Abstract
Magnesium deficiency (MgD) has been shown to impact numerous biological processes at the cellular and molecular levels. In the present review, we discuss the relationship between MgD and oxidative stress (OS). MgD is accompanied by increased levels of OS markers such as lipid, protein and DNA oxidative modification products. Additionally, a relationship was detected between MgD and a weakened antioxidant defence. Different mechanisms associated with MgD are involved in the development and maintenance of OS. These mechanisms include systemic reactions such as inflammation and endothelial dysfunction, as well as changes at the cellular level, such as mitochondrial dysfunction and excessive fatty acid production.
Keywords: Magnesium, Magnesium deficiency, Oxidative stress, Antioxidants, Reactive oxygen species, Lipid peroxidation
1. Introduction
It is firmly established that deficiencies of essential macro- and micronutrients are associated with the development of different diseases [1-3]. However, the pathological consequences of a nutrient deficiency often lack a clear or direct relationship with the functions of that nutrient in the body. Magnesium deficiency (MgD) is an excellent example of this scenario [4].
MgD can be caused by numerous factors including decreased dietary Mg intake, stress [5], high levels of alcohol consumption [6], and inherited renal magnesium transport disorders [7] that are associated with excessive Mg loss. Additionally, endocrine diseases (diabetes mellitus [8], metabolic syndrome [9]) and administration of some medical agents (diuretics, proton-pump inhibitors, cardiac glycosides, epidermal growth factor receptor inhibitors, calcineurin inhibitors [10], aminoglycoside antibiotics, amphotericin B, cisplatin, pentamidine, and cyclosporine [11]) can also result in MgD. Several review articles have been published on Mg metabolism and related disorders [12, 13].
Prior literature, particularly studies using animal models, suggests a correlation between MgD and the development of oxidative stress (OS) [14]. However, Mg is not an acknowledged functional component of the antioxidant defence system (AOS).
Therefore, mechanisms of OS associated with a lack of Mg are still a matter of debate. Furthermore, the role of Mg in oxidative damage to molecules, cells and tissues in the pathogenesis associated with MgD remains unclear. Here, we present a critical analysis of the relationship between OS and MgD and a mechanism explaining the interaction between them.
2. Origin and measurement of oxidative stress
Sies H. defined OS in the body as “an imbalance between oxidants and antioxidants in favour of the oxidants, potentially leading to damage” [15]. The above-mentioned oxidants are ‘reactive species’ (RS) (reactive oxygen (ROS)/nitrogen/chlorine) [16]; some RS are free radicals. Many RS play a critical physiological role, and their production is essential for the normal life cycle of an organism. However, overproduction of RS can cause oxidative damage to molecules, cells and tissues [17], which contributes to the development of many diseases [18-20].
OS can be assessed by measuring an imbalance between RS oxidant production and the functional activity of the AOS. Both the production and the damaging activity of RS are complicated and multifaceted. Additionally, there are many components in the AOS interact to regulate OS [21]. Therefore, several different markers are used to measure the production of RS oxidants and the ability of the AOS to detect OS [16, 22]. Many authors speculate that an imbalance between pro- and anti-oxidants results in an increased level of oxidative degradation of biomolecule products, such as lipid peroxidation products. Furthermore, an increased concentration of oxidative damage markers can also indicate OS [23]. A wide range of available analytical approaches allows the quantification of lipid peroxidation and free radical-based DNA or protein damage [24]. However, many of these techniques lack sensitivity or specificity, particularly when estimating oxidant stress levels in vivo. Currently, there is no gold standard for measuring OS, i.e., a specific marker whose level is consistently affected by OS of different origins [25]. A recently started multiinvestigator project (the Biomarkers of Oxidative Stress Study (BOSS)) aims provide such a marker1; however, currently none of the existing methods for OS detection can be considered absolutely reliable.
3. Oxidative stress and magnesium deficiency
Early clinical studies have provided evidence of the impact of the OS associated with MgD on human pathology. The gold standard for verifying MgD in clinical studies is the parenteral Mg tolerance test (low dose Mg load test) [26, 27]. Unfortunately, this test was rarely used in published studies; thus, there is a lack of reliable clinical data that provides evidence for the relationship between MgD and OS.
Diabetic patients only displayed an increased concentration of oxidised LDL in association with a reduced level of serum Mg. Patients with normal serum Mg levels did not demonstrate this increase in oxidised LDL concentrations [28]. It has been shown that low dietary Mg intake is accompanied by poor DNA repair capacity [29] and increased genomic instability [30].
Barbagallo et al. established a strong, direct correlation between RBC Mg levels and GSH/GSSG concentration (circulating reduced/oxidized glutathione) (r = 0.84, P < 0.0001) [31]. In another study, a negative correlation between Mg levels and OS stress markers (plasma superoxide anions and malondialdehyde) was observed in groups of the population chronically exposed to stress [32]. Interestingly, no correlation between low Mg intake and antioxidant capacity has been found among Korean adults [33].
Animal studies were conducted to obtain biologically relevant evidence of causal relationships between MgD and OS. Mgdeficient feed is used to induce dietary MgD in animals. It was demonstrated that the lipoprotein fractions (VLDL and LDL) from three-week old Mg-deficient rats were more susceptible to oxidative damage caused by CuSO4-induced oxidation than lipoprotein fractions from control rats. The triacylglycerol and alphatocopherol levels in plasma were significantly higher, whereas the level of alpha-tocopherol in the VLDL + LDL fraction was significantly lower in the Mg-deficient group compared to the control group. After exposing tissue homogenates to Fe-induced lipid peroxidation, the concentration of thiobarbituric acid-reactive substances was significantly higher in tissues from Mg-deficient rats than in those from control rats [34].
MgD was accompanied by a two-fold decrease in glutathione (GSH) concentration in RBCs [35]. In other types of cells, the overexpression of glutathione transferase has been suggested to be the cause of GSH depletion [36]. After six weeks, the MgD diet led to a significant decrease of both plasma and RBC Mg levels, followed by a marked increase in plasma malondialdehyde and a corresponding decrease in the total number of radical-trapping antioxidant markers [37]. In another study, rats fed the MgD diet displayed an impaired redox capacity, marked by increased levels of thiobarbituric acid-reactive substances and oxysterols in the plasma as well as an increased susceptibility of RBC to freeradical- induced haemolysis in vitro [38]. High levels of thiobarbituric acid-reactive substances in the aorta of rats fed the Mg deficient diet correlated with a significant reduction in the activity of superoxide dismutase and catalase as well as an increase in the net fractional rates of collagen synthesis [39]. In mice, hypomagnesaemia led to a decrease in GSH concentration and lowered the activity of superoxide dismutase, glutathione reductase, and glutathione S-transferase in RBCs. However, catalase activity increased and the activity of glutathione peroxidise was not significantly altered [40]. Boparai et al. found evidence for lipid peroxidation and protein oxidation in the plasma and liver of rats that received a low Mg diet [41]. Based on these findings, we investigated the effects of MgD on the intensity of protein oxidative damage. Fifty adult, female Wistar rats with weights between 200-250 g were divided into two groups. One group received a low Mg diet (Mg content ≤ 15 mg/kg) and demineralized water for two months to induce hypomagnesaemia. The other group was fed a basal control diet (Mg content ≈ 500 mg/kg) and water (with Mg content 20 mg/l) for an equal duration. To evaluate the Mg concentration, a two ml sample of heparinized venous blood was collected every week from the sublingual vein while the rats were under isoflurane anaesthesia [42]. RBC and plasma Mg levels were measured via a previously described colorimetric assay method in which Mg is stained using thiazole yellow [43]. After Mg concentration in rats fed the low Mg diet had decreased, the animals were treated with one of the following supplementations: Mg L-aspartate, Mg N-acetyltaurate, Mg chloride or Mg sulphate (50 mg of elementary Mg per kg body weight for 14 days). Protein carbonyls (PC) concentration was assessed using the reaction of carbonyl groups with 2, 4-dinitrophenylhydrazine (DNPH) and measuring the resulting protein-bound 2, 4-dinitrophenylhydrazones. The yellow product was quantified by spectrophotometry at 363 nm [44]. We then calculated the ratio between the concentrations of carbonyl products (mol/L) and total protein (g/L). We found that the increased level of PC in rats fed the low Mg diet was partially or completely reversed by treatment with certain organic and inorganic Mg salts [45].
Some research teams have focused on understanding these mechanisms on the cellular level. MgD promoted apoptosis in rat hepatocyte primary culture, which was accompanied by an accumulation of malondialdehyde and a decreased GSH concentration [46]. N-acetylcysteine partially attenuated the apoptosis of human and rat hepatocytes induced by low extracellular Mg concentrations, but surprisingly, also increased both caspase-3 activity and lipid peroxidation in hepatocytes exposed to physiological Mg concentrations [47]. Two hours after exposure to low Mg, human umbilical vein endothelial cells (HUVEC) were more sensitive to the oxidant action of H2O2 and demonstrated an increased level of the DNA damage marker 8-hydroxy-deoxyguanine compared to controls cultured in physiologic concentrations of Mg [48]. Dickens et al. found enhanced free radical-induced intracellular oxidation and cytotoxicity in bovine endothelial cells incubated in a low-Mg medium [49].
4. Mechanisms of oxidative stress caused by magnesium deficiency
MgD is believed to indirectly enhance oxidative damage of biomolecules by inducing a stress response (Figure 1). It is possible that a decreased ratio of Mg to Ca stimulates catecholamine release from the adrenal glands. However, catecholamines increase the production of ROS. This creates a vicious positive feedback cycle where, for example, elevated blood epinephrine levels result in a further reduction of the Mg concentration [50]. Contrastingly, MgD leads to the activation of the rennin-angiotensin system that also induces OS [51].
Fig. 1.
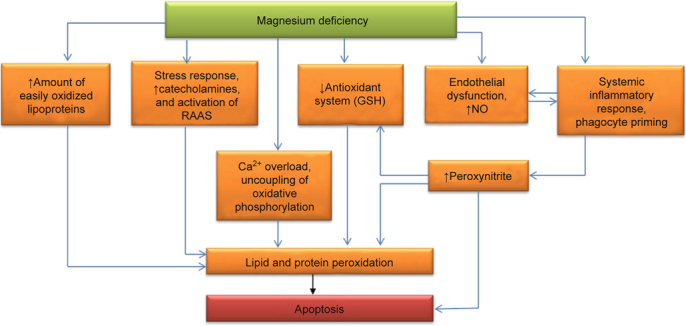
- Pathogenic relationship between magnesium deficiency and oxidative stress.
Inflammation is the other important cause of the OS that results from MgD [52]. MgD stimulates the production of acute phase proteins (e.g., C-reactive protein) [53]. The decrease of extra- and intracellular Mg concentrations sensitizes immunocompetent cells to proinflammatory stimuli. Collectively, factors that would not normally cause an immune response lead to an oxidative burden in phagocytes and neutrophil activation in Mgdeficient organisms. Furthermore, low a blood Mg concentration directly stimulates phagocyte priming and results in oxidative burden [54], possibly due to the rise of intracellular Ca levels [55]. Excessive amounts of RS, created by NADPH oxidase and myeloperoxidase, enter into the space around the neutrophils and macrophages [55] and damage biomolecules, particularly components of lipoproteins and the surrounding cells [56]. In contrast, Mg repletion therapy promotes an anti-inflammatory response and decreased levels of proinflammatory markers in initially Mg deficient rats [57, 58].
Another early marker of MgD is endothelial dysfunction [59]. Under physiological conditions, the endothelium produces signalling molecules, which maintain the dynamic balance between thrombin formation and fibrinolysis. These signalling molecules also control and inhibit excessive synthesis of proinflammatory cytokines [60]. The endothelial dysfunction linked to MgD has one important feature. Endothelial dysfunction is frequently associated with reduced NO production in endotheliocytes [61]. However, preclinical studies in animal and tissue models have demonstrated that MgD actually increased NO production in the endothelium and other cells via the activation of an inducible isoform of NO-synthase [62-65]. Elevated NO production can be a disadvantage because it is accompanied by a simultaneous increase of RS, such as superoxide [66]. Under these conditions, excessive NO does not cause vasodilation, but rather, reacts with superoxide to form peroxynitrite [67]. A potent vasoconstrictor, peroxynitrite easily causes oxidative damage to biomolecules and cellular structures [68, 69]. Mak et al. have shown that, in particular, excessive NO production is responsible for a decreased concentration of GSH in red blood cells [62]. Moreover, hyperproduction of NO can provoke the apoptosis of certain cell types [70]. Finally, endothelial dysfunction and a hyperactivated inflammatory response can potentiate each other [61].
Intracellular production of RS can be enhanced by impaired mitochondrial function. MgD facilitates the uncoupling of oxidative phosphorylation, which leads to electron loss in the electron transport chain [71]. Low Mg levels result in an accumulation of calcium in the cytosol [72, 73] that contributes to the uncoupling of oxidative phosphorylation as well as the stimulation of other peroxidation pathways [74-76]. An overproduction of peroxynitrite that also results from MgD further exacerbates mitochondrial dysfunction [77, 78].
Apart from the enhanced generation of ROS and free radicals, MgD also increases the amount of substrates that are available for radical oxidation. MgD promotes hypertriglyceridemia, in which numerous, easily-oxidized lipoproteins enter the blood stream [79] and the activity of lipoprotein lipase is down-regulated [80]. Additionally, MgD contributes to insulin resistance and the overproduction of contra-insulin hormones (epinephrine and cortisol) [81, 82]. The key factors implicated in hyperlipidaemia are: the activation of lipolysis in fat tissue, the excessive release of free fatty acids, the stimulation of lipogenesis in the liver followed by the hyperproduction of triglyceride-rich atherogenic lipoproteins and the inhibition of HDL synthesis [34, 83-85]. In cellular membranes, an increased ratio of Ca to Mg stimulates phospholipase A2 activity [86, 87], which is responsible for the mobilisation of unsaturated fatty acids (UFA) from phospholipids. Free UFA as well as those bound to triglycerides and phospholipids can be easily oxidized by ROS to form lipid hydroperoxides. These hydroperoxides can decompose to form new radicals, thus initiating branching chain reactions that lead to a self-sustaining peroxidation process [88, 89].
5. Suggestions for clinical application
As Mg is suggested to be an important player in the pathogenesis of diseases [2-13, 90-93] and is associated with disturbed antioxidant regulation [28, 31, 32, 37-40, 45, 48-50], estimation and correction of impaired magnesium status is highly recommended in MgD patients.
6. Conclusion
To summarise, MgD and OS are undoubtedly strongly linked together. Moreover, several well-established and also several emerging mechanisms of OS in Mg deficient organisms were described. Nevertheless, many aspects of the causal relationship between MgD and OS still remain fragmented. Therefore, further preclinical and clinical studies are necessary to clarify the mechanisms involved in relationship between MgD, OS and OSassociated diseases.
Acknowledgments
We gratefully acknowledge the financial support of the Ministry of Higher Education (Malaysia) under the project 600-RMI/ RAGS 5/3 (46/2014) and Universiti Teknologi MARA (Malaysia) under the project 600-IRMI/MyRA 5/3/LESTARI (0088/2016).
Abbreviations:
- Mg
Magnesium
- MgD
Magnesium Deficiency
- OS
Oxidative Stress
- AOS
Antioxidant Defence System
- RS/ROS
Reactive Species / Reactive Oxygen Species
- BOSS
Biomarkers of Oxidative Stress Study
- GSH/GSSG
Reduced/Oxidized Glutathione
- RBC
Red Blood Cell
- PC
Protein Carbonyls
- DNPH
2,4-Dinitrophenylhydrazine
- HUVEC
Human Umbilical Vein Endothelial Cells
- VLDL
Very-Low-Density Lipoprotein
- LDL
Low-Density Lipoprotein
- UFA
Unsaturated Fatty Acids
Footnotes
References
- [1].Park S, Johnson M, Fischer JG. Vitamin and mineral supplements: barriers and challenges for older adults. J Nutr Elder. 2008;27:297–317. doi: 10.1080/01639360802265855. [DOI] [PubMed] [Google Scholar]
- [2].Boy E, Mannar V, Pandav C, de Benoist B, Viteri F, Fontaine O, et al. Achievements, challenges, and promising new approaches in vitamin and mineral deficiency control. Nutr Rev. 2009; 67 Suppl 1: S24-S30. doi:10.1111/j.1753-4887.2009.00155.x. [DOI] [PubMed]
- [3].Suskind DL. Nutritional deficiencies during normal growth. Pediatr Clin North Am. 2009;56:1035–53. doi: 10.1016/j.pcl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- [4].Spasov AA, Orobinskaia TA, Smirnova LA. Magnesium salts in physiology and pathology: the potentials for their use in medicine. Usp Fiziol Nauk. 1997;28:79–93. [PubMed] [Google Scholar]
- [5].Seelig MS. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications (a review) J Am Coll Nutr. 1994;13:429–46. doi: 10.1080/07315724.1994.10718432. [DOI] [PubMed] [Google Scholar]
- [6].Elisaf M, Merkourpoulos M, Tsianos EV, Siamopoulos KC. Pathogenetic mechanisms of hypomagnesemia in alcoholic patients. J Trace Elem Med Biol. 1995;9:210–4. doi: 10.1016/S0946-672X(11)80026-X. [DOI] [PubMed] [Google Scholar]
- [7].Knoers NV. Inherited forms of renal hypomagnesemia: an update. Pediatr Nephrol. 2009;24:697–705. doi: 10.1007/s00467-008-0968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes. 2016;65:3–13. doi: 10.2337/db15-1028. [DOI] [PubMed] [Google Scholar]
- [9].Lima Mde L, Cruz T, Rodrigues LE, Bomfim O, Melo J, Correia R, et al. Serum and intracellular magnesium deficiency in patients with metabolic syndrome–evidences for its relation to insulin resistance. Diabetes Res Clin Pract. 2009;83:257–62. doi: 10.1016/j.diabres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- [10].de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- [11].Agus ZS. Hypomagnesemia. J Am Soc Nephrol. 1999;10:1616–22. doi: 10.1681/ASN.V1071616. [DOI] [PubMed] [Google Scholar]
- [12].Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24:47–66. [PMC free article] [PubMed] [Google Scholar]
- [13].Pham PC, Pham PA, Pham SV, Pham PT, Pham PM, Pham PT. Hypomagnesemia: a clinical perspective. Int J Nephrol Renovasc Dis. 2014;7:219–30. doi: 10.2147/IJNRD.S42054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barbagallo M, Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes Res. 2009;22(235):46. doi: 10.1684/mrh.2009.0187. [DOI] [PubMed] [Google Scholar]
- [15].Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- [16].Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–50. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- [18].Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002;227:671–82. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- [19].Heistad DD. Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler Thromb Vasc Biol. 2006;26:689–95. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- [20].Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zaĭtsev VG, Ostrovskiĭ OV. Markers of oxidative lesion and antioxidative system for the use in clinical laboratory diagnosis. Klin Lab Diagn. 2008;9:61. [Google Scholar]
- [23].Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- [24].Lee SH, Blair IA. Oxidative DNA damage and cardiovascular disease. Trends Cardiovasc Med. 2001;11:148–55. doi: 10.1016/S1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- [25].Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- [26].Ryzen E, Elbaum N, Singer FR, Rude RK. Parenteral magnesium tolerance testing in the evaluation of magnesium deficiency. Magnesium. 1985;4:137–47. [PubMed] [Google Scholar]
- [27].Elin RJ. Magnesium metabolism in health and disease. Dis Mon. 1988;34:161–218. doi: 10.1016/0011-5029(88)90013-2. [DOI] [PubMed] [Google Scholar]
- [28].Wegner M, Araszkiewicz A, Zozulińska-Ziółkiewicz D, Wierusz-Wysocka B, Pioruńska-Mikołajczak A, Pioruńska-Stolzmann M. The relationship between concentrations of magnesium and oxidized low density lipoprotein and the activity of platelet activating factor acetylhydrolase in the serum of patients with type 1 diabetes. Magnes Res. 2010;23:97–104. doi: 10.1684/mrh.2010.0207. [DOI] [PubMed] [Google Scholar]
- [29].Mahabir S, Wei Q, Barrera SL, Dong YQ, Etzel CJ, Spitz MR, Forman MR. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis. 2008; 29: 949-56. doi:10.1093/carcin/bgn043. [DOI] [PMC free article] [PubMed]
- [30].Hartwig A. Role of magnesium in genomic stability. Mutat Res. 2001;475:113–1121. doi: 10.1016/S0027-5107(01)00074-4. [DOI] [PubMed] [Google Scholar]
- [31].Barbagallo M, Dominguez LJ, Tagliamonte MR, Resnick LM, Paolisso G. Effects of glutathione on red blood cell intracellular magnesium: relation to glucose metabolism. Hypertension. 1999;34:76–82. doi: 10.1161/01.HYP.34.1.76. [DOI] [PubMed] [Google Scholar]
- [32].Cernak I, Savic V, Kotur J, Prokic V, Kuljic B, Grbovic D, et al. Alterations in magnesium and oxidative status during chronic emotional stress. Magnes Res. 2000;13:29–36. [PubMed] [Google Scholar]
- [33].Bae YJ, Choi MK. Magnesium intake and its relevance with antioxidant capacity in Korean adults. Biol Trace Elem Res. 2011; 143: 213 – 25. doi:10.1007/s12011-010-8883-y. [DOI] [PubMed]
- [34].Gueux E, Azais-Braesco V, Bussière L, Grolier P, Mazur A, Rayssiguier Y. Effect of magnesium deficiency on triacylglycerol-rich lipoprotein and tissue susceptibility to peroxidation in relation to vitamin E content. Br J Nutr. 1995;74:849–56. [PubMed] [Google Scholar]
- [35].Mak IT, Komarov AM, Wagner TL, Stafford RE, Dickens BF, Weglicki WB. Enhanced NO production during Mg deficiency and its role in mediating red blood cell glutathione loss. Am J Physiol. 1996;271:C385–390. doi: 10.1152/ajpcell.1996.271.1.C385. [DOI] [PubMed] [Google Scholar]
- [36].Wolf FI, Trapani V, Simonacci M, Boninsegna A, Mazur A, Maier JA. Magnesium deficiency affects mammary epithelial cell proliferation: involvement of oxidative stress. Nutr Cancer. 2009;61:131–6. doi: 10.1080/01635580802376360. [DOI] [PubMed] [Google Scholar]
- [37].Hans CP, Chaudhary DP, Bansal DD. Magnesium deficiency increases oxidative stress in rats. Indian J Exp Biol. 2002;40:1275–9. [PubMed] [Google Scholar]
- [38].Blache D, Devaux S, Joubert O, Loreau N, Schneider M, Durand P, et al. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic Biol Med. 2006;41:277–84. doi: 10.1016/j.freeradbiomed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [39].Shivakumar K, Kumar BP. Magnesium deficiency enhances oxidative stress and collagen synthesis in vivo in the aorta of rats. Int J Biochem Cell Biol. 1997;29:1273–8. doi: 10.1016/S1357-2725(97)00068-X. [DOI] [PubMed] [Google Scholar]
- [40].Kuzniar A, Mitura P, Kurys P, Szymonik-Lesiuk S, Florianczyk B, Stryjecka-Zimmer M. The influence of hypomagnesemia on erythrocyte antioxidant enzyme defence system in mice. Biometals. 2003;16:349–57. doi: 10.1023/A:1020632505289. [DOI] [PubMed] [Google Scholar]
- [41].Boparai RK, Kiran R, Bansal DD. Insinuation of exacerbated oxidative stress in sucrose-fed rats with a low dietary intake of magnesium: evidence of oxidative damage to proteins. Free Radic Res. 2007;41:981–9. doi: 10.1080/10715760701447892. [DOI] [PubMed] [Google Scholar]
- [42].Zeller W, Weber H, Panoussis B, Bürge T, Bergmann R. Refinement of blood sampling from the sublingual vein of rats. Lab Anim. 1998;32:369–76. doi: 10.1258/002367798780599910. [DOI] [PubMed] [Google Scholar]
- [43].Iezhitsa IN, Spasov AA, Kharitonova MV, Kravchenko MS. Effect of magnesium chloride on psychomotor activity, emotional status, and acute behavioural responses to clonidine, d-amphetamine, arecoline, nicotine, apomorphine, and L-5-hydroxytryptophan. Nutr Neurosci. 2011;14:10–24. doi: 10.1179/174313211X12966635733277. [DOI] [PubMed] [Google Scholar]
- [44].Hawkins CL, Morgan PE, Davies MJ. Quantification of protein modification by oxidants. Free Radic Biol Med. 2009; 46: 965-88. doi:10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed]
- [45].Kharitonova MV, Zheltova AA, Iezhitsa IN, Ozerov AA, Spasov AA. Effect of magnesium salts on oxidative protein modification in rats fed low magnesium diet. Abstracts of the 12th Meeting of the Asia Pacific Federation of Pharmacologists, 9-13 July, 2013, Shanghai, China, Acta Pharmacol Sin. 2013, 34 (Supplement), 151-151 (S13. 19).
- [46].Martin H, Richert L, Berthelot A. Magnesium deficiency induces apoptosis in primary cultures of rat hepatocytes. J Nutr. 2003;133:2505–11. doi: 10.1093/jn/133.8.2505. [DOI] [PubMed] [Google Scholar]
- [47].Martin H, Abadie C, Heyd B, Mantion G, Richert L, Berthelot A. N-acetylcysteine partially reverses oxidative stress and apoptosis exacerbated by Mg-deficiency culturing conditions in primary cultures of rat and human hepatocytes. J Am Coll Nutr. 2006;25:363–9. doi: 10.1080/07315724.2006.10719547. [DOI] [PubMed] [Google Scholar]
- [48].Wolf FI, Trapani V, Simonacci M, Ferré S, Maier JA. Magnesium deficiency and endothelial dysfunction: is oxidative stress involved? Magnes Res. 2008;21:58–64. [PubMed] [Google Scholar]
- [49].Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992;311:187–91. doi: 10.1016/0014-5793(92)81098-7. [DOI] [PubMed] [Google Scholar]
- [50].Joborn H, Akerström G, Ljunghall S. Effects of exogenous catecholamines and exercise on plasma magnesium concentrations. Clin Endocrinol (Oxf) 1985;23:219–26. doi: 10.1111/j.1365-2265.1985.tb00217.x. [DOI] [PubMed] [Google Scholar]
- [51].Rayssiguier Y, Libako P, Nowacki W, Rock E. Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnes Res. 2010;23:73–80. doi: 10.1684/mrh.2010.0208. [DOI] [PubMed] [Google Scholar]
- [52].Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys. 2007;458:48–56. doi: 10.1016/j.abb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- [53].King DE. Inflammation and elevation of C-reactive protein: does magnesium play a key role? Magnes Res. 2009;22:57–9. [PubMed] [Google Scholar]
- [54].Libako P, Nowacki W, Rock E, Rayssiguier Y, Mazur A. Phagocyte priming by low magnesium status: input to the enhanced inflammatory and oxidative stress responses. Magnes Res. 2010;23:1–4. doi: 10.1684/mrh.2009.0201. [DOI] [PubMed] [Google Scholar]
- [55].Bussière FI, Gueux E, Rock E, Mazur A, Rayssiguier Y. Protective effect of calcium deficiency on the inflammatory response in magnesium-deficient rats. Eur J Nutr. 2002;41:197–202. doi: 10.1007/s00394-002-0376-0. [DOI] [PubMed] [Google Scholar]
- [56].Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41:457–65. [PMC free article] [PubMed] [Google Scholar]
- [57].Spasov AA, Iezhitsa IN, Kravchenko MS, Kharitonova MV. Study of anti-inflammatory activity of some organic and inorganic magnesium salts in rats fed with magnesium-deficient diet. Vopr Pitan. 2007;76:67–73. [PubMed] [Google Scholar]
- [58].Kharitonova M, Iezhitsa I, Zheltova A, Ozerov A, Spasov A, Skalny A. Comparative angioprotective effects of magnesium compounds. J Trace Elem Med Biol. 2015; 29: 227-34. doi:10.1016/j.jtemb.2014.06.026. [DOI] [PubMed]
- [59].Maier JA, Malpuech-Brugère C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689:13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [60].Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary venoocclusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant. 2008; 41: 677-86. doi:10.1038/sj.bmt.1705990. [DOI] [PubMed]
- [61].Corti R, Fuster V, Badimon JJ. Pathogenetic concepts of acute coronary syndromes. J Am Coll Cardiol. 2003;41:7S–14S. doi: 10.1016/S0735-1097(02)02833-4. [DOI] [PubMed] [Google Scholar]
- [62].Mak IT, Komarov AM, Wagner TL, Stafford RE, Dickens BF, Weglicki WB. Enhanced NO production during Mg deficiency and its role in mediating red blood cell glutathione loss. Am J Physiol. 1996;271:C385–90. doi: 10.1152/ajpcell.1996.271.1.C385. [DOI] [PubMed] [Google Scholar]
- [63].Carlin Schooley M, Franz KB. Magnesium deficiency during pregnancy in rats increases systolic blood pressure and plasma nitrite. Am J Hypertens. 2002;15:1081–6. doi: 10.1016/S0895-7061(02)03064-9. [DOI] [PubMed] [Google Scholar]
- [64].Yokoyama T, Oono H, Miyamoto A, Ishiguro S, Nishio A. Magnesium-deficient medium enhances NO production in alveolar macrophages isolated from rats. Life Sci. 2003;72:1247–57. doi: 10.1016/S0024-3205(02)02371-8. [DOI] [PubMed] [Google Scholar]
- [65].Nagai N, Fukuhata T, Ito Y. Effect of magnesium deficiency on intracellular ATP levels in human lens epithelial cells. Biol Pharm Bull. 2007;30:6–10. doi: 10.1248/bpb.30.6. [DOI] [PubMed] [Google Scholar]
- [66].Rock E, Astier C, Lab C, Malpuech C, Nowacki W, Gueux E, et al. Magnesium deficiency in rats induces a rise in plasma nitric oxide. Magnes Res. 1995;8:237–422. [PubMed] [Google Scholar]
- [67].Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- [68].Obata T. Nitric oxide and depolarization induce hydroxyl radical generation. Jpn J Pharmacol. 2002;88:1–5. doi: 10.1254/jjp.88.1. [DOI] [PubMed] [Google Scholar]
- [69].Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–70. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- [70].Han W, Fu S, Wei N, Xie B, Li W, Yang S, et al. Nitric oxide overproduction derived from inducible nitric oxide synthase increases cardiomyocyte apoptosis in human atrial fibrillation. Int J Cardiol. 2008; 130: 165 – 73. doi:10.1016/j.ijcard.2008.02.026. [DOI] [PubMed]
- [71].Goubern M, Rayssiguier Y, Miroux B, Chapey MF, Ricquier D, Durlach J. Effect of acute magnesium deficiency on the masking and unmasking of the proton channel of the uncoupling protein in rat brown fat. Magnes Res. 1993;6:135–43. [PubMed] [Google Scholar]
- [72].Turlapaty PD, Altura BM. Extracellular magnesium ions control calcium exchange and content of vascular smooth muscle. Eur J Pharmacol. 1978;52:421–3. doi: 10.1016/0014-2999(78)90303-5. [DOI] [PubMed] [Google Scholar]
- [73].Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. 1995;41:347–59. [PubMed] [Google Scholar]
- [74].Malis CD, Bonventre JV. Mechanism of calcium potentiation of oxygen free radical injury to renal mitochondria. A model for postischemic and toxic mitochondrial damage. J Biol Chem. 1986;261:14201–8. [PubMed] [Google Scholar]
- [75].Kristián T, Siesjö BK. Calcium in ischemic cell death. Stroke. 1998;29:705–18. doi: 10.1161/01.STR.29.3.705. [DOI] [PubMed] [Google Scholar]
- [76].Brookes PS, Yoon Y, Robotham JL, Anders MW, and ROS. a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- [77].Boczkowski J, Lisdero CL, Lanone S, Samb A, Carreras MC, Boveris A, et al. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J. 1999;13:1637–46. doi: 10.1096/fasebj.13.12.1637. [DOI] [PubMed] [Google Scholar]
- [78].Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J Neurosci Res. 2007;85:2216–23. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- [79].Rayssiguier Y, Libako P, Nowacki W, Rock E. Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnes Res. 2010;23:73–80. doi: 10.1684/mrh.2010.0208. [DOI] [PubMed] [Google Scholar]
- [80].Spasov AA. Magnesium in medical practice. Volgograd, “Otrok”, 2000, 268. (Russian)
- [81].Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. Failure of beta-cell function for compensate variation in insulin sensitivity in hypomagnesemic subjects. Magnes Res. 2009;22:151–6. [PubMed] [Google Scholar]
- [82].Günther T. The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes Res. 2010;23:5–18. doi: 10.1684/mrh.2009.0195. [DOI] [PubMed] [Google Scholar]
- [83].Nassir F, Mazur A, Giannoni F, Gueux E, Davidson NO, Rayssiguier Y. Magnesium deficiency modulates hepatic lipogenesis and apolipoprotein gene expression in the rat. Biochim Biophys Acta. 1995;1257:125–322. doi: 10.1016/0005-2760(95)00065-K. [DOI] [PubMed] [Google Scholar]
- [84].King JL, Miller RJ, Blue JP, Jr, O’Brien WD, Jr, Erdman JW., Jr Inadequate dietary magnesium intake increases atherosclerotic plaque development in rabbits. Nutr Res. 2009;29:343–9. doi: 10.1016/j.nutres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Corica F, Corsonello A, Ientile R, Cucinotta D, Di Benedetto A, Perticone F, et al. Serum ionized magnesium levels in relation to metabolic syndrome in type 2 diabetic patients. J Am Coll Nutr. 2006;25:210–5. doi: 10.1080/07315724.2006.10719534. [DOI] [PubMed] [Google Scholar]
- [86].Chakraborti S, Michael JR, Patra SK. Protein kinase C dependent and independent activation of phospholipase A2 under calcium ionophore (A23187) exposure in rabbit pulmonary arterial smooth muscle cells. FEBS Lett. 1991;285:104–7. doi: 10.1016/0014-5793(91)80735-L. [DOI] [PubMed] [Google Scholar]
- [87].Murthy M, Rao GH, Robinson P, Reddy S. Influx of extracellular calcium and agonist-coupling appear essential for the activation of thromboxane A2-dependent phospholipase A2 in human platelets. Prostaglandins Leukot Essent Fatty Acids. 1995;53:31–9. doi: 10.1016/0952-3278(95)90080-2. [DOI] [PubMed] [Google Scholar]
- [88].Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–42. [PubMed] [Google Scholar]
- [89].Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–43. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Agarwal R, Iezhitsa L, Agarwal P. Pathogenetic role of magnesium deficiency in ophthalmic diseases. Biometals. 2014;27:5–18. doi: 10.1007/s10534-013-9684-5. [DOI] [PubMed] [Google Scholar]
- [91].Agarwal R, Iezhitsa IN, Agarwal P, Spasov AA. Mechanisms of cataractogenesis in the presence of magnesium deficiency. Magnes Res. 2013; 26: 2–8. http://www.jle.com/e-docs/00/04/86/AC/vers_alt/VersionPDF.pdf doi:10.1684/mrh.2013.0336 [DOI] [PubMed]
- [92].Iezhitsa IN, Spasov AA. Potassium magnesium homeostasis: physiology, pathophysiology, clinical consequences of deficiency and pharmacological correction. Usp Fiziol Nauk. 2008;39:23–41. [PubMed] [Google Scholar]
- [93].Iezhitsa IN. Potassium and magnesium depletions in congestive heart failure–pathophysiology, consequences and replenishment. Clin Calcium. 2005;15:123–33. [PubMed] [Google Scholar]


