Conspectus
Molecular spintronics (spin + electronics), which aims to exploit both the spin degree of freedom and the electron charge in molecular devices, has recently received massive attention. Our recent experiments on molecular spintronics employ chiral molecules which have the unexpected property of acting as spin filters, by way of an effect we call “chiral-induced spin selectivity” (CISS). In this Account, we discuss new types of spin-dependent electrochemistry measurements and their use to probe the spin-dependent charge transport properties of nonmagnetic chiral conductive polymers and biomolecules, such as oligopeptides, L/D cysteine, cytochrome c, bacteriorhodopsin (bR), and oligopeptide-CdSe nanoparticles (NPs) hybrid structures. Spin-dependent electrochemical measurements were carried out by employing ferromagnetic electrodes modified with chiral molecules used as the working electrode. Redox probes were used either in solution or when directly attached to the ferromagnetic electrodes. During the electrochemical measurements, the ferromagnetic electrode was magnetized either with its magnetic moment pointing “UP” or “DOWN” using a permanent magnet (H = 0.5 T), placed underneath the chemically modified ferromagnetic electrodes. The spin polarization of the current was found to be in the range of 5–30%, even in the case of small chiral molecules. Chiral films of the l- and d-cysteine tethered with a redox-active dye, toludin blue O, show spin polarizarion that depends on the chirality. Because the nickel electrodes are susceptible to corrosion, we explored the effect of coating them with a thin gold overlayer. The effect of the gold layer on the spin polarization of the electrons ejected from the electrode was investigated. In addition, the role of the structure of the protein on the spin selective transport was also studied as a function of bias voltage and the effect of protein denaturation was revealed. In addition to “dark” measurements, we also describe photoelectrochemical measurements in which light is used to affect the spin selective electron transport through the chiral molecules. We describe how the excitation of a chromophore (such as CdSe nanoparticles), which is attached to a chiral working electrode, can flip the preferred spin orientation of the photocurrent, when measured under the identical conditions. Thus, chirality-induced spin polarization, when combined with light and magnetic field effects, opens new avenues for the study of the spin transport properties of chiral molecules and biomolecules and for creating new types of spintronic devices in which light and molecular chirality provide new functions and properties.
Introduction
The field of spintronics (or spin-based electronics)1,2 uses both the spin and charge of electrons in logic and other electronics applications. The electron spin concept underlies our understanding of magnetism, and the spin properties of molecules and materials can be manipulated by applying a magnetic field. Moreover, it is commonly assumed that magnetic materials or materials possessing high spin–orbit coupling (SOC) are needed to observe spin-dependent charge transport. The control of spin currents by an applied magnetic field was practically implemented in 1988 through the discovery of the giant magnetoresistance (GMR)3,4 effect, and since then spintronic functionality has been implemented in solid state devices.5,9 These ideas have fostered the vision for a molecular spintronics, where spin current flows across organic/biomolecules (a spin-transporting medium).6−8 While the importance of electron spin in determining the electronic structure of atoms, molecules, and materials is well-known and the importance of spin selection rules in chemical reaction mechanisms is well appreciated,9−11 the role of spin in charge transfer through molecules and among molecules is less well studied. Here we overview our recent contributions to spin transport in molecules and its manifestation in electrochemical processes.
In 1999, we first observed that the interaction of electrons with chiral molecules is spin specific as probed by photoelectron spectroscopy;12 this effect is referred to as Chirality Induced Spin Selectivity (CISS).13,14 In experiments that measured the spin orientation of photoelectrons transmitted through self-assembled monolayers (SAMs) of double-strand DNA (dsDNA), surprising results were obtained.15 It was found that photoelectrons ejected from Au coated with dsDNA are spin polarized even when ejected with unpolarized light. Namely, the yield of photoelectrons changed with the light polarization, but the DNA molecules filtered for the same preferred spin orientation, independent of the light polarization. The dsDNA spin filtering ability was also proved by spin-dependent conductive-AFM measurements.16 More experimental17−22 and theoretical23−26 studies of the CISS effect have been performed since then. In recent experiments, it was demonstrated that electrons that pass through a layer of dsDNA, and their spin was therefore polarized, induce chiral-selective chemistry.27
This Account focuses on our recent developments in the study of the spin dependent transport through chiral thin films, as applied to spin-dependent electrochemistry.
Basics of Spin-Dependent Electrochemistry
Magnetic field effects in electrochemistry are a well-established and thoroughly studied topic. Typical studies mainly deal with the Lorentz force28 and Kelvin force effects;29 however, workers have reported additional effects, such as magnetic field induced conformational changes30 that change the charge transfer current, and magnetic field dependences of electrochemical reaction mechanisms when either radical intermediates31 or radical pairs32−34 are involved. In addition, external magnetic fields have been used to manipulate the morphology of electrodeposited films and create electrodes with enantiomeric selectivity.35 Nonetheless, before our work,36 no studies had explored whether the faradaic current is spin-polarized. Despite the fact that electrochemical experimental methods are intensively investigated and are constantly being developed,37 the spin polarization effects in electrochemistry have not been explored.
In order to examine the spin polarization, it is necessary to introduce charge carriers with a particular spin orientation and/or analyze the charge current for the spin orientation. In the studies described here, we use a ferromagnetic electrode which can be magnetized by a permanent magnet. Flipping of the magnetic field (by changing the magnet orientation just underneath the working electrode) allows for selection of spin orientation, so that it is possible to inject spin-polarized electrons from the electrodes.13,14 Alternatively, it is possible to coat a nonmagnetic electrode with self-assembled monolayers (SAMs) of chiral molecules, use the chiral molecules (via the CISS effect to select the spin of the electrons ejected into the electrochemical system. A possible alternative approach, allowing for spin-dependent electrochemical measurements, is to embed a Hall probe inside the working electrode and to measure the magnetization, which results from the spin current, by the Hall voltage.38 Although this new method is highly promising, the studies described herein use the ferromagnetic electrodes for investigating the CISS effect, as illustrated in Figure 1A. Namely, in the studies described here, a ferromagnetic electrode was coated with a SAM of chiral molecules. The electrode serves as the spin analyzer, that provides information on the spin filtering power of the chiral SAMs.6,7
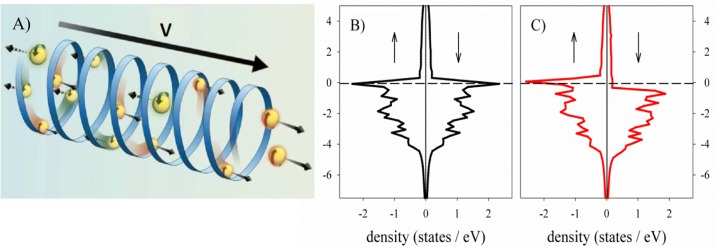
Figure 1.
(A) Schematic illustration of the CISS effect. Electron transmission through a chiral potential depends on their spin. The linear momenta of the electrons are coupled to their spins, and therefore electrons moving from left to right have the opposite spin than those moving from right to left. In this example, the spin is antiparallel to the electrons’ velocity. (B, C) Energy vs density of states (DOS), spin polarized plane wave GGA (generalized-gradient approximation) calculation for nickel (fcc). PAW (pseudo augmented-wave) PBE (Perdew–Burke–Ernzerhof) potential, energy cutoff 270 eV (VASP code).40 The dashed line indicates the Fermi level. (B) No magnetic moment and (C) collinear magnetic moment applied. Note that, for the magnetized substrate, the density of spin states (“UP” ↑ and “DOWN” ↓ arrows) near the Fermi levels are different.
Figure 1B shows the essential physics underlying the spin-dependent electrochemical measurements presented in this Account. When the electrode is magnetized in a specific direction, the “majority” spin electrons are stabilized relative to the electrons with the “minority” spin. Thus, the spin sublevels of the ferromagnetic electrode are populated to a different extent. In this specific example, just underneath the Fermi level (EF), the states are mainly populated with the majority spins, and hence the density of states of the majority spins (ρma) is high and most electrons ejected from the electrode will have this spin orientation. On the other hand, above the Fermi level the highest density of states is of the minority spins (ρmi) and therefore electrons having this spin orientation will be more efficiently injected into the electrode. Note that the orientation of the majority spin is antiparallel to the magnetic moment of the electrode.39 As a matter of convenience, we will refer throughout to the spin orientation as “UP” or “DOWN”. The choice is arbitrary in each experiment and does not relate to the actual orientation of the spin.
Let us assume that the rates of electron transfer through the adsorbed SAMs are given by kup and kdown for the two spin orientations. The current, I, at a steady state situation from the electrode to the solution is given by
| 1 |
in which C is a constant that depends
on various
experimental parameters of the cell, but is not affected by the magnetic
field direction. Hence, when the magnet is switched from the condition
in which the majority spins is “up” to the condition
in which the majority spin is “down”, the current will
change only if the rate constants change. Namely, the ratio between
the currents will be 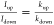 , and
we define the spin polarization, P, by the anisotropy
in the currents:
, and
we define the spin polarization, P, by the anisotropy
in the currents:
| 2 |
Therefore, the efficiency of injecting electrons from the electrode to the solution, or vice versa, depends on the chirality of the SAM that covers the ferromagnet; and for a given chirality, one spin orientation will be transmitted more efficiently through the chiral SAM than the other.
Spin Filtering through Chiral Conductive Polymer
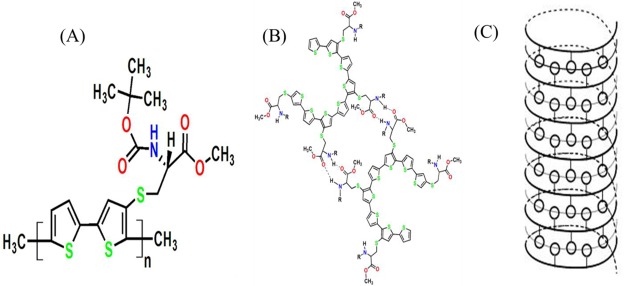
Ferromagnetic working electrodes (Ni, Co) are essential for manipulating the spin selectivity by an applied magnetic field, but their chemical reactivity toward ambient oxygen and water during monolayer formation can corrupt their ferromagnetic response.41,42 In particular, Ni (for Co the situation is even worse) forms an oxide layer which can deteriorate the coherence of spin in the current flowing through it. Thus, we developed a method to reduce the oxide film on Ni in situ with the assembly of a monolayer of chiral polymer.43 We grafted the polymer film on the Ni surface via electrochemical reduction, scanning the potential in the −0.1 to −0.8 V range vs saturated calomel electrode (SCE); the l-isomer of the chiral polymer, poly{[methyl N-(tert-butoxycarbonyl)-S-3-thienyl-l-cysteinate]-cothiophene} (PCT-L, Figure 2) forms a helical supramolecular structure via intermolecular H-bonding and π stacking among the thiophene rings. The PCT-L film (∼3 nm thick) was characterized by a number of different methods, including CD measurements, PMIRRAS, solid state magnetoresistance measurements, and enantioselectivity in the voltammetric response in the presence of a chiral ferrocene derivative. Other deposition methods, including spin coating or drop casting, which produced thicker films (∼40–60 nm) did not show any spin selectivity effect, presumably because of the scattering of electrons (including the loss of spin coherence) in such thick films. Thus, the working electrode consists of a ferromagnetic Ni film that is coated by a PCT-L layer, which acts as a spin-filter.
Figure 2.
(A) Chemical structure of L-polymer, PCT-L (see text), (B) intermolecular hydrogen bond-based interactions between the substituents, and (C) helical structure formed due to the hydrogen bonding between the polar substituents and the π stacking of the thiophene rings. The curved lines indicate the backbone, while the circles represent l-cysteine derivatives. Reproduced with permission from ref (43). Copyright 2015 WILEY-VCH.
The setup for the spin-dependent electrochemical measurements is shown in Figure 3A. The PCT-L coated nickel working electrode was magnetized, with magnetization pointing “UP” or “DOWN″, by placing a permanent magnet, H = 0.5 T, underneath the working electrode during the electrochemical measurements. The applied magnetic field H⃗ is oriented perpendicular to the electrode surface, and hence parallel to the electrical current j⃗, in order to minimize effects from the Lorentz force.
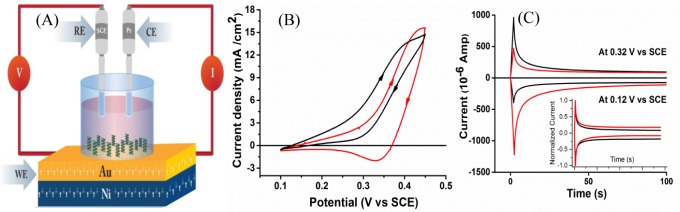
Figure 3.
(A) Schematic diagram illustrating the spin-dependent electrochemistry setup where a PCT/Ni electrode, platinum, and saturated calomel electrode (SCE) were used as the working electrode (WE), counter electrode (CE) and reference electrode (RE), respectively. The polymer grafted Ni electrode was magnetized by an external magnetic (H = 0.5 T) with its magnetic dipole pointing “UP” or “DOWN” in the course of electrochemical measurements. (B) Cyclic voltammograms of the L-polymer/Ni working electrode when the redox couple to an achiral ferrocene under magnetic field pointing “UP” (solid red curve), and “DOWN” (solid black curve). (C) Chronoamperometric measurements of the same system performed at two different potentials. In the inset, the normalized curves. Reproduced with permission from ref (43). Copyright 2015 WILEY-VCH.
Figure 3B shows the magnetic field effect on cyclic voltammograms obtained with the PCT-L polymer coated Ni working electrode in contact with an achiral ferrocene (Fc) redox couple. The red curve shows the case for the magnetic field direction oriented “UP”, for which the reduction current (electron flow from the ferromagnetic electrode to the ferrocenium) through the PCT-L is more kinetically facile (displays a well-defined faradaic peak) than the oxidation step.
In contrast, the black curve shows the voltammetry on the same electrode with the opposite magnetic field direction; in this case, the cathodic current is less facile and the cathodic peak is less well-defined. This effect was not observed for a gold electrode coated with the PCT-L -polymer. The spin selectivity in electron transport through the chiral polymer was demonstrated also by chronoamperometric measurements keeping the bias voltage fixed (Figure 3C).43 The spin-dependent chronoamperometric measurements showed a spin polarization, P, of +34% and −50% for potentials of +0.32 and +0.12 V, respectively; measured at a short time after the voltage pulse was initiated (2–3 s). However, at longer times (∼100 s) the spin polarization disappears. A comparison of the average decay times show that the relaxation of the current transient at 0.32 V versus SCE is larger for the UP magnetization than it is for the DOWN magnetization, implying that the UP magnetization has a higher resistance. In contrast, the chronoamperometric curve for the UP magnetization at 0.12 V, is shorter than that for the DOWN magnetization, implying that the DOWN magnetization has a higher resistance. These results are consistent with the current intensity measured and support the observation that the transport through the adsorbed film is spin dependent.
Spin Filtering Across Cysteine Coated Electrodes
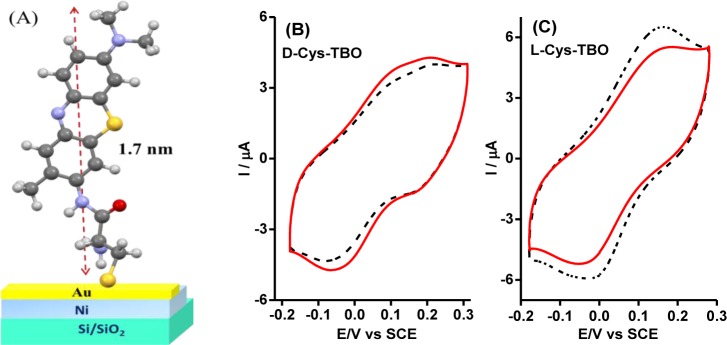
The redox probe toluidine blue O (TBO) was grafted, via covalent bond formation, to both L or D cysteine monolayer films about ∼1.7 nm thick (see Figure 4A). The cysteine being chiral acts as a spin filter.44 Nickel oxidation was prevented by coating the Ni surface with ultrathin Au overlayers of different thicknesses (5, 15, 20, 30 nm) upon which the SAMs of cysteine were prepared. The Au layer has excellent chemical stability, but its large spin–orbit coupling can destroy the spin polarization of the injected electrons, and this feature allowed us to examine how the spin polarization behavior changed as a function of the thickness of the Au overlayer. The voltammograms, which were obtained for a Ni/Au(10 nm)/d-Cys-TBO chiral working electrode, showed a dependence of the faradaic current on the direction of the nickel magnetization; and the spin polarization was found to be −6% for the oxidation peak potential at +0.185 V and to be +4.7% for the reduction peak potential at −0.056 V SCE (Figure 4B). On the other hand, the effective spin polarizations for the Ni/Au(10 nm)/l-Cys-TBO chiral electrode were found to have the opposite signs. For instance, the l-Cys-TBO produces a higher faradaic current (both cathodic and anodic) when the magnetization of the modified nickel electrode was “UP”, than when it was “DOWN”. As a result, the spin polarization measured was +9% for the oxidation peak potential at 0.160 V and it was −7.5% for the reduction peak potential at −0.024 V (Figure 4C). Note that the electrochemistry of the redox active achiral 6-(ferrocenyl)hexanethiol SAMs adsorbed onto the 10 nm Au/Ni does not show any magnetic field effect when measured under identical conditions.
Figure 4.
(A) Schematic diagram for covalently tethered toluidine blue O (TBO) to the working electrode via a cysteine SAMs (L or D). (B) Spin-dependent cyclic voltammogram recorded for d-cysteine-toluidine blue O, and (C) cyclic voltammogram for l-cysteine-toluidine blue O on a 200 nm Ni/10 nm Au electrode. The working electrode was magnetized with its magnetic moment pointing either “UP” (dashed black curve) or “DOWN” (solid red curve). Reproduced with permission from ref (44). Copyright 2015 American Chemical Society.
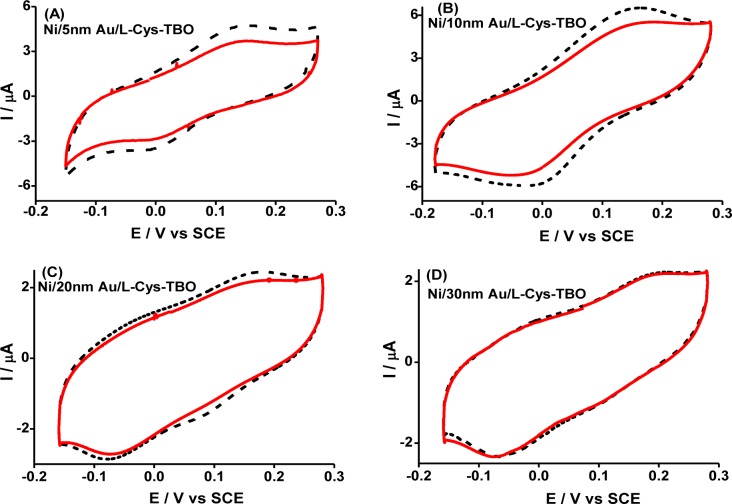
Figure 5 shows the effect of the Au overlayer thickness (5, 15, 20, 30 nm) on the spin polarization. Indeed the spin polarization decreases systematically as the Au layer thickness increases, from 11% for a 5 nm thick gold layer to being almost negligible at a 30 nm thick gold layer. This dependence of the spin polarization on the Au overlay thickness supports the conclusion that the asymmetry in the faradaic peak currents arises from spin polarized electrons ejected from the ferromagnetic metal electrode and transmitted spin-selectively through the chiral monolayer.
Figure 5.
Spin dependent cyclic voltammograms measured for l-Cys-TBO assembly adsorbed on gold-coated Ni electrodes. The voltammograms were recorded with four different gold overlayer thicknesses under otherwise identical conditions. Panels (A)–(D) represent 5, 10, 20, and 30 nm thick Au layers, respectively. The modified nickel electrode was magnetized with its magnetic moment pointing either “UP” (dashed black curve) or “DOWN” (solid red curve). Reproduced with permission from ref (44). Copyright 2015 American Chemical Society.
Spin Filtering through the Oligopeptides and the Effect of the Helix Length
Spin-dependent electrochemistry across helical oligopeptide films, which are directly adsorbed onto a metallic Ni electrode, was studied as a function of the peptide length (Figure 6).45 Thiol terminated oligopeptide SAMs were formed onto the nickel substrates that served as the working electrode. We examined the voltammetry with a ferricyanide/ferrocyanide redox couple for three different oligopeptides with the common formula (Boc)-Cys-(S-Acm)-(Ala-Leu)n-NH-(CH2)2–SH; referred to as AL5, AL6, and AL7 where n = 5, 6, and 7, respectively. While the current in the voltammograms decreased with increasing polypeptide length, the spin polarization increased. It was 8% for AL5 (2.2 nm thick), 12% for AL6 (2.5 nm thick), and 17% for AL7 (2.8 nm thick). This length dependence of the spin polarization was similar to that observed by photoelectron experiments which measure the electron spin direction via a Mott polarizer.45
Figure 6.
Spin-dependent voltammograms measured with Ni working electrodes chemically modified with the SAMs of (A) AL5, (B) AL6, and (C) AL7. The redox probes, Fe2+/Fe3+, were used in all cases to monitor the spin filtering property. Solid red and solid blue curves indicate magnetic field direction pointing “UP” and “DOWN”, respectively. Reproduced with permission from ref (45). Copyright 2015 American Chemical Society.
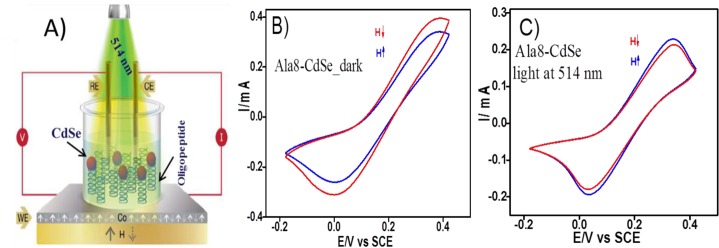
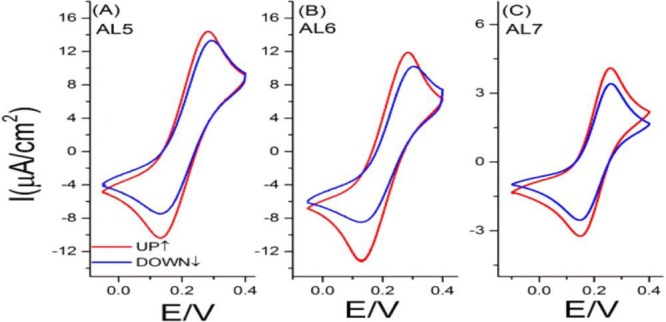
We examined the spin-polarization for photoinduced electron transfer through the same oligopeptides by tethering CdSe nanoparticles (NPs) to the terminus of the chiral oligopeptide.46 The CdSe NPs (5–6 nm diameter) were attached covalently to the outer surface of SAMs of the thiolated oligopeptide (AL8), which were formed on ferromagnetic cobalt electrodes (see Figure 7A). We observed spin-dependent charge-transfer across the SAMs of AL8 (not shown) and the AL8-CdSe NPs in the dark (Figure 7B), which showed a higher faradaic current with the magnetization pointing “DOWN″, and under 514 nm irradiation (Figure 7C), which showed a higher faradaic current with the magnetization pointing “UP”. For the dark measurements, the spin polarization for the chiral AL8 SAMs was found to be +11% at +0.050 and +7% at +0.32 V vs SCE. Under light irradiation, the spin polarization was reversed, having a value of −3% at +0.025 V (Figure 7C). This photoswitching of the spin polarization was confirmed by chronoamperometry measurements in the dark and in the light (for more details, see ref (46)).
Figure 7.
(A) Schematic illustrating the setup used for the light controlled spin-dependent electrochemical measurements across AL8-CdSe NPs assemblies. The working electrode was magnetized “UP” or “DOWN” (white and yellow arrows, respectively) during the electrochemical measurements. The CdSe NPs were excited by a green laser (λexc = 514 nm). (B) Spin-dependent voltammograms recorded for AL8-CdSe hybrid structures in the dark and (C) voltammograms obtained under 514 nm light. The modified cobalt was magnetized “UP” (solid blue line) or “DOWN” (solid red line). Voltammograms were recorded in the presence of a ferricyanide/ferrocyanide redox couple in solution. Reproduced with permission from ref (46). Copyright 2016 American Chemical Society.
The photoswitching in the spin polarization can be rationalized by the photoinduced change in the electric dipole across the chiral molecule. Before photoexcitation, the NPs are positively charged, as was confirmed by a 105 meV decrease in the work function of the electrode upon the NPs attachment to the SAMs of AL8 (see Figure 8A). Upon photoexcitation, the NPs transfer a hole from the excited NPs to the Co substrate and thus the NPs are more negatively charged (Figure 8B). This observation was confirmed by a chemically resolved electrical measurement technique which shows a decrease in the binding energy of the Cd line (Cd 3d5/2) in the AL8-CdSe assembly by 120 meV upon photoexcitation with a red laser of λ = 630 nm (details are given in the Supporting Information of ref (46)). This means that the electric field acting on the transferred electrons, within the chiral molecule, switches sign upon illumination. The switch in the spin transmitted can be explained by the fact that the spin preferred in electron transmission depends both on the handedness of the chiral molecule and on the direction of the electric field acting on the electron.38 Hence, for a given handedness, switching the direction of the electric field along the molecules is expected to switch the spin preferred in the transmission, as observed.
Figure 8.
Schematic diagram illustrating the effect of the light on the spin selectivity. (A) Before photoexcitation, the CdSe-NPs are positively charged and electrons are transferred with their spin aligned parallel to their velocity. (B) When the NPs are photoexcited, the NPs become negatively charged. The electric field on the AL8-CdSe is in the opposite direction and thus the electrons with spin antiparallel to their velocity are preferentially transferred.
Spin Filtering in Electron Transfer through Protein
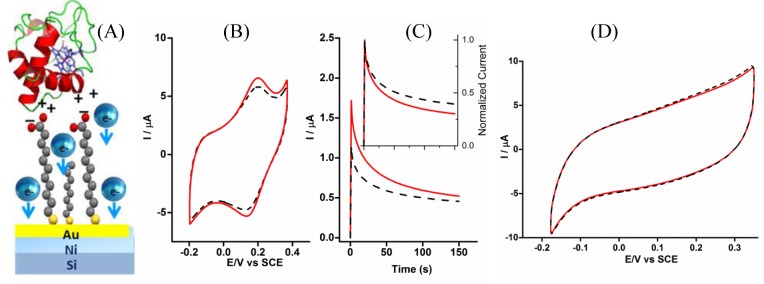
The redox active globular protein, cytochrome c, was adsorbed electrostatically through mixed SAMs of 11-mercaptoundecanoic acid and 1-octanethiol on 10 nm Au/Ni electrodes (Figure 9A), and voltammograms were measured for two different magnetic field directions. The redox peak positions were not affected by the magnetic field direction, but the magnitudes of the faradaic current peaks are affected. The voltammogram showed a higher current when the magnetic field is oriented “DOWN” than that found for the “UP” direction, indicating that the preferred spin orientation across the cytochrome c was antiparallel to the electrons’ direction of propagation.44
Figure 9.
(A) Scheme for immobilizing cytochrome c on a mixed SAM of 11-mercaptoundecanoic acid and 1-octanethiol over the Au coated (10 nm) Ni electrode. (B) Spin-dependent voltammograms of mixed-SAMs-cytochrome c. (C) Chronoamperometric measurements of the same system performed at a potential of 0.19 V; inset shows the normalized chronoamperometric curves. (D) Voltammograms that were obtained after denaturing the cytochrome c (applying −1 V vs SCE). Black dashed curve and red solid curve correspond to magnetic field pointing “UP” and “DOWN”, respectively. Reproduced with permission from ref (44). Copyright 2015 American Chemical Society.
Figure 9B shows the electrochemical data for these immobilized cytochrome c films. The spin polarization is −11%, calculated either at +0.13 V or at +0.19 V. The spin polarization was also confirmed by chronoamperometry which measures the current as a function of time; Figure 9C shows the case for a bias potential of 0.19 V. The chronoamperometry shows a higher current response for the “DOWN” magnetization at short times, but the current for the two magnetizations becomes almost equal with time, as the double layer is formed at the electrode–electrolyte interface. Note that the redox probe is grafted on the surface, and is not present in the bulk solution. Thus, the current measured in the long time transient regime is not related to the faradaic charge transfer (the amount of redox probe is fixed), rather it arises from the diffusion of the base electrolyte which is balancing the charge in the double layer. An analogous pattern can be recognized also in the cyclic voltammogram (Figure 9B) for which the spin polarization is larger at the potential corresponding to the current peak, where the faradaic charge transfer contribution is large compared to the double layer charging. The normalized curves (shown in the inset) indicate that the electrons with spin “UP” have a higher resistance in crossing the adsorbed layer than those with spin “DOWN”. Figure 9D shows the voltammetry that was obtained for the denatured form of cytochrome c. The adsorbed protein was denatured by applying a voltage (−1.0 V). In this case, the faradaic peaks disappeared and no difference was observed when the current was measured for the two different magnetic field directions. This effect was attributed to structural changes and charge redistribution in the protein backbone upon applying such a high bias.44
Light Controlled Spin Filtering Across the Bacteriorhodopsin
The existence of electron spin polarization for charge transfer and charge displacement processes in living organisms could also be manifest in photochemical and photobiological responses of biological systems.47 To examine this aspect, we performed spin-dependent electrochemistry measurements using the bacteriorhodopsin (bR) transmembrane protein.48 The faradaic current was monitored across bR films deposited onto a nickel electrode in the presence of a ferricyanide/ferrocyanide redox couple in the electrochemical cell. For this system, a higher current was observed when the working electrode was magnetized with its magnetic moment pointing in the “UP” direction. The native bR film exhibited a spin polarization of 21% when measured at +0.12 V (vs SCE) (Figure 10). Spin-dependent photoelectron transmission experiments with the bR films showed a similar spin polarization.48
Figure 10.
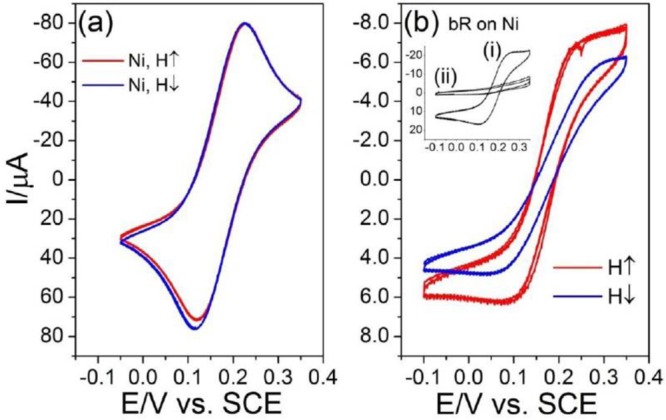
(a) CV for a Ni bare surface as a working electrode. (b) CV obtained using the bR thin film physisorbed on the Ni working electrode; arrows in the figures indicate the two possible directions (conventionally UP and DOWN) of the magnetic field (H = 0.35 T), which is orthogonal to the surface of the working electrode. Inset in panel (b) shows CVs of a bR/Ni thin film at H = 0: (i) freshly deposited bR on Ni and (ii) after the “electrochemical burning” of the bR. Reproduced with permission from ref (48). Copyright 2013 National Academy of Sciences.
Although the spin filtering across the native bR was not affected by light, we observed light activated spin filtering through a film of purple membrane that contained a mutant of bacteriorhodopsin (D96N) adsorbed on nickel substrates.49 Note that both for the wild bR and the mutant, the preferred spin direction (higher faradaic current) was observed when the modified nickel was magnetized in the “UP” direction.
Concluding Remarks
Our recent research activities have developed ferromagnetic/chiral architectures that allow one to unravel the fascinating physics underlying spin selective electron transmission across chiral films and to examine its impact on redox chemistry. Spin transport across chiral molecules gives rise to unusual electrical and magnetic properties that may form the basis for future chiral/nanoelectronic/spintronic devices. The novel spin-dependent electrochemistry methods, which have been developed, will allow us and others to study biorelated systems in solution, keeping the results as close as possible to physiological conditions. In the future, these methods will allow us to examine the importance of spin selectivity for electron transport in biology.
The chiral induced spin selectivity effect makes it possible to envision spintronic devices without using a ferromagnetic spin injector, since the chiral molecules themselves act as spin filters. The chirality induced electron transfer process, in combination with the application of light and magnetic fields could provide new strategies for light-controlled spintronics research.
Acknowledgments
P.C.M. acknowledges Alberta Innovates Technology Futures for financial support. R.N. acknowledges the funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant Agreement No. 338720 CISS, the VW Foundation, and the Israel Science Foundation. D.H.W. acknowledges the support from the NSF CHE-1464701. The authors thank several scientists worldwide who devoted to making the CISS project successful. Special thanks to Zeev Vager, Leeor Kronik, Sidney Cohen, Hagai Cohen, Karen Michaeli, Mordechai Sheves (WIS), Helmut Zacharias (Munster), Giovanni Cuniberti, Rafael Gutierrez (Dresden), Yossi Paltiel (HUJI), Richard Rosenberg (Argonne), Luisa Schenetti (Modena), Jerome Lacour (Geneva), Eric Fullerton (San Diego), Vladimiro Mujica (Arizona State), and Slavomir Sek (Warsaw).
Biographies
Prakash Chandra Mondal was born in Kultali, W.B., India. He completed a B.Sc. (2006) in Chemistry (Hons) from Jadavpur University, Kolkata, India, and a M.Sc (2008) in Chemistry from the Indian Institute of Technology (IIT), Kharagpur, India. He earned his Ph.D. (2013) from the University of Delhi. In 2013, he moved to the Weizmann Institute of Science (WIS), Israel to work with Prof. Ron Naaman. During his postdoctoral work at WIS, he developed several methodologies for spin-dependent electrochemistry using chiral polymers, amino acids, oligopeptides, and redox-proteins. In 2016, he joined Prof. Richard McCreery at National Institute for Nanotechnology (NINT), Alberta, Canada. His current research is focused on charge transport across the organic molecules on carbon electrodes.
Claudio Fontanesi completed his Doctor in Chemistry “magna cum laude” from the University of Modena, 1982. In 1987, he joined the University of Modena as an Assistant Professor, Department of Chemistry. In 2001, he became an Associate Professor of Physical Chemistry. His research interest includes electrochemistry of polymer films, and SAMs of chiral molecules, molecular spintronics. He served as a Vice-president of the “Divisione di ElettrochimicaItaliana”.
David H. Waldeck completed his B.Sc. in Chemistry at the University of Cincinnati, Cincinnati, OH, and his Ph.D. at the University of Chicago. He then moved to the University of California, Berkeley as an IBM postdoctoral Fellow for 2 years. In 1985, he joined the chemistry faculty at the University of Pittsburgh. His research program uses methods of spectroscopy, electrochemistry, and microscopy to investigate primary processes in the condensed phase, which includes liquids, solids, and liquid/solid interfaces. Current research includes the fundamental understanding of electron-transfer reactions, electron transport in supramolecular structures, and nanophotonics.
Ron Naaman completed his B.Sc. in Chemistry at the Ben Gurion University, Beer-Sheva, Israel, and Ph.D. at the Weizmann Institute, Israel. He then moved for a postdoctoral fellowship to Stanford, California, for 2 years and then spent 1 year at the Chemistry Department at Harvard. In 1980, he returned to Israel and became a faculty member at the Weizmann Institute. His work is focused on new electronic properties that emerge from the formation of supramolecular structures. He studies the effect of formation of clusters and van der Waals complexes on the reactivity of molecules. This work was followed by studies of the reactive properties and electronic properties of SAMs. In parallel, his research group explores the transfer of information through supramolecular systems and produces self-assembled electrical devices. Recently, he studies the spin-selective transfer properties of chiral molecules.
Author Present Address
∥ National Institute for Nanotechnology, University of Alberta, Edmonton T6G 2M9 Canada.
Author Contributions
The manuscript was written through contribution of all authors. All authors have given approval to the final version of the Account.
The authors declare no competing financial interest.
References
- Sinova J.; Zutic I. New Moves of the Spintronics Tango. Nat. Mater. 2012, 11, 368–371. 10.1038/nmat3304. [DOI] [PubMed] [Google Scholar]
- Pulizzi F. Spintronics. Nat. Mater. 2012, 11, 367. 10.1038/nmat3327. [DOI] [PubMed] [Google Scholar]
- Baibich M. N.; Broto J. M.; Fert A.; Van Dau F. N.; Petroff F.; Etienne P.; Creuzet G.; Friederich A.; Chazelas J. Giant Magnetoresistance of (001)Fe/(001)Cr Magnetic Superlattices. Phys. Rev. Lett. 1988, 61, 2472–2475. 10.1103/PhysRevLett.61.2472. [DOI] [PubMed] [Google Scholar]
- Binasch G.; Grünberg P.; Saurenbach F.; Zinn W. Enhanced Magnetoresistance in Layered Magnetic Structures with Antiferromagnetic Interlayer Exchange. Phys. Rev. B: Condens. Matter Mater. Phys. 1989, 39, 4828–4830. 10.1103/PhysRevB.39.4828. [DOI] [PubMed] [Google Scholar]
- Prinz G. A. Magnetoelectronics. Science 1998, 282, 1660–1663. 10.1126/science.282.5394.1660. [DOI] [PubMed] [Google Scholar]
- Sanvito S. Molecular Spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355. 10.1039/c1cs15047b. [DOI] [PubMed] [Google Scholar]
- Sanvito S. The Rise of Spinterface Science. Nat. Phys. 2010, 6, 562–564. 10.1038/nphys1714. [DOI] [Google Scholar]
- Santos T. S.; Lee J. S.; Migdal P.; Lekshmi I. C.; Satpati B.; Moodera J. S. Room-Temperature Tunnel Magnetoresistance and Spin-Polarized Tunneling through an Organic Semiconductor Barrier. Phys. Rev. Lett. 2007, 98, 016601. 10.1103/PhysRevLett.98.016601. [DOI] [PubMed] [Google Scholar]
- Okazaki M.; Toriyama K. Control of a Chemical Reaction by Spin Manipulation of the Transient Radical Pair As Demonstrated for Isotope Enrichment. J. Phys. Chem. 1995, 99, 489–491. 10.1021/j100002a005. [DOI] [Google Scholar]
- Schuler B.; Fatayer S.; Mohn F.; Moll N.; Pavliček N.; Meyer G.; Peña D.; Gross L. Reversible Bergman Cyclization by Atomic Manipulation. Nat. Chem. 2016, 8, 220–224. 10.1038/nchem.2438. [DOI] [PubMed] [Google Scholar]
- Li X.; Feng D.; Tong H.; Jia T.; Deng L.; Sun Z.; Xu Z. Hole Surface Trapping Dynamics Directly Monitored by Electron Spin Manipulation in CdS Nanocrystals. J. Phys. Chem. Lett. 2014, 5, 4310–4316. 10.1021/jz502340w. [DOI] [PubMed] [Google Scholar]
- Ray K.; Ananthavel S. P.; Waldeck D. H.; Naaman R. Asymmetric Scattering of Polarized Electrons by Organized Organic Films of Chiral Molecules. Science 1999, 283, 814–816. 10.1126/science.283.5403.814. [DOI] [PubMed] [Google Scholar]
- Naaman R.; Waldeck D. H. The Chiral Induced Spin Selectivity Effect. J. Phys. Chem. Lett. 2012, 3, 2178–2187. 10.1021/jz300793y. [DOI] [PubMed] [Google Scholar]
- Naaman R.; Waldeck D. H. Spintronics and Chirality: Spin Selectivity in Electron Transport Through Chiral Molecules. Annu. Rev. Phys. Chem. 2015, 66, 263–281. 10.1146/annurev-physchem-040214-121554. [DOI] [PubMed] [Google Scholar]
- Gohler B.; Hamelbeck V.; Markus T. Z.; Kettner M.; Hanne G. F.; Vager Z.; Naaman R.; Zacharias H. Spin Selectivity in Electron Transmission Through Self-Assembled Monolayers of Double-Stranded DNA. Science 2011, 331, 894–897. 10.1126/science.1199339. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Markus T. Z.; Cohen S. R.; Vager Z.; Gutierrez R.; Naaman R. Spin Specific Electron Conduction Through DNA Oligomers. Nano Lett. 2011, 11, 4652–4655. 10.1021/nl2021637. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. A.; Symonds J. M.; Kalyanaraman V.; Markus T.; Orlando T. M.; Naaman R.; Medina E. E.; Lopez F. A.; Mujica V. Kinetic Energy Dependence of Spin Filtering of Electrons Transmitted Through Organized Layers of DNA. J. Phys. Chem. C 2013, 117, 22307–22313. 10.1021/jp402387y. [DOI] [Google Scholar]
- Ravi S.; Sowmiya P.; Karthikeyan A. Magnetoresistance and Spin-filtering Efficiency of DNA-Sandwiched Ferromagnetic Nanostructures. SPIN 2013, 03, 1350003. 10.1142/S2010324713500033. [DOI] [Google Scholar]
- Alam K. M.; Pramanik S. Spin Filtering Through Single-Wall Carbon Nanotubes Functionalized with Single-Stranded DNA. Adv. Funct. Mater. 2015, 25, 3210–3218. 10.1002/adfm.201500494. [DOI] [Google Scholar]
- Niño M. Á.; Kowalik I. A.; Luque F. J.; Arvanitis D.; Miranda R.; de Miguel J. J. Enantiospecific Spin Polarization of Electrons Photoemitted Through Layers of Homochiral Organic Molecules. Adv. Mater. 2014, 26, 7474–7479. 10.1002/adma.201402810. [DOI] [PubMed] [Google Scholar]
- Ben Dor O.; Morali N.; Yochelis S.; Baczewski L. T.; Paltiel Y. Local Light-Induced Magnetization Using Nanodots and Chiral Molecules. Nano Lett. 2014, 14, 6042–6049. 10.1021/nl502391t. [DOI] [PubMed] [Google Scholar]
- Peer N.; Dujovne I.; Yochelis S.; Paltiel Y. Nanoscale Charge Separation Using Chiral Molecules. ACS Photonics 2015, 2, 1476–1481. 10.1021/acsphotonics.5b00343. [DOI] [Google Scholar]
- Gutierrez R.; Diaz E.; Naaman R.; Cuniberti G. Spin-Selective Transport Through Helical Molecular Systems. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 85, 081404. 10.1103/PhysRevB.85.081404. [DOI] [Google Scholar]
- Skourtis S. S.; Beratan D. N.; Naaman R.; Nitzan A.; Waldeck D. H. Chiral Control of Electron Transmission Through Molecules. Phys. Rev. Lett. 2008, 101, 238103. 10.1103/PhysRevLett.101.238103. [DOI] [PubMed] [Google Scholar]
- Guo A. M.; Sun Q. F. Spin-selective Transport of Electrons in DNA Double Helix. Phys. Rev. Lett. 2012, 108, 218102. 10.1103/PhysRevLett.108.218102. [DOI] [PubMed] [Google Scholar]
- Guo A. M.; Sun Q. F. Spin-dependent Electron Transport in Protein-like Single-helical Molecules. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 11658–11662. 10.1073/pnas.1407716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. A.; Mishra D.; Naaman R. Chiral Selective Chemistry Induced by Natural Selection of Spin-Polarized Electrons. Angew. Chem., Int. Ed. 2015, 54, 7295–7298. 10.1002/anie.201501678. [DOI] [PubMed] [Google Scholar]
- Monzon L. M. A.; Coey J. M. D. Magnetic Fields in Electrochemistry: The Lorentz Force. A Mini-Review. Electrochem. Commun. 2014, 42, 38–41. 10.1016/j.elecom.2014.02.006. [DOI] [Google Scholar]
- Monzon L. M. A.; Coey J. M. D. Magnetic Fields in Electrochemistry: The Kelvin Force. A Mini-Review. Electrochem. Commun. 2014, 42, 42–45. 10.1016/j.elecom.2014.02.005. [DOI] [Google Scholar]
- Saravanan G.; Ozeki S. Magnetic Field Control of Electron Tunneling Pathways in the Monolayer of (Ferrocenylmethyl)dodecyldimethylammonium Bromide on a Gold Electrode. J. Phys. Chem. B 2008, 112, 3–6. 10.1021/jp076663l. [DOI] [PubMed] [Google Scholar]
- Lee C.-C; Chou T.-C. Effects of Magnetic Field on the Reaction Kinetics of Electroless Nickel Deposition. Electrochim. Acta 1995, 40, 965–970. 10.1016/0013-4686(95)00007-2. [DOI] [Google Scholar]
- Scott A. M.; Miura T.; Ricks A. B.; Dance Z. E. X.; Giacobbe E. M.; Colvin M. T.; Wasielewski M. R. Spin-Selective Charge Transport Pathways Through p-Oligophenylene-Linked Donor-Bridge-Acceptor Molecules. J. Am. Chem. Soc. 2009, 131, 17655–17666. 10.1021/ja907625k. [DOI] [PubMed] [Google Scholar]
- Wasielewski M. R. Energy, Charge, and Spin Transport in Molecules and Self-Assembled Nanostructures Inspired by Photosynthesis. J. Org. Chem. 2006, 71, 5051–5066. 10.1021/jo060225d. [DOI] [PubMed] [Google Scholar]
- Verhoeven J. W. On the Role of Spin Correlation in the Formation, Decay, and Detection of Long-lived, Intramolecular Charge-Transfer States. J. Photochem. Photobiol., C 2006, 7, 40–60. 10.1016/j.jphotochemrev.2006.04.001. [DOI] [Google Scholar]
- Mogi I.; Watanabe K. Enantioselective Recognition of Tartaric Acid on Magneto-electrodeposited Copper Film Electrodes. Chem. Lett. 2012, 41, 1439–1441. 10.1246/cl.2012.1439. [DOI] [Google Scholar]
- Wei J. J.; Schafmeister C.; Bird G.; Paul A.; Naaman R.; Waldeck D. H. Molecular Chirality and Charge Transfer through Self-Assembled Scaffold Monolayers. J. Phys. Chem. B 2006, 110, 1301–1308. 10.1021/jp055145c. [DOI] [PubMed] [Google Scholar]
- Bard A. J.; Faulkner L. R.. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, 2001. [Google Scholar]
- Eckshtain-Levi M.; Capua E.; Refaely-Abramson S.; Sarkar S.; Gavrilov Y.; Mathew S. P.; Paltiel Y.; Levy Y.; Kronik L.; Naaman R. Cold Denaturation Induces Inversion of Dipole and Spin Transfer in Chiral Peptide Monolayers. Nat. Commun. 2016, 7, 10744. 10.1038/ncomms10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See, for example,Kittel C.Introduction to Solid State Physics, 7th ed.; Wiley: New York, 1996; pp 420–421. [Google Scholar]; Ashcroft N. W.; Mermin N. D.. Solid State Physics; Holt, Rinehart and Winston: New York, 1976; pp 654–661. [Google Scholar]
- Kresse G.; Furthmüller J. Efficient Iterative Schemes for ab initio Total-Energy Calculations using a Plane-Wave Basis Set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996, 54, 11169. 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- Mekhalif Z.; Laffineur F.; Couturier N.; Delhalle J. Elaboration of Self-Assembled Monolayers of n-alkanethiols on Nickel Polycrystalline Substrates: Time, Concentration, and Solvent Effects. Langmuir 2003, 19, 637–645. 10.1021/la020332c. [DOI] [Google Scholar]
- Fontanesi C.; Tassinari F.; Parenti F.; Cohen H.; Mondal P. C.; Kiran V.; Giglia A.; Pasquali L.; Naaman R. New One-Step Thiol Functionalization Procedure For Ni by Self-Assembled Monolayers. Langmuir 2015, 31, 3546–3552. 10.1021/acs.langmuir.5b00177. [DOI] [PubMed] [Google Scholar]
- Mondal P. C.; Kantor-Uriel N.; Mathew S. P.; Tassinari F.; Fontanesi C.; Naaman R. Chiral Conductive Polymers as Spin Filters. Adv. Mater. 2015, 27, 1924–1927. 10.1002/adma.201405249. [DOI] [PubMed] [Google Scholar]
- Mondal P. C.; Fontanesi C.; Waldeck D. H.; Naaman R. Field and Chirality Effects on Electrochemical Charge Transfer Rates: Spin-dependent Electrochemistry. ACS Nano 2015, 9, 3377–3384. 10.1021/acsnano.5b00832. [DOI] [PubMed] [Google Scholar]
- Kettner M.; Gohler B.; Zacharias H.; Mishra D.; Kiran V.; Naaman R.; Fontanesi C.; Waldeck D. H.; Sek S.; Pawlowski J.; Juhaniewicz J. Spin Filtering in Electron Transport Through Chiral Oligopeptides. J. Phys. Chem. C 2015, 119, 14542–14547. 10.1021/jp509974z. [DOI] [Google Scholar]
- Mondal P. C.; Roy P.; Kim D.; Fullerton E. E.; Cohen H.; Naaman R. Photo-spintronics: Magnetic Field Controlled Photoemission and Light Controlled Spin Transport in Hybrid Chiral Oligopeptides-Nanoparticles Structures. Nano Lett. 2016, 16, 2806–2811. 10.1021/acs.nanolett.6b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hore P. J. Are Biochemical Reactions Affected by Weak Magnetic Fields?. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 1357–1358. 10.1073/pnas.1120531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D.; Markus T. Z.; Naaman R.; Kettner M.; Göhler B.; Zacharias H.; Friedman N.; Sheves M.; Fontanesi C. Spin-dependent Electron Transmission Through Bacteriorhodopsin Embedded in Purple Membrane. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 14872–14876. 10.1073/pnas.1311493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einati H.; Mishra D.; Friedman N.; Sheves M.; Naaman R. Light-Controlled Spin Filtering in Bacteriorhodopsin. Nano Lett. 2015, 15, 1052–1056. 10.1021/nl503961p. [DOI] [PMC free article] [PubMed] [Google Scholar]