Abstract
Osmotic pressure (Π) induces the stretching of plasma membranes of cells or lipid membranes of vesicles, which plays various roles in physiological functions. However, there have been no experimental estimations of the membrane tension of vesicles upon exposure to Π. In this report, we estimated experimentally the lateral tension of the membranes of giant unilamellar vesicles (GUVs) when they were transferred into a hypotonic solution. First, we investigated the effect of Π on the rate constant, kp, of constant-tension (σex)-induced rupture of dioleoylphosphatidylcholine (DOPC)-GUVs using the method developed by us recently. We obtained the σex dependence of kp in GUVs under Π and by comparing this result with that in the absence of Π, we estimated the tension of the membrane due to Π at the swelling equilibrium, . Next, we measured the volume change of DOPC-GUVs under small Π. The experimentally obtained values of and the volume change agreed with their theoretical values within the limits of the experimental errors. Finally, we investigated the characteristics of the Π-induced pore formation in GUVs. The corresponding to the threshold Π at which pore formation is induced is similar to the threshold tension of the σex-induced rupture. The time course of the radius change of GUVs in the Π-induced pore formation depends on the total membrane tension, σt; for small σt, the radius increased with time to an equilibrium one, which remained constant for a long time until pore formation, but for large σt, the radius increased with time and pore formation occurred before the swelling equilibrium was reached. Based on these results, we discussed the and the Π-induced pore formation in lipid membranes.
Introduction
When cells or vesicles of lipid membranes are transferred into a hypotonic solution containing a lower concentration of solute compared with the solution inside the cells or vesicles, an osmotic pressure, Π, is applied to the cells or vesicles. This results in water molecules entering the cells or vesicles, causing their volume to increase (the cells or vesicles swell). In turn, this induces a lateral tension in the plasma membrane or lipid membrane, and this tension plays various important roles in the physiological functions and physicochemical properties of the membrane (1, 2, 3, 4). When the tension reaches a critical magnitude, pore formation occurs, causing lysis (rupture) of the vesicles which induces leakage (efflux) of their internal contents (5, 6). The Π-induced increase of vesicle volume was used to investigate the elastic properties of lipid bilayers of large unilamellar vesicles (LUVs) by measuring the change of average diameters of the LUVs under Π using dynamic light scattering (DLS) (7, 8, 9). The Π-induced leakage of fluorescent probes was also measured and analyzed (5, 10, 11). However, all measurements to date of the effects of Π on vesicles have focused only on the Π-induced volume increase of vesicles (6, 7, 8, 9, 10, 11, 12, 13, 14) and the leakage of their internal contents. There have been no experimental estimations of the membrane tension of vesicles of lipid membranes upon exposure to Π. Moreover, all previous experiments have been conducted using LUVs. The polydispersity of LUVs (i.e., large distribution in LUV diameters) affects analysis by DLS (11), and fluorescence intensity measurements of LUV suspensions provide an ensemble average of the fluorescence intensity of many LUVs (15). In contrast, giant unilamellar vesicles (GUVs) of lipid membranes with diameters >10 μm have the advantage that their structures and physical properties can be directly observed as a function of time using optical microscopy (16, 17, 18, 19). Measurement of the diameter and observation of the shape of each GUV allows the selection of GUVs with a specific shape and an appropriate diameter for each experiment. Using this advantage, osmotic-pressure-induced shape changes of GUVs were observed (20, 21). The shrinkage of a GUV after its transfer to a hypertonic environment was observed, and by analysis of the time course of the volume decrease of each GUV, the water permeability of lipid membranes was determined (22, 23). The advantage of this approach was that the osmotic shrinkage of each GUV could be measured individually, and not as an ensemble average of many vesicles, as had been the case with previous measurements using LUVs.
It is well known that external forces also induce lateral tension on the plasma membranes of cells or the lipid membranes of vesicles, causing rupture of the cell or vesicle or pore formation in the membrane. There has been recent significant progress in research on tension-induced pore formation in lipid membranes using GUVs (16, 24, 25, 26, 27, 28, 29). Using a viscous solvent, Brochard-Wyart et al. visualized directly at video rate the fast dynamics of pore closing in a GUV stretched using intense optical illumination (16, 24). Evans et al. investigated the rupture of a GUV under dynamic applied tension, in which ramps of tension with loading rates (i.e., tension/time) were applied using a micropipette (25, 26). Recently, we developed a method to determine the rate constant of constant-tension (σex)-induced rupture of a GUV as a function of σex using the concept of mean first passage time (MFPT) (27).
Understanding the effect of Π on the activities of membrane proteins and membrane active peptides such as antimicrobial peptides (19), and on the physicochemical properties of lipid membranes, requires information on the quantitative values of lateral tension due to Π. In this report, we estimated experimentally the lateral tension on the membranes of GUVs after transfer to a hypotonic solution. For this purpose, we followed a new idea. If we apply an external force to a GUV to induce a constant tension, σex, in the presence of Π, the total tension, σt, on the GUV membrane is increased by the lateral tension due to Π, and thus, the rate constant of σex-induced rupture of a GUV, kp, increases. Therefore, if we compare the σex dependence of kp in GUVs under Π with that in the absence of Π (27) to obtain the difference of the values of σex to induce the same kp value, we can estimate the membrane tension due to Π. Based on this idea, we made several kinds of experiments. First, we investigated the effect of Π on the kp of σex-induced rupture in GUVs composed of dioleoylphosphatidylcholine (DOPC) using the method developed by us recently (27). By obtaining the σex dependence of kp in GUVs under Π and comparing this result with that obtained in the absence of Π, we estimated the membrane tension of the GUVs at swelling equilibrium under Π, . Next, we measured the volume change of DOPC-GUVs under small Π. The experimentally obtained values of and the volume change were compared with the theoretical values. Finally, we investigated Π-induced pore formation in GUVs and compared this result with that of σex-induced rupture.
Materials and Methods
Preparation and observation of DOPC-GUVs
DOPC was purchased from Avanti Polar Lipids (Alabaster, AL). 6-Dodecanoyl-2- (dimethylamino)-naphthalene (Laurdan) was purchased from Invitrogen (Carlsbad, CA). Bovine serum albumin (BSA) was purchased from Wako Pure Chemical Industry Ltd. (Osaka, Japan). DOPC-GUVs were prepared by natural swelling in water (MilliQ) containing sucrose as follows (27). DOPC in chloroform (1.0 mM, 200 μL) in a small glass bottle (5 mL) was dried by N2 gas to produce a thin, homogeneous lipid film; then, residual chloroform in the film was removed by placing the bottle in a vacuum desiccator connected to a rotary vacuum pump for >12 h. MilliQ water (20 μL) was added and the bottle was sealed and incubated in 45–47°C water for 8 min (prehydration). Then, 1.0 mL of 100.0 mM sucrose in MilliQ was added gently, and the bottle was resealed and incubated in an incubator at 37°C for 2 h to produce a GUV suspension. The GUV suspension (20 μL, 98.0 mM sucrose aqueous solution) was diluted into 280 μL of various concentrations of glucose aqueous solution, and each mixture was transferred into a hand-made chamber (15). The GUVs were observed using an inverted fluorescence phase-contrast and differential interference contrast (DIC) microscope (IX-71, Olympus, Tokyo, Japan) at 25 ± 1°C using a stage thermocontrol system (Thermoplate, Tokai Hit, Shizuoka, Japan). Phase-contrast and DIC images of GUVs were recorded using a CCD camera (CS230B, Olympus) with a hard disk.
All experiments described below used DOPC-GUVs within 2 h of their preparation (i.e., after the incubation at 37°C) to increase the reproducibility of the data and the GUVs were placed and used in the chamber within 30 min of being transferred to the glucose solution to prevent any effect of water evaporation from the chamber.
Measurement of the rate constant of constant-tension-induced rupture of a GUV under Π
We used our recently developed method to measure the rate constant of the σex-induced rupture of a GUV (27). To apply Π on a GUV, first the GUV suspension was transferred into a chamber containing a lower concentration of glucose solution in MilliQ water compared to the solution inside the GUV. Swelling equilibrium was attained after >5 min incubation, then external tension was applied to a single GUV using a glass micropipette prepared by pulling a 1.0 mm glass capillary composed of borosilicate glass (G-1, Narishige, Tokyo, Japan) using a puller (PP-83 or PC-10, Narishige, Tokyo, Japan) (15). A single GUV was held for 2 min at the tip of a micropipette containing the same concentration of glucose solution as in the chamber by applying slight suction pressure (i.e., the difference in pressure between the outside and the inside of a micropipette, ΔPm), thus providing a tension of 0.5 mN/m on the bilayer. The GUV was then rapidly (in ∼10 s) aspirated to apply a specific tension on the GUV membrane, and this tension remained constant until the GUV was completely aspirated into the micropipette as a result of rupture of the GUV, or after 6 min, whichever occurred first. The time of rupture was defined as the time when the GUV was completely aspirated and was measured with a time resolution of <1 s. The σex of the GUV membrane due to an external force produced by the suction pressure can be described as a function of ΔPm as follows (17):
| (1) |
where dp is the internal diameter of the micropipette and Dv is the diameter of the spherical part of the GUV outside the micropipette. Micropipettes with dp = 7.0–8.0 μm were used. The micropipettes were coated with 0.5% (w/v) BSA in glucose solution and the glass surfaces in the chambers were coated with 0.1% (w/v) BSA in glucose solution; in all cases, excess BSA solution was removed by washing using glucose solution, leaving a BSA coating on the glass surfaces (15, 27, 28, 29). ΔPm was measured using a differential pressure transducer (DP15, Validyne, Northridge, CA), pressure amplifier (PA501, Validyne), and a digital multimeter (15).
Measurement of Π-induced volume changes of DOPC-GUVs
Here, we applied the similar method used to measure the volume decrease of a GUV upon transfer into a hypertonic solution (23, 30). To apply Π to a GUV held at the tip of a micropipette, the GUV was transferred from a chamber (chamber A) containing 98.0 mM glucose in MilliQ water, and thus isotonic against the 98.0 mM sucrose solution inside the GUV, to another chamber (chamber B) containing a lower concentration of glucose solution in MilliQ water that was thus hypotonic against the solution inside the GUV. A single GUV in chamber A was held by a micropipette using a tension of 0.5 mN/m for 2 min and then transferred into chamber B, which contained glucose solution with a lower osmolarity. To circumvent the small air gap between the two chambers, we used a glass capillary with a diameter of 1.0 mm filled with 98.0 mM glucose solution to transfer the GUV from chamber A to chamber B. After the transfer, the glass capillary was retracted from the GUV, and then the concentration of glucose solution in the vicinity of the GUV was rapidly changed to that of the bulk solution in chamber B. The volume change of the GUV, ΔV, was determined by the change in the projection length of the GUV inside the micropipette, ΔL, as follows (23):
| (2) |
where V and V0 are the volume of the GUV at t = t and t = 0, respectively (t is the time after the transfer of the GUV into chamber B). For the experiments described in Time Course of the Radius Change of DOPC-GUVs Induced by Π, membrane tensions >0.5 mN/m (4.0−5.0 mN/m) were applied by micropipette aspiration, whereas all other experimental procedures were as described above.
Measurement of Π-induced leakage of the internal content from DOPC-GUVs
Π was applied on a GUV using the method described in Measurement of Π-Induced Volume Changes of DOPC-GUVs. After transfer of the GUV from chamber A to chamber B, we decreased the suction pressure and released the GUV into the hypotonic solution in the chamber. The GUV sank gradually to the bottom of the chamber. We continuously observed the moving GUV by keeping it in focus under the microscope, and recorded the images of the GUV at video rate (i.e., 1 frame/33 ms).
Theory on the membrane tension of a GUV induced by Π
Here we consider the response of a GUV under Π. Before the application of Π, a GUV has an initial radius of r0, its initial surface area and initial volume are A0 and V0, respectively, and the initial sucrose concentration inside the GUV is Cin0. When we transfer the GUV into a hypotonic solution where the glucose concentration is Cout (< Cin0) (i.e., ΔC0 = Cin0 − Cout) (where the units of all the concentrations are mol/m3 (mM)), Π is applied to the GUV due to the difference in the chemical potential of water inside and outside of the GUV. It is reported that when the concentrations, C, of sucrose and glucose are low (i.e., C < 100 mM) the linearity between Π and C holds, and thus, the ideal equation (van’t Hoff’s law) for Π can be used (31, 32). We also measured osmolarity (mOsm/L) of sucrose and glucose solutions of <98 mM and obtained the linear relationship between the osmolarity and the molar concentration (Fig. S1 in the Supporting Material). Therefore, here we can use Π = RTΔC0, where R is the gas constant and T is the absolute temperature. Due to Π, water molecules start to enter the GUV and the radius and the volume of the GUV increase, inducing an increase in the surface area of the GUV, which creates a lateral tension, σosm, in the GUV membrane produced by its stretching. This produces the hydrostatic pressure difference between the inside and the outside of the GUV, ΔP (= Pin − Pout). The volume increase also induces a decrease in sucrose concentration inside the GUV. When the radius of the GUV increases by Δr (i.e., its radius is r0 + Δr, and its surface area and its volume are A0 + ΔA and V0 + ΔV, respectively), ΔP(Δr) can be expressed by the following equation using the Laplace law (33):
| (3) |
It is noted that σosm is a function of Δr, i.e., σosm(Δr). Π also decreases due to the dilution of the sucrose concentration inside the GUV until it reaches Π(Δr). If Π(Δr) > ΔP(Δr), water molecules continue to enter the GUV. When the swelling of the GUV reaches equilibrium, Δr, ΔA, and ΔV attain their equilibrium values, Δreq, ΔAeq, and ΔVeq, respectively, and ΔPeq = Πeq = RTΔCeq, where (here, Cineq is Cin at swelling equilibrium) and σosm(Δr) reaches its equilibrium value, . Therefore, we can obtain from ΔCeq as follows:
| (4) |
where
| (5) |
We used an approximation for ΔVeq/V0 ≈ 3(Δreq/r0), because the GUV is spherical and Δreq is very small. On the other hand, we can obtain using the elastic modulus of the bilayer of the GUV, Kbil, as follows (17):
| (6) |
Similarly, here we used an approximation for ΔAeq/A0 ≈ 2(Δreq/r0), because the GUV is spherical and Δreq is very small. From Eqs. 4−6, we obtained an equation to derive Δreq/r0 as follows:
| (7) |
where higher-order terms of (Δreq/r0)n with n ≥ 2 were neglected. The first term of the denominator in Eq. 7 represents the dependence of Δreq/r0 on Kbil and r0. From Eqs. 6 and 7, we can obtain . We measured the value of Kbil of a DOPC-GUV in 100 mM sucrose (inside the GUV)/100 mM glucose (outside the GUV) using the standard method (34, 35), and obtained its mean value and the standard deviation; Kbil = 218 ± 26 mN/m (n = 16) (Fig. S2), which is almost the same as reported values (17, 35). We consider the example where Cin0 = 98.0 mM, Cout = 96.0 mM (hence, ΔC0 = 2.0 mM), r0 = 10.0 μm, Kbil = 218 mN/m, and T = 298 K. From Eq. 7, we obtain Δreq/r0 = 6.2 × 10−3. Using Eq. 6, = 2.7 mN/m. Using Eq. 5, ΔCeq = Cineq − Cout = 98.2−98.0 = 0.2 mM. In this case, each term of the right side of Eq. 7 is as follows; 2500 Pa for the numerator, 44,000 Pa for the first term of the denominator, 360,000 Pa for the second term, and 2,500 for the third term. In most experiments done in this study, we used ΔC0 values in the range of 1.0 – 8.0 mM, which correspond to (mN/m) (Table S4).
Results and Discussion
Effect of Π on constant-tension-induced rupture in DOPC-GUVs
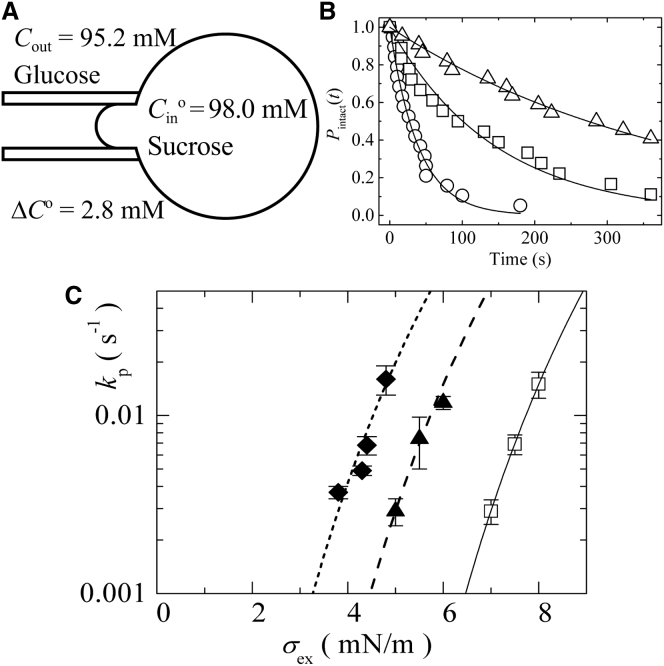
We investigated the effect of Π on the constant-tension-induced rupture of single DOPC-GUVs. First, we examined the effect of Π due to the initial difference in solute concentration between the inside and the outside of the GUV, ΔC0 (i.e., 2.8 mM). The external, constant tension, σex, of the GUV membrane due to aspiration was 3.8 mN/m. For this purpose, we first transferred GUVs containing 98.0 mM sucrose solution into a chamber containing 95.0 mM glucose solution (final solute concentration outside the GUVs were 95.2 mM) and incubated the suspension for >5 min so that the swelling of the GUV attained equilibrium against Π. Next, a single GUV was held at the tip of a micropipette for 2 min using only slight aspiration pressure to provide a tension of 0.5 mN/m on the bilayer to eliminate the problems of the hidden area (17, 27). Then the GUV was rapidly (in ∼10 s) aspirated to obtain a tension of 3.8 mN/m (Fig. 1 A). After a period of time, the GUV was suddenly aspirated into the micropipette. Based on our previous articles (27, 28), this sudden aspiration is due to a pore forming in the GUV membrane, causing rupture of the GUV and complete aspiration of the GUV into the micropipette due to the pressure difference between the inside and the outside of the micropipette. When we repeated the same experiment with 19 single GUVs (n = 19), we found that rupture of a GUV occurred stochastically at different times. The time course of the fraction of intact (unruptured) GUVs among all the GUVs, Pintact (t) (27), was fit to a single exponential decay function as follows (Fig. 1 B, open squares).
| (8) |
where kp is the rate constant of σex-induced rupture formation, which is the same as the rate constant of σex-induced pore formation, and t represents the duration of the tension applied to the GUV. This fitting provided a kp value of 6.9 × 10−3 s−1. Next, the same experiment was performed using 4.3 mN/m (n = 19). Pintact (t) decreased more rapidly with time (Fig. 1 B, open circles), and curve fitting provided a kp value of 2.5 × 10−2 s−1. To obtain the σex dependence of kp, we conducted three independent experiments, each using 18−22 GUVs, and obtained mean values and standard errors for kp. Fig. 1 C (solid rhombus) shows that the kp of DOPC-GUVs for ΔC0 = 2.8 mM increased with σex. Next, we investigated the effect of Π for ΔC0 = 1.9 mM (using a glucose concentration of 96.0 mM in the chamber, and final solute concentration outside the GUVs was 96.1 mM) on the σex-induced rupture of a DOPC-GUV. Fig. 1 C (solid triangle) shows that the kp value increased with σex. For comparison, the experimental data for kp of DOPC-GUVs without Π (ΔC0 = 0.0 mM) (27) is also shown in Fig. 1 C (open squares). Fig. 1 C indicates that as ΔC0 increased, lower tensions were required to produce the same values of kp. In other words, the application of Π to DOPC-GUVs greatly increased the kp of the GUV.
Figure 1.
Effect of Π on constant-tension-induced rupture of DOPC-GUVs. (A) A scheme of the measurement for ΔC0 = Cin0 − Cout = 2.8 mM, where Cin0 is the initial sucrose concentration inside a GUV and Cout is the glucose concentration on the outside of the GUV. (B) Time course of the fraction of intact DOPC-GUVs without rupture among all of the examined GUVs, Pintact(t), in the presence of Π due to ΔC0 = 2.8 mM and tension due to the micropipette aspiration: σex = 3.3 mN/m (open triangles), 3.8 mN/m (open squares), and 4.3 mN/m (open circles). The number of single GUVs examined was 18−22 in each experiment. The solid lines represent the best-fit curves of Eq. 8. (C) Dependence of kp on external tension for ΔC0 = 1.9 mM (solid triangle), ΔC0 = 2.8 mM (solid rhombus), and ΔC0 = 0 mM (open squares) (i.e., no osmotic pressure). Error bars show standard errors. The solid lines show the best fits in accordance with the theoretical curves corresponding to Eq. 9 using Γ = 10.5 pN and Dr = 165 nm2/s. The data for ΔC0 = 0.0 mM (open squares) and its fitting curve are reprinted from (27) with permission from the American Chemical Society. The dashed line and the dotted line correspond to the theoretical Eq. 9 using σt = σex + 2.6 mN/m and σt = σex + 3.8 mN/m, respectively.
Here, we experimentally estimated the membrane tension due to Π at swelling equilibrium, , by analyzing the results of Fig. 1 C. We can reasonably assume the additivity of the tension σex due to aspiration according to eq. 1 and the tension due to Π, , according to eq. 6, and hence the total tension, σt, can be expressed by . Moreover, the value of kp is determined by σt. When we compare σex values which induce the same values of kp under different conditions (Fig. 1 C), the difference between σex of the GUV in the presence of Π and σex of the GUV without Π corresponds to of the GUV in the presence of Π. The values of σt of the GUV without Π for specific values of kp were determined by the following theoretical equation, which fits well to the experimental results of kp versus σt (27):
| (9) |
where Dr is the diffusion coefficient of a particle in r-phase space, Γ is the line energy per unit length of a prepore rim in lipid bilayers (i.e., the line tension), and k is the Boltzmann constant. Here, we assumed a hydrophilic prepore and obtained the values of the parameters for the DOPC membrane by analysis of the relationship between kp and σt using Eq. 9: Γ = 10.5 pN and Dr = 165 nm2/s (27). For the value of Γ for the hydrophilic pore, Karatekin et al. obtained 6.9 – 20.7 pN depending on the provider of DOPC, as determined by analysis of the closure dynamics of transient pores (24). However, here we used Γ = 10.5 pN because it was obtained by the best-fitting data according to Eq. 9. Next, we explain an example of the analysis using Eq. 9. For ΔC0 = 1.9 mM, kp = 1.7 × 10−2 s−1 at σex = 5.5 mN/m (experimental data), and σex in the absence of Π (i.e., ΔC0 = 0 mM), which induced the same kp value (i.e., 1.7 × 10−2 s−1), was 8.1 mN/m according to Eq. 9. Hence, the difference between σex (ΔC0 = 0 mM) and σex (ΔC0 = 1.9 mM) is 2.6 mN/m, which corresponds to the experimentally determined , , for this condition. We also calculated for other conditions, such as kp and ΔC0 (Tables 1 and 2). The mean values of for different σex values were 2.6 ± 0.1 mN/m for ΔC0 = 1.9 mM and 3.8 ± 0.1 mN/m for ΔC0 = 2.8 mM. Therefore, for ΔC0 = 1.9 mM, σt = σex + 2.6 mN/m. Using this value of σt and the same values of Γ and Dr as in the absence of Π, we plotted the theoretical curve (Eq. 9) of kp versus σt in Fig. 1 C (dashed line). Similarly, for ΔC0 = 2.8 mM, σt = σex + 3.8 mN/m, and using this value of σt we plotted the theoretical curve of kp versus σt in Fig. 1 C (dotted line). Both theoretical curves fit well to the experimental results. To our knowledge, these data provide the first experimentally determined membrane tensions induced by Π.
Table 1.
Comparison of the External Tensions that Induced the Same kp Values in the Presence and Absence of Osmotic Pressure
| σex (mN/m) with ΔC0 | kp (s−1) | σex (mN/m) with ΔC0 = 0a | (mN/m) = σex (ΔC0 = 0) − σex (ΔC0) | (mN/m) | th (mN/m) | (μm) |
|---|---|---|---|---|---|---|
| 4.5 | 3.2 × 10−3 | 7.1 | 2.6 | 2.6 ± 0.1 | 2.6 ± 0.7 | 13.4 ± 0.2 |
| 5.0 | 9.4 × 10−3 | 7.7 | 2.7 | |||
| 5.5 | 1.7 × 10−2 | 8.1 | 2.6 |
Values are from the analysis of the data for ΔC0 = 1.9 mM in Fig. 1. and are mean values of and , respectively.
Values were obtained using Eq. 9.
Table 2.
Comparison of the External Tensions that Induced the Same kp Values in the Presence and Absence of Osmotic Pressure
| σex (mN/m) with ΔC0 | kp (s−1) | σex (mN/m) with ΔC0 = 0a | (mN/m) = σex (ΔC0 = 0) − σex (ΔC0) | (mN/m) | th (mN/m) | (μm) |
|---|---|---|---|---|---|---|
| 3.3 | 3.5 × 10−3 | 7.1 | 3.8 | 3.8 ± 0.1 | 3.9 ± 0.7 | 13.7 ± 0.2 |
| 3.8 | 9.3 × 10−3 | 7.7 | 3.9 | |||
| 4.3 | 1.5 × 10−2 | 8.0 | 3.7 |
Values are from the analysis of the data for ΔC0 = 2.8 mM in Fig. 1. and are mean values of and , respectively.
Values were obtained using Eq. 9.
On the other hand, using the theory described in Theory on the Membrane Tension of a GUV Induced by Π, we determined the theoretical values of , : 2.6 ± 0.7 mN/m for ΔC0 = 1.9 mM, and 3.9 ± 0.7 mN/m for ΔC0 = 2.8 mM. Here, we estimated the error of from the errors of ΔC0, Cout, and Kbil. The errors of ΔC0 and Cout were evaluated based on the method of preparation of the sucrose and glucose solutions: the errors of these concentrations are estimated to be ±0.2 mM for the 100 mM sucrose solution and ±0.5 mM for the 100 mM glucose solution, and hence, the error of ΔC0 is ±0.5 mM. The variation of the initial radius of a GUV, r0, does not affect the value of significantly. For the experiments at ΔC0 = 1.9 mM, we calculated for various sizes of GUVs under the same conditions: 2.7 mN/m for r0 = 20 μm and 2.6 mN/m for r0 = 10 μm. Therefore, we conclude that the values of agree with those of within the limits of the experimental errors, indicating that the theory described in Materials and Methods (Theory on the Membrane Tension of a GUV Induced by Π) is correct.
Increase in the volume of DOPC-GUVs induced by Π
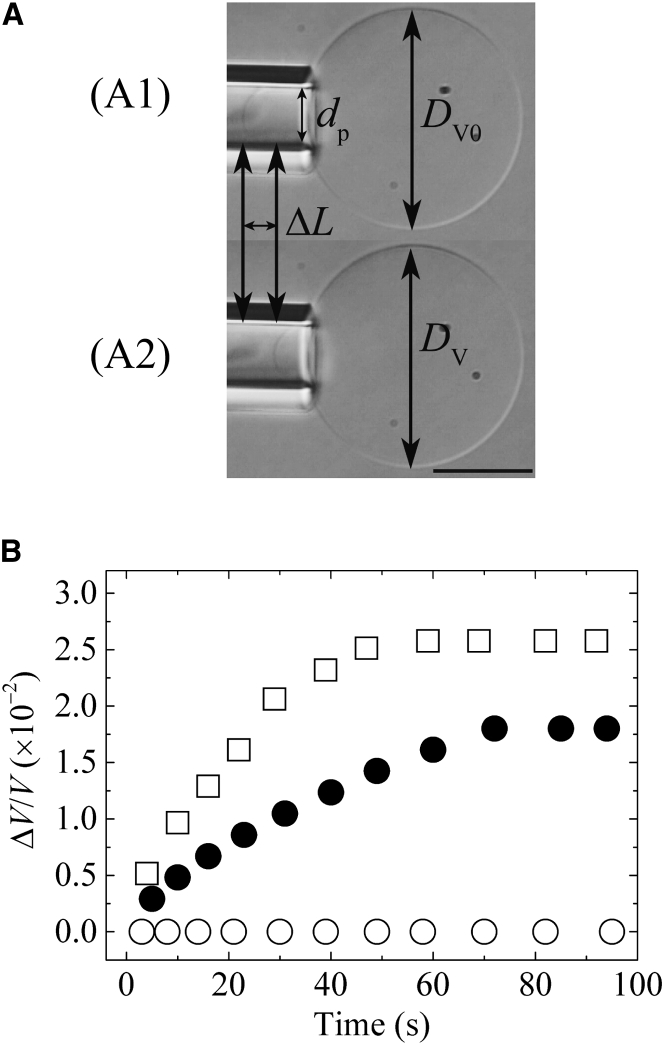
According to the above-referenced theory, if we can determine Π-induced Δreq experimentally, we can estimate indirectly. For this purpose, we measured the volume change of a single DOPC-GUV upon exposure to Π using the method developed by Evans and colleagues (23). A single GUV containing 98.0 mM sucrose solution was held at the tip of a micropipette for a few minutes using only slight aspiration pressure in chamber A, which contained 98.0 mM glucose solution (the tension applied to the bilayer was 0.5 mN/m). The GUV was then transferred to chamber B, which contained 96.0 mM glucose solution (i.e., ΔC0 = 2.0 mM). The projection length, ΔL, of the GUV inside the micropipette (Fig. 2 A) decreased with time and attained an equilibrium value, ΔLeq, after 70 s. Using Eq. 2, we calculated the volume change of the GUV, ΔV, to obtain the ratio of ΔV to its initial volume, V0, i.e., ΔV/V0. Fig. 2 B (solid circles) indicates that ΔV/V0 increased with time to an equilibrium value, ΔVeq/V0. The mean value of ΔVeq/V0 was (1.7 ± 0.1) × 10−2 (n = 14). The same experiments were performed using ΔC0 = 3.0 mM (Fig. 2 B, open squares); the time course of ΔV/V0 was similar to that for ΔC0 = 2.0 mM, and ΔVeq/V0 = (2.6 ± 0.1) × 10−2 (n = 17). In the control experiment (ΔC0 = 0.0 mM) (Fig. 2 B, open circles), no significant change in ΔV/V0 was observed for 20 min after transfer to chamber B.
Figure 2.
Increase in volume of DOPC-GUV induced by Π. (A1) A DIC image of a GUV held at the tip of a micropipette using a small aspiration pressure in chamber A, which contained an isotonic solution. (A2) A DIC image of a GUV held at the tip of a micropipette after the GUV was transferred into another chamber, chamber B, containing a hypotonic solution. dp is the internal diameter of the micropipette and ΔL is the change of the projection length. The bar corresponds to 10 μm. (B) Time course of volume change of a GUV after it was transferred into chamber B, which contained 96.0 mM (i.e., ΔC0 = 2.0 mM; solid circles), 95.0 mM (i.e., ΔC0 = 3.0 mM; open squares), and 98.0 mM (i.e., ΔC0 = 0.0 mM, open circles) glucose solutions.
As shown in Fig. 2 B, ΔVeq/V0 increased with ΔC0. This result indicates that higher Π induces larger swelling of GUVs, which can be reasonably explained by Eq. 7. Moreover, the values of ΔVeq/V0 allow us to experimentally estimate the membrane tension due to Π at swelling equilibrium of the GUV, . Since Δr is small, (Δreq/r0) ≈ (1/3)ΔVeq/V0 = (5.6 ± 0.1) × 10−3. Using Eq. 6, = 2.7 ± 0.1 mN/m. We can also obtain the values of using the above-referenced theory. For ΔC0 = 2.0 ± 0.5 mM, we obtained Δreq/r0 = (6.4 ± 1.6) × 10−3 using Eq. 7, and hence = 2.8 ± 0.7 mN/m using Eq. 6 (Table S3). For ΔC0 = 3.0 mM, = 4.1 ± 0.2 mN/m, since (Δreq/r0) = (8.6 ± 0.2) × 10−3. On the other hand, theoretically, Δreq/r0 = (9.8 ± 1.7) × 10−3 and = 4.3 ± 0.7 mN/m (Table S3). The dependence of the theoretical values of ΔVeq/V0 obtained by Eq. 6 on r0 is very small, and therefore, the experimental values of and Δreq/r0 agree with the theoretical values within the limits of the experimental errors.
Rapid, transient leakage of sucrose from DOPC-GUVs induced by Π
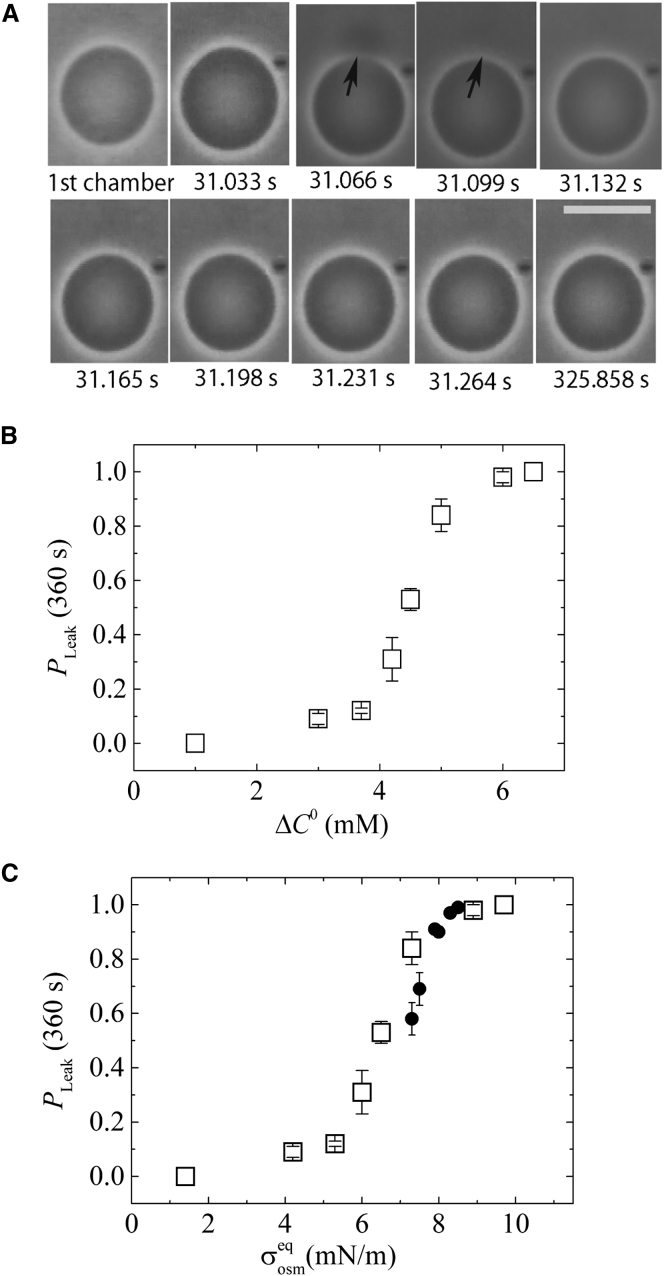
Next, we investigated Π-induced pore formation in a GUV to compare it with the constant-tension-induced pore formation where the tension in the GUV membrane is produced by an external force (27). For this purpose, we initially held a single GUV containing 98.0 mM sucrose solution in chamber A, which contained 98.0 mM glucose solution, at the tip of a micropipette for a few minutes using only slight aspiration pressure. The tension on the bilayer was 0.5 mN/m. The GUV was then transferred into chamber B, which contained 90.0 mM glucose solution (ΔC0 = 8.0 mM), the suction pressure was decreased, and the GUV was released into the hypotonic solution in chamber B. The GUV was continuously observed after release. Fig. 3 A shows phase-contrast images of the response of a GUV in chamber A: the diameter of the GUV in chamber B at 31.033 s after transfer into chamber B was larger than its diameter in chamber A in the absence of Π. Rapid leakage of sucrose solution was observed at 31.066 ± 0.033 s (Fig. 3 A, black arrow), and consequently, the diameter of the GUV rapidly decreased in 33 ms (i.e., from 31.033 s to 31.066 s in Fig. 3 A); then, after 31.099 s, it gradually increased, indicating that the leakage of sucrose stopped at 31.099 ± 0.033 s. The phase contrast of the GUV did not decrease significantly due to this leakage. These results can be considered as follows. First, sudden pore formation occurred in the GUV membrane due to the swelling of GUVs due to Π, inducing a transient leakage of a small amount of sucrose solution. Thus, the diameter of the GUV rapidly decreased, and then the leakage stopped within 99 ms after pore formation, indicating the resealing of the GUV membrane. Then, the GUV volume increased again due to Π. The time required for closure of the pore (here 99 ms) was determined by observation of visible sucrose leakage, and hence it is not accurate. This experiment was repeated using 21 GUVs and similar results were obtained.
Figure 3.
Large Π-induced pore formation in DOPC-GUVs. (A) Phase-contrast microscopic images of a GUV after it was transferred into chamber B, which contained 90.0 mM glucose solution (i.e., ΔC0 = 8.0 mM). The numbers below each image show the time in seconds after the transfer of the GUV into chamber B. Scale bar, 25 μm. The arrows show a rapid, transient leakage of sucrose from the DOPC-GUV. (B) The fraction of GUV where a transient leakage occurred during the first 6 min, PLeak (360 s), as a function of ΔC0. Mean values and standard errors of PLeak (360 s) for each ΔC0 were determined among three independent experiments using 10−15 GUVs for each experiment. Error bars show standard errors. (C) PLeak (360 s) as a function of membrane tension due to Π at equilibrium, (open squares). ΔC0 values in (B) were converted into . For comparison, the data in Fig. 1 were added (solid circles); the values of the fraction of ruptured GUVs during the first 6 min among all the examined GUVs, Ppore (360 s), were obtained for the theoretical values of for each condition of the experiments of Fig. 1.
We investigated the effect of ΔC0 on the rate of Π-induced pore formation in lipid membranes. For the experiments shown in Fig. 3 A, we cannot obtain the rate constant of Π-induced pore formation, which is different from the σex-induced rupture of GUVs (see Effect of Π on Constant-Tension-Induced Rupture in DOPC-GUVs). However, we can use one measure of this rate, namely, the fraction of GUVs in which transient leakage occurred during the first 6 min among all the examined GUVs, PLeak (360 s) (27). Fig. 3 B shows that PLeak (360 s) was negligible (≤0.12) at ΔC0 ≤ 3.7 mM, but at ΔC0 ≥ 4.2 mM, PLeak (360 s) increased with increasing ΔC0 and reached 1.0 at ΔC0 = 6.5 mM. This result indicates that the rate of Π-induced pore formation increased with an increase in ΔC0, i.e., Π. After conversion of ΔC0 to using Eqs. 6 and 7, we obtained the dependence of PLeak (360 s) on (Fig. 3 C; Table S4). At ≤ 5.3 mN/m, PLeak (360 s) was negligible (≤0.12). At = 6.0 mN/m, PLeak (360 s) became significant (= 0.31 ± 0.08), and at ≥ 6.0 mN/m, PLeak (360 s) increased with . This behavior is similar to the σex-induced rupture of DOPC-GUVs (27).
We observed two transient leakages from most GUVs within 6 min for higher ΔC0, such as ΔC0 = 20 mM (Fig. S3).
Time course of radius change of DOPC-GUVs induced by Π
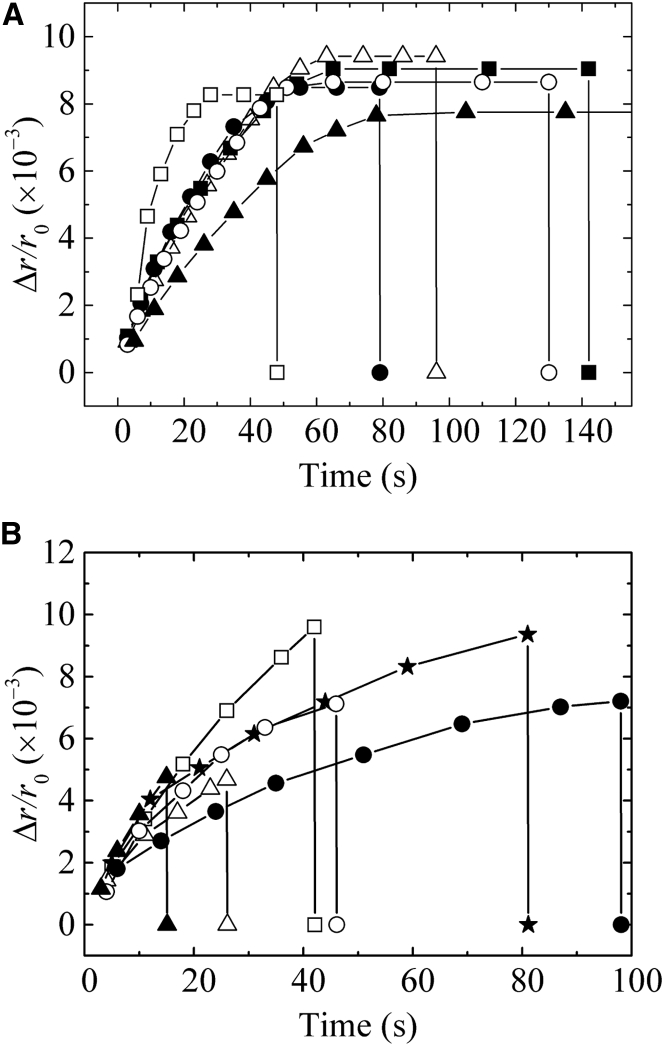
In the previous section, we could not obtain accurate values for Δr/r0 when pore formation occurred in a DOPC-GUV upon exposure to large Π, and therefore we applied the method used in Increase in the Volume of DOPC-GUVs Induced by Π. The first experiment used the condition ΔC0 = 3.0 mM and σex = 4.0 mN/m. A single GUV containing 98.0 mM sucrose solution was held at the tip of a micropipette using a tension on the bilayer of 4.0 mN/m in chamber A, containing 98.0 mM glucose solution, for 2 min; then, the GUV was transferred into chamber B, containing 95.0 mM glucose solution. The projection length, ΔL, of the GUV inside the micropipette decreased with time to an equilibrium value, ΔLeq, which remained constant for a long time, then suddenly the GUV was aspirated into the micropipette (i.e., pore formation occurred). Using Eq. 2, we obtained the ΔV/V0 ratio of the GUV, which was converted into Δr/r0 using ΔV/V0 ≈ 3(Δr/r0). Fig. 4 A shows the time course of Δr/r0 for several GUVs subjected to the same conditions. Δr/r0 increased with time to reach an equilibrium value, Δreq/r0, which remained constant for a long time, until sudden rupture occurred. The values of Δreq/r0 were similar for all examined GUVs (i.e., Δreq/r0 = (8.6 ± 0.2) × 10−3 (n =17)).
Figure 4.
The time courses of the fractional change of radius of a GUV, Δr/r0, under Π and σex. The curves represent the Δr/r0 results for several GUVs for (A) ΔC0 = 3.0 mM and σex = 4.0 mN/m and (B) ΔC0 = 4.0 mM and σex = 5.0 mN/m.
The second experiment used the condition ΔC0 = 4.0 mM and σex = 5.0 mN/m, which produces a larger membrane tension. Fig. 4 B shows the time course of Δr/r0 of several GUVs under the same conditions. After transfer of the GUV into chamber B, Δr/r0 increased with time and the GUV was aspirated into the micropipette at various values of Δr/r0 (0.0043–0.010) before reaching a constant value of Δr/r0 (i.e., the swelling equilibrium) for most GUVs. The rupture of the GUVs occurred stochastically, and the membrane tensions at the time of rupture were different.
Two different time courses of volume change (or radius change) of GUVs were observed in Π-induced pore formation (Fig. 4, A and B). In these experiments, there are two sources of membrane tension (σex and ); consequently, . For relatively small σt, the radius of the GUV increased to an equilibrium value, req, which remained constant for a long time, indicating that σt also remained constant for a long time after the swelling equilibrium, and then suddenly the GUV ruptured (Fig. 4 A). If we observe many GUVs under the same conditions, it is clear that the rupture of GUVs occurred stochastically (Fig. 4 A). These experimental results are different from the theoretical predictions of the effect of Π on the radius (or volume) of vesicles (i.e., immediately after the radius of a vesicle reaches a critical value, pore formation in the vesicle membrane occurs and the vesicle radius rapidly decreases) (6, 14), but are very similar to the phenomenon observed in the σex-induced rupture of a GUV (27, 28, 29). The mean value of obtained experimentally by Eq. 7 and Δreq/r0 = (8.6 ± 0.2)× 10−3 was 4.1 ± 0.1 mN/m, and therefore, = 8.1 mN/m. Furthermore, = 4.3 ± 0.7 mN/m (Table S5), which agrees with the experimental value. In contrast, for relatively large σt, as shown in Fig. 4 B, the radius of a GUV increased with time and the rupture of the GUV occurred at various values of Δr/r0 (0.0043−0.010) before reaching the swelling equilibrium. In this case ( = 15.7 μm), = 5.8 mN/m, and hence, the theoretical value of is 5.0 + 5.8 = 10.8 mN/m at swelling equilibrium, which is much larger than the σex values in the σex-induced rupture of DOPC-GUVs (27). Therefore, rupture occurred before the membrane tension of a GUV reached equilibrium. In this situation, the membrane tension increased with time because the radius of the GUV increased with time and then pore formation occurred suddenly. Membrane tension at the time of rupture of the GUV varied widely, similar to tension-induced rupture when tension changed with time (25, 26).
General discussion
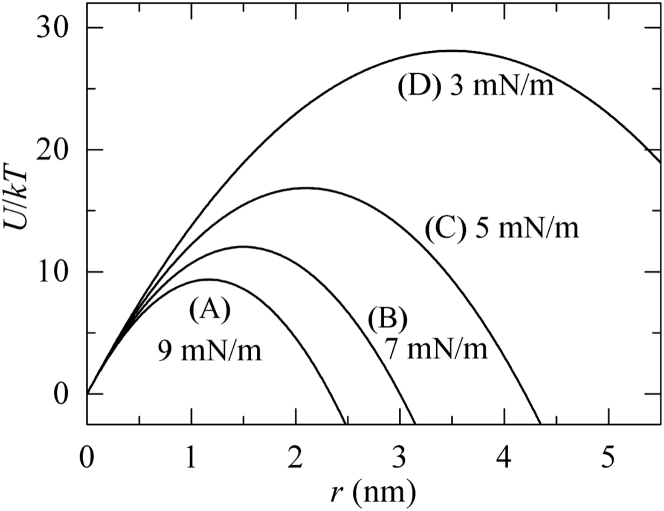
The analysis of the effect of Π on the σex-induced rupture of a DOPC-GUV (Fig. 1) provided the values of the membrane tension due to Π, . If ≥ 6.0 mN/m, pore formation occurred in the GUV membrane (Fig. 3 C). It is generally considered that thermal fluctuation in the lateral density of a lipid membrane induces a prepore (27). According to the classical theory of tension-induced pore formation (36, 37, 38), once a prepore with radius rp is formed in the membrane, the total free energy of the system changes by an additional free-energy component (called the free energy of a prepore, U (rp, σt)) consisting of two terms: one term (−πrp2σt) is associated with lateral tension (σt) and favors expansion of the prepore, and the other term (2πrpΓ) is associated with the line tension (Γ) of the prepore edge and favors prepore closure. The free energy of a prepore, U (rp, σt), can therefore be expressed as
| (10) |
U (rp, σt) has a maximum of Ua = U (rp∗) = πΓ2/σt at r = rp∗ (= Γ/σt) (Fig. 5). If the radius of a prepore is less than the critical radius, rp∗, it closes quickly. However, if the radius expands and reaches rp∗, the prepore transforms into a pore. In the case of σex-induced rupture, the radius of a prepore reaches rp∗ stochastically and hence, rupture occurs stochastically. Experimentally, the decrease in the fraction of intact GUVs fits a single-exponential decay function, indicating that the rupture can be considered as an irreversible two-state transition (27, 28, 29). This fitting also provided the rate constant of σex-induced pore formation, which increased with an increase in σex (26, 27, 28). Recently, the values of the activation energy of σex-induced pore formations were experimentally determined, and the analysis of the activation energy clearly indicated that the dependence of Ua on σex in the classical theory is correct (29, 39). On the basis of this theory, we consider the Π-induced pore formation in a GUV, where = σt. At ΔC0 ≤ 3.7 mM (i. e., ≤ 5.3 mN/m) (Table S4), the corresponding membrane tensions are so small that the activation energy is much higher than the thermal energy. At ΔC0 ≥ 4.2 mM (i, e., ≥ 6.0 mN/m), the corresponding membrane tension increases and therefore the activation energy becomes sufficiently low to induce pore formation.
Figure 5.
Dependence of the free-energy profile of a prepore in a DOPC-GUV on its radius for various tensions due to Π, : (A) 9.0, (B) 7.0, (C) 5.0, and (D) 3.0 mN/m. U(r) is calculated according to Eq. 10 using Γ = 10.5 pN.
Here, we consider whether and σex have the same effect on vesicle membranes as lateral tension on the membrane. For the experiments shown in Fig. 1, both σex and were applied to a GUV, and hence the total tension on the GUV membrane was . For these experiments, we can also use one measure of the rate of σt-induced rupture of GUVs, namely, the fraction of ruptured GUVs among all examined GUVs at 6 min, Ppore (360 s) (Tables S6 and S7). We consider that leakage of sucrose occurs when pore formation occurs in the GUV membrane, and therefore, Ppore (360 s) = PLeak (360 s). The theoretical values of σt for each condition of the experiments depicted in Fig. 1 were determined (Tables S6 and S7) and the relationship between Ppore (360 s) versus σt was plotted in Fig. 3 C (solid circles). If we compare the values of (open squares) and those of (σex = 3.3 – 5.5 mN/m) (solid circles), both agree with each other within the limits of experimental errors. Therefore, we can conclude that Ppore (360 s) is mainly determined by σt, irrespective of the contribution of σex. On the other hand, the results of the σex-induced rupture of a DOPC-GUV show that at σex = 7.0 mN/m, the rate constant of pore formation, kp, was (3.1 ± 0.4) × 10−3 s−1 (Fig. 1 C, open squares) (27), Ppore (360 s) = 0.55 ± 0.02, and at σex ≥ 7.0 mN/m, kp increased with σex. The agreement of PLeak (360 s) (Fig. 3 C) with Ppore (360 s) at σt = 7.0 mN/m, and the similarity of the dependence of the rate of pore formation (judging from PLeak (360 s) and kp) on membrane tension ( or σex), indicate that is equivalent to σex, supporting the conclusion that Ppore (360 s) is mainly determined by σt, irrespective of the contribution of σex. These results indicate that both and σex contribute to the total tension equivalently (i.e., ) and induce pore formation in the GUV membrane, i.e., cannot be distinguished from σex. Based on this conclusion, we can reasonably consider that σex can induce leakage of sucrose due to pore formation in the GUV membrane in a manner similar to -induced leakage, although this could not be observed directly in the experiments presented here due to the rapid aspiration of the GUVs (27, 28, 29).
If the radius of a prepore reaches rp∗ during the thermal fluctuation of the membrane, a pore is formed, inducing a rapid leakage of internal sucrose solution due to the Laplace pressure. This induces a rapid decrease in GUV volume (Fig. 3 A), and therefore, the tension due to stretching of the membrane disappears. Subsequently, the pore rapidly closes due to the line tension of the pore. Generally, the dynamics of closing of a large pore induced by tension have been investigated experimentally (16, 24, 40, 41), and the theories of the evolution of a pore induced by tension can explain well the results of closing of large pores (42, 43). Therefore, we can reasonably consider that the evolution of the Π-induced pore can be explained by these theories, although we did not get any quantitative information on the closing of the Π-induced pore in this report. During the decrease in GUV volume due to the efflux of sucrose solution, a submicroscopic daughter vesicle may be formed and subsequently released from the parent GUV, which cannot be observed by phase-contrast microscopy (22, 44). In this case, the area of the GUV also decreases. After the pore closes, the concentration of sucrose inside the GUV is still higher than that of glucose outside the GUV, and hence the GUV swells again. When the GUV reswelled to a large enough volume to produce a large membrane tension, the second rapid leakage of sucrose occurred (Fig. S3). It is noted that the phase contrast of the resealed GUVs was similar to that of the intact GUVs, because the amount of sucrose leakage was small. Therefore, it is difficult to distinguish intact GUVs from resealed GUVs after Π-induced pore formation.
It is noteworthy to consider the dependence of Π-induced tension, , on the radius of the vesicles. If the values of the parameters other than the radius are the same, according to Eq. 4, is proportional to the radius of the vesicles when ΔCeq is the same. For example, for GUVs with a radius of 10 μm is 100 times larger than for LUVs with a radius of 100 nm. However, for GUVs, ΔCeq is much smaller than that ΔC0, in contrast to LUVs, where ΔCeq ≈ ΔC0. For example, at Cin0 = 98.0 mM and Cout = 96.0 mM (hence ΔC0 = 2.0 mM), ΔCeq for GUVs with a radius of 10 μm is 0.2 mM, but ΔCeq for LUVs with a radius of 100 nm is 1.9 mM (as estimated theoretically using Eqs. 5 and 7). The final size dependence of is determined by Δreq/r0 according to Eq. 7. Under the above concentration conditions, Δreq/r0 for GUVs with a radius of 10 μm is 6.2 × 10−3, which is 13 times larger than that for LUVs with a radius of 100 nm (= 4.9 × 10−4), and hence, for the GUV is 13 times larger than for the LUV. To validate this theory on the radius dependence of , we measured a change in a physical property of lipid membranes induced by Π. It was recently reported that the membrane stretching due to lateral tension increases the fluidity of lipid membranes (45, 46) and the diffusion coefficient of lipid molecules (45, 46, 47). To monitor the fluidity of the membranes, we used generalized emission polarization value (GP) of Laurdan in membranes, because it is generally considered that with an increase in fluidity of lipid membranes the interaction of water molecules with a Laurdan molecule in the membrane interface increases, which causes the GP value to decrease (48, 49). Both GP values for DOPC-LUVs and DOPC-GUVs decreased with an increase in ΔC0, indicating that the fluidity of the membranes of both vesicles increased with Π, but to induce similar decrements of the GP values ∼10 times higher ΔC0 values were required for DOPC-LUVs compared with DOPC-GUVs (Fig. S4, B and D). These results suggest that the stretching of the membranes due to lateral tension increased with an increase in Π, but to induce similar amounts of stretching, ∼10 times higher ΔC0 values were required for DOPC-LUVs compared with DOPC-GUVs. After converting ΔC0 to , we found that the GP values were essentially the same in DOPC-GUVs and DOPC-LUVs at the same (Fig. S4 E). This result supports the theory on the radius dependence of .
As described above in Results and Discussion, when GUVs are transferred to a hypotonic solution, the volume of the GUVs increases due to the osmotic pressure. In the initial response of the GUVs, we have to consider the hidden-excess area (23). Thermal undulation motion of GUV membranes (50, 51) and long thin (submicron) tubular protrusions (52, 53) can be considered as sources of the hidden-excess membrane area of the GUVs. To prevent the influence of the hidden-excess area, for the experiment using micropipettes (e.g., Figs. 1, 2, and 4), we first applied a small aspiration pressure on a GUV to provide a tension of 0.5 mN/m for 2 min to incorporate the hidden-excess area, which is similar to the method of (17, 27). Based on (51), this is a sufficient condition to incorporate hidden-excess area.
Recently it was reported that sucrose or glucose may influence the bending modulus of lipid membranes, κ (54, 55, 56, 57). Some data indicate that the bending modulus decreased with sucrose (or glucose) concentration, and other data indicate no effects. Moreover, its mechanism is not clear yet. On the other hand, the bending modulus is related to the elastic modulus of the bilayer of the GUV, Kbil, and the bilayer thickness, d (e.g., κ ∝ Kbild2) (58). Hence, the value of Kbil may depend on sucrose (or glucose) concentration if the value of d does not depend on sucrose (or glucose) concentration. We measured the values of Kbil of DOPC-GUV in various sucrose/glucose concentrations from 50 to 200 mM and found that they were almost the same in this limited range of sucrose/glucose concentration (Fig. S2 B). In all experiments in this report, we used 78.0−98.0 mM sucrose/glucose, and hence we can consider that the effect of sucrose/glucose concentration on their results was negligible.
The above results clearly show that GUVs, which are cell-sized vesicles, are weak against Π. Pore formation in the plasma membrane of cells causes cell death, yet solute concentrations outside of cells can change easily. To prevent Π-induced cell death, cells have had to modify their membrane structure by incorporating cholesterol (34) and mechanosensitive channels (2) into their plasma membranes during the evolution of life. It is well known that cholesterol increases the line tension of pores or prepores and therefore the probability of pore formation in lipid membranes decreases (24, 25, 41), and also that mechanosensitive channels open when membranes are stretched by Π (3, 4). Therefore, cells are much stronger against Π than GUVs of lipid membranes lacking cholesterol.
Conclusions
In this report, we succeeded in determining the membrane tension, , of DOPC-GUVs under osmotic pressure (Π) experimentally for the first time, to the best of our knowledge, by analyzing the effects of Π on the constant tension-induced rupture of GUVs. We also estimated by analysis of the volume change of the GUVs under small Π. These experimentally estimated values of agreed with their theoretical values within the limits of their experimental errors. The total tension due to and the external tension determine the response of GUVs: at lower total tensions, the radius of the GUVs increased to an equilibrium value, req, which remained constant for a long time, and then stochastic rupture of the GUV occurred, as is the case for the constant-tension-induced rupture of GUVs. These results provide quantitative information on membrane tension due to Π, which is valuable for research on the effects of Π on the activities of membrane proteins and membrane active peptides.
Author Contributions
S.U.A.S. and M.Y. designed the research. S.U.A.S., C.G., and M.M. performed the experiments. S.U.A.S., C.G., M.A.S.K., and M.Y. analyzed data. S.U.A.S., C.G., and M.Y. wrote the article.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (No. 15H04361) from the Japan Society for the Promotion of Science to M.Y. Part of this research was carried out under the Cooperative Research Projectof the Research Institute of Electronics, Shizuoka University.
Editor: Ana-Suncana Smith.
Footnotes
Mohammad Abu Sayem Karal’s present address is Department of Physics, Bangladesh University of Engineering and Technology, Dhaka-1000, Bangladesh.
Supporting Materials and Methods, four figures, and seven tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30877-3.
Supporting Material
References
- 1.Sperelakis N., editor. Cell Physiology Source Book, 4th ed. Essentials of membrane biophysics. Academic press; London: 2012. [Google Scholar]
- 2.Sachs F. Stretch-activated ion channels: what are they? Physiology (Bethesda) 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukharev S.I., Blount P., Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 4.Levina N., Tötemeyer S., Booth I.R. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taupin C., Dvolaitzky M., Sauterey C. Osmotic pressure induced pores in phospholipid vesicles. Biochemistry. 1975;14:4771–4775. doi: 10.1021/bi00692a032. [DOI] [PubMed] [Google Scholar]
- 6.Koslov M.M., Markin V.S. A theory of osmotic lysis of lipid vesicles. J. Theor. Biol. 1984;109:17–39. doi: 10.1016/s0022-5193(84)80108-3. [DOI] [PubMed] [Google Scholar]
- 7.Sun S.T., Milon A., Nakatani Y. Osmotic swelling of unilamellar vesicles by the stopped-flow light scattering method. Elastic properties of vesicles. Biochim. Biophys. Acta. 1986;860:525–530. [Google Scholar]
- 8.Li W., Aurora T.S., Cummins H.Z. Elasticity of synthetic phospholipid vesicles and submitochondrial particles during osmotic swelling. Biochemistry. 1986;25:8220–8229. doi: 10.1021/bi00373a015. [DOI] [PubMed] [Google Scholar]
- 9.Rutkowski C.A., Williams L.M., Cummins H.Z. The elasticity of synthetic phospholipid vesicles obtained by photon correlation spectroscopy. Biochemistry. 1991;30:5688–5696. doi: 10.1021/bi00237a008. [DOI] [PubMed] [Google Scholar]
- 10.Ertel A., Marangoni A.G., Wood J.M. Mechanical properties of vesicles. I. Coordinated analysis of osmotic swelling and lysis. Biophys. J. 1993;64:426–434. doi: 10.1016/S0006-3495(93)81383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallett F.R., Marsh J., Wood J.M. Mechanical properties of vesicles. II. A model for osmotic swelling and lysis. Biophys. J. 1993;64:435–442. doi: 10.1016/S0006-3495(93)81384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mui B.L.-S., Cullis P.R., Madden T.D. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys. J. 1993;64:443–453. doi: 10.1016/S0006-3495(93)81385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin Y., Idiart M.A. Pore dynamics of osmotically stressed vesicles. Physica A. 2004;331:571–578. [Google Scholar]
- 14.Idiart M.A., Levin Y. Rupture of a liposomal vesicle. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2004;69:061922. doi: 10.1103/PhysRevE.69.061922. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki M. The single GUV method to reveal elementary processes of leakage of internal contents from liposomes induced by antimicrobial substances. Adv. Planar Lipid Bilayers and Liposomes. 2008;7:121–142. [Google Scholar]
- 16.Sandre O., Moreaux L., Brochard-Wyart F. Dynamics of transient pores in stretched vesicles. Proc. Natl. Acad. Sci. USA. 1999;96:10591–10596. doi: 10.1073/pnas.96.19.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawicz W., Olbrich K.C., Evans E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgart T., Hess S.T., Webb W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 19.Islam M.Z., Alam J.M., Yamazaki M. The single GUV method for revealing the functions of antimicrobial, pore-forming toxin, and cell-penetrating peptides or proteins. Phys. Chem. Chem. Phys. 2014;16:15752–15767. doi: 10.1039/c4cp00717d. [DOI] [PubMed] [Google Scholar]
- 20.Hotani H. Transformation pathways of liposomes. J. Mol. Biol. 1984;178:113–120. doi: 10.1016/0022-2836(84)90234-1. [DOI] [PubMed] [Google Scholar]
- 21.Ho J.C.S., Rangamani P., Parikh A.N. Mixing water, transducing energy, and shaping membranes: autonomously self-regulating giant vesicles. Langmuir. 2016;32:2151–2163. doi: 10.1021/acs.langmuir.5b04470. [DOI] [PubMed] [Google Scholar]
- 22.Boroske E., Elwenspoek M., Helfrich W. Osmotic shrinkage of giant egg-lecithin vesicles. Biophys. J. 1981;34:95–109. doi: 10.1016/S0006-3495(81)84839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olbrich K., Rawicz W., Evans E. Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 2000;79:321–327. doi: 10.1016/S0006-3495(00)76294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karatekin E., Sandre O., Brochard-Wyart F. Cascades of transient pores in giant vesicles: line tension and transport. Biophys. J. 2003;84:1734–1749. doi: 10.1016/S0006-3495(03)74981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans E., Heinrich V., Rawicz W. Dynamic tension spectroscopy and strength of biomembranes. Biophys. J. 2003;85:2342–2350. doi: 10.1016/s0006-3495(03)74658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans E., Smith B.A. Kinetics of hole nucleation in biomembrane rupture. New J. Phys. 2011;13:095010. doi: 10.1088/1367-2630/13/9/095010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levadny V., Tsuboi T.A., Yamazaki M. Rate constant of tension-induced pore formation in lipid membranes. Langmuir. 2013;29:3848–3852. doi: 10.1021/la304662p. [DOI] [PubMed] [Google Scholar]
- 28.Karal M.A.S., Levadnyy V., Yamazaki M. Electrostatic interaction effects on tension-induced pore formation in lipid membranes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2015;92:012708. doi: 10.1103/PhysRevE.92.012708. [DOI] [PubMed] [Google Scholar]
- 29.Karal M.A.S., Yamazaki M. Communication: activation energy of tension-induced pore formation in lipid membranes. J. Chem. Phys. 2015;143:081103. doi: 10.1063/1.4930108. [DOI] [PubMed] [Google Scholar]
- 30.Yoshitani T., Yamazaki M. Water permeability of lipid membranes of GUVs and its dependence on actin cytoskeletons inside the GUVs. Proc. IEEE Int. Symp. Micro-NanoMechatronics and Human Sci. 2008;2008:130–134. [Google Scholar]
- 31.Grattoni A., Merlo M., Ferrari M. Osmotic pressure beyond concentration restrictions. J. Phys. Chem. B. 2007;111:11770–11775. doi: 10.1021/jp075834j. [DOI] [PubMed] [Google Scholar]
- 32.Minkov I., Manev E.D., Kolikov K.H. Equilibrium and dynamic behavior of aqueous solutions with varied concentration at constant and variable volume. Sci. World J. 2013;2013:876897. doi: 10.1155/2013/876897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Gennes P.-G., Brochard-Wyart F., Quere D. Springer; New York: 2004. Capillarity and Wetting Phenomena; Drops, Bubbles, Pearls, Waves. [Google Scholar]
- 34.Needham D., Nunn R.S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 1990;58:997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karal M.A.S., Alam J.M., Yamazaki M. Stretch-activated pore of the antimicrobial peptide, magainin 2. Langmuir. 2015;31:3391–3401. doi: 10.1021/la503318z. [DOI] [PubMed] [Google Scholar]
- 36.Deryagin B.V., Gutop Y.V. Theory of the breakdown (rupture) of free films. Kolloidn. Zh. 1962;24:370–374. [Google Scholar]
- 37.Litster J.D. Stability of lipid bilayers and red blood cell membranes. Phys. Lett. A. 1975;53:193–194. [Google Scholar]
- 38.Fuertes G., Giménez D., Salgado J. A lipocentric view of peptide-induced pores. Eur. Biophys. J. 2011;40:399–415. doi: 10.1007/s00249-011-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karal M.A.S., Levadnyy V., Yamazaki M. Analysis of constant tension-induced rupture of lipid membranes using activation energy. Phys. Chem. Chem. Phys. 2016;18:13487–13495. doi: 10.1039/c6cp01184e. [DOI] [PubMed] [Google Scholar]
- 40.Srividya N., Muralidharan S., Tripp B. Determination of the line tension of giant vesicles from pore-closing dynamics. J. Phys. Chem. B. 2008;112:7147–7152. doi: 10.1021/jp7119203. [DOI] [PubMed] [Google Scholar]
- 41.Portet T., Dimova R. A new method for measuring edge tensions and stability of lipid bilayers: effect of membrane composition. Biophys. J. 2010;99:3264–3273. doi: 10.1016/j.bpj.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brochard-Wyart F., De Gennes P.-G., Sandre O. Transient pores in stretched vesicles: role of leak-out. Physica A. 2000;278:32–51. [Google Scholar]
- 43.Ryham R., Berezovik I., Cohen F.S. Aqueous viscosity is the primary source of friction in lipidic pore dynamics. Biophys. J. 2011;101:2929–2938. doi: 10.1016/j.bpj.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mavčič B., Babnik B., Kralj-Iglič V. Shape transformation of giant phospholipid vesicles at high concentrations of C12E8. Bioelectrochemistry. 2004;63:183–187. doi: 10.1016/j.bioelechem.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Muddana H.S., Gullapalli R.R., Butler P.J. Atomistic simulation of lipid and DiI dynamics in membrane bilayers under tension. Phys. Chem. Chem. Phys. 2011;13:1368–1378. doi: 10.1039/c0cp00430h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy A.S., Warshaviak D.T., Chachisvilis M. Effect of membrane tension on the physical properties of DOPC lipid bilayer membrane. Biochim. Biophys. Acta. 2012;1818:2271–2281. doi: 10.1016/j.bbamem.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butler P.J., Norwich G., Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am. J. Physiol. Cell Physiol. 2001;280:C962–C969. doi: 10.1152/ajpcell.2001.280.4.C962. [DOI] [PubMed] [Google Scholar]
- 48.De Vequi-Suplicy C.C., Benatti C.R., Lamy M.T. Laurdan in fluid bilayers: position and structural sensitivity. J. Fluoresc. 2006;16:431–439. doi: 10.1007/s10895-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 49.Lakowicz J.R. 2nd ed. Kluwer Academic; Dordrecht, the Netherlands: 1999. Principles of Fluorescence Spectroscopy. [Google Scholar]
- 50.Evans E., Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 1990;64:2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- 51.Vitkova V., Genova J., Bivas I. Permeability and the hidden area of lipid bilayers. Eur. Biophys. J. 2004;33:706–714. doi: 10.1007/s00249-004-0415-2. [DOI] [PubMed] [Google Scholar]
- 52.Mathivet L., Cribier S., Devaux P.F. Shape change and physical properties of giant phospholipid vesicles prepared in the presence of an AC electric field. Biophys. J. 1996;70:1112–1121. doi: 10.1016/S0006-3495(96)79693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perutková S., Kralj-Iglič V., Iglič A. Mechanical stability of membrane nanotubular protrusions influenced by attachment of flexible rod-like proteins. J. Biomech. 2010;43:1612–1617. doi: 10.1016/j.jbiomech.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Genova J., Zheliaskova A., Mitov M.D. The influence of sucrose on the elasticity of SOPC lipid membrane studied by the analysis of thermally induced shape fluctuation. Colloids Surf. A Physicochem. Eng. Asp. 2006;282–283:420–422. [Google Scholar]
- 55.Shchelokovskyy P., Tristram-Nagle S., Dimova R. Effect of the HIV-1 fusion peptide on the mechanical properties and leaflet coupling of lipid bilayers. New J. Phys. 2011;13:25004. doi: 10.1088/1367-2630/13/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drabik D., Przybyło M., Langner M. The modified fluorescence based vesicle fluctuation spectroscopy technique for determination of lipid bilayer bending properties. Biochim. Biophys. Acta. 2016;1858:244–252. doi: 10.1016/j.bbamem.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Nagle J.F., Jablin M.S., Tristram-Nagle S. Sugar does not affect the bending and tilt moduli of simple lipid bilayers. Chem. Phys. Lipids. 2016;196:76–80. doi: 10.1016/j.chemphyslip.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Marsh D. Lateral pressure in membranes. Biochim. Biophys. Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.