Abstract
Background
The objective of this analysis is to explore potential impact on operating room (OR) efficiency and incidence of residual neuromuscular blockade (RNMB) with use of sugammadex (Bridion™, Merck & Co., Inc., Kenilworth, NJ USA) versus neostigmine for neuromuscular block reversal in Canada.
Methods
A discrete event simulation (DES) model was developed to compare ORs using either neostigmine or sugammadex for NMB reversal over one month. Selected inputs included OR procedure and turnover times, hospital policies for paid staff overtime and procedural cancellations due to OR time over-run, and reductions in RNMB and associated complications with sugammadex use. Trials show sugammadex’s impact on OR time and RNMB varies by whether full neuromuscular recovery (train-of-four ratio ≥0.9) is verified prior to extubation in the OR. Scenarios were therefore evaluated reflecting varied assumptions for neuromuscular reversal practices.
Results
With use of moderate neuromuscular block, when full neuromuscular recovery is verified prior to extubation (93 procedures performed with sugammadex, 91 with neostigmine), use of sugammadex versus neostigmine avoided 2.4 procedural cancellations due to OR time over-run and 33.5 h of paid staff overtime, while saving an average of 62 min per OR day. No difference was observed between comparators for these endpoints in the scenario when full neuromuscular recovery was not verified prior to extubation, however, per procedure risk of RNMB at extubation was reduced from 60% to 4% (reflecting 51 cases prevented), with associated reductions in risks of hypoxemia (12 cases avoided) and upper airway obstruction (23 cases avoided).
Sugammadex impact in reversing deep neuromuscular block was evaluated in an exploratory analysis. When it was hypothetically assumed that 30 min of OR time were saved per procedure, the number of paid hours of staff over-time dropped from 84.1 to 32.0, with a 93% reduction in the per patient risk of residual blockade.
Conclusions
In clinical practice within Canada, for the majority of patients currently managed with moderate neuromuscular block, the principal impact of substituting sugammadex for neostigmine is likely to be a reduction in the risk of residual blockade and associated complications. For patients maintained at a deep level of block to the end of the procedure, sugammadex is likely to both enhance OR efficiency and reduce residual block complications.
Electronic supplementary material
The online version of this article (doi:10.1186/s12871-016-0281-3) contains supplementary material, which is available to authorized users.
Keywords: Neuromuscular block, Reversal, Sugammadex, Neostigmine, Residual blockade, Operating room, Efficiency
Background
Neuromuscular blocking agents (NMBAs) are often administered during surgical procedures to provide muscle relaxation, and to prevent patient movement, which may increase the risk of surgical complications. When neuromuscular block no longer needs to be maintained, patients may either be allowed to spontaneously recover neuromuscular function or be administered a reversal agent for more rapid recovery. The acetylcholinesterase inhibitor neostigmine is commonly used for reversal of moderate neuromuscular blockade (e.g., when at least the second twitch [T2] of a train-of-four stimulation is present). Due to the occurrence of muscarinic side effects with neostigmine such as nausea, vomiting and bradycardia, it is typically co-administered with an anti-muscarinic agent such as atropine or glycopyrrolate [1]. Recovery of neuromuscular function via either spontaneous reversal or use of neostigmine is neither rapid nor of predictable duration [2, 3]. Patients may therefore be inadvertently extubated while still experiencing residual neuromuscular paralysis (residual neuromuscular blockade), with accompanying respiratory and muscular complications [4].
Sugammadex (Bridion™, Merck & Co., Inc., Kenilworth, NJ USA, sponsor of the present analysis), a modified gamma-cyclodextrin, is a more recently developed reversal agent, recently approved for commercialization in Canada (and also licensed in the United States and European Union) to reverse neuromuscular blockade induced by the NMBAs rocuronium or vecuronium [5]. In clinical trials, sugammadex has been shown to produce much more rapid and predictable reversal of neuromuscular block than neostigmine, in the absence of anti-muscarinic side effects and, in trials where quantitative neuromuscular monitoring was not required, a steep reduction in the incidence of residual neuromuscular blockade (RNMB) [3, 5–8]. In addition, sugammadex is efficacious in rapidly reversing deep neuromuscular block (i.e., at re-appearance of 1–2 post-tetanic counts), [2, 9, 10] whereas acetylcholinesterase inhibitors such as neostigmine cannot adequately reverse deep levels of blockade because they reach a “ceiling” effect in which the increase in acetylcholine concentration is insufficient to displace enough NMBA molecules to reverse neuromuscular block [10, 11].
The objective of the present analysis is to explore potential impact on operating room (OR) efficiency and incidence of RNMB with use of sugammadex versus neostigmine for routine reversal of neuromuscular blockade in Canada. This paper describes a discrete event simulation (DES) model developed for this purpose. Outcomes explored include selected clinical events associated with residual neuromuscular blockade and OR efficiency-related measures.
Methods
Ethical committee approval was not required for the present study as modeling was based on secondary data sources and human or animal subjects were not enrolled.
Perspective
The model perspective is that of a Canadian hospital. An OR schedule and patient clinical outcomes associated with neuromuscular blockade were simulated over a 1 month period (21 working days) for ORs utilizing either sugammadex or neostigmine for neuromuscular block reversal.
Treatment comparators
The primary analyses evaluate reversal of moderate neuromuscular blockade, with sugammadex (2 mg/kg) compared to neostigmine (50 μg/kg) plus glycopyrrolate (10 μg/kg). Reversal of deep neuromuscular block is evaluated as an exploratory analysis utilizing a 4 mg/kg dose of sugammadex. Neuromuscular block was assumed to be maintained with either rocuronium or vecuronium (though in Canada vecuronium is not utilized), for which sugammadex is indicated for reversal.
Acetylcholinesterase inhibitors such as neostigmine cannot adequately reverse deep levels of blockade [10, 11]. In clinical practice, for procedures utilizing deep block, the level of neuromuscular blockade is generally allowed to fade to moderate, prior to administering neostigmine. This scenario was modeled as an exploratory analysis as there has not been a corresponding clinical trial of sugammadex to date in which all patients in the neostigmine arm were administered deep block which was allowed to fade to moderate prior to reversal agent administration, and hypothetical values for potential time savings with sugammadex use were therefore evaluated.
Structure
The DES model was developed in part with Arena Version 14.7 software (Rockwell Automation, Milwaukee, WI), with a Microsoft Excel 2010 (Redmond, WA) interface used for input entry and output reporting. The model compares ORs using either neostigmine or sugammadex for NMB reversal, for the same simulated schedule of procedures performed daily on 21 working days over 1 month. The impact of using sugammadex compared to neostigmine on OR procedure time (time from OR admission to OR discharge) within clinical trials has varied according to how neuromuscular recovery and extubation have been managed [12–16]. Because neuromuscular recovery practices may vary by institution, the model therefore evaluates a variety of different scenarios for the proportion of patients (0%, 5%, 10%, 25%, 50%, 75%, 100%) verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR.
Random durations are generated for the OR procedures utilizing the assumed mean duration for each procedure and its statistical distribution. Random values are generated similarly for other parameters influencing procedural flow, including time from the start of the OR day to initiation of the 1st procedure, turnover times between procedures, frequency of emergency procedures (immediately inserted into the OR schedule bumping the next scheduled procedure), frequency of semi-emergency procedures (added on at the end of the OR day), probability and impact of procedural cancellations due to reasons other than OR time over-run and time for staff clean-up of the OR at the end of the day. Additional model parameters include hospital policies for procedural cancellation when OR time over-run occurs, impact of sugammadex on OR time per procedure compared to neostigmine, risk of residual neuromuscular blockade and its clinical sequelae with neostigmine use and risk reductions with sugammadex and staff eligible for OR overtime pay. An overview of the model structure is shown in Fig. 1 for OR procedural flow and in Fig. 2 for residual neuromuscular blockade and associated complications.
Fig. 1.
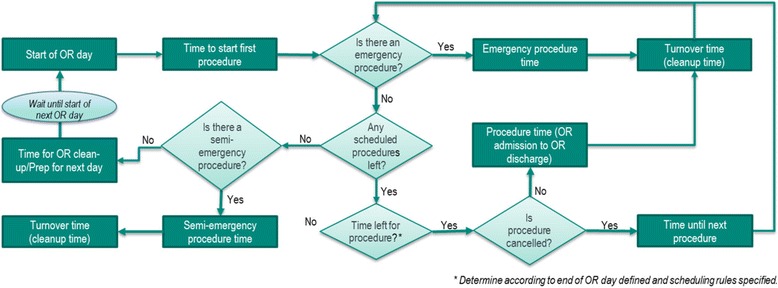
Diagram of operating room day and procedural flow
Fig. 2.
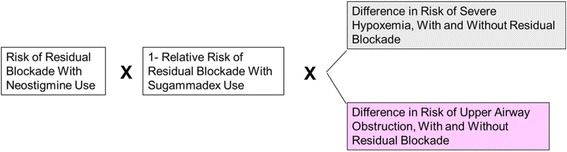
Model structure for reduction in risk of residual neuromuscular blockade and associated complications
The aforementioned parameters are used to estimate sugammadex’s impact versus neostigmine on OR times and procedural flow, staff overtime, procedural cancellation and risk of residual neuromuscular blockade and associated complications.
Inputs
A series of systematic literature searches of the US National Library of Medicine’s PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) were conducted to obtain data values for various model parameters. In addition, to the PubMed database, the Cochrane Library and University of York and Centre for Reviews and Dissemination (CRD) databases were also searched. Reference lists within publications of potential relevance were further searched to identify additional studies. Proprietary informational sources providing relevant data were also reviewed. For conciseness, the specific PubMed literature search strategies for each model input are described in detail in Additional file 1. PubMed searches were conducted for literature published up to August 21, 2015.
For operating room scheduling and staffing parameters (e.g., time to start of OR day, turnover times, time for OR clean-up, procedure duration, OR staff eligible for over-time pay), values would expected to be highly variable across operating rooms and institutions. With respect to scheduling, available publications reporting data often reflect examples where efficiency was less than desired and improvements were sought. Rather than conducting a formal meta-analysis across publications, data from available sources deemed as reasonable and credible by OR experts were included within the model and values were varied in reported sensitivity analyses.
In clinical practice, parameters for the OR day and procedural flow vary by hospital and OR. Illustrative examples of parameter values for these variables were gathered from the literature or based on assumption as described in Table 1. Estimation methods for other individual model parameters are described in greater detail within this section.
Table 1.
Base case model inputs
| Parameter | Default valuea | Source |
|---|---|---|
| Time Horizon | 1 month (21 working days) | Assumption |
| Operating Room | ||
| Start of OR day | 7:30 AM | Assumption |
| End of OR day | 4:00 PM | Assumption |
| Time from start of OR day to OR admission of first patient | 10 min | [36] |
| Time between procedures (turnover time) | 35.6 min | [37] |
| Time for OR clean-up/prep for next day | 15 min | Assumption |
| Procedure | ||
| Number of procedures per day | 5 | Assumption |
| Mean time per procedure with neostigmine use | 72.9 min | Analysis of RECITE Canada data (data on file) |
| Probability of cancellation of schedule procedure (unrelated to OR time over-run) | 10.7% | [38] |
| Is next procedure moved up when cancellation occurs? | No | Assumption |
| Can a procedure be cancelled because there is not enough OR time available? | Yes | Assumption |
| Cancellation policy | No procedures may begin after end of OR day | Assumption |
| Probability of emergency procedure insertion | 0% | Assumption |
| Probability of semi-emergency procedure insertion | 0% | Assumption |
| Residual Neuromuscular Block | ||
| Risk of residual block at extubation with neostigmine use | 60.0% | [6, 19, 20] |
| Absolute excess risk of hypoxemia with residual block | 24.5% | [33] |
| Absolute excess risk of upper airway obstruction with residual block | 44.2% | [6, 32] |
| Impact of Sugammadex vs. Neostigmine | ||
| Reduction in time from patient OR admission to OR discharge | ||
| All patients verified to have full neuromuscular recovery (TOF ratio ≥0.9) prior to extubation in the OR | 14 min | Grintescu et al. 2009 [16]; P318 2010 [13] |
| No patients verified to have full neuromuscular recovery (TOF ratio ≥0.9) prior to extubation in the OR | 0 min | P334 2009 [12]; P07981 2013 [14]; P07038 2014 [15] |
| Reduction in risk of residual neuromuscular blockade at extubation, among patients not verified to have full neuromuscular recovery (TOF ratio ≥0.9) prior to extubation | 93% | [6] |
| OR Staff Eligible for Overtime Pay | ||
| Registered nurses | 3 present | Assumption |
| Respiratory therapist | 1 present | Assumption |
| Nurse aide | 1 present | Assumption |
| Overtime pay policy | 30 min increments | Assumption |
OR Operating room
aAssumed Arena probability distributions [Mean time per procedure with neostigmine use (LOGN 72.9,29.2); Turnover time (10+EXPO{25.6}); Time to OR admission of 1st patient (DISC{0.5,5,1,15});OR clean-up time (TRIA{7.5,15,22.5})]
Impact of sugammadex vs. neostigmine on OR procedure time
Published clinical trials comparing sugammadex to neostigmine have typically reported information on time from administration of the reversal agent to full patient neuromuscular recovery to a TOF ratio of ≥ 0.9, measured via quantitative neuromuscular monitoring [17]. This is the preferred endpoint for evaluating sugammadex’s efficacy with respect to accelerating neuromuscular recovery, consistent with the direct impact of usage. However, of interest with respect to the present modeling, is the impact of accelerated neuromuscular recovery on the downstream endpoint of patient time in the OR. Comparisons between sugammadex and neostigmine of time from reversal agent administration to full neuromuscular recovery often do not well correlate with impact on overall OR time for several reasons.
First, in clinical practice, patients are often discharged from the OR prior to achieving full neuromuscular recovery, while residual neuromuscular blockade (TOF ratio < 0.9) is present [4, 18]. Second, even where patients have full neuromuscular recovery in the OR, that may not be a rate-limiting step to OR discharge if other necessary activities (e.g., wound suturing, medical equipment removal) occur during this time period currently with use of neostigmine, the time for which cannot be fully eliminated with accelerated neuromuscular recovery. Third, in clinical practice, recognizing the much shorter time to full neuromuscular recovery with sugammadex versus neostigmine, [17] the products may be administered at different time points within a procedure (neostigmine earlier due to longer lead-time needed for an effect and sugammadex later due to very rapid effect in reversing moderate block, and sugammadex earlier and neostigmine later in procedures ending in deep block). As a result, the shortened reversal time with sugammadex would not translate to a commensurate reduction in time to OR discharge. Thus, neuromuscular recovery time is likely to correlate least with patient time in the OR in procedures where there is not full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR, or where deep block is maintained through the end of the procedure, with the potential for a somewhat greater correlation in procedures utilizing a moderate level of block where patients are maintained in the OR through full neuromuscular recovery.
Data were therefore analyzed from available clinical trials reporting time from the endpoint of patient OR admission to OR discharge for subjects randomized to receive sugammadex or neostigmine (Table 2). This endpoint directly corresponds to patient time spent in the OR as evaluated within the DES model. Results were stratified by whether patients in the trial were verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR, as the impact of sugammadex on OR time has been found to vary according to how neuromuscular recovery is managed. Specifically, larger and statistically significant OR time savings with sugammadex use have been observed in trials where all patients were “verified” to have full neuromuscular recovery (train-of-four [TOF] ratio ≥ 0.9) prior to extubation via quantitative neuromuscular monitoring, whereas time savings have either not been observed or not achieved statistical significance in trials where verification of full neuromuscular recovery was not required [12–16]. Available data were insufficient for further stratification by use of rocuronium vs. vecuronium as the NMBA, or depth of neuromuscular block throughout the procedure or at reversal. Results across trials within each stratified group were pooled via a random effects meta-analysis.
Table 2.
Sugammadex impact vs. neostigmine on time from patient OR admission to OR discharge, per procedure
| A. Trials Requiring Verificationa of Full Neuromuscular Recovery (TOF ratio ≥ 0.9) Prior to Extubation in the OR | ||||||
| Sugammadex Arm | Neostigmine Arm | |||||
| N | Minutes from OR admission to discharge | N | Minutes from OR admission to discharge | SugammadexTime Savings | P-value | Source |
| 17 | 64 | 17 | 80 | 16 | 0.04 | [16] |
| 66 b | 158 | 64 | 169 | 11 | 0.23 | [13] |
| 83 | 81 | 14 | 0.02 | Meta-analysis | ||
| B. Trials Not Requiring Verification of Full Neuromuscular Recovery Prior to Extubation in the OR | ||||||
| Sugammadex Arm | Neostigmine Arm | |||||
| N | Minutes from OR admission to discharge | N | Minutes from OR admission to discharge | SugammadexTime Savings | P-value | Source |
| 48 | 183 | 46 | 167 | −16 | 0.22 | [12] |
| 290 | 167 | 315 | 167 | 0 | NA | [15] |
| 74 b | 242 | 77 | 253 | 11 | 0.40 | [14] |
| 412 | 438 | −1 | 0.89 | Meta-analysis | ||
NA Not applicable OR Operating room, TOF Train-of-four
aVerification of full neuromuscular recovery (TOF ratio ≥ 0.9) based on quantitative neuromuscular monitoring
bNumbers below this row reflect a pooling of data via meta-analysis
Risk of residual blockade at extubation and associated efficacy of sugammadex
A statistically significant reduction in the incidence of residual neuromuscular blockade at extubation with use of sugammadex versus neostigmine has been demonstrated in a randomized clinical trial where patients were not required to have verification of full neuromuscular recovery prior to extubation in the OR [6]. As additional studies have also reported a risk of residual blockade (TOF < 0.9) at extubation with neostigmine use, a literature search was conducted to estimate the background risk of residual blockade.
Studies describing the risk of residual blockade at extubation exclusively with rocuronium or vecuronium and neostigmine use were gathered from the literature based on two review articles, [4, 18] and a search of the PubMed database using terms of “residual blockade”, “residual block” or “curarization” for studies published from January 1, 2008 (updating from the literature canvassed in the review articles) up to July 10, 2015. Studies using quantitative neuromuscular monitoring to determine the time point for extubation were excluded. Three studies meeting review eligibility criteria were identified and meta-analyzed [6, 19, 20]. Rocuronium was used within each of the studies, and corresponding data were not found for patients receiving vecuronium. However, vecuronium is not utilized within Canada. The resultant average risk of residual block at extubation with neostigmine use was estimated to be 60% when patients are not required to have verification of full neuromuscular recovery (TOF ratio ≤ 0.9) prior to extubation in the OR. Additional details on the estimation are provided in Additional file 1.
The efficacy of sugammadex in preventing residual blockade, specified as a reduction in the risk of residual blockade at extubation with sugammadex usage (93% reduction) compared to neostigmine, was estimated based on data from the aforementioned randomized trial [6].
Risk of clinical sequelae of residual blockade
Clinical sequelae of residual blockade may include post-operative aspiration, hypoxemia, muscle weakness and upper airway obstruction.
In the case of aspiration, aspiration pneumonitis (and atelectasis) have been reported as potential outcomes of residual blockade [4, 21]. However, an elevated risk of their occurrence has been observed with long-acting NMBAs, but not with the intermediate-acting NMBAs modeled in this analysis [22] and, to date, a reduction in their incidence with sugammadex use has not been found [23, 24]. Therefore, these clinical events were not modeled as residual blockade outcomes. Uncomplicated aspiration, occurring to the level of, or above, the vocal cords has been reported in volunteer studies with TOF ratios < 0.9, but not below the vocal cords. Due to the rare documentation of aspiration during the post-extubation and recovery periods in general anesthetic settings, [25–27] and likely silent nature of events with lack of clinical management, aspiration was not modeled within the DES.
Muscle weakness, which may be characterized by general weakness, as well as difficulty with speaking and vision, is not uncommon post-operatively [21, 28], and occurs with greater frequency among patients with residual neuromuscular blockade [28, 29]. However, no studies could be identified documenting an excess cost associated with post-operative muscle weakness, nor did clinician consultation suggest this to be a major contributor to resource use. Therefore muscle weakness was also not modeled within the DES.
Published literature were available from which to construct estimates of the excess risk of hypoxemia and upper airway obstruction associated with the occurrence of residual neuromuscular blockade as reported in Table 1. As the estimation methods are fairly detailed, the derivation of these values is described in Additional file 1.
Reversal agent resource use
Consistent with prior Canadian health technology assessments (HTAs) and a UK National Institute for Health Research (NIHR) HTA, an average patient weight of 75 kg was assumed, with vial wastage [30]. Consistent with clinical trials evaluating sugammadex, it is assumed that a 50 μg/kg dose of neostigmine is administered, in combination with a 10 μg/kg dose of glycopyrrolate. For sugammadex, it is assumed that doses of 2 mg/kg and 4 mg/kg are used to reverse moderate and deep block, respectively.
Operating room overtime resource use
When OR procedures run over the regular OR day, it is assumed that, among the OR staff, registered nurses, respiratory therapists and nurse aides are eligible for overtime pay. Over-time is assumed to be paid in 30 min increments, rounded up to the nearest half hour. It is assumed that 3 registered nurses, one nurse aide and one respiratory therapist are present in a given OR.
Outputs
Model outputs for an OR, reported over a one month time horizon with respect to use of sugammadex versus neostigmine, include the number of procedures performed, procedural cancellations due to regular OR day over-run, hours of OR staff overtime and cases and complications of residual blockade avoided. Other primary outputs include the number of OR minutes saved per day, % of days all procedures are completed within the regular OR day and reduction in risk of RNMB. Each set of outputs is reported under various scenarios for the % of patients verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR.
Sensitivity analyses
Illustrative sensitivity analyses are reported varying the assumed policy for cancelling a procedure due to lack of OR time (cancel if < 50% of procedure can be completed within the regular OR day, never cancel), whether the next procedure is moved up when cancelling (fully move up next procedure), the proportion of procedures in OR which are emergency procedures (15%) and at 95% confidence interval values for sugammadex OR time savings per procedure versus neostigmine.
Exploratory analysis
An exploratory analysis is conducted evaluating results for the 4 mg/kg dose of sugammadex in reversing patients who are maintained at a deep level of neuromuscular block until the end of the procedure. Sugammadex is efficacious in rapidly reversing deep neuromuscular block (i.e., at re-appearance of 1–2 post-tetanic counts), [2, 10] whereas acetylcholinesterase inhibitors such as neostigmine cannot adequately reverse deep levels of blockade because they reach a “ceiling” in which the increase in acetylcholine concentration is insufficient to displace enough NMBA molecules to reverse neuromuscular block [10, 11]. For these patients in clinical practice, anesthesiologists wait until the depth of block has faded to a moderate level after the completion of the procedure before administering neostigmine. There has not been a corresponding clinical trial of sugammadex to date in which all patients in the neostigmine arm were administered deep block through the end of the procedure, which was allowed to fade to moderate block prior to reversal agent administration. Therefore, hypothetical OR time savings per procedure with sugammadex use of 15, 30, 45 and 60 min were explored.
Because the anesthesiologist must wait to reverse with neostigmine until the depth of block has faded from deep to moderate, it was implicitly assumed based on expert feedback that the procedures would be longer than in the base case when moderate block is used. To achieve an OR schedule of identical average expected duration as in the base case, it was assumed that 3 procedures of 145 min each, utilizing deep block to the end of the procedure, would be scheduled per OR day.
Simulation
To allow for variability within the OR schedule across OR days, procedure times were drawn from a lognormal distribution around mean values, with discrete, exponential and triangular distributions within ARENA used to draw values for time to first procedure start, turnover times and time for OR clean-up/next day prep, respectively. As point estimate values are varied within the model, a fixed “variability ratio” was chosen for each of the distributions, so that the standard deviation was adjusted according to the mean value entered. The model was run with 50 replications within each analysis, representing 1050 simulated OR days, upon which results converged to stable values.
Results
Primary analyses
Comparison of sugammadex and neostigmine in the reversal of moderate neuromuscular block with respect to OR efficiency and clinical outcomes in an OR over a 1 month period is reported in Table 3. The estimated average number of OR minutes saved per day with sugammadex use varies from 0 to 62 min as the percentage of patients verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR is varied between 0 and 100%. Correspondingly, the percent of days all procedures are completed within the regular OR day with sugammadex use varies from 40.6% (equivalent to with neostigmine use) up to 72.7%, when the percentage of patients verified to have full recovery in the OR is varied from 0 to 100%. Depending on the proportion of patients verified to have full recovery in the OR, the number of procedures performed over 1 month may be increased (range of 0 to 2.2 additional procedures), and number of procedures cancelled due to lack of OR time (range of 0 to 2.4 cancelled procedures avoided) and paid hours of staff over-time decreased with sugammadex use (range of 0 to 33.5 fewer hours).
Table 3.
Comparison of sugammadex and neostigmine on OR efficiency and clinical outcomes in an OR over 1 month in the reversal of moderate neuromuscular block
| Outcome Measure | Neostigmine | Sugammadex (2 mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| % of patients verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation | ||||||||
| 0% | 5% | 10% | 25% | 50% | 75% | 100% | ||
| OR efficiency outcomes | ||||||||
| Number of OR minutes saved per day | – | 0 | 3 | 6 | 15 | 31 | 46 | 62 |
| % of days all procedures are completed within the regular OR day | 40.6% | 40.6% | 40.8% | 42.7% | 49.0% | 58.0% | 65.0% | 72.7% |
| Number of procedures performed | 90.8 | 90.8 | 90.9 | 91.0 | 91.6 | 92.5 | 92.9 | 93.0 |
| Procedures cancelled due to lack of OR time | 3.5 | 3.5 | 3.4 | 3.3 | 2.8 | 1.9 | 1.4 | 1.1 |
| Procedures cancelled for other reasons | 10.7 | 10.7 | 10.7 | 10.8 | 10.7 | 10.6 | 10.8 | 11.0 |
| Paid hours of staff over-time | 57.8 | 57.8 | 57.4 | 54.9 | 47.9 | 38.9 | 31.9 | 24.3 |
| Clinical outcomes | ||||||||
| Cases of residual blockade avoided | – | 51 | 48 | 46 | 38 | 25 | 13 | 0 |
| Hypoxemia cases avoideda | – | 12 | 12 | 11 | 9 | 6 | 3 | 0 |
| Upper airway obstruction cases avoideda | – | 23 | 21 | 20 | 17 | 11 | 6 | 0 |
| Absolute reduction in risk of residual blockade, per patient | – | 56% | 53% | 50% | 42% | 28% | 14% | 0% |
OR Operating room
aIncludes both cases which are and are not clinically diagnosed and managed
The impact of sugammadex upon clinical outcomes of RNMB exhibits an opposite trend with respect to neuromuscular recovery practices. The number of cases of residual blockade avoided with sugammadex decreases from 51 to 0 as the percent of patients verified to have full recovery in the OR increases from 0 to 100%, with an absolute reduction in the risk of residual blockade per patient ranging from 56 to 0%. In the scenario where 0% of patients were verified to have full neuromuscular recovery prior to extubation, the numbers needed to treat (NNT) to prevent a case of residual blockade, hypoxemia and upper airway obstruction were 1.8, 7.6 and 3.9, respectively. The NNTs increase as a higher % of patients are verified to have full neuromuscular recovery.
Sensitivity analyses
Prior work has found OR resource use and costs with sugammadex use to be much less sensitive to offsets associated with residual blockade as compared to OR staff time/over-time [31]. It was therefore elected for illustrative purposes to focus the sensitivity analyses upon the scenario where 100% of patients are verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) in the OR prior to extubation, as OR staff over-time is most influential within this scenario (Table 4).
Table 4.
Sensitivity Analyses - For scenario where 100% of patients are verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR
| Number of OR minutes saved per day | % of days all procedures are completed within the regular OR day | Procedures cancelled due to lack of OR time | Paid hours of staff over-time | |
|---|---|---|---|---|
| Primary Analysisa | ||||
| Neostigmine | – | 40.6% | 3.5 | 57.8 |
| Sugammadex | 62 | 72.7% | 1.1 | 24.3 |
| Cancel if < 50% of procedures can be completed within the regular OR day | ||||
| Neostigmine | – | 39.5% | 6.8 | 36.0 |
| Sugammadex | 62 | 73.7% | 1.9 | 16.5 |
| Never cancel a procedure due to lack of OR time | ||||
| Neostigmine | – | 40.4% | 0.0 | 92.0 |
| Sugammadex | 63 | 74.3% | 0.0 | 31.4 |
| Fully move up next procedure if a cancellation occurs | ||||
| Neostigmine | – | 63.2% | 2.4 | 32.3 |
| Sugammadex | 62 | 85.2% | 0.7 | 12.9 |
| Assume 15% of procedures are emergency cases | ||||
| Neostigmine | – | 19.8% | 16.1 | 108.5 |
| Sugammadex | 57 | 45.0% | 9.6 | 66.6 |
| Sugammadex OR time saved at lower bound of 95% CI in trials (2 min) | ||||
| Neostigmine | – | 40.6% | 3.5 | 57.8 |
| Sugammadex | 9 | 44.9% | 2.8 | 54.0 |
| Sugammadex OR time saved at lower bound of 95% CI in trials (26 min) | ||||
| Neostigmine | – | 40.6% | 3.5 | 57.8 |
| Sugammadex | 116 | 92.2% | 0.3 | 5.8 |
CI Confidence interval OR Operating room
aIn the primary analysis, procedures are cancelled if they cannot begin within the regular OR day, when a procedure is cancelled for any reason, the next procedure is not moved up, and no emergency cases occur
In the primary analyses, it is assumed that no procedures may begin after the end of the regular OR day and are otherwise cancelled. When this is modified to assume that cancellation occurs if <50% of the anticipated duration of a procedure may be completed within the regular OR day (a relatively lower threshold for cancelling), the number of procedural cancellations avoided with sugammadex use increases, with a smaller absolute reduction in staff over-time hours. In contrast, if it is assumed that a procedure can never be cancelled due to a lack of OR time, the reduction in paid hours of staff over-time with sugammadex use nearly doubles as compared to within the primary analyses.
If it is assumed that 15% of procedures are emergency cases (as opposed to 0% in the primary analyses), which bump scheduled cases, the number of procedures cancelled due to lack of OR time and paid hours of staff over-time increase dramatically, with relatively larger absolute reductions in these outcomes with sugammadex use as compared to in the primary analyses.
Results were also sensitive to assumed OR time savings with sugammadex versus neostigmine, when varied across values for the 95% confidence interval for this parameter (2 to 26 min) as observed within pooled clinical trial data.
Exploratory analyses
In the exploratory analyses (Table 5), it is assumed that patients are maintained at a deep level of neuromuscular block through the end of the procedure and reversed with neostigmine or a 4 mg/kg dose of sugammadex. As described in the Methods section, as the amount of time saved with sugammadex versus neostigmine in clinical practice in these patients is unknown, hypothetical time savings of 15, 30, 45 and 60 min per procedure are explored. When the amount of OR time saved is fixed, the percentage of patients verified to have full neuromuscular recovery in the OR no longer influences sugammadex’s impact upon OR time within the analysis. For illustrative purposes, it was therefore elected to conduct the exploratory analysis for the scenario where 0% of patients are verified to have full neuromuscular recovery in the OR, as results would be very similar for the other scenarios, with the exception of the incidence of clinical outcomes of residual blockade.
Table 5.
Exploratory analyses - Comparison of sugammadex and neostigmine on OR efficiency and clinical outcomes in an OR over 1 month when deep block is maintained to the end of all procedures [0% of patients verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation]
| Outcome Measure | Neostigmine | Sugammadex (4 mg/kg) | |||
|---|---|---|---|---|---|
| Minutes of OR time saved per procedure | |||||
| 15 | 30 | 45 | 60 | ||
| OR efficiency outcomes | |||||
| Number of OR minutes saved per day | – | 39 | 79 | 118 | 158 |
| % of days all procedures are completed within the regular OR day | 46.7% | 61.9% | 77.1% | 86.9% | 91.4% |
| Number of procedures performed | 54.5 | 54.7 | 55.1 | 55.1 | 55.2 |
| Procedures cancelled due to lack of OR time | 1.2 | 0.7 | 0.4 | 0.4 | 0.3 |
| Procedures cancelled for other reasons | 7.3 | 7.6 | 7.6 | 7.6 | 7.6 |
| Paid hours of staff over-time | 84.1 | 54.9 | 32.0 | 18.3 | 11.5 |
| Clinical outcomes | |||||
| Cases of residual blockade avoided | – | 30 | 30 | 30 | 30 |
| Hypoxemia cases avoideda | – | 7 | 7 | 7 | 7 |
| Upper airway obstruction cases avoideda | – | 13 | 13 | 13 | 13 |
| Absolute reduction in risk of residual blockade, per patient | – | 56% | 56% | 56% | 56% |
OR Operating room
aIncludes both cases which are and are not clinically diagnosed and managed
In the deep block analysis, as the number of minutes of OR time saved per procedure with sugammadex increases, the number of procedures cancelled due to a lack of OR time decreases, along with paid hours of staff over-time. For instance, the number of paid hours of staff over-time drops from 84.1 to 32.0, when 30 min of OR time are saved per procedure. The number of paid hours of staff over-time with neostigmine use is greater than in the model base case due to the impact of the assumed increased variability in the duration of the procedures for the longer deep block procedures as compared to the shorter moderate block procedures.
Discussion
This analysis has shown that, depending upon the neuromuscular management and extubation practices at a given hospital, sugammadex can potentially reduce the risk of RNMB and/or enhance operating room efficiency.
For procedures ending with moderate neuromuscular block, when patients are not verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR, use of neostigmine results in a high incidence of RNMB [6], with associated clinical complications of upper airway obstruction and hypoxemia [32, 33]. To avoid these risks of respiratory complications, if patients administered neostigmine are maintained in the OR until full neuromuscular recovery is verified and they may be safely extubated, additional time is expended within the OR [13, 16]. Sugammadex can ameliorate this trade-off between OR efficiency and the occurrence of residual neuromuscular block by substantially accelerating the time to complete neuromuscular recovery [17] and safe extubation.
When patients are maintained with deep neuromuscular block to the end of the procedure, sugammadex is very likely to save time in the OR compared to neostigmine use, with reductions in the risk of RNMB dependent upon whether patients are verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR.
In clinical practice, patients currently are rarely verified to have full neuromuscular recovery (TOF ratio ≥ 0.9) prior to extubation in the OR as evidenced by low use of quantitative neuromuscular monitoring [34, 35] and high incidences of RNMB with neostigmine use [6, 19, 20]. Thus, for the majority of patients currently managed with moderate neuromuscular block in Canada, the principal impact of the substitution of sugammadex for neostigmine is likely to be a reduction in the risk of residual blockade and associated complications. Under these conditions, for patients managed with deep neuromuscular block through the end of the procedure, sugammadex is expected to both reduce OR procedure times and complications associated with residual blockade relative to use of neostigmine.
In interpreting the magnitude of OR time saved, avoided procedural cancellations, avoided staff overtime and avoided residual block, where applicable, within the present analysis, a number of caveats should be kept in mind. First, given the running time of the DES model to complete iterations for the base case, it was prohibitive to run a formal probabilistic sensitivity analysis (PSA) across model parameters. However, most of the model parameters for which results are most sensitive, as described in the Results section, are based on different scenario-based assumptions for quantitative neuromuscular monitoring, hospital cancellation and emergency procedure policies. These types of parameters are best explored in the one-way sensitivity analyses currently performed within the analysis, as they are not amenable to PSA.
Second, for illustrative purposes, it was assumed that all procedures within the OR day used NMBAs of rocuronium or vecuronium, and neostigmine or sugammadex for neuromuscular reversal. This assumption enables full evaluation of the potential for sugammadex to impact OR time-related outcomes. In clinical practice, however, there may be variation across procedures occurring within a given OR on a particular day in terms of whether NMBAs are used and, if so, whether neuromuscular block is reversed using a pharmaceutical agent, or allowed to spontaneously reverse. In ORs where this variation occurs, the total potential impact of sugammadex on outcomes related to OR time savings and residual block avoidance would be lessened. Also, the primary analysis has modeled 5 short procedures of 72.9 min each. For ORs where 2–3 longer procedures are performed within a given day, all else equal, the potential OR time savings and number of residual blockade cases prevented by sugammadex are also likely to be relatively less.
However, the resource use impact of OR time savings within the present analysis, which was limited to those staff members earning over-time pay due to OR time over-run, is likely to only partially account for the benefits to a hospital. One could also consider the intangible value of time saved for salaried OR staff members who are ineligible for over-time pay (e.g., surgeon and anesthesiologist) as well as non-overtime minutes saved for all OR staff members. Furthermore, when cancelled procedures are avoided, there are potentially impacts for the hospital and patient related to rescheduling, re-preparation for the procedure and patient time/work loss. Finally, if enough OR time saved can be accrued within a given day, and the OR is running at full capacity throughout the year, and there is a queue for procedures or opportunity to expand the demand for OR procedures, hospitals may be able to increase annual procedural throughput and decrease surgical waiting times.
The impact of clinical sequelae of residual blockade is a relatively under-researched area. It is important to note that available literature were insufficient for identifying where excess hospital resource use is incurred for rarer but more serious respiratory outcomes which could potentially occur (e.g., post-operative myocardial infarction in cardiovascular disease patients, aspiration pneumonia in emergency cases where patients are unable to be operated on with an empty stomach). Further research is needed to better understand the degree to which these rarer clinical events are linked to residual block and potentially avoidable with sugammadex use. It is evident, however, that both residual blockade and its sequelae are very common in the OR and PACU, with an estimated risk of residual blockade at extubation of 60% with neostigmine use as reported herein [6, 19, 20].
Conclusions
Sugammadex can potentially reduce the risk of RNMB, and/or enhance operating room efficiency, relative to use of neostigmine for routine reversal of neuromuscular block. In clinical practice within Canada, for the majority of patients currently managed with moderate neuromuscular block, the principal impact of using sugammadex instead of neostigmine is likely to be a reduction in the risk of residual blockade and associated complications. For patients maintained at a deep level of block to the end of the procedure, sugammadex is likely to both enhance OR efficiency and reduce complications of residual block. Where OR efficiency gains occur, potential benefits of sugammadex may include reduced procedural cancellations due to OR time over-run, avoided staff over-time and opportunity to evaluate if procedural throughput may be increased.
Acknowledgments
The authors would like to thank André Jacques, Trellisys Technologies, Inc. (Montreal, Canada) who provided support in the construction of the DES model. We also thank Susan Grant and Eric Lefrançois of Merck Canada and Jonathan Schelfhout of Merck U.S. for helpful input into earlier versions of the model.
Funding
The authors completed this work as employees of Merck & Co., Inc., which supported the publication charges for this manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file 1.
Authors’ contributions
RPI, CJ, AG and AG contributed to the design of the DES model. The initial manuscript draft was developed by RPI with input from CJ. All authors have read, provided input to and approved the final manuscript.
Competing interests
The authors are employed by Merck & Co., Inc. and may own company stock and/or stock options.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional file
Additional details on estimation of selected model input values. (DOC 96 kb)
Contributor Information
Ralph P. Insinga, Email: ralph_insinga@merck.com
Cédric Joyal, Email: cedric.joyal@merck.com.
Alexandra Goyette, Email: alexandra.goyette@merck.com.
André Galarneau, Email: andre.galarneau@merck.com.
References
- 1.Srivastava A, Hunter JM. Reversal of neuromuscular block. Br J Anaesth. 2009;103:115–29. doi: 10.1093/bja/aep093. [DOI] [PubMed] [Google Scholar]
- 2.A randomized, safety-assessor blinded trial comparing 4.0 Mg.Kg-1 Sugammadex with placebo in adult subjects scheduled for surgery requiring profound neuromuscular blockade. Clinical Trial Report on Protocol 19.4.316. Merck & Co., Inc. 2010.
- 3.Blobner M, Eriksson LI, Scholz J, Motsch J, Della RG, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27:874–81. doi: 10.1097/EJA.0b013e32833d56b7. [DOI] [PubMed] [Google Scholar]
- 4.Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111:120–8. doi: 10.1213/ANE.0b013e3181e33bd9. [DOI] [PubMed] [Google Scholar]
- 5.Rex C, Bergner UA, Puhringer FK. Sugammadex: a selective relaxant-binding agent providing rapid reversal. Curr Opin Anaesthesiol. 2010;23:461–5. doi: 10.1097/ACO.0b013e32833a5413. [DOI] [PubMed] [Google Scholar]
- 6.Sabo D, Jones RK, Berry J, Sloan T, Chen JY, Morte JB, Groudine S. Residual neuromuscular blockade at extubation: a randomized comparison of sugammadex and neostigmine reversal of rocuronium-induced blockade in patients undergoing abdominal surgery. J Anesthes Clin Res. 2011;2:140. doi: 10.4172/2155-6148.1000140. [DOI] [Google Scholar]
- 7.Flockton EA, Mastronardi P, Hunter JM, Gomar C, Mirakhur RK, Aguilera L, Giunta FG, Meistelman C, Prins ME. Reversal of rocuronium-induced neuromuscular block with sugammadex is faster than reversal of cisatracurium-induced block with neostigmine. Br J Anaesth. 2008;100:622–30. doi: 10.1093/bja/aen037. [DOI] [PubMed] [Google Scholar]
- 8.Khuenl-Brady KS, Wattwil M, Vanacker BF, Lora-Tamayo JI, Rietbergen H, Alvarez-Gomez JA. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: a multicenter, randomized, controlled trial. Anesth Analg. 2010;110:64–73. doi: 10.1213/ane.0b013e3181ac53c3. [DOI] [PubMed] [Google Scholar]
- 9.Geldner G, Niskanen M, Laurila P, Mizikov V, Hubler M, Beck G, Rietbergen H, Nicolayenko E. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia. 2012;67:991–8. doi: 10.1111/j.1365-2044.2012.07197.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109:816–24. doi: 10.1097/ALN.0b013e31818a3fee. [DOI] [PubMed] [Google Scholar]
- 11.Yang LP, Keam SJ. Sugammadex: a review of its use in anaesthetic practice. Drugs. 2009;69:919–42. doi: 10.2165/00003495-200969070-00008. [DOI] [PubMed] [Google Scholar]
- 12.A multi-center, randomized, parallel group, comparative, active controlled, safety assessor blinded, anesthesiologist-TOF-Watch® SX blinded trial comparing T4/T1 ratio at time of tracheal extubation using 4 mg.kg-1 sugammadex administered at 1–2 PTCs or better after the last dose of rocuronium bromide to 50 ?g.kg-1 neostigmine administered as per standard of care in adult subjects undergoing elective open abdominal procedures requiring neuromuscular blockade reversal. Clinical Trial Report on Protocol 19.4.334. Merck & Co., Inc. 2009.
- 13.A multi-center, randomized, parallel group, comparative, active controlled, safety assessor blinded trial in adult subjects comparing the efficacy and safety of sugammadex (SCH 900616, ORG 25969) administered at 1–2 PTC with neostigmine administered at reappearance of T2 in subjects undergoing laparoscopic cholecystectomy or appendectomy under propofol anesthesia. Clinical Trial Report on Protocol 19.4.318. Merck & Co., Inc. 2010.
- 14.Effect of sugammadex compared with usual care for reversal of neuromuscular blockade induced by rocuronium on incidence of residual blockade at PACU entry (Phase 5, Protocol No. P07981 [also known as MK 8616–064]). 2012.
- 15.A randomized, controlled, parallel-group, double-blind trial of sugammadex or usual care (neostigmine or spontaneous recovery) for reversal of rocuronium- or vecuronium-induced neuromuscular blockade in patients receiving thromboprophylaxis and undergoing hip fracture surgery or joint (hip/knee) replacement (P07038). 2014.
- 16.Grintescu I, Mirea L, Ologoiu D, Ungureanu R, Mekauvar S, Vasilescu M. Comparison of the cost-effectiveness of sugammadex and neostigmine during general anesthesia for laparascopic cholecystectomy. Br J Anaesth. 2009;103:917. [Google Scholar]
- 17.Partownavid P, Romito BT, Ching W, Berry AA, Barkulis CT, Nguyen KP, Jahr JS. Sugammadex: a comprehensive review of the published human science, including renal studies. Am J Ther. 2014;22:298–317. [DOI] [PubMed]
- 18.Naguib M, Kopman AF, Ensor JE. Neuromuscular monitoring and postoperative residual curarisation: a meta-analysis. Br J Anaesth. 2007;98:302–16. doi: 10.1093/bja/ael386. [DOI] [PubMed] [Google Scholar]
- 19.Kotake Y, Ochiai R, Suzuki T, Ogawa S, Takagi S, Ozaki M, Nakatsuka I, Takeda J. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg. 2013;117:345–51. doi: 10.1213/ANE.0b013e3182999672. [DOI] [PubMed] [Google Scholar]
- 20.Murphy GS, Szokol JW, Marymont JH, Franklin M, Avram MJ, Vender JS. Residual paralysis at the time of tracheal extubation. Anesth Analg. 2005;100:1840–5. doi: 10.1213/01.ANE.0000151159.55655.CB. [DOI] [PubMed] [Google Scholar]
- 21.Murphy GS, Szokol JW, Franklin M, Marymont JH, Avram MJ, Vender JS. Postanesthesia care unit recovery times and neuromuscular blocking drugs: a prospective study of orthopedic surgical patients randomized to receive pancuronium or rocuronium. Anesth Analg. 2004;98:193–200. doi: 10.1213/01.ANE.0000095040.36648.F7. [DOI] [PubMed] [Google Scholar]
- 22.Berg H, Roed J, Viby-Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, Krintel JJ. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–103. doi: 10.1111/j.1399-6576.1997.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 23.Ledowski T, Hillyard S, O’Dea B, Archer R, Vilas-Boas F, Kyle B. Introduction of sugammadex as standard reversal agent: impact on the incidence of residual neuromuscular blockade and postoperative patient outcome. Indian J Anaesth. 2013;57:46–51. doi: 10.4103/0019-5049.108562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledowski T, Falke L, Johnston F, Gillies E, Greenaway M, De Mel A, Tiong WS, Phillips M. Retrospective investigation of postoperative outcome after reversal of residual neuromuscular blockade: Sugammadex, neostigmine or no reversal. Eur J Anaesthesiol. 2014;31:423–9. doi: 10.1097/EJA.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 25.Rassam S, Sandbythomas M, Vaughan RS, Hall JE. Airway management before, during and after extubation: a survey of practice in the United Kingdom and Ireland. Anaesthesia. 2005;60:995–1001. doi: 10.1111/j.1365-2044.2005.04235.x. [DOI] [PubMed] [Google Scholar]
- 26.Sakai T, Planinsic RM, Quinlan JJ, Handley LJ, Kim TY, Hilmi IA. The incidence and outcome of perioperative pulmonary aspiration in a university hospital: a 4-year retrospective analysis. Anesth Analg. 2006;103:941–7. doi: 10.1213/01.ane.0000237296.57941.e7. [DOI] [PubMed] [Google Scholar]
- 27.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Cammu G, De Witte J, De Veylder J, Byttebier G, Vandeput D, Foubert L, Vandenbroucke G, Deloof T. Postoperative residual paralysis in outpatients versus inpatients. Anesth Analg. 2006;102:426–9. doi: 10.1213/01.ane.0000195543.61123.1f. [DOI] [PubMed] [Google Scholar]
- 29.Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Marymont JH, Vender JS, Gray J, Landry E, Gupta DK. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology. 2011;115:946–54. doi: 10.1097/ALN.0b013e3182342840. [DOI] [PubMed] [Google Scholar]
- 30.Chambers D, Paulden M, Paton F, Heirs M, Duffy S, Craig D, Hunter J, Wilson J, Sculpher M, Woolacott N. Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment. Health Technol Assess. 2010;14:1–211. doi: 10.3310/hta14390. [DOI] [PubMed] [Google Scholar]
- 31.Insinga RP, Konstantopoulou T, Athanasakis K, Argyris G. Budget impact of using sugammadex for the routine reversal of neuromuscular blockade in a Greek health care setting. ISPOR 15th Annual European Congress. Berlin, Germany, November 3–7, 2012.
- 32.Eikermann M, Groeben H, Husing J, Peters J. Accelerometry of adductor pollicis muscle predicts recovery of respiratory function from neuromuscular blockade. Anesthesiology. 2003;98:1333–7. doi: 10.1097/00000542-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Norton M, Xara D, Parente D, Barbosa M, Abelha FJ. Residual neuromuscular block as a risk factor for critical respiratory events in the post anesthesia care unit. Rev Esp Anestesiol Reanim. 2013;60:190–6. doi: 10.1016/j.redar.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Naguib M, Kopman AF, Lien CA, Hunter JM, Lopez A, Brull SJ. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111:110–9. doi: 10.1213/ANE.0b013e3181c07428. [DOI] [PubMed] [Google Scholar]
- 35.Phillips S, Stewart PA, Bilgin AB. A survey of the management of neuromuscular blockade monitoring in Australia and New Zealand. Anaesth Intensive Care. 2013;41:374–9. doi: 10.1177/0310057X1304100316. [DOI] [PubMed] [Google Scholar]
- 36.Parker M, Hattle R, Prejeant D, Stock G. Starting the First Surgical Case on Time to Cut Delays. Six Sigma case study. February 2010. Available at: www.isixsigma.com/new-to-six-sigma/dmaic/starting-first-surgical-case-time-cut-delays. Accessed: 7/9/2015.
- 37.Jerico MC, Perroca MG, da Penha VC. Measuring quality indicators in the operating room: cleaning and turnover time. Rev Lat Am Enfermagem. 2011;19:1239–46. doi: 10.1590/S0104-11692011000500023. [DOI] [PubMed] [Google Scholar]
- 38.Schofield WN, Rubin GL, Piza M, Lai YY, Sindhusake D, Fearnside MR, Klineberg PL. Cancellation of operations on the day of intended surgery at a major Australian referral hospital. Med J Aust. 2005;182:612–5. doi: 10.5694/j.1326-5377.2005.tb06846.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional file 1.


