Abstract
Background
Health-related quality of life (HRQOL) has been reported to be associated with cognitive function; however, whether or not this relationship involves causality is uncertain. This study aimed to determine whether HRQOL levels are associated with subsequent changes in cognitive function in elderly people requiring care.
Methods
Participants were 74 community-dwelling elderly people utilizing the long-term care service (69 % women) who underwent physical and psychological examinations at baseline and follow-up. The outcome was 2-year changes in Mini-Mental State Examination (∆MMSE) score. The potential predictor was HRQOL level assessed by the EuroQol 5 dimension (EQ-5D) score (utility value) at baseline; other variables were body mass index (BMI), Barthel index, grip strength, Geriatric Depression Scale, serum albumin, and serum hemoglobin. Associations between EQ-5D and ∆MMSE scores were assessed using correlation analysis, regression analysis, and analysis of covariance (ANCOVA).
Results
Mean age, BMI, and Barthel index at baseline were 81.6 years [standard deviation (SD) 8.2], 21.1 kg/m2 (SD 4.0), and 79 (SD 20), respectively; the mean ∆MMSE score was –2.2 (SD 5.1). EQ-5D was significantly correlated with ∆MMSE (partial r = 0.375, P = 0.0012). The mean ∆MMSE values of the 1st, 2nd, and 3rd EQ-5D quartiles were –4.2 (adjusted P = 0.0050), –2.6 (adjusted P = 0.0476), and –2.4 (adjusted P = 0.0298), respectively, which were lower than the –0.1 of the reference 4th quartile.
Conclusions
HRQOL as assessed by EQ-5D is associated with longitudinal cognitive decline in frail elderly people, and cognitive function may be maintained in individuals with high HRQOL levels.
Keywords: Cohort studies, Dementia, Frail elderly, Mild cognitive impairment, Quality of life
Introduction
The elderly population is rapidly growing in Japan. The proportion of people aged 65 years and older was 26.0 % in 2014, and is projected to be as high as 38.8 % by 2050 [1]. Accordingly, the number of frail elderly people requiring care is also increasing. The long-term care service system was implemented in Japan in 2000; since then, the number of elderly people requiring care has increased steeply from 2.2 to 5.9 million in 2014 [1].
Cognitive impairment is a common health problem in physically frail elderly people. An important challenge for these individuals is preventing a decrease in their cognitive function. A number of cohort studies have reported that higher levels of frailty predict cognitive decline, and that various frailty-related indicators, such as body mass index (BMI), muscle strength, activities of daily living (ADL), serum albumin concentration, and depression are also predictive of impaired cognitive function [2–4].
Health-related quality of life (HRQOL) is an important construct for elderly people, because it describes an individual’s overall health status [5]. Some epidemiologic studies have found that HRQOL is associated with cognitive function [5–7], but it is not clear whether causality is involved or not. We previously conducted a longitudinal study to determine factors that prevent diminished ADL performance in community-dwelling elderly people utilizing the long-term care service system [8, 9]. The present study used this framework to determine whether HRQOL levels are associated with longitudinal changes in cognitive function in frail elderly people requiring care.
Participants and methods
Study participants
This study targeted all 518 noninstitutionalized elderly persons using the long-term care insurance in 2002 October in Yamato town (currently a part of Minamiuonuma city), Niigata prefecture, Japan. Of the 518 individuals, 194 agreed to participate in baseline examinations [agreement rate 37.5 % (194/518)], of whom 156 completed the Mini-Mental State Examination (MMSE) [participation rate 30.1 % (156/518)]. Of these, 79 participated in the follow-up MMSE examination (22 died and 55 did not participate); we excluded five due to severe cognitive impairment (MMSE <18) [10] at baseline, giving the follow-up study a total of 74 participants [follow-up rate 47.4 % (74/156)].
Informed consent was obtained from all participants and their family members. Baseline and follow-up data were linked with subject-specific anonymous identification numbers, and the linked dataset was created without using any personal information. The study protocol was approved by the Ethics Committee of Niigata University School of Medicine.
Baseline examinations
The baseline examinations were conducted between January and April 2003, and included collection of demographic data and information on ADL levels through interviews, physical and psychological examinations, and collection of a blood sample. All subjects at baseline responded to the interviews. For 112 subjects who were using ambulatory care (day-service), examinations were conducted at each of the respective day-service facilities; for the remaining 44 subjects who were not using a day-service, we visited the subjects’ homes for the examination. Detailed information on the baseline study has been published previously [8].
Demographic data, including age, sex, and past and current disease histories were obtained from the subjects.
ADL levels were evaluated by the Barthel index [11], with zero points assigned to those requiring the maximum level of assistance and 100 points assigned to those requiring the minimum level of assistance. Cognitive function, the outcome of this study, was assessed using the MMSE [12]. These scores range from zero to 30, with lower scores indicating greater cognitive impairment; an MMSE score less than 24 was defined as cognitive impairment [13].
Body weight was measured, and body height was estimated as being twice the value of the left arm span [14], since height in the standing position could not be measured accurately in some subjects. Arm span-estimated height has been reported to highly correlate with body height (r = 0.93) [14], and thus can be used for calculating body mass index (BMI). The grip strength of each hand was measured once with a digital hand dynamometer (T.K.K.5401 Takei Scientific Instruments Co., Ltd., Niigata, Japan) and the higher value was adopted.
HRQOL was measured using the Japanese version of the EuroQol 5 dimension (EQ-5D) quality of life assessment tool [15]. The EQ-5D measures health status and expresses it in a descriptive profile and an index value [16]. This study used the EQ-5D descriptive system in face-to-face interviews. Interviewees describe their health state by choosing one of three levels (1, “extreme problems”; 2, “some problems”; and 3, “no problem”) for each of five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. From these, we obtained each EQ-5D score (utility values) using a conversion table developed with the Japanese value set [17]. The EQ-5D score ranges from 1 to –0.1110, whereby 1 denotes “perfectly healthy”, 0 denotes “dead”, and –0.1110 denotes “worse than dead/worst health state possible”. Mood was evaluated using the 15-item version of the Geriatric Depression Scale (GDS-15) [18], in which a high score indicates depression. Psychological assessments including EQ-5D and GDS-15 were performed through interviews by two trained nurses.
Serum albumin and blood hemoglobin concentrations, which have been reported as potential predictors of cognitive decline [4], were determined by the bromocresol green (BCG) and sodium lauryl sulphate–hemoglobin (SLS–HB) methods, respectively, from a non-fasting blood sample using a clinical biochemistry analyser (BioMajesty 12; Jeol, Tokyo, Japan).
Follow-up examination
MMSE was reassessed between January and April 2005 in the follow-up examination conducted in the same manner as the baseline examination [9]. Two-year changes in MMSE (∆MMSE) were considered to be an indicator of longitudinal cognitive decline.
Statistical analyses
Data for continuous variables were tested for normality. Because MMSE and ∆MMSE scores were skewed toward lower values, they were logarithmically transformed for the statistical tests. EQ-5D level was divided into quartiles. To compare characteristics between participants and non-participants in the follow-up examination, Student’s t test and χ2 test were used to test differences in mean values and proportions, respectively. To test associations between baseline characteristics and MMSE profiles, baseline variables and log-transformed MMSE or log-transformed ∆MMSE scores were assessed using Pearson’s correlation coefficients. Further, Pearson’s partial correlation coefficients adjusted for age and sex were also calculated. To test linear trends (P for trend) between baseline characteristics and EQ-5D quartiles, simple linear regression analysis was used. To test linear trend (P for trend) between EQ-5D quartiles and log-transformed ∆MMSE scores, multiple linear regression analysis adjusted for age, sex, baseline log-transformed MMSE, histories of cerebrovascular disease, cardiovascular disease, and diabetes was used. Furthermore, the mean values of ∆MMSE among EQ-5D quartiles and among levels of each dimension were compared by Dunnett multiple comparison of analysis of covariance (ANCOVA), adjusting for the same covariates as multiple linear regression analysis. To determine associations between EQ-5D quartiles and incidence of cognitive impairment, i.e., the proportion of persons with MMSE <24 at follow-up in participants who were cognitively normal at baseline (MMSE ≥24) and followed up for 2 years (n = 60), multiple logistic regression analysis was performed, adjusting for the same covariates as multiple linear regression analysis. Finally, correlation between the five dimensional scores of EQ-5D and ∆MMSE was assessed using Pearson’s partial correlation coefficients. Data were analysed using SAS statistical software (release 9.1.3, SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
Mean values [standard deviation (SD)] of age, BMI, grip strength, Barthel index, EQ-5D score, GDS-15 score, serum albumin, blood hemoglobin, and MMSE scores were 81.6 years (8.2), 21.1 kg/m2 (4.0), 16.8 kg (6.6), 79 (20), 0.56 (0.22), 4.1 (3.1), 4.0 g/dL (0.3), 13.1 g/dL (1.5), and 24.9 (2.8), respectively, in the 74 participants (51 women and 23 men) who completed follow-up. The same variables were 83.9 years (7.6), 19.1 kg/m2 (3.6), 14.4 kg (6.2), 64 (29), 0.46 (0.22), 4.8 (3.3), 3.8 g/dL (0.3), 12.2 g/dL (1.9), and 20.0 (6.0) in the other 82 participants (58 women and 24 men), respectively. Statistically significant differences between groups were noted for all variables except for age and GDS-15. Prevalence of cognitive impairment (MMSE <24) was 18.9 % in the 74 participants and 65.9 % in the 82 participants. Prevalence of past or current disease history of cerebrovascular disease, cardiovascular disease, diabetes was 17.6, 14.9, 8.1 % in the 74 participants, and 24.4 %, 15.9 %, and 6.1 % in the 82 participants, respectively, with no statistically significant difference.
Pearson’s correlation coefficients between variables at baseline and MMSE scores are shown in Table 1. Herein, we describe significant findings at baseline and 2-year changes in MMSE (∆MMSE) as analyzed using partial correlation coefficients, adjusted for age and sex. At baseline, grip strength (partial r = 0.207, P = 0.0106), Barthel index (partial r = 0.294, P = 0.0002), and serum albumin (partial r = 0.228, P = 0.0046) were significantly correlated with baseline MMSE scores. In the longitudinal analyses, Barthel index (partial r = 0.244, P = 0.0386) and EQ-5D score (partial r = 0.375, P = 0.0012) was significantly correlated with ∆MMSE, while BMI, grip strength, GDS-15, albumin, and haemoglobin were not.
Table 1.
Pearson’s correlation coefficients (upper line) and partial correlation coefficients (lower line) between variables at baseline and scores of Mini-Mental State Examination (MMSE)
| Baseline variables | MMSE score | |||
|---|---|---|---|---|
| Baseline (n = 156) | Follow-up (n = 74) | 2-year changes (n = 74) | ||
| BMI (kg/m2) | r | 0.112 (P = 0.1656) | –0.136 (P = 0.2463) | –0.053 (P = 0.6528) |
| partial r | 0.062 (P = 0.4482) | –0.178 (P = 0.1349) | –0.057 (P = 0.6371) | |
| Grip strength (kg) | r | 0.248† (P = 0.0020) | 0.120 (P = 0.3065) | 0.093 (P = 0.4331) |
| partial r | 0.207† (P = 0.0106) | 0.057 (P = 0.6364) | 0.067 (P = 0.5753) | |
| Barthel index | r | 0.269 (P = 0.0007) | 0.184 (P = 0.1157) | 0.191 (P = 0.1023) |
| partial r | 0.294 (P = 0.0002) | 0.261 (P = 0.0270) | 0.244 (P = 0.0386) | |
| EQ-5D score | r | 0.112 (P = 0.1622) | 0.289 (P = 0.0124) | 0.355 (P = 0.0019) |
| partial r | 0.137 (P = 0.0900) | 0.322 (P = 0.0058) | 0.375 (P = 0.0012) | |
| Mobility | r | 0.070 (P = 0.3826) | 0.245 (P = 0.0351) | 0.280 (P = 0.0156) |
| partial r | 0.090 (P = 0.2647) | 0.279 (P = 0.0175) | 0.302 (P = 0.0100) | |
| Self-care | r | 0.227 (P = 0.0044) | 0.321 (P = 0.0053) | 0.233 (P = 0.0461) |
| partial r | 0.248 (P = 0.0020) | 0.381 (P = 0.0010) | 0.280 (P = 0.0173) | |
| Usual activities | r | 0.313 (P < 0.0001) | 0.283 (P = 0.0146) | 0.195 (P = 0.0964) |
| partial r | 0.316 (P < 0.0001) | 0.304 (P = 0.0095) | 0.216 (P = 0.0689) | |
| Pain/discomfort | r | –0.297 (P = 0.0002) | 0.199 (P = 0.0893) | 0.356 (P = 0.0019) |
| partial r | –0.287 (P = 0.0003) | 0.185 (P = 0.1194) | 0.344 (P = 0.0031) | |
| Anxiety/depression | r | 0.137 (P = 0.0888) | 0.033 (P = 0.7831) | 0.179 (P = 0.1260) |
| partial r | 0.131 (P = 0.1045) | 0.026 (P = 0.8260) | 0.171 (P = 0.1514) | |
| GDS-15 score | r | –0.094* (P = 0.2446) | –0.234* (P = 0.0464) | –0.188* (P = 0.1112) |
| partial r | –0.121* (P = 0.1356) | –0.229* (P = 0.0549) | –0.183* (P = 0.1264) | |
| Serum albumin (g/dL) | r | 0.230 (P = 0.0040) | 0.127 (P = 0.2800) | 0.105 (P = 0.3720) |
| partial r | 0.228 (P = 0.0046) | 0.150 (P = 0.2073) | 0.131 (P = 0.2717) | |
| Blood hemoglobin (g/dL) | r | 0.078 (P = 0.3340) | –0.146 (P = 0.2153) | –0.120 (P = 0.3100) |
| partial r | 0.036 (P = 0.6577) | –0.181 (P = 0.1285) | −0.134 (P = 0.2605) | |
Partial correlation coefficients were adjusted for age and sex
r correlation coefficient
* 1 value missing
†3 values missing
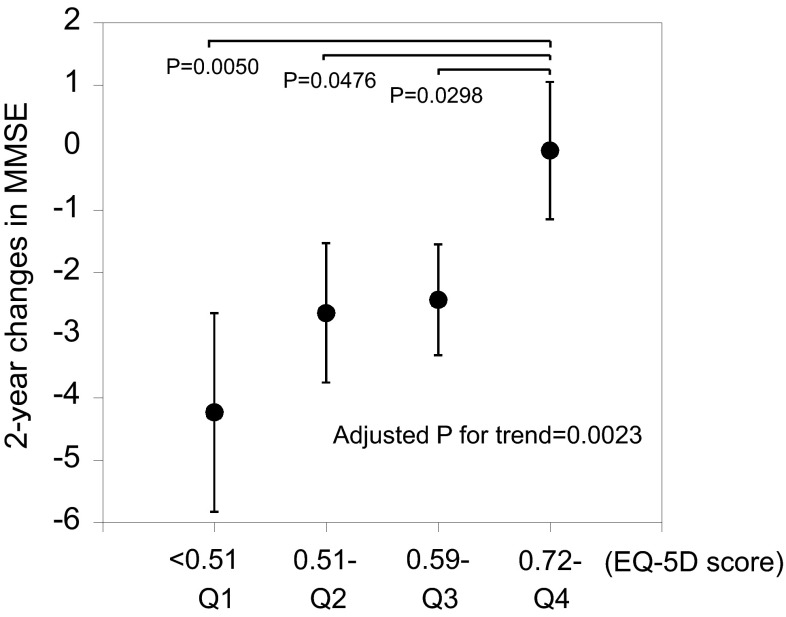
Demographic, physical, and biochemical characteristics at baseline according to baseline EQ-5D quartiles in 74 participants followed up are displayed in Table 2. Baseline Barthel index and serum albumin were positively (P < 0.0001 and P = 0.0171, respectively), and baseline GDS-15 was inversely (P < 0.0001) associated with EQ-5D. ∆MMSE according to EQ-5D quartiles at baseline are shown in Fig. 1. Participants with higher EQ-5D levels had smaller ∆MMSE (adjusted P for trend = 0.0023). The unadjusted mean ∆MMSE values were –4.2, –2.6, and –2.4 for the 1st, 2nd, and 3rd quartiles, respectively, showing significant differences (i.e., decreased) from the –0.1 of the 4th quartile reference group. We further explored if EQ-5D quartiles are associated with incidence (24 persons) of cognitive impairment in cognitively normal participants (n = 60). Multiple logistic regression analysis showed that the adjusted odds ratio for one unit increase of EQ-5D quartiles was 0.53 (95 % confidence interval 0.30–0.92; P = 0.0234).
Table 2.
Demographic, physical, and biochemical characteristics (mean with SD in parentheses) at baseline according to baseline EQ-5D quartiles in 74 participants who completed follow-up
| Baseline variables | EQ-5D quartiles | P for trend | |||
|---|---|---|---|---|---|
| 1st quartile <0.51 (n = 17) | 2nd quartile 0.51–0.58 (n = 14) | 3rd quartile 0.59–0.71 (n = 23) | 4th quartile ≥0.72 (n = 20) | ||
| Age (years) | 77.5 (9.6) | 84.0 (6.1) | 83.7 (6.3) | 81.0 (9.0) | 0.2372 |
| Body height (cm) | 154 (8) | 150 (7) | 151 (8) | 154 (7) | 0.9657 |
| Body weight (kg) | 47.8 (10.1) | 46.5 (8.2) | 49.3 (10.3) | 51.4 (9.9) | 0.1913 |
| BMI (kg/m2) | 20.0 (3.3) | 20.6 (3.8) | 21.7 (4.9) | 21.6 (3.7) | 0.1703 |
| Grip strength (kg) | 17.6 (8.8) | 14.6 (4.0) | 16.2 (5.8) | 18.3 (6.8) | 0.5823 |
| Barthel index | 55.9 (21.7) | 77.5 (13.4) | 84.3 (13.0) | 94.0 (5.0) | <0.0001 |
| GDS-15 score | 6.8* (4.0) | 4.1 (2.8) | 3.4 (1.8) | 2.8 (2.2) | <0.0001 |
| Serum albumin (g/dL) | 3.9 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 4.2 (0.3) | 0.0171 |
| Blood hemoglobin (g/dL) | 13.0 (1.6) | 12.8 (1.1) | 13.1 (1.8) | 13.3 (1.4) | 0.4467 |
| MMSE score | 25.1 (3.0) | 24.2 (4.0) | 24.3 (2.6) | 25.9 (1.7) | 0.8231 |
* 1 value missing
Fig. 1.
Unadjusted mean values of 2-year changes in the Mini-Mental State Examination (MMSE) according to quartiles of baseline EQ-5D. Mean values across ED-5Q quartiles were compared by ANCOVA with Dunnett multiple comparison test. P for trend was adjusted for age, sex, baseline log-transformed MMSE, and histories of cerebrovascular disease, cardiovascular disease, and diabetes. Bars indicate standard errors
We analysed the correlation between the five dimensional scores of the EQ-5D and ∆MMSE (Table 1). When correlation coefficients were adjusted for age and sex, “pain/discomfort” had the highest coefficient (partial r = 0.344, P = 0.0031), followed by “mobility” (partial r = 0.302, P = 0.0100) and “self-care” (partial r = 0.2798, P = 0.0173), but the coefficients for “usual activities” or “anxiety/depression” were not statistically significant. Regarding “pain/discomfort”, the mean ∆MMSE of the “some problems” group (n = 41) or “extreme problems” group (n = 2) was –3.3 (adjusted P = 0.0054), which was significantly different from the –0.3 of the reference “no problems” group (n = 36).
Discussion
This study first reported a longitudinal association between EQ-5D levels and subsequent cognitive decline in frail elderly people. This association was maintained across various statistical methods including simple correlation, multiple linear regression analysis, ANCOVA, and multiple logistic regression analysis.
Studies have reported a cross-sectional association between HRQOL and cognitive function. Wolfs et al. [6] showed a significant correlation between EQ-5D and MMSE scores in elderly patients with cognitive impairment. Two additional cross-sectional studies reported associations between HRQOL (assessed with scales other than the EQ-5D) and cognitive function in elderly people [5, 7].
Wolfs et al. [6] also showed a significant correlation between score changes of both EQ-5D and MMSE, but did not analyse the causality between the two variables. To the best of our knowledge, however, no cohort studies have been conducted. The present cohort study is the first to report EQ-5D-assessed HRQOL associated with subsequent cognitive decline in frail elderly people; that is, cognitive function is maintained in individuals with high levels of HRQOL. This finding is supported by our additional finding that those with normal cognition and higher EQ-5D had a lower risk of cognitive impairment, as analysed by multiple logistic regression analysis.
We also showed that the “pain/discomfort” dimension of the EQ-5D had a higher coefficient correlated with cognitive decline than ADL-related dimensions, such as “mobility” and “self-care”. This finding implies a role for pain and discomfort in deteriorating cognitive function. This hypothesis is partly supported by a recently published paper reporting that elderly people with more severe chronic pain show a decline in cognitive function, e.g., poorer performance on memory tests and executive functioning [19]. Further research is needed to verify this hypothesis.
Generalization of our findings should be made with caution. We studied physically dependent elderly people requiring some care at home, who are considered to be at high risk for cognitive impairment. Therefore, our results can be generalized to frail elderly people in the community; however, they may not be applicable to independent elderly people at relatively low risk for cognitive impairment. This is a limitation of this study.
This study was conducted in 2003–2005. As such, some situations may have differed from those at present. Nationwide number of users of long-term care insurance in 2005 was 4.1 million, while that in 2014 is 5.9 million, or nearly 1.5 times more [1]. This may indicate that characteristics of the users are becoming diversified. In that context, it is interesting to confirm whether the association between HRQOL and cognitive decline in the population at present is similar to that of the present study.
Some other limitations are worth noting in this study. First, our results may have been influenced by selection bias, given the low agreement rate (37.5 %). In addition, the prevalence of cognitive impairment (MMSE <24) in the 82 dropouts (65.9 %) was much higher than that of the 74 non-dropouts (18.9 %), suggesting that our results may reflect frail individuals with better cognition. Second, we adjusted for basic confounding factors such as age and sex, but could not control for many other potential confounders, including environmental and lifestyle factors of the participants.
In conclusion, EQ-5D-assessed HRQOL is associated with longitudinal cognitive decline in frail elderly people. Cognitive function may be maintained in individuals with high levels of HRQOL. Further studies will be needed to understand whether intervention for achieving high levels of HRQOL prevent cognitive decline in frail elderly people.
Acknowledgments
The authors wish to thank Ms. M. Hasegawa for her help in data collection. This study was funded partly by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (C) 14572208.
Compliance with ethical standards
Conflict of interest
None declared.
References
- 1.Health and Welfare Statistics Association 2015/2016 . Kokumin-Eisei-no-Doko. Tokyo: Health and Welfare Statistics Association; 2015. [Google Scholar]
- 2.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment: a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Rajan KB, Hebert LE, Scherr PA, Mendes de Leon CF, Evans DA. Disability in basic and instrumental activities of daily living is associated with faster rate of decline in cognitive function of older adults. J Gerontol A Biol Sci Med Sci. 2013;68:624–630. doi: 10.1093/gerona/gls208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taniguchi Y, Shinkai S, Nishi M, Murayama H, Nofuji Y, Yoshida H, et al. Nutritional biomarkers and subsequent cognitive decline among community-dwelling older Japanese: a prospective study. J Gerontol A Biol Sci Med Sci. 2014;69:1276–1283. doi: 10.1093/gerona/glt286. [DOI] [PubMed] [Google Scholar]
- 5.Davis JC, Bryan S, McLeod R, Rogers J, Khan K, Liu-Ambrose T. Exploration of the association between quality of life, assessed by the EQ-5D and ICECAP-O, and falls risk, cognitive function and daily function, in older adults with mobility impairments. BMC Geriatr. 2012;12:65. doi: 10.1186/1471-2318-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfs CA, Dirksen CD, Kessels A, Willems DC, Verhey FR, Severens JL. Performance of the EQ-5D and the EQ-5D+C in elderly patients with cognitive impairments. Health Qual Life Outcomes. 2007;5:33. doi: 10.1186/1477-7525-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almomani FM, McDowd JM, Bani-Issa W, Almomani M. Health-related quality of life and physical, mental, and cognitive disabilities among nursing home residents in Jordan. Qual Life Res. 2014;23:155–165. doi: 10.1007/s11136-013-0461-2. [DOI] [PubMed] [Google Scholar]
- 8.Nishiwaki T, Nakamura K, Ueno K, Fujino K, Yamamoto M. Health characteristics of elderly Japanese requiring care at home. Tohoku J Exp Med. 2005;205:231–239. doi: 10.1620/tjem.205.231. [DOI] [PubMed] [Google Scholar]
- 9.Nishiwaki T, Ueno K, Hasegawa M, Nakamura K. The usefulness of day-service in maintaining general nutritional status in elderly Japanese: a longitudinal study. Tohoku J Exp Med. 2007;211:15–21. doi: 10.1620/tjem.211.15. [DOI] [PubMed] [Google Scholar]
- 10.Weissman MM, Myers JK, Tischler GL, Holzer CE, 3rd, Leaf PJ, Orvaschel H, et al. Psychiatric disorders (DSM-III) and cognitive impairment among the elderly in a US urban community. Acta Psychiatr Scand. 1985;71:366–379. doi: 10.1111/j.1600-0447.1985.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Lin JS, O’Connor E, Rossom RC, Perdue LA, Burda BU, Thompson M, et al. Screening for cognitive impairment in older adults: an evidence update for the US preventive services task force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 14.Kwok T, Whitelaw MN. The use of armspan in nutritional assessment of the elderly. J Am Geriatr Soc. 1991;39:492–496. doi: 10.1111/j.1532-5415.1991.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 15.Japanese EuroQol Translation Team The development of the Japanese EuroQol instrument. J Health Care Soc (Iryo to Shakai) 1998;8:109–123. [Google Scholar]
- 16.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11:341–353. doi: 10.1002/hec.673. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann N, Mittmann N, Silver IL, Shulman KI, Busto UA, Shear NH, et al. A validation study of the Geriatric Depression Scale short form. Int J Geriatr Psychiatry. 1996;11:457–460. doi: 10.1002/(SICI)1099-1166(199605)11:5<457::AID-GPS325>3.0.CO;2-2. [DOI] [Google Scholar]
- 19.van der Leeuw G, Eggermont LH, Shi L, Milberg WP, Gross AL, Hausdorff JM, et al. Pain and cognitive function among older adults living in the community. J Gerontol A Biol Sci Med Sci. 2016;71:398–405. doi: 10.1093/gerona/glv166. [DOI] [PMC free article] [PubMed] [Google Scholar]