Abstract
Background
Aspirin has been shown to lower the incidence and the mortality of vascular disease and cancer but its wider adoption appears to be seriously impeded by concerns about gastrointestinal (GI) bleeding. Unlike heart attacks, stroke and cancer, GI bleeding is an acute event, usually followed by complete recovery. We propose therefore that a more appropriate evaluation of the risk-benefit balance would be based on fatal adverse events, rather than on the incidence of bleeding. We therefore present a literature search and meta-analysis to ascertain fatal events attributable to low-dose aspirin.
Methods
In a systematic literature review we identified reports of randomised controlled trials of aspirin in which both total GI bleeding events and bleeds that led to death had been reported. Principal investigators of studies in which fatal events had not been adequately described were contacted via email and asked for further details. A meta-analyses was then performed to estimate the risk of fatal gastrointestinal bleeding attributable to low-dose aspirin.
Results
Eleven randomised trials were identified in the literature search. In these the relative risk (RR) of ‘major’ incident GI bleeding in subjects who had been randomised to low-dose aspirin was 1.55 (95% CI 1.33, 1.83), and the risk of a bleed attributable to aspirin being fatal was 0.45 (95% CI 0.25, 0.80). In all the subjects randomised to aspirin, compared with those randomised not to receive aspirin, there was no significant increase in the risk of a fatal bleed (RR 0.77; 95% CI 0.41, 1.43).
Conclusions
The majority of the adverse events caused by aspirin are GI bleeds, and there appears to be no valid evidence that the overall frequency of fatal GI bleeds is increased by aspirin. The substantive risk for prophylactic aspirin is therefore cerebral haemorrhage which can be fatal or severely disabling, with an estimated risk of one death and one disabling stroke for every 1,000 people taking aspirin for ten years. These adverse effects of aspirin should be weighed against the reductions in vascular disease and cancer.
Introduction
Cardiovascular disease (CVD) and cancer are leading causes of death and disability in the world, with a combined treatment cost and global economic impact of approximately 2 trillion USD annually [1–5]. Low-dose aspirin (70–325 mg per day) is effective in the secondary prevention of vascular events in patients who have previously experienced heart attacks and strokes in [6,7,8], and more recently the US Preventative Services Task Force Agency has recommended low dose aspirin for the primary prevention of colorectal (CRC), and vascular disease in healthy individuals age 50 to 59, who have a 10-year CVD risk of 10% or greater.[9]
After a latency period of about five years, low-dose aspirin also appears to reduce the risk and mortality of colorectal and other cancers.[9–13] Furthermore, there is growing evidence that aspirin may be useful as an adjunct treatment in established colorectal and other cancers.[14]
The widespread adoption of aspirin for primary cancer and vascular prevention is however limited by concerns of toxicity, in particular major gastrointestinal (GI) bleeding, [8,9,15] ‘major’ being usually defined as bleeds that require blood transfusion together with those that lead to death. In a recent systematic overview aspirin was associated with a relative increase in GI risk (RR) of 1.4 (95% confidence intervals (CI) of 1.2–1.7), equivalent to an extra 0.5–3.6 bleeds per 1,000 person-years.[16] An increase in cerebral bleeding has also been attributed to low-dose aspirin, the RR estimated to be 1.4 (95% CI 1.2–1.7)[11] equivalent to one or two events per 10,000 subject-years.[17]
Nevertheless, low dose aspirin has been shown to have a favourable risk-benefit ratio when its cancer and vascular benefits are combined. Thun et al concluded that: ‘even a 10% reduction in overall cancer incidence [by aspirin] beginning during the first 10 years of treatment could tip the balance of benefits and risks favourably in average-risk populations’.[18] Pignone et al states: ‘aspirin appears beneficial for a large proportion of middle-aged men at low-moderate CHD risk’.[19] In a cautious meta-analysis Cuzick et al judged the benefit-harm balance to be favourable, with benefits increasing the longer aspirin is taken.[20] Two other evaluations which are limited to colorectal cancer and ignore the reduction in vascular disease also concluded that aspirin as a primary prevention strategy is cost-effective particularly when recommended alongside colorectal screening.[21,22] The most recent evaluation to be reported concludes that within the general population there would be benefit from aspirin use within the ages of 40–85 years.[23] In all these studies however, estimates of harm from aspirin are based on the frequency of ‘major’ GI together with cerebral bleeding.
Iatrogenic adverse events are problematic in healthy subjects, and especially in preventive interventions. Yet in the evaluation of the risk-benefit balance it is important to take account of the severity of the disease events prevented and caused by the intervention, and not just the frequencies of the events. Thus GI bleeds, which are sometimes severe, are acute events usually followed by recovery without sequelae, while strokes can leave residual physical and/or cognitive impairments in those who survive, and those who survive heart attacks or cancer may require complex and lifelong interventions.[24] It would therefore seem to be reasonable to consider fatal bleeds attributable to aspirin to be comparable in severity to the disease events prevented.
The distinction between GI bleeding and fatal bleeding is not trivial. A number of studies have already shown that GI bleeds attributable to aspirin carry a risk of death that is lower than that of spontaneous GI bleeding.[7,25–28].
If there is to be a genuine relationship of co-production in health promotion, the best available evidence should be shared with healthy subjects and with patients. Citizens involved in a ‘citizens’ jury’ have urged that the evidence relating to aspirin prophylaxis should be promoted and made easily available to the wider public [29] and it was this contact with the public that inspired this present research study.
The objective set for our study was a systematic review and meta-analysis of fatal GI bleeding attributable to low-dose aspirin. A specific aim was to estimate the frequency of fatal GI bleeds in subjects randomised to low-dose aspirin, relative to the fatality rate of spontaneous bleeds in those randomised not to take aspirin.
Methods
Literature searches
From pilot literature searches, using text words for aspirin and fatal bleeding, it became clear that a number of relevant studies known to the research team were not identified. This was because, although relevant information was contained in the body of some of the papers, words indicative of fatal bleeding were not included in the title or in the abstract.
A two part search was therefore developed and tested against known relevant papers, to ensure a highly sensitive search for publications with data on fatal bleeding events: (i) The subject search terms (for title, abstract and subject headings) were combined with a specific search developed to identify papers looking at aspirin adverse events. This enhanced search was run in Medline and Embase. The search combined subject headings (MeSH and Emtree as appropriate) for aspirin adverse event combined with (fatal* or death or mortality). (ii) A key word search to identify any mention of aspirin and fatal bleeding in the body of the text was conducted in Medline and CINAHL databases with full text capability using the terms (aspirin* or acetylsalicylic acid) and ((bleed* or bled* or haemorrhage or hemorrhage) and (fatal* or death or mortality)). Searches were completed in July 2016 and there were no date or language restrictions. Search strategies are summarised in S1 Table.
Given the challenges of finding papers with data on fatal bleeding events, supplementary search strategies were also employed. The reference lists of all included papers were examined in detail and emails requesting further data relevant to our purposes were requested from the authors when necessary.
Three of the authors (PCE, AW, DM) carried out the literature searching and identification of reports with relevant data. Papers were selected at title and abstract, and full text, stage by PCE and GM independently in duplicate. PCE extracted data from each report and the selection of data, and the correctness of every datum was checked by GM. An appraisal of bias in these studies [30] was conducted by ALW and DM independently in duplicate and is detailed in S2 Table. Discrepancies were resolved by discussion with PCE.
Selection criteria
Studies were included in the main meta-analysis if they were randomised controlled trials of primary prevention or secondary prevention of further events, in which aspirin had been compared with a placebo; if ‘major’ gastrointestinal bleeding was reported; if the number of fatal GI bleeds was recorded or was ascertained either in correspondence with an author, or occasionally in another independent report. Intervention trials in general surgery, coronary artery procedures, stent insertion etc. in which aspirin had been randomised were excluded, being short-term, usually with multiple interventions additional to aspirin.
Analysis plan
We analysed three outcomes: the risk of GI bleed attributable to aspirin, the risk of fatal GI bleed among those who bled, and the risk of a fatal bleed in subjects who had been randomised to receive aspirin. We used this last as a measure of risk of low-dose aspirin prophylaxis which is comparable to the disease risks reduced by the use of aspirin, namely a vascular event or a cancer. We also examined and comment upon estimates of fatal GI bleeding made by other authors, both in overviews and in observational studies.
Statistics
Standard methods as recommended in Egger et al [31] were used throughout. Thus meta-analysis were conducted using random effects estimates, weighted by the method of DerSimonian and Laird,[32] with estimates of heterogeneity taken from the Mantel and Haenszel fixed effects model.[33] The summary statistic risk ratio (RR) was derived, and 95% confidence intervals estimated and heterogeneity assessed using the Q statistic. The random effects model was used throughout to incorporate an estimate of between study variation into the calculation of common effects. Begg’s funnel plots and Egger’s tests were examined as indicators of publication bias. Sensitivity analyses were conducted whereby each study was omitted in turn and the summary estimate recalculated to determine the influence of each study. All analyses were carried out using the statistical package STATA.
Results
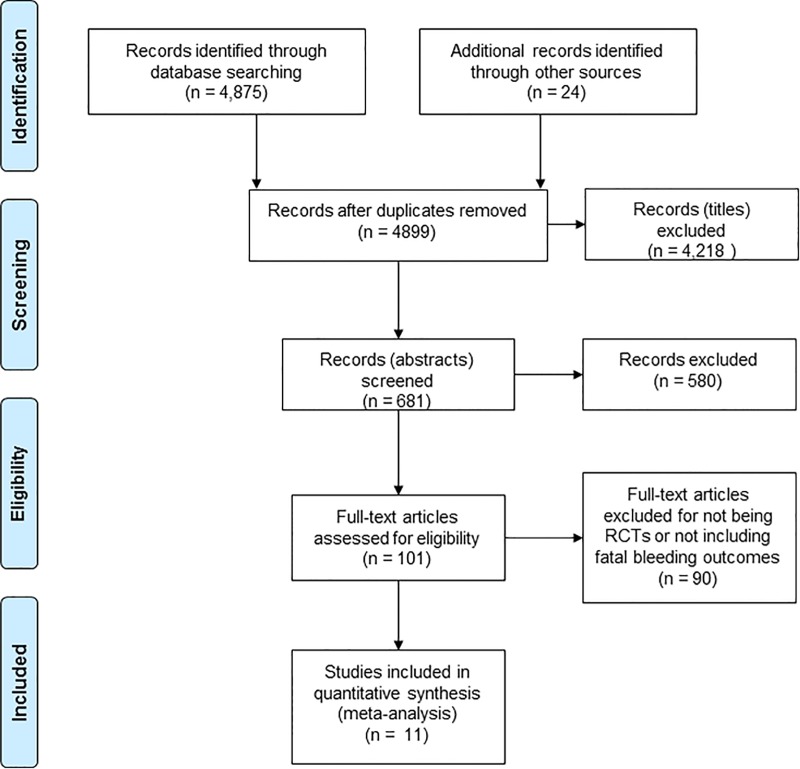
A flow diagram of the search and study selection process can be seen in Fig 1. Authors of 38 papers were contacted by email to request further data. Eleven long-term randomised controlled trials of aspirin prophylaxis with data on fatal adverse effects were identified that met our selection criteria.[34–44]
Fig 1. Flow diagram of the search and study selection.
Table 1 summarises data from these and S2 Table summarises aspects of the trials of relevance to quality and to possible bias. Nine trials were assessed as having low risk of bias [35–41,43,44] and two with unclear risk of bias. [34,42]
Table 1. Details of randomised trials.
| Source | Dose of aspirin and duration of follow-up (range, mean or median) | Number of subjects | Number of bleeds | Fatal bleeds | ||||
|---|---|---|---|---|---|---|---|---|
| Aspirin | No aspirin | Aspirin | No aspirin | Aspirin | No aspirin | |||
| Peto et al (1988)[34] | 5,139 healthy male doctors | 500mg daily or 300 enteric coated for 5–6 years | 3,429 | 1,710 | 89 | 27 | 3 | 3 |
| Physicians’ Health Study (1989)[35] | 22,071 healthy physicians (United States) | 325 mg alternate days for 60.2 months | 11,037 | 11,034 | 13 | 6 | 1 | 0 |
| Swedish Angina Trial (1992)[36] | 1,360 patients with a transient ischaemic attack or myocardial infarction | 75 mg daily for 32 months | 676 | 684 | 9 | 4 | 1 | 1 |
| Internat. Stroke Trial (1997)[37] | 19,435 Patients with ischaemic stroke | 300 mg daily for 6 months | 4,858 | 4,860 | 23 | 14 | 4 | 2 |
| Thrombosis Prevention trial (1998)[38] | 5499 men at increased risk of vascular disease | 75 mg daily for 6.8 years | 1,268 | 1,272 | 7 | 4 | 1 | 2 |
| Hansson et al (1998)[30] | Hypertensive patients on ‘optimal’ treatment | 75 mg daily for 3.8 years | 9,399 | 9,391 | 129 | 70 | 5 | 5 |
| Primary Prev. Project (2001)[40] | 4,495 selected from general practitioner lists | 100 mg daily for 3–6 years | 2,226 | 2,269 | 17 | 5 | 1 | 3 |
| Baron et al (2003)[41] | 1,121 patients selected at colo-rectal screening | 81 mg daily 325 mg daily each for 3 years | 377/372 | 372 | 2/4 | 3 | 0/1 | 0/0 |
| Ridker (2005)[42] | 39,876 women | 100 mg alternate days for 10 years | 19,934 | 19,942 | 127 | 91 | 2 | 3 |
| Belch et al (2008)[43] | 1,276 diabetic patients with arterial disease | 1900 mg daily for 6.7 years | 638 | 638 | 28 | 31 | 0 | 2 |
| Brighton et al (2012)[44] | 822 patients with venous thrombosis | 100 mg daily for 37 months | 411 | 411 | 8 | 6 | 0 | 2 |
| Risk of a GI bleed | ||||||||
| On aspirin | 8/1000 | RR = 1.55 (1.32, 1.83) heterogeneity p = 0.401 | ||||||
| On placebo | 5/1000 | |||||||
| Risk of a bleed being fatal | ||||||||
| On aspirin | 4% | RR = 0.45 (0.25, 0,80) heterogeneity p = 0.783 | ||||||
| On placebo | 10% | |||||||
| Risk of a fatal bleed if randomised to aspirin | ||||||||
| On aspirin | 3.7/10,000 | RR = 0.77 (0.41, 1.43) heterogeneity p = 0.908 | ||||||
| On placebo | 4.7/10,000 | |||||||
Abbreviations: CI: confidence interval; mg: milligrams; RCT: randomised controlled trial; RR: risk ratio.
A meta-analysis gives no evidence of heterogeneity between the trials, and shows that aspirin increases the frequency of GI bleeds by about sixty percent, with a relative risk (RR) of 1.55 (95% confidence limits (CI) 1.32, 1.83; heterogeneity p = 0.401).
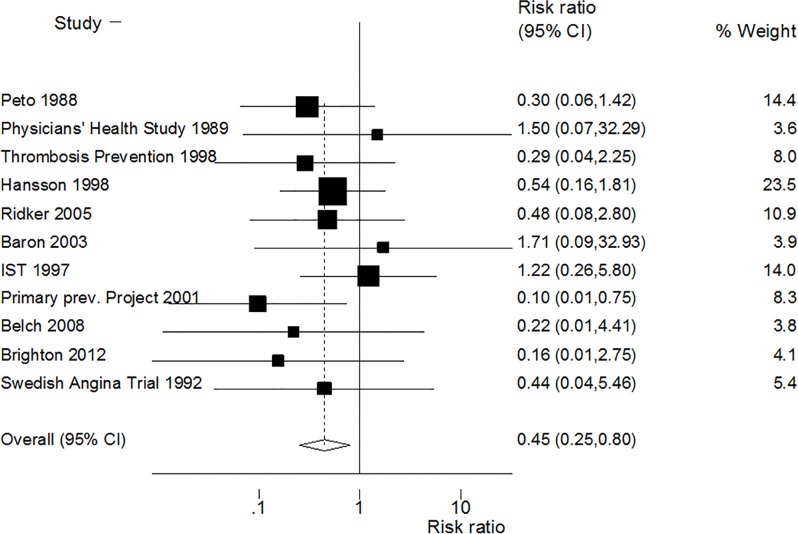
The forest plot in Fig 2 is based on data in Table 1 and shows that GI bleeds which led to death appear to be reduced in subjects who had been taking aspirin (RR 0.45; 95% CI 0.25, 0.80; heterogeneity p = 0.783). The sensitivity analysis (S1 Fig) shows that no individual study affected the overall RR dominantly. This procedure confirmed the stability of our overall result. We found no evidence of publication bias influencing this result (Egger’s test P = 0.754, see funnel plot in S2 Fig).
Fig 2. Forest plot of GI bleeds that led to death.
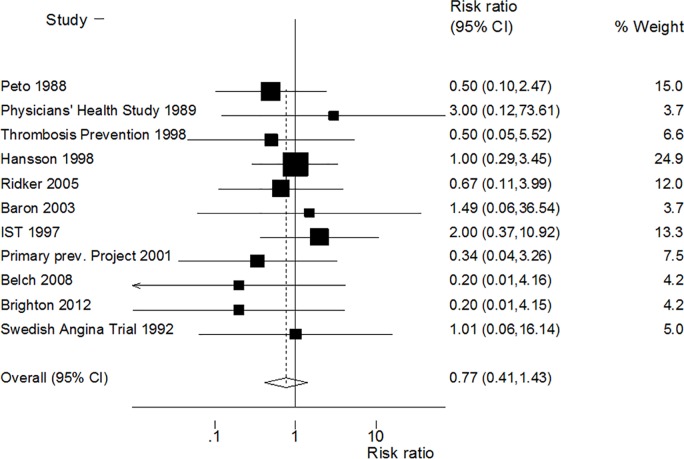
Of greatest relevance to the risk of prophylactic aspirin is however the risk of a fatal GI bleed in subjects who take low-dose aspirin, relative to the risk in those who take no aspirin. This risk was estimated in the randomised trials and is shown in a forest plot (Fig 3). There is no evidence of any significant increase in fatal GI bleeds attributable to aspirin. The risk of death from a bleed is: 3.7 ± 1.6 per 10,000 in subjects randomised to aspirin, and 4.7 ± 1.8 per 10,000 in subjects who had been randomised not to receive aspirin, the risk ratio (RR) being 0.77 (95% CI 0.41, 1.43 heterogeneity p = 0.908). The sensitivity analysis (S3 Fig) shows that, again, no individual study affected the overall RR dominantly, confirming the stability of the overall result. We found no evidence of publication bias influencing this result (Egger’s test P = 0.506, see funnel plot in S4 Fig).
Fig 3. Forest plot of risk of subjects randomised to aspirin.
Discussion
Our meta-analysis, incorporating new data from direct email contact with the authors of some of the randomised aspirin studies, indicates that although aspirin increases the risk of GI bleeding by around 60%, low-dose aspirin is associated with a lower risk of fatality amongst patients who developed GI bleeding (RR 0.45; 95% CI 0.25, 0.80; heterogeneity 0.783). It is important to note that in no report was mention made of the use of gastric protection by a proton pump inhibitor (PPI) or other treatment as their use could have reduced some of the serious bleeding events caused by aspirin. Nor does our study take account of the use of enteric-coated aspirin, as this has not been shown to reduce risk of upper gastrointestinal complications.[45]
Furthermore, and most important, amongst the totality of all the subjects who had been randomised to take aspirin there was no increase in death from GI bleeding compared with subjects who had been randomised to take no aspirin (RR 0.77; 95% CI 0.41, 1.43; heterogeneity 0.908).
These findings are consistent with those from several earlier overviews of trials which have also suggested a reduction in the fatality of bleeds attributed to aspirin (Table 2). Data from these overviews were not included in our meta-analysis due to partial overlap with studies included in our analyses, together with the inclusion by these authors of some small and short term trials in patients undergoing surgery etc. These overviews all estimate the risk of death in subjects who bled, with the exception of the recent overview conducted for the U.S. Preventive Services Task Force in which fatal bleeds were examined within the totality of subjects,[9] and showed, as we have, no significant increase in fatal GI bleeds in subjects randomised to aspirin (OR 1.00; 95% CI 0.43, 2.36).
Table 2. Overviews of trials of GI bleeding and bleeds that were fatal in studies reported by other authors.
| Source | Details | No. of subjects | No. of bleeds | No. of fatal bleeds | Risk of a GI bleed attributable to aspirin leading to death (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| On aspirin | On placebo | On aspirin | On placebo | On aspirin | On placebo | |||
| ATT (2009)[7] | 6 RCTs | 47,293 | 45,618 | 335 | 219 | 9 | 20 | OR 0.48 (0.17, 1.34) |
| Rothwell et al (2012)[25] | 34 RCTs | 40,269 | 40363 | 203 | 132 | 8 | 15 | OR 0.32 (0.12, 0.83) |
| Lanas et al (2011)[26] | 28 RCTs | 42,0891 | 42,0891 | ? | ? | 16 | 17 | OR 0.94 (0.47, 1.87) |
| McQuaid et al (2006)[27] | 14 RCTs | 25,964 | 25,993 | 48 | 28 | Na2 | Na2 | RR 1.23 (0.45, 3.41) |
| Wu et al (2016)[28] | 12 RCTs | 616 | 3,640 | 43 | 18 | 0 | 3 | RR 0.29 (0.03, 1.22) |
| Present study | 14 RCTs | 56,654 | 55,016 | 468 | 285 | 24 | 29 | RR 0.45 (0.25, 0.80) |
1 Only total numbers are given, equal numbers on aspirin and placebo assumed.
2 Na = Not available.
3 Estimated from data in the published paper.
Abbreviations: CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio.
Randomised trials are a valid source of evidence on these issues [19] and the above conclusions are therefore based entirely on randomised trials. Observational studies are prone to selection bias, are likely to be confounded, and polypharmacy is frequent. It is important however to consider how applicable our findings are likely to be to the broader community, since subjects involved in trials may have been selected to be at a low risk for GI bleeding, and subjects with gastric symptoms are likely to have been excluded.
Nevertheless, in an extensive review of observational community-based studies it was judged that: ‘The risks of major bleeding with low-dose aspirin in real-world settings are of a similar magnitude to those reported in randomised trials.[16] This confirms our own finding that the fatality rate of GI bleeds in our selected randomised trials (10.2%) and fatal bleeding rate (6.8%) in a selection of community based observational studies are reasonably similar.[46–53] In fact, it can very reasonably be argued on a practical level that clinical practice in the general community should be no less careful than selection of subjects for a trial.[24]
The strengths of our study lie in the fact that it is based on a systematic literature survey, supplemented with correspondence with 38 authors of studies of possible relevance. On the other hand, the number of available relevant data is small, and therefore the estimates we have made have a considerable degree of uncertainty. It should be noted however that the scarcity of fatal bleeding in contrast to the number of disease events prevented, is in itself an answer to the issue examined in our report.
The basic conclusion from our study is that although aspirin increases risk of GI bleeding, the overall risk of fatal bleeding is not significantly elevated, and the fatality rate, should GI bleeding occur, is significantly reduced.
How may these seemingly contradictory findings be reconciled? We suggest that the most likely explanation is that aspirin, through its anti-thrombotic effect may unmask gastrointestinal pathology early in its natural history, when the anatomical extent of the lesion is more limited, and the risk of massive uncontrolled bleeding lower and medical intervention is associated with the highest likelihood of success. A similar process may also occur with bleeding caused by Helicobacter pylori associated ulceration, or oesophageal varices.
A number of authors of observational studies of patients admitted with a GI bleed have recorded whether or not the patients had been taking aspirin, or were taking neither aspirin nor any other antiplatelet or anticoagulant drug. Thus Abu Daya et al commented that ‘Aspirin may have beneficial effects in patients with upper GI bleeding’.[51] Mose et al wrote: ‘low-dose aspirin… may even be associated with an improved outcome [in patients with peptic ulcer bleeding] compared with non-use’.[48] Ahsberg et al state ‘we found decreasing incidence … of non-variceal upper and lower GI bleeding during a period when prescription rates of drugs that increase the risk of GI bleed have increased several-fold. The increased use of these ‘high-risk drugs’ causes more severe bleeding but has had no influence on the risk of fatal outcome.’[49] Finally, Wehbeh et al gave their report the title: ‘Aspirin has a protective effect against adverse outcomes in patients with non-variceal upper gastrointestinal bleeding’.[50]
The main concern about intestinal bleeding focuses upon the upper GI tract. Bleeding also occurs from the lower intestinal tract, but the literature on this is sparse and most of the reports identified had been based on patients who had undergone colonoscopy, often with polypectomy, in whom large bowel disease appeared to account for the increase in lower GI bleeding attributable to aspirin.[54–57]
The few randomised studies which give evidence on lower GI bleeding from aspirin, show an increase, but the evidence is inconsistent,[16] and most of the evidence comes from the general population. A study conducted in Spain during 1996–2005 estimated the mortality of lower GI events to be 8.8%, compared to 5.5% for upper GI events.[58] Two further studies, also conducted in Spain, were based on the hospital records of 41 and 50 thousand patients admitted with a gastrointestinal event in 2001. In both the mortality of upper GI events was 5%, both in the total groups of patients and in the patients who had been taking aspirin or an NSAID.[46] Lower GI events accounted for 11–13% of the admissions, and 4–5% of these patients died. Amongst patients taking aspirin or an NSAID the mortality of lower GI bleeds was 4% in one of the studies and almost 12% in the other. A further study in Sweden examined the records of 731 patients admitted with non-variceal GI bleeding, and reported that the mortality of 440 patients with upper GI bleeding, and 289 with lower GI bleeding was 3% in both.[59]
These reports do not give adequate evidence on the relevance of aspirin to the bleeding reported and we therefore feel that the evidence on lower GI bleeding is too limited and too inconsistent to support any reliable conclusion relevant to the issues examined in our report.
Unlike a gastrointestinal bleed, the consequences of a cerebral bleed, whether or not fatal, are of a severity comparable to the effects of a myocardial infarct or a cancer. Mortality is around 30–50% [7,60–62] and severe physical and psychological disablement are possible in those who survive.[17] It would therefore seem to be reasonable to include all cerebral bleeds, fatal and non-fatal, in an evaluation of the risk-benefit balance of aspirin prophylaxis.
Fortunately cerebral bleeds attributable to aspirin are rare. The risk ratio associated with aspirin is about 1.4 (95% CI 1.2–1.7),[16] equivalent to one or two haemorrhagic strokes per year in every 10.000 subjects on aspirin.[17] Gorlick & Wiseman however comment on the probable reduction in cerebral bleeding if blood pressure is measured before aspirin prophylaxis is started, and hypertension, if present, is adequately treated.[17] Evidence to support this comes from a trial based on more than 18,000 hypertensive patients, all of whom were receiving optimal antihypertensive treatment. Amongst almost ten thousand patients randomised to aspirin there seven fatal bleeds and eight (P<0.001) in those on placebo.[39]
An important issue in the evaluation of prophylactic aspirin is the duration of aspirin taking as this is relevant both to the benefits, and to the risks of the drug. While reductions in vascular disease appear to commence immediately, almost all studies of cancer prevention show a 3-5-year delay before benefit from aspirin is clinically apparent, and thereafter the reduction increases.[25,63] This delay had been predicted on the grounds that prevention by aspirin occurs at a cellular level and there is an inevitable delay before an effect upon a cell becomes apparent in the absence of a tumour.[64]
On the other hand, the incidence of GI bleeding attributable to aspirin appears to decrease over time. Within the first month of aspirin taking the risk is increased more than four-fold,[63,65] but it then reduces rapidly and after about three to five years of aspirin taking there appears to be no excess in GI bleeds (OR 0.63; 95% CI 0.34, 1.16).[25] Likewise, most of the deaths from bleeding occur within the first month of aspirin taking,[66] implying the presence of underlying, untreated gastric pathology.
Additional to these changes, reports from a number of countries indicate that gastrointestinal bleeding and fatal bleeding have decreased substantially over time, probably because of better patient care, increased use of gastroprotective drugs, lower doses of aspirin and less use of other NSAIDs, and more effective treatment of the bleeding. These reductions have been large: almost 50% in Scotland over an 18 year period,[67] to almost half in Spain over ten years,[58] by almost 20% over 18 years in the USA,[68] and over 25% within seven years in Wales.[69]
Conclusions
Gastrointestinal bleeds constitute the majority of the adverse events caused by aspirin. The increase is about 60% overall, but there appears to be no increase in fatal GI bleeds attributable to low-dose aspirin, indeed prophylactic aspirin appear to be associated with a reduction in the fatality of GI bleeds.
The undesirable effect of prophylactic aspirin which is of a severity comparable to a vascular disease event or a cancer is a bleed that leads to death, and low-dose aspirin appears to be associated with one death and one disabling haemorrhagic stroke per year in every 10,000 people taking low-dose aspirin. The available evidence makes it seems likely that these cerebral events would be reduced if hypertension is identified and adequately treated.[7,17,39]
In addition, there will be one or two non-fatal GI bleeds per 1,000 people each year, but the frequency of these bleeds appears to fall rapidly, and there is no evidence of any increase in GI bleeds attributable to aspirin after three or four years of prophylaxis
All these conclusions are relevant to the risk-benefit balance of aspirin prophylaxis and should be communicated to subjects at risk of vascular disease and/or cancer, to enable them to make an informed decision about the protection of their own health.[29,70]
Supporting Information
The middle vertical axis indicates the overall RR and the two vertical axes indicate its 95% CI. Every hollow circle indicates the pooled RR when the left study was omitted in this meta-analysis. The two ends of every broken line represent the 95% CIs.
(TIF)
(TIF)
The middle vertical axis indicates the overall RR and the two vertical axes indicate its 95% CI. Every hollow circle indicates the pooled RR when the left study was omitted in this meta-analysis. The two ends of every broken line represent the 95% CIs.
(TIF)
(TIF)
(DOCX)
[28]
(DOCX)
Acknowledgments
We are most grateful to the authors of papers to whom we wrote, many of whom supplied us with new data, some requiring new analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funders – who were in effect the Universities and Health Services who paid the salaries of the authors - had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Cancer Society and Livestrong Report. The Global Economic Impact of Cancer. http://www.cancer.org/acs/grpups/conmtent@internationalaffairs/documents/document/acspc-026203.pdf.
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of Cancer Care in the United States. J Cancer Inst 2011, 10.1093/jnci/djg495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramon LF, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population based cost analysis. Lancet Oncol 2013;14:1165–74. 10.1016/S1470-2045(13)70442-X [DOI] [PubMed] [Google Scholar]
- 4.Bloom DE, Cafiero ET., Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S. et al. The Global Economic Burden of noncommunicable diseases World Economic Forum. [Google Scholar]
- 5.Feigin FL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA et al. Global and regional burden of stroke during the 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayden M, Pignone M, Phillips C, Mulrow C. US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern med 2009;150:396–404. [DOI] [PubMed] [Google Scholar]
- 7.Antithrombotic Trialists’ Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2012;33:1635–701. 10.1093/eurheartj/ehs092 [DOI] [PubMed] [Google Scholar]
- 9.Chubak J, Kamineni A, Buist DSM, Anderson ML, Whitlock EP Aspirin Use for the Prevention of Colorectal Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses, No. 133 Kaiser Permanente Research Affiliates Evidence-based Practice Center, Rockville (MD): Agency for Healthcare Research and Quality; Report No.: 15-05228-EF-1); USSep 2015 [PubMed]
- 10.Agra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastases: a systematic comparison of evidence from observational studies versus randomised trials. Lancet oncology 2012;13:518–27 10.1016/S1470-2045(12)70112-2 [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality and non-vascular death: analysis of the time course of risks and benefits in 51 randomised trials. Lancet. 2012. April 28;379(9826):1602–12. 10.1016/S0140-6736(11)61720-0 Epub 2012 Mar 21. [DOI] [PubMed] [Google Scholar]
- 12.Mills EJ, Wu P, Alberton M, Kanters S, Lanas A, Lester R. Low-dose aspirin and cancer mortality: a meta-analyisi of randomised trials. Am J Med. 2012. June;125(6):560–7. 10.1016/j.amjmed.2012.01.017 Epub 2012 Apr 17. [DOI] [PubMed] [Google Scholar]
- 13.Battistoni A, Mastomarino V, Volpe M. Reducing cardiovascular and cáncer risk: how to address global prevention in clinical practice. Clin Cardiol 2015. 10.1002/cic.22394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, Kelson M. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analysis of published studies. Published: April 20, 2016. 10.1371/journal.pone.0152402 [DOI] [PMC free article] [PubMed]
- 15.Baigent C. Aspirin for all over 50?. Brit. Med. J. 2005;330, 1442–3. 10.1136/bmj.330.7505.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez LAG, Martin-Perez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PLOS ONE 10.1371/journal.pone.0160046 August4,2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelick PB, Weisman SM. Risk of Hemorrhagic Stroke With Aspirin Use. An Update. Stroke.2005; 36: 1801–7. 10.1161/01.STR.0000174189.81153.85 [DOI] [PubMed] [Google Scholar]
- 18.Thun M, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev. Clin. Oncol. 2012;9:259–67. 10.1038/nrclinonc.2011.199 [DOI] [PubMed] [Google Scholar]
- 19.Pignone M, Earnshaw S, McDade C, Pletcher MJ. Effect of including cancer mortality on the cost-effectiveness of aspirin for primary prevention in men. J Gen Intern Med May 2013. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015 Jan;26(1):47–57. 10.1093/annonc/mdu225 Epub 2014 Aug 5. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan C, Rex DK, Cooper GS,Zuillo A, Launois R, Benamouzig R. Primary prevention of colortectal cancer with low-dose aspirin in combination with endoscopy: a cost effective analysis. Gut 2012;61:1172–9. 10.1136/gutjnl-2011-300206 [DOI] [PubMed] [Google Scholar]
- 22.Soon SS, Chia WK, Chan ML, HO GF, Jian X, Deng YH et al. Cost-effectiveness of aspirin adjuvant therapy in early stage colorectal cancer in older patients. PLoS One. 2014. September 24;9(9):e107866 10.1371/journal.pone.0107866 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stegman I, Bossuyt PM, Tsung Yu, Boyd C, Puhan MA. Aspirin for primary prevention of cardiovascular disease and cancer. A benefit and harm analysis. PLoS ONE 10(7)e0127194 10.1371/journal.pone.0127194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pignone M. Aspirin for primary prevention of cardiovascular events in people with diabetes. Circulation 2010;121:2694–701. 10.1161/CIR.0b013e3181e3b133 [DOI] [PubMed] [Google Scholar]
- 25.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality and non-vascular death: analysis of the time course of risks and benefits in 51 randomised trials. Lancet. 2012. April 28;379(9826):1602–12. 10.1016/S0140-6736(11)61720-0 Epub 2012 Mar 21. [DOI] [PubMed] [Google Scholar]
- 26.Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clinial Gastroenterol Hepatol 2011;9:762–8. [DOI] [PubMed] [Google Scholar]
- 27.McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomised controlled trials. Amer J Med 2006;119:624–38. 10.1016/j.amjmed.2005.10.039 [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Alotaibi GS, Alsaleh K, Linkins LA, McMurtry MS. Case-fatality of recurrent venous thromboenbolism and major bleeding associated with aspirin, warfarin, and direct oral anticoagulants for secondary prevention. Thromb Res. 2015. February;135(2):243–8. 10.1016/j.thromres.2014.10.033 Epub 2014 Dec 2. [DOI] [PubMed] [Google Scholar]
- 29.Elwood PC, Longley MJ. My Health–Whose Responsibility–a jury decides. J Epidemiol Comm Hlth. 2010;64:761–4. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Collaboration. Available: http://ohg.cochrane.org/sites/ohg.cochrane.org/files/uploads/Risk%20of%20bias%20assessment%20tool.pdf.
- 31.Egger M, Davey Smith G, Altman DG. Systematic Reviews in Health Care Meta-analysis in context Edited by BMJ Books; 1995 [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 33.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–48. [PubMed] [Google Scholar]
- 34.Peto R, Gray R, Collins R, Wheatley K, Hennekens C, Jamrozik K, et al. Randomised trial of prophylactic daily aspirin in Bristish male doctors. Br Med J (Clin Res Ed). 1988. January 30;296(6618):313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steering Committee of the Physicians Health Study Research Group. Final report on the aspirin component of the ongoing physicians’ health study. NEJM 1989;321:129–35 10.1056/NEJM198907203210301 [DOI] [PubMed] [Google Scholar]
- 36.The SALT collaborative Gropup. Swedish aspirin low-dose trial (SALT) of 75mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet 1991;338:1345–49. [PubMed] [Google Scholar]
- 37.International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both or neither among 19435 patients with acute ischaemic stroke, International Stroke Trial Collaboratifve Group. Lancet 1997;349:1569–81 [PubMed] [Google Scholar]
- 38.ANON Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998;351:233–41. [PubMed] [Google Scholar]
- 39.Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, Ménard J, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) trial. HOT study group. Lancet. 1998. June 13;351(9118):1755–62. [DOI] [PubMed] [Google Scholar]
- 40.Collaborative Group of the Primary Prevent Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet 2001;357:89–95 [DOI] [PubMed] [Google Scholar]
- 41.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomised trial of Aspirin to prevent colorectal adenomas. N Engl J Med 2003; 348:891–899 March 6, 2003 10.1056/NEJMoa021735 [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomised trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005. March 31;352(13):1293–304. Epub 2005 Mar 7. 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 43.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. Brit Med J 2008;337:1030–34. 2008;337:a1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012. November 22;367(21):1979–87. 10.1056/NEJMoa1210384 Epub 2012 Nov 4. [DOI] [PubMed] [Google Scholar]
- 45.Abajo FJ, Rodriguez LAG. Risk of upper gastrointestinal bleeding and perforation associated with low-dose aspirin as plain and enteric-coated formulations. BMC Clinical Pharmacology 2001;1:1 10.1186/1472-6904-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanas A, Perez-Aisa MA, Feu F, Ponce J, Saperas E, Santolaria S, et al. A nationwide study of mortality associated with hospital admissions due to severe gastrointestinal events and those associated with non-steroidal anti-inflammatory drug use. Am J Gastroenterol. 2005. August;100(8):1685–93. 10.1111/j.1572-0241.2005.41833.x [DOI] [PubMed] [Google Scholar]
- 47.Roussomoustakaki M, Potamianos S, Koutroubakis I, Matrella E, Manousos O, Kouroumalis E. Low mortality and morbidity of upper gastrointestinal bleeding in Crete. The role of individual non-steroidal anti-inflammatory drugs (NSAIDs). Ann Gastroenterol 2000;13:225–232. [Google Scholar]
- 48.Mose H, Larsen M, Riis A. Thirty-day mortality after peptic ulcer bleeding in hospitalised patients receiving low-dose aspirin at time of admission. Amer J Geriatr Pharmacol 2006;4:244–50. [DOI] [PubMed] [Google Scholar]
- 49.Ashberg K, Hoglund P, Kim WH, von Holstein CS. Impact of Aspirin, NSAIDs, warfarin, corticosteroids and SSRIs on the site and outcome of non-variceal upper and lower gastrointestinal bleeding. Scand J Gastroenterol. 2010;45:1404–15. 10.3109/00365521.2010.510567 [DOI] [PubMed] [Google Scholar]
- 50.Wehbeh A, Tamim HM, Abu Daya H, Abou Mrad R, Badreddine RJ, Eloubeidi MA et al. Aspirin has a protective effect against adverse outcomes in patients with nonvarical upper gastrointestinal bleeding. Dig Dis Sci 2015 July 2015;60(7)2077–2087 First online: 03 March 2015 10.1007/s10620-015-3604-1 [DOI] [PubMed] [Google Scholar]
- 51.Abu Daya H, Eloubeidi M, Tamim H, Halawi H, Malli AH, Rockey DC et al. Opposing effects of aspirin and anticoagulants on morbidity and mortality in patients with upper gastrointestinal bleeding. J Dig Dis. 2014. June;15(6):283–92. 10.1111/1751-2980.12140 [DOI] [PubMed] [Google Scholar]
- 52.Kaviani MJ, Pirastehfar M, Azari A, Saberifiroozi M. Etiology and outcome of patients with upper gastrointestinal bleeding: a study from South of Iran. Saudi J Gastroenterol. 2010. Oct-Dec;16(4):253–9. 10.4103/1319-3767.70608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sung JJ, Tsoi KK, Ma TK, Yung MY, Lau JY, Chiu PW. Causes of mortality in patients with peptic ulcer bleeding: a prospective study of 10,428 cases. Am J Gastroenterol. 2010. January;105(1):84–9. 10.1038/ajg.2009.507 Epub 2009 Sep 15 [DOI] [PubMed] [Google Scholar]
- 54.Hreinsson JP, Gumundsson S, Kalaitzakis E, Bjorn ES. Lower gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Eur J Gastroenterol Heaptol 2013;25:37–43. [DOI] [PubMed] [Google Scholar]
- 55.Hreinsson JP, Palsdottir S, Bjornsson ES. The association of drugs with severity and specific causes of acute lower gastrointestinal bleeding: a prospective study. J Clin Gawstroenterol 2015; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 56.Peura DA, Lanza FL, Gostout CJ, Foutch PG. The Americal Collegeo of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997. June;92(6):924–8. [PubMed] [Google Scholar]
- 57.Liberman D: Progress and challenges in colorectal screening and surveillance. Gastroenterology 2010;138:2115–26. 10.1053/j.gastro.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 58.Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009. July;104(7):1633–41. 10.1038/ajg.2009.164 Epub 2009 May 5. [DOI] [PubMed] [Google Scholar]
- 59.Ahsberg K, Hoglund P, von Holstein CS. Mortality from peptic ulcer bleeding: the impact of comorbidity and the use of drugs that promote bleeding. Aliment Pharmacol Ther 2010;32:801–10. 10.1111/j.1365-2036.2010.04399.x [DOI] [PubMed] [Google Scholar]
- 60.Narum S, Solhaug V, Myhr K, Brørs O, Kringen MK. Characterization of non-warfarin-associated bleeding events reported to the Norwegian spontaneous reporting system. Eur J Clin Pharmacol. 2013. Jul;69(7):1445–52. 10.1007/s00228-013-1479-7 Epub 2013 Feb 20. [DOI] [PubMed] [Google Scholar]
- 61.Ivanusa M, Ivanusa Z. Risk factors and hospital outcomes in stroke and myocardial infarction. BMC Public Health. 2004;5:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzales-Perez A, Gaist D, Wallander MA, McFeat G, García-Rodríguez LA l. Mortality after haemorrhagic stroke: data from general practice (The Health Improvement Network). Neurology. 2013. August 6;81(6):559–65. 10.1212/WNL.0b013e31829e6eff Epub 2013 Jul 10. [DOI] [PubMed] [Google Scholar]
- 63.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010. November 20;376(9754):1741–50. 10.1016/S0140-6736(10)61543-7 [DOI] [PubMed] [Google Scholar]
- 64.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC et al. : Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995. September 7;333(10):609–14. 10.1056/NEJM199509073331001 [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez LAG, Hermandez-Diaz S, de Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol 2001;52:563–71. 10.1046/j.0306-5251.2001.01476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, et al. Predictors of bleeding and time dependence of association of bleeding with mortality. Circulation. 2011;14;123(23):2681–9. 10.1161/CIRCULATIONAHA.110.002683 [DOI] [PubMed] [Google Scholar]
- 67.Kang JY, Elders A, Majeed A, Maxwell JD, Bardhan KD. Recent trends in hospital admissions and mortality rates for peptic ulcer in Scotland 1982–2002. Aliment Pharmacol Ther. 2006. July 1;24(1):65–79. 10.1111/j.1365-2036.2006.02960.x [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y, Encinosa W. Hospitalizations for Gastrointestinal Bleeding in 1998 and 2006 Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality; Rockville, MD: Available: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb44.pdf. [PubMed] [Google Scholar]
- 69.Button LA, Roberts SE, Evans PA, Goldacre MJ, Akbari A, Dsilva R et al. Hospitalized incidence and case fatality for upper gastrointestinal bleeding from 1999 to 2007: a record linkage study. Aliment Pharmacol Ther. 2011. January;33(1):64–76. 10.1111/j.1365-2036.2010.04495.x [DOI] [PubMed] [Google Scholar]
- 70.Kassirer JP. Incorporating patients’ preferences into medical decisions. New Eng J Med 1994;330:1895–6. 10.1056/NEJM199406303302611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The middle vertical axis indicates the overall RR and the two vertical axes indicate its 95% CI. Every hollow circle indicates the pooled RR when the left study was omitted in this meta-analysis. The two ends of every broken line represent the 95% CIs.
(TIF)
(TIF)
The middle vertical axis indicates the overall RR and the two vertical axes indicate its 95% CI. Every hollow circle indicates the pooled RR when the left study was omitted in this meta-analysis. The two ends of every broken line represent the 95% CIs.
(TIF)
(TIF)
(DOCX)
[28]
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.