Abstract
Schizophrenia is a chronic and debilitating neuropsychiatric disorder affecting approximately 1% of the world’s population. This disease is associated with considerable morbidity placing a major financial burden on society. Antipsychotics have been the mainstay of the pharmacological treatment of schizophrenia for decades. The traditional typical and atypical antipsychotics demonstrate clinical efficacy in treating positive symptoms, such as hallucinations and delusions, while are largely ineffective and may worsen negative symptoms, such as blunted affect and social withdrawal, as well as cognitive function. The inability to treat these latter symptoms may contribute to social function impairment associated with schizophrenia. The dysfunction of multiple neurotransmitter systems in schizophrenia suggests that drugs selectively targeting one neurotransmission pathway are unlikely to meet all the therapeutic needs of this heterogeneous disorder. Often, however, the unintentional engagement of multiple pharmacological targets or even the excessive engagement of intended pharmacological targets can lead to undesired consequences and poor tolerability. In this article, we will review marketed typical and atypical antipsychotics and new therapeutic agents targeting dopamine receptors and other neurotransmitters for the treatment of schizophrenia. Representative typical and atypical antipsychotic drugs and new investigational drug candidates will be systematically reviewed and compared by reviewing structure-activity relationships, pharmacokinetic properties, drug metabolism and safety, pharmacological properties, preclinical data in animal models, clinical outcomes and associated side effects.
Keywords: Keywords: Antipsychotic, Aripiprazole, Clozapine, Dopamine, Haloperidol, ITI-007, Risperidone, Schizophrenia.
1. INTRODUCTION
1.1. Schizophrenia
Schizophrenia is a chronic neuropsychiatric illness affecting approximately 1% of the global population [1, 2]. Individuals with the disorder may experience hallucinations, delusions, suspiciousness, and conceptual disorganization among other so called positive symptoms. These positive symptoms can be accompanied by social withdrawal, blunted affect, emotional withdrawal and asociality, collectively referred as negative symptoms. Cognitive impairment is also a core feature of schizophrenia [3]. Together with other residual symptoms including depression, the broad array of symptoms associated with schizophrenia results in significant impairment of social function with the inability to fully integrate into one’s family and into the workplace. Although the etiology and pathology of schizophrenia remain largely un-resolved [4-6], dopamine system dysfunction clearly contributes to the pathophysiology of this disorder [7-9]. Subsequent research suggests that the serotonergic pathway also plays an important role [10]. Recent molecular genetic studies conducted by an international schizophrenia consortium strongly support the fact that schizophrenia is a polygenic disease [11].
1.2. Current Treatments for Schizophrenia
It is well accepted that the positive symptoms of schizophrenia are associated with hyperdopaminergic neurotransmission in the brain, particularly in the mesolimbic dopamine pathway, while the negative symptoms and cognitive deficits associated with schizophrenia may be caused by hypodopaminergic activity in the mesocortical pathway [12-16]. Dopamine D2 receptor antagonists, such as chlorpromazine and haloperidol, have demonstrated clinical efficacy in the reduction of positive symptoms. However, these first generation antipsychotics are ineffective and may exacerbate negative symptoms and cognitive deficits associated with schizophrenia. As detailed below, they bind to a number of receptor systems, many of which contribute to serious side effects. Although modulating dopamine neurotransmission
has been a dominant therapeutic approach, findings from both clinical and preclinical research have suggested that dysregulation of other neurotransmitter systems including serotonin, glutamate, gamma-aminobutyric acid (GABA) and acetylcholine also contribute to the pathophysiology of schizophrenia [17]. The dysfunction of multiple neurotransmitter systems in schizophrenia indicated that drugs selectively targeting one neurotransmission pathway are unlikely to meet the therapeutic needs of this heterogeneous disorder. Treatment involving the modulation of multiple targets may be more effective to address social dysfunction as well as positive symptoms associated with schizophrenia. In this article, we will review marketed typical and atypical antipsychotics as well as novel therapeutic agents targeting dopamine D2 receptors and other neurotransmitters systems for the treatment of schizophrenia. The structure-activity relationships (SAR) and receptor binding profiles of these compounds will be discussed. Representative compounds from each generation of antipsychotics will be compared in various aspects including pharmacological profiles, efficacy in behavioral models, clinical outcomes and associated side effects.
2. DEVELOPMENT OF ANTIPSYCHOTICS FOR THE TREATMENT OF SCHIZOPHRENIA
Since the first antipsychotic, chlorpromazine (1), was introduced in the 1950s [18], many other antipsychotic drugs have been discovered and marketed during the past six decades. While the chemical structures of these antipsychotics are quite diverse, they all have somewhat similar pharmacological action, mainly dopamine D2 receptor blockade. These compounds generally can be classified as “typical” and “atypical” antipsychotics based upon both pattern of clinical effects and mechanism of action [19, 20]. The typical antipsychotic drugs, represented by haloperidol (2) and chlorpromazine (1), are also called the first generation antipsychotics (FGA) or neuroleptics. The FGAs are effective at reducing positive symptoms associated with schizophrenia, but are largely limited by extrapyramidal motor side effects (EPS), hyperprolactinemia and cognitive dulling. These adverse effects are likely mediated by high dopamine D2 receptor occupancy. The serendipitous discovery of compounds such as clozapine (3) defined a new generation of antipsychotic medications, referred to as the atypical antipsychotics. The atypical antipsychotics, including clozapine (3) and risperidone (4), are considered as the second generation antipsychotics (SGA). Serotonin 5-HT2A receptor antagonism in combination with D2 receptor antagonism is thought to be the hallmark pharmacology of the SGAs. The SGAs have reduced EPS liability compared to the FGAs, but can be associated with increased weight gain and metabolic burden mediated by unintended off-target pharmacological interactions [21]. Many are still associated with high rates of akathisia even though rates of parkinsonism have been reduced in some compounds. Clozapine, arguably the most effective antipsychotic, has a myriad of safety issues that precludes it from being used as a first-line therapy. The more recently marketed antipsychotics with D2 receptor partial agonist effects rather than D2 antagonism, as exemplified by aripiprazole (5) and brexpiprazole (6), are still considered as atypical antipsychotics, though these drugs were marketed as the third generation antipsychotics. Presynaptic partial agonism at D2 receptors has allowed for a further reduction in EPS and hyperprolactinemia, but postsynaptic partial agonism at D2 receptors has been associated with relatively high levels of akathisia and more recently with uncontrollable pathological urges to gamble, binge eat, shop and have sex [22]. Currently, new investigational drugs with novel mechanisms are being developed in order to identify better therapeutic agents that can treat not only the positive symptoms of schizophrenia but also enhance social function and improve safety and tolerability. For instance, ITI-007 (7) represents a first-in-class small molecule therapeutic agent interacting with serotonergic, dopaminergic and glutamatergic neurotransmitter targets in a complex, unique and regionally selective manner [23, 24]. We will briefly review the first and second generation antipsychotics since these antipsychotics have been reviewed extensively [25-27]. Newer antipsychotic drug candidates targeting dopamine along with other neurotransmitter systems will be discussed in detail. Representative antipsychotic drugs, haloperidol (2), clozapine (3), risperidone (4), aripiprazole (5), and ITI-007 (7), representing a new investigational drug for schizophrenia, will be systematically reviewed.
2.1. Haloperidol and Other First-Generation Antipsychotics (FGA)
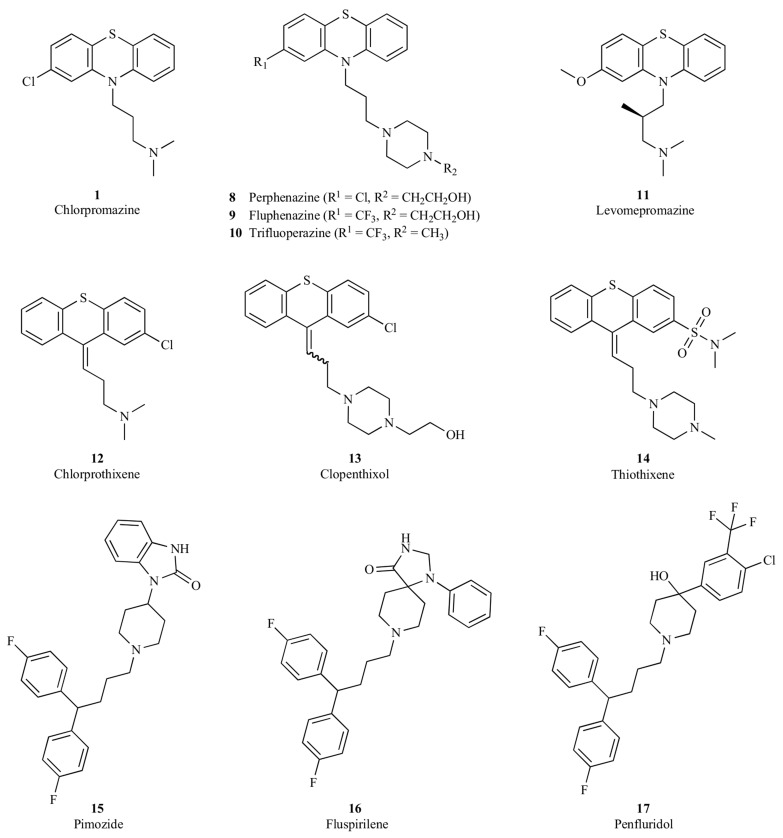
Typical antipsychotics exert their action predominantly through dopamine D2 receptor antagonism. As exemplified in (Fig. 1), these first generation antipsychotics may be generally categorized into several chemical classes, including (a) phenothiazines, such as chlorpromazine (1) [28, 29], perphenazine (8) [30, 31], fluphenazine (9) [32, 33], trifluoperazine (10) [34, 35] and levomepromazine (11) [36], (b) thioxanthenes, such as chlorprothixene (12) [37, 38], clopenthixol (13) [39] and thiothixene (14) [40], and (c) diphenylbutylpiperidines, such as pimozide (15) [41, 42], fluspirilene (16) [43] and penfluridol (17) [44].
Fig. (1).
Representative first-generation antipsychotics from the phenothiazine, thioxanthene and diphenylbutylpiperidine series.
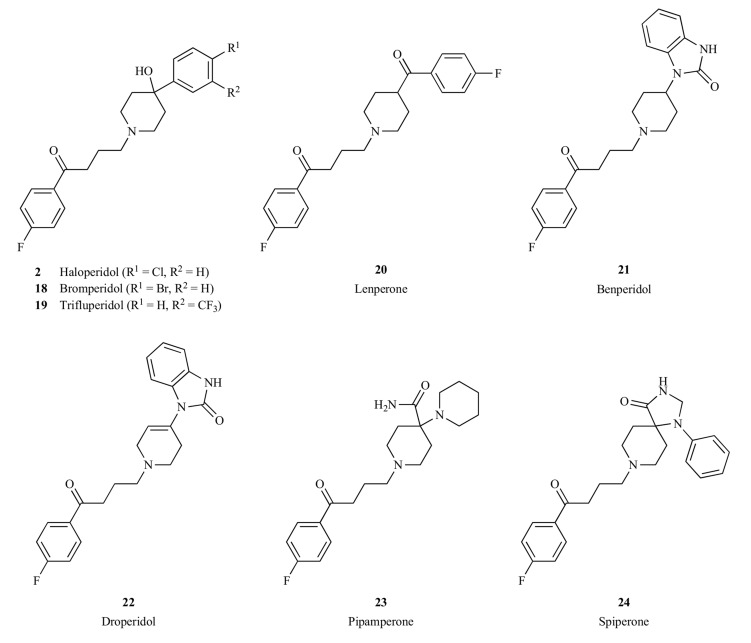
Butyrophenones are another important class of compounds with antipsychotic activities. Haloperidol (2) is a well-known typical antipsychotic drug that belongs to this chemical class [45, 46]. Other drugs, such as bromperidol (18) [47, 48], trifluperidol (19) [49], lenperone (20) [50], benperidol (21) [51, 52], droperidol (22) [53], pipamperone (23) [54, 55] and spiperone (24) [56] are also from this family (Fig. 2 ). As summarized in Table 1, these butyrophenones have different receptor binding profiles. Each of these compounds exhibited different clinical efficacy. For instance, bromperidol (18) possesses a dopamine D2 receptor binding affinity similar to that of haloperidol, yet it has an apparent elimination half-life of approximately 24 h, supporting a once-daily dose regimen [57]. Trifluperidol (19) is a more potent neuroleptic drug than haloperidol and has been studied in withdrawn and autistic patients with schizophrenia. Benperidol (21) and spiperone (24) are two of the most potent antipsychotic drugs in the butyrophenone family, though not approved for use in the United States. Spiperone has shown efficacy in treating drug-resistant schizophrenia [58]. Droperidol is a short-acting neuroleptic drug with pronounced antiemetic and anti-shock properties [59], though it is not used for the treatment of schizophrenia due to its short duration of action. Pipamperone is generally classified as a
Fig. (2).
Representative first-generation antipsychotics from the butyrophenone chemical class.
Table 1.
Receptor binding affinity of the first generation antipsychotics in the butyrophenones chemical class.
|
Receptor
Ki (nM) 1 |
Haloperidol (2) | Bromperidol (18) | Trifluperidol (19) | Benperidol (21) | Droperidol (22) | Pipamperone (23) | Spiperone (24) |
|---|---|---|---|---|---|---|---|
| D2 | 2.0 | 2.1 | 0.4 | 0.027 | 0.25 | 120 | 0.053 |
| D1 | 83 | 600 | 740 | 4100 | 880 | 4900 | 577 |
| D3 | 4.0 | 2.3 | 4.2 | NA | NA | 250 | 0.28 |
| D4 | 15 | 48 | 326 | 0.066 | 0.84 | 5.1 | 1.4 |
| D5 | 147 | NA2 | NA | NA | NA | NA | 4500 |
| 5-HT1A | 1200 | NA | NA | NA | NA | 2770 | 209 |
| 5-HT2A | 70 | 26 | 5.4 | 3.7 | 4.6 | 5.4 | 1.41 |
| 5-HT2C | 5000 | NA | NA | NA | NA | 227 | 1108 |
| α1 adrenergic | 12 | NA | NA | NA | NA | 66 | 25 |
| H1 histaminergic | 3000 | NA | NA | NA | NA | 2400 | 476 |
| M1 muscarinic | >10000 | 7600 | NA | NA | NA | NA | NA |
1Receptor binding affinities were obtained from the NIMH Psychoactive Drug Screening Program (PDSP) Database [61].2 NA stands for not available.
first-generation typical antipsychotic, yet it possessed prominent serotonin 5-HT2A binding affinity (Table 1), and was considered as a forerunner of atypical antipsychotics [60].
Among these typical antipsychotics, haloperidol (2) is the most commonly used, so its properties will be extensively reviewed in this article and compared with representative examples of newer generations of antipsychotics. Haloperidol was first synthesized by Bert Hermans at the Janssen Laboratories in Belgium in February, 1958. It was given the generic name of haloperidol because of the two halogenated substituents incorporated into the molecule [62]. Under the brand name Haldol®, haloperidol was marketed in Belgium in 1959, and later marketed in United States and other countries. It is currently on the World Health Organization (WHO) Model List of Essential Medicines [63]. This compound preferentially binds to dopamine and α1-adrenergic receptors with negligible affinity for serotonin 5-HT2C, histamine H1 and muscarinic M1 receptors (Table 1), which are thought to be associated with adverse effects of marketed antipsychotics [64-66].
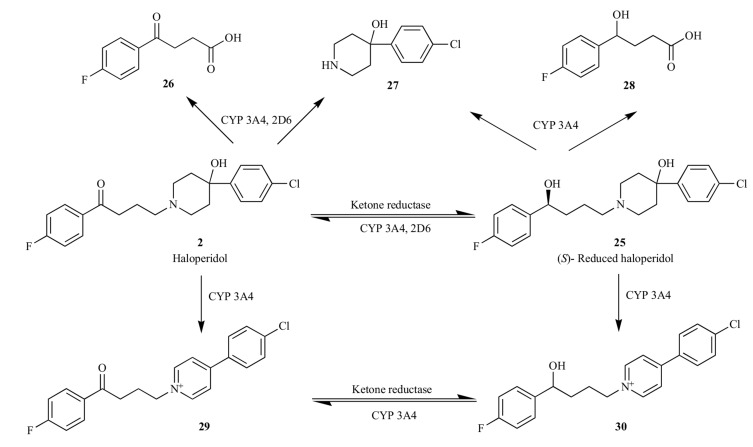
Haloperidol is extensively metabolized in the liver. In human, the compound primarily undergoes glucuronidation [67], ketone reduction to stereoselectively generate the (S)-enantiomer of reduced haloperidol (25) via a ketone reductase [68], and N-dealkylation to give dealkylated metabolites (26 – 28) via cytochrome P450 3A4 and 2D6 [69, 70]. The reduced haloperidol (25) can also be back converted to haloperidol via CYP 3A4, as shown in Fig. (3) [71, 72]. It has been suggested that haloperidol (2) is also subjected to cytochrome P450 mediated metabolism to form pyridinium metabolites (29 and 30), structural analogues of MPP+ (1-methyl-4-phenylpyridinium) [73-75]. In psychiatric patients treated with haloperidol chronically, the severity of tardive dyskinesia and parkinsonism appears to be associated with an increased ratio of pyridinium (28) to haloperidol [76]. To prevent the formation of pyridinium metabolites, new chemical series of antipsychotic agents designed based upon
Fig. (3).
Primary metabolic pathways of haloperidol (2). The glucuronidation of haloperidol catalyzed by uridine 5'-diphospho-glucuronosyltransferase (UGT) was not shown in this Figure.
the haloperidol scaffold have been reported in recent years [77, 78].
2.2. Clozapine, Risperidone, Aripiprazole and other Second-Generation Antipsychotics (SGA)
Unlike the typical antipsychotics, which preferentially block dopamine D2 receptors, the second-generation antipsychotic drugs not only reduce dopamine neurotransmission, but also act on serotonin receptors, especially 5-HT2A receptors and typically as antagonists [79]. Biochemical, electrophysiological and behavioral studies have shown that 5-HT2A receptor antagonists have antipsychotic-like activity [80]. The highly selective 5-HT2A antagonist, MDL-100907 (31) exhibited antipsychotic activity in several preclinical animal models [81, 82], but it failed, like other selective 5-HT2A antagonists, to exhibit sufficient efficacy in clinical trials to be approved for the treatment of schizophrenia [83]. To achieve better efficacy, blockade of both serotonin 5-HT2A and dopamine D2 receptors is warranted at clinically effective doses. There is now considerable preclinical and some clinical evidence that effects on 5-HT receptors contribute to the low risk of producing EPS, which is the defining characteristic of the atypical antipsychotics, compared to typical antipsychotics [84, 85].
Importantly, the data suggest that 5-HT2A receptor antagonism potentiates mesolimbic D2 receptor antagonist-mediated efficacy, but does not alter nigrostriatal D2 receptor antagonist-mediated motor side effects. Ritanserin, a 5-HT2A/C receptor antagonist, enhanced raclopride-induced dopamine concentrations in the medial prefrontal cortex and raclopride-induced increases in accumbal dopamine signal, but not in the striatum [86]. Ritanserin potentiated raclopride’s antipsychotic-like efficacy without increasing raclopride-induced catalepsy [87]. Similarly, pimavanserin (ACP-103), a 5-HT2A receptor inverse agonist, potentiated haloperidol’s antipsychotic-like efficacy in animal models, but did not increase haloperidol-induced catalepsy [88]. Pimavanserin was also shown to significantly decrease haloperidol- or risperidone-induced hyperprolactinemia in animal models [88]. As adjunctive to antipsychotics in patients with schizophrenia, pimavanserin enhanced the efficacy of a low subtherapeutic dose of risperidone without increasing motor side effects, but did not enhance the efficacy of a low therapeutic dose of haloperidol [89]. Pimavanserin is not approved for use in schizophrenia. To date, no selective 5-HT2A receptor antagonists or inverse agonists have shown convincing antipsychotic efficacy as a monotherapy for the treatment of schizophrenia, but the pharmacological mechanism of blocking 5-HT2A receptors is thought to play an important role, together with D2 receptor blockade, in the efficacy of atypical antipsychotics.
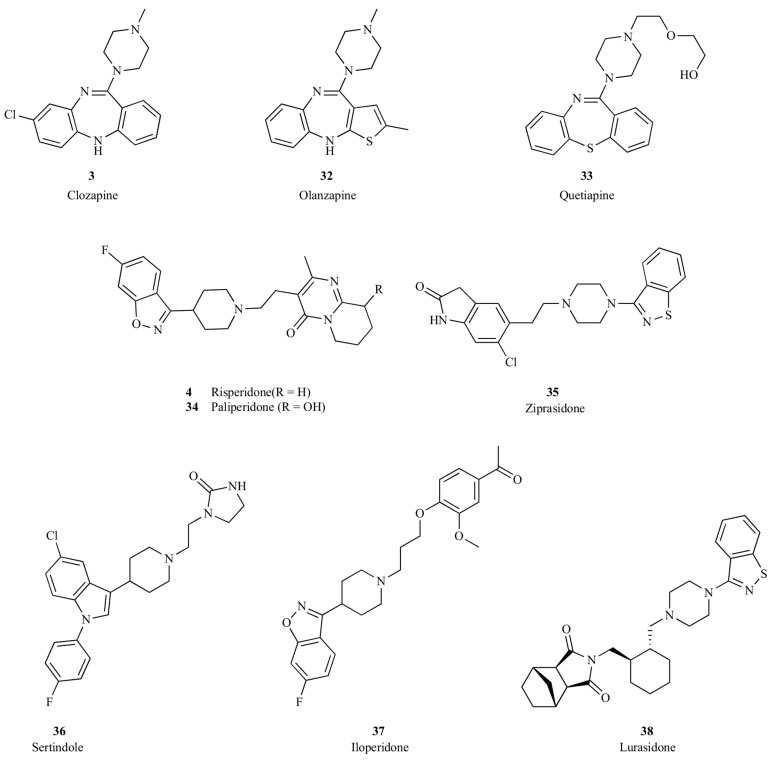
The serendipitous discovery of clozapine (3) in the 1960s opened the second major chapter in the pharmacological treatment of schizophrenia. Clozapine exerted antipsychotic effects in humans with a markedly reduced risk of EPS or hyperprolactinemia at efficacious doses. This profile was sufficiently different from the first generation of antipsychotics that clozapine became the prototype of the so-called atypical antipsychotic drugs. A number of second-generation antipsychotics were developed based upon clozapine, such as risperidone (4), olanzapine (32), quetiapine (33), paliperidone (34), ziprasidone (35), sertindole (36), iloperidone (37) and lurasidone (38), as shown in Fig. (4). These atypical antipsychotics have somewhat different pharmacological profiles, yet they all have antagonistic activity on dopaminergic D2 and serotoninergic 5-HT2A receptors. These atypical antipsychotics can be divided mechanistically into those that bind to multiple other neuroreceptors including modest affinity to D2 and 5-HT2A, such as clozapine, olanzapine and quetiapine, and those that exhibit potent D2 and 5-HT2A antagonistic activities, such as risperidone, paliperidone, sertindole and lurasidone, as summarized in Table 2. Among these SGA, clozapine (3) and risperidone (4) are the most widely used, and so these two antipsychotics were selected for extensive review and comparison with other generations of antipsychotic drugs.
Fig. (4).
Clozapine, risperidone and other second-generation antipsychotic drugs.
Table 2.
Receptor Binding Affinity of Representative Second-Generation Antipsychotic Drugs.
|
Receptor
Ki (nM)1 |
Clozapine
(3) |
Olanzapine (32) | Quetiapine (33) | Risperidone (4) | Paliperidone (34) | Sertindole (36) | Lurasidone 3 (38) |
|---|---|---|---|---|---|---|---|
| D2 | 144 | 21 | 245 | 4.9 | 2.8 | 2.7 | 1.0 |
| D1 | 189 | 58 | 1277 | 147 | 41 | 12 | 262 |
| D3 | 270 | 49 | 240 | 3.6 | 6.9 | 2.5 | 15.7 |
| D4 | 39 | 14 | 2000 | 4.4 | 54 | 9.0 | 29.7 |
| D5 | 235 | 90 | 1738 | 563 | 29 | NA 2 | NA |
| 5-HT1A | 105 | 2063 | 431 | 427 | 638 | 280 | 6.4 |
| 5-HT2A | 5.2 | 2.65 | 135 | 0.17 | 1.2 | 0.28 | 0.47 |
| 5-HT2C | 10.7 | 14 | 1184 | 12 | 48 | 0.90 | 415 |
| α1A adrenergic | 1.6 | 109 | 22 | 5.0 | 2.5 | 1.8 | NA |
| α1B adrenergic | 7.0 | 263 | 39 | 9.0 | 0.70 | NA | NA |
| H1 histaminergic | 2.0 | 4.9 | 7.5 | 15 | 5.6 | 130 | >1000 |
| M1 muscarinic | 14 | 24 | 120 | >10,000 | >10,000 | NA | >1000 |
Clozapine (3) has a very rich pharmacology, targeting a wide range of receptors including adrenergic, muscarinic, histaminergic, dopaminergic and serotonergic receptors. Clozapine was developed by Sandoz in 1961 and first introduced in Europe in the 1970s. It was later withdrawn from the market after reports of clozapine-induced agranulocytosis that led to death in some patients [90]. Clozapine is known to cause weight gain and metabolic disturbances and is also associated with an increased incidence of seizures and myocarditis [91]. Despite the tremendous safety burden, clozapine is arguably the most efficacious antipsychotic drug and was reintroduced into the US market in 1990, and used only for treatment-resistant schizophrenia [92, 93]. Clozapine is eliminated by oxidation in the liver, predominantly by CYP1A2 [94, 95]. Smoking, a potent inducer of CYP1A2 enzyme activity, results in significantly lower clozapine serum concentrations in smokers compared with non-smokers [96]. N-Desmethylclozapine (norclozapine) and clozapine N-oxide are major metabolites of clozapine [97]. Studies have indicated that clozapine may be transformed by bioactivation to a chemically reactive nitrenium ion, which may play an important role in the pathogenesis of clozapine-induced agranulocytosis [98, 99]. Olanzapine (32) and clozapine (3) have very similar structures, and olanzapine was also found to be oxidized to a reactive nitrenium intermediate by hypochlorous acid (HOCl), which is a major oxidant produced by activated neutrophils. However, the incidence of agranulocytosis caused by olanzapine is much lower than that of clozapine [100]. Through the substitution of sulfur for the bridging nitrogen in the dibenzodiazepine-type antipsychotic clozapine, the dibenzothiazepine-type compound quetiapine (33) does not directly form a nitrenium ion when incubated with myeloperoxidase. However, quetiapine was found to be metabolized to 7-hydroxyquetiapine, which can subsequently be oxidized by human myeloperoxidase to form a reactive quinone-imine and a reactive radical. This drug metabolism was reported to lead to continued, although reduced, neutrophil toxicity [101].
Risperidone (4) represents another group of atypical antipsychotics. It has potent dopaminergic D2 and serotonergic 5-HT2A antagonistic activities with high affinity to adrenergic and histaminergic receptors. Risperidone was developed by Janssen-Cilag between 1988 and 1992 and was first approved by the FDA in 1993 for the treatment of schizophrenia in adults. Later, it was approved for the short-term treatment of acute manic or mixed episodes associated with bipolar disorder and the treatment of irritability associated with autistic disorder. It is currently on the WHO Model List of Essential Medicines, along with chlorpromazine (1), haloperidol (2), clozapine (3) and fluphenazine (9), for the treatment of mental and behavioral disorders [63]. The treatment-related adverse effects of risperidone, such as weight gain, orthostatic hypotension and sedation, are reported to be caused by the high binding affinity of the compound to 5-HT2C, adrenergic α1 and histaminergic H1 receptors, which will be discussed and compared with other antipsychotics in another section. Risperidone in humans is metabolized to 9-hydroxy risperidone (paliperidone (34)) by CYP2D6 and, to a lesser extent, CYP3A4 [104].
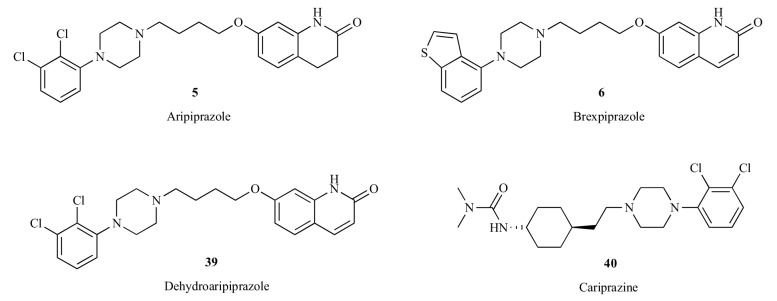
Aripiprazole (5) is a relatively new antipsychotic drug representing the third group of atypical antipsychotics [105, 106]. Unlike clozapine (3) and risperidone (4), which are pure antagonists of dopaminergic and serotonergic receptors, aripiprazole acts as a partial agonist at the serotonin 5-HT1A receptors and the pre- and postsynaptic dopamine D2 receptors, and as an antagonist at the serotonin 5-HT2A receptors [107-109]. It was marketed as the prototype of the third-generation of antipsychotics, the so-called dopamine system stabilizers [26, 110, 111]. Aripiprazole is primarily metabolized by CYP3A4 and CYP2D6, forming mainly an active metabolite, dehydroaripiprazole (39) [112]. The concentration of this active metabolite typically accumulated to about 40% of the parent compound concentration [113]. Brexpiprazole (6) may be considered as an analog of the dehydration metabolite of aripiprazole. The dichlorophenyl group substituted on the piperazinyl ring in dehydroaripiprazole (39) was replaced with a benzothiophenyl group in brexpiprazole (6), as shown in Fig (5). Brexpiprazole functions a partial agonist at pre- and post synaptic dopamine D2 and D3 receptors and is more potent at serotonin 5-HT1A (partial agonist) and 5-HT2A (antagonist) and adrenergic α1B (antagonist) receptors in comparison with aripiprazole (Table 3) [114, 115]. On July 10, 2015, brexpiprazole (Rexulti) was approved by FDA to treat adults with schizophrenia and as an add-on treatment to an antidepressant medication to treat adults with major depressive disorder (MDD) [116]. Cariprazine (40) is another pre- and post synaptic dopamine D2 and D3 partial agonist that was recently approved by FDA for the treatment of schizophrenia and bipolar disorder in adults [117]. It has a higher affinity for dopamine D3 receptors versus D2 receptors, and exhibited low affinity at human serotonin 5-HT2A receptors [118]. All three of these atypical antipsychotics contain characteristic (4-arylpiperazin-1-yl)alkyl groups. The differences and similarities of these drugs as dopamine receptor partial agonists have been systematically reviewed [119]. In this article, we will focus on the comparisons of aripiprazole (5), a representative antipsychotic drug from this class, with other classes of typical and atypical antipsychotics.
Fig. (5).
Aripiprazole, its metabolite and other second-generation antipsychotics acting as dopamine partial agonists.
Table 3.
Receptor binding profiles of aripiprazole (5) and other dopamine partial agonists as atypical antipsychotics.
| Receptor Ki (nM) 1 | Aripiprazole (5) 3 | Brexpiprazole (6) 4 | Cariprazine (40) 5 |
|---|---|---|---|
| D2 | 0.34 | 0.30 | 0.49 |
| D1 | 1960 | 160 | NA |
| D3 | 0.8 | 1.1 | 0.085 |
| D4 | 44 | 6.3 | NA 2 |
| D5 | 2590 | NA | NA |
| 5-HT1A | 1.7 | 0.12 | 2.6 |
| 5-HT2A | 3.4 | 0.47 | 18.6 |
| 5-HT2C | 15 | 34 | 135 |
| α1A adrenergic | 26 | 3.8 | 132 |
| α1B adrenergic | 35 | 0.17 | >1000 |
| H1 histaminergic | 61 | 19 | 23.4 |
| M1 muscarinic | 6780 | 67% inhibition at 10 μM | NA |
1Receptor binding affinities were obtained from the NIMH Psychoactive Drug Screening Program (PDSP) Database [61].2NA stands for not available. 3 Data from the cited literatures [108, 120]. 4 Data from the cited literatures [121, 122]. 5 Data calculated based upon the pKi reported in the cited literature [118].
2.3. ITI-007 and Other Investigational New Drugs Targeting Dopamine
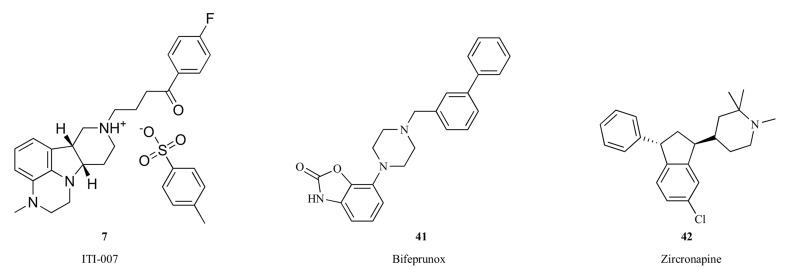
In addition to the approved antipsychotic drugs described above, a few new drug candidates targeting dopamine and other neuroreceptors, such as ITI-007 (7), bifeprunox (41), zicronapine (42), Lu AF35700 (43) and RP5063 (44), entered late stage clinical development for schizophrenia and other neuropsychiatric disorders.
ITI-007 (7) represents a new approach to the treatment of schizophrenia, targeting an improvement in social function in addition to antipsychotic efficacy and an associated highly favorable safety and tolerability profile. ITI-007 is a small molecule therapeutic agent interacting with serotonergic, dopaminergic and glutamatergic neurotransmitter targets in a complex, unique and regionally selective manner [23, 24]. ITI-007 is designed specifically to combine potent serotonin 5-HT2A receptor antagonism with modulation of phosphoprotein pathways downstream of dopamine receptors and with serotonin reuptake inhibition. ITI-007 has dual properties, acting as a post-synaptic antagonist and as a pre-synaptic partial agonist at dopamine D2 receptors in vivo with mesolimbic/mesocortical selectivity [23]. Though the effect of ITI-007 at pre-synaptic D2 receptors resembles that of aripiprazole [106, 107], the post-synaptic D2 interactions are different. Whereas aripiprazole is a partial agonist at pre-synaptic and post-synaptic receptors, ITI-007 is an antagonist at post-synaptic receptors. The structural features of the compound responsible for this unique interaction at D2 receptors for ITI-007 have not been defined. ITI-007 also indirectly modulates glutamatergic activity by increasing the phosphorylation of the NR2B (or GluN2B) subunit of N-methyl-D-aspartate (NMDA) channels in extrastriatal dopamine-rich brain regions (e.g. nucleus accumbens). The precise molecular pathway underlying this effect of ITI-007 has not been elucidated, though phosphorylation of NR2B at the tyrosine-1472 (Y1472) residue is known to be regulated through a pathway downstream of dopamine D1 receptor activation impacting Fyn kinase [123]. The spectrum of biochemical actions is referred to as dopamine receptor protein phosphorylation modulation (DPPM). The combination of ITI-007's high potency blockade of 5-HT2A receptors, efficient dopamine modulation, serotonin reuptake inhibition, and indirect enhancement of glutamatergic neurotransmission has been shown to yield antipsychotic efficacy without motor side effects or cardiometabolic safety issues. ITI-007 demonstrated a reduction of positive symptoms in patients with schizophrenia comparable to risperidone, but with significantly lower blood levels of biomarkers indicative of potential metabolic dysfunction (i.e., insulin, glucose, cholesterol and triglycerides) and prolactin [124]. ITI-007 was also associated with lower rates of motor side effects, such as akathisia, and cardiovascular side effects, such as tachycardia, than risperidone. Moreover, ITI-007 showed a greater efficacy in the improvement of negative symptoms and prosocial behavior than risperidone. The efficacy of ITI-007 for the reduction in psychosis and improvement in social function in patients with schizophrenia was confirmed in a phase III clinical trial [125].
Bifeprunox (41) is partial agonist of dopamine D2 and serotonin 5-HT1A receptors [126]. Activation of serotonin 5-HT1A receptors has been shown to reduce extrapyramidal symptoms in rodent models [127, 128]. Based upon data in rats, the balance of activity at 5-HT1A and D2 receptors may bring potential benefit to some of the negative symptoms of schizophrenia [129]. In a randomized, double-blind, placebo-controlled clinical study, 20 mg of bifeprunox demonstrated efficacy and produced a reduction in the positive and negative syndrome scale (PANSS) total score that was significantly different from placebo in patients with an acute exacerbation of schizophrenia [130]. However, this compound showed inadequate efficacy in the subsequent multinational phase III clinical trials [131], and so the development of bifeprunox has been terminated [132].
Zicronapine (42, Lu 31-130) exhibited potent antagonistic activities at dopamine D1, D2 and serotonin 5-HT2A receptors [133], and showed efficacy in patients with schizophrenia in phase II clinical studies [134, 135]. In 2014, Lundbeck removed zicronapine from its development portfolio due to the development of Lu AF35700 (43), which was claimed to have a better drug-like profile than zicronapine [136]. Lu AF35700 has a novel pharmacological profile with predominant D1 vs. D2 dopamine receptor occupancy combined with high 5-HT6 receptor occupancy [137]. A phase III clinical trial has been initiated using Lu AF35700 to treat patients with treatment resistant schizophrenia [138].
RP5063 (44) is a novel dopamine-serotonin system stabilizer exhibiting potent partial agonist activity at the dopamine D2, D3, D4, serotonin 5-HT1A and 5-HT2A receptors, and antagonist activity at the serotonin 5-HT6 and 5-HT7 receptors [139]. RP5063 showed efficacy in patients with acute schizophrenia in a placebo controlled phase II clinical study [140] . Currently, the phase III trials of this investigational drug for the treatment of schizophrenia are in preparation [141].
Among the few investigational drug candidates targeting dopamine and other neuroreceptors, ITI-007 (7) represents a new approach to the treatment of schizophrenia and demonstrated positive top-line results in a recent phase III clinical trial in patients with schizophrenia [125]. Therefore, ITI-007 was selected as a representative new drug candidate to compare with the first- and second-generation antipsychotics, haloperidol (2), clozapine (3), risperidone (4) and aripiprazole (5).
3. COMPARISON OF REPRESENTATIVE ANTIPSYCHOTICS, HALOPERIDOL, CLOZAPINE, RISPERIDONE, ARIPIPRAZOLE AND ITI-007
In this section, haloperidol (2) from the first generation of antipsychotics, clozapine (3), risperidone (4) and aripiprazole (5) from the second-generation atypical antipsychotics, and the investigational new drug ITI-007 (7) representing a new approach for the treatment of schizophrenia were compared side-by-side in terms of receptor binding profile, mechanism of action, behavioral results in animal models, clinical outcomes, dopamine D2 receptor occupancy, treatment related side effects and other aspects.
3.1. Comparison of Receptor Binding Profiles and Functional Selectivity
Early, or first-generation treatments for schizophrenia, including haloperidol (2), are characterized by high affinity (i.e., nanomolar) binding to dopamine D2 receptors [157]. Haloperidol functions as a potent antagonist of D2 receptor-mediated inhibition of adenylyl cyclase and cellular accumulation of the second messenger molecule, cAMP [158, 159]. Second-generation antipsychotic medications, such as clozapine (3) and risperidone (4), also display high affinity binding to dopamine D2 receptors (Table 4). Antagonism of
Table 4.
Comparison of biochemical and pharmacological properties of representative antipsychotics, haloperidol, clozapine, risperidone, aripiprazole and ITI-007.
| Haloperidol (2) | Clozapine (3) | Risperidone (4) | Aripiprazole (5) | ITI-007 (7) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Receptor binding (Ki, nM) | |||||||||
| D2 | 2.0 | 144 | 4.9 | 0.34 | 32 | ||||
| D1 | 83 | 189 | 147 | 1960 | 52 | ||||
| D3 | 4.0 | 270 | 3.6 | 0.8 | > 100 | ||||
| D4 | 15 | 39 | 4.4 | 44 | 108 | ||||
| D5 | 147 | 235 | 563 | 2590 | > 100 | ||||
| 5-HT1A | 1202 | 105 | 427 | 1.7 | 1480 | ||||
| 5-HT2A | 70 | 5.2 | 0.17 | 3.4 | 0.54 | ||||
| 5-HT2C | 5000 | 10.7 | 12 | 15 | 173 | ||||
| α1A adrenergic | 12 | 1.6 | 5.0 | 26 | 73 | ||||
| α1B adrenergic | 8 | 7.0 | 9.0 | 35 | 31 | ||||
| H1 histamine | 3002 | 2.0 | 15 | 61 | > 1000 | ||||
| M1 muscarinic | >10000 | 14 | >10,000 | 6780 | > 1000 | ||||
| SERT | 3256 | 1624 | > 10,000 | 1080 [120] | 61 | ||||
| NET | 2112 | 3168 | 5454 | 2093 | > 1000 | ||||
| DAT | > 10,000 | > 10,000 | > 10,000 | 3215 | > 1000 | ||||
| Ratio of Ki values | |||||||||
| D2/5-HT2A | 0.029 | 28 | 29 | 0.10 | 59 | ||||
| 5-HT2C/5-HT2A | 71 | 2.1 | 71 | 4.4 | 320 | ||||
| H1/5-HT2A | 43 | 0.38 | 88 | 18 | > 1850 | ||||
| Mechanism of action (receptor functionality) | D2 and D3 inverse agonist [142] | Antagonist at dopaminergic, adrenergic, cholinergic, histaminergic and serotonergic receptors [143] |
Antagonist at serotonin 5-HT2, dopamine D2, adrenergic α1 and α2, and histaminergic H1 receptors [144] | D2 and 5-HT1A partial agonist; 5-HT2A antagonist [108] |
5-HT2A antagonist; post synaptic antagonist and pre-synaptic partial agonist at dopamine D2 receptors; glutamatergic phosphoprotein modulator; serotonin reuptake inhibitor [23] | ||||
| Blockade of Amphetamine-induced hyperlocomotion ED50 (mg/kg, PO) | 0.04 [23] | 4.27 [145] | 0.33 [23] | 4.65 [23] | 0.95 [23] | ||||
| Ratio of effective dose for catalepsy induction/effective dose for blockade of hyperlocomotion | 1 [146, 147] | 10 [146] | 5 [146] | 12 [146, 148] | >30 [23] | ||||
| Target dosage for the treatment of adult schizophrenia | 2- 5 mg every 4- 8 hours [149] | 300 – 450 mg/day; 900 mg/day maximum | 4 – 8 mg/day; 16 mg/day maximum | 10 – 15 mg/day recommended dose; 30 mg/day maximum | 40 – 60 mg/day [124, 125] |
||||
| Haloperidol (2) | Clozapine (3) | Risperidone (4) | Aripiprazole (5) | ITI-007 (7) | |||||
| Dose frequency | Every 4- 8 hours | Three times a day | once or twice daily | Once daily | Once daily | ||||
| Dose titration requirement | Required | Required to achieve efficacious dose | Required to achieve efficacious dose | Required to achieve maximum dose | None | ||||
| Dopamine D2 receptor occupancy at therapeutic doses | 53 – 74% at 2 mg/day [150]; 53 – 88% at 1 – 5 mg/day [151] |
24.5% at 250 mg/day; < 60% at 400 – 600 mg/day [152, 174] | 73% at 4 mg/day [153, 182] | 83.5 ± 3.2% at 15 mg/day; 96.8 ± 5.3% at 40 mg/day [154, 155] | ~ 40% at 60 mg/day [125, 156] | ||||
| Approved indications | Schizophrenia; control of tics and vocal utterances of Tourette’s Disorder [149] | Treatment-Resistant Schizophrenia; reduction in the Risk of Recurrent Suicidal Behavior in Schizophrenia or Schizoaffective Disorders [143] | Schizophrenia; acute manic or mixed episodes associated with bipolar I disorder; treatment of irritability associated with autistic disorder [144] |
Schizophrenia; acute treatment of manic and mixed episodes associated with bipolar I disorder; adjunctive treatment of MDD; irritability associated with autistic Disorder; treatment of Tourette’s disorder [108] | Investigational new drug currently in phase III clinical development | ||||
| Other indications in phase III clinical trials | Schizophrenia; bipolar depression | ||||||||
D2 receptor activity is recognized as an effective treatment for reducing positive symptoms (i.e., hallucinations and delusions) of psychosis in schizophrenic patients. Unfortunately, this activity also limits the utility of agents, such as haloperidol, due to the propensity to induce motor abnormalities including acute Parkinson-like movement deficits and dystonia, referred to generally as extrapyramidal motor syndromes, and chronic tardive dyskinesia mediated through blockade of dopamine D2 receptors in motor pathways of the basal ganglia [160]. Further, such drugs are relatively ineffective in alleviating the negative symptoms associated with schizophrenia, including asociality and depression, and also induce other troubling side effects including an elevation of serum prolactin levels [161].
As a class, second-generation antipsychotics (SGA) suffer from a variety of debilitating side effects, including excessive weight gain (i.e., as much as 50 pounds/year), type II diabetes, cognitive impairment, sedation, blurred vision, orthostatic hypotension, constipation, dizziness, and loss of bladder control. The side effects appear to be associated with non-selective interactions of these medications with receptors that are unrelated to antipsychotic efficacy, including serotonergic 5-HT2C, histaminergic H1, alpha-adrenergic and muscarinic receptors [162-165]. For example, clozapine (3) has a high affinity for the H1 histamine receptor (Table 4), and meta-analyses show a strong correlation between risk of weight gain and H1 receptor affinity [65]. In fact, risperidone (4) and clozapine (3), which induce significant weight gain in patients, display high affinity binding to two receptors implicated in excessive weight gain, namely the H1 histamine receptor [162, 166], and the 5-HT2C serotonin receptor [164]. The moderate binding affinity of aripiprazole (5) for the human H1 histamine receptor (Ki = 61 nM) is consistent with aripiprazole exhibiting a minimal short-term weight gain [162]. Aripiprazole acts as a partial agonist at the 5-HT2C receptor. In the presence of antidepressants with high serotonergic activity, aripiprazole has been reported to act as an antagonist at the 5-HT2C receptor, resulting in significant weight gain [167].
ITI-007, currently in Phase III human clinical studies, displays a receptor binding profile that predicts minimal risk for many of side effects associated with antipsychotic drugs. ITI-007 has negligible binding affinity as an antagonist at M1 muscarinic receptors implicated in the cognitive dulling effects common in antipsychotic drugs [163]. In addition, ITI-007 possesses minimal binding affinity for 5-HT2C serotonin receptors (Ki = 173 nM) and the H1 histamine receptors (Ki > 1000 nM) [23] implicated in both weight gain and the aberrant metabolic side effects leading to type II diabetes in patients treated with antipsychotic drugs [162, 164, 165]. Results of human clinical trials of ITI-007, to date [124, 125] have not shown significant weight gain in patients with schizophrenia, suggesting that the compound’s unique receptor binding profile is key in alleviating this side effect.
3.2. Comparison of In Vivo Efficacy in Preclinical Animal Models
In the absence of an animal model that effectively encapsulates the symptoms of schizophrenia, screening of new antipsychotic medications continues to focus on models that detect activity of a particular neurotransmitter receptor subtype. The various animal models traditionally employed have been reviewed in depth by Arnt and Skarsfeldt [146]. Here, we briefly compare the activity of representative agents, including haloperidol (2), clozapine (3), risperidone (4), aripiprazole (5) and ITI-007 (7), in two assays that utilize motor readouts, namely hyperlocomotion and catalepsy. Both of these assays report on the activities at brain dopamine D2 receptors, but in a regionally-selective manner. They offer insight into the relative effects of compounds on efficacy compared with motor side effects. Amphetamine (AMPH)-stimulated hyperlocomotion has been demonstrated to preferentially involve activation of dopamine D2 receptors located in limbic dopamine pathways (e.g., nucleus accumbens) [168, 169], whereas drug-induced catalepsy involves blockade of dopamine D2 receptors in the striatum [146]. Thus, the comparative potency of compounds in hyperlocomotion and catalepsy assays provides a valuable measure of their therapeutic windows for successful control of schizophrenia symptoms without adverse motor side effects.
Of the five representative compounds, haloperidol (2) displays the greatest potency (i.e., sub-nanomolar ED50) for blockade of amphetamine-induced hyperlocomotion (Table 4) and for induction of catalepsy [146]. This observation is correlated with haloperidol’s potent binding affinity for and functional antagonism of dopamine D2 receptors (Ki = 2 nM). Notably, the ratio of the effective dose for induction of catalepsy and that for blockade of hyperlocomotion is ~1.0 [146, 147], supporting a high liability of haloperidol for extrapyramidal motor side effects at dose levels required for antipsychotic activity. Risperidone (4), which possesses similar high affinity binding and functional antagonism of dopamine D2 receptors (Ki = 4.9 nM) is less potent (~10-fold) in inhibiting amphetamine hyperlocomotion and less effective in causing catalepsy with a ratio of effective doses for catalepsy compared to hyperlocomotion blockade of ~5.0 [146]. Like risperidone, both clozapine (3) and aripiprazole (5) display less potent blockade of AMPH-induced hyperlocomotion than haloperidol (Table 4). They also display a lower propensity to induce catalepsy than either haloperidol or risperidone. The ratio of effective doses for induction of catalepsy compared to blockade of hyperlocomotion is ~10 for clozapine [146] and 12 for aripiprazole [146, 148]. The improved motor side effect profile of these compounds may owe, in part, to the presumed effects of all three compounds (risperidone, clozapine, and aripiprazole) on cortical serotonin 5-HT2A receptors. Significantly, clozapine and other newer molecules, in particular, aripiprazole, are also believed to demonstrate preferential effects on dopamine D2 receptors in limbic dopamine pathways, compared with catalepsy-producing receptors in the nigrostriatal pathways [170-172]. Further, aripiprazole has been shown to exhibit partial agonist activity at pre- and postsynaptic dopamine D2 receptors in the nigrostriatal system, further reducing the expression of motor side effects [172].
ITI-007 (7) demonstrates a preclinical profile that includes potent blockade of AMPH-induced hyperlocomotion (Table 4) consistent with its high-affinity for D2 dopamine receptors (Ki = 32nM). Evidence also indicates ITI-007 displays limbic dopamine system selectivity based on its ability to increase dopamine release in prefrontal cortex, but not striatum. Further, ITI-007 administration to animals does not increase striatal dopamine metabolism, indicating partial D2 receptors agonist activity at presynaptic striatal D2 receptors [23]. ITI-007 results in minimal motor side effects in animals as the ratio of the effective drug doses for the appearance of catalepsy, compared to blockade of hyperlocomotion, is ~30 [23], The absence of significant motor side effects seen in animals given ITI-007 likely owes to its unique combination of dopamine and serotonin receptor activities [23].
In summary, SGA medications and newer candidate therapeutics, like ITI-007, show an evolution from the pronounced motor side effect profile of FGA molecules, like haloperidol, to the most benign motor profile of ITI-007, without a significant loss of antipsychotic activity, as predicted from blockade of AMPH-induced locomotion (Table 4). This evolution appears due, in part to design features introduced into new molecules, including serotonin receptor activities and dopamine receptor partial agonist properties.
3.3. Comparison of Dopamine D2 Receptor Occupancy at Therapeutic Doses
Positron emission tomography (PET) imaging studies have demonstrated that clinical antipsychotic response is usually associated with at least 65% occupancy of striatal D2 dopamine receptors, while 50-73% occupancy can be associated with hyperprolactinemia and over 80% occupancy is associated with extrapyramidal side effects [173-180]. Therefore, it is difficult to achieve antipsychotic efficacy without concomitant motoric disturbances and hyperprolactinemia with both typical and atypical antipsychotic drugs. This relationship between D2 receptor occupancy, clinical response and side effects, even in first episode patients, is well-established [179].
Haloperidol, for example, often gives high (>80%) striatal D2 receptor occupancy at therapeutic doses and results in motor disturbances such as parkinsonism and akathisia [174]. Similarly, risperidone, across its effective dose range of 4 to 12 mg/day, is associated with 72 – 81% striatal D2 receptor occupancy [152, 175, 181-183]. With aripiprazole, D2 and D3 receptor occupancy levels are high, with average levels ranging between ~71% at a low, sub-therapeutic dose of 2 mg/day to ~96% at 40 mg/day [155, 184]. Although aripiprazole is associated with relatively less liability for parkinsonism, other motoric side effects such as akathisia occur at relatively high rates. Clozapine is an exception with relatively low striatal D2 receptor occupancy (<60%) at antipsychotic doses and a low liability for parkinsonism, akathisia, and hyperprolactinemia [152, 174, 183, 185, 186]. At a low single dose of 10 mg in healthy volunteers, ITI-007 demonstrated low (~12%) striatal D2 receptor occupancy and high (>80%) cortical 5-HT2A receptor occupancy [156]. ITI-007 demonstrated an average of 29% (peak of 39%) D2 occupancy at the highest evaluated dose in healthy volunteers. At a 40 mg dose, ITI-007 also demonstrated occupancy of serotonin transporters in healthy volunteers in a range similar to that of its D2 receptor occupancy, consistent with its in vitro pharmacological profile and antidepressant-like effects. In a double-blind, placebo-controlled efficacy trial, a dose of 60 mg ITI-007 was evaluated in patients with acute schizophrenia and demonstrated antipsychotic efficacy with a placebo-like motor side effect profile and no hyperprolactinemia [124]. Subsequently, 60 mg ITI-007 demonstrated approximately 40% striatal D2 receptor occupancy at plasma steady state after two weeks of administration in patients with schizophrenia who had been washed off their previous antipsychotic medications for at least two weeks prior to a within-subject baseline PET scan [125]. These data suggest that ITI-007 achieves antipsychotic efficacy at low levels of striatal D2 receptor occupancy yielding a lower risk for extrapyramidal side effects and hyperprolactinemia.
In summary, all clinically effective antipsychotics approved for the treatment of schizophrenia to date exhibit dopamine D2 receptor occupancy. High striatal D2 receptor occupancy was once thought to be required for antipsychotic efficacy, with clozapine as an exception. Growing evidence, including the recent example with ITI-007, suggests that sustained high levels of striatal D2 receptor occupancy are not required to achieve antipsychotic efficacy. A reduction in striatal D2 receptor occupancy also seems to provide safety and tolerability advantages, with reduced risk for motor side effects and hyperprolactinemia.
3.4. Clinical Outcomes and Treatment-Emergent Side Effects
Clinical efficacy of marketed antipsychotic drugs, including haloperidol (2), clozapine (3), risperidone (4) and aripiprazole (5), has been extensively reviewed [187-192]. Examples from each generation of antipsychotics have demonstrated sufficient clinical efficacy compared to placebo in acute treatment trials to gain approval by regulatory authorities. The investigational drug candidate ITI-007 (7) also has demonstrated antipsychotic efficacy. ITI-007 (60 mg) exhibited efficacy with statistically significant superiority over placebo at Week 4 (study endpoint) as measured by the change from baseline on the PANSS total score (p=0.022) in a randomized, double-blind, placebo-controlled, multi-center phase III study for the treatment of patients with schizophrenia [125, 193]. ITI-007 showed a dose-related improvement in symptoms of schizophrenia with ITI-007 40 mg dose also demonstrating efficacy on several measures. Moreover, ITI-007 60 mg showed significant antipsychotic efficacy as early as week 1, which was maintained throughout the entire study. Patients treated with ITI-007 60 mg and 40 mg showed statistically significant improvement on the Clinical Global Impression Scale for Severity of Illness (CGI-S; p=0.003 for 60 mg, p=0.025 for 40 mg) [125]. Importantly, ITI-007 improved prosocial behavior and psychosocial function [124].
While only modest differences in efficacy have been observed among currently available antipsychotic drugs, their side effect profiles differ greatly [192]. Treatment decisions often are based on a weighing of risks and benefits of the various available antipsychotic drugs in light of individual history of treatment response and tolerability of particular side effects. The treatment-emergent side effects of the marketed antipsychotic drugs are different and mostly correlated with the receptor binding profiles of these compounds. Extrapyramidal syndrome is a commonly observed side effect, especially for the first generation antipsychotics, such as haloperidol, which generally require high striatal D2 receptor occupancy for efficacy and consequently result in motor disturbances such as parkinsonism and akathisia [174, 190]. It has been suggested for first-episode patients that aripiprazole may be considered as the preferred choice over risperidone; however, if the potential for akathisia and poor impulse control is a concern, low-dose risperidone may be a better choice over aripiprazole [191]. Clozapine demonstrated less severe motoric side effects, which is likely due to the relatively lower D2 receptor occupancy at therapeutic doses [194]. However, clozapine’s safety risks limit its use to patients with treatment-resistant schizophrenia with close safety monitoring. Additionally, the currently available antipsychotic drugs exhibit various degrees of metabolic and endocrine abnormalities. Clozapine and risperidone are in particular associated with significant weight gain, especially in children and adolescents [195]. Aripiprazole exhibits relatively less metabolic side-effects than other commonly used atypical antipsychotic drugs [196], but at an increased risk for akathisia. In addition to motoric and metabolic adverse effects, various cardiovascular side effects are associated with the marketed antipsychotic drugs [197-199]. Clozapine is known to cause myocarditis and cardiomyopathy [200].
In clinical trials to date, ITI-007 (7) is well-tolerated and demonstrates a favorable safety profile that does not differ from placebo [124]. At therapeutic doses, ITI-007 did not show significant difference from placebo on weight gain, prolactin levels and metabolic parameters including cholesterol, triglycerides, glucose and insulin. ITI-007 was associated with low rates of motor side effects, including akathisia, similar to placebo. Key measures of cardiovascular function, including heart rate, QTc intervals and other ECG parameters, were also similar between ITI-007 and placebo [124]. These clinical observations suggest that ITI-007 is not associated with the usual side effects of existing medications for schizophrenia. An effective dose of 60 mg ITI-007 is associated with approximately 40% striatal D2 receptor occupancy, substantially lower than that required by other antipsychotic drugs [125, 156]. This sparing of D2 receptor occupancy likely contributes to ITI-007’s reduced liability for motor side effects and hyperprolactinemia.
CONCLUSION
Currently available antipsychotic treatments demonstrate efficacy for the treatment of positive symptoms associated with schizophrenia, but do not address the wide array of symptoms and psychosocial impairment experienced by people living with this disorder. Moreover, people living with schizophrenia and their treating psychiatrists often have to choose between varying side effect profiles, trading off cardiometabolic disturbances for motor dysfunction or vice versa. The vast majority (74%) of patients discontinue their study medication within 18 months [163]. Psychosocial functioning is at best modestly improved with currently available treatments, likely related to high early treatment discontinuation rates [201]. Better treatment options are clearly needed. Improving tolerability while maintaining efficacy would likely result in increased treatment adherence and improved psychosocial outcome. Dissociation of antipsychotic efficacy from high dopamine D2 receptor occupancy is one strategy for improving tolerability by reducing motoric liability. Limiting off-target pharmacological interactions associated with cardiometabolic burden will improve overall safety and tolerability profiles. Engagement across essential dopaminergic, serotonergic and glutamatergic targets may offer relief of a broad array of symptoms associated with schizophrenia, including a primary improvement in social function. Novel drug candidates designed to provide such improvements are currently in development for the treatment of schizophrenia.
Fig. (6).
Structures of ITI-007 (7), bifeprunox (41) and zicronapine (42).
ACKNOWLEDGEMENTS
The authors wish to thank colleagues at Intra-Cellular Therapies, Inc. for their thoughtful comments on the manuscript.
LIST OF ABBREVIATIONS
- cAMP
3',5'-Cyclic adenosine monophosphate
- CYP
Cytochrome p450
- DPPM
Dopamine receptor protein phosphorylation modulation
- EPS
Extrapyramidal syndrome
- FDA
U.S. Food and Drug Administration
- FGA
First-generation antipsychotics
- MDD
Major depressive disorder
- MPP+
1-Methyl-4-phenylpyridinium
- PANSS
Positive and Negative Syndrome Scale
- PET
Positron emission tomography
- SGA
Second-generation antipsychotics
- UGT
Uridine 5'-diphospho-glucuronosyltransferase
- WHO
World Health Organization
CONFLICT OF INTEREST
Peng Li, Gretchen L. Snyder and Kimberly E. Vanover are full-time employees of Intra-Cellular Therapies, Inc.
REFERENCES
- 1.Wickelgren I. A new route to treating schizophrenia? Science. 1998;281(5381):1264–1265. doi: 10.1126/science.281.5381.1264. [DOI] [PubMed] [Google Scholar]
- 2.Marino M.J., Knutsen L.J., Williams M. Emerging opportunities for antipsychotic drug discovery in the postgenomic era. J. Med. Chem. 2008;51(5):1077–1107. doi: 10.1021/jm701094q. [DOI] [PubMed] [Google Scholar]
- 3.Remington G., Agid O., Foussias G. Schizophrenia as a disorder of too little dopamine: implications for symptoms and treatment. Expert Rev. Neurother. 2011;11(4):589–607. doi: 10.1586/ern.10.191. [DOI] [PubMed] [Google Scholar]
- 4.Hosak L., Hosakova J. The complex etiology of schizophrenia - general state of the art. Neuroendocrinol. Lett. 2015;36(7):631–637. [PubMed] [Google Scholar]
- 5.Radulescu A. A multi-etiology model of systemic degeneration in schizophrenia. J. Theor. Biol. 2009;259(2):269–279. doi: 10.1016/j.jtbi.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Walker E., Kestler L., Bollini A., Hochman K.M. Schizophrenia: etiology and course. Annu. Rev. Psychol. 2004;55:401–430. doi: 10.1146/annurev.psych.55.090902.141950. [DOI] [PubMed] [Google Scholar]
- 7.Kim D.H., Maneen M.J., Stahl S.M. Building a better antipsychotic: receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics. 2009;6(1):78–85. doi: 10.1016/j.nurt.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grace A.A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 9.Dean B. Neurochemistry of schizophrenia: the contribution of neuroimaging postmortem pathology and neurochemistry in schizophrenia. Curr. Top. Med. Chem. 2012;12(21):2375–2392. doi: 10.2174/156802612805289935. [DOI] [PubMed] [Google Scholar]
- 10.Dean B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J. Neurochem. 2003;85(1):1–13. doi: 10.1046/j.1471-4159.2003.01693.x. [DOI] [PubMed] [Google Scholar]
- 11.International Schizophrenia C.; Purcell, S. M.; Wray, N. R.; Stone, J. L.; Visscher, P. M.; O'Donovan, M. C.; Sullivan, P. F.; Sklar, P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray J.A., Feldon J., Rawlins J.N., Hemsley D.R., Smith A.D. The neuropsychology of schizophrenia. Behav. Brain Sci. 1991;14(01):1–20. [Google Scholar]
- 13.Kapur S., Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am. J. Psychiatry. 1996;153(4):466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- 14.Juckel G., Schlagenhauf F., Koslowski M., Wustenberg T., Villringer A., Knutson B., Wrase J., Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Lindenmayer J.P., Nasrallah H., Pucci M., James S., Citrome L. A systematic review of psychostimulant treatment of negative symptoms of schizophrenia: challenges and therapeutic opportunities. Schizophr. Res. 2013;147(2-3):241–252. doi: 10.1016/j.schres.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman J.A., Bymaster F.P., Meltzer H.Y., Deutch A.Y., Duncan G.E., Marx C.E., Aprille J.R., Dwyer D.S., Li X.M., Mahadik S.P., Duman R.S., Porter J.H., Modica-Napolitano J.S., Newton S.S., Csernansky J.G. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol. Rev. 2008;60(3):358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ban T.A. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr. Dis. Treat. 2007;3(4):495–500. [PMC free article] [PubMed] [Google Scholar]
- 19.Stockmeier C.A., DiCarlo J.J., Zhang Y., Thompson P., Meltzer H.Y. Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. J. Pharmacol. Exp. Ther. 1993;266(3):1374–1384. [PubMed] [Google Scholar]
- 20.Meltzer H.Y., Matsubara S., Lee J.C. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 1989;251(1):238–246. [PubMed] [Google Scholar]
- 21.Kinon B.J., Lieberman J.A. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology (Berl.) 1996;124(1-2):2–34. doi: 10.1007/BF02245602. [DOI] [PubMed] [Google Scholar]
- 22.FDA News Release
- 23.Snyder G.L., Vanover K.E., Zhu H., Miller D.B., O'Callaghan J.P., Tomesch J., Li P., Zhang Q., Krishnan V., Hendrick J.P., Nestler E.J., Davis R.E., Wennogle L.P., Mates S. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl.) 2015;232(3):605–621. doi: 10.1007/s00213-014-3704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P., Zhang Q., Robichaud A.J., Lee T., Tomesch J., Yao W., Beard J.D., Snyder G.L., Zhu H., Peng Y., Hendrick J.P., Vanover K.E., Davis R.E., Mates S., Wennogle L.P. Discovery of a tetracyclic quinoxaline derivative as a potent and orally active multifunctional drug candidate for the treatment of neuropsychiatric and neurological disorders. J. Med. Chem. 2014;57(6):2670–2682. doi: 10.1021/jm401958n. [DOI] [PubMed] [Google Scholar]
- 25.Meltzer H.Y. Update on typical and atypical antipsychotic drugs. Annu. Rev. Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 26.Mailman R.B., Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr. Pharm. Des. 2010;16(5):488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Citrome L. Oral antipsychotic update: a brief review of new and investigational agents for the treatment of schizophrenia. CNS Spectr. 2012;17(Suppl. 1):1–9. doi: 10.1017/S1092852912000727. [DOI] [PubMed] [Google Scholar]
- 28.Samara M.T., Cao H., Helfer B., Davis J.M., Leucht S. Chlorpromazine versus every other antipsychotic for schizophrenia: a systematic review and meta-analysis challenging the dogma of equal efficacy of antipsychotic drugs. Eur. Neuropsychopharmacol. 2014;24(7):1046–1055. doi: 10.1016/j.euroneuro.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Adams C.E., Rathbone J., Thornley B., Clarke M., Borrill J., Wahlbeck K., Awad A.G. Chlorpromazine for schizophrenia: a Cochrane systematic review of 50 years of randomised controlled trials. BMC Med. 2005;3:15. doi: 10.1186/1741-7015-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi H., Fervaha G., Uchida H., Suzuki T., Bies R.R., Gronte D., Remington G. Impact of once- versus twice-daily perphenazine dosing on clinical outcomes: an analysis of the CATIE data. J. Clin. Psychiatry. 2014;75(5):506–511. doi: 10.4088/JCP.13m08695. [DOI] [PubMed] [Google Scholar]
- 31.Haran T. Perphenazine (fentazin) in the management of chronic schizophrenia. J. Ir. Med. Assoc. 1960;46:135–138. [PubMed] [Google Scholar]
- 32.Matar H.E., Almerie M.Q., Sampson S. Fluphenazine (oral) versus placebo for schizophrenia. Schizophr. Bull. 2013;39(6):1187–1188. doi: 10.1093/schbul/sbt140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reznikoff L. The use of fluphenazine (Prolixin) in rehabilitation of chronic schizophrenic patients. Am. J. Psychiatry. 1960;117:457–458. doi: 10.1176/ajp.117.5.457. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald R., Watts T.P. Trifluoperazine dihydrochloride (stelazine) in paranoid schizophrenia. BMJ. 1959;1(5121):549–550. doi: 10.1136/bmj.1.5121.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janicak P.G., Javaid J.I., Sharma R.P., Comaty J.E., Peterson J., Davis J.M. Trifluoperazine plasma levels and clinical response. J. Clin. Psychopharmacol. 1989;9(5):340–346. [PubMed] [Google Scholar]
- 36.Suzuki H., Gen K., Takahashi Y. A naturalistic comparison study of the efficacy and safety of intramuscular olanzapine, intramuscular haloperidol, and intramuscular levomepromazine in acute agitated patients with schizophrenia. Hum. Psychopharmacol. 2014;29(1):83–88. doi: 10.1002/hup.2376. [DOI] [PubMed] [Google Scholar]
- 37.Kurland A.A., Yazicioglu E. Effect of chlorprothixene on schizophrenic patients. Dis. Nerv. Syst. 1961;22:636–638. [PubMed] [Google Scholar]
- 38.Fux M., Belmaker R.H. A controlled comparative study of chlorprothixene vs. haloperidol in chronic schizophrenia. Isr. J. Psychiatry Relat. Sci. 1991;28(1):37–40. [PubMed] [Google Scholar]
- 39.Simpson G.M., Arengo A.D., Angus J.W., Beckles E.D., Rochlin D. A one-year trial of clopenthixol in chronic schizophrenia. Can. Psychiatr. Assoc. J. 1972;17(4):321–324. doi: 10.1177/070674377201700409. [DOI] [PubMed] [Google Scholar]
- 40.Hollister L.E., Lombrozo L., Huang C.C. Plasma concentrations of thiothixene and clinical response in treatment-resistant schizophrenics. Int. Clin. Psychopharmacol. 1987;2(1):77–82. doi: 10.1097/00004850-198701000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Gunduz-Bruce H., Oliver S., Gueorguieva R., Forselius-Bielen K., D'Souza D.C., Zimolo Z., Tek C., Kaliora S., Ray S., Petrides G. Efficacy of pimozide augmentation for clozapine partial responders with schizophrenia. Schizophr. Res. 2013;143(2-3):344–347. doi: 10.1016/j.schres.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Chouinard G., Lehmann H.E., Ban T.A. Pimozide in the treatment of chronic schizophrenic patients. Curr. Ther. Res. Clin. Exp. 1970;12(9):598–603. [PubMed] [Google Scholar]
- 43.Chouinard G., Annable L., Steinberg S. A controlled clinical trial of fluspirilene, a long-acting injectable neuroleptic, in schizophrenic patients with acute exacerbation. J. Clin. Psychopharmacol. 1986;6(1):21–26. [PubMed] [Google Scholar]
- 44.Vaidyalingam N. Evaluation of penfluridol in hospitalised chronic schizophrenic. J. Postgrad. Med. 1990;36(2):100–103. [PubMed] [Google Scholar]
- 45.Stewart A., Lafave H.G., Segovia G. Haloperidol--new addition to the drug treatment of schizophrenia. Behav. Neuropsychiatry. 1969;1(7):23–28. [PubMed] [Google Scholar]
- 46.Leucht C., Kitzmantel M., Chua L., Kane J., Leucht S. Haloperidol versus chlorpromazine for treatment of schizophrenia. Schizophr. Bull. 2008;34(5):813–815. doi: 10.1093/schbul/sbn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki H., Gen K., Inoue Y. Comparison of the anti-dopamine D(2) and anti-serotonin 5-HT(2A) activities of chlorpromazine, bromperidol, haloperidol and second-generation antipsychotics parent compounds and metabolites thereof. J. Psychopharmacol. 2013;27(4):396–400. doi: 10.1177/0269881113478281. [DOI] [PubMed] [Google Scholar]
- 48.Yasui-Furukori N., Kondo T., Ishida M., Tanaka O., Mihara K., Kaneko S., Otani K. The characteristics of side-effects of bromperidol in schizophrenic patients. Psychiatry Clin. Neurosci. 2002;56(1):103–106. doi: 10.1046/j.1440-1819.2002.00936.x. [DOI] [PubMed] [Google Scholar]
- 49.Menon M.S., Ramachandran V. A controlled clinical trial of trifluperidol on a group of chronic schizophrenic patients. Curr. Ther. Res. Clin. Exp. 1972;14(1):17–21. [PubMed] [Google Scholar]
- 50.Woggon B., Franke A., Hucker H., Ruether E., Athen D., Angst J., Hippius H. Antipsychotic effects, side effects and effective dosis of the butyrophenone lenperone (AHR 2277). Int. Pharmacopsychiatry. 1977;12(2):113–126. doi: 10.1159/000468295. [DOI] [PubMed] [Google Scholar]
- 51.Eisenstein S.A., Antenor-Dorsey J.A., Gredysa D.M., Koller J.M., Bihun E.C., Ranck S.A., Arbelaez A.M., Klein S., Perlmutter J.S., Moerlein S.M., Black K.J., Hershey T. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse. 2013;67(11):748–756. doi: 10.1002/syn.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schonfeldt-Lecuona C., Juengling F.D., Connemann B.J., Reske S.N., Spitzer M., Kassubek J. Complete dopamine D2 receptor occupancy without extrapyramidal side effects under benperidol. J. Clin. Psychopharmacol. 2004;24(1):97–98. doi: 10.1097/01.jcp.0000106227.36344.ca. [DOI] [PubMed] [Google Scholar]
- 53.Langer G., Puhringer W. Haloperidol and droperidol treatment in schizophrenics. Clinical application of the “prolactin-model”. Acta Psychiatr. Belg. 1980;80(5):574–583. [PubMed] [Google Scholar]
- 54.Paraschakis A. Pipamperone augmentation of clozapine and sodium valproate in refractory schizophrenia: a case report. Clin. Neuropharmacol. 2014;37(2):60–61. doi: 10.1097/WNF.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 55.Squelart P., Saravia J. Pipamperone (Dipiperon), a useful sedative neuroleptic drug in troublesome chronic psychotic patients. Acta Psychiatr. Belg. 1977;77(2):284–293. [PubMed] [Google Scholar]
- 56.Halbach M., Henning U. Abnormal glucocorticoid dependent increase of spiperone binding sites on lymphocytes from schizophrenics in vitro. Pharmacopsychiatry. 1989;22(5):169–173. doi: 10.1055/s-2007-1014601. [DOI] [PubMed] [Google Scholar]
- 57.Dubinsky B., McGuire J.L., Niemegeers C.J., Janssen P.A., Weintraub H.S., McKenzie B.E. Bromperidol, a new butyrophenone neuroleptic: a review. Psychopharmacology (Berl.) 1982;78(1):1–7. doi: 10.1007/BF00470578. [DOI] [PubMed] [Google Scholar]
- 58.Ackenheil M., Stille G., Hoffmeister F. Psychotropic agents. Berlin, New York: Springer-Verlag; 1980. [Google Scholar]
- 59.Ahmad-Sabry M.H., Shareghi G. Long-term use of intrathecal droperidol as an excellent antiemetic in nonmalignant pain--a retrospective study. Middle East J. Anaesthesiol. 2012;21(6):857–862. [PubMed] [Google Scholar]
- 60.Awouters F.H., Lewi P.J. Forty years of antipsychotic Drug research--from haloperidol to paliperidone--with Dr. Paul Janssen. Arzneimittelforschung. 2007;57(10):625–632. doi: 10.1055/s-0031-1296660. [DOI] [PubMed] [Google Scholar]
- 61.Besnard J., Ruda G.F., Setola V., Abecassis K., Rodriguiz R.M., Huang X.P., Norval S., Sassano M.F., Shin A.I., Webster L.A., Simeons F.R., Stojanovski L., Prat A., Seidah N.G., Constam D.B., Bickerton G.R., Read K.D., Wetsel W.C., Gilbert I.H., Roth B.L., Hopkins A.L. Automated design of ligands to polypharmacological profiles. Nature. 2012;492(7428):215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez-Munoz F., Alamo C. The consolidation of neuroleptic therapy: Janssen, the discovery of haloperidol and its introduction into clinical practice. Brain Res. Bull. 2009;79(2):130–141. doi: 10.1016/j.brainresbull.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization WHO Model List of Essential Medicines.
- 64.Reynolds G.P., Kirk S.L. Metabolic side effects of antipsychotic drug treatment--pharmacological mechanisms. Pharmacol. Ther. 2010;125(1):169–179. doi: 10.1016/j.pharmthera.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Deng C., Weston-Green K., Huang X.F. The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(1):1–4. doi: 10.1016/j.pnpbp.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Kirk S.L., Glazebrook J., Grayson B., Neill J.C., Reynolds G.P. Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl.) 2009;207(1):119–125. doi: 10.1007/s00213-009-1639-8. [DOI] [PubMed] [Google Scholar]
- 67.Kato Y., Nakajima M., Oda S., Fukami T., Yokoi T. Human UDP-glucuronosyltransferase isoforms involved in haloperidol glucuronidation and quantitative estimation of their contribution. Drug Metab. Dispos. 2012;40(2):240–248. doi: 10.1124/dmd.111.042150. [DOI] [PubMed] [Google Scholar]
- 68.Eyles D.W., Pond S.M. Stereospecific reduction of haloperidol in human tissues. Biochem. Pharmacol. 1992;44(5):867–871. doi: 10.1016/0006-2952(92)90117-2. [DOI] [PubMed] [Google Scholar]
- 69.Pan L., Belpaire F.M. In vitro study on the involvement of CYP1A2, CYP2D6 and CYP3A4 in the metabolism of haloperidol and reduced haloperidol. Eur. J. Clin. Pharmacol. 1999;55(8):599–604. doi: 10.1007/s002280050679. [DOI] [PubMed] [Google Scholar]
- 70.Fang J., Baker G.B., Silverstone P.H., Coutts R.T. Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell. Mol. Neurobiol. 1997;17(2):227–233. doi: 10.1023/a:1026317929335. [DOI] [PubMed] [Google Scholar]
- 71.Kudo S., Odomi M. Involvement of human cytochrome P450 3A4 in reduced haloperidol oxidation. Eur. J. Clin. Pharmacol. 1998;54(3):253–259. doi: 10.1007/s002280050455. [DOI] [PubMed] [Google Scholar]
- 72.Chakraborty B.S., Hubbard J.W., Hawes E.M., McKay G., Cooper J.K., Gurnsey T., Korchinski E.D., Midha K.K. Interconversion between haloperidol and reduced haloperidol in healthy volunteers. Eur. J. Clin. Pharmacol. 1989;37(1):45–48. doi: 10.1007/BF00609423. [DOI] [PubMed] [Google Scholar]
- 73.Avent K.M., DeVoss J.J., Gillam E.M. Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites. Chem. Res. Toxicol. 2006;19(7):914–920. doi: 10.1021/tx0600090. [DOI] [PubMed] [Google Scholar]
- 74.Wiemerslage L., Schultz B.J., Ganguly A., Lee D. Selective degeneration of dopaminergic neurons by MPP(+) and its rescue by D2 autoreceptors in Drosophila primary culture. J. Neurochem. 2013;126(4):529–540. doi: 10.1111/jnc.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eyles D.W., McGrath J.J., Pond S.M. Formation of pyridinium species of haloperidol in human liver and brain. Psychopharmacology (Berl.) 1996;125(3):214–219. doi: 10.1007/BF02247331. [DOI] [PubMed] [Google Scholar]
- 76.Ulrich S., Sandmann U., Genz A. Serum concentrations of haloperidol pyridinium metabolites and the relationship with tardive dyskinesia and parkinsonism: a cross-section study in psychiatric patients. Pharmacopsychiatry. 2005;38(4):171–177. doi: 10.1055/s-2005-871240. [DOI] [PubMed] [Google Scholar]
- 77.Sampson D., Bricker B., Zhu X.Y., Peprah K., Lamango N.S., Setola V., Roth B.L., Ablordeppey S.Y. Further evaluation of the tropane analogs of haloperidol. Bioorg. Med. Chem. Lett. 2014;24(17):4294–4297. doi: 10.1016/j.bmcl.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peprah K., Zhu X.Y., Eyunni S.V., Setola V., Roth B.L., Ablordeppey S.Y. Multi-receptor drug design: Haloperidol as a scaffold for the design and synthesis of atypical antipsychotic agents. Bioorg. Med. Chem. 2012;20(3):1291–1297. doi: 10.1016/j.bmc.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mocci G., Jimenez-Sanchez L., Adell A., Cortes R., Artigas F. Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology. 2014;79:49–58. doi: 10.1016/j.neuropharm.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt C.J., Sorensen S.M., Kehne J.H., Carr A.A., Palfreyman M.G. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995;56(25):2209–2222. doi: 10.1016/0024-3205(95)00210-w. [DOI] [PubMed] [Google Scholar]
- 81.Sorensen S.M., Kehne J.H., Fadayel G.M., Humphreys T.M., Ketteler H.J., Sullivan C.K., Taylor V.L., Schmidt C.J. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J. Pharmacol. Exp. Ther. 1993;266(2):684–691. [PubMed] [Google Scholar]
- 82.Kehne J.H., Baron B.M., Carr A.A., Chaney S.F., Elands J., Feldman D.J., Frank R.A., van Giersbergen P.L., McCloskey T.C., Johnson M.P., McCarty D.R., Poirot M., Senyah Y., Siegel B.W., Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J. Pharmacol. Exp. Ther. 1996;277(2):968–981. [PubMed] [Google Scholar]
- 83.de Paulis T. M-100907 (Aventis). Curr. Opin. Investig. Drugs. 2001;2(1):123–132. [PubMed] [Google Scholar]
- 84.Meltzer H.Y., Massey B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011;11(1):59–67. doi: 10.1016/j.coph.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Melnik T., Soares B.G., Puga M.E., Atallah A.N. Efficacy and safety of atypical antipsychotic drugs (quetiapine, risperidone, aripiprazole and paliperidone) compared with placebo or typical antipsychotic drugs for treating refractory schizophrenia: overview of systematic reviews. Sao Paulo Med. J. 2010;128(3):141–166. doi: 10.1590/S1516-31802010000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andersson J.L., Nomikos G.G., Marcus M., Hertel P., Mathe J.M., Svensson T.H. Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn Schmiedebergs Arch. Pharmacol. 1995;352(4):374–385. doi: 10.1007/BF00172774. [DOI] [PubMed] [Google Scholar]
- 87.Wadenberg M.L., Salmi P., Jimenez P., Svensson T., Ahlenius S. Enhancement of antipsychotic-like properties of the dopamine D2 receptor antagonist, raclopride, by the additional treatment with the 5-HT2 receptor blocking agent, ritanserin, in the rat. Eur. Neuropsychopharmacol. 1996;6(4):305–310. doi: 10.1016/s0924-977x(96)00035-1. [DOI] [PubMed] [Google Scholar]
- 88.Gardell L.R., Vanover K.E., Pounds L., Johnson R.W., Barido R., Anderson G.T., Veinbergs I., Dyssegaard A., Brunmark P., Tabatabaei A., Davis R.E., Brann M.R., Hacksell U., Bonhaus D.W. ACP-103, a 5-hydroxytryptamine 2A receptor inverse agonist, improves the antipsychotic efficacy and side-effect profile of haloperidol and risperidone in experimental models. J. Pharmacol. Exp. Ther. 2007;322(2):862–870. doi: 10.1124/jpet.107.121715. [DOI] [PubMed] [Google Scholar]
- 89.Meltzer H.Y., Elkis H., Vanover K., Weiner D.M., van Kammen D.P., Peters P., Hacksell U. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr. Res. 2012;141(2-3):144–152. doi: 10.1016/j.schres.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 90.Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist. Psychiatry. 2007;18(1):39–60. doi: 10.1177/0957154X07070335. [DOI] [PubMed] [Google Scholar]
- 91.Nielsen J., Correll C.U., Manu P., Kane J.M. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J. Clin. Psychiatry. 2013;74(6):603–613. doi: 10.4088/JCP.12r08064. [DOI] [PubMed] [Google Scholar]
- 92.El-Badri S., Mellsop G. Clozapine use and outcomes among patients with treatment resistant schizophrenia. Australas. Psychiatry. 2011;19(5):410–414. doi: 10.3109/10398562.2011.602078. [DOI] [PubMed] [Google Scholar]
- 93.Kane J., Honigfeld G., Singer J., Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 94.Buur-Rasmussen B., Brosen K. Cytochrome P450 and therapeutic drug monitoring with respect to clozapine. Eur. Neuropsychopharmacol. 1999;9(6):453–459. doi: 10.1016/s0924-977x(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 95.Fang J., Coutts R.T., McKenna K.F., Baker G.B. Elucidation of individual cytochrome P450 enzymes involved in the metabolism of clozapine. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358(5):592–599. doi: 10.1007/pl00005298. [DOI] [PubMed] [Google Scholar]
- 96.Rostami-Hodjegan A., Amin A.M., Spencer E.P., Lennard M.S., Tucker G.T., Flanagan R.J. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J. Clin. Psychopharmacol. 2004;24(1):70–78. doi: 10.1097/01.jcp.0000106221.36344.4d. [DOI] [PubMed] [Google Scholar]
- 97.Heusler P., Bruins Slot L., Tourette A., Tardif S., Cussac D. The clozapine metabolite N-desmethylclozapine displays variable activity in diverse functional assays at human dopamine D(2) and serotonin 5-HT(1)A receptors. Eur. J. Pharmacol. 2011;669(1-3):51–58. doi: 10.1016/j.ejphar.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 98.Ng W., Kennar R., Uetrecht J. Effect of clozapine and olanzapine on neutrophil kinetics: implications for drug-induced agranulocytosis. Chem. Res. Toxicol. 2014;27(7):1104–1108. doi: 10.1021/tx500183x. [DOI] [PubMed] [Google Scholar]
- 99.Williams D.P., Pirmohamed M., Naisbitt D.J., Maggs J.L., Park B.K. Neutrophil cytotoxicity of the chemically reactive metabolite(s) of clozapine: possible role in agranulocytosis. J. Pharmacol. Exp. Ther. 1997;283(3):1375–1382. [PubMed] [Google Scholar]
- 100.Tolosa-Vilella C., Ruiz-Ripoll A., Mari-Alfonso B., Naval-Sendra E. Olanzapine-induced agranulocytosis: a case report and review of the literature. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(2):411–414. doi: 10.1016/s0278-5846(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 101.Li X., Cameron M.D. Potential role of a quetiapine metabolite in quetiapine-induced neutropenia and agranulocytosis. Chem. Res. Toxicol. 2012;25(5):1004–1011. doi: 10.1021/tx2005635. [DOI] [PubMed] [Google Scholar]
- 102.Ishibashi T., Horisawa T., Tokuda K., Ishiyama T., Ogasa M., Tagashira R., Matsumoto K., Nishikawa H., Ueda Y., Toma S., Oki H., Tanno N., Saji I., Ito A., Ohno Y., Nakamura M. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 2010;334(1):171–181. doi: 10.1124/jpet.110.167346. [DOI] [PubMed] [Google Scholar]
- 103.U.S. Food and Drug Administration. Lurasidone Pharmacology Review(s). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200603O rig1s000PharmR.pdf. Accessed January 26. 2016.
- 104.Berecz R., Dorado P., De La Rubia A., Caceres M.C., Degrell I. A, L. L., The role of cytochrome P450 enzymes in the metabolism of risperidone and its clinical relevance for drug interactions. Curr. Drug Targets. 2004;5(6):573–579. doi: 10.2174/1389450043345263. [DOI] [PubMed] [Google Scholar]
- 105.Burris K.D., Molski T.F., Xu C., Ryan E., Tottori K., Kikuchi T., Yocca F.D., Molinoff P.B. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 2002;302(1):381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 106.Lieberman J.A. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251–267. doi: 10.2165/00023210-200418040-00005. [DOI] [PubMed] [Google Scholar]
- 107.Hirose T., Kikuchi T. Aripiprazole, a novel antipsychotic agent: dopamine D2 receptor partial agonist. J. Med. Invest. 2005;52(Suppl.):284–290. doi: 10.2152/jmi.52.284. [DOI] [PubMed] [Google Scholar]
- 108.U.S. Food and Drug Administration. ABILIFY (aripiprazole) drug label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/ 021436s038,021713s030,021729s022,021866s023lbl.pdf . Accessed September 4, 2015.
- 109.Stark A.D., Jordan S., Allers K.A., Bertekap R.L., Chen R., Mistry Kannan T., Molski T.F., Yocca F.D., Sharp T., Kikuchi T., Burris K.D. Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT 2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacology (Berl.) 2007;190(3):373–382. doi: 10.1007/s00213-006-0621-y. [DOI] [PubMed] [Google Scholar]
- 110.de Araujo A.N., de Sena E.P., de Oliveira I.R., Juruena M.F. Antipsychotic agents: efficacy and safety in schizophrenia. Drug Healthc. Patient Saf. 2012;4:173–180. doi: 10.2147/DHPS.S37429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zajdel P., Partyka A., Marciniec K., Bojarski A.J., Pawlowski M., Wesolowska A. Quinoline- and isoquinoline-sulfonamide analogs of aripiprazole: novel antipsychotic agents? Future Med. Chem. 2014;6(1):57–75. doi: 10.4155/fmc.13.158. [DOI] [PubMed] [Google Scholar]
- 112.Winans E. Aripiprazole. Am. J. Health Syst. Pharm. 2003;60(23):2437–2445. doi: 10.1093/ajhp/60.23.2437. [DOI] [PubMed] [Google Scholar]
- 113.Kirschbaum K.M., Muller M.J., Malevani J., Mobascher A., Burchardt C., Piel M., Hiemke C. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J. Biol. Psychiatry. 2008;9(3):212–218. doi: 10.1080/15622970701361255. [DOI] [PubMed] [Google Scholar]
- 114.Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int. J. Clin. Pract. 2015;69(9):978–997. doi: 10.1111/ijcp.12714. [DOI] [PubMed] [Google Scholar]
- 115.Citrome L. Brexpiprazole: a new dopamine D2 receptor partial agonist for the treatment of schizophrenia and major depressive disorder. Drugs Today (Barc) 2015;51(7):397–414. doi: 10.1358/dot.2015.51.7.2358605. [DOI] [PubMed] [Google Scholar]
- 116.FDA News Release. FDA approves new drug to treat schizophrenia and as an add on to an antidepressant to treat major depressive disorder.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm454647.htm . Accessed September 20, 2015.
- 117.FDA News Release. FDA approves new drug to treat schizophrenia and bipolar disorder.http://www.fda.gov/newsevents/newsroom/ pressannouncements/ucm463103.htm . Accessed October 12,2015.
- 118.Kiss B., Horvath A., Nemethy Z., Schmidt E., Laszlovszky I., Bugovics G., Fazekas K., Hornok K., Orosz S., Gyertyan I., Agai-Csongor E., Domany G., Tihanyi K., Adham N., Szombathelyi Z. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J. Pharmacol. Exp. Ther. 2010;333(1):328–340. doi: 10.1124/jpet.109.160432. [DOI] [PubMed] [Google Scholar]
- 119.Citrome L. The ABC's of dopamine receptor partial agonists - aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int. J. Clin. Pract. 2015 doi: 10.1111/ijcp.12752. [DOI] [PubMed] [Google Scholar]
- 120.Shapiro D.A., Renock S., Arrington E., Chiodo L.A., Liu L.X., Sibley D.R., Roth B.L., Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 121.Maeda K., Sugino H., Akazawa H., Amada N., Shimada J., Futamura T., Yamashita H., Ito N., McQuade R.D., Mork A., Pehrson A.L., Hentzer M., Nielsen V., Bundgaard C., Arnt J., Stensbol T.B., Kikuchi T. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 2014;350(3):589–604. doi: 10.1124/jpet.114.213793. [DOI] [PubMed] [Google Scholar]
- 122.U.S. Food and Drug Administration. REXULTI brexpiprazole drug label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205422 s000lbl.pdf . Accessed September 4, 2015.
- 123.Carty N.C., Xu J., Kurup P., Brouillette J., Goebel-Goody S.M., Austin D.R., Yuan P., Chen G., Correa P.R., Haroutunian V., Pittenger C., Lombroso P.J. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl. Psychiatry. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lieberman J.A., Davis R.E., Correll C.U., Goff D.C., Kane J.M., Tamminga C.A., Mates S., Vanover K.E. ITI-007 for the Treatment of Schizophrenia: A 4-Week Randomized, Double-Blind, Controlled Trial. Biol. Psychiatry. 2016;79(12):952–961. doi: 10.1016/j.biopsych.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 125.Intra-Celular Therapies Press Release. Intra-Cellular Therapies Announces Positive Top-Line Results From the First Phase 3 Trial of ITI-007 in Patients With Schizophrenia and Confirms the Unique Pharmacology of ITI-007 in a Separate Positron Emission Tomography Study. http://ir.intracellulartherapies.com/ releasedetail.cfm?ReleaseID=931821 . Accessed March 05, 2016.
- 126.Newman-Tancredi A., Assie M.B., Leduc N., Ormiere A.M., Danty N., Cosi C. Novel antipsychotics activate recombinant human and native rat serotonin 5-HT1A receptors: affinity, efficacy and potential implications for treatment of schizophrenia. Int. J. Neuropsychopharmacol. 2005;8(3):341–356. doi: 10.1017/S1461145704005000. [DOI] [PubMed] [Google Scholar]
- 127.Newman-Tancredi A. The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr. Opin. Investig. Drugs. 2010;11(7):802–812. [PubMed] [Google Scholar]
- 128.Kleven M.S., Barret-Grevoz C., Bruins Slot L., Newman-Tancredi A. Novel antipsychotic agents with 5-HT(1A) agonist properties: role of 5-HT(1A) receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology. 2005;49(2):135–143. doi: 10.1016/j.neuropharm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 129.Bruins Slot L.A., Kleven M.S., Newman-Tancredi A. Effects of novel antipsychotics with mixed D(2) antagonist/5-HT(1A) agonist properties on PCP-induced social interaction deficits in the rat. Neuropharmacology. 2005;49(7):996–1006. doi: 10.1016/j.neuropharm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 130.Casey D.E., Sands E.E., Heisterberg J., Yang H.M. Efficacy and safety of bifeprunox in patients with an acute exacerbation of schizophrenia: results from a randomized, double-blind, placebo-controlled, multicenter, dose-finding study. Psychopharmacology (Berl.) 2008;200(3):317–331. doi: 10.1007/s00213-008-1207-7. [DOI] [PubMed] [Google Scholar]
- 131.ClinicalTrial.Gov. Efficacy of Bifeprunox in patients With schizophrenia. https://clinicaltrials.gov/ct2/show/ https://clinicaltrials.gov/ct2/show/ Accessed February 27, 2016.
- 132.Lundbeck Press Release. Pipeline update - following an interim analysis the studies with bifeprunox for the treatment of schizophrenia is discontinued. http://investor.lundbeck.com/ releasedetail.cfm?releaseid=608617 . Accessed February 27, 2016.
- 133.Bogeso K.P., Arnt J., Frederiksen K., Hansen H.O., Hyttel J., Pedersen H. Enhanced D1 affinity in a series of piperazine ring substituted 1-piperazino-3-arylindans with potential atypical antipsychotic activity. J. Med. Chem. 1995;38(22):4380–4392. doi: 10.1021/jm00022a004. [DOI] [PubMed] [Google Scholar]
- 134.Lundbeck Press Release. Zicronapine shows significant positive data in clinical phase II in the treatment of patients with schizophrenia - planning for continued clinical work. http://investor.lundbeck.com/releasedetail.cfm?ReleaseID=608605 . Accessed February 27, 2016.
- 135.ClinicalTrial.Gov. Efficacy of Lu 31-130 in Patients With Schizophrenia. https://www.clinicaltrials.gov/ct2/show. Accessed February 27. 2016.
- 136.Lundbeck Corporate Release. Performance in 2014 positions Lundbeck well for 2015 and beyond. http://www.euroinvestor.dk/ pdf/cse/2015/02/13101242/Lundbeck%20FY2014%20Results.pdf. Accessed February 27. 2016.
- 137.ClinicalTrial.Gov. Positron Emission Tomography (PET) Study Investigating Dopamine and Serotonin Receptor Occupancy After Multiple Oral Dosing of Lu AF35700. https://www. clinicaltrials.gov/ct2/show. Accessed February 27. 2016.
- 138.Lundbeck Press Release. Lundbeck starts clinical phase III program with Lu AF35700 in patients with treatment resistant schizophrenia. http://investor.lundbeck.com. Accessed March 11. 2016.
- 139.Cantillon M. EFFICACY AND SAFETY OF NOVEL DOPAMINE SEROTONIN STABILIZER RP 5063 IN ACUTE SCHIZOPHRENIA AND SCHIZOAFFECTIVE DISORDER. Schizophr. Res. 2014;153(Suppl. 1):S22. doi: 10.1016/j.schres.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 140.ClinicalTrial.Gov. https://www.clinicaltrials. gov/ct2/show/results. Accessed February 27. 2016.
- 141.Reviva Pharmaceuticals Press Release: Reviva Pharmaceuticals Reports RP5063 Positive Efficacy Results for Memory Deficits. http://revivapharma.com/wp-content/uploads/2015/03/2015-1022- RS-Reviva-News-Release.pdf. Accessed February 27. 2016.
- 142.Burstein E.S., Ma J., Wong S., Gao Y., Pham E., Knapp A.E., Nash N.R., Olsson R., Davis R.E., Hacksell U., Weiner D.M., Brann M.R. Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. J. Pharmacol. Exp. Ther. 2005;315(3):1278–1287. doi: 10.1124/jpet.105.092155. [DOI] [PubMed] [Google Scholar]
- 143.U.S. Food and Drug Administration CLOZAPINE drug label.http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203039 Orig1s000lbl.pdf. Accessed January 24. 2016.
- 144.U.S. Food and Drug Administration. RISPERDAL (risperidone) drug label.http://www.accessdata.fda.gov/ drugsatfda_docs/label/ 2014/020272s073,020588s062,021444s048lbl.pdf. Accessed January 26. 2016.
- 145.Lapish C.C., Belardetti F., Ashby D.M., Ahn S., Butts K.A., So K., Macrae C.M., Hynd J.J., Miller J.J., Phillips A.G. A preclinical assessment of d.l-govadine as a potential antipsychotic and cognitive enhancer. Int. J. Neuropsychopharmacol. 2012;15(10):1441–1455. doi: 10.1017/S146114571100157X. [DOI] [PubMed] [Google Scholar]
- 146.Arnt J., Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18(2):63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 147.Sánchez C., Arnt J., Dragsted N., Hyttel J., Lembøl H.L., Meier E., Perregaard J., Skarsfeldt T. Neurochemical and in vivo pharmacological profile of sertindole, a limbic-selective neuroleptic compound. Drug Dev. Res. 1991;22(3):239–250. [Google Scholar]
- 148.Kikuchi T., Tottori K., Uwahodo Y., Hirose T., Miwa T., Oshiro Y., Morita S. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinon e (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J. Pharmacol. Exp. Ther. 1995;274(1):329–336. [PubMed] [Google Scholar]
- 149.U.S. Food and Drug Administration. HALDOL (haloperidol) drug label.http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/ 015923s084lbl.pdf. Accessed January 19. 2016.
- 150.Kapur S., Remington G., Jones C., Wilson A., DaSilva J., Houle S., Zipursky R. High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am. J. Psychiatry. 1996;153(7):948–950. doi: 10.1176/ajp.153.7.948. [DOI] [PubMed] [Google Scholar]
- 151.Kapur S., Zipursky R., Roy P., Jones C., Remington G., Reed K., Houle S. The relationship between D2 receptor occupancy and plasma levels on low dose oral haloperidol: a PET study. Psychopharmacology (Berl.) 1997;131(2):148–152. doi: 10.1007/s002130050277. [DOI] [PubMed] [Google Scholar]
- 152.Tauscher J., Hussain T., Agid O., Verhoeff N.P., Wilson A.A., Houle S., Remington G., Zipursky R.B., Kapur S. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am. J. Psychiatry. 2004;161(9):1620–1625. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- 153.Farah A. Atypicality of atypical antipsychotics. Prim. Care Companion J. Clin. Psychiatry. 2005;7(6):268–274. doi: 10.4088/pcc.v07n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kegeles L.S., Slifstein M., Frankle W.G., Xu X., Hackett E., Bae S.A., Gonzales R., Kim J.H., Alvarez B., Gil R., Laruelle M., Abi-Dargham A. Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacology. 2008;33(13):3111–3125. doi: 10.1038/npp.2008.33. [DOI] [PubMed] [Google Scholar]
- 155.Yokoi F., Grunder G., Biziere K., Stephane M., Dogan A.S., Dannals R.F., Ravert H., Suri A., Bramer S., Wong D.F. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248–259. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 156.Davis R.E., Vanover K.E., Zhou Y., Brasic J.R., Guevara M., Bisuna B., Ye W., Raymont V., Willis W., Kumar A., Gapasin L., Goldwater D.R., Mates S., Wong D.F. ITI-007 demonstrates brain occupancy at serotonin 5-HT(2)A and dopamine D(2) receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl.) 2015;232(15):2863–2872. doi: 10.1007/s00213-015-3922-1. [DOI] [PubMed] [Google Scholar]
- 157.Creese I., Burt D.R., Snyder S.H. Dopamine receptors and average clinical doses. Science. 1976;194(4264):546. doi: 10.1126/science.194.4264.546. [DOI] [PubMed] [Google Scholar]
- 158.Kebabian J.W., Calne D.B. Multiple receptors for dopamine. Nature. 1979;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 159.Stoof J.C., Kebabian J.W. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294(5839):366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- 160.Miyamoto S., Miyake N., Jarskog L.F., Fleischhacker W.W., Lieberman J.A. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry. 2012;17(12):1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 161.Meltzer H.Y., Fatemi S.H. The role of serotonin in schizophrenia and the mechanism of action of antipsychotic drugs. In: Kane J.M., Möller H-J., Awouters F., editors. Serotonin in antipsychotic treatment: Mechanisms and clinical practice. New York: Marcel Dekker; 1996. pp. 77–107. [Google Scholar]
- 162.Kroeze W.K., Hufeisen S.J., Popadak B.A., Renock S.M., Steinberg S., Ernsberger P., Jayathilake K., Meltzer H.Y., Roth B.L. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 163.Lieberman J.A., Stroup T.S., McEvoy J.P., Swartz M.S., Rosenheck R.A., Perkins D.O., Keefe R.S., Davis S.M., Davis C.E., Lebowitz B.D., Severe J., Hsiao J.K. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 164.Nasrallah H.A. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol. Psychiatry. 2008;13(1):27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- 165.Matsui-Sakata A., Ohtani H., Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab. Pharmacokinet. 2005;20(5):368–378. doi: 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- 166.Wirshing D.A., Wirshing W.C., Kysar L., Berisford M.A., Goldstein D., Pashdag J., Mintz J., Marder S.R. Novel antipsychotics: comparison of weight gain liabilities. J. Clin. Psychiatry. 1999;60(6):358–363. [PubMed] [Google Scholar]
- 167.Nguyen C.T., Rosen J.A., Bota R.G. Aripiprazole partial agonism at 5-HT2C: a comparison of weight gain associated with aripiprazole adjunctive to antidepressants with high versus low serotonergic activities. Prim. Care Companion CNS Disord. 2012;14(5) doi: 10.4088/PCC.12m01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Costall B., Naylor R.J. A comparison of the abilities of typical neuroleptic agents and of thioridazine, clozapine, sulpiride and metoclopramide to antagonise the hyperactivity induced by dopamine applied intracerebrally to areas of the extrapyramidal and mesolimbic systems. Eur. J. Pharmacol. 1976;40(1):9–19. doi: 10.1016/0014-2999(76)90348-4. [DOI] [PubMed] [Google Scholar]
- 169.Costall B., Fortune D.H., Hui S.C., Naylor R.J. Neuroleptic antagonism of the motor inhibitory effects of apomorphine within the nucleus accumbens: drug interaction at presynaptic receptors? Eur. J. Pharmacol. 1980;63(4):347–358. doi: 10.1016/0014-2999(80)90265-4. [DOI] [PubMed] [Google Scholar]
- 170.Ichikawa J., Ishii H., Bonaccorso S., Fowler W.L., O'Laughlin I.A., Meltzer H.Y. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J. Neurochem. 2001;76(5):1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 171.Ichikawa J., Li Z., Dai J., Meltzer H.Y. Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res. 2002;956(2):349–357. doi: 10.1016/s0006-8993(02)03570-9. [DOI] [PubMed] [Google Scholar]
- 172.Nakai S., Hirose T., Uwahodo Y., Imaoka T., Okazaki H., Miwa T., Nakai M., Yamada S., Dunn B., Burris K.D., Molinoff P.B., Tottori K., Altar C.A., Kikuchi T. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur. J. Pharmacol. 2003;472(1-2):89–97. doi: 10.1016/s0014-2999(03)01857-0. [DOI] [PubMed] [Google Scholar]
- 173.Farde L., Nordstrom A.L. PET analysis indicates atypical central dopamine receptor occupancy in clozapine-treated patients. Br. J. Psychiatry Suppl. 1992;(17):30–33. [PubMed] [Google Scholar]
- 174.Farde L., Nordstrom A.L., Wiesel F.A., Pauli S., Halldin C., Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch. Gen. Psychiatry. 1992;49(7):538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 175.Nyberg S., Eriksson B., Oxenstierna G., Halldin C., Farde L. Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am. J. Psychiatry. 1999;156(6):869–875. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- 176.Nordstrom A.L., Farde L., Wiesel F.A., Forslund K., Pauli S., Halldin C., Uppfeldt G. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol. Psychiatry. 1993;33(4):227–235. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- 177.Nordstrom A.L., Farde L. Plasma prolactin and central D2 receptor occupancy in antipsychotic drug-treated patients. J. Clin. Psychopharmacol. 1998;18(4):305–310. doi: 10.1097/00004714-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 178.Wong D.F., Kuwabara H., Brasic J.R., Stock T., Maini A., Gean E.G., Loebel A. Determination of dopamine D(2) receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology (Berl.) 2013;229(2):245–252. doi: 10.1007/s00213-013-3103-z. [DOI] [PubMed] [Google Scholar]
- 179.Kapur S., Zipursky R., Jones C., Remington G., Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am. J. Psychiatry. 2000;157(4):514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 180.Tsuboi T., Bies R.R., Suzuki T., Mamo D.C., Pollock B.G., Graff-Guerrero A., Mimura M., Uchida H. Hyperprolactinemia and estimated dopamine D2 receptor occupancy in patients with schizophrenia: analysis of the CATIE data. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:178–182. doi: 10.1016/j.pnpbp.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 181.Farde L., Nyberg S., Oxenstierna G., Nakashima Y., Halldin C., Ericsson B. Positron emission tomography studies on D2 and 5-HT2 receptor binding in risperidone-treated schizophrenic patients. J. Clin. Psychopharmacol. 1995;15(1) Suppl. 1:19S–23S. doi: 10.1097/00004714-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 182.Kapur S., Remington G., Zipursky R.B., Wilson A.A., Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci. 1995;57(10):PL103–PL107. doi: 10.1016/0024-3205(95)02037-j. [DOI] [PubMed] [Google Scholar]
- 183.Kapur S., Zipursky R.B., Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am. J. Psychiatry. 1999;156(2):286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 184.Mamo D., Graff A., Mizrahi R., Shammi C.M., Romeyer F., Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am. J. Psychiatry. 2007;164(9):1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- 185.Nordstrom A.L., Farde L., Nyberg S., Karlsson P., Halldin C., Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am. J. Psychiatry. 1995;152(10):1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- 186.Kessler R.M., Ansari M.S., Riccardi P., Li R., Jayathilake K., Dawant B., Meltzer H.Y. Occupancy of striatal and extrastriatal dopamine D2 receptors by clozapine and quetiapine. Neuropsychopharmacology. 2006;31(9):1991–2001. doi: 10.1038/sj.npp.1301108. [DOI] [PubMed] [Google Scholar]
- 187.Volavka J., Czobor P., Sheitman B., Lindenmayer J.P., Citrome L., McEvoy J.P., Cooper T.B., Chakos M., Lieberman J.A. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am. J. Psychiatry. 2002;159(2):255–262. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]
- 188.Bitter I., Czobor P., Dossenbach M., Volavka J. Effectiveness of clozapine, olanzapine, quetiapine, risperidone, and haloperidol monotherapy in reducing hostile and aggressive behavior in outpatients treated for schizophrenia: a prospective naturalistic study (IC-SOHO). Eur. Psychiatry. 2005;20(5-6):403–408. doi: 10.1016/j.eurpsy.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 189.Volavka J., Czobor P., Cooper T.B., Sheitman B., Lindenmayer J.P., Citrome L., McEvoy J.P., Lieberman J.A. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. J. Clin. Psychiatry. 2004;65(1):57–61. doi: 10.4088/jcp.v65n0109. [DOI] [PubMed] [Google Scholar]
- 190.Carlson C.D., Cavazzoni P.A., Berg P.H., Wei H., Beasley C.M., Kane J.M. An integrated analysis of acute treatment-emergent extrapyramidal syndrome in patients with schizophrenia during olanzapine clinical trials: comparisons with placebo, haloperidol, risperidone, or clozapine. J. Clin. Psychiatry. 2003;64(8):898–906. doi: 10.4088/jcp.v64n0807. [DOI] [PubMed] [Google Scholar]
- 191.Robinson D.G., Gallego J.A., John M., Petrides G., Hassoun Y., Zhang J.P., Lopez L., Braga R.J., Sevy S.M., Addington J., Kellner C.H., Tohen M., Naraine M., Bennett N., Greenberg J., Lencz T., Correll C.U., Kane J.M., Malhotra A.K. A Randomized Comparison of Aripiprazole and Risperidone for the Acute Treatment of First-Episode Schizophrenia and Related Disorders: 3-Month Outcomes. Schizophr. Bull. 2015;41(6):1227–1236. doi: 10.1093/schbul/sbv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Leucht S., Cipriani A., Spineli L., Mavridis D., Orey D., Richter F., Samara M., Barbui C., Engel R.R., Geddes J.R., Kissling W., Stapf M.P., Lassig B., Salanti G., Davis J.M. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 193.ClinicalTrial.Gov A Trial to Assess the Antipsychotic Efficacy of ITI-007.
- 194.Kurz M., Hummer M., Oberbauer H., Fleischhacker W.W. Extrapyramidal side effects of clozapine and haloperidol. Psychopharmacology (Berl.) 1995;118(1):52–56. doi: 10.1007/BF02245249. [DOI] [PubMed] [Google Scholar]
- 195.Fleischhaker C., Heiser P., Hennighausen K., Herpertz-Dahlmann B., Holtkamp K., Mehler-Wex C., Rauh R., Remschmidt H., Schulz E., Warnke A. Weight gain associated with clozapine, olanzapine and risperidone in children and adolescents. J. Neural Transm (Vienna) 2007;114(2):273–280. doi: 10.1007/s00702-006-0602-7. [DOI] [PubMed] [Google Scholar]
- 196.Citrome L., Kalsekar I., Baker R.A., Hebden T. A review of real-world data on the effects of aripiprazole on weight and metabolic outcomes in adults. Curr. Med. Res. Opin. 2014;30(8):1629–1641. doi: 10.1185/03007995.2014.908280. [DOI] [PubMed] [Google Scholar]
- 197.Buckley N.A., Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215–228. doi: 10.2165/00002018-200023030-00004. [DOI] [PubMed] [Google Scholar]
- 198.Correll C.U., Joffe B.I., Rosen L.M., Sullivan T.B., Joffe R.T. Cardiovascular and cerebrovascular risk factors and events associated with second-generation antipsychotic compared to antidepressant use in a non-elderly adult sample: results from a claims-based inception cohort study. World Psychiatry. 2015;14(1):56–63. doi: 10.1002/wps.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sahlberg M., Holm E., Gislason G.H., Kober L., Torp-Pedersen C., Andersson C. Association of Selected Antipsychotic Agents With Major Adverse Cardiovascular Events and Noncardiovascular Mortality in Elderly Persons. J. Am. Heart Assoc. 2015;4(9):e001666. doi: 10.1161/JAHA.114.001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wooltorton E. Antipsychotic clozapine (Clozaril): myocarditis and cardiovascular toxicity. CMAJ. 2002;166(9):1185–1186. [PMC free article] [PubMed] [Google Scholar]
- 201.Swartz M.S., Perkins D.O., Stroup T.S., Davis S.M., Capuano G., Rosenheck R.A., Reimherr F., McGee M.F., Keefe R.S., McEvoy J.P., Hsiao J.K., Lieberman J.A. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am. J. Psychiatry. 2007;164(3):428–436. doi: 10.1176/ajp.2007.164.3.428. [DOI] [PubMed] [Google Scholar]