Abstract
Prenatal maternal infection contributes to the etiology of schizophrenia, with D-serine, an endogenous co-agonist of the N-methyl-D-aspartate (NMDA) receptor, playing a role in the pathophysiology of this disease. We examined whether supplementation with D-serine during juvenile and adolescent stages could prevent the onset of cognitive deficits, prodromal and the core symptoms of schizophrenia in adult offspring after maternal immune activation (MIA). Juvenile offspring exposed prenatally to poly(I:C) showed reduced expression of NMDA receptor subunits in the hippocampus. Supplementing drinking water with D-serine (600 mg/L from P28 to P56) prevented the onset of cognitive deficits in adult offspring after MIA, in a significant manner. This study shows that supplementing offspring with D-serine during juvenile and adolescent stages could prevent the onset of psychosis in adulthood, after MIA. Therefore, early intervention with D-serine may prevent the occurrence of psychosis in high-risk subjects.
Multiple lines of evidence suggest that hypofunction of glutamatergic neurotransmission via the N-methyl-D-aspartate (NMDA) receptor plays a crucial role in the pathophysiology of schizophrenia1,2,3,4,5,6,7,8,9,10,11. D-serine, an obligatory co-agonist at the NMDA receptor, is integral to neurotransmission via NMDA signaling throughout development and into adulthood12,13. A number of clinical studies have highlighted disturbed NMDA receptor neurotransmission due to decreased D-serine levels as a causative factor in the pathophysiology of schizophrenia4,5,6,7,8,9,14,15,16,17. First, there are reports showing lower levels of D-serine in the blood, cerebrospinal fluid (CSF), and postmortem brain tissue from patients with schizophrenia, relative to normal controls18,19,20,21,22,23. Secondly, treatment with D-serine is beneficial for alleviating several symptoms associated with schizophrenia24,25,26, even in treatment-resistant disease27,28. Meta-analyses support these findings that D-serine is effective in treating schizophrenia29,30, although D-serine is not approved as therapeutic drug for schizophrenia. Thirdly, mRNA expression and the activity of D-amino acid oxidase (DAAO), which metabolizes D-serine, is increased in postmortem brains of schizophrenic patients21,31,32. Endogenous D-serine is synthesized from L-serine by serine racemase (SRR)33. Levels of SRR protein in the prefrontal cortex and hippocampus of schizophrenia cohorts were lower than those of control groups21. Finally, the G72 gene located at chromosome 13q is significantly associated with schizophrenia34,35. This gene has been designated a DAAO activator, since the G72 protein interacts physically with DAAO34. Meta-analyses provided evidence of significant association between G72/G30 genes and schizophrenia35,36,37. Interestingly, there are two reports showing increased G72 protein levels in the blood of patients with schizophrenia38,39. A subsequent largest GWAS study of schizophrenia demonstrated the SRR gene as a susceptible gene40.
Multiple epidemiological studies support the neurodevelopmental hypothesis for the pathogenesis of schizophrenia41. Maternal immune activation (MIA) is an environmental risk factor for the development of psychiatric disorders, such as schizophrenia, and a prenatal immune challenge by the viral mimetic, poly(I:C) is capable of inducing long-lasting behavioral abnormalities in adulthood42,43,44,45. It is thought that prenatal poly(I:C) exposure attenuated the expression of GluN1, a subtype of the NMDA receptor in the brains of P21 rat offspring45, implicating NMDA receptor hypofunction in juvenile offspring after MIA. These findings point to the possibility that hypofunction at this receptor in juvenile offspring after MIA could interfere with normal fetal brain neurodevelopment, and that these deficits promote the onset of schizophrenia in adulthood.
Cognitive impairment is detectable in subjects at high-risk for psychosis several years preceding onset of frank disease46,47. Interestingly, high-risk subjects who later developed psychosis showed poorer neurocognitive functioning compared with those who did not develop a psychotic disorder47, indicating that cognitive impairment could be a risk factor for conversion to psychosis. It is clear that providing early intervention at the prodromal phase of psychosis is one of the most important and challenging tasks in psychiatry. This study was undertaken to examine whether D-serine supplementation from juvenile stages (P28) to adolescence (P56) could prevent the onset of cognitive deficits in adult offspring (<P70), after MIA.
Results
Cognitive deficits in juvenile offspring after MIA
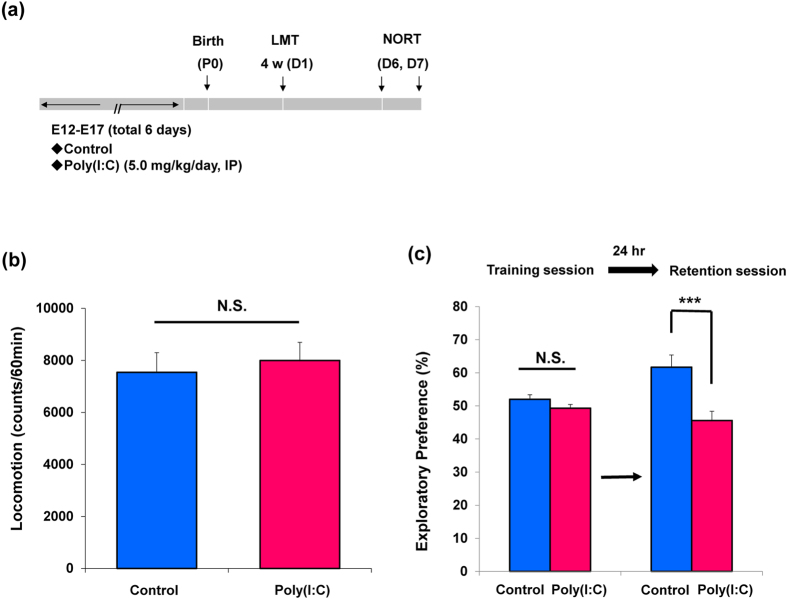
Behavioral tests of juvenile offspring were performed during P28-P35 after prenatal poly(I:C)(5 mg/kg/day from E12 to E17) injections (Fig. 1a). In the open field test, spontaneous locomotion was unchanged (P = 0.670) between control group and poly(I:C)-treated group (Fig. 1b). In the novel object recognition test (NORT), there was no difference (P = 0.141) between two groups in the training session. However, in the retention session, the exploratory preference of poly(I:C) group was significantly (P = 0.001) lower than that of control (Fig. 1c). These results imply that prenatal poly(I:C) exposure induces cognitive deficits in juvenile offspring.
Figure 1. Behaviors in the juvenile offspring after prenatal poly(I:C) exposure.
(a): Schedule of treatment and behavioral tests. Saline or poly(I:C)(5 mg/kg/day from E12 to E17) was injected into pregnant mice. Behavioral tests including locomotion (LMT: D1) and novel object recognition test (NORT: D6 and D7) were performed. (b): Locomotion: There was no difference between ploy(I:C) offspring group and control group at juvenile stage. The value is expressed as the mean ± SEM. (n = 13 for control group, n = 19 for poly(I:C) group). (c): Novel object recognition test (NORT): the exploratory preferences were significantly lower in the poly(I:C) offspring than controls in the retention session, but there was no difference between the two groups in the training session. ***P < 0.001 compared with control group. The value is expressed as the mean ± SEM (n = 13 for control group, n = 18 for poly(I:C) group).
Levels of amino acids and their ratios in the brain regions of juvenile offspring after MIA
We measured tissue levels of amino acids (glutamate, glutamine, glycine, L-serine, D-serine, γ-amino butylic acid (GABA)) in the frontal cortex, hippocampus, and striatum at juvenile stage (P28). Treatment with poly(I:C) significantly increased levels of glutamate and glutamine in the frontal cortex, but significantly decreased levels of GABA in the frontal cortex (Table 1). Furthermore, treatment with poly(I:C) significantly decreased levels of glutamate in the hippocampus, but significantly increased levels of glycine in the hippocampus (Table 1). Moreover, treatment with poly(I:C) significantly increased levels of glycine and L-serine in the striatum, whereas other amino acids were not altered (Table 1). Levels of D-serine in the three regions remained the same (Table 1).
Table 1. Levels of amino acids and their ratios in the frontal cortex, hippocampus and striatum of the juvenile offspring after MIA.
| Glutamate | Glutamine | Glycine | L-Serine | D-Serine | GABA | |
|---|---|---|---|---|---|---|
| Frontal cortex | ||||||
| Control | 9.882 ± 0.141 | 5.446 ± 0.133 | 0.665 ± 0.011 | 0.674 ± 0.015 | 0.357 ± 0.008 | 2.683 ± 0.079 |
| Poly(I:C) | 10.713 ± 0.223** | 5.871 ± 0.075** | 0.724 ± 0.025 | 0.727 ± 0.023 | 0.367 ± 0.011 | 1.991 ± 0.035*** |
| Hippocampus | ||||||
| Control | 8.905 ± 0.153 | 5.433 ± 0.083 | 0.712 ± 0.012 | 0.656 ± 0.015 | 0.268 ± 0.008 | 2.415 ± 0.057 |
| Poly(I:C) | 8.507 ± 0.120* | 5.399 ± 0.090 | 0.835 ± 0.042* | 0.687 ± 0.017 | 0.265 ± 0.006 | 2.447 ± 0.054 |
| Striatum | ||||||
| Control | 8.326 ± 0.148 | 5.985 ± 0.131 | 0.739 ± 0.015 | 0.666 ± 0.018 | 0.282 ± 0.007 | 2.659 ± 0.110 |
| Poly(I:C) | 8.257 ± 0.176 | 6.055 ± 0.104 | 0.883 ± 0.053* | 0.745 ± 0.022* | 0.296 ± 0.006 | 2.742 ± 0.061 |
| Glutamine/Glutamate | L-Serine/Glycine | D-Serine/L-Serine | GABA/Glutamate | |||
| Frontal cortex | ||||||
| Control | 1.825 ± 0.041 | 1.016 ± 0.025 | 0.530 ± 0.007 | 0.272 ± 0.007 | ||
| Poly(I:C) | 1.824 ± 0.027 | 1.012 ± 0.027 | 0.506 ± 0.005** | 0.187 ± 0.005*** | ||
| Hippocampus | ||||||
| Control | 1.640 ± 0.022 | 0.925 ± 0.024 | 0.409 ± 0.011 | 0.271 ± 0.005 | ||
| Poly(I:C) | 1.578 ± 0.014* | 0.851 ± 0.033 | 0.386 ± 0.006 | 0.288 ± 0.005* | ||
| Striatum | ||||||
| Control | 1.396 ± 0.030 | 0.905 ± 0.026 | 0.424 ± 0.007 | 0.320 ± 0.014 | ||
| Poly(I:C) | 1.363 ± 0.017 | 0.872 ± 0.031 | 0.400 ± 0.006** | 0.334 ± 0.009 | ||
Data (nmol/mg tissue) are expressed as the mean ± SEM (Control: n = 13, Poly(I:C): n = 19). *P < 0.05, **P < 0.01, ***P < 0.001 compared to control group (Student’s t test).
The ratio of glutamine to glutamate in the hippocampus of poly(I:C) group was significantly lower than that of control group, suggesting abnormalities in glutamine-glutamate cycle in the hippocampus of juvenile offspring after prenatal poly(I:C) injections (Table 1). Furthermore, the ratio of D-serine to L-serine in the frontal cortex and striatum of poly(I:C) group was significantly lower than that of control group, suggesting reduced production of D-serine from L-serine in these regions (Table 1). Moreover, the ratio of GABA to glutamate in the frontal cortex of poly(I:C) group was significantly lower than that of control group whereas this ratio in the hippocampus of poly(I:C) group was slightly higher than that of control group (Table 1). These findings suggest abnormalities in the NMDA receptor neurotransmission in the brain of juvenile offspring after MIA.
Alterations in the gene expression of SRR, DAO, and NMDA receptor subunits in the brain from juvenile offspring after MIA
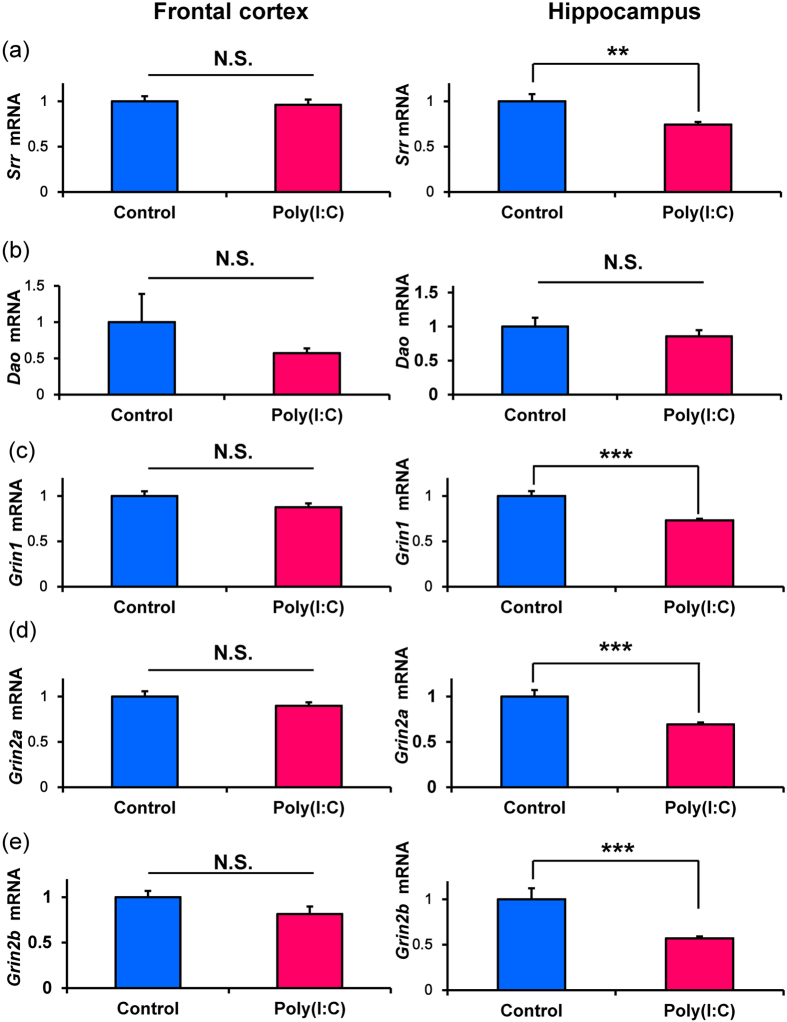
We measured gene expression of serine racemase (Srr), DAO (Dao), and the NMDA receptor subunits (Grin1, Grin2a, Grin2b) in the frontal cortex and hippocampus. Expression of Srr in the hippocampus of poly(I:C) group was significantly (P = 0.002) lower than that of control group although expression of Srr in the frontal cortex was not different (Fig. 2a). Furthermore, expression of Dao in the PFC and hippocampus was not different (P = 0.357) for two groups (Fig. 2b). Expressions of Grin1 (P < 0.001), Grin2a (P < 0.001), and Grin2b (P < 0.001) in the hippocampus of poly(I:C) group were significantly lower than those of control group (Fig. 2c–e). In contrast, expressions of Grin1, Grin2a, and Grin2b in the frontal cortex were not different for two groups (Fig. 2c–e). These findings suggest alterations in the NMDA receptor function in the hippocampus of juvenile offspring after prenatal poly(I:C) injections.
Figure 2. Gene expression in the frontal cortex and hippocampus from the juvenile offspring after prenatal poly(I:C) exposure.
(a): Serine racemase (Srr). (b): D-amino acid oxidase (Dao). (c): GluN1 subtype of the NMDA receptor (Grin1). (d): GluN2A subtype of NMDA receptor (Grin2a). (e): GluN2B subtype of the NMDA receptor (Grin2b). Data represent the mean ± S.E.M. (n = 10 for control group, n = 14 for poly(I:C) group). *P < 0.05, **P < 0.01, *** P < 0.001 compared with control group.
Cognitive deficits in adult offspring after MIA
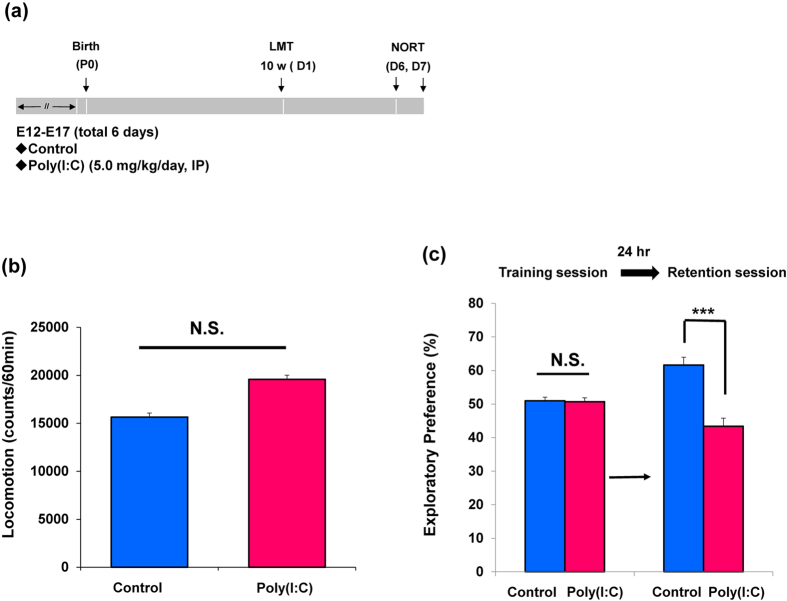
Behavioral tests of juvenile offspring were performed during P70-P84 after prenatal poly(I:C)(5 mg/kg/day from E12 to E17) injections (Fig. 3a). In the open field test, locomotion was significantly unchanged (P = 0.088) between two groups (Fig. 3b). In the NORT, there was no difference (P = 0.850) between two groups in the training session. However, in the retention session, the exploratory preference of poly(I:C) group was significantly (P < 0.001) lower than that of control (Fig. 3c). These findings indicate that prenatal poly(I:C) exposure caused cognitive in adult offspring.
Figure 3. Behaviors in the adult offspring after prenatal poly(I:C) exposure.
(a): Schedule of treatment and behavioral tests. Saline or poly(I:C)(5 mg/kg/day from E12 to E17) was injected into pregnant mice. Behavioral tests including locomotion (LMT: 10 W (D1)) and novel object recognition test (NORT: D6 and D7) were performed. (b): Locomotion: There was no difference between ploy(I:C) offspring and controls at juvenile stage. The value is expressed as the mean ± SEM. (n = 21). (c): NORT: The exploratory preferences were significantly lower in the poly(I:C) offspring than controls in the retention session, but there was no difference between the two groups in the training session. ***P < 0.001 compared with control group. The value is expressed as the mean ± SEM (n = 22 for control group, n = 20 for poly(I:C) group).
Levels of amino acids and their ratios in the brain regions of adult offspring after MIA
Treatment with poly(I:C) significantly decreased levels of D-serine in the frontal cortex, whereas other amino acids were not altered (Table 2). Furthermore, treatment with poly(I:C) significantly decreased levels of glutamate, L-serine, and D-serine in the hippocampus (Table 2). Moreover, treatment with poly(I:C) significantly decreased levels of L-serine, and D-serine in the striatum whereas glycine levels were increased in the poly(I:C) group (Table 2). Interestingly, levels of D-serine in the three regions were significantly lower than those of control group (Table 2).
Table 2. Levels of amino acids and their ratios in the frontal cortex, hippocampus and striatum of the adult offspring after MIA.
| Glutamate | Glutamine | Glycine | L-Serine | D-Serine | GABA | |
| Frontal cortex | ||||||
| Control | 9.394 ± 0.201 | 5.114 ± 0.162 | 0.728 ± 0.022 | 0.742 ± 0.022 | 0.370 ± 0.011 | 2.492± 0.050 |
| Poly(I:C) | 8.953 ± 0.140 | 4.777 ± 0.111 | 0.722 ± 0.015 | 0.697 ± 0.020 | 0.333 ± 0.009* | 2.544 ± 0.062 |
| Hippocampus | ||||||
| Control | 9.816 ± 0.147 | 5.165 ± 0.113 | 0.909 ± 0.016 | 0.860 ± 0.018 | 0.354 ± 0.009 | 2.562 ± 0.054 |
| Poly(I:C) | 9.361 ± 0.134* | 4.911 ± 0.094 | 0.967 ± 0.026 | 0.735 ± 0.014*** | 0.284 ± 0.007*** | 2.675 ± 0.071 |
| Striatum | ||||||
| Control | 8.128 ± 0.189 | 5.537 ± 0.154 | 0.921 ± 0.029 | 0.815± 0.022 | 0.320 ± 0.009 | 2.973 ± 0.093 |
| Poly(I:C) | 8.562 ± 0.172 | 5.636 ± 0.127 | 1.015 ± 0.025* | 0.745 ± 0.012* | 0.288 ± 0.007** | 3.182 ± 0.101 |
| Glutamine/Glutamate | L-Serine/Glycine | D-Seri/L-Serinene | GABA/Glutamate | |||
| Frontal cortex | ||||||
| Control | 1.848 ± 0.043 | 1.027 ± 0.037 | 0.499 ± 0.005 | 0.267 ± 0.008 | ||
| Poly(I:C) | 1.883 ± 0.040 | 0.967 ± 0.021 | 0.478 ± 0.007* | 0.285 ± 0.007 | ||
| Hippocampus | ||||||
| Control | 1.909 ± 0.043 | 0.949 ± 0.020 | 0.412 ± 0.008 | 0.262 ± 0.006 | ||
| Poly(I:C) | 1.911 ± 0.031 | 0.765 ± 0.021*** | 0.387 ± 0.007* | 0.286 ± 0.006* | ||
| Striatum | ||||||
| Control | 1.475 ± 0.033 | 0.892 ± 0.027 | 0.394 ± 0.009 | 0.369 ± 0.016 | ||
| Poly(I:C) | 1.523 ± 0.026 | 0.738 ± 0.016*** | 0.386 ± 0.004 | 0.373 ± 0.013 | ||
Data (nmol/mg tissue) are expressed as the mean ± SEM (Control: n = 13, Poly(I:C): n = 13). *P < 0.05, **P < 0.01, ***P < 0.001 compared to control group (Student’s t test).
The ratio of glutamine to glutamate in the three regions was not different (Table 2). Furthermore, the ratio of L-serine to glycine in the hippocampus and striatum of poly(I:C) group was significantly lower than that of control group, suggesting alterations in the L-serine – glycine conversion in these regions (Table 2). Moreover, the ratio of D-serine to L-serine in the frontal cortex and hippocampus of poly(I:C) group was significantly lower than that of control group, suggesting alterations in the D-serine – L-serine conversion in these regions (Table 2). The ratio of GABA to glutamate in the hippocampus of poly(I:C) group was significantly higher than that of control group (Table 2). These findings suggest abnormalities in the NMDA receptor neurotransmission in the brain regions of adult offspring after MIA.
Supplementation of D-serine in drinking water prevents cognitive deficits in adult offspring after MIA
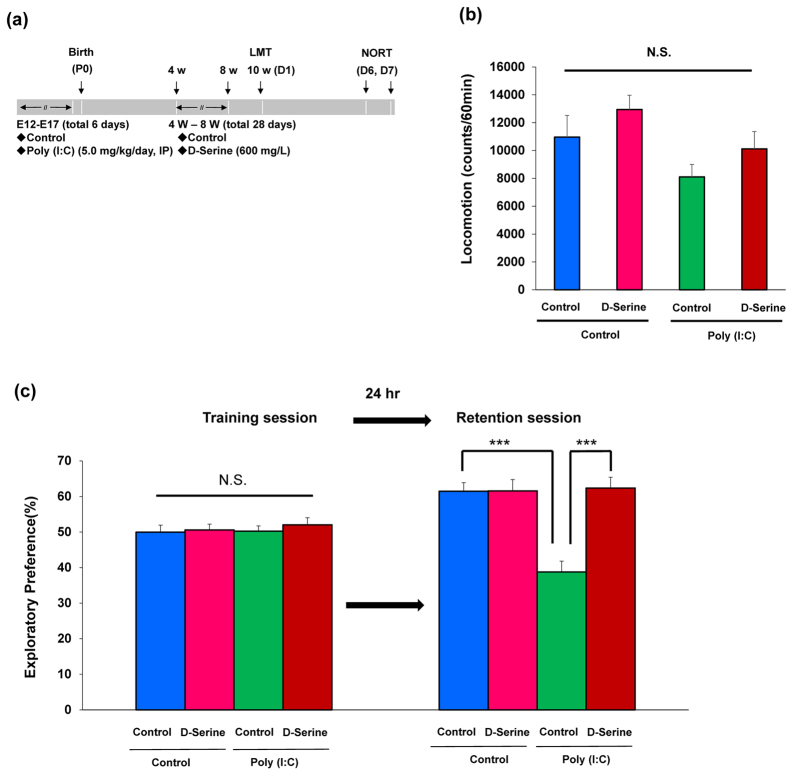
We examined whether D-serine was capable of preventing cognitive deficits in adult offspring after MIA. From P28 to P56, D-serine (600 mg/L) or a vehicle in drinking water was given into mice. To exclude the acute effects of D-serine, water in drinking water was given into all mice for 2-weeks (from P57 to P70) before behavioral tests (from P70 to P84)(Fig. 4a). Two-way ANOVA of locomotion data revealed no difference (poly(I:C): F1,43 = 5.467, P = 0.024, D-serine: F1,43 = 2.698, P = 0.108, Interaction: F1,43 = 0.000, P = 0.987) among the four groups (Fig. 4b). In the training session of NORT, there was no difference (poly(I:C): F1,42 = 0.230, P = 0.634, D-serine: F1,42 = 0.450, P = 0.506, Interaction: F1,42 = 0.110, P = 0.742) between four groups (Fig. 4c). In the retention session, two-way ANOVA of NORT data revealed statistical significance (poly(I:C): F1,42 = 13.58, P = 0.001, D-serine: F1,42 = 15.83, P < 0.001, Interaction: F1,42 = 15.66, P < 0.001) among the four groups (Fig. 4c). The exploratory preference of poly(I:C) group was significantly lower than that of control, and supplementation of D-serine significantly improved poly(I:C)-induced cognitive deficits in adult offspring (Fig. 4c).
Figure 4. Effects of D-serine supplementation on cognitive deficits in the adult offspring after prenatal poly(I:C) exposure.
(a): Schedule of treatment and behavioral tests. Saline or poly(I:C)(5 mg/kg/day from E12 to E17) was injected into pregnant mice. Vehicle or D-serine (600 mg/L) in drinking water was given into mice from 4-week to 8-week olds. Behavioral tests including locomotion (LMT: 10 W (D1)) and novel object recognition test (NORT: D6 and D7) were performed. (b): Locomotion: there was no significant difference among the four groups in the locomotor activity. The value is expressed as the mean ± SEM (n = 10–15). N.S.: not significant. (c): NORT: The exploratory preferences were significantly lower in the poly(I:C) offspring than controls in the retention session, but there was no difference between the two groups in the training session. ***P < 0.001 compared with control group. The value is expressed as the mean ± SEM (n = 9–13).
Discussion
In this study, we found that prenatal exposure to poly(I:C) caused cognitive deficits in juvenile and adult offspring. Furthermore, it also caused alterations in the levels and the ratio of crucial amino acids (glutamate, glutamine, glycine, D-serine, L-serine, GABA) in the brains of juvenile and adult offspring. These amino acids are related to the glutamine-glutamate-GABA cycle in the brain7,8,48(Fig. 5). Moreover, gene expression of Srr, Grin1, Grin2a, and Grin2b in the hippocampus of poly(I:C) treated animals was significantly lower than that of control groups, suggesting NMDA receptor hypofunction in the hippocampus of juvenile offspring after MIA. Finally, supplementation with D-serine during juvenile and adolescent stages could prevent cognitive deficits in adult offspring after MIA. Considering the crucial role of NMDA receptors in brain development, it is likely that prenatal poly(I:C) exposure causes NMDA receptor hypofunction in the brains of juvenile offspring, giving rise to the later life behavioral abnormalities seen in adult offspring after MIA. It is therefore possible that treatment with D-serine could prevent the onset of psychosis in high-risk subjects.
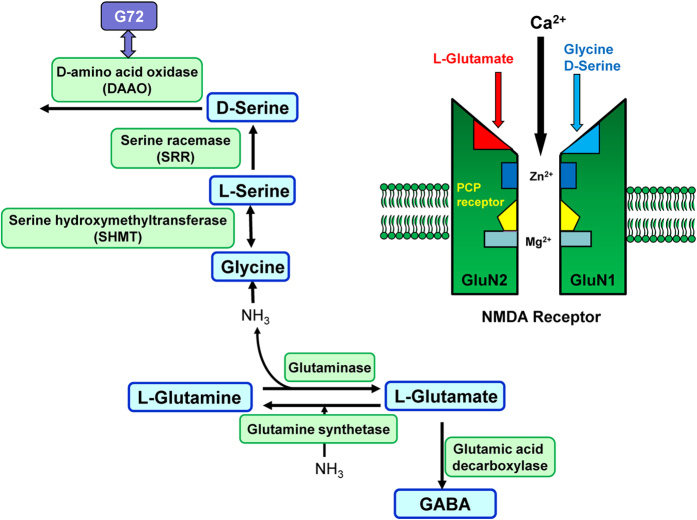
Figure 5. Synthetic and metabolic pathway of amino acids and NMDA receptor.
L-Glutamate, an excitatory amino acid, is synthesized from L-glutamine by glutaminase, and metabolized to L-glutamine by glutamine synthetase. In addition, L-glutamate is metabolized to γ-aminobutyric acid (GABA), an inhibitory amino acid, by glutamic acid decarboxylase. D-Serine is synthesized from L-serine by serine racemase (SRR), and is metabolized by D-amino acid oxidase (DAAO). L-Serine is converted to glycine by serine hydroxymethyltransferase (SHMT). Phencyclidine (PCP) is an ion-channel blocker of the NMDA receptor. Glycine and D-serine are endogenous co-agonists of the glycine modulatory site on the GluN1 subunit. Glutamate is an endogenous agonist at glutamate sites on the GluN2 subunit. Thus, glutamine-glutamate-GABA cycle plays a key role in the NMDA receptor neurotransmission. (A slight modification with ref. 8).
We found reduced expression of the Srr gene in the hippocampus of juvenile offspring after prenatal poly(I:C) exposure, although levels of D-serine and L-serine and the ratio of D- to L-serine in the hippocampus remained the same. We also found reduced gene expression of NMDA receptor subtypes,Grin1, Grin2a, and Grin2b in the hippocampus of juvenile offspring after prenatal poly(I:C) exposure. Thus, it seems that disturbance of NMDA receptor function in the hippocampus might play a role in the cognitive deficits seen in juvenile offspring after MIA. It was shown that prenatal poly(I:C)(10 mg/kg/day on days E14, E16 and E18) exposure caused a reduction of Grin1 in rat brains from P21 offspring45. Other research suggested that prenatal poly(I:C) (5 mg/kg on gestation day 17) exposure significantly reduced GluN1 protein levels in the dorsal hippocampus of adult offspring49. Taken together, it is likely that maternal activation of the immune system can interfere with NMDA receptor function during brain development, inducing cognitive deficits in juvenile and adult offspring. Further detailed studies on how prenatal poly(I:C) exposure induces the NMDA receptor hypofunction and behavioral abnormalities in juvenile and adulthood are needed.
In this study, we found significant alterations in the D-serine levels in three brain regions of adult offspring after MIA although D-serine levels were not altered in juvenile offspring, indicating neurodevelopmental changes of D-serine in the poly(I:C) model. Furthermore, we found significant alterations in GABA levels and GABA/glutamate ratio in the frontal cortex from juvenile offspring after MIA although these findings were recovered to control levels at adult offspring after MIA. Together, these findings suggest neurodevelopmental changes in the synthesis and metabolism of amino acids in the brain regions after MIA.
Patients with schizophrenia show non-psychotic and non-specific prodromal symptoms, such as cognitive impairment, for several years preceding the onset of frank psychosis46,47. A meta-analysis of 27 studies showed that the average rate of transition to full psychosis among such patients is 22 percent within the first year and 36 percent within three years47. Therefore, providing early intervention at the prodromal phase of schizophrenia and related psychosis is one of the most important and challenging tasks in psychiatry50. Here, we found that prenatal poly(I:C) exposure induced cognitive deficits in juvenile offspring, suggesting that these offspring may show prodromal, or at risk of psychosis symptoms. Interestingly, we found that supplementation with D-serine from juvenile to adolescent stages prevented cognitive deficits in adult offspring after MIA. Previously, we also reported that chronic administration of D-serine (900 mg/kg/day from P35 to P70) significantly prevented the onset of behavioral abnormalities after neonatal exposure to phenazine methosulfate (a SRR inhibitor)51. Very interestingly, a recent double-blind, placebo-controlled, randomized study showed that D-serine (60 mg/kg/day for 16 weeks) could prevent the conversion to psychosis in individuals at clinical high risk of schizophrenia52. These findings make D-serine an attractive prophylactic amino acid for early intervention in the onset of schizophrenia53, mainly because D-serine is effective for treating several symptoms in schizophrenia24,25,26,27,28,29,52.
In conclusion, our results suggest that prenatal poly(I:C) exposure causes cognitive deficits relevant to prodromal symptoms, during juvenile and adult stages. Interestingly, supplementation with D-serine from juvenile to adolescent stages could prevent cognitive deficits in adult offspring after MIA, indicating that D-serine may serve as an early intervention for psychosis.
Methods and Materials
Animals
Pregnant ddY mice (E5, 9–10 weeks old) were purchased from Japan SLC Inc. (Hamamatsu, Shizuoka, Japan). The mice were housed in clear polycarbonate cages (22.5 × 33.8 × 14.0 cm), under a controlled 12/12 hour light-dark cycle (lights on from 07:00 am to 07:00 pm), with room temperature at 23 ± 1 °C and humidity at 55 ± 5%. The mice were given free access to water and food pellets. All experiments were carried out in accordance with the Guide for Animal Experimentation of Chiba University. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee.
Prenatal administration of poly(I:C)
Treatment schedule of poly(I:C) was performed according to our previous reports42,54. Every six consecutive days from E12 to E17, the pregnant mice were injected intraperitoneally (i.p.) with poly(I:C)(5.0 mg/kg, Sigma-Aldrich Co. Ltd., USA) dissolved in physiological saline, or an equivalent volume of saline. The male mice of offspring were separated from their mothers after 3 weeks, and mice were caged in separate groups.
Supplementation of D-serine as drinking water
To examine whether D-serine supplementation during juvenile and adolescence could prevent the onset of behavioral abnormalities in adult mice of offspring after MIA, D-serine (600 mg/L, Sigma-Aldrich, St. Louis, MO, USA) or vehicle (water) were administered as drinking water from P28 to P56; this period is thought to represent juvenile to adolescence. The dose resulted in a daily dose of approximately 100 mg/kg D-serine per body weight (average weight: 30 g, average drinking volume: 5 mL/day). From P57, all mice received water. Behavioral tests were performed at adulthood (P70-P84).
Measurement of amino acids in the brain
At juvenile (P28), and adult (P70) stages, mice were sacrificed, and their brains were removed for measurement of amino acids. The frontal cortex, hippocampus and striatum were quickly dissected on ice from whole brain. The dissected tissues were weighed and stored at −80°C until assayed.
Briefly, brain tissues were homogenized in 1.5 mL of methanol (HPLC grade) on ice. The homogenates were centrifuged at 3000 g for 6 min at 4 °C, and 20 μL of supernatant was evaporated to dryness at 40 °C. To the residue, 20 μL of H2O (HPLC grade), 20 μL of 0.1 M borate buffer (pH 8.0), and 60 μL of 50 mM 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F; Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan) in CH3CN (HPLC grade) were added. The reaction mixture was then heated to 60 °C for 2 min, and immediately supplemented with 100 μL of H2O/acetonitrile (90/10) containing 0.1% trifluoroacetic acid (TFA) to stop the reaction. Levels of amino acids (D-serine, L-serine, glycine, glutamine, glutamate, GABA) were measured using high performance liquid chromatography (HPLC) system (Shimadzu Corporation, Kyoto, Japan), as previously reported48,55. Fluorescence detection was performed at 530 nm with an excitation wavelength of 470 nm.
Measurement of gene expression in the brain
At juvenile (P28) stage, mice were sacrificed, and their brains were removed for measurement of gene expression of Srr, Dao, Grin1, Grin2a, and Grin2b. The frontal cortex and hippocampus were quickly dissected on ice from whole brain. A quantitative RT-PCR system (Step One Plus, Thermo Fisher Scientific, Yokohama, Japan) was used to measure mRNAs. The specific mRNA transcripts were quantified by TaqManGene Expression assays (Thermo Fisher Scientific, Yokohama, Japan). Expression levels of Srr (Mm00489123_m1), Dao (Mm00438378_m1), Grin1 (Mm00433790_m1), Grin2a (Mm00433802_m1), and Grin2b (Mm00433820_m1) were measured in brain tissue. Total RNA was extracted by use of an RNeasy Mini Kit (Qiagen, Hilden, Germany). The purity of total RNA was assessed by Biophotometer plus (Eppendorf, Hamburg, Germany). the RNA samples were used in the first strand cDNA synthesis with High Capacity cDNA Reverse Transcription Kit (#4368813 Thermo Fisher Scientific, Yokohama, Japan). All samples were tested in triplicate and average values were used for quantification. The average values were normalized to Vic-labeled Actb mRNA (#4352341E: pre-developed TaqMan Assay Reagents, Thermo Fisher Scientific, Yokohama, Japan).
Locomotor activity in mice
Both horizontal and rearing activity were monitored by an infrared ray passive sensor system (SCANET-SV10, Melquest Ltd, Toyama, Japan), and activity was integrated every 10 minutes, as previously reported51,54,56. Individual mice were placed in activity chambers and allowed 2 hours of free exploration as spontaneous activity.
Novel object recognition test (NORT)
The NORT was performed as previously reported51,54,57,58. Before testing, mice were habituated in the box for 3 days. During a training session, two objects (differing in shape and color but of similar size) were placed in the box 35.5 cm apart (symmetrically), and each animal was allowed to explore in the box for 5 minutes. The animals were considered to be exploring the object when the head of the animal was both facing and within 2.54 cm of the object or when any part of the body, except for the tail was touching the object. The time that mice spent exploring each object was recorded. After training, mice were immediately returned to their home cages, and the box and objects were cleaned with 75% ethanol, to avoid any possible instinctive odorant cues. Retention tests were carried out at one-day intervals, following the respective training. During the retention test, each mouse was reintroduced into their original test box, and one of the training objects was replaced by a novel object. The mice were then allowed to explore freely for 5 minutes, and the time spent exploring each object was recorded. Throughout the experiments, the objects were counter-balanced, in terms of their physical complexity and emotional neutrality. A preference index, that is, the ratio of time spent exploring either of the two objects (training session) or the novel object (retention test session) over the total time spent exploring both objects, was used.
Statistical analysis
All data are shown as mean ± standard error of the mean (S.E.M.). The data of amino acids, locomotion, and NORT were analyzed by Student’s t-test, or two-way analysis of variance (ANOVA), followed Bonferroni test. Significance for results was set at P < 0.05.
Additional Information
How to cite this article: Fujita, Y. et al. Supplementation with D-serine prevents the onset of cognitive deficits in adult offspring after maternal immune activation. Sci. Rep. 6, 37261; doi: 10.1038/srep37261 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.H.).
Footnotes
Author Contributions Y.F. and T.I. performed the experiments and analyzed the data; K.H. designed and coordinated overall experimental goals and wrote the manuscript. All authors have read and commented on the final manuscript and have agreed to its submission.
References
- Javitt D. C. & Zukin S. R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 148, 1301–1308 (1991). [DOI] [PubMed] [Google Scholar]
- Krystal J. H. et al. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv. Rev. Psychiatry 7, 125–143 (1999). [PubMed] [Google Scholar]
- Heresco-Levy U. N-methyl-D-aspartate (NMDA) receptor-based treatment approaches in schizophrenia: the first decade. Int. J. Neuropsychopharmacol. 3, 243–258 (2000). [DOI] [PubMed] [Google Scholar]
- Coyle J. T. & Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 174, 32–38 (2004). [DOI] [PubMed] [Google Scholar]
- Javitt D. C. Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr. Bull. 38, 911–913 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. et al. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 263, 367–377 (2013). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Abnormalities of the glutamine-glutamate-GABA cycle in the schizophrenia brain. Schizophr. Res. 156, 281–282 (2014). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Targeting of NMDA receptors in the new treatment of schizophrenia. Expert Opin. Ther. Targets 18, 1049–1063 (2014). [DOI] [PubMed] [Google Scholar]
- Ohgi Y., Futamura T. & Hashimoto K. Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr. Mol. Med. 15, 206–221 (2015). [DOI] [PubMed] [Google Scholar]
- Steullet P. et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr. Res. 176, 41–51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadi M. P., Behrens M. M. & Sejnowski T. J. Abnormal gamma oscillations in N-methyl-D-aspartate receptor hypofunction models of schizophrenia. Biol. Psychiatry 79, 716–726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard J. M. D-Amino acids in brain neurotransmission and synaptic plasticity. Amino Acids 43, 1851–1860 (2012). [DOI] [PubMed] [Google Scholar]
- Mothet J. P., Le Bail M. & Billard J. M. Time and space profiling of NMDA receptor co-agonist functions. J. Neurochem. 135, 210–225 (2015). [DOI] [PubMed] [Google Scholar]
- Labrie V., Wong A. H. & Roder J. C. Contributions of the D-serine pathway to schizophrenia. Neuropharmacology 62, 1484–1503 (2012). [DOI] [PubMed] [Google Scholar]
- Javitt D. C. et al. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr. Bull. 38, 958–966 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H., Lane H. Y. & Tsai G. E. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol. Biochem. Behav. 100, 665–677 (2012). [DOI] [PubMed] [Google Scholar]
- Van der Auwera S. et al. The inverse link between genetic risk for schizophrenia and migraine through NMDA (N-methyl-D-aspartate) receptor activation via D-serine. Eur. Neuropsychopharmacol. 26, 1507–1515 (2016). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch. Gen. Psychiatry 60, 572–576 (2003). [DOI] [PubMed] [Google Scholar]
- Yamada K. et al. Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol. Psychiatry 57, 1493–1503 (2005). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 767–769 (2005). [DOI] [PubMed] [Google Scholar]
- Bendikov I. et al. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr. Res. 90, 41–51 (2007). [DOI] [PubMed] [Google Scholar]
- Calcia M. A. et al. Plasma levels of D-serine in Brazillian individuals with schizophrenia. Schizophr. Res. 142, 83–87 (2012). [DOI] [PubMed] [Google Scholar]
- Fukushima T. et al. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS One 9, e101652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G. et al. D-serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 44, 1081–1089 (1998). [DOI] [PubMed] [Google Scholar]
- Lane H. Y. et al. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int. J. Neuropsychopharmacol. 13, 451–460 (2010). [DOI] [PubMed] [Google Scholar]
- Kantrowitz J. T. et al. High dose of D-serine in the treatment of schizophrenia. Schizophr. Res. 121, 125–130 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U. et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol. Psychiatry 57, 577–585 (2005). [DOI] [PubMed] [Google Scholar]
- Ermilov M. et al. A pilot double-blind comparison of D-serine and high-dose olanzapine in treatment-resistant patients with schizophrenia. Schizophr. Res. 150, 604–605 (2013). [DOI] [PubMed] [Google Scholar]
- Tsai G. E. & Lin P. Y. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia. A critical review and meta-analysis. Curr. Pham. Des. 16, 522–537 (2010). [DOI] [PubMed] [Google Scholar]
- Singh S. P. & Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs 25, 859–885 (2011). [DOI] [PubMed] [Google Scholar]
- Verrall L. et al. D-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur. J. Neurosci. 26, 1657–1669 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira C. et al. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr. Res. 101, 76–83 (2008). [DOI] [PubMed] [Google Scholar]
- Wolosker H. Serine racemase and the serine shuttle between neurons and astrocytes. Biochem. Biophys. Acta. 1814, 1558–1566 (2011). [DOI] [PubMed] [Google Scholar]
- Chumakov I. et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc. Natl. Acad. Sci. USA 99, 13675–13680 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detetra-Wadleigh S. D. & McMahon F. J. G72/G30 in schizophrenia and bipolar disorder: review and meta-analysis. Biol. Psychiatry 60, 106–114 (2006). [DOI] [PubMed] [Google Scholar]
- Li D. & He L. G72/G30 genes and schizophrenia: a systemic meta-analysis of association studies. Genetics 175, 917–922 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J. et al. Allelic association of G72/G30 with schizophrenia and bipolar disorder: a comprehensive meta-analysis. Schizophr. Res. 98, 89–97 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H. et al. Distinctively higher plasma G72 protein levels in patients with schizophrenia than in healthy individuals. Mol. Psychiatry 19, 636–637 (2014). [DOI] [PubMed] [Google Scholar]
- Akyol E. S. et al. Increased serum G72 protein levels in patients with schizophrenia: a potential candidate biomarker. Acta. Neuropsychiatr. doi: 10.1017/neu.2016.34. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group for the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. J. et al. The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann. Med. 35, 86–93 (2009). [DOI] [PubMed] [Google Scholar]
- Ozawa K. et al. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol. Psychiatry 59, 546–554 (2006). [DOI] [PubMed] [Google Scholar]
- Yoshimi N., Futamura T. & Hashimoto K. Prenatal immune activation and subsequent peripubertal stress as a new model of schizophrenia. Expert Rev. Neurother. 13, 747–750 (2013). [DOI] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 75, 307–315 (2014). [DOI] [PubMed] [Google Scholar]
- Forrest C. M. et al. Prenatal activation of Toll-like receptors-3 by administration of the viral mimetic poly(I:C) changes synaptic proteins, N-methyl-D-aspartate receptors and neurogenesis markers in offspring. Mol. Brain 5, 22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión R. E. et al. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am. J. Psychiatry 168, 806–813 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P. et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry 69, 562–571 (2012). [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Sawa A. & Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry 62, 1310–1316 (2007). [DOI] [PubMed] [Google Scholar]
- Meyer U. et al. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 22, 469–486 (2008). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Can the sigma-1 receptor agonist fluvoxamine prevent schizophrenia? CNS Neurol. Disord. Drug Targets 8, 470–474 (2009). [DOI] [PubMed] [Google Scholar]
- Hagiwara H., Iyo M. & Hashimoto K. Neonatal disruption of serine racemase causes schizophrenia-like behavioral abnormalities in adulthood: clinical rescue by D-serine. PLoS One 8, e62438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz J. T. et al. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry 2, 403–412 (2015). [DOI] [PubMed] [Google Scholar]
- Dong C. & Hashimoto K. Early intervention for psychosis with N-methyl-D-aspartate receptor modulators. Clin. Psychopharmacol. Neurosci. 13, 328–329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M. et al. Intake of 7,8-dihydroxyflavone during juvenile and adolescent stages prevents onset of psychosis at adult offspring after maternal immune activation. Sci. Rep. in press, 6, 36087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K. et al. Depressive-like behavior in adrenocorticotropic hormone-treated rats blocked by memantine. Pharmacol. Biochem. Behav. 102, 329–334 (2012). [DOI] [PubMed] [Google Scholar]
- Matsuura A. et al. Effects of sodium benzoate on pre-pulse inhibition deficits and hyperlocomotion in mice after administration of phencyclidine. Acta. Neuropsychiat. 27, 159–167 (2015). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur. J. Pharmacol. 519, 114–117 (2005). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology 32, 514–521 (2007). [DOI] [PubMed] [Google Scholar]