Abstract
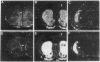
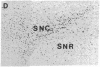
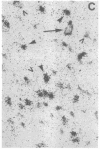
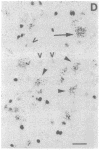
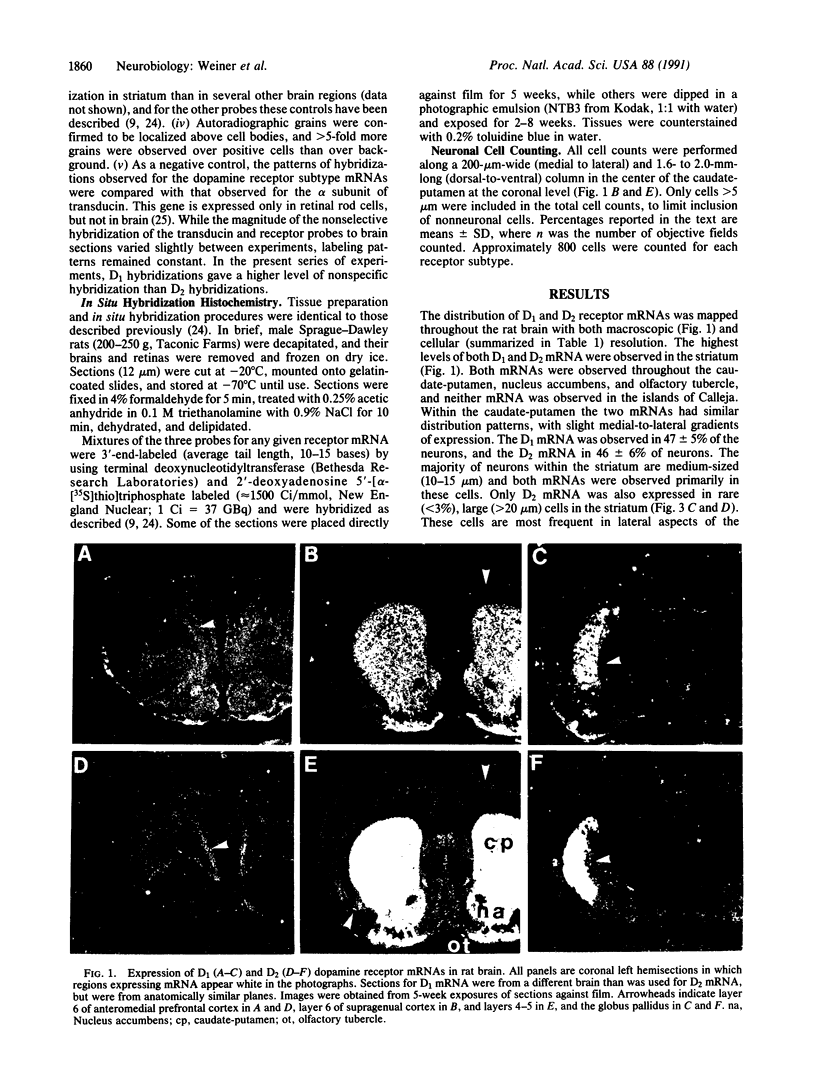
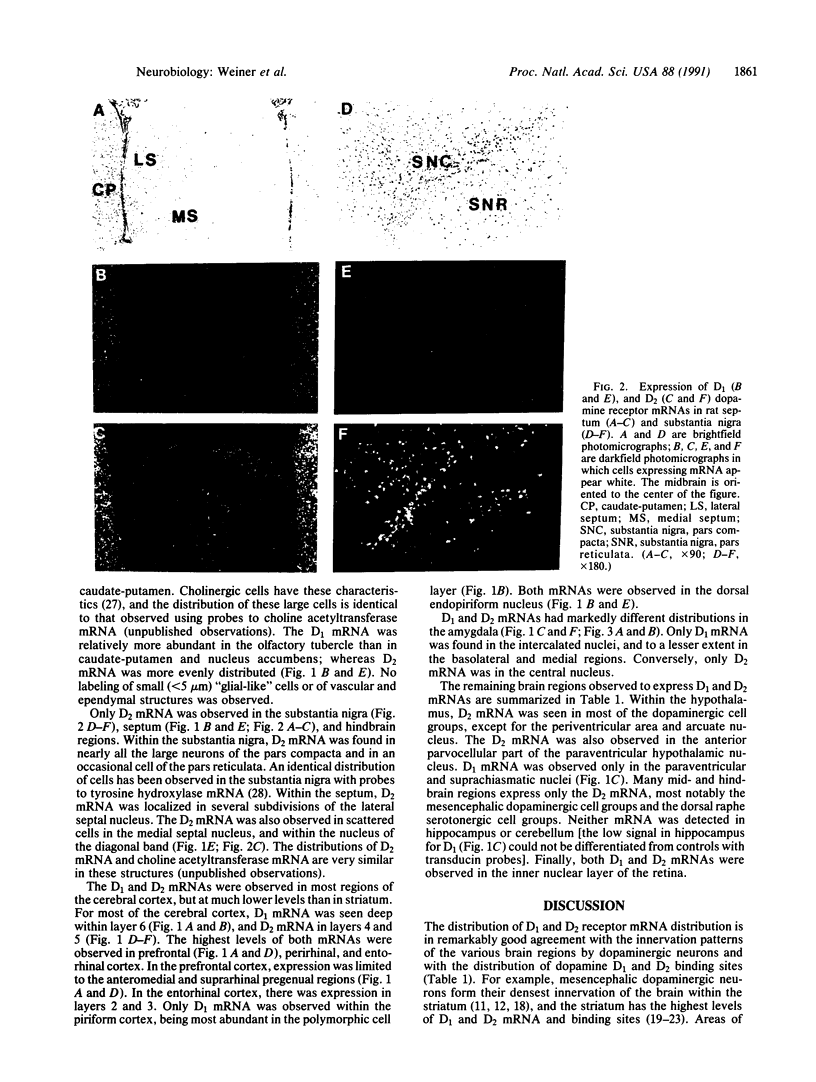
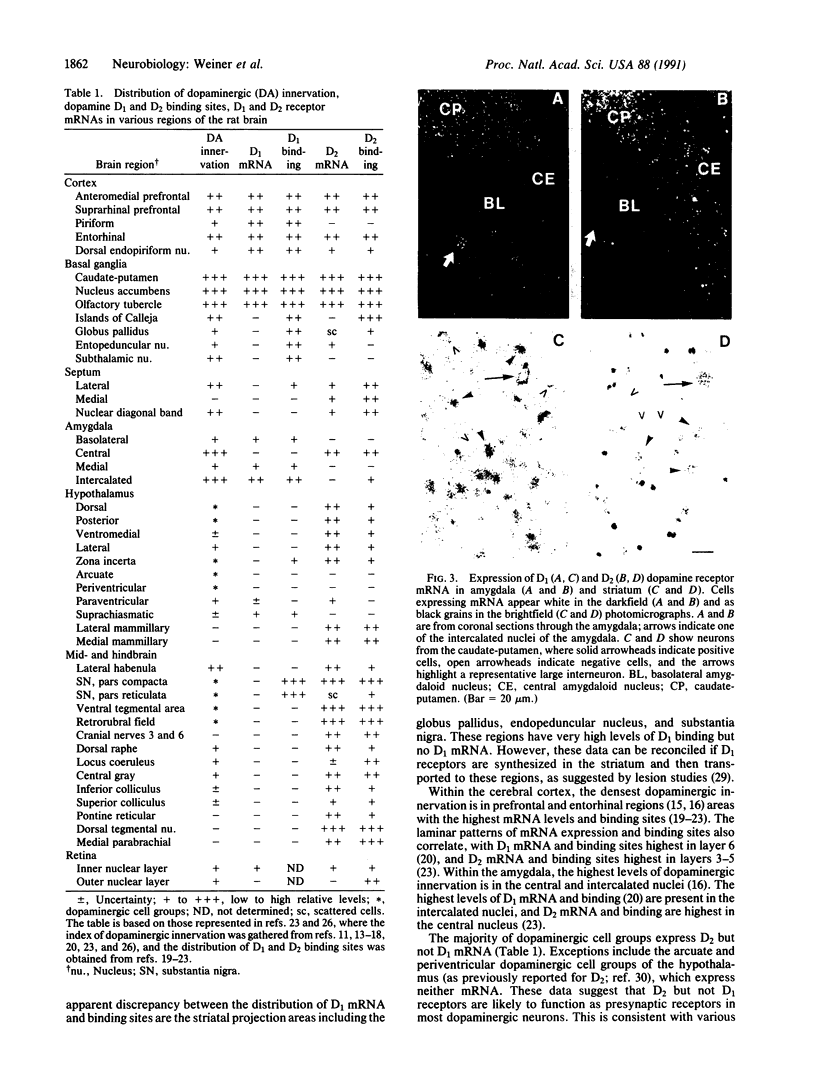
Physiological and pharmacological criteria have divided dopamine receptors into D1 and D2 subtypes, and genes encoding these subtypes have recently been cloned. Based on the sequences of the cloned receptors, we prepared oligodeoxynucleotide probes to map the cellular expression of the corresponding mRNAs in rat brain by in situ hybridization histochemistry. These mRNAs showed largely overlapping yet distinct patterns of expression. The highest levels of expression for both mRNAs were observed in the caudate-putamen, nucleus accumbens, and olfactory tubercle. Within the caudate-putamen, 47 +/- 6% and 46 +/- 5% of the medium-sized neurons (10-15 microns) expressed the D1 and D2 mRNAs, respectively, and only the D2 mRNA was observed in the larger neurons (greater than 20 microns). The D1 and D2 mRNAs were expressed in most cortical regions, with the highest levels in the prefrontal and entorhinal cortices. Within neocortex, D1 mRNA was observed primarily in layer 6 and D2 mRNA in layers 4-5. Within the amygdala, D1 mRNA was observed in the intercalated nuclei, and D2 mRNA in the central nucleus. Within the hypothalamus, D1 mRNA was observed in the suprachiasmatic nucleus and D2 mRNA in many of the dopaminergic cell groups. Within the septum, globus pallidus, superior and inferior colliculi, mammillary bodies, and substantia nigra only D2 mRNA was detected. These data provide insight into the neuroanatomical basis of the differential effects of drugs that act on D1 or D2 receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. H., Gingrich J. A., Bates M. D., Dearry A., Falardeau P., Senogles S. E., Caron M. G. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci. 1990 Jun;11(6):231–236. doi: 10.1016/0165-6147(90)90249-8. [DOI] [PubMed] [Google Scholar]
- Battaglia G., Norman A. B., Hess E. J., Creese I. Forskolin potentiates the stimulation of rat striatal adenylate cyclase mediated by D-1 dopamine receptors, guanine nucleotides, and sodium fluoride. J Neurochem. 1986 Apr;46(4):1180–1185. doi: 10.1111/j.1471-4159.1986.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Bianchine J. R. Drug therapy of parkinsonism. N Engl J Med. 1976 Oct 7;295(15):814–818. doi: 10.1056/NEJM197610072951505. [DOI] [PubMed] [Google Scholar]
- Björklund A., Lindvall O., Nobin A. Evidence of an incerto-hypothalamic dopamine neurone system in the rat. Brain Res. 1975 May 16;89(1):29–42. doi: 10.1016/0006-8993(75)90131-6. [DOI] [PubMed] [Google Scholar]
- Björklund A., Moore R. Y., Nobin A., Stenevi U. The organization of tubero-hypophyseal and reticulo-infundibular catecholamine neuron systems in the rat brain. Brain Res. 1973 Mar 15;51:171–191. doi: 10.1016/0006-8993(73)90371-5. [DOI] [PubMed] [Google Scholar]
- Bouthenet M. L., Martres M. P., Sales N., Schwartz J. C. A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience. 1987 Jan;20(1):117–155. doi: 10.1016/0306-4522(87)90008-x. [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann M. R., Young W. S., 3rd Dopamine receptors are located on rods in bovine retina. Neurosci Lett. 1986 Sep 12;69(3):221–226. doi: 10.1016/0304-3940(86)90483-0. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Charuchinda C., Supavilai P., Karobath M., Palacios J. M. Dopamine D2 receptors in the rat brain: autoradiographic visualization using a high-affinity selective agonist ligand. J Neurosci. 1987 May;7(5):1352–1360. doi: 10.1523/JNEUROSCI.07-05-01352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D., White F. J. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1(4):347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Gehlert D. R., McCabe R. T., Barnett A., Wamsley J. K. D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci. 1986 Aug;6(8):2352–2365. doi: 10.1523/JNEUROSCI.06-08-02352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon J. H., Koziell D. A., Moore R. Y. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978 Aug 1;180(3):509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol. 1978 Aug 1;180(3):533–544. doi: 10.1002/cne.901800309. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978 Aug 1;180(3):545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Gale K., Guidotti A., Costa E. Dopamine-sensitive adenylate cyclase: location in substantia nigra. Science. 1977 Feb 4;195(4277):503–505. doi: 10.1126/science.13499. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Levey A. I., Wainer B. H., Mufson E. J., Mesulam M. M. Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience. 1983 May;9(1):9–22. doi: 10.1016/0306-4522(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A., Divac I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978 Feb 17;142(1):1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff J. H., Mansour A., Bunzow J. R., Van Tol H. H., Watson S. J., Jr, Civelli O. Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7625–7628. doi: 10.1073/pnas.86.19.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R., Wickens J. R., Beninger R. J. Dopamine D-1 and D-2 receptors in relation to reward and performance: a case for the D-1 receptor as a primary site of therapeutic action of neuroleptic drugs. Prog Neurobiol. 1990;34(2):143–183. doi: 10.1016/0301-0082(90)90005-2. [DOI] [PubMed] [Google Scholar]
- Morelli M., Mennini T., Di Chiara G. Nigral dopamine autoreceptors are exclusively of the D2 type: quantitative autoradiography of [125I]iodosulpride and [125I]SCH 23982 in adjacent brain sections. Neuroscience. 1988 Dec;27(3):865–870. doi: 10.1016/0306-4522(88)90189-3. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Lefkowitz R. J., Caron M. G. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- Plaznik A., Stefanski R., Kostowski W. Interaction between accumbens D1 and D2 receptors regulating rat locomotor activity. Psychopharmacology (Berl) 1989;99(4):558–562. doi: 10.1007/BF00589908. [DOI] [PubMed] [Google Scholar]
- Raport C. J., Dere B., Hurley J. B. Characterization of the mouse rod transducin alpha subunit gene. J Biol Chem. 1989 May 5;264(13):7122–7128. [PubMed] [Google Scholar]
- Robinson S. E., Malthe-Sørenssen D., Wood P. L., Commissiong J. Dopaminergic control of the septal-hippocampal cholinergic pathway. J Pharmacol Exp Ther. 1979 Mar;208(3):476–479. [PubMed] [Google Scholar]
- Savasta M., Dubois A., Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986 Jun 11;375(2):291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- Scatton B. Effect of dopamine agonists and neuroleptic agents on striatal acetylcholine transmission in the rat: evidence against dopamine receptor multiplicity. J Pharmacol Exp Ther. 1982 Jan;220(1):197–202. [PubMed] [Google Scholar]
- Stormann T. M., Gdula D. C., Weiner D. M., Brann M. R. Molecular cloning and expression of a dopamine D2 receptor from human retina. Mol Pharmacol. 1990 Jan;37(1):1–6. [PubMed] [Google Scholar]
- Sunahara R. K., Niznik H. B., Weiner D. M., Stormann T. M., Brann M. R., Kennedy J. L., Gelernter J. E., Rozmahel R., Yang Y. L., Israel Y. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990 Sep 6;347(6288):80–83. doi: 10.1038/347080a0. [DOI] [PubMed] [Google Scholar]
- Wamsley J. K., Gehlert D. R., Filloux F. M., Dawson T. M. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J Chem Neuroanat. 1989 May-Jun;2(3):119–137. [PubMed] [Google Scholar]
- Weiner D. M., Brann M. R. The distribution of a dopamine D2 receptor mRNA in rat brain. FEBS Lett. 1989 Aug 14;253(1-2):207–213. doi: 10.1016/0014-5793(89)80960-3. [DOI] [PubMed] [Google Scholar]
- Weiner D. M., Levey A. I., Brann M. R. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. J., Wang R. Y. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984 Nov;231(2):275–280. [PubMed] [Google Scholar]
- Young W. S., 3rd, Bonner T. I., Brann M. R. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]