Abstract
Sarcopenia is the subclinical loss of skeletal muscle and strength and has been extensively studied in both the cancer and surgical literature. Specifically, sarcopenia has gained significant recognition as an important prognostic factor for both complications and survival in cancer patients. Herein, we review the current literature to date highlighting the specific impact of sarcopenia in patients undergoing oncologic procedures.
Keywords: sarcopenia, adenocarcinoma, complications, survival
INTRODUCTION
First introduced by Rosenberg in 1989, sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength [1]. Sarcopenia is commonly accepted as an age related process and, in that setting is an important predictor of surgical outcomes, discharge destination, and independence following admissions for trauma alerts [2,3]. There is increasing evidence that the elderly and frail are not the only populations, which suffer from sarcopenia. With an increase in fatty tissue mass: lean tissue mass ratio, patients experience sarcopenic obesity. This population is vulnerable to both the adverse health consequences of excess adipose tissue as well as to the complications associated with a decrease in muscle mass [4–6]. Perhaps most striking is the cohort of patients suffering from a malignancy and cancer-related cachexia. Loss of lean tissue mass attributed to malignancy is a well-established complication and has been the focus of a great deal of clinical investigation [7].
Malignancy can result in a hypercatabolic state caused by tumor metabolism, systemic inflammation, and other tumor-mediated effects [8]. This derangement in an individual's homeostasis combined with other cancer-mediated effects such as anorexia, fatigue, decreased functional status, and immobility leads to a depletion of skeletal muscle and the development of sarcopenia. The impact of sarcopenia in cancer patients has been studied across a broad range of malignancies. In patients treated with chemotherapeutic agents, it has been shown to predict drug toxicity [9,10], time to tumor progression [9], and mortality [11]. Muscle loss is also exacerbated by the administration of cytotoxic chemotherapy [12]. Moreover, sarcopenia is independently associated with postoperative outcomes following resection of malignancy in colorectal cancer [13–15], colorectal liver metastasis [16], esophageal carcinoma [17], hepatocellular carcinoma [18,19], melanoma [20], pancreatic adenocarcinoma [21–23], and bladder cancer [24–26]. It has also been shown to be an independent prognostic indicator in cancer patients undergoing palliative therapy [27]. While the stepwise progression towards sarcopenia is not yet clearly defined, there is no question of the deleterious effects that it has on clinical outcomes in cancer populations.
The decision to undergo any surgical intervention is based on weighing the clinical benefits versus potential complications. Patients with sarcopenia are particularly vulnerable to major physiologic stressors including surgery and surgical complications [28]. Englsbe et al. demonstrated that core muscle size is independently predictive of mortality and complications following major elective general or vascular surgery [29]. Sarcopenia has also been shown to correlate with mortality after liver transplantation, length of stay after colon resection, and surgical site infections following midline laparotomies and colon resections [30–32]. Several factors are considered when evaluating a patient's preoperative status including medical co-morbidities and nutritional status. Concomitant with these objective data, the surgeon also uses a more subjective “eyeball test” to evaluate for the patient's expected physiologic reserve [33]. The association between sarcopenia and surgical outcomes provides the surgeon a more impartial tool for assessing for the ability to tolerate surgery.
The aim of this review was to evaluate the current literature for an association between sarcopenia and surgical outcomes following resection of malignancy. Sarcopenia is a component of body habitus that can be quantified preoperatively and altered over time. The assessment of sarcopenia can lead to changes in management strategy, patient selection, and improved informed consent prior to surgical resection of malignancy.
MATERIALS AND METHODS
A systematic review was conducted by querying the PubMed Database for manuscripts that included the following key words [“sarcopenia”] AND [“surgery” OR “operation”] AND [“cancer” OR “malignancy”] AND [“outcome” OR “complications” OR “survival”]. Search parameters were set to articles published within the last 5 years in the English language; the site was last accessed on May 4th 2015. Relevant manuscripts were selected and each “References” section was screened for additional pertinent studies not yielded in the original search. Study eligibility criteria included articles discussing sarcopenia, or depletion of muscle mass, in patients with known malignancy who underwent surgical intervention. The initial query of the PubMed database yielded 110 studies, of which 14 met eligibility criteria. The full texts of appropriate studies were reviewed and analyzed for definition of sarcopenia, method of sarcopenia quantification, type of malignancy, surgical intervention, and postoperative outcomes.
RESULTS
Methods of Quantifying Sarcopenia
While the broader definition of sarcopenia has been widely accepted, there remains no standardized methodology for both the assessment and classification of sarcopenia in the clinical setting. The current framework for quantification involves imaging of skeletal muscle and the determination of cutoff values based on individual study populations. Table I lists methods used to measure and quantify sarcopenia in published studies.
TABLE I.
Modalities and Methodology for Quantifying Sarcopenia in Clinical Studies
| Imaging modality | Methodology for Hounsfield unit measurements | Skeletal muscle quantification | How Sarcopenia is defined |
|---|---|---|---|
| Computed Tomography scan Dual Energy X-ray Absorptiometry Bioelectrical Impedance Assay MRI |
Aquarius iNtuition Aquarius NET Server Inbody ImageJ Manual MATLAB Osirix SliceOmatic SYNAPSE VINCENT Utravisual |
Cross sectional area of skeletal muscles at L3–L5 Cross sectional area of psoas muscles at L3–L4 and umbilicus Density of Psoas muscles at L3–L4 Multifidus muscle and subcutaneous fat at umbilicus Appendicular Skeletal Muscle Mass |
Lowest gender specific quartile Optimum stratification to attain the most significant p-value to define sex specific cutoffs associated with mortality or outcome in patients Categorical cutoff value based on optimum stratification through a series of sensitivity analysis Continuous variable 2 Standard deviations below Gender-specific mean |
In the studies evaluated, imaging modalities used included Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI), Dual Energy X-Ray Absorptiometry [34], and Bioelectrical Impedance Assay [17]. A majority of studies used CT scans [13,14,19–24,35,36]. This can be attributed to the fact that preoperative CT scans are the standard of care for patients undergoing resection of a malignancy. Most studies employed a semi-automated method for taking measurements from the scans; the intended musculature was manually outlined with a preset Hounsfield Unit density threshold. This technique allows for more precise calculation of the muscle area while excluding fat and vasculature that fall outside the preset Hounsfield Unit range [37]. The Hounsfield Unit parameters set by most studies was within −30 to 150 HU [13,14,16,18,19,22,24,35,36].
There are several different musculature measurements that are used to quantify sarcopenia. In general, measurements are taken at a particular level of the lumbar spine (primarily L3), or the value is obtained by averaging measurements from two consecutive lumbar vertebral levels (e.g., L4 and L5; Fig. 1, Panels A–C). A majority of the studies reviewed obtained the cross sectional area of the abdominal skeletal musculature (including bilateral psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal oblique, and rectus abdominis) [13,14,18,19,26,35,36] or the cross sectional area of the psoas muscles [16,20–24]. A few studies defined sarcopenia based on both psoas muscle area and psoas muscle density, expressed in Hounsfield Units [20,21]. Psoas muscle density is a proxy for muscle quality as it accounts for fatty infiltration of muscle tissue. This is also known as the Hounsfield Unit Average Calculation, or HUAC [21]. Other measurements included the appendicular skeletal muscle mass [34], and the multifidus muscle with subcutaneous fat [23].
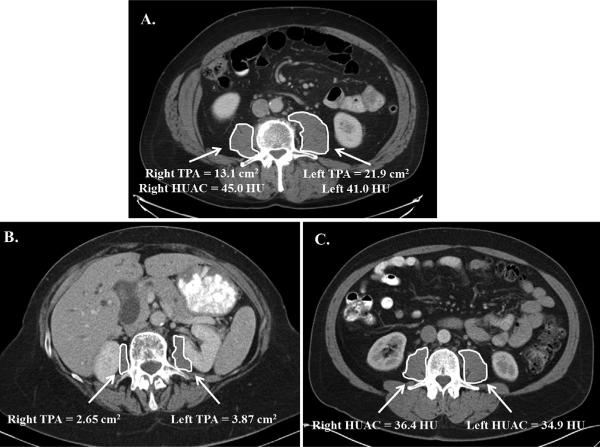
Fig. 1.
Quantification of sarcopenia on contrast enhanced computed tomography in patients with pancreatic adenocarcinoma being considered for pancreatectomy. Panel A. Normal patient. Computed Tomography Image taken at the level of the third lumbar vertebral body. The Total Psoas Index is as measure of muscle mass that accounts for patient height and is calculated as (right psoas area + left psoas area/height2). The Hounsfield Unit Average Calculation is a measurement of radiation attenuation thus muscle density and fatty infiltration. The combination of the right and left Hounsfield Unit Measurement is utilized for the HUAC. HUAC = (right HUAC + left HUAC)/2. Right HUAC, Right Hounsfield Unit* Right Psoas Area/Total Psoas Area). Left HUAC is calculated in the same fashion using the left third vertebral body. TPA, Total Psoas Area; HUAC, Hounsfield Unit Average Calculation; HU, Hounsfield Units. Panel B. Patient with Sarcopenia—low Total Psoas Area. Computed Tomography Scan taken at the level of the third lumbar vertebral body. The highlighted area clearly shows small psoas muscle area. Note that the TPA does not account for muscle density. This patient's Total Psoas Index was calculated using the patient's height and met the lowest 25th percentile for sarcopenia [21]. Panel C. Patient with Sarcopenia—low Hounsfield Unit Average Calculation. Computed Tomography Scan taken at the level of the third lumbar vertebral body. The highlighted area demonstrates a patient with a normal TPA (good muscle mass), yet with poor muscle quality. This patient is an example of where muscle mass may not be predictive of complications yet the patient does have sarcopenia. HUAC has been shown to be an independent predictor of complications following pancreatectomy for adenocarcinoma [21]. TPA, Total Psoas Area; HUAC, Hounsfield Unit Average Calculation; HU, Hounsfield Units.
An example how sarcopenia is quantified by CT imaging is illustrated from a recent study published by Joglekar and co-workers [21]. In this study, sarcopenia was defined as meeting the lower 25th percentile for gender-specific Total Psoas Index (TPI) and HUAC (Fig. 1). The TPI was calculated by measuring the right and left absolute psoas muscle mass (Total Psoas Area, TPA) at L3 and accounting for patient height (TPI = right psoas area + left psoas area/height2). The combination of the right and left Hounsfield Unit Measurement is utilized for the HUAC. HUAC = (right HUAC + left HUAC)/2. Right HUAC = Right Hounsfield Unit*Right Psoas Area/Total Psoas Area). Left HUAC is calculated in the same fashion using the left third vertebral body. Figure 1, Panel A demonstrates a patient with a normal TPA as seen by the substantial muscle mass. The patient shown in Panel B is illustrative of someone with very small TPA and therefore a low TPI (sarcopenia). The patient shown in Panel C has a substantial muscle mass as shown by visual estimation, but the quality of the muscle is low based on the low HUAC and met criteria for sarcopenia [21].
In many of the studies reviewed, sarcopenia was largely defined as a dichotomous variable by establishing cutoff points for the muscle index used. Cutoff values were commonly determined by lowest gender specific quartile [13,21,22], optimum stratification to obtain gender specific cutoffs [14,18,19,23,24,35,36], or two standard deviations below the gender specific mean [34]. Of note, numerous studies obtained their cutoff values by using the optimum stratification model outlined by Prado and co-workers [35]. Gender specific cutoffs were used due to the baseline variability in body habitus between males and females. Despite the variability in the specifics of the method for quantifying sarcopenia, the studies included in this review all used an imaging modality to obtain measurements of skeletal muscle mass or density and defined cutoff values based on the skeletal muscle index calculated.
Studies Evaluating the Impact of Sarcopenia in Surgical Oncology Patients
The studies that met criteria for evaluation of sarcopenia and postoperative outcomes following resection of cancer are summarized in Table II. A total of 3,046 patients were included in these studies across numerous solid tumor types including colorectal cancer [13,16,38], colorectal cancer liver metastases [16], esophageal cancer [17], hepatocellular carcinoma [18,19], melanoma [20], pancreatic cancer [21–23], and bladder cancer [24–26]. The percentage of patients with sarcopenia across the studies ranged from 11.1% to 68.8%. This broad range may be attributed to the lack of a standardized definition of sarcopenia, as well as innate differences in the patient populations evaluated. As shown in Table II, many of the studies evaluated demonstrated the significant prognostic role of sarcopenia for both cancer-related survival and complications following oncologic procedures. The significant findings across both sites of primary disease and type of procedure highlights the importance of sarcopenia as a predictive measure in surgical oncology patients.
TABLE II.
Major Outcomes Evaluated in Surgical Oncology Patients with and without Sarcopenia
| Author (year) | Malignancy | N | Patients with Sarcopenia N (%) | Main outcomes measured | Conclusion regarding Sarcopenia |
|---|---|---|---|---|---|
| Miyamoto (2015) | Colorectal Cancer | 220 | 55 (25%) | Recurrence free survival Overall survival | Negative prognostic factor after colectomy |
| Reisinger (2015) | Colorectal Cancer | 310 | 148 (44.7%) | 30-day mortality | Independent predictor of operative mortality from colectomy |
| VanVugt (2015) | Peritoneal Carcinomatosis of Colorectal Cancer | 206 | 90 (43.7%) | Severe postoperative complications | Associated with an increased risk of severe postoperative complications following CRS/HIPEC |
| Peng (2011) | Colorectal Liver Metastasis | 259 | 41 (16%) | Postoperative complications Disease free survival | Independently associated with increased risk of postoperative complications following hepatic resection |
| Ida (2015) | Esophageal Squamous Cell Carcinoma | 138 | 61 (44.2%) | Postoperative respiratory complications | Independent risk factor for postoperative respiratory complications following esophagectomy |
| Harimoto (2013) | Hepatocellular Carcinoma | 186 | 75 (40.3%) | Overall survival Recurrence free survival | Independent prognostic factor for overall and recurrence-free survival following hepatic resection for hepatoma |
| Iritani (2015) | Hepatocellular Carcinoma | 45* | 24 (11.1%) | Overall survival Recurrence rate | Negative prognostic factor for survival following hepatectomy for hepatoma |
| Sabel (2011) | Melanoma | 101 | – | Disease free survival complications | Predicts disease free survival and complications following therapeutic lymph node dissection for stage III melanoma |
| Joglekar (2015) | Pancreatic Adenocarcinoma | 118 | 31 (26.3%) | Postoperative complications survival | Significant predictor of complications following pancreatectomy for adenocarcinoma |
| Peng (2011) | Pancreatic Adenocarcinoma | 557 | BMI ≤ 24.9 36.2% BMI ≥ 30 13.3% |
Postoperative complications Survival | Prognostic factor for survival following pancreatectomy for adenocarcinoma |
| Okumura (2015) | Pancreatic Adenocarcinoma | 230 | – | Overall survival Recurrence free survival | Prognostic factor for survival following pancreatectomy for adenocarcinoma |
| Smith (2014) | Muscle Invasive Bladder Cancer | 224 | – | Postoperative complications Survival | Predicts major complications following radical cystectomy in women |
| Psutka (2014) | Urothelial Cancer of the Bladder | 205 | 141 (68.8%) | Cancer-specific survival Overall survival | Increased cancer specific and overall mortality following radical cystectomy |
| Wan (2014) | Bladder Cancer | 247 | – | Early complication rates | Lower skeletal muscle index is an independent predictor of early complications following radical cystectomy |
BMI, Body Mass Index; CRS/HIPEC, cytoreductive surgery and heated intraperitoneal chemotherapy.
Impact of Sarcopenia on Postoperative Complications in Surgical Oncology Patients
The incidence and severity of postoperative complications as related to sarcopenia are shown in Table III. Sarcopenia predicted complications following a broad range of surgical interventions including cytoreductive surgery with heated intraperitoneal chemotherapy (CRS/HIPEC) for peritoneal carcinomatosis of colorectal cancer [38], hepatic resection for colorectal liver metastasis [16], esophagectomy for squamous cell carcinoma [17], therapeutic lymph node dissection for stage III melanoma [20], pancreatectomy for pancreatic adenocarcinoma [21], and radical cystectomy for bladder cancer [24].
TABLE III.
Impact of Sarcopenia on Postoperative Complications in Surgical Oncology Patients
| Author (year) | Malignancy | Surgery | Complications grading system | Findings on multivariate analysis |
|---|---|---|---|---|
| VanVugt (2015) | Peritoneal Carcinomatosis from Colorectal Cancer | Cytoreductive Surgery/HIPEC | Clavien–Dindo | Sarcopenia is independently associated with severe postoperative complications |
| Peng (2011) | Colorectal Liver Metastasis | Hepatic Resection | Clavien–Dindo | Sarcopenia is independently associated with increased risk of major complications |
| Ida (2015) | Esophageal Squamous Cell Carcinoma | Esophagectomy | Clavien–Dindo | Sarcopenia is independently associated with respiratory complications |
| Sabel (2011) | Stage III Melanoma | Therapeutic Lymph Node Dissection | – | 8.1% increase in complication rate for every 10 Hounsfield Unit decrease in muscle density |
| Joglekar (2015) | Pancreatic Adenocarcinoma | Pancreatectomy | Common Toxicity Criteria for Adverse Events International Study Group for Pancreatic Surgery | Sarcopenia is a significant independent predictor of length of stay, ICU stay, any complication, major Grade III complications, delayed gastric emptying, infectious, gastrointestinal, pulmonary, and cardiac complications |
| Smith (2014) | Muscle Invasive Bladder Cancer | Radical Cystectomy | Clavien–Dindo | Sarcopenia was a predictor of major complications in women |
| Wan (2014) | Bladder Cancer | Radical Cystectomy | Clavien–Dindo | Lower skeletal muscle index was predictive of severe complication. Each 1 cm2/m2 increase in skeletal muscle index decreased the odds of severe morbidity by 4.8% |
Grading of complications is a critical aspect of any study evaluating outcomes across patient groups in surgical oncology patients [39]. The most mature and established complications reporting system available is that for pancreatectomy, where there is a specific grading system that has been published and validated across institutions [40,41]. When an organ-specific grading system is not available, there are other excellent scoring systems that have been validated across institutions. In this review, the majority of the studies graded complications according to the Clavien–Dindo classification scheme, with a severe complication defined as Clavien–Dindo Grade ≥3 [42].
Numerous studies published the rate of complications between sarcopenic versus non-sarcopenic patients (Table III). VanVugt and co-workers reported that the rate of complications following CRS/HIPEC was 54.4% in sarcopenic patients versus 41.4% in normal patients, and the rate of severe postoperative complications to be 33.3% versus 21.6% [38]. In an evaluation of sarcopenia in patients having esophagectomy, the rate of postoperative respiratory complications following operation was 15.2% in sarcopenic patients versus 6.5% in the normal population [17]. The rate of a major complications following radical cystectomy was 30% (sarcopenic) versus 16% (non-sarcopenic) [24]. These significant differences highlight the importance of sarcopenia in patient selection for surgery, especially in light of alternative options for treatment. Two studies demonstrated an increased risk of complications based on incremental changes in the muscle index used to quantify sarcopenia. In the study by Sabel and co-workers, there was an 8.1% increase in complication rate for every 10 HU decrease in muscle density [20]. Wan and co-workers found that each 1cm2/m2 increase in skeletal muscle index decreased the odds of severe morbidity by 4.8% following radical cystectomy [26].
When evaluating pancreatectomy for adenocarcinoma, Joglekar and co-workers utilized two methods for quantification of sarcopenia (Fig. 1). The complications were graded according to Common Toxicity for Adverse Events or the International Study Group for Pancreatic Surgery where applicable. The TPI only predicted length of hospital stay on multivariate analysis. However, the HUAC, a measure of muscle quality, was also an independent predictor of length of stay and ICU admission, but also of grade 3 complications, overall complications, delayed gastric emptying, and infectious, gastrointestinal, and cardiopulmonary complications. Survival was not found to be different based on the TPI or HUAC in this study. The authors concluded that not only muscle mass, but muscle quality is an important variable in assessment of sarcopenia that should be considered when evaluating patients for pancreatectomy.
Sarcopenia was a predictive of early operative mortality in two studies. Reisinger and co-workers reported that the 30-day mortality was 8.8% in patients with sarcopenia versus 0.7% in non-sarcopenic patients [14]. Similarly, Pstuka and co-workers reported a trend towards an increase in 90-day all-cause mortality in those patients with sarcopenia (7.8% vs. 1.6%, P = 0.07) [25]. One study evaluated sarcopenia and sarcopenic obesity in patients undergoing partial hepatectomy for colorectal liver metastasis, and found that the presence of neither clinical scenario impacted survival or complications following resection [43]. This study, among others, also highlighted the importance of further research in this area along with need for uniform definitions of reporting, including more clinical data on malnutrition, fitness, and frailty [14,44].
Impact of Sarcopenia on Postoperative Survival in Surgical Oncology Patients
Several studies evaluated the impact of sarcopenia on postoperative cancer-specific and overall survival and was shown to be independently associated with survival in several of the studies reviewed. Table IV highlights the differences in survival data observed between the sarcopenic and non-sarcopenic patients. Psutka and co-workers found on multivariate analysis that sarcopenia was associated with a >2 fold increased risk of death from bladder cancer (both overall and cancer-specific survival) [25]. The study by Peng and co-workers observed a 63% increased risk of death at 3 years in sarcopenic patients with pancreatic ductal adenocarcinoma [22]. Consistent with these findings, Sabel and co-workers found that with every 10 HU decrease in psoas muscle density there was a 28% decrease in disease-free survival [20]. The findings in these studies controlled for complications and other significant prognostic factors using multivariate analysis. Therefore, these findings demonstrate that sarcopenia is a significant prognostic factor for survival. Whether sarcopenia is a determinant or merely predictor associated with survival remains unknown, and future studies may help clarify the significance of this novel biomarker of complications and survival.
TABLE IV.
Impact of Sarcopenia on Postoperative Oncologic Outcomes in Surgical Oncology Patients
| Postoperative survival based on Sarcopenia status* |
||||
|---|---|---|---|---|
| Author (year) | Type of malignancy | Survival outcome measured | Sarcopenic (%) | Non-sarcopenic (%) |
| Psutka (2014) | Urothelial Bladder Cancer | 5-year overall survival | 39 | 70 |
| 5-year cancer specific survival | 49 | 72 | ||
| Miyamoto (2015) | Colorectal Cancer | 5-year overall survival | 68 | 85 |
| 5-year recurrence free survival | 56 | 79 | ||
| Harimoto (2013) | Hepatocellular Carcinoma | 5-year overall survival | 71 | 83.7 |
| 5-year recurrence free survival | 13 | 33.2 | ||
| Peng (2012) | Pancreatic Adenocarcinoma | 3-year survival (men) | 20.3 | 39.2 |
| 3-year survival (women) | 26.1 | 40.8 | ||
| Okumura (2015) | Pancreatic Adenocarcinoma | Mean survival time | 17.7 months | 33.2 months |
All survival differences shown had a P-value of <0.05.
DISCUSSION
Sarcopenia is a process of normal aging that can be exacerbated by the hypercatabolic state and inflammatory response caused by malignancy [4]. It is an objective, subclinical measure of patient frailty and nutritional status that can be used to gauge an individual's preoperative condition. Although numerous quantification methods exist, the preponderance of data were obtained from measurements derived from preoperative CT scans. Sarcopenia has been previously studied in the context of cancer, surgery, and surgical oncology [35]. Herein is a systematic review and in depth analysis of studies investigating the impact of sarcopenia on outcome following surgical resection of cancer. The studies demonstrated that sarcopenia is an independent prognostic factor for both complications and survival following surgical resection of known malignancy.
The prognostic value of sarcopenia on postoperative complications and survival is clinically relevant as it can be objectively and reliably measured and is a potentially modifiable risk factor. While a standard first line therapy for remediating sarcopenia has not yet been identified, several studies have suggested potential interventions. Commonly proposed strategies include a combination of high-protein nutritional support, early physical therapy, and alternative muscle stimulation for the non-ambulatory population [8,45]. The University of Michigan created the Michigan Surgical Home and Optimization Program motivated by their research in sarcopenia. More than 350 patients were enrolled in their preoperative training program that included physical exercise, cessation of smoking, stress reduction, nutritional support, and daily spirometer exercises. The preliminary results note a 2-day reduction in postoperative length of stay and a reduction in cost for both the payer and hospital [28]. Determining a patient's extent of sarcopenia can also alter the management strategy of a cancer patient. The decision to pursue adjuvant versus neoadjuvant therapy may be influenced by the potential to improve the patient's fitness preoperatively. In addition, it allows for improved selection of surgical candidates and better risk stratification for expected postoperative outcomes.
The findings in this study are consistent with the conclusions in the review by Gibson and co-workers [46], which evaluated the available evidence on the role of CT scans in both the identification of sarcopenia in patients with abdominal malignancies as well as the predictive value of body composition analysis in clinical outcomes. They concluded that CT scans can identify reduced muscle mass and predict negative cancer outcomes in patients with abdominal malignancies [46]. The current review included all patients undergoing surgical intervention for cancer and was not limited to abdominal malignancies. The primary outcomes evaluated were postoperative complications and survival. In contrast, Gibson and co-workers evaluated for the incidence of sarcopenia determined by CT scan, clinical outcomes, and the incidence of malnutrition established by traditional methods of nutritional assessment and therefore are complimentary reports [46].
The clinical applicability of this review encompasses patients with known malignancy undergoing surgical resection. Tumors assessed in this review included multiple solid tumors of many sites included colorectal cancer, liver metastasis, esophageal cancer, hepatocellular carcinoma, melanoma, pancreatic cancer, and bladder cancer. Given the unique qualities of each cancer, it is impossible to conjecture whether sarcopenia will have the same prognostic value in all types of malignancy. Further, nutritional rehabilitation may be more readily applied preoperatively in those patients who are found to be sarcopenic. Ultimately, the ability to modify a patient's risk factors for poor outcomes improves the opportunity for improved patient care.
The primary limitation of this review was that the methodology used to quantify sarcopenia was not consistent across the studies. This necessarily results in variability in the reported results. The publications measured different musculature at different spinal levels, used diverse muscle indices, and had varied sarcopenia cut off values. In addition, a majority of the manuscripts were based on single center studies; introducing inherent bias in patient selection, surgical technique, postoperative management, and complications reporting. Most studies were conducted retrospectively leading to increased selection bias, information bias, and variability in assessing survival data. Nevertheless, the consistency of the data demonstrates the importance of sarcopenia in this patient population.
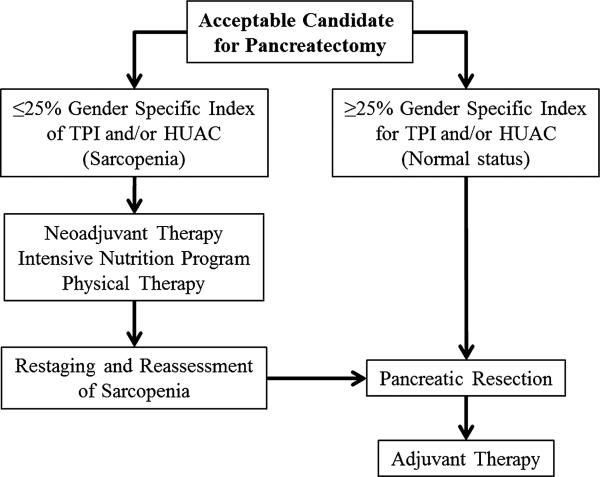
So what should the Surgical Oncologist do with these data moving forward? A theoretical algorithm for the sarcopenic patient is shown in Figure 2. The use of an intervention program would be an ideal option for patients with severe sarcopenia. The data presented herein are convincing enough to support a novel, tailored approach to these patients. This example is a patient with resectable pancreatic head adenocarcinoma who has been deemed an operative candidate and yet has severe sarcopenia. Several studies have shown a worse survival outcome and an increase in postoperative complications based on body composition measurement in patients with pancreatic adenocarcinoma, regardless of stage or treatment modality [21,22,27,47,48]. This raises the question of how to proceed with patients who have sarcopenia. The proposed algorithm is not evidence-based, but theoretically shows an option for patients with resectable disease who are operative candidates yet who have sarcopenia (Fig. 2). Informed consent is improved with the knowledge that a patient has sarcopenia, but it would be better to use these data to improve the patient outcome rather than just inform the patient of the risk for complications. With more centers publishing on improved outcomes with neoadjuvant therapy for pancreatic adenocarcinoma, this may be an option for the sarcopenic patient [49,50]. It is possible that sarcopenic patients may not tolerate therapy as well as non-sarcopenic patients, but this is not known. Ideally, the neoadjuvant therapy could be combined with an intensive program of nutrition and exercise, followed by restaging and reassessment of sarcopenia [28,51,52].
Fig. 2.
Theoretical algorithm for a patient deemed to have resectable pancreatic head adenocarcinoma. A patient with a resectable pancreatic head adenocarcinoma deemed to be an operative candidate with sarcopenia is at significant increased risk for complications and death following resection. An alternative treatment algorithm for initiation of systemic therapy along with a protocol to improve nutrition and fitness preoperatively is proposed. This protocol is theoretical and is not supported by level I evidence. TPI, Total Psoas Index; HUAC, Hounsfield Unit Average Calculation.
For future studies, it would be valuable to have a universal method for quantifying sarcopenia and determining standardized cutoff values that can be reliably reproduced across institutions. A majority of studies have defined sarcopenia as a dichotomous variable, but it can also be utilized as a continuous variable to optimize the cutoffs for each individual study. The use of a standardized gender specific cutoff value would potentially reduce bias across studies but may not be practical due to the heterogeneity of imaging modalities, patients, and cancer subtypes.
In addition to imaging measurements, the European Consensus Definition enlists the criteria for the diagnosis of sarcopenia as the presence of low muscle mass and one of the following—low muscle strength or low physical performance [1]. This is a critical aspect of the evaluation of patients that must be considered. Future prospective studies may more accurately assess sarcopenia by utilizing both imaging and clinical data, such as frailty [44]. Therefore, clinical data combined with imaging criteria for sarcopenia should be combined to guide patient selection for treatment. There is also a need for the development of a therapeutic strategy to improve the extent of a patient's sarcopenia. If a preoperative protocol were developed, prospective studies analyzing patients in treatment versus control arms might determine whether treating sarcopenia alters a patient's postoperative clinical outcome.
CONCLUSIONS
Sarcopenia is an independent prognostic factor for complications and survival following surgical resection of malignancy. It is a subclinical objective measure of skeletal muscle loss that can be easily quantified using preoperative CT scans. Given its significant prognostic value, it can be used to alter the clinical management of sarcopenic cancer patients. Introducing an intensive perioperative nutritional and exercise program is not novel. However, quantifiable measurements, like sarcopenia, have not been available to guide programs in an evidence-based fashion. This valuable clinical information may help improve the assessment of a patient's preoperative caliber, selection for surgical resection, and the determination of timing of multimodality therapy.
Acknowledgments
Funding and support: none.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairchild B, Webb TP, Xiang Q, et al. Sarcopenia and frailty in elderly trauma patients. World J Surg. 2015;39:373–379. doi: 10.1007/s00268-014-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y, Karvellas CJ, Baracos V, et al. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014;156:521–527. doi: 10.1016/j.surg.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Prado CM, Wells JC, Smith SR, et al. Sarcopenic obesity: A critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Chung JY, Kang HT, Lee DC, et al. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch Gerontol Geriatr. 2013;56:270–278. doi: 10.1016/j.archger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Ormsbee MJ, Prado CM, Ilich JZ, et al. Osteosarcopenic obesity: The role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle. 2014;5:183–192. doi: 10.1007/s13539-014-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3:269–275. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 8.Roubenoff R, Hughes VA. Sarcopenia: Current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–M724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 9.Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 10.Antoun S, Baracos VE, Birdsell L, et al. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21:1594–1598. doi: 10.1093/annonc/mdp605. [DOI] [PubMed] [Google Scholar]
- 11.Dodson RM, Firoozmand A, Hyder O, et al. Impact of sarcopenia on outcomes following intra-arterial therapy of hepatic malignancies. J Gastrointest Surg. 2013;17:2123–2132. doi: 10.1007/s11605-013-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoun S, Birdsell L, Sawyer MB, et al. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: Results from a placebo-controlled study. J Clin Oncol. 2010;28:1054–1060. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22:2663–2668. doi: 10.1245/s10434-014-4281-6. [DOI] [PubMed] [Google Scholar]
- 14.Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 15.Sabel MS, Terjimanian M, Conlon AS, et al. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J Surg Oncol. 2013;108:169–175. doi: 10.1002/jso.23366. [DOI] [PubMed] [Google Scholar]
- 16.Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. doi: 10.1111/j.1477-2574.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ida S, Watanabe M, Yoshida N, et al. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4559-3. PMID 25862583. [DOI] [PubMed] [Google Scholar]
- 18.Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 19.Iritani S, Imai K, Takai K, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. 2015;50:323–332. doi: 10.1007/s00535-014-0964-9. [DOI] [PubMed] [Google Scholar]
- 20.Sabel MS, Lee J, Cai S, et al. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann Surg Oncol. 2011;18:3579–3585. doi: 10.1245/s10434-011-1976-9. [DOI] [PubMed] [Google Scholar]
- 21.Joglekar S, Asghar A, Mott SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adeno-carcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088–1098. doi: 10.1016/j.surg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Smith AB, Deal AM, Yu H, et al. Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol. 2014;191:1714–1720. doi: 10.1016/j.juro.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 25.Psutka SP, Carrasco A, Schmit GD, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer. 2014;120:2910–2918. doi: 10.1002/cncr.28798. [DOI] [PubMed] [Google Scholar]
- 26.Wan F, Zhu Y, Gu C, et al. Lower skeletal muscle index and early complications in patients undergoing radical cystectomy for bladder cancer. World J Surg Oncol. 2014;12:14. doi: 10.1186/1477-7819-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 28.Friedman J, Lussiez A, Sullivan J, et al. Implications of sarcopenia in major surgery. Nutr Clin Pract. 2015;30:175–179. doi: 10.1177/0884533615569888. [DOI] [PubMed] [Google Scholar]
- 29.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 30.Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JS, Terjimanian MN, Tishberg LM, et al. Surgical site infection and analytic morphometric assessment of body composition in patients undergoing midline laparotomy. J Am Coll Surg. 2011;213:236–244. doi: 10.1016/j.jamcollsurg.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Englesbe MJ, Terjimanian MN, Lee JS, et al. Morphometric age and surgical risk. J Am Coll Surg. 2013;216:976–985. doi: 10.1016/j.jamcollsurg.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 35.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 36.Baracos VE, Reiman T, Mourtzakis M, et al. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 37.Tyagi M, Bukrinsky M. Human immunodeficiency virus (HIV) latency: The major hurdle in HIV eradication. Mol Med. 2012;18:1096–1108. doi: 10.2119/molmed.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Vugt JL, Braam HJ, van Oudheusden TR, et al. Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4429-z. PMID 25672564. [DOI] [PubMed] [Google Scholar]
- 39.Grobmyer SR, Pieracci FM, Allen PJ, et al. Defining morbidity after pancreaticoduodenectomy: Use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–364. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Mezhir JJ. Management of complications following pancreatic resection: An evidence-based approach. J Surg Oncol. 2013;107:58–66. doi: 10.1002/jso.23139. [DOI] [PubMed] [Google Scholar]
- 42.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 43.Lodewick TM, van Nijnatten TJ, van Dam RM, et al. Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB (Oxford) 2015;17:438–446. doi: 10.1111/hpb.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: A prospective cohort study. Ann Surg. 2014;259:960–965. doi: 10.1097/SLA.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanna JS. Sarcopenia and critical illness: A deadly combination in the elderly. JPEN J Parenter Enteral Nutr. 2015;39:273–281. doi: 10.1177/0148607114567710. [DOI] [PubMed] [Google Scholar]
- 46.Gibson DJ, Burden ST, Strauss BJ, et al. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: A systematic review. Eur J Clin Nutr. 2015:18. doi: 10.1038/ejcn.2015.32. [DOI] [PubMed] [Google Scholar]
- 47.Aslani A, Gill AJ, Roach PJ, et al. Preoperative body composition is influenced by the stage of operable pancreatic adenocarcinoma but does not predict survival after Whipple's procedure. HPB (Oxford) 2010;12:325–333. doi: 10.1111/j.1477-2574.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wigmore SJ, Plester CE, Richardson RA, et al. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997;75:106–109. doi: 10.1038/bjc.1997.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadot E, Doussot A, O'Reilly EM, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4647-4. PMID 26065868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arvold ND, Ryan DP, Niemierko A, et al. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer. 2012;118:3026–3035. doi: 10.1002/cncr.26633. [DOI] [PubMed] [Google Scholar]
- 51.Collins JT, Noble S, Chester J, et al. Association of sarcopenia and observed physical performance with attainment of multidisciplinary team planned treatment in non-small cell lung cancer: An observational study protocol. BMC Cancer. 2015;15:544. doi: 10.1186/s12885-015-1565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paddon-Jones D, R asmussen BB Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]