive therapy found that treatment-resistant patients who were less severely depressed responded better to electroconvulsive therapy treatment than more severely depressed patients.48 Thus, it is conceivable that severity of depression may not correlate with severity of systemic manifestations, and that the severity of systemic symptoms may independently predict a positive response to ketamine. Additional studies are required to fully examine this question.
Ketamine rapidly lowered levels of resistin, a profound pro-inflammatory compound.26,29,30,49 As noted above, resistin is not strongly related to insulin sensitivity.50 Because inflammatory mediators consistently promote insulin resistance,51 some have suggested that resistin’s impact on insulin sensitivity is mediated by its ability to promote cytokine secretion. Indeed, data emerging over the past several years directly implicate resistin as an inflammatory mediator with pronounced effects in human inflammation and illnesses where inflammation has a significant role, including diabetes, coronary artery disease, inflammatory bowel disease and rheumatic diseases such as rheumatoid arthritis and systemic lupus erythematosis.52–55 Given its wide role in a broad and disparate range of inflammatory diseases, it would not be surprising to find that resistin is elevated in patients with depressive disorders. The ability of ketamine to lower resistin levels could indicate that ketamine has substantial anti-inflammatory effects in patients with depressive illness, many of whom seem to be in a clear pro-inflammatory state.
Despite its pronounced possible relevance to affective illness, we found that plasma leptin levels shed no light on the potential role of adipokines on ketamine response, or as possible transducers of ketamine’s pronounced therapeutic effects. It should be noted that acute therapeutic response to ketamine in this patient group was greater in those with higher BMIs,7 which consistently correlates positively with leptin levels.
With regard to differences between diagnostic groups, resistin levels were significantly lower in BD compared with MDD patients at day 1. This result might be associated with the add-on use of mood stabilizers in the BD group, or might represent a finding specific to diagnosis. Furthermore, higher leptin and resistin levels were found in individuals described as having ‘atypical depression’, which supports the presence of increased metabolic dysfunction and inflammation in ‘atypical’ compared with ‘melancholic’ depression.56 Other studies have noted increased triglycerides and low-density lipoprotein levels in subjects with bipolar depression compared with those with MDD,42 which may underlie the selective decrease in resistin levels induced by ketamine observed in BD patients in this study.
The current data have several potential clinical implications. First, low plasma adiponectin levels predicted a positive acute antidepressant response to ketamine in patients with treatment-resistant depression. In addition, the fact that lower adiponectin levels correlated with number of previous clinical episodes indirectly suggests that a prolonged clinical course might be associated with greater likelihood of response to ketamine. Furthermore, strong evidence suggests that resistin has a role in multiple inflammatory illnesses. Thus, it may be a common factor in many inflammatory diseases. These data suggest that resistin may partially contribute to the peripheral inflammation associated with depression. In light of data demonstrating that resistin at the hypothalamic locus also increases the secretion of interleukin-6,57 resistin could also partially contribute to the CNS inflammation associated with affective illness. As noted above, depressive symptoms in patients with atypical depression correlate positively with resistin levels, and resistin levels fall significantly after antidepressant treatment.58 It is noteworthy that ketamine is capable of quickly and significantly decreasing plasma resistin levels. These data suggest, but do not prove, that adipokines participate in the mechanism of acute ketamine-induced antidepressant effects. All of these possibilities are amenable to further clinical and basic investigation.
The potential clinical implications of the present data include: (1) that ketamine may work best in those with more severe systemic manifestations, and (2) that ketamine’s known anti-inflammatory properties may be associated with its ability to lower levels of pro-inflammatory adipokines like resistin. To date, our studies have administered a single dose of ketamine. In the near future, we will be able to give repeated doses ketamine to assess whether antidepressant response can be sustained. This will allow us to better evaluate the role of adipokines in response to ketamine and/or its therapeutic effects.
Acknowledgments
Funding for this work (NCT00088699) was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857), by a NARSAD Independent Investigator to CAZ and by a Brain and Behavior Mood Disorders Research Award to CAZ. We thank the 7SE research unit and staff for their support.
Footnotes
CONFLICT OF INTEREST
CAZ is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. He has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government. The remaining authors declare no conflict of interest.
References
- 1.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20:32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22:1023–1031. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caselli C. Role of adiponectin system in insulin resistance. Mol Genet Metab. 2014;113:155–160. doi: 10.1016/j.ymgme.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92:1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 5.Une K, Takei YA, Tomita N, Asamura T, Ohrui T, Furukawa K, et al. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur J Neurol. 2011;18:1006–1009. doi: 10.1111/j.1468-1331.2010.03194.x. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 7.Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75:e417–e423. doi: 10.4088/JCP.13m08698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8:283–290. doi: 10.4103/0259-1162.143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thannikary L, Naik B. Ketamine. In: Atlee JL, editor. Complications in Anesthesia. Saunders Elsevier; Philadelphia, PA: 2007. pp. 78–79. [Google Scholar]
- 10.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi K, Yuasa D, Shibata R, Murohara T, Ouchi N. Adiponectin as a target in obesity-related inflammatory state. Endocr Metab Immune Disord Drug Targets. 2015;15:145–150. doi: 10.2174/1871530315666150316122709. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker-Duffen JL, Walsh K. Cardiometabolic effects of adiponectin. Best Pract Res Clin Endocrinol Metab. 2014;28:81–91. doi: 10.1016/j.beem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15:221. doi: 10.1186/cc10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Handelsman Y, Oral EA, Bloomgarden ZT, Brown RJ, Chan JL, Einhorn D, et al. The clinical approach to the detection of lipodystrophy - an AACE consensus statement. Endocr Pract. 2013;19:107–116. doi: 10.4158/endp.19.1.v767575m65p5mr06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 18.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song J, Kang SM, Kim E, Kim CH, Song HT, Lee JE. Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study. Cell Death Dis. 2015;6:e1844. doi: 10.1038/cddis.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 22.Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165:313–327. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cizza G, Nguyen VT, Eskandari F, Duan Z, Wright EC, Reynolds JC, et al. Low 24- hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: the Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry. 2010;71:1079–1087. doi: 10.4088/JCP.09m05314blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber-Hamann B, Kratzsch J, Kopf D, Lederbogen F, Gilles M, Heuser I, et al. Resistin and adiponectin in major depression: the association with free cortisol and effects of antidepressant treatment. J Psychiatr Res. 2007;41:344–350. doi: 10.1016/j.jpsychires.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Guo M, Zhang D, Cheng SY, Liu M, Ding J, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci USA. 2012;24:12248–12253. doi: 10.1073/pnas.1202835109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010 doi: 10.1155/2010/748048. pii: 748048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology. 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Guo M, Zhang D, Cheng SY, Liu M, Ding J, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci USA. 2012;109:12248–12253. doi: 10.1073/pnas.1202835109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, et al. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Qasim AN, Metkus TS, Tadesse M, Lehrke M, Restine S, Wolfe ML, et al. Resistin gene variation is associated with systemic inflammation but not plasma adipokine levels, metabolic syndrome or coronary atherosclerosis in nondiabetic Caucasians. Clin Endocrinol. 2009;70:698–705. doi: 10.1111/j.1365-2265.2008.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cell Mol Immunol. 2006;3:29–34. [PubMed] [Google Scholar]
- 32.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 33.De Kock M, Loix S, Lavand’homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–410. doi: 10.1111/cns.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumor necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esel E, Ozsoy S, Tutus A, Sofuoglu S, Kartalci S, Bayram F, et al. Effects of antidepressant treatment and of gender on serum leptin levels in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:565–570. doi: 10.1016/j.pnpbp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Zupancic ML, Mahajan A. Leptin as a neuroactive agent. Psychosom Med. 2011;73:407–414. doi: 10.1097/PSY.0b013e31821a196f. [DOI] [PubMed] [Google Scholar]
- 37.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eikelis N, Esler M, Barton D, Dawood T, Wiesner G, Lambert G. Reduced brain leptin in patients with major depressive disorder and in suicide victims. Mol Psychiatry. 2006;11:800–801. doi: 10.1038/sj.mp.4001862. [DOI] [PubMed] [Google Scholar]
- 39.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 40.Stieg MR, Sievers C, Farr O, Stalla GK, Mantzoros CS. Leptin: a hormone linking activation of neuroendocrine axes with neuropathology. Psychoneuroendocrinology. 2015;51:47–57. doi: 10.1016/j.psyneuen.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, et al. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl Psychiatry. 2012;2:e83. doi: 10.1038/tp.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wysokinski A, Strzelecki D, Kloszewska I. Levels of triglycerides, cholesterol, LDL, HDL and glucose in patients with schizophrenia, unipolar depression and bipolar disorder. Diabetes Metab Syndr. 2015;9:168–176. doi: 10.1016/j.dsx.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Khan A, Leventhal RM, Khan SR, Brown WA. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol. 2002;22:40–45. doi: 10.1097/00004714-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 44.DiazGranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62:10–17. [PubMed] [Google Scholar]
- 47.Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry. 2015;76:1374–1384. doi: 10.4088/JCP.14r09528. [DOI] [PubMed] [Google Scholar]
- 49.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 50.Owecki M, Miczke A, Nikisch E, Pupek-Musialik D, Sowinski J. Serum resistin concentrations are higher in human obesity but independent from insulin resistance. Exp Clin Endocrinol Diabetes. 2011;119:117–121. doi: 10.1055/s-0030-1263111. [DOI] [PubMed] [Google Scholar]
- 51.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 52.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 54.Konrad A, Lehrke M, Schachinger V, Seibold F, Stark R, Ochsenkuhn T, et al. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007;19:1070–1074. doi: 10.1097/MEG.0b013e3282f16251. [DOI] [PubMed] [Google Scholar]
- 55.Migita K, Maeda Y, Miyashita T, Kimura H, Nakamura M, Ishibashi H, et al. The serum levels of resistin in rheumatoid arthritis patients. Clin Exp Rheumatol. 2006;24:698–701. [PubMed] [Google Scholar]
- 56.Gold PW, Chrouso GP. Melancholic and atypical subtypes of depression represent distinct pathophysiological entities: CRH, neural circuits, and the diathesis for anxiety and depression. Mol Psychiatry. 2013;18:632–634. doi: 10.1038/mp.2013.5. [DOI] [PubMed] [Google Scholar]
- 57.Benomar Y, Gertler A, De Lacy P, Crepin D, Ould Hamouda H, Riffault L, et al. Central resistin overexposure induces insulin resistance through toll-like receptor 4. Diabetes. 2013;62:102–114. doi: 10.2337/db12-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehto SM, Huotari A, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, et al. Serum adiponectin and resistin levels in major deprssive disorder. Acta Psychiatr Scand. 2010;121:209–215. doi: 10.1111/j.1600-0447.2009.01463.x. [DOI] [PubMed] [Google Scholar]