Abstract
Background
VRC 012 was a Phase I study of a prototype recombinant adenoviral-vector serotype-35 (rAd35) HIV vaccine, the precursor to two recently published clinical trials, HVTN 077 and 083. On the basis of prior evaluation of multiclade rAd5 HIV vaccines, Envelope A (EnvA) was selected as the standard antigen for a series of prototype HIV vaccines to compare various vaccine platforms. In addition, prior studies of rAd5-vectored vaccines suggested pre-existing human immunity may be a confounding factor in vaccine efficacy. rAd35 is less seroprevalent across human populations and was chosen for testing alone and in combination with a rAd5-EnvA vaccine in the present two-part phase I study.
Methods
First, five subjects each received a single injection of 109, 1010, or 1011 particle units (PU) of rAd35-EnvA in an open-label, dose-escalation study. Next, 20 Ad5/Ad35-seronegative subjects were randomized to blinded, heterologous prime-boost schedules combining rAd5-EnvA and rAd35-EnvA with a three month interval. rAd35-EnvA was given at 1010 or 1011 PU to ten subjects each; all rAd5-EnvA injections were 1010 PU. EnvA-specific immunogenicity was assessed four weeks post-injection. Solicited reactogenicity and clinical safety were followed after each injection.
Results
Vaccinations were well tolerated at all dosages. Antibody responses measured by ELISA were detected at 4 weeks in 30% and 50% of subjects after single doses of 1010 or 1011 PU rAd35, respectively, and in 89% after a single rAd5-EnvA 1010 PU injection. EnvA-specific IFN-γ ELISpot responses were detected at four weeks in 0%, 70%, and 50% of subjects after the respective rAd35-EnvA dosages compared to 89% of subjects after rAd5. T cell responses were higher after a single rAd5-EnvA 1010 PU injection than after a single rAd35-EnvA 1010 PU injection, and humoral responses were low after a single dose of either vector. Of those completing the vaccine schedule, 100% of rAd5-EnvA recipients and 90% of rAd35-EnvA recipients had both T cell and humoral responses after boosting with the heterologous vector. ELISpot response magnitude was similar in both regimens and comparable to a single dose of rAd5. A trend toward more robust CD8 T cell responses using rAd5-EnvA prime and rAd35-EnvA boost was observed. Humoral response magnitude was also similar after either heterologous regimen, but was several fold higher than after a single dose of rAd5. Adverse events (AEs) related to study vaccines were in general mild and limited to one episode of hematuria, Grade two. Activated partial thromboplastin time (aPTT) AEs were consistent with an in vitro effect on the laboratory assay for aPTT due to a transient induction of anti-phospholipid antibody, a phenomenon that has been reported in other adenoviral vector vaccine trials.
Conclusions
Limitations of the rAd vaccine vectors, including the complex interactions among pre-existing adenoviral immunity and vaccine-induced immune responses, have prompted investigators to include less seroprevalent vectors such as rAd35-EnvA in prime-boost regimens. The rAd35-EnvA vaccine described here was well tolerated and immunogenic. While it effectively primed and boosted antibody responses when given in a reciprocal prime-boost regimen with rAd5-EnvA using a three-month interval, it did not significantly improve the frequency or magnitude of T cell responses above a single dose of rAd5. The humoral and cellular immunogenicity data reported here may inform future vaccine and study design.
Trial Registration
ClinicalTrials.gov NCT00479999
Introduction
Despite three decades of research, the acquired immunodeficiency syndrome (AIDS) pandemic continues. Development of an effective human immunodeficiency virus (HIV) vaccine remains an urgent global health priority. The modest degree of protection reported in the RV144 vaccine trial demonstrated proof-of-concept that a vaccine can offer some protection against HIV infection [1]. Based upon subsequent analysis of the those results, which specified that the antibody component of the vaccine response was an important mediator of protection [2], the attention among the HIV vaccine field has been refocused on the quality and profile of antibody responses elicited by candidate vaccines. However, T cell-mediated immunity is still considered a useful complement to antibody neutralization for clearing HIV-infected cells and controlling HIV viral loads. Therefore, an important aspect of effective HIV vaccine development includes the creation of T cell immunogens delivered by recombinant vectors that can both elicit CD8 T cell responses and provide CD4 T cells capable of priming antibody responses. Thoughtfully designed recombinant adenovirus vectored vaccines have the potential to elicit such broad humoral and cellular immune responses [3, 4].
Adenovirus-based vaccines produce broad antibody responses and potent cellular immunity, and the Vaccine Research Center (VRC) has developed a recombinant adenoviral-vector (rAd) serotype-5-based preventive HIV vaccine that has been studied in numerous Phase I and II trials [3–7]. The utility of rAd5 vectors has been limited by the prevalence and magnitude of pre-existing, vector-specific neutralizing antibodies (NAbs), particularly in sub-Saharan Africa and Southeast Asia [8], which may reduce vaccine immunogenicity [3, 9]. Although two HIV vaccine efficacy studies employing rAd5 vaccines did not demonstrate efficacy in prevention of HIV [10–13], recombinant, non-replicating vaccine vectors remain of interest for the development of HIV vaccines, contingent upon development of methods to overcome known limitations of the rAd vector platforms. One such method is a prime-boost strategy with heterologous vectors, combining a low seroprevalent adenoviral serotype vector with another rAd vector that may or may not need to be a low seroprevalent serotype. Previous work has shown that vectors derived from immunologically distinct adenoviral serotypes may be able to overcome the suppressive effects of pre-existing anti-Ad immunity [14–16].
Previously, preclinical studies with adenoviral vector vaccines have included an observation of transient, mild elevations in the activated partial thromboplastin time (aPTT) noted in vaccinated rabbits compared to controls [17]. aPTT is a measure of blood clotting, specifically the intrinsic and common pathways of coagulation. A prolongation in the time of clot formation in this in vitro reaction suggests an individual has either a deficiency of one of several clotting factors or the presence of a clotting inhibitor, such as an antibody against a clotting factor. The normal range of aPTT depends upon the specific laboratory performing the test, but generally falls between 25 and 37 seconds. A contemporaneous Phase I clinical trial of the VRC’s Ebola-rAd5 vaccine observed that two subjects had prolonged aPTT measurements noted after vaccination, that were determined to be due to the transient induction of an anti-phospholipid antibody (APA) that interacts in vitro with the accelerant used to activate the partial thromboplastin time reaction [18]. This phenomenon has been reported previously in a study of an adenoviral vector product studied for the treatment of prostate cancer [19], and in both that trial and the series of Ebola-rAd5 vaccine trials performed at the VRC, there have been no clinically significant findings associated with this in vitro artifact [20, 21]. Although aPTT is not routinely performed in HIV vaccine studies, and was not required for evaluation of safety, post-vaccination aPTT assessments were added to VRC 012 by amendment prior to the beginning of Part II of the study. This would confirm whether the preclinical and clinical aPTT changes reported with other adenoviral vector vaccines would be observed in the present study, characterize the severity and duration of the phenomenon, and confirm that there were no clinically relevant consequences.
In 2005, the AIDS Vaccine Research Subcommittee (AVRS), chose a single antigen to carry forward in iterative vaccine development in order to facilitate the comparison of various vaccine vectors without the expense and time required to test multiple antigens iteratively [22]. Prior evaluation of a multiclade rAd5 HIV vaccine demonstrated EnvA immunogenicity and ease of manipulation [3–7]. Therefore, EnvA was selected as the gene-based antigen to evaluate a serotype 35 adenoviral vaccine vector (rAd35-EnvA) prototype in combination with rAd5 (rAd5-EnvA). The vaccine component described in the present study, an experimental HIV-1 EnvA-expressing recombinant human adenovirus B, rAd35, was one of the first of these alternative adenovirus serotypes to be studied in humans.
The VRC 012 study was designed to evaluate a prototype vaccine strategy using a then-novel rAd35 with the single encoded HIV-1 antigen, EnvA. The rAd35-EnvA vaccine was evaluated alone in a dose escalation evaluation (Part I) and then as a prime or boost for rAd5-EnvA (Part II), in seronegative adults. The study was the precursor to two recently published clinical trials, HVTN 077 and 083, that used the same vaccines with similar prime/boost regimens [23, 24]. Here, we report the results of the first Phase I study of this rAd35-EnvA HIV vaccine, evaluated alone and in a heterologous prime-boost combination with a rAd5 vector expressing the same vaccine antigen.
Methods
Study design
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see S1 CONSORT Checklist and S1 Protocol.
VRC 012 (NIH 07-I-0167, NCT00479999) was a single-site, Phase I, two part study to examine dose, safety, tolerability and immunogenicity of a rAd35 prototype vaccine encoding for an HIV-1 envelope sequence from clade A (EnvA). This study was conducted at the National Institutes of Health (NIH), Bethesda, MD by the Vaccine Research Center (VRC) and approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board (IRB) and was performed in accordance with 45 CFR Part 46, U.S. Food and Drug Administration regulations for investigational products. All subjects provided written informed consent. Study subjects were healthy adults, aged 18–50, recruited through an IRB-approved screening protocol (NIH 02-I-0127, NCT 00031304) with informed consent to be screened for an HIV vaccine clinical trial. All participants were tested for Ad35 antibody within 12 weeks of enrollment and, if enrolled in Part II, were tested for Ad5 antibody as well within the same timeframe. Funding for the conduct of this clinical trial was provided by the National Institute of Allergy and Infectious Diseases (NIAID) intramural research program.
In Part I, three open-label dosage groups of five participants each were sequentially enrolled. Subjects in Groups one, two, and three received one injection of rAd35-EnvA at 109 particle units (PU), 1010 PU and 1011 PU, respectively and were followed for 24 weeks with no long-term follow-up requirements. In Part II, 20 subjects enrolled in Group four were blindly randomized to subgroups A, B, C, or D, and received a heterologous prime-boost vaccination series with a twelve week interval, as shown in the vaccination schema Table 1. The randomization sequence was obtained by computer-generated random numbers and provided to the study pharmacist by the statistician. The pharmacist and the statistician were responsible for maintaining security of the treatment assignments. To maintain blinding, any discussion of the treatment assignment between the VRC clinicians and the pharmacy staff or statistician was prohibited until after the assignments were permitted to be known to all. To decrease the potential for participant dropouts during the period between randomization and initial vaccination, randomization occurred on Day 0 after the study consent was signed and eligibility was confirmed. The study number was assigned through completion of the eligibility checklist in the electronic study database and was the next sequential number in the study number sequence. The Group four assignments were unblinded when safety data collection through 4 weeks after the booster injection for all subjects were completed. Full details of sample size determination and power to detect serious adverse events may be found in the clinical protocol located in the supplementary information, S1 Protocol.
Table 1. Study Schema.
| Part I: Open Label Sequentially Enrolled Dose Escalation | |||
| N | Day 0 rAd35-EnvA VACCINATION | ||
| Group 1 | 5 | 109 PU IM | |
| Group 2 | 5 | 1010 PU IM | |
| Group 3 | 5 | 1011 PU IM | |
| Total | 15 | ||
| Part II: Heterologous Prime-Boost Randomized and Completed Injection Schedules | |||
| Randomized/Completed | Day 0 PRIME | Week 12 (-7/+21 days) BOOST | |
| Group 4A | 5/5 | rAd35-EnvA 1010 PU IM | rAd5-EnvA 1010 PU IM |
| Group 4B | 5/3 | rAd5-EnvA 1010 PU IM | rAd35-EnvA 1010 PU IM |
| Group 4C | 5/5 | rAd35-EnvA 1011 PU IM | rAd5-EnvA 1010 PU IM |
| Group 4D | 5/5 | rAd5-EnvA 1010 PU IM | rAd35-EnvA 1011 PU IM |
| Total | 20/18 | First 10 subjects blindly randomized to 4A or 4B; second 10 subjects blindly randomized to 4C or 4D. | |
All rAd5-EnvA injections contained 1010 PU administered intramuscularly (IM). rAd35-EnvA was given at 1010 or 1011 PU to ten subjects each, also IM. The Part II follow-up plan included clinic visits through 52 weeks after enrollment, with subsequent annual contact through five years after enrollment. The primary endpoint was safety and tolerability of the different doses of rAd35-EnvA alone and in combination with rAd5-EnvA. Secondary objectives included evaluating the magnitude and frequency of the immune responses to the heterologous prime-boost vaccine regimen as measured by intracellular cytokine staining, ELISpot, vaccine antigen-specific ELISA, neutralization assays, and neutralizing antibody titers to Ad5 and Ad35 at Study Weeks four and 16.
Clinical safety evaluations included laboratory tests, physical assessments and solicited reactogenicity reports via diary card for 5 days post injection. Research blood samples were collected at enrollment, baseline, and Weeks four, 12, and 24 for Groups one-three (Part I), and for Group four at enrollment, baseline, Weeks four, 12, 16, 24, 36, 52, then yearly to five years.
Vaccines
The same gp140(A) gene construct that was used in the rAd5 product VRC-HIVADV014-00-VP [3, 25], was used in the construction of both the rAd5-EnvA and rAd35-EnvA adenoviral vector vaccines in this study, as previously described [26]. In brief, the sequence from 92rw020 clade A strain (CCR5-tropic, GenBank accession number U08794) was used to create the synthetic gp140 versions of the clade A envelope gene truncated at the transmembrane domain of gp41. The cleavage site and fusion peptide at the junction of envelope gp120 and gp41 regions were deleted, and a portion of the interspace between the two heptad repeat regions in gp41 was deleted. The rAd35 adenovector (VRC-HIVADV027-00-VP) consisted of the Ad35 genome with a deletion of the E1 region, rendering the adenovector replication-deficient. The rAd5 adenovector (VRC-HIVADV038-00-VP) was similarly altered. Vaccines were tested in compliance with good manufacturing practices before release for use in clinical trials.
T cell and antibody assays
Peripheral blood mononuclear cells (PBMC) were isolated for cryopreservation within six hours of each blood draw. All T cell assays were performed on cryopreserved cells. The antibodies, peptides, and methods used for cell stimulation have been previously reported [7]. Intracellular cytokine staining (ICS), IFN-γ ELISpot analysis, and analysis of serum antibody levels by ELISA were performed and analyzed by previously reported methods using stimulation with peptide pools (15-mers overlapping by 11) matched to the EnvA vaccine antigen, also as previously described [7].
Statistical Methods
All data from participants who received at least one vaccination were analyzed. The analyses used for the primary objectives of safety and tolerability were solely descriptive, with percentages and exact confidence intervals reported. For the secondary analyses for immunogenicity, we used assay-specific pre-defined positivity criteria to categorize each individual as a responder or non-responder at each time point. For ELISA, a positive response was defined as any end point titer of 30 or greater, after raw OD correction based upon pre-vaccination samples. Volunteer sera were tested in duplicate serial 3-fold dilutions covering a dilution range from 1:30 to 1:21870. End point titers were based upon the mean sample OD for each dilution, which were corrected for the mean OD of the same dilution of the preimmunization sample. Endpoint titers for each sample/antigen were established as the last dilution with a pre-immunization corrected OD > 0.2. For ELISpot, a response was defined to be peptide-stimulated number of spots per million of at least 59 and greater than four fold above background. For ICS, a positive response was defined to be one in which the proportion of positive cells was statistically higher in the peptide-stimulated condition as compared to the background-stimulated condition by a one-sided Fisher’s exact test, and the difference in the percentages was at least 0.05% for all CD4 cytokine producers and IL-2 producing CD8s, and at least 0.08% for IFN-γ and TNF-α producing CD8s.
Analyses of immunological data included descriptive statistics as well as comparisons between the randomized subgroups of Group four. Between group comparisons use Fisher’s Exact Test for binary data and Wilcoxon Rank Sum tests for the magnitude of the response. Statistical analyses were performed using SAS 9.2 and R 3.1.1.
Results
Study conduct and population
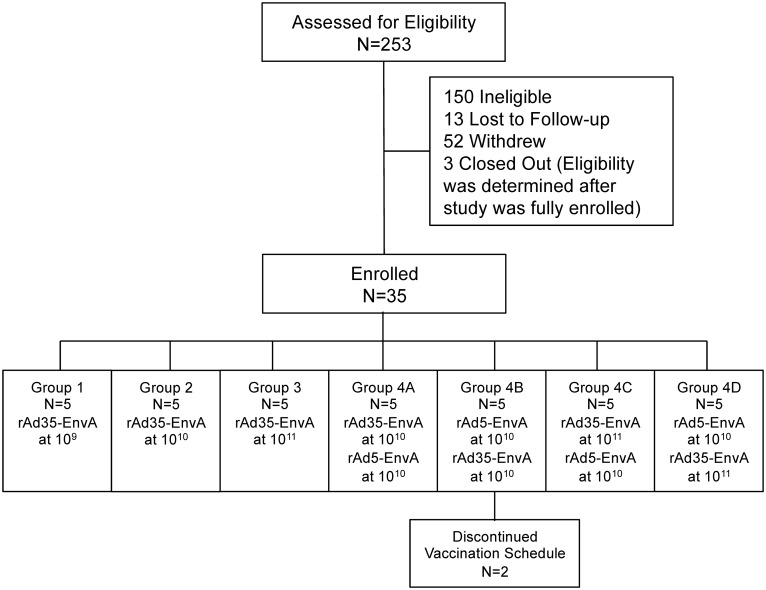
The 15 subjects in Part I were enrolled between June 18, 2007 and March 31, 2008. The last Part I visit occurred September 15, 2008. The 20 subjects in Part II were enrolled between December 1, 2008 and January 6, 2010. The last Part II visit occurred 1/5/2011. The trial ended at the completion of the last long-term follow-up contact on 1/24/2014. Among the 15 subjects enrolled in Part I, all received the single planned study injection, and 14 completed the protocol through Week 24; one subject was lost to follow-up after the Study Week two visit (Table 1). Among the 20 subjects in Part II, 18 completed both planned study injections on their schedules and two received a prime but not a boost injection. Both subjects were in Group 4B and, therefore, neither received the 1010 rAd35-EnvA boost injection (Table 1). Neither participant discontinued due to adverse reaction. Fig 1 shows the disposition of subjects.
Fig 1. CONSORT Flow Diagram.
Number of individuals assessed for eligibility, enrolled and followed up. In Part I of the study, the first 15 subjects were enrolled into the rAd35-EnvA dose escalation cohorts and in Part II, the last 20 subjects were randomized to the blinded prime-boost regimens.
Demographics
Study subject demographic characteristics are shown in Table 2. The 35 study enrollments included 14 (40%) male and 21 (60%) female subjects, with a mean age of 29.7 years (range 18 to 49 years). In addition to the 23 (66%) white subjects, eight (23%) were black or African American, one was American Indian/Alaskan native, one was Asian, and two were multiracial. All subjects were Non-Hispanic/Latino.
Table 2. Subject Demographics.
| VRC 012 Subject Demographic Characteristics | Part I: Dose Escalation (N = 15) | Part II: Prime/Boost (N = 20) | Overall (N = 35) | ||||
|---|---|---|---|---|---|---|---|
| Gender | Male, N (%) | 9 | (60) | 5 | (25) | 14 | (40) |
| Female, N (%) | 6 | (40) | 15 | (75) | 21 | (60) | |
| Age | Years, Mean [Range] | 27.7 | [18, 49] | 31.2 | [23,46] | 29.7 | [18,49] |
| Race | American Indian/Alaskan Native, N (%) | 0 | (0) | 1 | (5) | 1 | (2.9) |
| Asian, N (%) | 1 | (6.7) | 0 | (0) | 1 | (2.9) | |
| Black or African American, N (%) | 4 | (26.7) | 4 | (20) | 8 | (22.9) | |
| White, N (%) | 8 | (53.3) | 15 | (75) | 23 | (65.7) | |
| Multiracial, N (%) | 2 | (13.3) | 0 | (0) | 2 | (5.7) | |
| Ethnicity | Non-Hispanic Latino, N (%) | 15 | (100) | 20 | (100) | 35 | (100) |
| Hispanic/Latino, N (%) | 0 | (0) | 0 | (0) | 0 | (0) | |
| B.M.I. | Kg/m2, Mean [Range] | 25.3 | [19.3, 32.7] | 25.3 | [19.0, 39.1] | 25.3 | [19.0, 39.1] |
Vaccine Safety
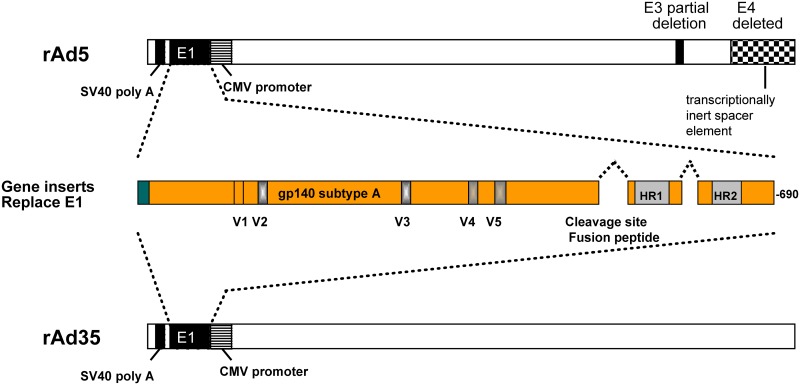
The vaccine construct is depicted in Fig 2. Vaccinations were well tolerated. Across all subjects, the worst severity of local reactogenicity was reported as none by ten (28.6%) subjects, mild by 24 (68.6%) and moderate by one (2.9%), while the worst severity of systemic reactogenicity was reported as none by 17 (48.6%) subjects, mild by 15 (42.9%) and moderate by three (8.6%), as shown in Table 3 and S1 and S2 Tables. Each subject was counted once at worst severity for any reactogenicity parameter on five-day solicited diary cards. The top section of Table 3 summarizes across local parameters (pain/tenderness, erythema, induration). The lower section of Table 3 summarizes across systemic parameters (malaise, myalgia, headache, chills, nausea, fever). Some of the subjects who experienced systemic symptoms had a pattern of acute flu-like symptoms (malaise, myalgia, headache, chills) in the first 24 hours after injection with improvement over the next few days, consistent with that observed in previous adenoviral vector vaccine studies, although only two of 35 (5.7%) recorded a mildly elevated temperature. The three occurrences that were reported as moderate in severity were in association with rAd5-EnvA boosts. In general, rAd35-EnvA was perceived to be clinically less reactogenic than rAd5.
Fig 2. Schema of gp140(A) Gene Construct.
The sequence from 92rw020 clade A strain (CCR5-tropic, GenBank accession number U08794) was used to create the synthetic gp140 versions of the clade A envelope gene truncated at the transmembrane domain of gp41. The cleavage site and fusion peptide at the junction of envelope gp120 and gp41 regions were deleted and a portion of the interspace between the two heptad repeat regions in gp41 was deleted. A portion of the E1 region was deleted from both vectors, rendering them replication-deficient.
Table 3. Frequency of Local and Systemic Reactogenicity Following Injections.
| Day 0030 | Day 0 | Day 0 | Day 0 | Week 12 | Week 12 | Week 12 | All Subjects | |
|---|---|---|---|---|---|---|---|---|
| rAd35-EnvA 109 | rAd35-EnvA 1010 | rAd35-EnvA 1011 | rAd5-EnvA 1010 | rAd35-EnvA 1010 | rAd35-EnvA 1011 | rAd5-EnvA 1010 | ||
| Symptoms Intensity | (N = 5) | (N = 10) | (N = 10) | (N = 10) | (N = 3) | (N = 5) | (N = 10) | (N = 35) |
| ANY LOCAL SYMPTOM (Pain/Tenderness, Swelling, Redness) | ||||||||
| None | 4 (80.0%) | 6 (60.0%) | 1 (10.0%) | 2 (20.0%) | 0 (0.0%) | 1 (20.0%) | 2 (20.0%) | 10 (28.6%) |
| Mild | 1 (20.0%) | 4 (40.0%) | 9 (90.0%) | 8 (80.0%) | 3 (100.0%) | 4 (80.0%) | 7 (70.0%) | 24 (68.6%) |
| Moderate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (10.0%) | 1 (2.9%) |
| ANY SYSTEMIC SYMPTOM (Malaise, Myalgia, Headache, Chills, Nausea, Temperature) | ||||||||
| None | 3 (60.0%) | 7 (70.0%) | 5 (50.0%) | 7 (70.0%) | 1 (33.3%) | 3 (60.0%) | 5 (50.0%) | 17 (48.6%) |
| Mild | 2 (40.0%) | 3 (30.0%) | 5 (50.0%) | 3 (30.0%) | 2 (66.7%) | 2 (40.0%) | 2 (20.0%) | 15 (42.9%) |
| Moderate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (30.0%) | 3 (8.6%) |
There were two serious adverse events during the study, neither of which was deemed related to the study vaccines. One was a case of breast cancer (described as moderately aggressive, BRCA negative, estrogen and progesterone receptor positive) in a 38-year-old female participant diagnosed 281 days after the second study injection and subsequently treated with outpatient lumpectomy, radiation and Tamoxifen. The other was a case of ductal carcinoma in situ in a 39-year-old female participant diagnosed 72 days after the second study injection and subsequently treated with mastectomy.
Transient prolonged aPTT was noted after five vaccinations in four of the 25 subjects in Part II, as shown in S1 Fig.[27] Three events occurred after rAd5-EnvA and two after rAd35-EnvA. In three of the four subjects, the aPTT elevation reached a grade one event and resolved within 14 days. The fourth subject experienced an initial grade one elevation in the aPTT after the rAd35-EnvA vaccination and then experienced a recurrence, with a grade three prolongation of aPTT, the highest recorded at 57.9 sec, after rAd5-EnvA boost and then slow normalization over the subsequent five months. This phenomenon has been documented before in a prior study evaluating another rAd5 vector [19] where the anti-phospholipid antibody response creates an artifact in the in vitro aPTT analysis. In the current study, the bleeding time and other coagulation parameters were not affected by rAd immunization. The single episode of grade 2 hematuria noted in a subject 14 days after rAd35-Env prime occurred a subject who did not have prolonged aPTT. The hematuria was noted on a single urinalysis and resolved without intervention.
One of 15 subjects in Part I and 16 of 20 in Part II had one or more test results indicating the presence of vaccine-induced seropositivity (VISP). These widely used clinical diagnostic HIV serological tests that measure antibody to HIV, including antibody to the vaccine antigen used in this trial, reverted to negative over the course of 9–12 months. A positive result on such a test should be interpreted as a false-positive and the subject’s HIV status confirmed by PCR. An example of the time course of VISP is shown as S2 Fig. All of the subjects remained HIV-negative by diagnostic PCR testing throughout the study.
Vaccine-induced antibody responses
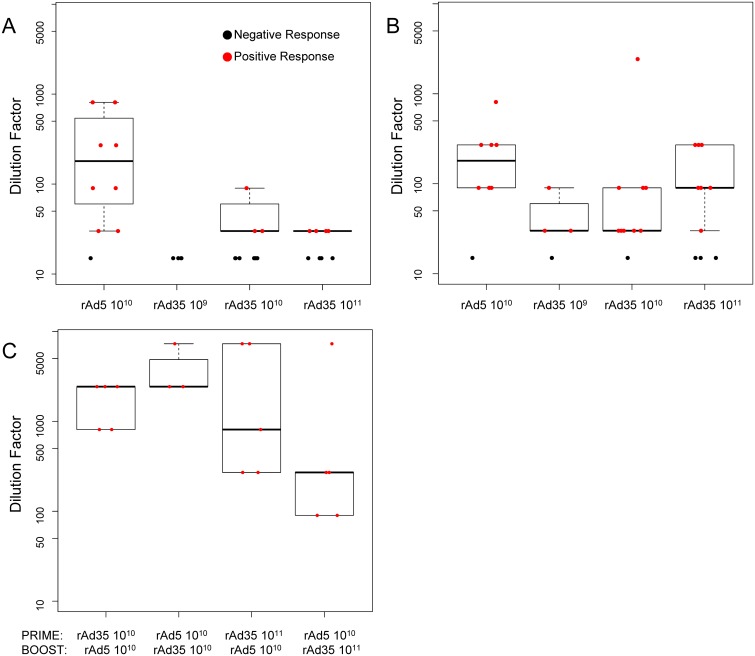
EnvA-specific antibody (Ab) responses measured by ELISA were detected at four weeks in 30% and 50% of subjects after single doses of 1010 or 1011 rAd35-EnvA, respectively, and in 89% after a single rAd5-EnvA 1010 PU injection, combining results from all subjects across parts 1 and 2 of the study. The magnitude of these Ab responses was low after a single dose of either vector and are shown in Fig 3A. At 12 weeks, positive ELISA responses were detected in 3/4 (75%), 9/10 (90%), and 7/10 (70%) subjects after single doses of 109, 1010, or 1011 PU of rAd35-EnvA, respectively, as shown in Fig 3B. When measured after boosting, positive titers were seen in 5/5 (100%) subjects in Group 4A, 3/4 (75%) in Group 4B, 5/5 (100%) in Group 4C, and 5/5 (100%) in Group 4D, shown in Fig 3C.[28]
Fig 3. ELISA Responses Against EnvA Measured At Two Timepoints Post Prime and Four Weeks Post Boost.
Endpoint titers/dilution were calculated after priming by vaccine and dose indicated on the X-axis (A & B), combined from Parts I and II, and each individual prime-boost combination indicated on the X-axis from Part II (C). Part A shows data 4 weeks post prime, while 4B shows data 12 weeks post prime. Part C shows data 4 weeks post boost. The responders are represented on the plot with red dots and are used to construct the boxplots showing the interquartile range; black dots represent the non-responders and are not included in the response range.
Vaccine-induced T cell responses
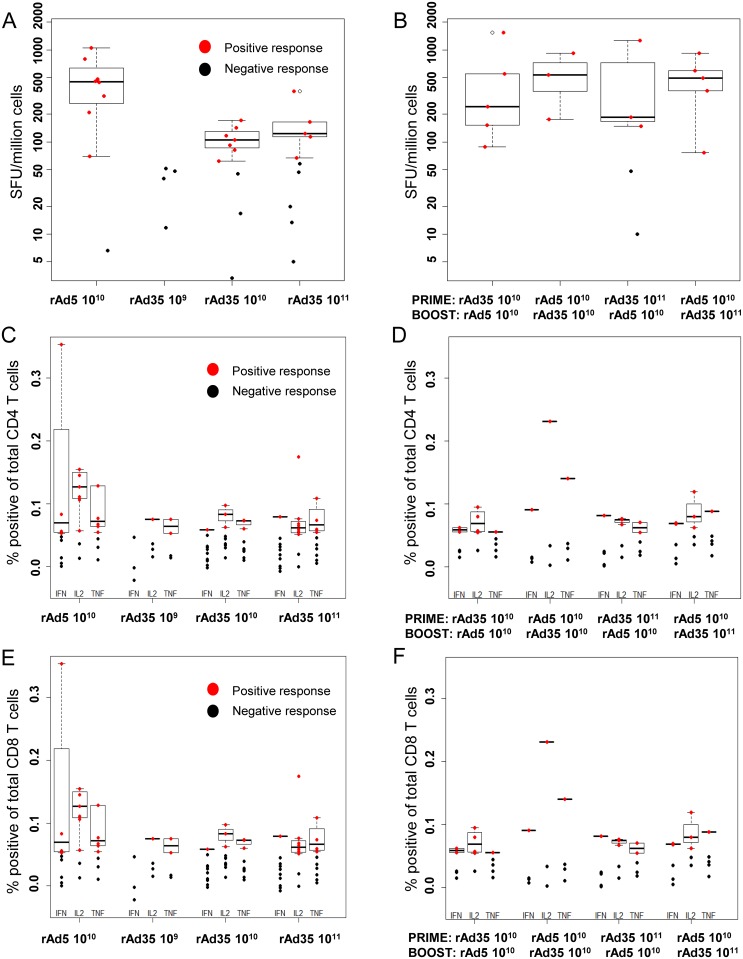
T cell responses as measured by ELISpot were higher after a single rAd5-EnvA injection than after a single rAd35-EnvA injection at any dose, as shown in Fig 4A. IFN-γ ELISpot responses were detected in 0/5 (0%), 7/10 (70%), and 5/10 (50%) of subjects after single doses of 109, 1010, or 1011 rAd35-EnvA, respectively, compared to 8/9 (89%) of subjects after a single rAd5-EnvA 1010 injection. T cell responses were detected in the majority of Group four rAd35-EnvA or rAd5-EnvA recipients after boosting with the heterologous vector (16/18, 89%), as demonstrated in Fig 4B. ELISpot and CD4 and CD8 T cell response magnitude was similar in both regimens and comparable to a single dose of rAd5-EnvA, as demonstrated in Fig 4C–4F. CD8 T cell polyfunctional responses shown in S3 Fig suggest that rAd5-EnvA is more effective than rAd35-EnvA as a CD8 T cell prime for a given antigen. Both dose groups of rAd35, 1010 and 1011, were combined for this analysis.
Fig 4. ELISpot and Intracellular Cytokine Staining for CD4 and CD8 T cell Responses Measured Four Weeks Post Prime and Boost.
T cell assays were measured after priming by vaccine and dose indicated on the X-axis, combining subjects from Parts I and II in the left panels, and each individual prime-boost combination indicated on the X-axis from Part II in the right panels. A & B show ELISpot results in spot forming units (SFU) per million cells. The lower panels represent intracellular cytokine staining of CD4 T cells (C & D), and CD8 T cells (E & F) for the indicated cytokines, and are reported in percentage of positive cells of total CD4 or CD8 positive T cells. Analysis was performed on samples from 4 weeks post injection (prime or boost). The responders are represented on the plot with red dots and are used to construct the boxplots showing the interquartile range; black dots represent the non-responders and are not included in the response range.
Discussion
Here we show the rAd35-EnvA vaccine VRC-HIVADV027-00-VP was well tolerated and immunogenic. While effectively priming and boosting antibody responses when given in a reciprocal prime-boost regimen with rAd5-EnvA, the rAd35-EnvA vaccine did not significantly improve the magnitude of the T cell response above a single dose of rAd5 in seronegative healthy volunteers when given at a three-month interval. HVTN 077 used these vaccines with a six-month prime/boost interval and HVTN 083 used a three-month interval, and both showed similar antibody response levels [23, 24]. After two large clinical trials failed to demonstrate protection or reduction of viral load with Ad5 vectored HIV vaccines [10–13], it is unlikely that a similar Ad5-based regimen will be carried forward into further HIV vaccine trials [29]. However, regimens including alternate serotype Ad vectors continue to be studied both in HIV and other diseases, and several regimens induce equal or greater immunogenicity than that described in the present study [30].
In this proof-of-concept study, we used the EnvA insert as a prototype antigen because it is safe, immunogenic, and easily detected in evaluation assays. In future vaccine designs, expressing an antigen that presents a native trimer conformation, more similar to what is presented on the surface of HIV, would be more likely to induce antibody responses with sufficient potency and breadth for protection. One advantage of rAd vectors is the ability to induce both innate and adaptive immune responses; future investigations of alternative boosts, including protein boosts or alternate classes of viral vectors such as poxvirus vectors including modified vaccinia Ankara (MVA), NYVAC, or avipox vectors may help optimize antibody responses to maximize the protective effect [31–36].
As a Group B Ad vector, Ad35 binds to CD46, an inhibitory complement receptor expressed on all nucleated human cells [37]. The specificity of this binding has limited the study of rAd35 vectors in animal models, which lack endogenous CD46 expression. Animals expressing chimeric CD46, particularly transgenic mice, have been shown to be susceptible to other viruses that use CD46, including human herpesvirus 6 [38] and measles [39], but require modifications that complicate studying vaccines and immunogenicity. Previous work in humans has shown that rAd35, but not rAd5, stimulates maturation of dendritic cells and high interferon (IFN) α production [40], both important components of innate immunity. Because of the ability of Ad35 to induce dendritic cell maturation and facilitate antigen presentation, as well as the shortened duration of antigen production post-vaccination compared to rAd5 vectors, rAd35 vectors have been proposed as the boost in heterologous prime-boost vaccination strategies, taking advantage of the higher precursor frequency of B cells and T cells specific for the recombinant insert [41–43]. The current study does not include a rAd5-EnvA prime-only control, or a rAd5-EnvA/rAd5-EnvA prime/boost, therefore we are unable to make a direct comparison or draw firm conclusions about the utility of rAd35-EnvA as a boost. Several other groups have tested rAd35-EnvA as a boost, some with encouraging results.
Lower seroprevalent adenoviral vectors have been employed with some success in HIV, malaria, HCV, and Ebola vaccine trials [44]. HVTN 083 employed the same rAd35/rAd5 heterologous prime boost vaccines described in the present study, and they induced improved T cell responses by ELISpot as well as T cell responses to a larger number of epitopes over homologous regimens [24]. HVTN 077 demonstrated that a DNA/rAd35-EnvA prime/boost vaccination strategy did elicit detectable immune responses in individuals with pre-existing Ad5 immunity [23]. That data complements our finding that rAd35-EnvA is immunogenic as a boost in a heterologous regimen in subjects seronegative for Ad5, and suggests that rAd35 may indeed be useful in overcoming pre-existing adenoviral immunity. Adjuvants and alternate heterologous prime/boost regimens also show promise. Ad35 vectors were employed to study immune responses to multigenic HIV-1 vaccines with or without rAd35 EnvA vaccine and with or without an adjuvanted protein vaccine in Phase I clinical trials, and induced moderate immunogenicity with some dose-related reactogenicity [45–47]. In comparing prime-boost regimens using rAd35 followed by adjuvanted protein or the opposite regimen, Omosa-Manyonyi et. al. concluded that response rates as measured by IFN-γ ELISpot were improved when rAd35 was used as the prime rather than the boost [47]. A rAd35 vector was used in Phase Ia and Ib trials of a circumsporozoite surface antigen malaria vaccine, in the USA and sub-Saharan Africa, and was modestly immunogenic in both settings [48, 49]. A series of studies employing two doses of an Ad35 TB vaccine in BCG-primed individuals demonstrated vaccine-induced multifunctional CD4 and CD8 T cell responses by intracellular cytokine staining [50–52].
In addition to these efforts with rAd35, several groups have used other lower seroprevalent rAds. A rAd26-EnvA HIV vaccine demonstrated good safety and immunogenicity in a Phase I study, eliciting both humoral and T cell responses against EnvA [53–55]. When rAd26/rAd35 heterologous regimens, employing a different rAd35 vector and EnvA insert, were evaluated in the presence or absence of pre-existing vector immunity, using rAd26 as the prime proved more effective than priming with rAd35 at producing EnvA binding ELISA titers. Both heterologous regimens produced higher EnvA ELISA titers than homologous regimens, and there were no significant differences noted between vector-naïve and vector pre-immune individuals [56]. A unique strategy to circumvent pre-existing Ad5 immunity but still reap the immunological benefits of the Ad5 response involved creating a chimeric Ad5/Ad48 HIV EnvA immunogen [57]. Hypervariable regions from Ad48 replaced corresponding Ad5 sequences, and the vaccine induced EnvA ELISA and ELISpot responses while producing higher neutralizing antibody titers to Ad48 than to Ad5. Further efforts to avoid pre-existing vector immunity included use of chimpanzee adenoviral (ChAd) vectors. A ChAd63 vaccine utilizing conserved epitopes of multiple HIV proteins was used in several prime boost regimens with DNA or MVA, and elicited broadly specific, polyfunctional CD4 and CD8 T cell responses in humans. [58] A ChAd63 vaccine was given with or without MVA boost to successfully induce high levels of CD4 and CD8 T cells specific for the preerythrocytic malaria antigen, multiple epitope thrombospondin-related adhesion protein (ME-TRAP) [59]. A ChAd3 vectored vaccine was used in a heterologous prime/boost vaccine regimen with human Ad6 to elicit potent, long-lasting CD4 and CD8 T cell responses specific for non-structural proteins from Hepatitis C Virus (HCV)[60, 61].
Part II of this study was the first time that aPTT has been routinely evaluated after vaccinations in a clinical study with HIV adenoviral vector vaccines. The aPTT prolongation observed here was consistent with an in vitro effect on the laboratory assay for aPTT due to a transient induction of anti-phospholipid antibody (APA) as described previously in preclinical (rabbit) toxicology studies with both Ad5 and Ad35 vector vaccines and in humans with both a rAd5 Ebola vaccine and an investigational rAd5 prostate cancer vaccine [17–19, 62]. We were able to further describe the decay of this transiently expressed antibody, and confirm that in our trial there were no clinical manifestations associated with what was operationally an in vitro laboratory phenomenon, and not an in vivo coagulation abnormality.
As this expanding body of work on lower seroprevalence adenoviral vectored vaccines demonstrates, improvements in the combination of platform and strategy have, in some cases, produced more robust humoral and cellular immune responses to candidate vaccines than those observed in the present study. However, the goal of broad and potent protection against infection with multiple pathogens, including HIV, remains elusive. Thoughtful and detailed immunologic analysis of human clinical trial data from studies such as this one may inform the further refinement of immunogens, vectors, and vaccination strategies.
Supporting Information
(DOC)
The time course of abnormal aPTT values in four subjects shows that abnormalities peaked around two weeks post prime and boost, and had begun to return to normal two weeks later. Subjects whose aPTT values met the definition of an adverse event (AE) were followed until resolution of the AE.
(PDF)
Antibody responses to EnvA detected by Abbot ELISA in study subject 019, who received rAd5-EnvA prime and rAd35-EnvA 1010 boost. The subject developed detectable EnvA responses at day 29 post prime, which declined prior to boost, and then peaked at day 112 (28 days after rAd35-EnvA boost), then declined slowly over the next 247 days to return to a level below the assay background.
(PDF)
Percentage of CD8 T cells elicited from trial volunteers immunized with either (A). rAd35 prime/rAd5 boost or (B). rAd5 prime/rAd35 boost liberating 1 (green), 2 (blue) or 3 (red) cytokines (IFNγ, IL2 or TNFα) following stimulation with EnvA specific overlapping 15mer peptides. Overall, the CD8 EnvA specific immune response was higher in those individuals following prime with rAd5. Moreover, the frequency of CD8 T cells producing 1, 2 or 3 cytokines was also greater following this regimen. Subjects receiving both doses of rAd35-EnvA were combined for this analysis.
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors would like to thank the study volunteers of VRC 012. We also thank NIH Clinical Center staff, the NIAID Intramural Institutional Review Board, EMMES Corporation, and other current and past VRC and NIH colleagues including Abe Mittelman, Philip Gomez, Charla Andrews, Rebecca Sheets, Judy Stein, Brenda Hartman, Michelle Conan-Cibotti, Adam Sherwat, Hope DeCederfelt and Judith Starling.
The VRC 012 Study Team
In addition to those listed as authors: Joseph Casazza, Ingelise Gordon, Lasonji Holman, Sarah Plummer, Pamela Costner, Floreliz Mendoza, Jamie Saunders, Kathy Zephir, Galina Yamshchikov, Brenda Larkin, and Sandra Sitar (Vaccine Research Center, NIAID, NIH), and Phyllis Renehan (The EMMES Corporation). These clinical trials were funded by the NIH Intramural Research Program. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency or collaborators.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for the conduct of this clinical trial was provided by the National Institute of Allergy and Infectious Diseases (NIAID) intramural research program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Wenjuan Gu: Ms. Gu is an employee of Clinical Research Directorate/Clinical Monitoring Research Program, Leidos Biomedical Research, Inc., NCI Campus at Frederick, Maryland 21702. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361(23):2209–20. 10.1056/NEJMoa0908492 . [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366(14):1275–86. 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. The Journal of infectious diseases. 2006;194(12):1638–49. 10.1086/509258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catanzaro AT, Roederer M, Koup RA, Bailer RT, Enama ME, Nason MC, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25(20):4085–92. 10.1016/j.vaccine.2007.02.050 . [DOI] [PubMed] [Google Scholar]
- 5.Enama ME, Ledgerwood JE, Novik L, Nason MC, Gordon IJ, Holman L, et al. Phase I randomized clinical trial of VRC DNA and rAd5 HIV-1 vaccine delivery by intramuscular (i.m.), subcutaneous (s.c.) and intradermal (i.d.) administration (VRC 011). PloS one. 2014;9(3):e91366 10.1371/journal.pone.0091366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. The Journal of infectious diseases. 2006;194(12):1650–60. 10.1086/509259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, et al. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PloS one. 2010;5(2):e9015 10.1371/journal.pone.0009015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29(32):5203–9. 10.1016/j.vaccine.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. Journal of virology. 2007;81(12):6594–604. 10.1128/JVI.02497-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–93. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Current opinion in HIV and AIDS. 2010;5(5):357–61. 10.1097/COH.0b013e32833d2d2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. The Lancet infectious diseases. 2011;11(7):507–15. 10.1016/S1473-3099(11)70098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. The New England journal of medicine. 2013;369(22):2083–92. 10.1056/NEJMoa1310566 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. Journal of immunology. 2004;172(10):6290–7. . [DOI] [PubMed] [Google Scholar]
- 15.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of virology. 2007;81(9):4654–63. 10.1128/JVI.02696-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28(4):950–7. 10.1016/j.vaccine.2009.10.145 . [DOI] [PubMed] [Google Scholar]
- 17.Sheets RL, Stein J, Bailer RT, Koup RA, Andrews C, Nason M, et al. Biodistribution and toxicological safety of adenovirus type 5 and type 35 vectored vaccines against human immunodeficiency virus-1 (HIV-1), Ebola, or Marburg are similar despite differing adenovirus serotype vector, manufacturer's construct, or gene inserts. Journal of immunotoxicology. 2008;5(3):315–35. 10.1080/15376510802312464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29(2):304–13. 10.1016/j.vaccine.2010.10.037 . [DOI] [PubMed] [Google Scholar]
- 19.Malaeb BS, Gardner TA, Margulis V, Yang L, Gillenwater JY, Chung LW, et al. Elevated activated partial thromboplastin time during administration of first-generation adenoviral vectors for gene therapy for prostate cancer: identification of lupus anticoagulants. Urology. 2005;66(4):830–4. 10.1016/j.urology.2005.04.041 . [DOI] [PubMed] [Google Scholar]
- 20.Kibuuka H, Berkowitz NM, Millard M, Enama ME, Tindikahwa A, Sekiziyivu AB, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet. 2015;385(9977):1545–54. 10.1016/S0140-6736(14)62385-0 . [DOI] [PubMed] [Google Scholar]
- 21.Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, et al. Chimpanzee Adenovirus Vector Ebola Vaccine—Preliminary Report. The New England journal of medicine. 2014. 10.1056/NEJMoa1410863 . [DOI] [PubMed] [Google Scholar]
- 22.Subcommittee AVR. AVRS Meeting Archive 2005 [updated 6/27/2011; cited 2014 3/23]. Available from: http://www.niaid.nih.gov/topics/HIVAIDS/Research/vaccines/advisory/avrs/AVRSarchive/Pages/avrwgAug05.aspx.
- 23.Fuchs JD, Bart PA, Frahm N, Morgan C, Gilbert PB, Kochar N, et al. Safety and Immunogenicity of a Recombinant Adenovirus Serotype 35-Vectored HIV-1 Vaccine in Adenovirus Serotype 5 Seronegative and Seropositive Individuals. J AIDS Clin Res. 2015;6(5). 10.4172/2155-6113.1000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh SR, Moodie Z, Fiore-Gartland AJ, Morgan C, Wilck MB, Hammer SM, et al. Vaccination with heterologous HIV envelope sequences and heterologous adenovirus vectors increases T cell responses to conserved regions (HVTN 083). The Journal of infectious diseases. 2015. 10.1093/infdis/jiv496 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peiperl L, Morgan C, Moodie Z, Li H, Russell N, Graham BS, et al. Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegative persons: a randomized clinical trial (HVTN 054). PloS one. 2010;5(10):e13579 10.1371/journal.pone.0013579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, et al. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. Journal of virology. 2002;76(11):5357–68. 10.1128/JVI.76.11.5357-5368.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enama ME NL, Hendel CS, Sheets R, Ledgerwood JE, Graham BS., editor Induction of False-Positive PTT Elevations by Investigational Adenoviral Vector Vaccines. 14th Annual Conference on Vaccine Research; 2011 May 16–18, 2011; Baltimore, Maryland, U. S. A. http://www.nfid.org/professional-education/archives/acvr/: National Foundation for Infectious Diseases; 2011.
- 28.Graham BSL, J.E.; Novik, L.; Enama, M.E.; Hendel, C.S.; Nason, M.C.; Koup, R.A.; Mascola, J.R.; Nabel, G.J., editor Safety and immunogenicty of a rAd35-EnvA prototype HIV-1 vaccine in combination with rAd5-EnvA in healthy adults (VRC012) AIDS Vaccine 2010; 2010 October 15, 2010; Atlanta, Georgia, U. S. A. AIDS Research and Human Retroviruses2010.
- 29.McMichael A, Picker LJ, Moore JP, Burton DR. Another HIV vaccine failure: where to next? Nat Med. 2013;19(12):1576–7. 10.1038/nm.3413 . [DOI] [PubMed] [Google Scholar]
- 30.Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev Vaccines. 2010;9(9):1055–69. 10.1586/erv.10.106 . [DOI] [PubMed] [Google Scholar]
- 31.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89–93. 10.1038/nature10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimani D, Jagne YJ, Cox M, Kimani E, Bliss CM, Gitau E, et al. Translating the immunogenicity of prime-boost immunisation with ChAd63 and MVA ME-TRAP from malaria naive to malaria-endemic populations. Molecular therapy: the journal of the American Society of Gene Therapy. 2014. 10.1038/mt.2014.109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perdiguero B, Gomez CE, Di Pilato M, Sorzano CO, Delaloye J, Roger T, et al. Deletion of the vaccinia virus gene A46R, encoding for an inhibitor of TLR signalling, is an effective approach to enhance the immunogenicity in mice of the HIV/AIDS vaccine candidate NYVAC-C. PloS one. 2013;8(9):e74831 10.1371/journal.pone.0074831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perdiguero B, Gomez CE, Najera JL, Sorzano CO, Delaloye J, Gonzalez-Sanz R, et al. Deletion of the viral anti-apoptotic gene F1L in the HIV/AIDS vaccine candidate MVA-C enhances immune responses against HIV-1 antigens. PloS one. 2012;7(10):e48524 10.1371/journal.pone.0048524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chmielewska AM, Naddeo M, Capone S, Ammendola V, Hu K, Meredith L, et al. Combined adenovirus vector and hepatitis C virus envelope protein prime-boost regimen elicits T cell and neutralizing antibody immune responses. Journal of virology. 2014;88(10):5502–10. 10.1128/JVI.03574-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bart PA, Huang Y, Karuna ST, Chappuis S, Gaillard J, Kochar N, et al. HIV-specific humoral responses benefit from stronger prime in phase Ib clinical trial. J Clin Invest. 2014;124(11):4843–56. 10.1172/JCI75894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen RF, Lee CY. Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection. International reviews of immunology. 2014;33(1):45–53. 10.3109/08830185.2013.823420 . [DOI] [PubMed] [Google Scholar]
- 38.Reynaud JM, Horvat B. Animal models for human herpesvirus 6 infection. Frontiers in microbiology. 2013;4:174 10.3389/fmicb.2013.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellin CI, Horvat B. Current animal models: transgenic animal models for the study of measles pathogenesis. Current topics in microbiology and immunology. 2009;330:111–27. . [DOI] [PubMed] [Google Scholar]
- 40.Lore K, Adams WC, Havenga MJ, Precopio ML, Holterman L, Goudsmit J, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. Journal of immunology. 2007;179(3):1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson MJ, Petrovas C, Yamamoto T, Lindsay RW, Lore K, Gall JG, et al. Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. Journal of immunology. 2012;188(12):6109–18. 10.4049/jimmunol.1103717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn KM, Da Costa A, Yamamoto A, Berry D, Lindsay RW, Darrah PA, et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. Journal of immunology. 2013;190(6):2720–35. 10.4049/jimmunol.1202861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn KM, Zak DE, Costa A, Yamamoto A, Kastenmuller K, Hill BJ, et al. Antigen expression determines adenoviral vaccine potency independent of IFN and STING signaling. J Clin Invest. 2015;125(3):1129–46. 10.1172/JCI78280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nat Rev Microbiol. 2014;12(11):765–71. 10.1038/nrmicro3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keefer MC, Gilmour J, Hayes P, Gill D, Kopycinski J, Cheeseman H, et al. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PloS one. 2012;7(8):e41936 10.1371/journal.pone.0041936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kopycinski J, Hayes P, Ashraf A, Cheeseman H, Lala F, Czyzewska-Khan J, et al. Broad HIV epitope specificity and viral inhibition induced by multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PloS one. 2014;9(3):e90378 10.1371/journal.pone.0090378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omosa-Manyonyi G, Mpendo J, Ruzagira E, Kilembe W, Chomba E, Roman F, et al. A Phase I Double Blind, Placebo-Controlled, Randomized Study of the Safety and Immunogenicity of an Adjuvanted HIV-1 Gag-Pol-Nef Fusion Protein and Adenovirus 35 Gag-RT-Int-Nef Vaccine in Healthy HIV-Uninfected African Adults. PloS one. 2015;10(5):e0125954 10.1371/journal.pone.0125954 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Creech CB, Dekker CL, Ho D, Phillips S, Mackey S, Murray-Krezan C, et al. Randomized, placebo-controlled trial to assess the safety and immunogenicity of an adenovirus type 35-based circumsporozoite malaria vaccine in healthy adults. Human vaccines & immunotherapeutics. 2013;9(12):2548–57. 10.4161/hv.26038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouedraogo A, Tiono AB, Kargougou D, Yaro JB, Ouedraogo E, Kabore Y, et al. A phase 1b randomized, controlled, double-blinded dosage-escalation trial to evaluate the safety, reactogenicity and immunogenicity of an adenovirus type 35 based circumsporozoite malaria vaccine in Burkinabe healthy adults 18 to 45 years of age. PloS one. 2013;8(11):e78679 10.1371/journal.pone.0078679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Churchyard GJ, Snowden MA, Hokey D, Dheenadhayalan V, McClain JB, Douoguih M, et al. The safety and immunogenicity of an adenovirus type 35-vectored TB vaccine in HIV-infected, BCG-vaccinated adults with CD4(+) T cell counts >350 cells/mm(3). Vaccine. 2015;33(15):1890–6. 10.1016/j.vaccine.2015.02.004 . [DOI] [PubMed] [Google Scholar]
- 51.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med. 2010;181(12):1407–17. 10.1164/rccm.200910-1484OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoft DF, Blazevic A, Stanley J, Landry B, Sizemore D, Kpamegan E, et al. A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine. 2012;30(12):2098–108. 10.1016/j.vaccine.2012.01.048 . [DOI] [PubMed] [Google Scholar]
- 53.Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). The Journal of infectious diseases. 2013;207(2):240–7. 10.1093/infdis/jis670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barouch DH, Liu J, Peter L, Abbink P, Iampietro MJ, Cheung A, et al. Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). The Journal of infectious diseases. 2013;207(2):248–56. 10.1093/infdis/jis671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baden LR, Liu J, Li H, Johnson JA, Walsh SR, Kleinjan JA, et al. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. The Journal of infectious diseases. 2015;211(4):518–28. 10.1093/infdis/jiu485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baden LR, Karita E, Mutua G, Bekker LG, Gray G, Page-Shipp L, et al. Assessment of the Safety and Immunogenicity of 2 Novel Vaccine Platforms for HIV-1 Prevention: A Randomized Trial. Ann Intern Med. 2016;164(5):313–22. 10.7326/M15-0880 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baden LR, Walsh SR, Seaman MS, Johnson JA, Tucker RP, Kleinjan JA, et al. First-in-human evaluation of a hexon chimeric adenovirus vector expressing HIV-1 Env (IPCAVD 002). The Journal of infectious diseases. 2014;210(7):1052–61. 10.1093/infdis/jiu217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, Ebrahimsa U, et al. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22(2):464–75. 10.1038/mt.2013.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. The Journal of infectious diseases. 2012;205(5):772–81. 10.1093/infdis/jir850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4(115):115ra1 10.1126/scitranslmed.3003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capone S, Naddeo M, D'Alise AM, Abbate A, Grazioli F, Del Gaudio A, et al. Fusion of HCV nonstructural antigen to MHC class II-associated invariant chain enhances T-cell responses induced by vectored vaccines in nonhuman primates. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22(5):1039–47. 10.1038/mt.2014.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koeneman KS, Kao C, Ko SC, Yang L, Wada Y, Kallmes DF, et al. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World journal of urology. 2000;18(2):102–10. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
The time course of abnormal aPTT values in four subjects shows that abnormalities peaked around two weeks post prime and boost, and had begun to return to normal two weeks later. Subjects whose aPTT values met the definition of an adverse event (AE) were followed until resolution of the AE.
(PDF)
Antibody responses to EnvA detected by Abbot ELISA in study subject 019, who received rAd5-EnvA prime and rAd35-EnvA 1010 boost. The subject developed detectable EnvA responses at day 29 post prime, which declined prior to boost, and then peaked at day 112 (28 days after rAd35-EnvA boost), then declined slowly over the next 247 days to return to a level below the assay background.
(PDF)
Percentage of CD8 T cells elicited from trial volunteers immunized with either (A). rAd35 prime/rAd5 boost or (B). rAd5 prime/rAd35 boost liberating 1 (green), 2 (blue) or 3 (red) cytokines (IFNγ, IL2 or TNFα) following stimulation with EnvA specific overlapping 15mer peptides. Overall, the CD8 EnvA specific immune response was higher in those individuals following prime with rAd5. Moreover, the frequency of CD8 T cells producing 1, 2 or 3 cytokines was also greater following this regimen. Subjects receiving both doses of rAd35-EnvA were combined for this analysis.
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.