Abstract
Background Endoscopic skull base approaches are being used to address complicated neurovascular pathology. These approaches are safest when proximal vascular control of the cavernous carotid artery (CavCA) can be obtained.
Methods We present a cadaver-based anatomic simulation study showing the feasibility of clip placement for the CavCA as it courses through the cavernous sinus. The arterial vessels were injected with red microfil (Flow Tech, Carver, Massachusetts) to enhance visibility. The endoscope was directed through a precaruncular transorbital approach and instrumentation was managed through an endonasal transsphenoidal approach.
Results The dual approach minimized the “coning down” and instrument “sword fighting” that occurs as the rod lens endoscope and instruments are used laterally and posterior toward the clivus and brainstem. The precaruncular transorbital approach improved visualization of the clip application and improved the functional working area. The transorbital port allowed better appreciation of the distal clip tines, and the laterally positioned cranial nerves.
Conclusions The advantages may be most realized in the setting of endoscopic endonasal resection of highly vascular lesions and/or bleeding from a ruptured aneurysm being clipped. Simulated training provides an excellent opportunity to enhance skill sets and increase familiarity with anatomical visualization before entering the operative arena.
Keywords: cavernous carotid artery, CavCA, aneurysm clipping, simulation training, endonasal transsphenoidal approach, precaruncular transorbital approach
Introduction
Endoscopic endonasal approaches for sellar, parasellar, cavernous sinus, middle fossa, and petrous apex pathology have increased significantly.1 The increase application of the expanded endoscopic endonasal approach places the cavernous carotid artery (CavCA) at increased risk of injury during the resection of the pathology that affects these regions. Highly vascular, large, calcified lesions that obstruct visualization of the normal anatomic landmarks can limit the extent of resection and lead to poor visualization of the carotid artery subjecting it to injury. Limitations of traditional endoscopic approaches show the potential advantage for a dual port approach that may be used to access these lesions and improve visualization. The dual port approach can also increase the functional working area and facilitate microsurgical techniques. If the CavCA is not visualized, iatrogenic injury can lead to the formation of pseudoaneurysms. Treatment of trigeminal schwannomas and tuberculum sella meningiomas may require control of the proximal CavCA leaving it susceptible to injury.2 In addition to iatrogenic causes, CavCA aneurysms have been associated with fungal infections and chemotherapy treatment.3 4 These aneurysms and pseudoaneurysms are traditionally treated with endovascular techniques.5 When endovascular techniques are not sufficient, neurosurgical clipping must be performed.6
Endoscopic approaches have been employed to clip aneurysms of the anterior circulation. Perneczky and coworkers pioneered the keyhole approach for the treatment of anterior circulation aneurysms via the supraorbital minicraniotomy.7 More recently, Sawarkar and coworkers demonstrated a purely endoscopic clipping of anterior communicating artery, middle cerebral artery bifurcation, internal carotid artery bifurcation, and posterior cerebral artery aneurysms via this approach. They concluded that endoscopic visualization could help to reduce chances of an incompletely clipped aneurysm/residual neck and the risk of parent vessel/perforator occlusion.8 Germanwala, Zanation, and coworkers furthermore demonstrated effective clipping of a ruptured superior hypophyseal aneurysm.9 Recently, Ceylan et al used the endoscopic transsphenoidal approach to clip a carotid-cavernous aneurysm that was protruding into the sellae.10 These approaches provided excellent visualization and minimized extensive incisions. Important considerations are that pseudoaneurysms are associated with high-vascular flow, therefore, increasing the risk of hemorrhage.11 Furthermore, reports have suggested a high coexistence of CavCA aneurysms and pituitary adenomas necessitating wide angle visualization for treatment.12 It is, therefore, important that neurosurgeons in training develop sufficient technique and experience with endoscopic approaches before entering the operative arena.
In this article, we evaluated the potential benefits of an endoscopic dual port, transorbital and endonasal technique, to optimize the resection of vascular lesions within the bilateral CavCAs, sellar, parasellar, medial and lateral cavernous sinus, clivus, middle fossa, and petrous apex regions. We highlight a feasible, cost-effective, and replicable cadaveric simulation model for clipping the CavCA and gaining access to the aforementioned regions. This facilitates a four-handed microsurgical dissection, improved visualization, and enhanced functional working area. Simulated training for the treatment of aneurysms has provided enhanced trainee comfort with endoscopic instrumentation and greater appreciation of relevant intraoperative anatomical landmarks.13 The benefits for neurosurgical and otolaryngology residents is apparent in that skill set can be learned and improved upon in a safe environment without jeopardizing patient care.14 The literature supports that improvements can be obtained through endoscopic simulation exercises and that these improvements are maintained in the operative arena.15 Using dual transsphenoidal and precaruncular transorbital approaches further optimizes the visualization of the critical structures of the bilateral CavCAs, sellar, parasellar, medial and lateral cavernous sinus, clivus, middle fossa, and petrous apex regions. In addition to improving the functional working area, the dual port approach demonstrated clip application and vascular control of the C4 segment of the CavCA. The benefits of the dual approach are discussed in the context of improving simulated training exercises.
Methods
Preparation
Eight adult cadaveric heads were acquired and prepared in accordance with the Oregon Health and Science University body donation program. All procedures involving cadaveric specimens adhered to the code of ethics approved by the institutional review board and VirtuOHSU laboratories. The heads were frozen and thawed. After thawing, a 1:100 solution of ACD-A anticoagulant citrate dextrose solution (John B. Pierce Laboratory, New Haven, Connecticut) was mixed with warm water. The solution was used to wash out the carotid and vertebral arteries and jugular veins. Each vessel was clamped and the solution perfused for 10 minutes. The solution was washed out of the head with warm water and the head was stored at 5°C overnight. The following morning the heads were embalmed and placed in a bucket of fixative solution until use and after each simulation.
Microfil Injections
Red microfil (Flow Tech, Carver, Massachusetts) was mixed at concentrations of 40 µL red microfil dye, 50 µL diluent, and 4.5 µL curative agent. The right carotid artery was injected with 20 µL of the red microfil solution. To avoid injection into the peripheral circulation, the external carotid artery was isolated and clamped shut on both the left and right side. The red microfil solution was perfused through the intracerebral vessels. Once the solution reached the contralateral carotid, the left and right carotid arteries were clamped to allow vessel dilation with the microfil. The right vertebral was injected with 10 µL of microfil until flow reached the left vertebral artery. Both vertebral arteries were then clamped. The eight heads were injected in a similar manner with sufficient flow through all intracerebral vessels.
Dissection and Clip Placement
The head was placed in a flexed position to allow good visualization of the nasal passages. Endoscopes of 4 mm diameter and 18 cm length were used for the study with 0 and 30 degree lenses (Karl Storz, Tuttlingen, Germany). A fiberoptic light source with the camera was hooked to a Storz tower (Karl Storz) to power the endoscopes. The exposure of the sphenoid sinus was done as previously described.16 Briefly, the middle turbinates were lateralized and a wide bilateral sphenoidotomy was performed. The incision was made at the articulation of the rostrum and vomer. A periosteal elevator was used to clear the mucoperiosteum and the rostral bone was removed bilaterally with a kerrison 2 and drilled flush to the floor of the sphenoid sinus. Identification of the bone impressions of the clivus, sella, cavernous carotid arteries, and opticocarotid recesses were made. The bone was removed overlying these critical structures. The pituitary gland was retracted and the clivus was drilled out with a 5 mm coarse diamond burr drill. A kerrison was used to clear remaining bone fragments until the CavCAs were exposed. The dura over the pituitary gland was preserved and its dural attachments dissected free laterally through the proximal dural ring.
The precaruncular transorbital approach was performed as previously described.16 The transorbital port was placed contralateral to the CavCA being clipped (Fig. 1). Briefly, an incision was made medial to the caruncle into the avascular plane. The incision was widened superiorly and inferiorly using iris scissors. The frontoethmoidal suture was identified along the medial orbital wall. This suture and the anterior and posterior ethmoidal arteries are used as key anatomic landmarks. The transorbital port was created in the lamina papyracea below the level of the frontoethmoidal suture and through the ethmoid air cells more medially. The CavCA, clivus, and sella were visualized. The binares endonasal approach was used as the instrumentation port in all specimens. The comparisons of the functional working area, ergonomics, and visualization was made between using the expanded endonasal approach (EEA) alone, which included the endoscope, verse removing the endoscope from the endonasal port and placing it through the transorbital port while all other instrumentation and aneurysm clip appliers remained in the endonasal port. The clip applicator was used to show feasibility of clip placement for proximal and distal CavCA control.
Fig. 1.
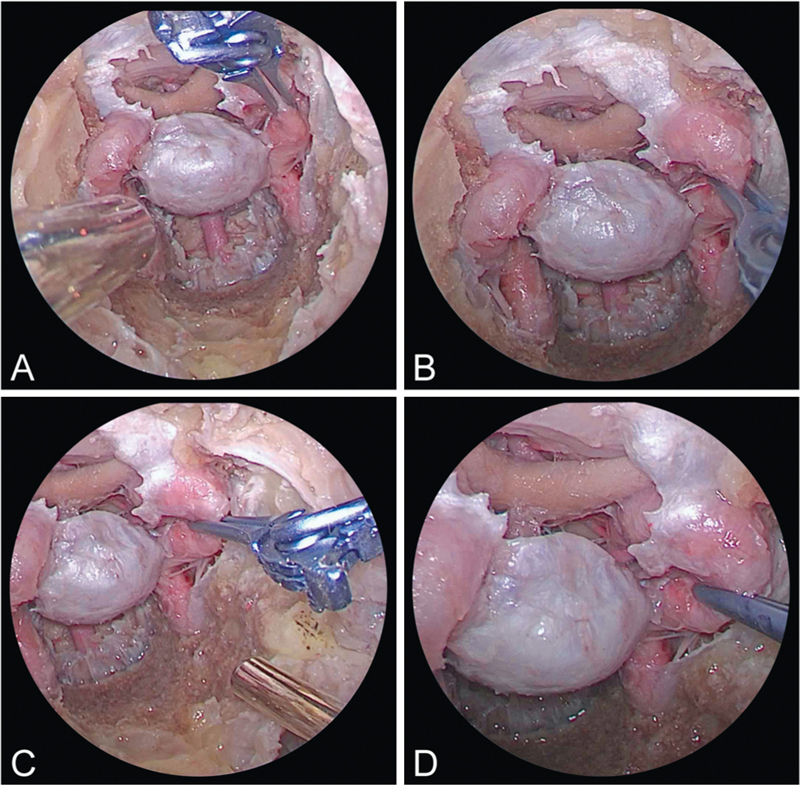
(A and B) Right endonasal view of combined endoscopic multiportal transorbital and endonasal approaches the left cavernous carotid and clipping. (C and D) Right transorbital view of combined endoscopic multiportal transorbital and endonasal approaches the left cavernous carotid and clipping.
Results
The endoscopic dissection revealed detailed carotid anatomy as the arteries coursed through the cavernous sinus. Approaches and complications of CavCA aneurysm clipping are outlined in (Table 1). The genu of the CavCA is the site most prone to iatrogenic injury. Using the dual transsphenoidal and precaruncular approaches, the genu of CavCA was visualized (Fig. 1). Other landmarks of interest were the bilateral CavCAs, sellar, parasellar, medial and lateral cavernous sinus, clivus, middle fossa, and petrous apex regions. Adequate working space was obtained with these dual approaches that allowed placement of the endoscope as well as surgical instrumentation (Fig. 1). The four-handed technique was used to provide sufficient working room for placement of the clip applicator (Fig. 1). The aneurysm clip was applied at various locations along the CavCA with particular feasibility at the genu (Fig. 1). The bone overlying the C4 segment of the CavCA was thickest at the horizontal to vertical transition of the CavCA distal to the petroclival ligament and proximal to the genu of the CavCA. This is important for the surgical team in preparation for the approach in understanding that more medial and lateral drilling was necessary at this segment of C4 to enable clip application. The bone overlying genu of the CavCA was the thinnest. The genu of the CavCA also has an anterior projection relative to the segment distal to the proximal dural ring and proximal to the genu. This makes the genu the most vulnerable segment for injury.
Table 1. Cavernous carotid aneurysm and injury.
| Study | Approach | Artery segment | Considerations |
|---|---|---|---|
| Aboud et al, 200221 | Microsurgical approaches | Anterior circulation arteries | Model developed for practice of bypass, clipping of aneurysms, and treatment of vascular dissections |
| Adeel et al, 201222 | Endovascular and endoscopy | Cavernous carotid pseudoaneurysm | In cases of severe trauma, cavernous carotid pseudoaneurysm may require neurosurgical treatment |
| Azar et al, 20153 | Transsphenoidal | Cavernous sinus and internal carotid artery | Fungal mycotic aneurysm and sphenoid sinusitis |
| Cappabianca et al, 200123 | Endoscopic transsphenoidal | Intracavernous carotid artery | Iatrogenic injury causing pseudoaneurysm formation and requiring endovascular intervention |
| Ceylan et al, 201310 | Endoscopic transsphenoidal | Cavernous carotid aneurysm | Cavernous carotid aneurysms may compress cranial nerves and lead to neuroopthalmologic progression if untreated |
| Cobb et al, 201524 | Endoscopic endonasal | Cavernous carotid artery | Iatrogenic injury during resection of osteoblastoma. Repair needed to be performed |
| Kalinin et al, 201325 | Endonasal transsphenoidal | Cavernous carotid artery | Iatrogenic injury during pituitary adenoma resection in 0.13% of patients |
| Koitschev et al, 200626 | Endonasal microsurgery | Sinus surgery for chronic sinusitis | Iatrogenic injury of cavernous carotid artery requiring endovascular intervention |
| Park et al, 199827 | Endoscopic endonasal | Functional endoscopic sinus surgery | Cavernous carotid portion of artery is most susceptible to injury. Training can help prevent unnecessary risk during surgeries |
| Pawar et al, 201028 | Endoscopic transsphenoidal | Sphenoid mucocele marsupialization | Iatrogenic injury of cavernous carotid and formation of pseudoaneurysm |
| Rangel-Castilla et al, 20145 | Endoscopic transsphenoidal | Cavernous carotid artery | Iatrogenic injury and repair |
| Ronchetti et al, 201311 | Endovascular and endoscopic | Aneurysm in sphenoid sinus | Epistaxis may be the presenting sign. Treatment may require dual endovascular and endoscopic approaches |
| Roopesh et al, 20124 | Microsurgical trapping of the aneurysms | Giant cavernous carotid aneurysm | Epistaxis may be presenting syndrome. Important to rule out aneurysm in suspected cases of fungal granulomas |
| Xia et al, 201212 | Endonasal endoscopic | Cavernous carotid aneurysm | Pituitary macroadenomas may present with concurrent aneurysms |
This dual port technique, transorbital endoscope port and endonasal instrumentation port approach, verse the endoscopic endonasal approach alone was found to provide improved visualization, functional working area, and ergonomics. The transorbital port allows for the removal of the rod lens endoscope from the endonasal binares port, thereby minimizing the “sword fighting” effect between the instruments and the endoscope that occurs more frequently as the working area “cones down” posterior to the sphenoidotomy commonly seen with more lateral EEA. The contralateral transorbital endoscopic port relative to the C4 segment of the CavCA (Fig. 1) optimized visualization of clip application, thereby obviating the incorporation of the cranial nerves in the clip. In addition, more accurate clip placement was accomplished. The transorbital port was significantly more advantageous to the purely endoscopic endonasal approach given, the more offset view of the clip tines relative to the artery itself and surrounding critical structures with a 0-degree endoscope. Multiangled endoscopes were not required to gain the perspective and visualization necessary to perform the task of clip application to all sites along the C4 segment of the CavCA. The single endonasal binares technique requires angled endoscopes to gain the offset view and perspective necessary for visualization of the targeted structures. The addition of the transorbital endoscopic port afforded surgeons the benefits of improved visualization without the potential disorientation of angled endoscopes. This significantly improved the functional working area of the instruments as well as the ergonomics. The use of an adjustable aneurysm clip applier and clip facilitated the clip placement, readjustment, and removal at different angles. It was found to decrease the observed torque on the clipped vessel and optimize clip placement given the narrow working area of this region of the skull base.
Discussion
The simulation continues to improve and offers a viable training experience for residents. Benet et al pioneered a three-dimensional-printed aneurysm model to practice intracranial clipping in cadavers.13 Other models have employed virtual reality models to improve resident training in intraoperative anatomy.17 Recent evidence suggests that residents who train with cadaver aneurysm models are more competent in handling intraoperative ruptures compared with residents without such training.18 Cadaver aneurysm simulation has also been used by experienced surgeons to practice complicated techniques for difficult aneurysms.19 In regards to clipping CavCA aneurysms and pseudoaneurysms, unique challenges are present due to high vascular flow and the likelihood of hemorrhage. Additionally, these types of aneurysms can cause cranial nerve deficits and variations in systolic blood flow.20 Simulation, therefore, offers a valuable opportunity for familiarizing with techniques and instrumentation as well as anticipating potential complications.
The benefit of this simulation model is that it is cost-effective and highly replicable. The heads have been used numerous times for training and setup in minimal. By supplying actual endoscopic instruments used in the operating room, the learners can become more familiar with instrumentation in a safe environment. The site of carotid aneurysms can be appreciated and the application of the aneurysm clip can be practiced. Bone removal allows appreciation of intraoperative anatomy. Residents and junior faculty can engage in repetitive training experiences under the guidance of trained faculty. The participants described the experience as highly beneficial and a much needed component of training. One resident even noted that the experience was eye opening in that it introduces participants to the complexity of skull-based endoscopic procedures. All residents have requested further training experiences through simulated cadaveric models.
The microfilm-injected arteries provided enhanced visualization of the vessels to allow the appreciation of intraoperative anatomy. The genu of the CavCA is the most likely site for iatrogenic injury as emphasized in (Table 1), and was readily visualized with the dual transsphenoidal and precaruncular transorbital approaches. The dual approaches combined with the four-handed technique provided ample room for manipulation of instrumentation and a wide angle for visualization of the CavCA. The endoscopic applier and adjustable aneurysm clip were found to be advantageous. The adjustable clip and applier expanded the positions in which clip application and removal could be performed. This can prove to be advantageous in the live clinical setting given that the more torque applied by the clip applier to the clip translates to the artery and can place it at more risk of iatrogenic injury. Thereby minimizing this manipulation the risk appears reduced. The advantage of the model is that it can be used numerous times, is cost-effective, and reproducible. The setup time is minimal and the arrangement allows focused instruction between the trainer and trainee. The trainee can practice the technique numerous times in a safe environment. This allows the trainee to familiarize with the endoscope and instrumentation before entering the operative arena. Several residents have described their simulated endoscopic skull base experience as “humbling” and gained an appreciation of the complexity of skull base endoscopic procedures.
Conclusion
Cadaveric simulation models have emerged as a valuable component of neurosurgical training. In this article, we demonstrate the feasibility of the dual transsphenoidal and precaruncular transorbital approaches to address complex pathology involving the sellar, parasellar, cavernous sinus, middle fossa, and petrous apex regions. We specifically demonstrated a novel dual portal approach to optimize visualization, functional working area, and ergonomics while endoscopically addressing complex and highly vascular lesions that affect patients in these locations. The dual transorbital endoscopic port and endonasal binares approach was ideal for gaining vascular control of the C4 segment of the CavCA via adjustable clipping. The model provides trainees with important instruction regarding the proper use of instrumentation, appreciation for intraoperative anatomy, and a safe environment to practice technique. The model is cost-effective, easy to setup, and is highly replicable. As flexible and digital endoscopy has become available, dual and multiportal, techniques to the skull base will likely be more widely utilized. The clinical application of this novel dual approach may expand our capabilities when addressing the pathology of this region.
Acknowledgments
Brandon Lucke-Wold received funding from a Neurosurgery Research and Education Foundation Medical Student Summer Research Fellowship, an American Foundation of Pharmaceutical Education Predoctoral Fellowship, and an American Medical Association Foundation Seed Grant.
Footnotes
Conflict of Interest None.
References
- 1.Zacharia B E, Romero F R, Rapoport S K, Raza S M, Anand V K, Schwartz T H. Endoscopic Endonasal Management of Metastatic Lesions of the Anterior Skull Base: Case Series and Literature Review. World Neurosurg. 2015;84(5):1267–1277. doi: 10.1016/j.wneu.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 2.Sekhar L N, Schramm V L Jr, Jones N F. et al. Operative exposure and management of the petrous and upper cervical internal carotid artery. Neurosurgery. 1986;19(6):967–982. doi: 10.1227/00006123-198612000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Azar M M Assi R Patel N Malinis M F Fungal Mycotic Aneurysm of the Internal Carotid Artery Associated with Sphenoid Sinusitis in an Immunocompromised Patient: A Case Report and Review of the Literature Mycopathologia 2015. (e-pub ahead of print). doi: 10.1007/s11046-015-9975-1 [DOI] [PubMed] [Google Scholar]
- 4.Roopesh Kumar V R Madhugiri V S Sasidharan G M Gundamaneni S K Giant cavernous carotid artery aneurysm mimicking a fungal granuloma and presenting with massive epistaxis BMJ Case Rep 2012. (e-pub ahead of print). doi: 10.1136/bcr-2012-006876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangel-Castilla L McDougall C G Spetzler R F Nakaji P Urgent cerebral revascularization bypass surgery for iatrogenic skull base internal carotid artery injury Neurosurgery 20141004640–647., discussion 647–648 [DOI] [PubMed] [Google Scholar]
- 6.Grigorian A, Rajaraman V, Hunt C D. Traumatic intracranial aneurysms complicating anterior skull base surgery. J Craniomaxillofac Trauma. 1998;4(4):10–14. [PubMed] [Google Scholar]
- 7.Reisch R Perneczky A Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision Neurosurgery 200557(4, Suppl)242–255., discussion 242–255 [DOI] [PubMed] [Google Scholar]
- 8.Sharma B S, Kumar A, Sawarkar D. Endoscopic controlled clipping of anterior circulation aneurysms via keyhole approach: Our initial experience. Neurol India. 2015;63(6):874–880. doi: 10.4103/0028-3886.170095. [DOI] [PubMed] [Google Scholar]
- 9.Heiferman D M, Somasundaram A, Alvarado A J, Zanation A M, Pittman A L, Germanwala A V. The endonasal approach for treatment of cerebral aneurysms: A critical review of the literature. Clin Neurol Neurosurg. 2015;134:91–97. doi: 10.1016/j.clineuro.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceylan S, Anik I, Koc K, Ciftci E, Cabuk B. Endoscopic approach to cavernous sinus aneurysm. Turk Neurosurg. 2013;23(3):404–406. doi: 10.5137/1019-5149.JTN.5503-11.1. [DOI] [PubMed] [Google Scholar]
- 11.Ronchetti G, Panciani P P, Cornali C. et al. Ruptured aneurysm in sphenoid sinus: which is the best treatment? Case Rep Neurol. 2013;5(1):1–5. doi: 10.1159/000346347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia X, Ramanathan M, Orr B A. et al. Expanded endonasal endoscopic approach for resection of a growth hormone-secreting pituitary macroadenoma coexistent with a cavernous carotid artery aneurysm. J Clin Neurosci. 2012;19(10):1437–1441. doi: 10.1016/j.jocn.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Benet A, Plata-Bello J, Abla A A, Acevedo-Bolton G, Saloner D, Lawton M T. Implantation of 3D-Printed Patient-Specific Aneurysm Models into Cadaveric Specimens: A New Training Paradigm to Allow for Improvements in Cerebrovascular Surgery and Research. Biomed Res Int. 2015;2015:939387. doi: 10.1155/2015/939387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicdonmez T Hamamcioglu M K Tiryaki M Cukur Z Cobanoglu S Microneurosurgical training model in fresh cadaveric cow brain: a laboratory study simulating the approach to the circle of Willis Surg Neurol 2006661100–104., discussion 104 [DOI] [PubMed] [Google Scholar]
- 15.Davies J, Khatib M, Bello F. Open surgical simulation—a review. J Surg Educ. 2013;70(5):618–627. doi: 10.1016/j.jsurg.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Ciporen J N, Moe K S, Ramanathan D. et al. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 2010;73(6):705–712. doi: 10.1016/j.wneu.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Alaraj A, Luciano C J, Bailey D P. et al. Virtual reality cerebral aneurysm clipping simulation with real-time haptic feedback. Neurosurgery. 2015;11 02:52–58. doi: 10.1227/NEU.0000000000000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aboud E, Aboud G, Al-Mefty O. et al. “Live cadavers” for training in the management of intraoperative aneurysmal rupture. J Neurosurg. 2015;123(5):1339–1346. doi: 10.3171/2014.12.JNS141551. [DOI] [PubMed] [Google Scholar]
- 19.Marinho P, Vermandel M, Bourgeois P, Lejeune J P, Mordon S, Thines L. Preoperative simulation for the planning of microsurgical clipping of intracranial aneurysms. Simul Healthc. 2014;9(6):370–376. doi: 10.1097/SIH.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 20.Lanza G, Vinciguerra L, Puglisi V. et al. Acute isolated trochlear nerve palsy in a patient with cavernous carotid aneurysm and visit-to-visit variability in systolic blood pressure. Int J Stroke. 2015;10(6):E61. doi: 10.1111/ijs.12552. [DOI] [PubMed] [Google Scholar]
- 21.Aboud E, Al-Mefty O, Yaşargil M G. New laboratory model for neurosurgical training that simulates live surgery. J Neurosurg. 2002;97(6):1367–1372. doi: 10.3171/jns.2002.97.6.1367. [DOI] [PubMed] [Google Scholar]
- 22.Adeel M Ikram M Post-traumatic pseudoaneurysm of internal carotid artery: a cause of intractable epistaxis BMJ Case Rep 2012. (e-pub ahead of print). doi: 10.1136/bcr.02.2012.5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappabianca P, Briganti F, Cavallo L M, de Divitiis E. Pseudoaneurysm of the intracavernous carotid artery following endoscopic endonasal transsphenoidal surgery, treated by endovascular approach. Acta Neurochir (Wien) 2001;143(1):95–96. doi: 10.1007/s007010170144. [DOI] [PubMed] [Google Scholar]
- 24.Cobb M I Nimjee S Gonzalez L F Jang D W Zomorodi A Direct Repair of Iatrogenic Internal Carotid Artery Injury During Endoscopic Endonasal Approach Surgery With Temporary Endovascular Balloon-Assisted Occlusion: Technical Case Report Neurosurgery 20151103E483–E486., discussion E486–E487 [DOI] [PubMed] [Google Scholar]
- 25.Kalinin P L Sharipov O I Shkarubo A N et al. Damage to the cavernous segment of internal carotid artery in transsphenoidal endoscopic removal of pituitary adenomas (report of 4 cases) [in Russian] Vopr Neirokhir 201377628–37., discussion 38 [PubMed] [Google Scholar]
- 26.Koitschev A, Simon C, Löwenheim H, Naegele T, Ernemann U. Management and outcome after internal carotid artery laceration during surgery of the paranasal sinuses. Acta Otolaryngol. 2006;126(7):730–738. doi: 10.1080/00016480500469578. [DOI] [PubMed] [Google Scholar]
- 27.Park A H, Stankiewicz J A, Chow J, Azar-Kia B. A protocol for management of a catastrophic complication of functional endoscopic sinus surgery: internal carotid artery injury. Am J Rhinol. 1998;12(3):153–158. doi: 10.2500/105065898781390154. [DOI] [PubMed] [Google Scholar]
- 28.Pawar S S, Loehrl T A, Michel M A, Fitzsimmons B F. Cavernous carotid pseudoaneurysm after endoscopic sphenoid mucocele marsupialization. Arch Otolaryngol Head Neck Surg. 2010;136(4):407–410. doi: 10.1001/archoto.2010.29. [DOI] [PubMed] [Google Scholar]


