Abstract
AIM
To evaluate whether implant design, glenoid positioning, and other factors influenced instability and scapular notching in reverse total shoulder arthroplasty.
METHODS
We retrospectively reviewed records of patients who had undergone reverse total shoulder arthroplasty by the senior author from July 2004 through October 2011 and who had at least 24 mo of follow-up. The 58 patients who met the criteria had 65 arthroplasties: 18 with a Grammont-type prosthesis (Grammont group) and 47 with a lateral-based prosthesis (lateral-design group). We compared the groups by rates of scapular notching and instability and by radiographic markers of glenoid position and tilt. We also compared glenoid sphere sizes and the number of subscapularis tendon repairs between the groups. Rates were compared using the Fisher exact test. Notching severity distribution was compared using the χ2 test of association. Significance was set at P < 0.05.
RESULTS
The Grammont group had a higher incidence of scapular notching (13 of 18; 72%) than the lateral-design group (11 of 47; 23%) (P < 0.001) and a higher incidence of instability (3 of 18; 17%) than the lateral-design group (0 of 47; 0%) (P = 0.019). Glenoid position, glenoid sphere size, and subscapularis tendon repair were not predictive of scapular notching or instability, independent of implant design. With the lateral-based prosthesis, each degree of inferior tilt of the baseplate was associated with a 7.3% reduction in the odds of developing notching (odds ratio 0.937, 95%CI: 0.894-0.983).
CONCLUSION
The lateral-based prosthesis was associated with less instability and notching compared with the Grammont-type prosthesis. Prosthesis design appears to be more important than glenoid positioning.
Keywords: Arthroplasty, Reverse, Instability, Scapular notching, Shoulder
Core tip: In reverse total shoulder arthroplasty (RTSA), we found that a Grammont-type prosthesis was associated with higher rates of instability and scapular notching and more severe notching compared with a prosthesis with a lateralized center of rotation. This study also suggests that some inferior tilt of the baseplate may decrease the notching rate. For the 2 prosthesis designs studied, neither glenoid sphere size nor repair of the subscapularis tendon was associated with rates of instability, rates of scapular notching, or severity of scapular notching. These findings are important to surgeons considering whether to use a Grammont-type prosthesis or a lateral-based implant when performing RTSA.
INTRODUCTION
Since reverse total shoulder arthroplasty (RTSA) was approved by the United States Food and Drug Administration in December 2003 for the treatment of arthritis associated with rotator cuff disease, it has rapidly gained popularity for treating patients with various shoulder conditions, including rotator cuff tear arthropathy, degenerative arthritis with rotator cuff deficiency, and pseudoparalysis associated with anterosuperior escape syndrome. Although RTSA provides pain relief in most patients, its associated rate and variety of complications are higher than those for anatomical total shoulder arthroplasty[1,2].
There are 2 designs of reverse total shoulder prosthesis. One is a Grammont-type prosthesis, which has a center of rotation at the level of the glenoid where the baseplate meets the bone[1]. The other, available from various manufacturers, has a center of rotation in a more lateral position, which theoretically increases the shear forces across the baseplate-to-glenoid bone interface[3].
The most common complications of RTSA are instability and scapular notching. Reported rates of instability range from 0%-31%[4-9]. Instability has been associated with component malposition[10], inadequate tensioning of the soft-tissue envelope[11-13], insufficient subscapularis tendon for repair[14], and use of the deltopectoral approach vs the superolateral approach[11,15]. Scapular notching is a concern because of its potential effect on long-term loosening of the prosthesis and on clinical results[16]. Reported rates of inferior scapular notching for a Grammont-type prosthesis range from 13%-67%[12,15-18].
Studies have compared the severity of scapular notching associated with different RTSA designs[19,20]. However, these studies included patients with a variety of diagnoses, as well as patients who underwent revision arthroplasty, which is associated with higher complication rates than primary RTSA[19,20]. They also included patients with a minimum follow-up of only 12 mo[19,20]. Although these studies addressed notching associated with RTSA, they did not address instability factors that might also be related to prosthesis positioning and design[18,20].
In patients with rotator cuff tear arthropathy, osteoarthritis with a rotator cuff tear, or osteoarthritis with glenoid bone loss, we sought to: (1) establish and compare the instability rates of those treated with a Grammont-type prosthesis vs a lateral-based prosthesis; (2) establish and compare the rates and severity of scapular notching between the 2 groups; and (3) determine in both groups whether glenoid baseplate position, repair of the subscapularis tendon, and glenoid sphere size were associated with different rates and severity of scapular notching.
MATERIALS AND METHODS
Institutional review board approval was obtained for this retrospective study.
Study population
From July 2004 through October 2011, 324 RTSAs were performed by the senior author, 196 of which had at least 2 years of follow-up. We included only patients undergoing their first RTSA with the diagnosis of rotator cuff tear arthropathy, osteoarthritis with a rotator cuff tear, or osteoarthritis with glenoid bone loss. Of those 196 RTSAs, 131 were excluded for the following reasons: 57 that were revised with a diagnosis of failed arthroplasty (based on clinical history, physical examination, and supporting radiographic studies); 37 for fractures and malunion; 17 for rheumatoid arthritis; 7 for inadequate follow-up data; 5 for avascular necrosis; 5 for dislocation arthroplasty; 2 for psoriatic arthritis; and 1 for hemophilic arthropathy. Therefore, our study group comprised 65 shoulders in 58 consecutive patients with a mean follow-up of 35 mo (range, 24-66 mo). Patients had surgery at a mean age of 70 ± 8.1 years. According to the glenoid bone loss classification system of Walch et al[21], there were 27 A2 glenoids, 15 B1 glenoids, 10 B2 glenoids, and 13 C glenoids.
From 2004 to 2007, we used a Grammont-type prosthesis (Tornier Inc., Stafford, Texas, United States) (the Grammont group, n = 18), and from 2007 to 2011 we used a prosthesis with a lateral-based center of rotation (DJO/Encore Medical Corporation, Austin, Texas, United States) (the lateral-design group, n = 47) (Table 1). There was no significant difference in mean age between groups (P = 0.722), but mean length of follow-up was longer in the Grammont group (P = 0.0004).
Table 1.
Characteristics of 58 adults who underwent 65 reverse total shoulder arthroplasties, 2004-2011
| Characteristic |
Grammont group (n = 18) |
Lateral-design group (n = 47) |
P-value | ||
| Mean ± SD | n (%) | Mean ± SD | n (%) | ||
| Male sex | 121 (67) | 311 (66) | NA1 | ||
| Age (yr) | 69 ± 7.3 | 70 ± 8.4 | 0.722 | ||
| Follow-up (mo) | 43 ± 15 | 32 ± 7.9 | 0.0004 | ||
| Dominant side affected | 12 (67) | 21 (45) | NA | ||
| Workers compensation | 02 (0) | 22 (3.4) | NA | ||
| Glenoid sphere diameter | |||||
| 32 mm | 01 (0) | 28 (60) | NA | ||
| ≥ 36 mm | 181 (100) | 19 (40) | NA | ||
Number of shoulders;
Number of patients. NA: Not applicable; SD: Standard deviation.
Surgical and postoperative details
Surgery was performed with patients in a beach chair position. All patients received general anesthesia with a scalene block or indwelling scalene catheter, as well as perioperative antibiotics. All surgical procedures were performed with a deltopectoral approach.
The glenoid was exposed circumferentially, and the glenoid component position was determined with guides provided by the prosthesis manufacturer. An attempt was made to place the glenoid component in approximately 10° of inferior inclination, but this was done visually with no measurement. The size of the glenoid sphere was chosen to best fit the glenoid size and the soft-tissue tension in each patient. In the Grammont group, the glenoid sphere diameters were 36 mm in 16 shoulders, 38 mm in 1 shoulder, and 42 mm in 1 shoulder. In the lateral-design group, the glenoid sphere diameters were 32 mm in 28 shoulders and 36 mm in 19 shoulders. In all patients, regardless of implant type, the humeral components were inserted in 30° of retroversion, and all components were cemented. Stability of the implants after reduction of the humeral component on the sphere was verified by moving the arm in rotation and also with axial distraction. The subscapularis tendon or anterior capsule was secured back to the proximal humerus when possible [in 9 (50%) of 18 shoulders in the Grammont group and 39 (83%) of 47 shoulders in the lateral-design group]. A biceps tenodesis was performed in all shoulders in which the biceps tendon was present.
After surgery, each shoulder was placed in an immobilizer. Unlimited motion was allowed in elevation and internal rotation, but external rotation was limited for 6 wk. Patients were not allowed to lift more than 0.45 kg for 3 mo. No patient had a structured rehabilitation program, but all were encouraged to use the arm for activities of daily living. Radiographs were obtained every 3 mo for the first year and then yearly.
At each follow-up evaluation, 3 conventional radiographs were obtained (a true anteroposterior view in external rotation (Grashey view)[22], an anteroposterior view in internal rotation, and an axillary view). Fluoroscopy was not used for any radiographs.
The presence of notching was evaluated by 2 observers who reached agreement upon the degree of notching using the system of Sirveaux et al[15]. Grade 1 was notching limited to the scapular pillar; grade 2 was notching in contact with the inferior screw of the baseplate; grade 3 was notching beyond the inferior screw; and grade 4 was notching that extends under the baseplate approaching the central peg.
Evaluation
Instability was determined using clinical examination and radiographic evidence of component dislocation. Radiographic analysis was performed by an independent observer. Scapular notching was graded according to the 4-grade classification system of Sirveaux et al[15] (Figure 1). The vertical position of the glenoid sphere was evaluated using 2 similar methods. First, using a method proposed by Lévigne et al[23], we measured the distance between the inferior glenoid osseous rim and the lowest point of the glenoid sphere on the external rotation anteroposterior view (Figure 2). Second, as proposed by Simovitch et al[16], we measured the peg-glenoid rim distance on the same view using a point marking the radiographic superior intersection of the central peg (or central screw in the lateral-based implant) and the glenoid sphere, and a point referencing the most inferior bone of the inferior glenoid rim adjacent to the medial surface of the glenoid sphere (Figure 3).
Figure 1.
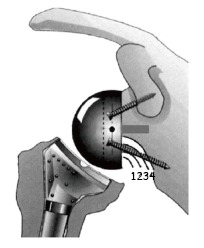
Scapular notching according to the 4-grade Sirveaux classification. Reproduced with permission and copyright of the British Editorial Society of Bone and Joint Surgery (Reprinted with permission from Sirveaux F et al[15], Figure 3).
Figure 2.
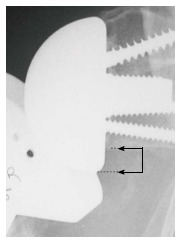
Distance between the inferior glenoid osseous rim (upper arm of the arrow) and the lowest point of the glenoid sphere (lower arm of the arrow) on the external rotation anteroposterior view. Reprinted with permission from Levigne C et al[23], Figure 8.
Figure 3.
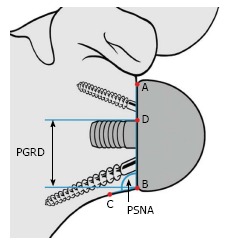
The prosthesis-scapular neck angle (PSNA) is the angle subtended by the intersection of line AB and line BC. Point C is located 1 cm medial to the junction of the glenosphere and the most inferior and lateral bone of the inferior glenoid rim or scapular neck. The peg-glenoid rim distance (PGRD) is the distance between points B and D. PGRD: Peg-glenoid rim distance; PSNA: Prosthesis-scapular neck angle. Reprinted with permission from Simovitch et al[16], Figure 4A.
The inclination of the glenoid sphere was measured in 3 ways. The first method, described by Levigne et al[23], uses a horizontal line placed on the most superior aspect of the glenoid on the anteroposterior radiograph. The angle formed between the horizontal line and a line parallel to the back surface of the glenoid sphere is measured; if it is > 90°, it is considered superiorly tilted, and if it is ≤ 90°, it is considered inferiorly tilted (Figure 4). The second method, described by Simovitch et al[16], defines the prosthesis-scapular neck angle as the angle between a line from superior to inferior along the glenoid baseplate and a line from the most inferior point of the baseplate’s prosthesis-bone interface to a point 1 cm medially along the inferior scapular neck (Figures 3 and 5A). The third method was described by Kempton et al[24], who noted that scapular neck anatomy can be highly variable at 1 cm from the baseplate and may be altered by eccentric reaming or previous surgery. Therefore, they defined a point 6 cm medial along the scapular border to which one draws the second line, which defines the prosthesis-scapular bone angle (Figure 5B).
Figure 4.
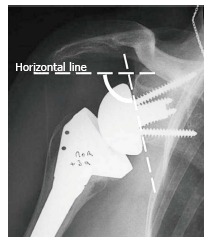
Glenoid inclination is the angle formed between a horizontal line and a line parallel to the back surface of the glenoid sphere. If it is > 90° it is classified as superiorly tilted, and if it is ≤ 90° it is classified as inferiorly tilted. Reprinted with permission from Levigne et al[23], Figure 11.
Figure 5.
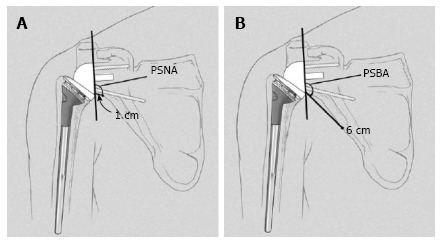
Illustration of measurement of the prosthesis-scapular neck angle and the prosthesis-scapular bone angle. A: The prosthesis-scapular neck angle is the angle between a line from superior to inferior along the glenoid baseplate and a line from the most inferior point of the baseplate’s prosthesis-bone interface to a point 1 cm medially along the inferior scapular neck; B: The prosthesis-scapular bone angle uses a point 6 cm medial along the scapular border to draw the second line. PSNA: Prosthesis-scapular neck angle; PSBA: Prosthesis-scapular bone angle. Reprinted with permission from Kempton et al[24], Figure 3.
Statistical analysis
To determine the association of prosthesis design with instability, we used a stepwise logistic regression model that included inferior glenoid notching, glenoid position[23], glenoid inclination[23], peg-glenoid rim distance[16], prosthesis-scapular neck angle[24], and prosthesis-scapular bone angle[24]. Variable selection was made on the basis of a forward stepwise selection method, with marginal significance levels set at 5% for entry and 10% for removal. This approach to model building was selected to minimize collinearity (redundancy) among variables[25].
We calculated the likelihood of instability as a function of subscapularis repair, glenoid sphere diameter, and inferior inclination using logistic regression within each prosthetic group and in the overall cohort. Receiver operating characteristic (ROC) curves were used to determine the optimal inferior tilt according to presence of instability and scapular notching. ROC curves plot sensitivity vs 1 - specificity to determine the discrimination threshold.
Rates between design groups were compared using the Fisher exact test. Distribution of notching type was compared between design groups using the χ2 test of association. Statistical analysis was performed using SAS, version 9.3, software (SAS Institute, Cary, North Carolina, United States) and SPSS, version 20.0, software (SPSS, Chicago, IL, United States). Statistical significance was set at P < 0.05.
An individual with advanced training in biostatistics was involved in the design or analysis of this work. No additional data are available.
RESULTS
There were 3 dislocations in the Grammont group and none in the lateral-design group, which was a significant difference (P = 0.014). There was a significantly higher rate of subscapularis tendon repair in the lateral-design group (P = 0.008). However, there was no association between dislocation and the presence of a subscapularis repair in either group (P = 0.170). Smaller glenoid spheres were used in the lateral-design group compared with the Grammont group (P < 0.001).
The rate of scapular notching was significantly higher in the Grammont group (13 of 18 shoulders, 72%) than in the lateral-design group (11 of 47 shoulders; 23%) (P < 0.001) (Table 2). Patients in the Grammont group had higher odds of developing notching [odds ratio (OR), 7.2; 95%CI: 2.1-24.7] and had significantly more severe notching than patients in the lateral-design group (P = 0.003). This association between the rate and severity of notching between the 2 implant systems persisted even after adjustment for length of follow-up using a general linear model (P = 0.001).
Table 2.
Scapular notching and instability by prosthesis design in 65 cases of reverse total shoulder arthroplasty with minimum 2-year follow-up, 2004-20111
| Parameter | Grammont group (n = 18), n (%) | Lateral-design group (n = 47), n (%) | P-value |
| Scapular notching | 13 (72) | 11 (23) | < 0.0012 |
| Notching severity5 | |||
| Grade 1 | 7 (39) | 8 (17) | > 0.0013 |
| Grade 2 | 2 (11) | 1 (2.1) | NA4 |
| Grade 3 | 2 (11) | 2 (4.3) | NA4 |
| Grade 4 | 2 (11) | 0 (0) | NA4 |
| Instability6 | 3 (17) | 0 (0) | 0.019 |
NA: Not applicable;
P-value from two-tailed Fisher exact test;
Adjusted for length of follow-up using general linear model;
Small values prevented determination of significant differences;
Notching severity was measured according to Sirveaux classification[15];
Instability was determined using clinical examination and radiographic evidence of component dislocation.
According to the method of Levigne et al[23] there were significant differences in glenoid position between the lateral-design group and the Grammont group (P = 0.004). There was significantly more glenoid inclination in the lateral-design group than the Grammont group (P = 0.027). However, there were no significant differences between the 2 groups for prosthesis-scapular neck angle (P = 0.368), prosthesis-scapular bone angle (P = 0.219), or peg-glenoid rim distance (P = 0.066) (Table 3).
Table 3.
Glenoid position by prosthesis design in 65 cases of reverse total shoulder arthroplasty, 2004-2011
| Parameter | Grammont group (n = 18), mean ± SD | Lateral-design group (n = 47), mean ± SD | P-value |
| Glenoid position (mm) | |||
| Inferior glenoid osseous rim to lowest point of glenoid sphere[23] | 1.5 ± 2.1 | -0.6 ± 3.3 | 0.004 |
| Peg-glenoid rim distance[16] | 22.6 ± 1.7 | 21.4 ± 3.3 | 0.066 |
| Glenoid inclination (°) | |||
| Inclination angle[23] | 93.2 ± 15.3 | 101 ± 11.7 | 0.027 |
| Prosthesis-scapular neck angle[16] | 102 ± 21.3 | 106 ± 17.1 | 0.368 |
| Prosthesis-scapular bone angle[24] | 126 ± 16.9 | 132 ± 11.0 | 0.219 |
We found no factors to be significantly associated with glenoid notching in the Grammont group. However, in the lateral-design group, glenoid inclination, prosthesis-scapular bone angle, and peg-glenoid rim distance were associated with the rate of notching. In the lateral-design group, for each 1° increase in the angle of glenoid inclination, there was a 7.3% reduction in the odds of developing notching (OR, 0.94; 95%CI: 0.89-0.98); for each 1° increase in the prosthesis-scapular bone angle, there was a 9.7% reduction in the odds of developing notching (OR, 0.09; 95%CI: 0.83-0.98); and for each 1-mm increase in the peg-glenoid rim distance, there was a 34% increase in the odds of developing notching (OR, 1.3; 95%CI: 1.0-1.7). A ROC curve analysis revealed that a glenoid inclination angle of < 99.8° tends to be associated with more notching than an angle of ≥99.8° (sensitivity, 66%; specificity, 75%).
Using ordinal logistic regression, we found that as the glenoid inclination angle increased in the lateral-based prosthesis, the likelihood of more severe scapular notching decreased (OR, 0.95; 95%CI: 0.91-0.98).
Based on the ROC curve, an inferior tilt of < 100.5° maximizes the sensitivity (66%) and specificity (79%) of discriminating between shoulders that develop scapular notching and those that do not (area under the curve, 0.74; 95%CI: 0.62-0.87) (Figure 6).
Figure 6.
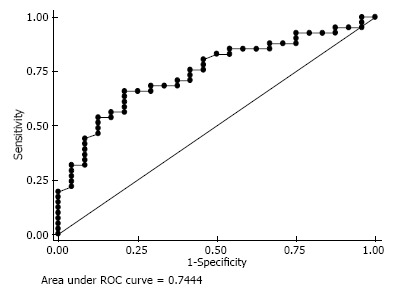
The receiver operating characteristic curve reveals the sensitivity and specificity for predicting the inferior glenoid tilt.
DISCUSSION
We sought to determine whether the design of the RTSA prosthesis and other factors such as subscapularis tendon repair, glenoid positioning, and glenoid sphere size were associated with differences in the rate of prosthesis instability, the rate of scapular notching, and the severity of scapular notching. We found that implant design was a significant factor in the development of instability, the rate of notching, and the severity of notching. Subscapularis repair and glenoid sphere size were not associated with differences in instability rates for either prosthesis design. The baseplate in the lateral-based prosthesis should be placed with some inferior tilt, and a more inferior baseplate position is associated with lower rates of scapular notching.
We observed a 4.5% instability rate (all occurrences in the Grammont group), which is similar to those of previous reports (2.4%-31%)[4,5,7]. However, those studies included revision cases and a wider range of preoperative diagnoses[4,5,7]. Glenoid sphere size and offsets in designs other than the Grammont-type prosthesis may play a crucial role in prosthesis stability; it has been postulated that proper glenoid sphere offset allows the deltoid to provide a compressive force that keeps the ball pressed into the socket[26]. Subsequent prosthetic designs have created offset by increasing the diameter of the glenoid sphere, placing a humeral neck extension beneath the polyethylene cup, and/or increasing the thickness of the polyethylene cup[27]. Our study supports the observation of Clark et al[28], who found that repair of the subscapularis tendon does not influence dislocation rates of the Grammont-type prosthesis.
We observed a 72% rate of scapular notching in the Grammont group, which is similar to those reported in the literature (range, 13%-67%)[12,15-17]. However, we found a 23% rate of scapular notching in the lateral-design group, which is higher than those reported in the literature (range, 0%-13%)[6,26,29]. Two factors have been suggested for the lower rates of notching in the lateral-based prosthesis: (1) the design of the glenoid side of the prosthesis; and (2) the humeral head-neck angle. Until a reverse prosthesis is developed that allows surgeons to choose between a lower or higher humeral head-neck angle, it is unlikely that we will know which factor is responsible for lower rates of notching.
Several studies have suggested that prosthesis design and baseplate and glenoid sphere position may influence scapular notching rates[19,20,30]. Our study is consistent with that of Gutiérrez et al[31], who found in a biomechanical model that tilting the glenoid component inferiorly might prevent notching by decreasing contact of the humeral component to the scapula. We found that glenoid tilt of < 100° was associated with more, and more severe, notching. We also found that inferior placement of the baseplate was associated with less notching for the lateral-based prosthesis, similar to the findings of Simovitch et al[16]. Berhouet et al[32] found the most effective way to prevent scapular notching was by using large-diameter glenoid spheres, but our study did not support that finding.
Our study had several limitations. It was neither prospective nor randomized, and because it was a consecutive series, the mean length of follow-up differed between the 2 groups. The rate and severity of notching in the lateral-design group might have been higher had the follow-up been longer. There were fewer shoulders in the Grammont group than in the lateral-design group, making type-2 error possible. We limited our study to patients undergoing primary RTSA for rotator cuff tear arthropathy, osteoarthritis with a rotator cuff tear, or osteoarthritis with glenoid bone loss. Therefore, our results may not be generalizable to RTSA for other causes or for revisions. It is possible that another design feature-the humeral head-neck angle-is partly responsible for our results. The humeral head-neck angle is 135° in the Grammont-type prosthesis and 155° in the lateral-based prosthesis. It was impossible for us to determine whether the humeral head-neck angle or the location of the center of rotation was the most important factor in our results. In addition, we evaluated only 2 implant systems, so our results may not apply to other systems. Also, variables not studied here such as body mass index, Charlson Comorbidity Index, or other measures of patient health might influence the results.
The surgery was performed by 1 surgeon in a referral practice, which may not be generalizable to other surgical practices. Also, the surgeon changed arthroplasty systems, and the learning curve might have affected the results. Wierks et al[33] suggested that the learning curve for a new operation is approximately 10 cases, but Kempton et al[34] suggested it might be as high as 40 cases.
Another limitation is that we used standard radiographs not obtained with fluoroscopy. The routine use of fluoroscopy for shoulder radiography has not been the practice at our institution for ethical and financial reasons. Several radiographic measures are described in the literature to assess glenoid position and tilt, but their relation to scapular notching has not been thoroughly studied[16,23,24]. To our knowledge, ours is the first study to correlate the peg-glenoid rim distance and the prosthesis-scapular neck angle with the rate of inferior scapular notching between the Grammont-type prosthesis and the lateral-based prosthesis. Furthermore, unlike previous reports[16], we found no single statistically significant radiographic factor related to glenoid notching in the Grammont group.
In conclusion, we found the Grammont-type of RTSA was associated with significantly higher rates of instability and scapular notching, as well as more severe scapular notching compared with a prosthesis with a lateralized glenoid sphere center of rotation and a decreased humeral head-neck angle. These findings are important to the surgeon when considering whether to use a Grammont-type prosthesis or a lateral-based implant when performing RTSA. This study also suggests that some inferior tilt of the baseplate may decrease the scapular notching rate and that, for the 2 prosthesis designs studied, neither glenoid sphere size nor repair of the subscapularis tendon was associated with different rates of instability, rates of scapular notching, or severity of scapular notching.
COMMENTS
Background
Since reverse total shoulder arthroplasty (RTSA) was approved by the United States Food and Drug Administration in December 2003 for the treatment of arthritis associated with rotator cuff disease, it has rapidly gained popularity for treating patients with various shoulder conditions, including rotator cuff tear arthropathy, degenerative arthritis with rotator cuff deficiency, and pseudoparalysis associated with anterosuperior escape syndrome.
Research frontiers
Although RTSA provides pain relief in most patients, its associated rate and variety of complications are higher than those for anatomical total shoulder arthroplasty.
Innovations and breakthroughs
The authors found the Grammont-type of RTSA was associated with significantly higher rates of instability and scapular notching, as well as more severe scapular notching compared with a prosthesis with a lateralized glenoid sphere center of rotation and a decreased humeral head-neck angle.
Applications
The findings are important to the surgeon when considering whether to use a Grammont-type prosthesis or a lateral-based implant when performing RTSA. This study also suggests that some inferior tilt of the baseplate may decrease the scapular notching rate and that, for the 2 prosthesis designs studied, neither glenoid sphere size nor repair of the subscapularis tendon was associated with different rates of instability, rates of scapular notching, or severity of scapular notching.
Peer-review
As a retrospective cohort study, this is low level evidence. However, it does provide clinically relevant information for surgeons who perform RTSA surgery and is worthy of publication.
Footnotes
Institutional review board statement: Institutional review board approval was obtained for this retrospective study.
Informed consent statement: Patients were not required to give the informed consent for the study.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest concerning this article.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 13, 2016
First decision: May 19, 2016
Article in press: August 18, 2016
P- Reviewer: Metzger P, Sarda P S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
References
- 1.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 2.Scarlat MM. Complications with reverse total shoulder arthroplasty and recent evolutions. Int Orthop. 2013;37:843–851. doi: 10.1007/s00264-013-1832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henninger HB, Barg A, Anderson AE, Bachus KN, Burks RT, Tashjian RZ. Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21:1128–1135. doi: 10.1016/j.jse.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92:535–539. doi: 10.1302/0301-620X.92B4.22450. [DOI] [PubMed] [Google Scholar]
- 5.Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19:439–449. [PubMed] [Google Scholar]
- 6.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 7.Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17:284–295. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 9.Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:652–658. doi: 10.1016/j.jse.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Affonso J, Nicholson GP, Frankle MA, Walch G, Gerber C, Garzon-Muvdi J, McFarland EG. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157–168. [PubMed] [Google Scholar]
- 12.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Gallo RA, Gamradt SC, Mattern CJ, Cordasco FA, Craig EV, Dines DM, Warren RF. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20:584–590. doi: 10.1016/j.jse.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620x.86b3.14024. [DOI] [PubMed] [Google Scholar]
- 16.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 17.Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469:2512–2520. doi: 10.1007/s11999-010-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14:524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of prosthesis design on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:571–576. doi: 10.1016/j.jse.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21:1430–1441. doi: 10.1016/j.jse.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 21.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–760. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 22.Koh KH, Han KY, Yoon YC, Lee SW, Yoo JC. True anteroposterior (Grashey) view as a screening radiograph for further imaging study in rotator cuff tear. J Shoulder Elbow Surg. 2013;22:901–907. doi: 10.1016/j.jse.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:968–974. doi: 10.1016/j.jse.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Weisberg S. 2nd ed. New York: John Wiley & Sons; 1985. Applied Linear Regression. [Google Scholar]
- 26.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87:1697–1705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 27.Frankle M, Levy JC, Pupello D, Siegal S, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. a minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:178–190. doi: 10.2106/JBJS.F.00123. [DOI] [PubMed] [Google Scholar]
- 28.Clark JC, Ritchie J, Song FS, Kissenberth MJ, Tolan SJ, Hart ND, Hawkins RJ. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21:36–41. doi: 10.1016/j.jse.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez S, Levy JC, Frankle MA, Cuff D, Keller TS, Pupello DR, Lee WE. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17:608–615. doi: 10.1016/j.jse.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16:S9–S12. doi: 10.1016/j.jse.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Berhouet J, Garaud P, Favard L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:151–158. doi: 10.1016/j.jse.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Wierks C, Skolasky RL, Ji JH, McFarland EG. Reverse total shoulder replacement: intraoperative and early postoperative complications. Clin Orthop Relat Res. 2009;467:225–234. doi: 10.1007/s11999-008-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469:2496–2504. doi: 10.1007/s11999-011-1811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


