Abstract
Fibronectin is a glycoprotein of the extracellular matrix, and regulates the processes of self-renewal and cell cycle progression. This study aimed to investigate fibronectin expression in colorectal cancer (CRC) and elucidate the effects of fibronectin on CRC by using a knockdown approach. Immunohistochemistry was used to evaluate the expression of fibronectin in 107 CRC patient tissues and gene expression was detected by real-time quantitative PCR (qPCR) and western blot analysis. Based on the above findings, the association among fibronectin expression, clinicopathological features and prognosis was analyzed. Next, fibronectin expression was silenced by small-interfering RNAs (siRNAs) and the effects of fibronectin siRNA transfection on CRC cells and tumor growth in nude mice were assessed. Expression of genes in the NF-κB/p53-apoptosis signaling pathway were analyzed after fibronectin siRNA transfection both in vitro and in vivo. Based on the results, high expression of fibronectin was observed both in the CRC tissues and CRC cell lines. The expression level was positively correlated with TNM stage (P=0.0025) and distant metastasis (P=0.0013). By Kaplan-Meier analysis, the patients with low fibronectin expression had a longer survival time comparing to those with relatively high expression. Knockdown of fibronectin suppressed SW480 cell proliferation, migration and invasion. In addition, knockdown of fibronectin led to S phase cell cycle arrest. The following study showed that the NF-κB/p53-apoptosis signaling pathway in CRC was affected by fibronectin knockdown. Tumor formation was also depressed by fibronectin siRNA transfection of CRC cells. These results showed the significant role of fibronectin in CRC tissues and cell lines. Therefore, fibronectin may be regarded as a potential target for CRC treatment.
Keywords: NF-κB/p53-apoptosis signaling pathway, fibronectin, colorectal cancer, cell cycle, nude mice
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in humans around the world and accounts for approximately 9% of all cancer-related deaths (1). Early diagnosis of CRC is critical to perform surgical resection and contributes to improvement in the CRC survival rate of patients (2). However, to date, the long-term survival rate of patients with CRC remains poor. Although molecular alterations including epigenetic and genetic changes have been widely reported (3), knowledge of the molecular mechanisms in the progression of CRC is still limited and further studies are required to achieve a more complete understanding of CRC pathogenesis.
Fibronectin is a glycoprotein of the extracellular matrix which plays a crucial role in cell adhesion, growth, migration and differentiation (4,5). New findings suggest that fibronectin also participates in wound healing and embryonic development (6). Fibronectin has been found highly expressed in tumor vasculature and mediates angiogenesis during tumorigenesis showing a potential role in cancer progression (7,8). Actually, fibronectin has been implicated in the development of several types of cancers (9,10). Fibronectin participates in cell migration and invasion in metastatic cancer cells (11). Moreover, fibronectin has an anti-apoptotic function in standard chemotherapy (12). In breast cancer, high expression of fibronectin has been observed when compared to that in normal breast parenchyma (9). The molecular mechanisms of fibronectin in the progression of cancer cells have been identified as gene expression changes induced by fibronectin. For instance, fibronectin was found to enhance expression of MMPs which are also key factors in the promotion of cancer invasion and metastasis (13,14). Meanwhile, fibronectin was found to stimulate expression of inflammatory factors including IL-8, CXCL-3 and Toll-like receptor (TLR)2 indicating the regulatory influence of fibronectin on major inflammatory cells in the tumor microenvironment (15,16). However, the function of fibronectin in CRC demands to be resolved.
This study investigated the significance and therapeutic potential of fibronectin as well as the effects of fibronectin knockdown on the prognosis, cell proliferation and malignancy of CRC. Our findings demonstrated that silencing of fibronectin mediated by RNAi promoted G1 cell cycle arrest, leading to apoptosis in CRC cells which could be a potential treatment strategy.
Materials and methods
Patients
In total, 107 patients, who were diagnosed with CRC at The Second Xiangya Hospital of Central South University, were involved in the present study. The characteristics of these patients are shown in Table I. Normal control tissues were collected from 115 patients without CRC. The tissues were embedded in formalin-fixed paraffin. The follow-up study was performed for 60 months. All of the experiments were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and informed consent was provided by the patients themselves or their relatives.
Table I.
Correlation between characteristics of the CRC patients and fibronectin expression.
| Fibronectin expression | ||||
|---|---|---|---|---|
| Characteristics | n | Low | High | P-value |
| Total | 107 | 49 | 58 | |
| Age (years) | ||||
| <60 | 55 | 32 | 23 | 0.459 |
| ≥60 | 52 | 27 | 25 | |
| Gender | ||||
| Male | 58 | 28 | 30 | 0.542 |
| Female | 49 | 27 | 22 | |
| TNM stage | ||||
| I | 7 | 3 | 4 | 0.0025 |
| II | 47 | 17 | 30 | |
| III | 33 | 10 | 23 | |
| VI | 20 | 3 | 17 | |
| Distant metastasis | ||||
| No | 32 | 21 | 11 | 0.0013 |
| Yes | 75 | 18 | 57 | |
Immunohistochemistry and scoring
Protein expression was assayed by immunohistochemistry using the paraffin-embedded sections from the patients. The sections were first deparaffinized by xylene (Shanghai Sangon, Shanghai, China). Then, the sections were incubated with rabbit polyclonal anti-fibronectin antibody (1:500, ab2413; Abcam, Cambridge, MA, USA) at 4°C for 24 h. After washing with TBST buffer (Shanghai Sangon) for three times, the sections were incubated with goat anti-rabbit IgG H&L (HRP, 1:500, ab6721; Abcam) at room temperature for 1 h. Then, protein immunostaining was performed using DAB Plus substrate (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's instructions. The scoring of the stained tumor cells were divided into five grades: 0 (positive cells <5%), 1 (positive cells between 5 and 25%), 2 (positive cells between 26 and 50%), 3 (positive cells between 51 and 75%), and 4 (positive cells between 75 and 100%). The cells were observed using a light microscope (Axioskop; Zeiss, Germany). We designated grade 1 and 2 as low expression of fibronectin and grade 3 and 4 as high expression of fibronectin for the prognosis analysis.
Cell culture and transfection
CCD-18Co (normal colon cells), HT-29 (CRC cells), CaCo2 (CRC cells) and SW480 (CRC cells) cell lines were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). The cells were cultured in RPMI-1640 medium (Gibco-BRL, Paisley, UK) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA) at 37°C in 5% CO2. The fibronectin siRNA (sc-29315) and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Transfection was performed using Lipofectamine® 2000 (Invitrogen Life Technologies, Shanghai, China) following the manufacturer's instructions. Negative small interfering RNAs (siRNAs) and scrambled siRNA were synthesized by Shanghai Sangon.
Real-time quantitative PCR (qPCR)
RNA isolation and reverse transcription were performed using RNAiso Plus kit and PrimeScript™ II First Strand cDNA Synthesis kit (both from Takara, Dalian, China) according to the manufacturer's instructions, respectively. The qPCR primers were: fibronectin: forward, 5′-TTATG ACGAC GGGAA GACCT-3′ and reverse, 5′-GCTGG ATGGA AAGAT TACTC-3′; GADPH forward, 5′-AATCC CATCA CCATC TTCCA-3′ and reverse, 5′-TGGAC TCCAC GACGT ACTCA-3′; caspase-3 forward, 5′-TTAAT AAAGG TATCC ATGGA GAACA CT-3′ and reverse, 5′-TAGAG TTCTT TTGTG AG-3′; p53 forward, 5′-AACGG TACTC CGCCA CC-3′ and reverse, 5′-CGTGT CACCG TCGTG GA-3′; PARP forward, 5′-AGGCT GCTTT GTCAA GAA-3′ and reverse, 5′-CTTGC TGCTT GTTGA AGAT-3′; Bax forward, 5′-ACCAA GCTGA GCGAG TGTC-3′ and reverse, 5′-ACAAA GATGG TCACG GTCTG CC-3′; cytochrome c forward, 5′-CGTCG CATTC CAGAT TATCC A-3′ and reverse, 5′-CAACT ACGGA TATAT AAGAG CCAAA ACTG-3′; and NF-κB forward, 5′-ACCTG AGTCT TCTGG ACCGC TG-3′ and reverse 5′-CCAGC CTTCT CCCAA GAGTC GT-3′. The reaction was performed on Applied Biosystems® ABI 7500 system (Thermo Fisher Scientific). The reaction system SYBR® Premix Ex Taq™ II (Takara) was used according to the manufacturers instructions. The reaction condition was: 95°C for 15 min and 40 cycles of 95°C for 10 sec and 60°C for 10 sec. Melting curve analysis was used to determine the specific amplification.
Western blot analysis
SW480 cells were collected at the treatment time-points with reagents as suggested in each experiment. Cells were lysed with RIPA buffer (Thermo Fisher Scientific) and 15% electrophoresis (Thermo Fisher Scientific) was conducted under 90 V for 2 h. After undergoing electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes. Primary antibodies, rabbit polyclonal anti-fibronectin antibody (1:500), rabbit polyclonal caspase-3 antibody (1:500, ab2302), rabbit polyclonal NF-κB antibody (1:500, ab7971), rabbit polyclonal p53 antibody (1:500, ab1431), rabbit polyclonal PARP (1:500, ab6079), rabbit polyclonal Bax antibody (1:500, ab53154), rabbit polyclonal cytochrome c antibody (1:500, ab90529) and rabbit polyclonal GAPDH antibody (1:500, ab9485) (all from Abcam) were incubated with the membranes for 24 h at 4°C. After washing with TBST buffer three times, the secondary antibody goat anti-rabbit IgG H&L (HRP) was incubated at room temperature for 1 h. Immunostaining was carried out using DAB Plus substrate and chemiluminescence system (Amersham Biosciences, Freiburg, Germany). The results were analyzed using chemiluminescence Molecular Imager® ChemiDoc™ XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
MTT assay
In order to analyze the viability of the cells, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed. After undergoing transfection, the cells were seeded in 96-well plates at a density of 1×104 cells/well for 24, 48 and 72 h. Consequently, 20 µl MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to the wells and incubated at 37°C for 4 h. Then, the supernatant was removed, and the cells were dissolved in 200 µl of dimethyl sulfoxide (Sigma-Aldrich). The optical density was observed at 490 nm via a spectrophotometer (SpectraMax Plus 384; Molecular Devices, Sunnyvale, CA, USA). The experiments were performed in triplicate.
Cell migration and invasion assays
Transwell assays were performed using a 24-well insert (Corning, Inc., Corning, NY, USA) to analyze the effect of fibronectin on CRC cells. After transfection, the cells (1×104 cells/well) were seeded in the top of the chambers in triplicate for 48 h. The lower chambers with 10% fetal bovine serum were co-cultured for another 72 h. For the invasion assay, extracellular matrix gel (BD Biosciences, Bedford, MA, USA) was used. The cells located on the top surface of the membrane were discarded and the cells on the bottom surface were stained with 0.1% crystal violet (Shanghai Sangon). The number of cells on each insert were calculated in five visual fields randomly and calculated using a light microscope (Axioskop; Zeiss).
Flow cytometry
The cell cycle distribution was assessed by flow cytometry. The control and transfected cells (48 h after transfection) were collected and washed with PBS buffer (Shanghai Sangon). After fixation in 70% ethanol, the cells were stained using 20 µg/ml propidium iodide (PI) containing 20 µg/ml RNase (DNase-free) (both from Shanghai Sangon) for 2 h. Then the cells were assessed using flow cytometer Partec-PAS (Partec GmbH, Muenster, Germany). The cells in the G0/G1, S, G2/M, and sub-G1 phases were defined via Multicycle Cell Cycle software (Phoenix Flow System, San Diego, CA, USA).
Tumor formation assays
Female nude mice (6 to 8-weeks old) were provided by The Second Xiangya Hospital of Central South University. To obtain the tumor in vivo model, equal numbers of SW480 cells (1×106) or SW480 cells transfected with control siRNAs and fibronectin-siRNA were injected into the nude mice (four mice per group). After injection, the mice were maintained under a 12-h light/12-h dark cycle and fed with standard mouse diet for four weeks. The tumor volume was measured and calculated as (width2 × length)/2. The mice were then sacrificed using sodium pentobarbital (Sigma-Aldrich) and the tumor tissues were collected for further analysis. The study was performed following the recommendations of the Guide for the Care and Use of Laboratory Animals of The Second Xiangya Hospital of Central South University.
Statistical analysis
All the results are presented as mean ± standard deviation. Data analyses were processed via the two-tailed Student's t-test and the log-rank test. P<0.05 was considered as significant. SPSS v17.0 software was used to perform these analyses (SPSS Inc., Chicago, IL, USA).
Results
Upregulation of fibronectin in CRC tissues
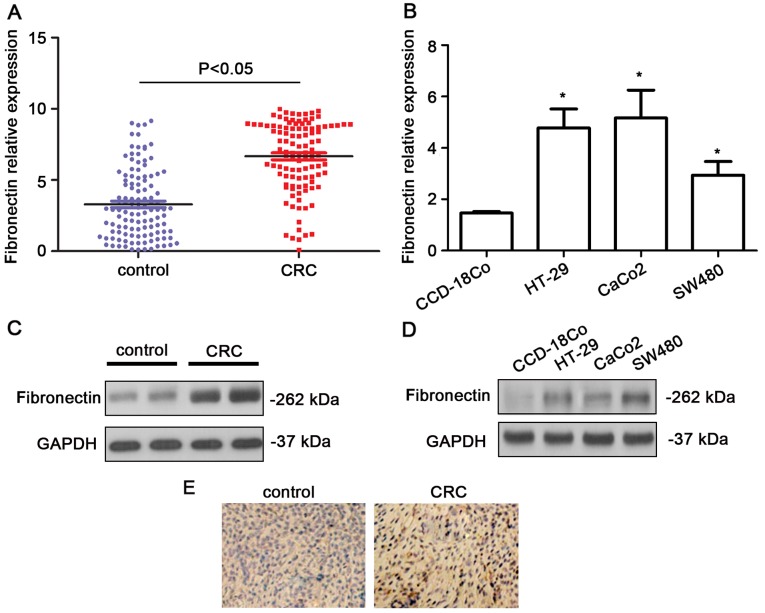
The expression levels of fibronectin were shown to be upregulated in the CRC tissues in comparison with that in the normal colon tissues using qPCR (P<0.05, Fig. 1A). Higher expression levels of fibronectin were also noted in the CRC cell lines (P<0.05, Fig. 1B). Western blot analysis indicated that the fibronectin blot was remarkably denser in the CRC samples (Fig. 1C). In the CRC cell lines, the protein levels of fibronectin showed a significant increase (Fig. 1D). In addition, as shown in the immunohistochemistry results, the expression level of fibronectin was obviously higher in the CRC tissues than that in the non-cancerous, normal colorectal tissues (Fig. 1E). The fibronectin protein expression was assessed in 107 CRC tissue samples by immunohistochemistry. In the CRC tissues, 54.21% (58/107) of the cases had high fibronectin expression (grade >2). Additionally, we observed a marked difference in fibronectin expression in tumors with different TNM stage (P=0.0025) and distant metastasis (P=0.0013) (Table I).
Figure 1.
Elevated expression of fibronectin in CRC patient tissues and cell lines. (A) qPCR analysis indicated higher expression of fibronectin in the CRC tissues compared to that noted in the control tissues. (B) qPCR analysis indicated higher expression of fibronectin in CRC cell lines including HT-29, CaCo2 and SW480 compared to that noted in the CCD-18co cells (normal colon cells) (*P<0.05). (C) Western blot analysis indicated higher expression of fibronectin in CRC tissues compared to that noted in the control tissues. (D) qPCR analysis indicated higher expression of fibronectin in CRC cell lines including HT-29, CaCo2 and SW480 compared to that noted in the CCD-18co cells (normal colon cells). (E) Immunohistochemistry indicated higher expression of fibronectin in the CRC tumor tissues.
Correlation between fibronectin expression and prognosis in CRC patients
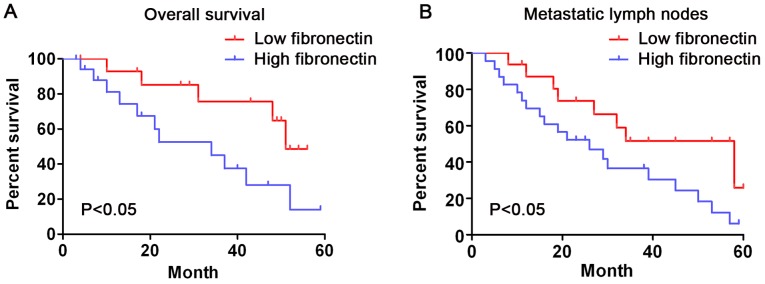
In short, the survival analysis showed that the survival time of patients whose fibronectin expression was low (grade 1–2) was significantly longer than that of those whose fibronectin expression was high (grade 3–4) (log-rank test, P<0.05, Fig. 2A). Patients with metastatic lymph nodes whose fibronectin expression was low showed a longer overall survival time than that in the patients whose fibronectin expression was high (Fig. 2B, log-rank test, P<0.05). These outcomes indicate that fibronectin expression is significantly related to the survival rate of CRC patients.
Figure 2.
Kaplan-Meier curves indicating the correlation of fibronectin expression and overall survival in patients with CRC. (A) Patients with higher fibronectin expression had poorer overall survival compared to the survival rate of patients with low fibronectin expression. (B) Patients with metastatic lymph nodes with higher fibronectin expression had poorer overall survival compared with patients with metastatic lymph nodes with low fibronectin expression.
Fibronectin-siRNA inhibits fibronectin expression in the SW480 cells
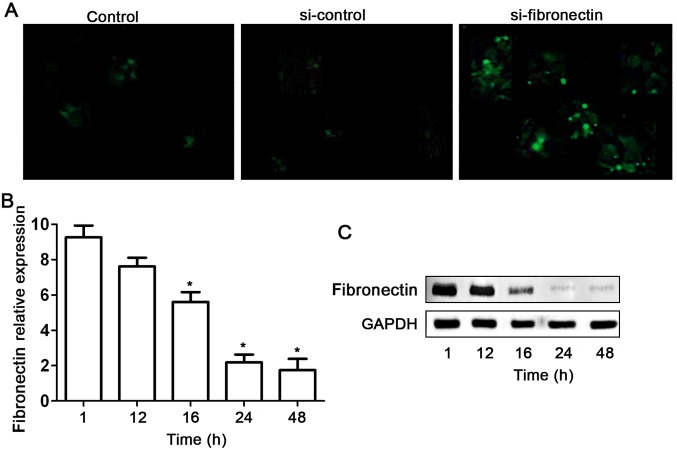
Based on our preliminary data of the high expression level of fibronectin in SW480 cell lines, we used RNAi technique to silence this gene in the SW480 cells. Fibronectin-siRNA (si-fibronectin) effectively suppressed fibronectin expression in the SW480 cells (Fig. 3). First, si-fibronectin was successfully transfected into the SW480 cells as indicated by fluorescent labels (Fig. 3A). Meanwhile the efficiency of the transfection had a time-dependent effect (Fig. 3B). Although no obvious decrease in expression of fibronectin was found after si-fibronectin transfection of SW480 cells for 12 h, fibronectin mRNA and protein levels were significantly suppressed at 16, 24 and 48 h (Fig. 3B and C).
Figure 3.
Knockdown of fibronectin expression by siRNA approach in SW480 cells. (A) Transfection with fibronectin-labeled siRNA was analyzed by fluorescence microscopy. (B) qPCR results indicated that 16, 24 and 48 h of transfection depressed fibronectin expression significantly (*P<0.05). (C) Western blot result indicated that 16, 24 and 48 h of transfection suppressed fibronectin expression.
Knockdown of fibronectin suppresses CRC cell proliferation, migration and invasion
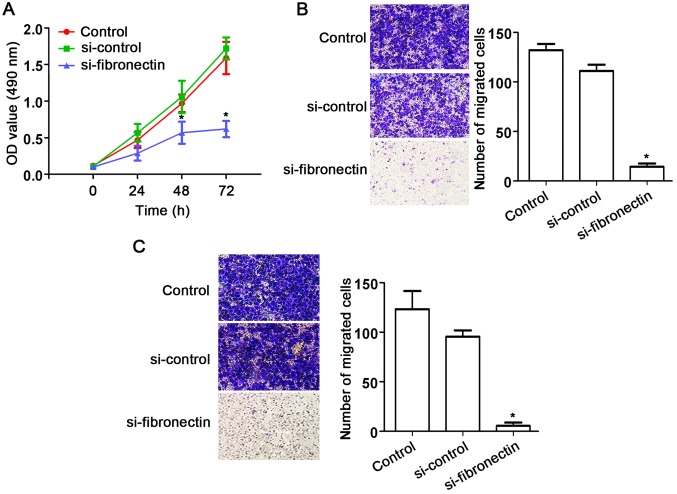
The effects of fibronectin on CRC SW480 cells were assessed using siRNA approach. After transfection with si-fibronectin, cell proliferation was significantly decreased compared to that observed in the control and si-control groups (Fig. 4A). In addition, fibronectin knockdown also suppressed cell migration (Fig. 4B) and cell invasion (Fig. 4C) of the SW480 cells. These results indicate the suppression of cell activities by fibronectin knockdown.
Figure 4.
Knockdown of fibronectin inhibits the proliferation, migration and invasion of SW480 cells. (A) MTT assay indicated that si-fibronectin transfection inhibited cell proliferation compared to that noted in the control and si-control groups (*P<0.05). (B) Transwell assay indicated that si-fibronectin transfection significantly inhibited cell migration compared to that noted in the control and si-control groups (*P<0.05). (C) After si-fibronectin transfection, cell invasion was significantly inhibited compared to that noted in the control and si-control groups (*P<0.05).
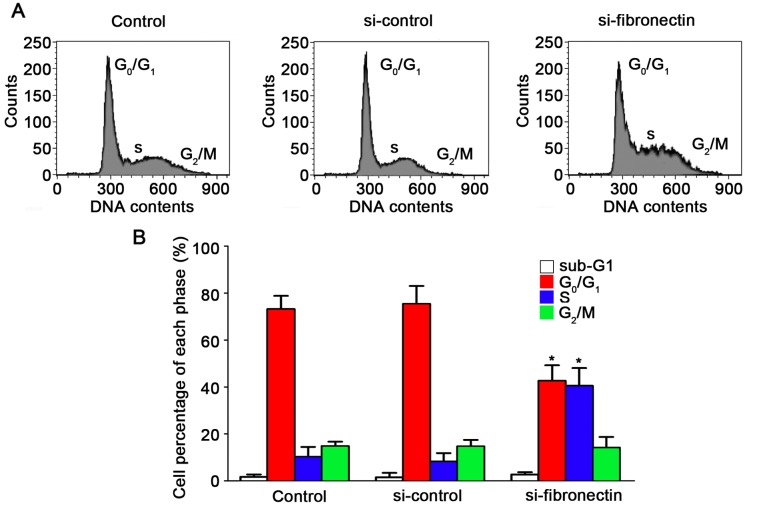
Knockdown of fibronectin causes S-phase cell cycle arrest in CRC cells
Effects of the silencing of fibronectin on the suppression of cell progression have been proven. To demonstrate the effect of fibronectin knockdown on the cell cycle, flow cytometry was performed. The results for the SW480 cells are shown in Fig. 5A after transfection with si-fibronectin, si-control and in the untreated control. After transfection with si-fibronectin, the percentage of cells in the S phase increased to 40.54% which was significantly higher compared to the control and si-control groups (Fig. 5B). The percentage of cells in the G0/G1 phases after si-fibronectin transfection was decreased to 42.62% (Fig. 5B). Hence, these results showed that the si-fibronectin transfection suppressed CRC cell viability. The cells could not proliferate normally by arrest in the S phase of the cell cycle and S-phase arrest may induce cell death and morphological changes during progression of CRC.
Figure 5.
Knockdown of fibronectin affects the cell cycle distribution of the SW480 cells. (A) Flow cytometry was used to analysis the cell cycle of the cells in the si-fibronectin, control and si-control groups. (B) Analysis of the cell cycle showed that the cells were arrested in the S phase after si-fibronectin transfection compared to the control and si-control groups (*P<0.05).
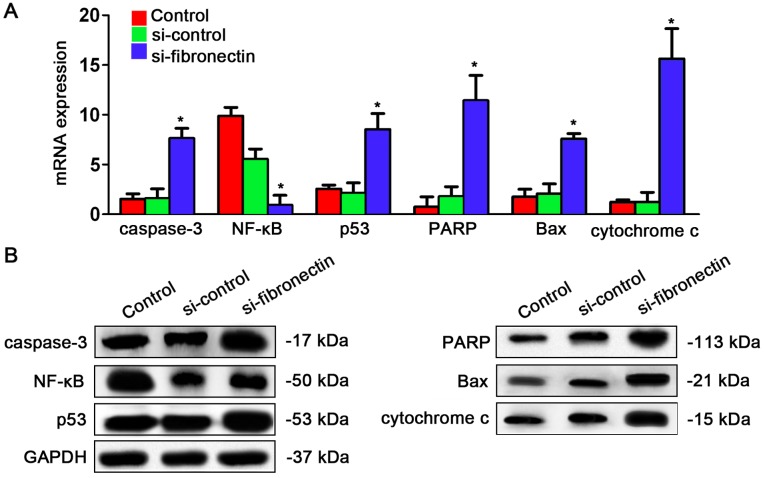
Fibronectin-siRNA transfection affects expression of genes in the NF-κB/p53-apoptosis signaling pathway in CRC cells
After understanding the effects of si-fibronectin transfection on the cell cycle, we explored whether the NF-κB/p53-apoptosis signaling pathway is regulated by fibronectin. The qPCR results showed that caspase-3, p53, PARP, Bax and cytochrome c were significantly increased after si-fibronectin transfection while only NF-κB was significantly depressed by si-fibronectin transfection (Fig. 6A). Similar results were observed for the protein expression levels. Caspase-3, p53, PARP, Bax and cytochrome had higher expression after si-fibronectin transfection while NF-κB had lower expression following si-fibronectin transfection (Fig. 6B).
Figure 6.
Knockdown of fibronectin affects gene expression of the NF-κB/p53-apoptosis signaling pathway in the SW480 cells. (A) qPCR results indicated that after si-fibronectin transfection, caspase-3, p53, PARP, Bax and cytochrome c were increased and NF-κB was decreased significantly compared to the control and si-control groups (*P<0.05). (B) Western blot results indicated that caspase-3, p53, PARP, Bax and cytochrome c were increased and NF-κB was decreased after si-fibronectin transfection compared to the control and si-control groups.
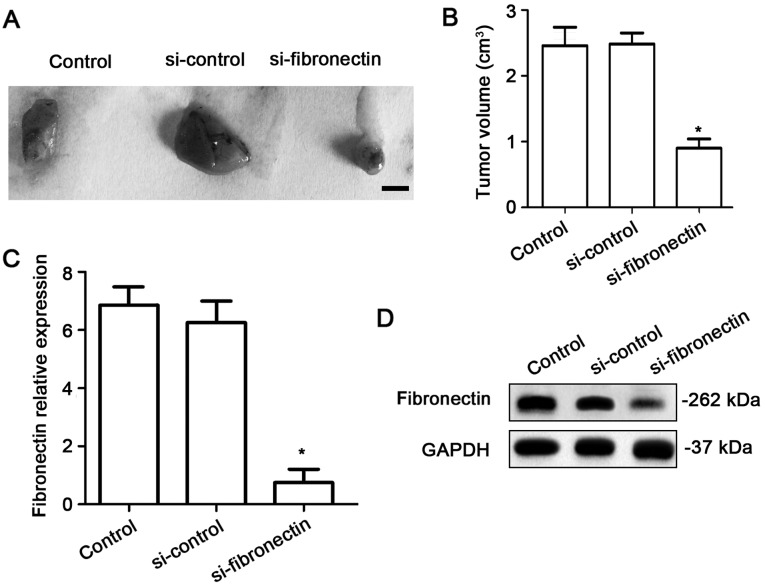
Fibronectin-siRNA transfection decreases tumor growth in vivo
To investigate the effects of fibronectin on tumor growth in vivo, the nude mice were injected using equal numbers of SW480 cells (1×106) or SW480 cells transfected with si-control or si-fibronectin. After 4 weeks, tumors significantly appeared in the mice. The results showed that si-fibronectin inhibited tumor growth in vivo (Fig. 7A and B). The expression of fibronectin was measured using qPCR and western blotting. Both at the RNA (Fig. 7C) and protein levels (Fig. 7D), the fibronectin expression was significantly decreased in the tumor tissue after transfection with si-fibronectin.
Figure 7.
Knockdown of fibronectin decreases tumor growth in vivo. (A) Tumor growth was significantly inhibited in a xenograft model by SW480 cells transfected with si-fibronectin compared to the control and si-control groups. (B) The volume of the tumors was significantly lower in the si-fibronectin group compared to the control and si-control groups (*P<0.05). (C) qPCR indicated suppression of the expression of fibronectin by si-fibronectin transfection compared to the control and si-control groups (*P<0.05). (D) Western blot results indicated that following si-fibronectin transfection, the fibronectin protein expression level was decreased.
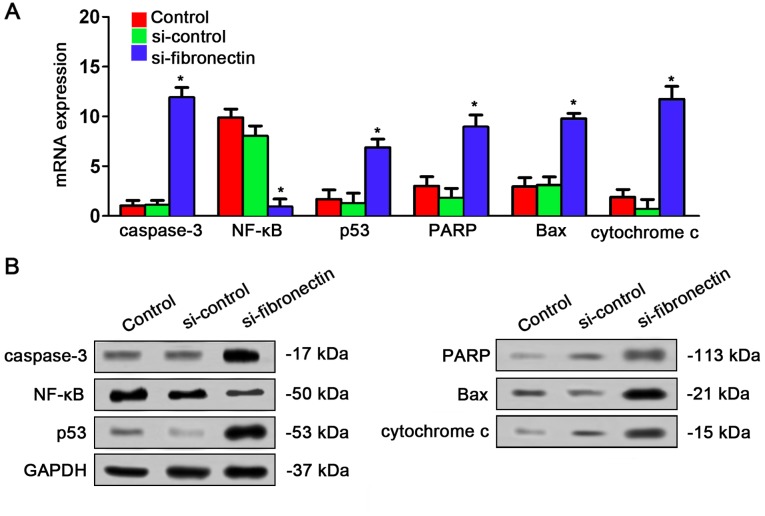
Fibronectin-siRNA affects expression of the NF-κB/p53-apoptosis signaling pathway in vivo
The effects of si-fibronectin on expression of NF-κB/p53-apoptosis signaling pathway in nude mice were assayed by qPCR and western blot analysis. The result was very similar to the results in vitro. qPCR indicated that caspase-3, p53, PARP, Bax and cytochrome c were significantly increased by si-fibronectin transfection in the tumor tissues from the nude mice while NF-κB was decreased after transfection (Fig. 8A). The result of western blotting also showed that caspase-3, p53, PARP, Bax and cytochrome c had higher expression after si-fibronectin transfection while NF-κB had lower expression after si-fibronectin transfection (Fig. 8B).
Figure 8.
Gene expression of the NF-κB/p53-apoptosis signaling pathway in xenograft nude mice. (A) In the tumor tissues, si-fibronectin transfection increased caspase-3, p53, PARP, Bax and cytochrome c mRNA expression and decreased NF-κB mRNA expression indicated by qPCR result (*P<0.05). (B) In the tumor tissues, si-fibronectin transfection stimulated expression of caspase-3, p53, PARP, Bax and cytochrome c protein levels and decreased NF-κB protein levels as indicated by western blot results.
Discussion
CRC is one of the most dangerous cancers; thus, the studies of CRC hold great value for human health. To date, the CRC incidence and mortality worldwide have increased dramatically over the past few decades (17). Early detection and new treatment strategies to decrease death rates are needed. Thus, the survival will be increased. However, this approach is limited due to the lack of screening tools with high specificity and sensitivity, appropriate for early-stage tumors (18,19). In this way, understanding the pathogenic mechanisms of CRC is urgently needed at present. It has been indicated that fibronectin is a multifunctional, extracellular matrix glycoprotein and takes part in regulating self-renewal, cell cycle progression and proliferation in cancer cells (20,21). To investigate the role of fibronectin in CRC, we assayed the expression of fibronectin in CRC patient tissues and cell lines. As far as we known, studies on the significant role of fibronectin in CRC are limited. We found that fibronectin was highly expressed in HT-29, CaCo2 and SW480 cells. This result is in line with previous studies on the basis of fibronectin overexpression in other types of human cancer cell lines (20,22).
In the present study, we found that expression of fibronectin was associated with cancer cell metastasis, TNM stage and survival. Moreover, no correlations were significant for age or gender of the patients. These results suggest that high expression of fibronectin could be regarded as a prognostic marker for CRC diagnosis. Remarkably high fibronectin expression has been observed in various human cancers, such as lung (21), bladder (23), ovarian (12) and breast cancers (12). Nevertheless, the mechanism involved in the modulation of CRC by fibronectin remains unexplained. In our study, fibronectin had higher expression in CRC tissues in comparison with its expression level in non-cancerous tissues, which indicates a similar expression pattern of fibronectin in different cancers.
In human breast cells, fibronectin overexpression has been found to promote chemotaxis of human malignant plasma cell lines and to stimulate cell invasion as well as migration (24). Additionally, in the present study, fibronectin was found to be a suitable potential prognostic marker for CRC. Meanwhile, high expression of fibronectin was negatively related with the prognosis of patients with breast cancer (9). Based on these results, a worse outcome was hypothesized in CRC patients who had higher fibronectin expression. The multivariate analysis demonstrated that high expression of fibronectin could be an independent prognostic parameter for CRC patients. In the cell lines, fibronectin-siRNA transfection remarkably depressed proliferation and invasion of CRC cells. Taken together, apart from acting as a prognostic marker, fibronectin could be a candidate therapeutic target.
In the present study, the functions of fibronectin in the progression of the cell cycle and apoptosis were defined by fibronectin-siRNA. These oligos resulted in a remarkable reduction in fibronectin mRNA expression. Knockdown of fibronectin suppressed the increase in CRC cells after transfection. Apoptosis was also induced by transfection. Silencing of fibronectin in SW480 cells led to suppression of growth. All of these findings were coincident with the cell cycle results, in which we detected an aggregation in the S phase after fibronectin-siRNA transfection. To understand apoptosis-related pathways, we detected the effects of fibronectin-siRNA transfection on the NF-κB/p53 apoptosis signaling pathway. The results showed that except for NF-κB, all the genes were significantly increased by fibronectin-siRNA transfection. NF-κB is a key factor which has transcriptional activation to regulate multiple gene expression and participates in cell proliferation, vasculogenesis and tumor metastasis (25,26). Suppression of NF-κB activity could induce the sensitivity of cancer cells to chemotherapy and radiotherapy (27). In addition, inhibition of NF-κB increased p53 and promoted apoptosis in cancer cells by increasing DNA damage (27). On the contrary, caspase-3, p53, PARP, Bax and cytochrome c were upregulated by fibronectin-siRNA transfection. These five genes are markers for apopotsis. Caspase-3 has a typical role in apoptosis by affecting chromatin condensation and DNA fragmentation (28). p53, an anticancer protein, plays a role in apoptosis in cancers cells and inhibition of angiogenesis (29). PARP maintains the structural stability of chromosomes and helps DNA replication and transcription (30). Bax participates in the p53 pathway which also induces apoptosis in cancer cells (31). Cytochrome c is an intermediary of apoptosis which controls cell death in response to DNA damage (32). These genes control the cell cycle and apoptosis of cancer cells. The present findings suggest that fibronectin-siRNA transfection suppressed CRC cell growth via the NF-κB/p53-apoptosis signaling pathway and arrested the cells in the S phase. Knockdown of fibronectin could be a candidate treatment strategy.
Taken together, fibronectin has the potential to be a diagnostic biomarker of CRC and high fibronectin is related to the poor prognosis of CRC patients. Knockdown of fibronectin may be helpful for the treatment of CRC. Further investigations are needed to identify the gene therapy strategies and efficacy of these approaches. The mechanisms of the modulation of CRC development by fibronectin need to be elucidated in more detail.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Xiang J, Li Z, Lu S, Hu J, Gao X, Yu L, Wang L, Wang J, Wu Y, et al. A plasma microRNA panel for early detection of colorectal cancer. Int J Cancer. 2015;136:152–161. doi: 10.1002/ijc.28136. [DOI] [PubMed] [Google Scholar]
- 3.Nishihara R, Morikawa T, Kuchiba A, Lochhead P, Yamauchi M, Liao X, Imamura Y, Nosho K, Shima K, Kawachi I, et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol. 2013;178:84–100. doi: 10.1093/aje/kws431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 5.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 6.Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of α5β1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.ATV.0000016045.93313.F2. [DOI] [PubMed] [Google Scholar]
- 7.Rybak J-N, Roesli C, Kaspar M, Villa A, Neri D. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67:10948–10957. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 8.Santimaria M, Moscatelli G, Viale GL, Giovannoni L, Neri G, Viti F, Leprini A, Borsi L, Castellani P, Zardi L, et al. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clin Cancer Res. 2003;9:571–579. [PubMed] [Google Scholar]
- 9.Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ, Pavlidis N. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: Their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–2370. doi: 10.1016/S0959-8049(02)00210-1. [DOI] [PubMed] [Google Scholar]
- 10.Saad S, Gottlieb DJ, Bradstock KF, Overall CM, Bendall LJ. Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer Res. 2002;62:283–289. [PubMed] [Google Scholar]
- 11.Meng XN, Jin Y, Yu Y, Bai J, Liu GY, Zhu J, Zhao YZ, Wang Z, Chen F, Lee KY, et al. Characterisation of fibronectin-mediated FAK signalling pathways in lung cancer cell migration and invasion. Br J Cancer. 2009;101:327–334. doi: 10.1038/sj.bjc.6605154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing H, Weng D, Chen G, Tao W, Zhu T, Yang X, Meng L, Wang S, Lu Y, Ma D. Activation of fibronectin/PI-3K/Akt2 leads to chemoresistance to docetaxel by regulating survivin protein expression in ovarian and breast cancer cells. Cancer Lett. 2008;261:108–119. doi: 10.1016/j.canlet.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Stanton H, Gavrilovic J, Atkinson SJ, d'Ortho MP, Yamada KM, Zardi L, Murphy G. The activation of ProMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of MT1-MMP (MMP-14) to a 45 kDa form. J Cell Sci. 1998;111:2789–2798. doi: 10.1242/jcs.111.18.2789. [DOI] [PubMed] [Google Scholar]
- 14.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Márquez A, Yagüe J, Cid MC. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999;94:2754–2766. [PubMed] [Google Scholar]
- 15.La Fleur M, Beaulieu AD, Kreis C, Poubelle P. Fibronectin gene expression in polymorphonuclear leukocytes. Accumulation of mRNA in inflammatory cells. J Biol Chem. 1987;262:2111–2115. [PubMed] [Google Scholar]
- 16.Hines KL, Kulkarni AB, McCarthy JB, Tian H, Ward JM, Christ M, McCartney-Francis NL, Furcht LT, Karlsson S, Wahl SM. Synthetic fibronectin peptides interrupt inflammatory cell infiltration in transforming growth factor beta 1 knockout mice. Proc Natl Acad Sci USA. 1994;91:5187–5191. doi: 10.1073/pnas.91.11.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava S, Verma M, Henson DE. Biomarkers for early detection of colon cancer. Clin Cancer Res. 2001;7:1118–1126. [PubMed] [Google Scholar]
- 20.Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, Altieri DC, Languino LR. Fibronectin protects prostate cancer cells from tumor necrosis factor-α-induced apoptosis via the AKT/survivin pathway. J Biol Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 21.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 22.Shibata K, Kikkawa F, Nawa A, Thant AA, Naruse K, Mizutani S, Hamaguchi M. Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 1998;58:900–903. [PubMed] [Google Scholar]
- 23.Eissa S, Swellam M, Sadek M, Mourad MS, El Ahmady O, Khalifa A. Comparative evaluation of the nuclear matrix protein, fibronectin, urinary bladder cancer antigen and voided urine cytology in the detection of bladder tumors. J Urol. 2002;168:465–469. doi: 10.1016/S0022-5347(05)64659-9. [DOI] [PubMed] [Google Scholar]
- 24.Shibayama H, Tagawa S, Hattori H, Inoue R, Katagiri S, Kitani T. Laminin and fibronectin promote the chemotaxis of human malignant plasma cell lines. Blood. 1995;86:719–725. [PubMed] [Google Scholar]
- 25.Baldwin AS., Jr The NF-κB and IκB proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 26.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 27.Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol. 2009;19:404–413. doi: 10.1016/j.tcb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z, Hu W, Rajagopal G, Levine AJ. The tumor suppressor p53: Cancer and aging. Cell Cycle. 2008;7:842–847. doi: 10.4161/cc.7.7.5657. [DOI] [PubMed] [Google Scholar]
- 30.Curtin NJ. PARP inhibitors for cancer therapy. Expert Rev Mol Med. 2005;7:1–20. doi: 10.1017/S146239940500904X. [DOI] [PubMed] [Google Scholar]
- 31.Mackey TJ, Borkowski A, Amin P, Jacobs SC, Kyprianou N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology. 1998;52:1085–1090. doi: 10.1016/S0090-4295(98)00360-4. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]