Abstract
The aggregation of the microtubule-associated protein tau is a seminal event in many neurodegenerative diseases, including Alzheimer’s disease. The inhibition or reversal of tau aggregation is therefore a potential therapeutic strategy for these diseases. Fungal natural products have proven to be a rich source of useful compounds having wide varieties of biological activities. We have previously screened Aspergillus nidulans secondary metabolites for their ability to inhibit tau aggregation in vitro using an arachidonic acid polymerization protocol. One aggregation inhibitor identified was asperbenzaldehyde, an intermediate in azaphilone biosynthesis. We therefore tested 11 azaphilone derivatives to determine their tau assembly inhibition properties in vitro. All compounds tested inhibited tau filament assembly to some extent, while four of the 11 compounds had the advantageous property of disassembling preformed tau aggregates in a dose-dependent fashion. The addition of these compounds to the tau aggregates reduced both the total length and numbers of tau polymers. The most potent compounds were tested in in vitro reactions to determine whether they interfere with tau’s normal function of stabilizing microtubules (MTs). We found that they did not completely inhibit MT assembly in the presence of tau. These derivatives are very promising lead compounds for tau aggregation inhibitors and, more excitingly, for compounds that can disassemble pre-existing tau filaments. They also represent a new class of anti-tau aggregation compounds with a novel structural scaffold.
Keywords: Tau, microtubule-associated protein, aggregation inhibitor, Alzheimer’s disease, azaphilone, natural products, Aspergillus, Aspergillus nidulans
Graphical Abstract
Introduction
Alzheimer’s disease (AD) is the most common form of dementia. This devastating condition is made worse by the relative lack of therapies available for its treatment. Current therapeutics largely target cholinergic pathways and do little to slow or reverse the accumulation of aggregates of either beta amyloid or the microtubule-associated protein tau into senile plaques or neurofibrillary tangles respectively. The location and amount of tau aggregation into neurofibrillary tangles directly correlates with the type and severity of the disease progression1. Therefore, there is great interest in identifying small molecules that may inhibit or reverse tau aggregation. Recently a tau aggregation inhibitor, a stable, reduced form of methylthioninium chloride, has reached phase 3 clinical trials2, validating the potential of tau aggregation as a target, but there is certainly a need for additional lead anti-tau aggregation compounds for further development into therapeutics.
An ideal tau aggregation inhibitor should inhibit the assembly of tau aggregates and disassemble pre-formed tau aggregates as well. Inhibitors of tau aggregation also should not impair the normal function of tau to bind and stabilize microtubules. Previously identified tau aggregation inhibitors, including molecules belonging to the anthraquinone class, have had widely diverse structures, with fused ring structures being a commonly occurring structural motif3. Recent emphasis has been placed on identifying natural products with novel scaffolds that may have useful properties for the treatment of AD by inhibiting tau aggregation4, 5.
Fungi have historically been a good source of secondary metabolites, which have useful pharmacological properties such as antibiotics, immunosuppressants, and cholesterol-lowering drugs, among others6. We have identified numerous biosynthetic pathways in Aspergillus nidulans that lead to production of a wide range of secondary metabolites6–9. In a previous study, we tested several A. nidulans secondary metabolites for their ability to inhibit tau aggregation in vitro and found that several were active inhibitors at micromolar concentrations, although they did not have tau disaggregation properties10. Among these, two, ω-dihydroxyemodin and asperthecin, belonged to the anthraquinone class of compounds, a class that includes compounds shown to inhibit tau aggregation. A third compound, asperbenzaldehyde, however, was structurally distinct from previously identified tau aggregation inhibitors. Asperbenzaldehyde is also interesting in that it is an intermediate in the biological synthesis of azaphilone compounds11.
Azaphilones are known to exhibit a great variety of biologically important activities including inhibitions of gp120–CD4 binding12 and heat shock protein 90 (Hsp90)13–15, among others. Several azaphilones have been shown to have lipoxygenase inhibitor activity11. Inhibition of lipoxygenases may help reduce fatty acid metabolite levels that are elevated in AD16. Azaphilones, including lipoxygenase-inhibiting azaphilones, can be obtained from asperbenzaldehyde using a 2–3 step semisynthetic route11. We therefore sought to determine whether azaphilones derived from asperbenzaldehyde inhibit tau aggregation, hoping that they might be a useful step in finding compounds with two biological targets relevant to treating AD.
Beginning with asperbenzaldehyde, which was purified from a fungal strain engineered to overproduce this compound, the azaphilones were prepared as previously described using two schemes11. The first employs p-toluenesulfonic acid to form the 2-benzopyrilium salt followed by oxidation by lead tetraacetate with or without halogenation. The second scheme employs the hypervalent-iodine-mediated phenol oxidative dearomatization of the 2-benzopyrilium salt with o-iodoxy-benzoic acid followed by halogenation and/or a Wittig olefination with carbethoxymethylenetriphenylphosphorane.
Using standard biochemical assays, we investigated the ability of these compounds to alter the aggregation of tau and its stabilization of microtubules. We found that while all compounds inhibited tau aggregation, a smaller subset had the added activity of disassembling pre-formed tau aggregates. The compounds most effective at inhibiting tau aggregation and disassembling pre-formed tau filaments also allowed tau to retain the majority of its microtubule stabilizing functions.
Results
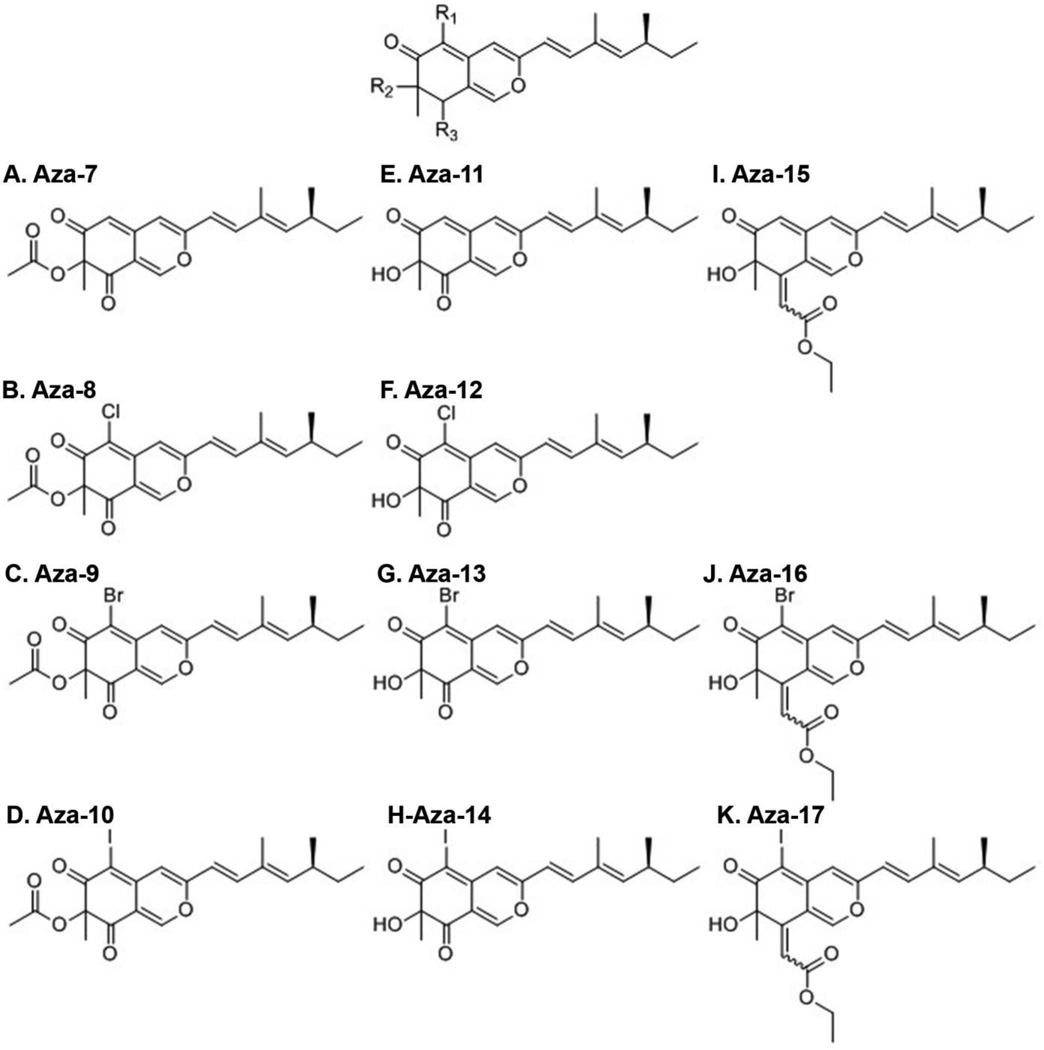
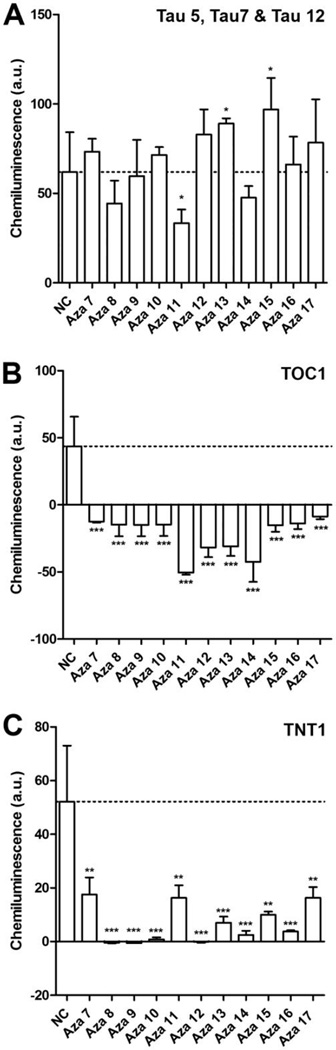
Eleven compounds with the same azaphilone backbone differing at three points of diversity (R1, R2, and R3) were used in this study (Figure 1). Tau polymerization was initiated in vitro using a standard arachidonic acid induction assay17. To determine whether the compounds could inhibit assembly of tau filaments, each of the compounds, at a final concentration of 200 µM, was preincubated with 2 µM tau for 20 min before the addition of 75 µM arachidonic acid. The degree of tau aggregation inhibition for each compound was determined using a membrane filter assay18. This assay has been used previously to screen A. nidulans secondary metabolites including anthraquinones, xanthones, polyketides, a benzophenone and the asperbenzaldehyde compound that was the parent compound for the synthesis of the azaphilones used in this study10. A mixture of antibodies to the amino terminal region, central region and carboxy terminal region of tau (tau 12, tau 5, and tau 7 respectively) was used to detect tau aggregates. In this assay, only compound aza-11 significantly reduced the amount of tau aggregation detected (Figure 2A). Compounds aza-13 and aza-15 significantly increased the amount of tau aggregation and the remaining compounds had no significant effect (Figure 2A). However, when antibodies against toxic species of tau were employed for detection, very different results were observed. All aza compounds completely abolished recognition by the TOC1 antibody, which recognizes toxic oligomers in vitro and in Alzheimer’s disease tissue19, as compared to controls without compound (Figure 2B). Similarly, significant reductions in recognition by TNT1, an antibody that recognizes the phosphatase-activating domain of tau and is exposed in pathological forms of tau20, were observed for all aza compounds as compared to controls without compound (Figure 2C).
Figure 1. Compounds used in this study.
The core structure of the azaphilone compounds is shown with the positions of modications indicated by R1, R2 and R3. A.-K.: The structures of the 11 compounds used in this study: A) aza-7, B) aza-8, C) aza-9, D) aza-10, E) aza-11, F) aza-12, G) aza-13, H) aza-14, I) aza-15, J) aza-16, and K) aza-17.
Figure 2. Filter trap assay of tau filament formation.
Tau polymerization reactions in A), B), and C) were performed with 2 µM tau and 75 µM arachidonic acid either with or without 200 µM compound. The compounds used are listed on the X- axis. The resulting amount of tau filament formation was determined using a filter trap assay. The values for tau filament formation were normalized to the amount of aggregation in the absence of compound (dashed line). Negative values indicate that there was less detectable tau on the filter after treatment with a compound than was observed with monomeric tau in the absence of arachidonic acid. The amount of tau on the filter was detected using A) a mixture of antibodies tau 5, tau 7 and tau 12, B) antibody TOC1, and C) antibody TNT1. The observation that the values are lower in B) and C) suggests that the tau aggregates that are retained on the filters are not in the toxic oligomeric form recognized by the TOC1 antibody and the phosphatase domain recognized by TNT1 antibody is not exposed. Values are the average of three trials ± SD. *P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001
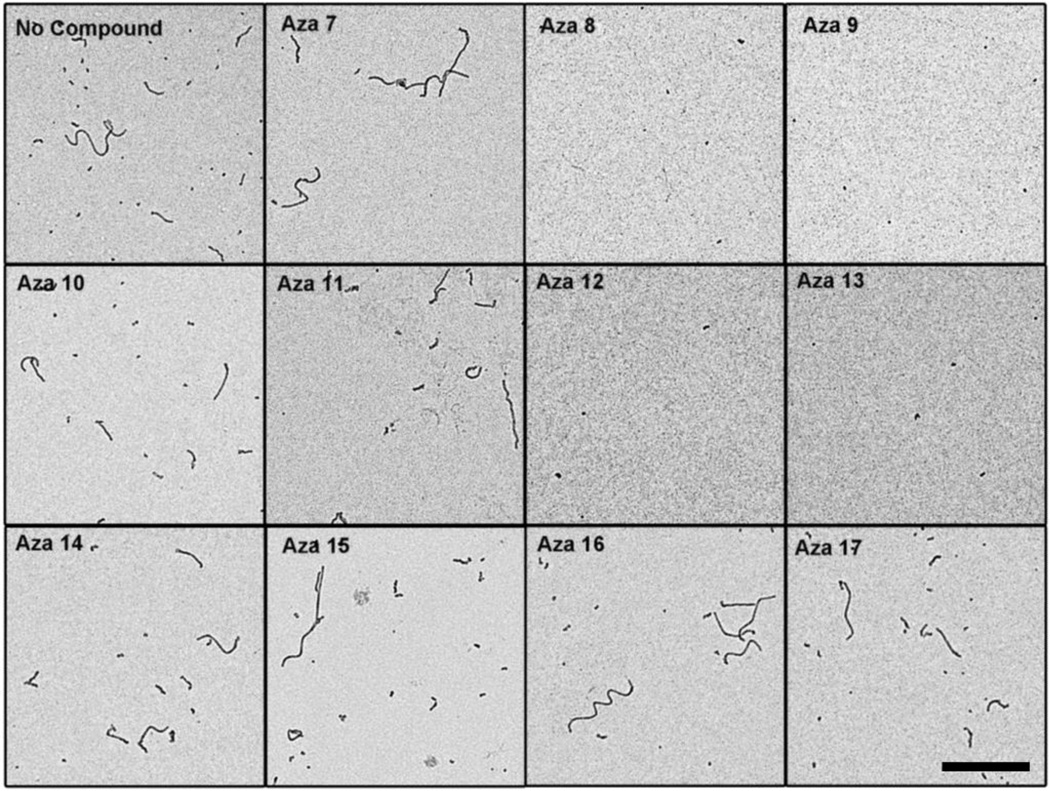
When the resulting tau aggregates from the inhibition reactions were visualized by electron microscopy, there were abundant numbers of long filaments in the absence of added compound (Figure 3). Surprisingly, all the azaphilones inhibited the formation of long tau filaments that were observed in the absence of compounds. Instead, amorphous small aggregates were observed after treatment with the compounds (Figure 3). This degree of tau aggregation inhibition was similar to what was observed by electron microscopy for asperbenzaldehyde and asperthecin, and stronger than what was observed for 2,ω-dihydroxyemodin in a prior study10. Because tau aggregation inhibitors that inhibit filament formation have previously been shown to stabilize off-pathway soluble oligomers that are large enough to be trapped in the membrane filter assay21, we believe that the mixture of tau antibodies to normal tau was detecting these aggregates in the filter trap assay (Figure 2A). These aggregates do not seem to be toxic because of their lack of reactivity to TOC1 (Figure 2B) and TNT1 (Figure 2C).
Figure 3. Electron microscopy of tau filament formation in the presence of azaphilone derivatives.
Tau polymerization reactions were performed with 2 µM tau and 75 µM arachidonic acid either with or without 200 µM compound. Aliquots of the reactions were prepared for negative stain electron microscopy. Representative images are shown for A) no compound control, B) aza-7, C) aza-8, D) aza-9, E) aza-10, F) aza-11, G) aza-12, H) aza-13, I) aza-14, J) aza-15, K) aza-16, and L) aza-17. The scale bar in the lower right panel represents 1 µm and is applicable to all images.
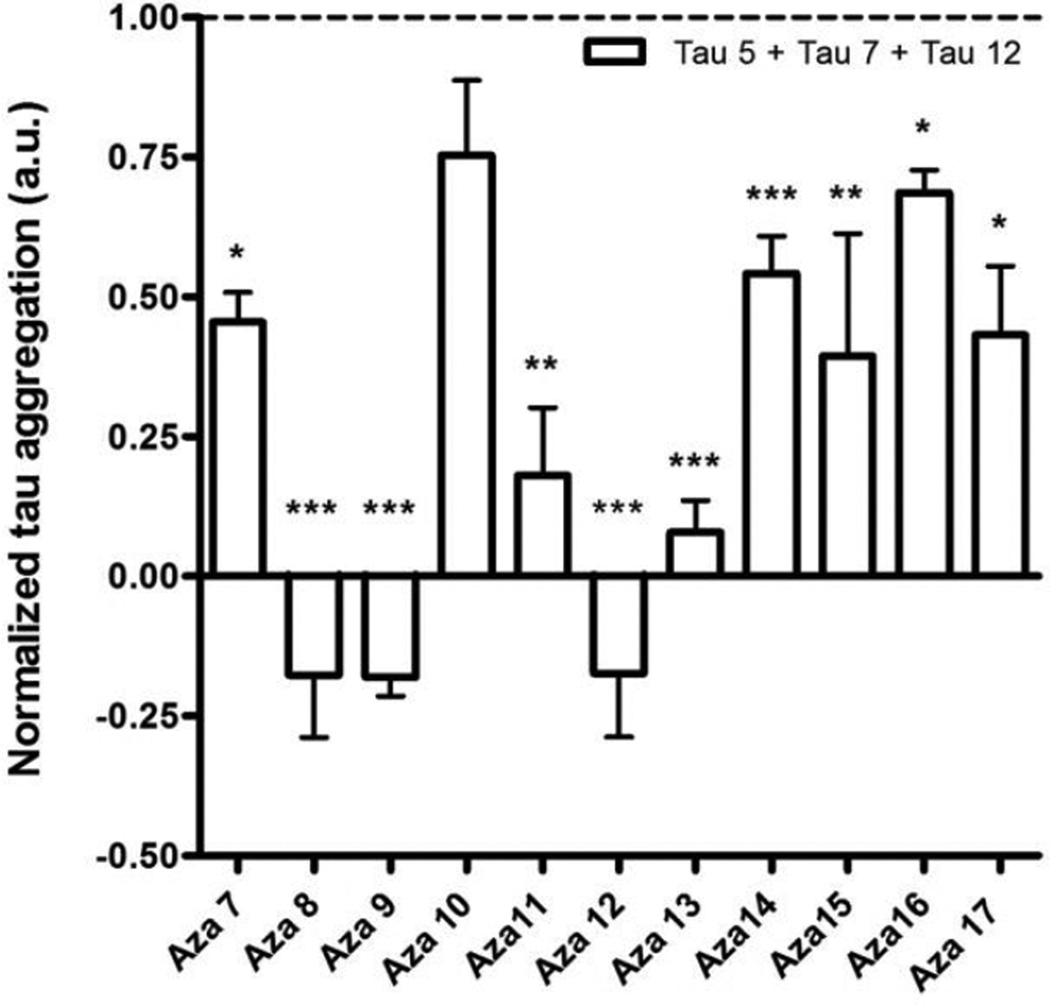
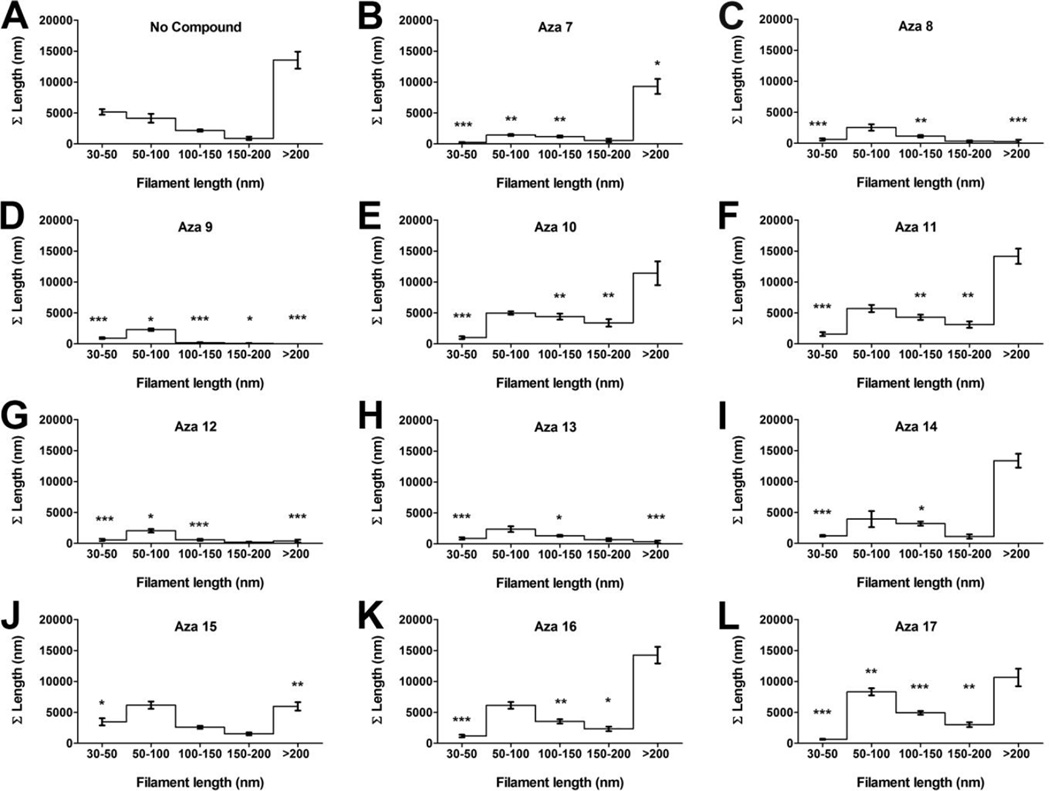
To determine whether these compounds can disassemble pre-formed tau aggregates, tau aggregation was allowed to proceed for six hours before the addition of compounds to a final concentration of 200 µM. After 12 hours the effect of compounds on the tau aggregation were examined by filter trap assay using the mixture of antibodies against normal tau (Figure 4). All compounds reduced the amount of preformed tau filaments, with compounds aza-8, aza-9, aza-11, aza-12, and aza-13 having the greatest activity. Electron microscopy was used to validate and extend the results from the filter trap assay. Compounds aza-8, aza-9, aza-12 and aza-13 substantially reduced the preexisting filament mass while the other compounds had less effect (Figure 5). To test whether compounds aza-8, aza-9, aza-12, and aza-13 were not simply blocking the adherence of tau filaments to the electron microscopy grids, compounds were added to pre-formed tau filaments and were immediately prepared for electron microscopy without allowing time for disassembly to occur. Under these conditions, none of the compounds blocked the binding of the filaments to the grid (Supplemental Figure S2). Quantitative analysis of the filament lengths in the presence and absence of compounds confirmed that compounds aza-8, aza-9, aza-12 and aza-13 had fewer aggregates overall compared to reactions without compound and virtually no filaments remaining greater than 200 nm in length (Figure 6).
Figure 4. Filter trap assay of tau filament disassembly.
Tau polymerization reactions were performed with 2 µM tau and 75 µM arachidonic acid at room temperature. After 6 hours, 200 µM compound or an equal volume of DMSO was added to the reactions. The compounds used are listed on the X-axis. The resulting amount of tau filament formation was determined using a filter trap assay. The values for tau filament formation were normalized to the amount of aggregates detected in the absence of compound (dashed line). Negative values indicate that there was less detectable tau on the filter after treatment with a compound than was observed with monomeric tau in the absence of arachidonic acid. The amount of tau on the filter was detected using a mixture of antibodies tau 5, tau 7 and tau 12. Values are the average of three trials ± SD. *P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001
Figure 5. Electron microscopy of tau filament disassembly in the presence of azaphilone derivatives.
Tau polymerization reactions were performed with 2 µM tau and 75 µM arachidonic acid at room temperature. After 6 hours, 200 µM compound or equal volume of DMSO was added to the reactions. Aliquots of the reactions were prepared for negative stain electron microscopy. Representative images are shown for A) no compound control, B) aza-7, C) aza-8, D) aza-9, E) aza-10, F) aza-11, G) aza-12, H) aza-13, I) aza-14, J) aza-15, K) aza-16, and L) aza-17. The scale bar in the lower right panel represents 1 µm and is applicable for all images.
Figure 6. Filament length distributions.
Tau disassembly reactions were performed and viewed by electron microscopy. The filaments remaining following incubation with or without compound were measured, and placed into bins according to their length (30–50 nm, 50–100 nm, 100–150 nm, 150–200 nm and > 200 nm). The lengths within a bin were summed to determine the total amount of filament length in each bin. A) Represents the length distributions for filaments in the control reaction without compound. The length distributions are also shown for filaments remaining following incubation in the presence of B) aza-7, C) aza-8, D) aza-9, E) aza-10, F) aza-11, G) aza-12, H) aza-13, I) aza-14, J) aza-15, K) aza-16, and L) aza-17. Each point is the average distribution for images of 5 different fields ± SD.*P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
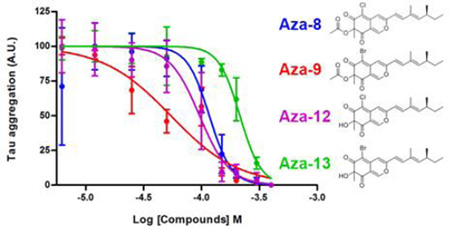
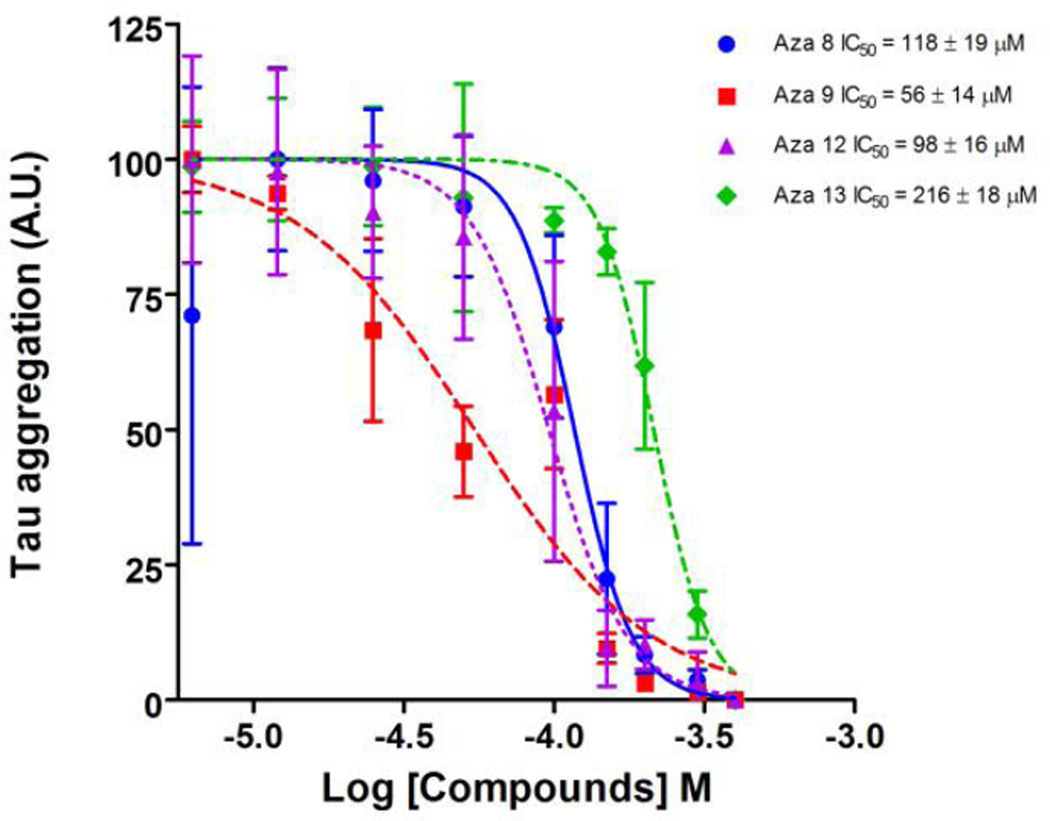
The IC50 of the four most potent compounds was determined using the filter trap assay. The amount of pre-formed filaments remaining following treatment with compounds for 12 hours was reduced in a concentration dependent manner for all four compounds tested (Figure 7). Compound aza-9 had an IC50 of 56 ± 14 µM, compared to 118 ± 19 µM, 98 ± 16 µM and 216 ± 18 µM for compounds aza-8, aza-12 and aza-13 respectively, indicating that aza-9 has the most activity for dissolving pre-formed tau filaments in vitro (Figure 7).
Figure 7. IC50 determination tau filament disassembly.
Polymerization reactions at 2 µM tau and 75 µM arachidonic acid were performed at room temperature. After 6 hours, compounds were added to these reactions at several different concentrations and incubated an additional 12 hours. The resulting amount of tau filaments in the reaction were determined by a filter trap assay detected by a mixture of antibodies to normal tau (Tau 5, Tau 7 and Tau 12). The amount of polymerization was normalized to controls in the absence of compound (100%). The normalized data was plotted against the log of the inhibition concentration and fit to a dose-response curve to determine the IC50 for A) Aza-8, B) Aza-9, C) Aza-12 and D) Aza-13. Data points are the average of three trials ± SD.
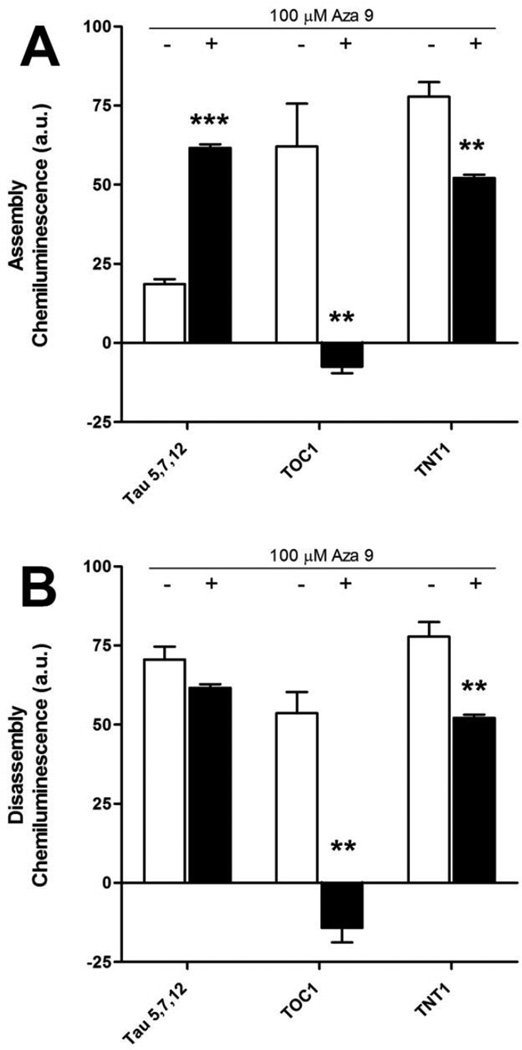
In a number of studies heparin has been used to induce tau aggregation. Because heparin-induced tau filaments might be different from arachidonic acid induced filaments, we wished to determine if aza 9 inhibited heparin-induced tau aggregation or disassembled heparin assembled tau aggregates. We chose aza 9 because it was the most potent compound among the 11 azaphilones. Filter trap assays were performed using the mixture of antibodies against normal tau, the TOC1 antibody and the TNT1 antibody. Aza 9 significantly reduced the assembly of TOC1 and TNT1 positive aggregates (Figure 8A). The addition of aza 9 also resulted in the significant disassembly of pre-formed filaments recognized by the TOC1 and TNT1 antibodies (Figure 8B).
Figure 8. Filter trap assay for filament assembly and disassembly of heparin-induced tau filaments.
A) For assembly inhibition, 100 µM aza 9 was incubated with 2 µM tau for 20 minutes before the addition 0.6 µM heparin. 16 hours after induction, the degree of aggregation was determined using the filter trap assay detected by a mixture of antibodies to normal tau (Tau 5, Tau 7 and Tau 12), an antibody to oligomeric tau (TOC1) and an antibody to a toxic conformation of tau (TNT1). The average of three independent trials ± SD is shown for no compound controls (white bars) and 100 µM aza 9 (black bars). B) 2 µM tau and 0.6 µM heparin were incubated together for 21 hours prior to the addition of 100 µM aza 9 or an equal volume of DMSO. Disassembly reactions proceeded for an additional 12 hours and the degree of aggregation was determined using the filter trap assay detected by a mixture of antibodies (Tau 5, Tau 7 and Tau 12), TOC1 and TNT1. The average of three independent trials ± SD is shown for no compound controls (white bars) and 100 µM aza 9 (black bars). *P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
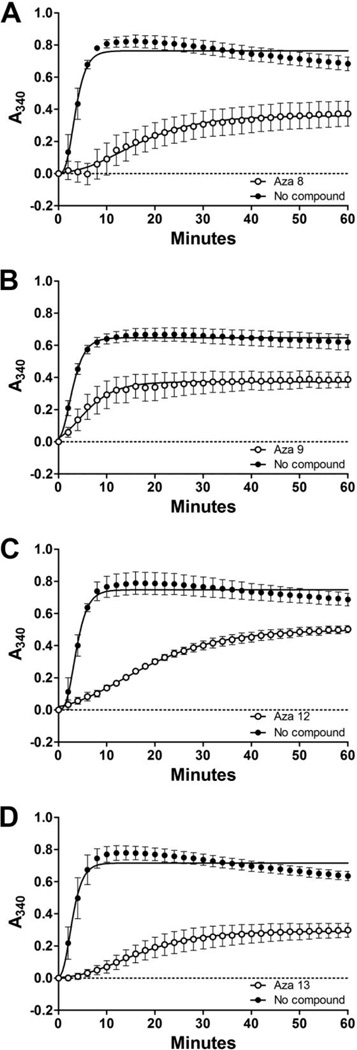
We chose the most potent azaphilone derivatives aza-8, aza-9, aza-12 and aza-13 to determine their effects on the normal function of tau to stabilize microtubules. Tubulin was mixed with tau in the presence or absence of 90 µM compound and the resulting microtubule formation was monitored by turbidity. All polymerization curves were fit using a Gompertz growth equation (Figure 9). While all four compounds affected the apparent rate and maximum amount of microtubule formation at the concentration tested (Table 1), tau still retained a significant ability to stabilize microtubule formation.
Figure 9. Microtubule assembly in the presence of the most potent azaphilone tau aggregation inhibitors.
Tubulin was incubated with either tau protein alone or tau in the presence of A) aza-8, B) aza-9, C) aza-12, or D) aza-13 at a concentration of 90 µM. Microtubule assembly was monitored by absorbance at 340 nm (y- axis) over time (x-axis). Each point is the average of three independent trails ± SD. All data were fit to Gompertz growth curve (dashed and solid lines for no compound and 90 µM azaphilones respectively). For clarity, only every second time point is shown on the graph.*P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
Table 1.
Statistical analysis of the effects of compounds aza-8, aza-9, aza-12, and aza-13 on the stabilization of microtubules.
| ti (min) | kapp (min−1) | Max (A340) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aza-8 | − | 3.08 | ± | 1.28 | 0.87 | ± | 0.14 | 0.76 | ± | 0.36 |
| + | 13.42 | ± | 6.59 | 0.12 | ± | 0.02*** | 0.36 | ± | 0.14* | |
| Aza-9 | − | 2.26 | ± | 0.65 | 0.62 | ± | 0.08 | 0.65 | ± | 0.38 |
| + | 5.03 | ± | 1.93 | 0.26 | ± | 0.19* | 0.38 | ± | 0.10* | |
| Aza-12 | − | 3.11 | ± | 1.22 | 0.78 | ± | 0.09 | 0.75 | ± | 0.10 |
| + | 12.83 | ± | 1.35*** | 0.09 | ± | 0.02*** | 0.50 | ± | 0.04* | |
| Aza-13 | − | 2.58 | ± | 1.29 | 0.86 | ± | 0.17 | 0.72 | ± | 0.06 |
| + | 15.65 | ± | 7.72* | 0.12 | ± | 0.05** | 0.30 | ± | 0.07** | |
Max (A340) is the maximum amount of microtubule polymerization, ti is the point of inflection of the curve at the time of maximum growth rate in minutes, and b is inversely proportional to the apparent rate of polymerization (kapp, min−1) as determined from the fit of three individual microtubule polymerization curves for each condition to a nonlinear Gompertz growth function (see Methods).
Discussion
Tau based therapeutic strategies have recently been gaining additional attention largely due to the major role tau pathology plays in many neurological disorders including Alzheimer’s disease. Several tau-directed therapeutic strategies with disease-modifying potential have been identified including modulating tau phosphorylation, microtubule stabilization, tau aggregation inhibitors and tau clearance using antibodies22–30. Conversion of soluble monomeric tau into insoluble tau aggregates could, potentially, result in both loss of function and gain of function toxicities31. Therefore, inhibiting aggregation of tau might prevent formation of the toxic oligomers or tangles. Inhibiting aggregation could also increase the levels of monomeric tau, thereby increasing the chances for its clearance through chaperone mediated processes32. Previous studies have identified several tau aggregation inhibitor (TAI) molecules including those belonging to the class of anthraquinones, phenothiazines, and a benzothiazolidine derivatives among others3, 33, 34. One TAI, a stable, reduced form of methylthioninium chloride, is currently in Phase III clinical trials2, indicating this approach has promise and it is, consequently, worthwhile to identify additional structural backbones with this activity. Many of the previously identified TAIs are comprised of fused ring structures believed to be capable of interacting with the β-sheet structures formed in tau aggregates, thereby inhibiting formation of tau filaments10, 21.
Fungal extracts are known to include pharmaceutically important secondary metabolites35. We therefore previously screened 17 secondary metabolites obtained from the fungus Aspergillus nidulans for TAIs due to their structural similarity to previously identified TAIs10. From this screen we identified 3 compounds that inhibited tau aggregation at micromolar concentrations. Two of these compounds belonged to the anthraquinone class of compounds and one was structurally unique from all the previously identified TAIs. We were particularly interested in this compound – asperbenzaldehyde. Asperbenzaldehyde is a precursor to an important class of natural products called azaphilones. Azaphilones are a structurally diverse group of polyketides that share a highly oxygenated bicyclic core and chiral quaternary center36. The azaphilones used in this study were obtained by semi-synthetic diversification of asperbenzaldehyde.
All eleven azaphilones inhibited the formation of tau filaments but some of them produced small amorphous tau aggregates, which can be seen in the electron micrographs in Figure 3. These aggregates were not recognized by TOC119 and TNT120 antibodies, which bind to toxic forms of tau, therefore we believe these compounds promote the formation of small off-pathway aggregates of tau which are not toxic and do not act as seeds for further tau filament assembly. The induction of these aggregates could be similar to soluble aggregates of tau induced by porphyrin pthalocyanine tetrasulfonate that have a different conformation from insoluble toxic tau oligomers21.
From a therapeutic point of view, TAIs would be more useful if they could also dissolve pre-formed tau filaments because they could theoretically be beneficial to patients that already demonstrate cognitive impairments. We found that a subset of the compounds, aza-8, aza-9, aza-12, and aza-13, showed this property. These four compounds have Br or Cl at position R1, while the other compounds have either I or H at R1 (Figure 1). Therefore, halogenation at position R1 may be not necessarily important for inhibition of tau filament formation, but electron-withdrawing groups at R1 specifically seem to enhance disassembly of tau filaments. Cl and Br are more electronegative (3.0 and 2.8 respectively), than I (2.5) and H (2.1) indicating that increased electronegativity at position R1 could have a significant impact on the activity of tau aggregation inhibitors with this scaffold. The four disassembly causing compounds have a ketone at position R3, while presence of the CHCO2Et moiety at the same position seems to virtually eliminate disassembly, even with halogenation. The impact of the chemical groups at the R2 position seems to be dependent upon the substitution at R1. Compounds aza-8 and aza-12 both have Cl at R1, but possess acetate and hydroxyl groups at R2, respectively. Despite this structural difference, there is no significant difference in their activity levels. However compounds aza-9 and aza-13 both containing Br at R1, and acetate and hydroxyl groups at R2, respectively differ in their levels of activity. Aza-9 is more potent than aza-13, therefore positioning of the acetate group at R2 in the presence of a Br at R1 might be important for compound activity.
Additionally, all four disassembly causing compounds have lipoxygenase-1 inhibitory activity in the low micromolar range (IC50 of 2–8µm)11. Inhibition of LOX-1 may help reduce fatty acid metabolites of arachidonic acid and docasahexanoic acid that are elevated in Alzheimer’s disease16. These compounds could therefore have two positive therapeutic activities in tau dementias. The relatively high IC50 values of our compounds indicate that they are unlikely to be of therapeutic value and we do not know if they have suitable bioavailability or pharmacokinetic properties. It is important, however, to identify new scaffolds with the appropriate biological activity for further development. We believe these compounds provide a novel TAI scaffold with the added features that they inhibit LOX-1 and some of them disassemble pre-formed tau aggregates.
In conclusion, this study shows that these compounds inhibit assembly of tau aggregates, disassemble preformed tau aggregates, and partially preserve tau’s ability to bind to tubulin and promote microtubule assembly. These compounds provide a promising novel scaffold for TAI molecules. The structure-activity relationship studies give us several leads for the probable important chemical groups required in this scaffold structure required for the anti-tau aggregation activity of the compounds. Further studies on the interaction between the compounds and tau will help to determine the precise mechanism of action of these compounds.
Methods
Chemicals and Reagents
Full length 2N4R tau (441 amino acids) was expressed in E. coli and purified as described previously37. Arachidonic acid (ARA) was purchased from Cayman Chemicals (Ann Arbor, MI). Heparin sodium salt was purchased from SIGMA (St. Louis, MO). Asperbenzaldehyde was purified from Aspergillus nidulans and converted to the compounds aza-7 through aza-17 (Figure 1) as described previously11 with the following minor modifications. We constructed a number of strains with various promoter combinations and used a variety of induction conditions to maximize asperbenzaldehyde production. Our best yields were obtained with strain LO8355 (pyrG89, pyroA4, riboB2, nkuA::argB, stcJ::AfriboB, AN1029(p):: AfpyrG-alcA(p), AN1033::AfpyroA, alcR(p)::ptrA-gpdA(p)). In this strain, the promoter of asperfuranone biosynthesis transcription factor AN1029 (using the AspGD nomenclature (http://aspergillusgenomes.org/)) is replaced with the highly inducible alcA promoter and the alcR promoter is replaced with the strong, constitutive gpdA promoter (−1241 to −1). AN1033 is replaced with the AfpyroA gene to interrupt the asperfuranone biosynthesis pathway causing asperbenzaldehyde to accumulate. Growth was in lactose minimal medium (20 g/L lactose, 6 g/L NaNO3, 0.52 g/L KCl, O.52 g/L MgSO4,·7H2O, 1.52 g/L KH2PO4, 1 mL/L trace elements solution)38. Spores were inoculated at 106/mL into 500 mL of medium in a 2L flask. Incubation was at 37 °C on a gyratory shaker and induction was with 30 mM methyl-ethyl-ketone, added 55 hours after inoculation. Cultures were harvested six days after inoculation. Yields of purified asperbenzaldehyde were greater than 2 g/L representing an approximate conversion of 10% of the carbon source to final product. Additional information on the purity of the compounds can be found in supplemental materials.
Inhibition of tau aggregation
75 µM arachidonic acid was used to initiate the aggregation of 2 µM tau in polymerization buffer (PB, 10 mM HEPES (pH 7.64), 5 mM DTT, 100 mM NaCl, 0.1 mM EDTA, and 3.75% ethanol) in vitro as previously described17. Compounds dissolved in DMSO were added to a final concentration of 200 µM and incubated with tau protein in PB 20 minutes prior to the addition of arachidonic acid. The reactions were allowed to proceed for 16 h at room temperature before analysis10. For heparin induced tau assembly inhibition reactions, 0.6 µM heparin was used to initiate aggregation on 2 µM tau in polymerization buffer (PB, 10mM HEPES (pH 7.64), 5 mM DTT, 17.7 mM NaCl) in vitro. Compounds dissolved in DMSO were added to a final concentration of 200 µM and incubated with tau protein in PB 20 minutes prior to the addition of heparin. The reactions were allowed to proceed for 16 h at 37 °C before analysis.
Disassembly of pre-formed filaments
Pre-formed tau filaments were generated with 2 µM tau and 75 µM ARA in PB as described above for 6 h at room temperature. Compounds dissolved in DMSO were added to the tau solution at final concentrations indicated in the Results section and figure legends. The reactions were allowed to proceed at room temperature for 12 h before analysis. For heparin induced tau aggregation assays, pre- formed tau filaments were generated with 2 µM tau and 0.6 µM heparin in PB as described above for 12 h at 37 °C. Compounds dissolved in DMSO were added to the tau solution at final concentrations indicated in the Results section and figure legends. The reactions were allowed to proceed at 37 °C for 24 h before analysis.
Filter trap assay
The amount of tau aggregates following assembly or disassembly reactions was determined by filter trap assay as described previously10. Reactions were diluted into TBS such that they contained 20 ng protein in 300 µL. For heparin induced tau aggregation reactions, the reactions were diluted into TBS such that they contained 60 ng protein in 300 µL. Solutions were passed through a nitrocellulose membrane using house vacuum in a dot-blot apparatus. The aggregates trapped on the membrane were detected by either general antibodies (a mixture of Tau 539 at 1:50,000 dilution, Tau 1240 at 1:250,000 dilution and Tau 741 at 1:250,000 dilution), or antibodies to toxic conformations (TNT119 at 1:200,000 or TOC120 at 1:7,000). All antibodies were a kind gift from Drs. Nick Kanaan and Lester I. Binder. HRP-linked Goat anti-mouse IgG (general antibodies and TNT1) or HRP-linked Goat anti-mouse IgM (TOC1) (Thermo Scientific, Rockford, IL) were used as the secondary antibodies and blots were developed using ECL (enhanced chemiluminescence) Western Blotting Analysis System (GE Healthcare, Buckinghamshire, UK). Images were captured with a Kodak Image Station 4000R or ChemiDoc-It2 Imager and were quantified using the histogram function of Adobe Photoshop 7.0. Statistical analyses were performed using unpaired t-tests to compare the triplicate values to control values.
Transmission electron microscopy
Polymerization reaction samples were diluted 1:10 in PB and fixed with 2% glutaraldehyde for 5 min. Fixed samples were placed on formvar carbon-coated grids and stained with uranyl acetate as previously described10. Images were captured with a Technai F20 XT Field emission transmission electron microscope (FEI Co., Hillsboro, OR) and Gatan Digital Micrograph imaging system (Gatan, Inc., Pleasanton, CA). The filaments were quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD) as previously described10. For quantitative analysis, filament lengths were placed into bins as described in results. Statistical analyses were performed using unpaired t-tests to compare 4 or 5 replicates for each bin size with the no compound data serving as reference values.
Tubulin Polymerization Assay
Polymerization of tubulin was measured using a Tubulin Polymerization Assay kit BK006P (Cytoskeleton, Inc., Denver, CO) following the manufacturer’s protocol. Briefly, 2 mg/ml porcine tubulin was incubated with 1.5 µM tau and 90 µM aza-8, aza-9, aza-12 or aza-13 compounds. Tubulin polymerization was monitored by turbidity at 340 nm in a Varian 50 MPR microplate reader at 37 °C for 1 h. Experiments were performed in triplicate, averaged and fit to a Gompertz growth equation as previously described42 using the equation:
Where y is the amount of microtubule polymerization measured at time t, a is the maximum amount of microtubule polymerization observed at an absorbance of 340 nm (Max), ti is the point of inflection of the curve at the time of maximum growth rate in minutes, and b is inversely proportional to the apparent rate of polymerization (kapp, min−1). The average values for the parameters a, b and ti were determined and compared to the no-compound control using a paired t-test to determine statistical significance *p≤ 0.05; **p≤ 0.01; ***p≤ 0.001.
Supplementary Material
Acknowledgments
We thank Adam Miltner for assistance with protein expression and purification. Funding was provided in part by P01-GM084077 from the National Institute of General Medical Sciences (CCCW and BRO), T32 GM008545 from the National Institute of General Medical Sciences (APR), R01-NS083391 from the National Institute of Neurological Disorders and Stroke (TCG) and by the H.L. Snyder Medical Foundation (BRO and TCG). We thank Drs. Nick Kanaan and Lester I. Binder for their kind gift of the antibodies used in this study.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
SRP carried out the tau assembly and disassembly experiments and drafted the manuscript. APR purified asperbenzaldehyde, generated and purified azaphilone derivatives, and assisted with analysis of structure/activity relationships. ADS purified asperbenzaldehyde, generated and purified derivatives of azaphilones. CEO constructed the A. nidulans strains for asperbenzaldehyde over-production and cultured the strains for overproduction. CCCW and BRO conceived of the generation and purification of derivatives of asperbenzaldehyde and azaphilones. TEP assisted with analysis of structure/activity relationships. BRO and TCG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 2.Wischik CM, Harrington CR, Storey JM. Tau-aggregation inhibitor therapy for Alzheimer's disease. Biochemical pharmacology. 2014;88:529–539. doi: 10.1016/j.bcp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Pickhardt M, Gazova Z, von Bergen M, Khlistunova I, Wang Y, Hascher A, Mandelkow EM, Biernat J, Mandelkow E. Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells. The Journal of biological chemistry. 2005;280:3628–3635. doi: 10.1074/jbc.M410984200. [DOI] [PubMed] [Google Scholar]
- 4.Calcul L, Zhang B, Jinwal UK, Dickey CA, Baker BJ. Natural products as a rich source of tau-targeting drugs for Alzheimer's disease. Future medicinal chemistry. 2012;4:1751–1761. doi: 10.4155/fmc.12.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang L, Gou S, Fang X, Cheng L, Fleck C. Current progresses of novel natural products and their derivatives/ analogs as anti-Alzheimer candidates: an update. Mini reviews in medicinal chemistry. 2013;13:870–887. doi: 10.2174/1389557511313060009. [DOI] [PubMed] [Google Scholar]
- 6.Yaegashi J, Oakley BR, Wang CC. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. Journal of industrial microbiology & biotechnology. 2014;41:433–442. doi: 10.1007/s10295-013-1386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics. 2006;172:1557–1566. doi: 10.1534/genetics.105.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakley CE, Edgerton-Morgan H, Oakley BR. Tools for manipulation of secondary metabolism pathways: rapid promoter replacements and gene deletions in Aspergillus nidulans. Methods in molecular biology. 2012;944:143–161. doi: 10.1007/978-1-62703-122-6_10. [DOI] [PubMed] [Google Scholar]
- 9.Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. Fusion PCR and gene targeting in Aspergillus nidulans. Nature protocols. 2006;1:3111–3120. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- 10.Paranjape SR, Chiang YM, Sanchez JF, Entwistle R, Wang CC, Oakley BR, Gamblin TC. Inhibition of Tau Aggregation by Three Aspergillus nidulans Secondary Metabolites: 2,omega-Dihydroxyemodin, Asperthecin, and Asperbenzaldehyde. Planta medica. 2014;80:77–85. doi: 10.1055/s-0033-1360180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somoza AD, Lee KH, Chiang YM, Oakley BR, Wang CC. Reengineering an azaphilone biosynthesis pathway in Aspergillus nidulans to create lipoxygenase inhibitors. Organic letters. 2012;14:972–975. doi: 10.1021/ol203094k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki K, Tahara H, Inokoshi J, Tanaka H, Masuma R, Omura S. New brominated and halogen-less derivatives and structure-activity relationship of azaphilones inhibiting gp120-CD4 binding. The Journal of antibiotics. 1998;51:1004–1011. doi: 10.7164/antibiotics.51.1004. [DOI] [PubMed] [Google Scholar]
- 13.Clark RC, Lee SY, Searcey M, Boger DL. The isolation, total synthesis and structure elucidation of chlorofusin, a natural product inhibitor of the p53-mDM2 protein-protein interaction. Natural product reports. 2009;26:465–477. doi: 10.1039/b821676b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso L, Dallavalle S, Merlini L, Bava A, Nasini G, Penco S, Giannini G, Giommarelli C, De Cesare A, Zuco V, Vesci L, Pisano C, Castorina M, Milazzo F, Cervoni ML, Dal Piaz F, De Tommasi N, Zunino F. Natural and semisynthetic azaphilones as a new scaffold for Hsp90 inhibitors. Bioorganic & medicinal chemistry. 2010;18:6031–6043. doi: 10.1016/j.bmc.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 15.Nam JY, Kim HK, Kwon JY, Han MY, Son KH, Lee UC, Choi JD, Kwon BM. 8-O-Methylsclerotiorinamine, antagonist of the Grb2-SH2 domain, isolated from Penicillium multicolor. Journal of natural products. 2000;63:1303–1305. doi: 10.1021/np0001169. [DOI] [PubMed] [Google Scholar]
- 16.Chu J, Pratico D. Pharmacologic blockade of 5-lipoxygenase improves the amyloidotic phenotype of an Alzheimer's disease transgenic mouse model involvement of gamma-secretase. The American journal of pathology. 2011;178:1762–1769. doi: 10.1016/j.ajpath.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Carlson SW, Branden M, Voss K, Sun Q, Rankin CA, Gamblin TC. A complex mechanism for inducer mediated tau polymerization. Biochemistry. 2007;46:8838–8849. doi: 10.1021/bi700403a. [DOI] [PubMed] [Google Scholar]
- 18.Chang E, Kuret J. Detection and quantification of tau aggregation using a membrane filter assay. Analytical biochemistry. 2008;373:330–336. doi: 10.1016/j.ab.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward SM, Himmelstein DS, Ren Y, Fu Y, Yu XW, Roberts K, Binder LI, Sahara N. TOC1: a valuable tool in assessing disease progression in the rTg4510 mouse model of tauopathy. Neurobiology of disease. 2014;67:37–48. doi: 10.1016/j.nbd.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, Binder LI. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akoury E, Gajda M, Pickhardt M, Biernat J, Soraya P, Griesinger C, Mandelkow E, Zweckstetter M. Inhibition of tau filament formation by conformational modulation. Journal of the American Chemical Society. 2013;135:2853–2862. doi: 10.1021/ja312471h. [DOI] [PubMed] [Google Scholar]
- 22.Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 23.Lagoja I, Pannecouque C, Griffioen G, Wera S, Rojasdelaparra VM, Van Aerschot A. Substituted 2-aminothiazoles are exceptional inhibitors of neuronal degeneration in tau-driven models of Alzheimer's disease. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2011;43:386–392. doi: 10.1016/j.ejps.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Brunden KR, Yao Y, Potuzak JS, Ferrer NI, Ballatore C, James MJ, Hogan AM, Trojanowski JQ, Smith AB, 3rd, Lee VM. The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer's disease and related tauopathies. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;63:341–351. doi: 10.1016/j.phrs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB, 3rd, Lee VM, Brunden KR. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang E, Congdon EE, Honson NS, Duff KE, Kuret J. Structure-activity relationship of cyanine tau aggregation inhibitors. Journal of medicinal chemistry. 2009;52:3539–3547. doi: 10.1021/jm900116d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honson NS, Jensen JR, Darby MV, Kuret J. Potent inhibition of tau fibrillization with a multivalent ligand. Biochemical and biophysical research communications. 2007;363:229–234. doi: 10.1016/j.bbrc.2007.08.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickhardt M, Larbig G, Khlistunova I, Coksezen A, Meyer B, Mandelkow EM, Schmidt B, Mandelkow E. Phenylthiazolyl-hydrazide and its derivatives are potent inhibitors of tau aggregation and toxicity in vitro and in cells. Biochemistry. 2007;46:10016–10023. doi: 10.1021/bi700878g. [DOI] [PubMed] [Google Scholar]
- 29.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnamurthy PK, Deng Y, Sigurdsson EM. Mechanistic Studies of Antibody-Mediated Clearance of Tau Aggregates Using an ex vivo Brain Slice Model. Frontiers in psychiatry. 2011;2:59. doi: 10.3389/fpsyt.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahara N, Maeda S, Takashima A. Tau oligomerization: a role for tau aggregation intermediates linked to neurodegeneration. Current Alzheimer research. 2008;5:591–598. doi: 10.2174/156720508786898442. [DOI] [PubMed] [Google Scholar]
- 32.Blair LJ, Zhang B, Dickey CA. Potential synergy between tau aggregation inhibitors and tau chaperone modulators. Alzheimer's research & therapy. 2013;5:41. doi: 10.1186/alzrt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chirita C, Necula M, Kuret J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. doi: 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- 34.Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller NP, Turner G, Bennett JW. Fungal secondary metabolism - from biochemistry to genomics. Nature reviews. Microbiology. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 36.Gao JM, Yang SX, Qin JC. Azaphilones: chemistry and biology. Chemical reviews. 2013;113:4755–4811. doi: 10.1021/cr300402y. [DOI] [PubMed] [Google Scholar]
- 37.Rankin CA, Sun Q, Gamblin TC. Pseudo-phosphorylation of tau at Ser202 and Thr205 affects tau filament formation. Brain research. Molecular brain research. 2005;138:84–93. doi: 10.1016/j.molbrainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Vishniac W, Santer M. The thiobacilli. Bacteriological reviews. 1957;21:195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horowitz PM, LaPointe N, Guillozet-Bongaarts AL, Berry RW, Binder LI. N-terminal fragments of tau inhibit full-length tau polymerization in vitro. Biochemistry. 2006;45:12859–12866. doi: 10.1021/bi061325g. [DOI] [PubMed] [Google Scholar]
- 42.Combs B, Voss K, Gamblin TC. Pseudohyperphosphorylation has differential effects on polymerization and function of tau isoforms. Biochemistry. 2011;50:9446–9456. doi: 10.1021/bi2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.