Abstract
Objectives
To characterize patients who received tracheostomies for airway compromise or were initiated on long-term ventilation for chronic respiratory failure in pediatric intensive care units (PICU), and to examine variation in the incidence of initiation, patient characteristics, and modalities across sites.
Design
Retrospective cross-sectional analysis.
Settings
Seventy three North American PICUs that participated in the Virtual Pediatric Intensive Care Unit Performance System.
Patients
PICU patients admitted between 2009 and 2011.
Interventions
None.
Measurements and Main Results
Among 115,437 PICU patients, 1.8% received a tracheostomy or were initiated on long-term ventilation; 1034 received a tracheostomy only, 717 were initiated on invasive ventilation (IV), and 381 were initiated on noninvasive ventilation (NIV). Ninety percent had substantial chronic conditions and comorbidities, including more than 50% with moderate or worse cerebral disability upon discharge. Seven percent were initiated after a catastrophic injury/event. Across sites, there was variation in incidence of tracheotomy and initiation of long-term ventilation, ranging 0–4.6%. There also was variation in patient characteristics, time to tracheotomy, number of extubations prior to tracheostomy, and the use of IV versus NIV.
Conclusions
While the PICU incidence of initiation of tracheostomies and long-term ventilation was relatively uncommon, it suggests that thousands of children and young adults receive these interventions each year in North American PICUs. The majority of them have conditions and comorbidities that impose on-going care needs, beyond those required by artificial airways and long-term ventilation themselves.
Keywords: Tracheostomy, Respiratory Insufficiency, Chronic Disease, Intensive Care Units, Pediatric
Introduction
Technology-dependent children require a device to compensate for the loss of a vital body function and daily skilled care to avert further disability, hospitalization, and death (1). Tracheostomies for airway patency and invasive ventilation (IV) and noninvasive ventilation (NIV) for chronic respiratory failure (CRF) may be the most demanding and precarious of dependencies. In many instances, these interventions improve and prolong the child’s life. However, these dependencies and their associated care needs can also impact their families and the healthcare system (2–5). Determining which children are appropriate candidates for tracheostomies or long-term ventilation (LTV) can sometimes be controversial, especially when the children have profound disabilities and/or life-limiting conditions (6–7). Because children who require these interventions are often survivors of critical illness, they are often initiated in intensive care settings, such as pediatric intensive care units (PICU).
Most studies of children receiving tracheostomies or initiated on LTV have been from a single-institution (8–10) or focused on patients receiving tracheostomies (11–14). Previous studies often group patients that received tracheostomies together without differentiating those supported by IV or patients receiving LTV without differentiating those using IV and NIV. They also group these complex patients into overly broad diagnostic categories and provide limited detail about their comorbidities and the circumstances around their initiation. Thus, relatively little is known about how frequently these interventions are initiated in patients, about what types of patients are initiated, or about the variation of practice around their initiation. Examining these topics is necessary to better understand practice patterns, be capable of examining trends, and gauge the scale of potential controversial scenarios. Therefore, we conducted a multi-institutional, retrospective cross-sectional analysis of PICU patients who received tracheostomies or were initiated on long-term IV or NIV. We present their demographic and clinical characteristics, including their underlying and comorbid conditions. To explore variation of practice, we describe how the incidence of initiation and severity of disabilities among initiated patients varied across institutions and how different modalities of LTV were used. This information provides a detailed picture of a major subset of these patients and the current state of practice.
Material and Methods
Data Source and Hospitals
We performed a cross-sectional analysis of patients discharged from North American PICUs that participated in the Virtual Pediatric Intensive Care Unit Performance System (VPS, LLC, Los Angeles, CA) (15) between January 2009 and December 2011. Participating institutions submitted demographic and clinical data for every discharge. Many sites voluntarily submitted additional data for every discharge. We included sites that voluntarily reported the use of tracheostomies and noninvasive ventilation. Mechanical ventilation was a mandatory data element. To identify patients with diagnoses associated with CRF, we excluded sites that did not report secondary diagnoses. Institution-specific data on the number of licensed pediatric and PICU beds, the proportion affiliated with medical schools and pediatric critical care fellowship programs, and the quarters of data that were contributed was reported.
Tracheostomies and Initiation of Long-Term Ventilation
Included VPS sites recorded every utilization of tracheostomies, assisted mechanical ventilation (and whether it was via endotracheal tube or tracheostomy), and NIV for every respective patient. Each utilization included start and end dates/times, as well as “Present on Admission” and “Present on Discharge” flags. Criteria used to identify patients initiated on tracheostomy alone, long-term IV, or long-term NIV are listed in Table 1. While there is no universally accepted definition of CRF, we ascribed to the one in Rogers’ Textbook of Pediatric Intensive Care—“The diagnosis of CRF is usually made once repeated attempts to wean from assisted ventilation have failed for at least 1 month in a child without superimposed acute respiratory disease or a patient who has a diagnosis with no prospect of being weaned from the ventilator (such as high spinal cord injury)” (16). To establish if patients met the month of assisted ventilation criterion, length of ventilation prior to PICU discharge was determined using start and end dates/times of assisted ventilation. Patients discharged to home, chronic care facilities, non-pulmonary rehabilitation facilities, or hospice on IV or NIV were considered to be supported by LTV, regardless of their PICU length of ventilation. Presumably, patients were not aggressively weaned from assisted ventilation in these settings. We presumed that patients did not have a superimposed acute respiratory disease because the PICU team determined them to be clinically appropriate for transfer to lower acuity care. To identify diagnoses conferring “no prospect of being weaned from the ventilator,” three co-authors (JDE, TGK, HBP) scrutinized a priori over 2000 VPS diagnosis codes. Seven diagnoses for patients using IV and four for those using NIV were agreed upon as meeting this criterion (Table 1).
Table 1.
Criteria used for identifying patients receiving tracheostomies or initiated on chronic ventilation
| Group | Criteria |
|---|---|
| Tracheostomy alone |
|
| Chronic respiratory failure |
|
| Invasive ventilation |
|
| Noninvasive ventilation |
|
Considered as implying “no prospect of being weaned” for patients using invasive and noninvasive ventilation
Considered as implying “no prospect of being weaned” for patients using invasive ventilation only
Patients and their characteristics
We included all patients regardless of age, as adults are cared for in PICUs (17). We reported characteristics of patients grouped by those who received a tracheostomy alone, those initiated on IV, those initiated on NIV, and all interventions combined. Demographic and baseline characteristics included sex, age, race (selected by data entry personnel at sites), insurance, number of complex chronic conditions (CCC), and baseline disability. Kernel Density plots were made to illustrate the overall distribution pattern of age at PICU admission for patients initiated on technologies. CCCs were defined using Feudtner’s definition (18) and identified among VPS diagnoses codes using a list developed by Edwards et al (19). For functionality/disability status, VPS used Pediatric Overall and Cerebral Performance Categories (POPC and PCPC) (20). Categories range from 1 (normal function) to 6 (brain death). Scores of 2, 3, and 4 indicate mild, moderate, and severe disability, respectively; 5 indicates coma or vegetative state. Admission and discharge characteristics included planned, perioperative, patient origin and disposition, risk of PICU mortality as a proxy for severity of illness at admission, length of PICU stay, and discharge disability. Predicted mortality was estimated using Paediatric Index of Mortality (PIM) 2 (21). We reported the size of the PICUs and presence of a pediatric critical care fellowship program where patients were initiated. To identify patients likely receiving tracheostomies or initiated on LTV due to catastrophic events, we reported the number admitted for head/neck/face/spinal cord injuries, cardiac arrest, cerebrovascular events, and burns.
Next, we identified the proportion of patients with specific chronic conditions. Conditions relevant to our study population were selected based on our experience, with an emphasis on neuromuscular and pulmonary conditions. “Less” relevant conditions were combined into their organ subcategories. As a comparison group, we reported the prevalence of these same conditions among PICU patients who were not initiated/already using a tracheostomy or LTV.
Data are presented as proportions +/− 95% confidence intervals, medians with ranges, or means with standard deviations. When information was available for only a subgroup of the patients, we noted this in the text or tables.
Variation of practice
To examine variation in the incidence of initiation of tracheostomies and LTV across sites, we calculated the median proportion and ranges of patients initiated on each technology. We constructed box plots of the time from PICU admission to tracheotomy and number of planned and unplanned extubations prior to tracheotomy to explore variability across sites. To examine the prevalence and variability of prevalence of static encephalopathy and/or generalized developmental delay in initiated patients across sites, we calculated their median proportion and ranges. More granularly, we assessed the distribution of baseline and discharge POPC and PCPC by sites; only sites that initiated ≥5 patients on a respective device were represented in order to avoid skewing data with sites that initiated very few patients. Next, we examined the utilization of IV and NIV among PICUs by depicting the number of patients initiated on IV versus NIV by site. We repeated this analysis focusing on children <3 years with neuromuscular disease and no craniofacial/airway abnormalities (ie, conditions that would make caregivers favor IV over NIV). Whether to offer LTV and what modality to offer these children are debated (7, 22–24). To help ensure counts could be reasonably compared across sites, these latter two analyses included sites that contributed 10 or more quarters of data. In these analyses, sites were grouped by ICU bed size, as a surrogate marker of more tertiary units that might care for more complex patients.
Because data were deidentified, this study qualified for exemption status by the University of California, San Francisco Committee on Human Research. Stata version 13 (StataCorp LP, College Station, TX) was used for analyses and figures.
Results
Sites and patients
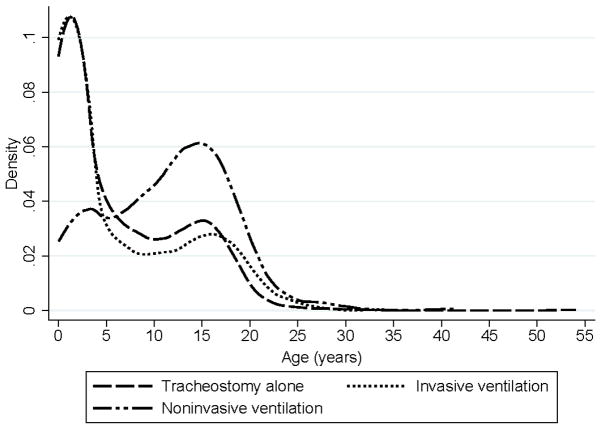
After excluding 66,185 patients (81,910 admissions) from 35 units, 73 PICUs contributed the required data on 115,437 patients (140,927 discharges) for analysis. Institutional characteristics of included PICUs are shown in Supplemental Digital Content Table 1. Of those patients, 1034 (0.9%) received a tracheostomy alone, 717 were initiated on IV (0.6%; 168 had a tracheostomy already in place, 549 had a tracheostomy placed during the same admission as initiation of IV), and 381 (0.3%) began NIV. Their demographic and baseline clinical characteristics are presented in Table 2. Among all three groups, 68% had ≥2 CCC, and 62% were enrolled in public insurance. There was a bimodal distribution of age (infants/toddlers and adolescents) for all three groups (Figure 1). Those receiving a tracheostomy alone or initiated on IV were generally younger than those initiated on NIV.
Table 2.
Demographic and baseline clinical characteristics of patients receiving tracheostomies or initiated on chronic ventilation
| Characteristic, % (95% CI) | Tracheostomy alone n=1,034 | Invasive ventilation n=717 | Noninvasive ventilation n=381 | Any device n=2,132 |
|---|---|---|---|---|
| Male sex | 59.8 (56.7–62.7) | 55.9 (52.2–59.5) | 60.1 (55.1–64.9) | 58.6 (56.5–60.6) |
| Age, months, median (IQR) | 42 (9–138) | 27 (6–143) | 143 (74–192) | 54 (10–158) |
| Racea | ||||
| Caucasian | 49.8 (46.5–53) | 51.9 (48–55.8) | 60.6 (55.1–65.9) | 52.4 (50.2–54.7) |
| African American | 22.6 (20–25.4) | 16.6 (13.8–19.7) | 16.8 (13.1–21.4) | 19.6 (17.8–21.4) |
| Hispanic | 17.9 (15.5–20.5) | 20.6 (17.6–23.9) | 13.7 (10.3–17.9) | 18.1 (16.4–19.9) |
| Asian/Indian/Pacific Islander | 2.3 (1.5–3.5) | 2.3 (1.3–3.8) | 3.8 (2.2–6.6) | 2.5 (1.9–3.4) |
| Other/mixed | 6.1 (4.8–7.9) | 6.1 (4.5–8.3) | 3.5 (1.9–6.2) | 5.6 (4.6–6.7) |
| Unspecified | 1.3 (0.7–2.3) | 2.6 (1.6–4.2) | 1.6 (0.7–3.8) | 1.8 (1.3–2.5) |
| Number of complex chronic conditions | ||||
| None | 15.1 (13–17.4) | 5.4 (4–7.4) | 5.5 (3.6–8.3) | 10.1 (8.9–11.5) |
| 1 | 26.2 (23.6–29) | 13.2 (11–15.9) | 25.2 (21.1–29.8) | 21.6 (19.9–23.4) |
| 2 | 17.6 (15.4–20) | 19.2 (16.5–22.3) | 21.3 (17.4–25.7) | 18.8 (17.2–20.6) |
| ≥ 3 | 41.1 (38.1–44.1) | 62.1 (58.4–65.6) | 48 (43–53.1) | 49.5 (47.3–51.6) |
| Baseline POPCb | ||||
| No disability | 19.6 (16–23.9) | 16.8 (12.9–21.7) | 4.7 (2.5–8.9) | 15.4 (13.2–18) |
| Mild disability | 21.9 (18.1–26.3) | 15.8 (11.9–20.6) | 8.9 (5.6–14) | 17.1 (14.7–19.7) |
| Moderate disability | 37 (32.3–41.9) | 31.5 (26.3–37.3) | 33.2 (26.8–40.2) | 34.4 (31.3–37.6) |
| Severe disability | 20.9 (17.2–25.2) | 35.8 (30.4–41.7) | 53.2 (46–60.2) | 32.9 (29.8–36.1) |
| Coma/vegetative state | 0.5 (0.1–2) | – | – | 0.2 (0.1–0.9) |
| Baseline PCPCb | ||||
| No disability | 43.6 (38.8–48.6) | 39 (33.5–45) | 28.9 (22.9–35.9) | 38.9 (35.7–42.2) |
| Mild disability | 18.1 (14.6–22.3) | 20.4 (16.1–25.6) | 21.1 (15.8–27.5) | 19.5 (17–22.3) |
| Moderate disability | 19.6 (16–23.9) | 19.7 (15.4–24.8) | 12.6 (8.6–18.2) | 18.1 (15.7–20.8) |
| Severe disability | 18.1 (14.6–22.3) | 20.8 (16.4–26) | 37.4 (30.7–44.5) | 23.2 (20.5–26.2) |
| Coma/vegetative state | 0.5 (0.1–2) | – | – | 0.2 (0.1–0.9) |
| Insurancec | ||||
| Medicaid/Medicare/government | 60.8 (56.7–64.8) | 65.7 (60.6–70.4) | 61.3 (54.9–67.2) | 62.4 (59.6–65.2) |
| Commercial | 33.1 (29.3–37.1) | 30.7 (26.2–35.7) | 36.7 (30.8–43) | 33.1 (30.4–35.9) |
| Self-Pay | 3 (1.9–4.8) | 1.7 (0.7–3.7) | 0.4 (0–2.9) | 20.7 (13.9–30.7) |
| Other | 3 (1.9–4.8) | 1.9 (0.9–4) | 1.7 (0.6–4.4) | 2.4 (1.7–3.5) |
CI, confidence interval; IQR, interquartile range; PCPC, Pediatric Cerebral Performance Categories; POPC, Pediatric Overall Performance Categories
Race data available for 1851 (87%) patients from 61 (82%) sites
Pediatric Performance Categories available for 863 (40%) patients from 28 (38%)
Insurance data available for 1162 (54%) patients from 36 (47%) sites
Figure 1.
Kernel Density plots of age of patients receiving tracheostomies or initiated on chronic invasive or noninvasive ventilation at PICU admission.
Admission and discharge characteristics of initiated patients are presented in Table 3. For all three groups, 72% of patients had unplanned PICU admissions. Eighteen patients in the tracheostomy group (1.7%) and 8 in the IV group (1%) had their tracheostomy placed within the first two days of a planned PICU admission, suggesting their initiation was planned. Fifty-seven patients who received a tracheostomy alone (5.5%) were admitted for head/neck/facial/spinal cord injuries; 19 (1.8%) for cardiac arrest; 16 (1.6%) for cerebrovascular events; and 5 (0.5%) with burns. Thirty-two (4.5%) of those initiated on IV were admitted for head/neck/facial/spinal cord injuries; 8 (1.1%) for cardiac arrest; 13 (1.8%) for cerebrovascular events; and 2 (0.3%) for burns. No patients initiated on NIV were admitted for similar injuries or events. Ten patients in the tracheostomy alone group and 23 in the IV group were previously supported by long-term NIV.
Table 3.
Admission and discharge characteristics of patients receiving tracheostomies or initiated on chronic ventilation
| Characteristic, % (95% CI) | Tracheostomy alone n=1,034 | Invasive ventilation n=717 | Noninvasive ventilation n=381 | Any technology n=2.132 |
|---|---|---|---|---|
| Unplanned admission | 68.7 (65.8–71.4) | 75.6 (72.3–78.6) | 75.9 (71.3–79.9) | 72.2 (70.3–74.1) |
| Peri-operative | 48.6 (45.6–51.7) | 25.5 (22.5–28.9) | 28.6 (24.3–33.4) | 37.3 (35.3–39.4) |
| Origin | ||||
| Emergency department | 28 (25.4–30.9) | 37.2 (33.8–40.8) | 41.2 (36.3–46.2) | 33.5 (31.5–35.5) |
| General ward | 18.7 (16.4–21.2) | 15.8 (13.3–18.6) | 18.1 (14.5–22.3) | 17.6 (16–19.2) |
| OR/PACU/procedure suite | 38 (35.1–41) | 18.4 (15.7–21.4) | 25.2 (21.1–29.8) | 29.2 (27.3–31.1) |
| Intermediate unit | 4.2 (3.2–5.7) | 7.3 (5.6–9.4) | 2.4 (1.2–4.5) | 4.9 (4–5.9) |
| Another ICU | 8.6 (7–10.4) | 16 (13.5–18.9) | 5.2 (3.4–8) | 10.5 (9.2–11.8) |
| Chronic/rehabilitation facility | 0.5 (0.2–1.2) | 2.1 (1.3–3.4) | 0.5 (0.1–2.1) | 1 (0.7–1.6) |
| Pulmonary rehabilitation facility | 0.1 (0–0.7) | 0.1 (0–1) | 0.3 (0–1.9) | 0.1 (0–0.4) |
| Home/outpatient | 1.6 (1–2.6) | 2.5 (1.6–4) | 6.3 (4.2–9.2) | 2.8 (2.2–3.6) |
| Other | 0.3 (0.1–0.9) | 0.6 (0.2–1.5) | 0.8 (0.3–2.4) | 0.5 (0.3–0.9) |
| Admitted from another hospital | 17.9 (15.7–20.4) | 23.3 (20.3–26.5) | 16.5 (13.1–20.6) | 19.4 (17.8–21.2) |
| PIM2 risk of mortality, %, mean (SD) | 6 (13.2) | 8.4 (15) | 5 (8.2) | 6.6 (13.2) |
| Admitted to PICU with | ||||
| ICU beds | ||||
| ≤17 | 17.9 (15.7–20.4) | 21.8 (18.9–24.9) | 17.8 (14.3–22) | 19.2 (17.5–20.9) |
| 18–24 | 46.8 (43.8–49.9) | 47.1 (43.5–50.8) | 59.3 (54.3–64.2) | 49.2 (47.1–51.4) |
| ≥25 | 35.3 (32.4–38.3) | 31.1 (27.8–34.6) | 22.8 (18.9–27.3) | 31.6 (29.7–33.6) |
| Affiliated fellowship program | 66.2 (63.2–69) | 61.6 (58–65.1) | 68.2 (63.4–72.7) | 65 (62.9–67) |
| Disposition | ||||
| General ward | 51 (47.9–54) | 24.3 (21.3–27.5) | 24.5 (21.3–30.1) | 37.4 (35.4–39.5) |
| Intermediate unit | 19.5 (16.7–22.8) | 17 (14.4–20) | 6.6 (4.5–9.5) | 16.4 (14.7–18.5) |
| OR | 0.2 (0–0.8) | 0.6 (0.2–1.5) | – | 0.3 (0.1–0.6) |
| Another ICU | 5 (3.4–7.3) | 5.6 (3.6–8.6) | 4.4 (2.4–7.6) | 5.2 (3.9–6.7) |
| Home | 9.4 (7.7–11.3) | 30.1 (26.9–33.6) | 45.9 (40.9–50.8) | 24.7 (22.9–26.6) |
| Chronic/rehabilitation facility | 7.4 (5.9–9.1) | 17.4 (14.8–20.4) | 12.9 (10.6–15.2) | 9.9 (8.7–11.3) |
| Pulmonary rehabilitation | 0.9 (0.5–1.7) | 3.1 (2–4.6) | 3.4 (2–5.8) | 2.1 (1.5–2.8) |
| Hospice | – | 0.8 (0.4–1.9) | 0.8 (0.3–2.4) | 0.4 (0.2–0.8) |
| Morgue | 6.2 (4.9–7.8) | – | – | – |
| Other | 0.6 (0.3–1.3) | 0.3 (0.1–1.1) | 0.5 (0.1–2.1) | 0.5 (0.3–0.9) |
| Discharged to another hospital | 3 (2.1–4.2) | 3.3 (2.3–4.9) | 1 (0.4–2.8) | 2.8 (2.2–3.6) |
| Discharge POPCa | ||||
| No disability | 3 (1.7–5.4) | 0.7 (0.2–2.8) | 3.2 (1.4–6.9) | 2.2 (1.4–3.4) |
| Mild disability | 16.7 (13.2–20.7) | 8.2 (5.5–12.1) | 7.4 (4.4–12.1) | 11.4 (9.4–13.7) |
| Moderate disability | 42.9 (37.9–48) | 37.3 (31.8–43.1) | 35.8 (29.2–42.9) | 38.2 (35–41.5) |
| Severe disability | 32.8 (28.1–.37.8) | 50.6 (44.5–56.4) | 53.7 (46.5–60.7) | 42 (38.8–45.4) |
| Coma/vegetative state | 4.6 (2.9–7.4) | 3.2 (1.6–5.9) | – | 2.9 (2–4.3) |
| Discharge PCPCa | ||||
| No disability | 25.4 (21.2–30.1) | 21.1 (16.7–26.4) | 28.4 (22.4–35.3) | 23.9 (21.2–26.9) |
| Mild disability | 17.2 (13.7–21.5) | 23.7 (19–29) | 20 (14.9–26.4) | 19.4 (16.9–22.2) |
| Moderate disability | 24.6 (20.4–29.3) | 24.4 (19.7–29.8) | 13.2 (9–18.8) | 21.3 (18.6–24.1) |
| Severe disability | 28.1 (23.8–33) | 27.6 (22.6–33.1) | 38.4 (31.7–45.6) | 29.3 (26.3–32.4) |
| Coma/vegetative state | 4.6 (2.9–7.4) | 3.2 (1.6–5.9) | – | 2.9 (2–4.3) |
| Discharged on CPAPb | – | 6.1 (4.6–8.2) | 15.7 (12.4–19.8) | – |
| Length of PICU stay, median days (IQR) | 18 (8–31) | 33 (16–57) | 20 (15–32) | 25 (14–46) |
CI, confidence interval; CPAP, continuous positive airway pressure; PACU, post-anesthesia care unit; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; MV, mechanical ventilation; OR, operating room; PIM, Paediatric Index of Mortality; PCPC, Pediatric Cerebral Performance Categories; POPC, Pediatric Overall Performance Categories; PPV, positive pressure ventilation; SD, standard deviation
Pediatric Performance Categories available for 863 (40%) patients from 28 (38%), but reported for 837 patients because of 26 tracheostomy alone patients that died prior to discharge.
As opposed to conventional mechanical ventilation for patients on chronic IV or BiPAP for patients on chronic NIV.
Twenty percent of IV patients and 16% of NIV patients were discharged to a chronic care or rehabilitation facility from a PICU. Just <1% of IV and NIV patients were discharged to a hospice facility. Six percent of patients in the tracheostomy group died during their PICU admission; 30% of whom met criteria for CRF. Of the 40% of patients with Pediatric Performance Categories reported, 66% and 83% had moderate or worse overall disability at baseline/admission and upon discharge, respectively. About a quarter had no cerebral disability, whereas 54% had moderate or worse cerebral disability upon discharge. Four percent of patients receiving a tracheostomy alone and 3% of those initiated on IV were discharged in a coma or vegetative state.
Chronic conditions of children receiving a tracheostomy or initiated on LTV are presented in Table 4. Forty percent of patients with tracheostomies alone and 22% of those initiated on IV had a craniofacial/airway abnormality. A quarter of patients initiated on NIV had obstructive apnea. More patients with spinal muscular atrophy and muscular dystrophies were initiated on NIV than IV. Relatively common comorbidities among all three groups included genetic syndromes, cerebral palsy, epilepsy, static encephalopathy/generalized developmental delay, and spine deformities.
Table 4.
Chronic conditions of PICU patients receiving tracheostomies or initiated on chronic ventilation
| Condition, % (95% CI) | Tracheostomy alone n=1034 | Invasive ventilation n=717 | Noninvasive ventilation n=381 | Admissions without tracheostomy or chronic ventilationa n=130,847 |
|---|---|---|---|---|
| Cardiac | ||||
| Congenital heart disease, non-complex | 6.1 (4.8–7.7) | 8.1 (6.3–10.3) | 3.4 (2–5.8) | 5.5 (5.4–5.6) |
| Congenital heart disease, complex | 9.1 (7.5–11) | 13.9 (11.6–16.7) | 7.1 (4.9–10.2) | 10.5 (10.3–10.7) |
| Pulmonary hypertension | 4.4 (3.3–5.9) | 10.7 (8.7–13.2) | 3.7 (2.2–6.1) | 1.8 (1.8–1.9) |
| Other cardiac condition | 1.4 (0.8–2.3) | 4.5 (3.2–6.2) | 3.9 (2.4–6.4) | 1.3 (1.3–1.4) |
| Endocrinologic | 6.6 (5.2–8.3) | 11 (8.9–13.5) | 10.5 (7.8–14) | 8 (7.9–8.2) |
| Immunologic | 2.7 (1.9–3.9) | 3.6 (2.5–5.3) | 4.5 (2.8–7.1) | 1.8 (1.7–1.9) |
| Gastroenterologicb | 4.9 (3.8–6.4) | 7.9 (6.2–10.2) | 14.7 (11.5–18.6) | 3.7 (3.6–3.8) |
| Genetic syndrome | 15.3 (13.2–17.6) | 18.7 (16–21.7) | 15 (11.7–18.9) | 6.7 (6.6–6.9) |
| Hematologic | 2.1 (1.4–3.2) | 2.2 (1.4–3.6) | 2.6 (1.4–4.8) | 2.7 (2.6–2.8) |
| Metabolic | 5 (3.9–6.5) | 6.7 (5.1–8.8) | 10 (7.3–13.4) | 2.7 (2.6–2.8) |
| Neuromusclar | ||||
| CCHS | 0.4 (0.1–1) | 1.1 (0.6–2.2) | 1 (0.4–2.8) | 0 (0–0) |
| Cerebral palsy | 9.9 (8.2–11.8) | 8.4 (6.5–10.6) | 22.6 (18.6–27.1) | 3.9 (3.8–4) |
| Chiari malformation | 0.6 (0.2–1.3) | 1.7 (1–2.9) | 1 (0.4–2.8) | 0.6 (0.5–0.6) |
| Ventricular shunt or other CNS device | 4.6 (3.5–6.1) | 5.3 (3.9–7.2) | 6.8 (4.7–9.8) | 2.7 (2.6–2.8) |
| Epilepsy | 18.4 (16.1–20.9) | 16.3 (13.8–19.2) | 31.2 (26.8–36.1) | 8.9 (8.7–9) |
| Hydrocephalus | 6.6 (5.2–8.2) | 8.2 (6.4–10.5) | 7.6 (5.3–10.8) | 4 (3.9–4.1) |
| Muscular dystrophy | 0.9 (0.5–1.7) | 8.1 (6.3–10.3) | 21.3 (17.4–25.7) | 0.4 (0.4–0.5) |
| Spina bifida | 1.6 (1–2.6) | 4.6 (3.3–6.4) | 5.8 (3.8–8.6) | 1.7 (1.6–1.7) |
| Spinal muscular atrophy type 1 | 0.6 (0.3–1.3) | 5.7 (4.2–7.7) | 13.1 (10.1–16.9) | 0.3 (0.2–0.3) |
| Static encephalopathy or generalized developmental delay | 20.2 (17.9–22.8) | 18.7 (16–21.7) | 22.3 (18.4–26.8) | 7.2 (7–7.3) |
| Other neuromuscular condition | 9.2 (7.6–11.1) | 10.3 (8.3–12.8) | 17.6 (14.1–21.8) | 3.9 (3.8–4) |
| Oncologic | ||||
| CNS tumor | 4.3 (3.2–5.7) | 3.2 (2.1–4.8) | 1.3 (0.5–3.1) | 3.5 (3.4–3.6) |
| Other oncologic conditionsc | 6.5 (5.1–8.2) | 3.3 (2.3–4.9) | 1.8 (0.9–3.8) | 5.9 (5.7–6) |
| Orthopedic | ||||
| Scoliosis/kyphosis | 5.9 (4.6–7.5) | 11.2 (9–13.7) | 33.6 (29–38.5) | 4.5 (4.4–4.6) |
| Otolaryngologic | ||||
| Craniofacial or airway abnormalityd | 39.7 (36.8–42.8) | 22.2 (19.3–25.4) | 9.7 (7.1–13.1) | 4.6 (4.5–4.7) |
| Obstructive sleep apneae | 6.6 (5.2–8.3) | 4.2 (2.9–5.9) | 26.5 (22.3–31.2) | 2.2 (2.1–2.3) |
| Prematurity | 7.5 (6.1–9.3) | 9.3 (7.4–11.7) | 2.4 (1.2–4.5) | 4.2 (4.1–4.3) |
| Pulmonary | ||||
| Asthma | 5.6 (4.4–7.2) | 6.1 (4.6–8.2) | 18.9 (15.3–23.2) | 11.1 (11–11.3) |
| Bronchopulmonary dysplasia | 5 (3.9–6.5) | 9.5 (7.4–11.9) | 1 (0.4–2.8) | 1.2 (1.1–1.3) |
| Cystic fibrosis | 0.3 (0.1–0.9) | 1.3 (0.7–2.4) | 0.8 (0.3–2.4) | 0.5 (0.4–0.5) |
| Diaphragm paralysis | 0.6 (0.3–1.3) | 3.3 (2.3–4.9) | 1.6 (0.7–3.5) | 0.2 (0.2–0.2) |
| Pulmonary hypoplasia | 0.3 (0.1–0.9) | 1.7 (1–2.9) | 1 (0.4–2.8) | 0.2 (0.2–0.3) |
| Other pulmonary conditions | 8.7 (7.1–10.6) | 14.2 (11.9–17) | 22 (18.1–26.5) | 2.6 (2.5–2.7) |
| Renal | 2.1 (1.4–3.2) | 3.1 (2–4.6) | 4.2 (2.6–6.8) | 2 (2–2.1) |
| Rheumatologic | 0.3 (0.1–0.9) | 0.6 (0.2–1.5) | 1.6 (0.7–3.5) | 0.6 (0.5–0.6) |
CCHS, congenital central hypoventilation syndrome; CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; PICU, pediatric intensive care unit Patients may have had more than one condition
Excludes patients who were admitted with a tracheostomy or already on chronic ventilation and/or died during their PICU admission
Does not include patients with feeding intolerance or dependence on feeding tubes
Includes “benign” oncologic conditions
Includes patients with upper and lower airway malacia
Excludes patients with documented anatomical upper airway abnormality
Variation of practice
While the median incidence of initiating tracheostomies and LTV across sites was low, there was variability in the ranges (0–4.6%) of incidences (Table 5). Thirteen sites initiated <1% on any device; 25 sites initiated >2%. Also there was no discernable pattern of initiation comparing PICUs of different sizes or by having affiliated fellowship programs. There was variability among sites regarding the timing of tracheotomy and number of extubations prior to tracheotomy (Supplemental Digital Content Figures 1 and 2).
Table 5.
Incidence of tracheotomy and initiation of chronic ventilation by ICU characteristic
| Technology by ICU bed size | Median (%) | Interquartile range | Range |
|---|---|---|---|
| Tracheostomy alone | |||
| ≤17 beds | 0.6 | 0.4 – 0.9 | 0 – 1.3 |
| 18–24 beds | 0.8 | 0.4 – 1.1 | 0.2 – 2.5 |
| ≥25 beds | 0.8 | 0.6 – 1.2 | 0.1 – 1.7 |
| No PCCM fellowship | 0.7 | 0.5 – 0.9 | 0 – 2.5 |
| Affiliated PCCM fellowship | 0.7 | 0.5 – 1.1 | 0.1 – 2.1 |
| Invasive ventilation | |||
| ≤17 beds | 0.5 | 0.2 – 0.8 | 0 – 2.5 |
| 18–24 beds | 0.6 | 0.5 – 0.9 | 0.2 – 1.7 |
| ≥25 beds | 0.6 | 0.4 – 0.9 | 0.3 – 1.8 |
| No PCCM fellowship | 0.6 | 0.4 – 0.7 | 0 – 1.7 |
| Affiliated PCCM fellowship | 0.6 | 0.4 – 0.9 | 0 – 2.5 |
| Noninvasive ventilation | |||
| ≤17 beds | 0.2 | 0 – 0.4 | 0 – 0.9 |
| 18–24 beds | 0.3 | 0.1 – 0.4 | 0 – 2 |
| ≥25 beds | 0.3 | 0.1 – 0.5 | 0 – 1.7 |
| No PCCM fellowship | 0.2 | 0 – 0.4 | 0 – 0.9 |
| Affiliated PCCM fellowship | 0.2 | 0.1 –0.4 | 0 – 0.2 |
| Any technology | |||
| ≤17 beds | 1.3 | 0.9 – 2.1 | 0 – 3 |
| 18–24 beds | 1.8 | 1.4 – 2.4 | 0.5 – 4.6 |
| ≥25 beds | 1.8 | 1.2 – 2.3 | 0.6 – 3 |
| No PCCM fellowship | 1.5 | 1.1 – 1.8 | 0 – 4.6 |
| Affiliated PCCM fellowship | 1.9 | 1.2 – 2.4 | 0.5 – 3.4 |
Percentiles are of PICU patients (as opposed to admissions)
The prevalence of static encephalopathy/generalized developmental delay among initiated patients varied across sites. The median proportion of tracheostomy patients with these comorbidities was 12.5% (IQR 0–28.6%; range 0–100%); the median proportion among IV patients was 14.3% (IQR 0–29.3%, range 0–100%); and the median proportion among NIV patients was 9.8% (IQR 0–33.3%, range 0–100%). Supplemental Digital Content Figure 3 and 4 shows the prevalence of overall and cerebral disabilities at baseline and upon discharge, respectively, among patients for whom Performance Categories were reported. There was variation between units in the severity of disabilities in their initiated patients.
Supplemental Digital Content Figure 5 depicts the number of patients initiated on long-term IV versus NIV by sites grouped by ICU bed size, as well as the subgroup analysis of patients with early-onset neuromuscular disease. There were differences between sites in the total number of patients initiated on LTV and the number initiated on IV versus NIV. Most units more commonly initiated patients on IV, but a few initiated more on NIV.
Discussion
With improving care of critically/chronically ill children, increasing numbers are living with tracheostomies and on LTV to support airway compromise and CRF, respectively (5, 9, 25–26). Increasingly, pediatric critical care medicine is focusing on morbidity after critical illness as an outcome of interest (27). Our analysis of patients in 73 PICUs who received tracheostomies or were initiated on LTV provides details of how often these interventions occur and to whom in PICUs. Among our cohort, 1.8% of PICU patients received a tracheostomy or was initiated on LTV. Relatively few patients were initiated after catastrophic events. Very few patients were admitted for a planned tracheostomy. Rather, the majority had CCC that presumably led or contributed to their airway compromise or CRF, and most were initiated during unplanned PICU admissions and after acute/acute-on-chronic critical illness. Many chronic conditions that are commonly associated with these CRF (eg, spinal muscular atrophy and muscular dystrophy) were not highly prevalent, suggesting that the conditions that put children at risk for CRF are heterogeneous. The bimodal distribution of age of initiation of LTV is consistent with the pathophysiology of CRF where increased respiratory load or diminished ventilation capacity manifest especially in young childhood and adolescence (16, 28). Certain comorbidities (eg, epilepsy, static encephalopathy, and spine deformities) were more prevalent. Subgroup analysis showed that about a third to a half of initiated patients had severe or worse disability upon discharge, depending on the intervention. Our findings also suggest that there may have been variation in practice around how and in whom these interventions were initiated across PICUs. Collectively, this means thousands of chronically ill children and young adults receive tracheostomies or are initiated on LTV each year in North American PICUs.
Previous studies of children who receive tracheostomies or LTV reported comparable findings. Lewis et al estimated that 4861 tracheotomies were performed in U.S. pediatric patients in 1997 (0.07% of all pediatric admissions) and found that practice varied by region (11). A study of United Kingdom PICUs found a 2% incidence of tracheostomy; institutional incidence varied from 0.13 to 5.66% (12). Wakeham et al. studied tracheostomies in children in PICUs who required mechanical ventilation for ≥3 days (13). They found 6.6% of these patients received a tracheostomy (48% of whom were also discharged on mechanical ventilator support) and significant variation in the use and timing of tracheostomy across units. Berry et al found that 48% of patients who received a tracheostomy at major children’s hospitals had neurological impairment (14). Our study augments previous ones by 1) including children and young adults cared for in larger and smaller PICUs/institutions; 2) differentiating patients who received a tracheostomy alone and those initiated on long-term IV; 3) differentiating patients who were initiated on long-term NIV from those on IV; 4) being the first to apply criteria for CRF, as opposed to including all patients receiving mechanical ventilation for days or discharged on it; 4) providing greater detail on patients’ chronic and comorbid conditions; and 5) providing greater detail on the variation around who and how patients were initiated.
It is important to highlight that, for many patients, these interventions improve and prolong life and are sometimes temporary (eg, patients with isolated upper airway abnormalities, bronchopulmonary dysplasia, and congenital central hypoventilation syndrome). For others, these dependencies are lifelong, do not mitigate the patients’ other conditions, confer their own risks (29–30), and, in some cases, can impose substantial care demands on families and the healthcare system (2–5). As a result, questions of who are appropriate candidates sometimes arise (7, 23, 31–33). Understanding who are initiated is a necessary first step to scrutinize these concerns and the care patients/families receive, as well as to analyze future trends.
This study has several limitations. First, VPS cannot identify patients with airway compromise or CRF for whom these interventions were not chosen or offered. These data are especially needed to further enlighten variation of practice. Second, we could only comment on patients initiated in PICUs. But many neonates receive tracheostomies or undergo initiation of LTV in neonatal ICUs (34–35), and many children using long-term NIV are initiated in non-ICU settings (9, 36). Nevertheless, our study captured a large, important subset of these patients. Third, unless they had previous admissions to reference, patients admitted with a tracheostomy, requiring acute mechanical ventilation, and discharged using long-term IV were considered already supported by long-term IV because we could not be certain they were not using long-term IV on admission. Similarly, patients admitted and discharged using NIV were considered to be already dependent on long-term NIV. Fourth, we could not discern between patients initiated on full- and part-time LTV. Fifth, some rehabilitation facilities may aggressively wean patients off positive pressure ventilation, but in VPS only pulmonary rehabilitation is differentiated from more general rehabilitation and chronic care facilities. Sixth, variation in who was initiated (and how) certainly reflects many PICU and non-PICU factors, including patient factors and institutional practices to transfer patients to other hospitals with pediatric otolaryngological expertise and home mechanical ventilation programs, that could not be studied. Small numbers of patients admitted could make substantive changes in incidence numbers. Variation may also reflect differences in patient populations that presented to different sites. We attempted to address this by picking one major subgroup (children with early-onset neuromuscular disease) and stratifying sites by institutional characteristics (eg, ICU bed size, associated pediatric critical care fellowship). Seventh, discharge POPC may have been biased towards worse disability because patients were discharged dependent on a tracheostomy and/or a ventilator. Eighth, multiple sites and many admissions were excluded because they did not voluntarily report on the use of tracheostomies and noninvasive ventilation. These sites tended to be smaller units that started participating in VPS towards the end of the study period. These exclusions may bias our results towards patients seen in larger PICUs. Finally, because this was not a longitudinal cohort study, the numbers of patients who were transitioned from natural to artificial airway or one ventilation modality to another are likely under represented.
Conclusions
Tracheotomy and initiation of LTV among PICU patients was relatively uncommon, but other PICU outcomes are similarly rare (eg, mortality). Given the increasing attention on morbidity as a PICU outcome (27), these interventions, which can prolong the child’s life but can also substantially impact their families and the healthcare system, are worthy of further study. This study provides multi-institutional descriptive and incidence data on children and young adults receiving tracheostomies or initiated on LTV in PICUs that may be used as a foundation for future examinations of trends of these interventions and deeper examinations of variation of practice.
Supplementary Material
Figure 1. Box plots of time from PICU admission to tracheotomy by PICU sites. A) Tracheostomy alone group. B) Chronic invasive ventilation group.
Figure 2. Box plots of number of planned and unplanned extubations prior to tracheotomy among patients initiated on chronic invasive ventilation.
Figure 3. Bar graphs of the prevalence of baseline disabilities upon PICU admission among patients receiving tracheostomies or initiated on chronic ventilation by PICU sites. Overall disability (ie, POPC) is on the left; cerebral disability (ie, PCPC) is on the right. Sites (ie, bars) are sorted by proportion of initiated patients with no disability for each graph. A) Tracheostomy alone group. Includes 372 patients from 17 sites and only patients discharged alive from the PICU. B) Chronic invasive ventilation group. Includes 260 patients from 17 sites. C) Chronic noninvasive ventilation group. Includes 165 patients from 9 sites.
Figure 4. Bar graphs of the prevalence of disabilities upon PICU discharge among the same patients receiving tracheostomies or initiated on chronic invasive or noninvasive ventilation by PICU sites. Overall disability (ie, POPC) is on the left; cerebral disability (ie, PCPC) is on the right. Sites (ie, bars) are sorted by proportion of initiated patients with no disability for each graph. A) Tracheostomy alone group. B) Chronic invasive ventilation group. C) Chronic noninvasive ventilation group.
Figure 5. Bar graphs of the number of patients initiated on chronic invasive ventilation versus chronic noninvasive ventilation by PICU sites between July 2009 and December 2011. A) All patients. B) Patients who were <3 years with neuromuscular disease and no craniofacial/airway abnormalities.
Supplemental Digital Content Table 1 Hospital and unit characteristics of participating sites
Acknowledgments
Funding/Support: Dr. Edwards was supported by a National Institutes of Health K23 grant (K23 HD 082361) and by the Pediatric Critical Care and Trauma Scientist Development Program (K12 HD 047349).
We thank the Virtual Pediatric Intensive Care Unit Systems for providing the data for this study and especially for the assistance of VPS, LLC members Christine Gall and Casey Lauer. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors have been implied or stated by VPS, LLC. We also thank the Pediatric Critical Care Scientist and Trauma Development Program for the grant support that permitted this work to be started and Dr. Eli Grunstein for his otolaryngological expertise.
Abbreviations
- CCC
complex chronic condition
- CPAP
continuous positive airway pressure
- CRF
chronic respiratory failure
- IQR
interquartile range
- IV
invasive ventilation
- LOS
length of stay
- NIV
noninvasive ventilation
- PCPC
Pediatric Cerebral Performance Category
- PIM
Paediatric Index of Mortality
- PICU
pediatric intensive care unit
- POPC
Pediatric Overall Performance Category
- VPS
Virtual Pediatric Intensive Care Unit Systems
Footnotes
The study was performed at University of California, San Francisco and Columbia University Medical Center.
Conflict of Interest Disclosures: The authors have no potential conflicts of interest to disclose.
References
- 1.U.S. Congress Office of Technology Assessment. OTA-TM-H-38. Washington, DC: U. S. Government Printing Office; 1987. Technology-Dependent Children: Hospital v. Home Care. [Google Scholar]
- 2.Carnevale FA, Alexander E, Davis M, et al. Daily living with distress and enrichment: the moral experience of families with ventilator-assisted children at home. Pediatrics. 2006;117:e48–60. doi: 10.1542/peds.2005-0789. [DOI] [PubMed] [Google Scholar]
- 3.Kuo DZ, Cohen E, Agrawal R, et al. A national profile of caregiver challenges among more medically complex children with special health care needs. Arch Pediatr Adolesc Med. 2011;165:1020–1026. doi: 10.1001/archpediatrics.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noyes J, Godfrey C, Beecham J. Resource use and service costs for ventilator-dependent children and young people in the UK. Health Soc Care Community. 2006;14:508–522. doi: 10.1111/j.1365-2524.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- 5.Benneyworth BD, Gebremariam A, Clark SJ, et al. Inpatient health care utilization for children dependent on long-term mechanical ventilation. Pediatrics. 2011;127:e1533–1541. doi: 10.1542/peds.2010-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilfond BS. Tracheostomies and assisted ventilation in children with profound disabilities: navigating family and professional values. Pediatrics. 2014;133(Suppl 1):S44–49. doi: 10.1542/peds.2013-3608H. [DOI] [PubMed] [Google Scholar]
- 7.Benson RC, Hardy KA, Gildengorin G, Hsia D. International survey of physician recommendation for tracheostomy for Spinal Muscular Atrophy Type I. Pediatr Pulmonol. 2012;47:606–611. doi: 10.1002/ppul.21617. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Heffernan C, Saluja S, et al. Indications, Hospital Course, and Complexity of Patients Undergoing Tracheostomy at a Tertiary Care Pediatric Hospital. Otolaryngol Head Neck Surg. 2014;151:232–239. doi: 10.1177/0194599814531731. [DOI] [PubMed] [Google Scholar]
- 9.Amin R, Sayal P, Syed F, et al. Pediatric long-term home mechanical ventilation: twenty years of follow-up from one Canadian center. Pediatr Pulmonol. 2014;49:816–824. doi: 10.1002/ppul.22868. [DOI] [PubMed] [Google Scholar]
- 10.Chatwin M, Tan HL, Bush A, et al. Long Term Non-Invasive Ventilation in Children: Impact on Survival and Transition to Adult Care. PLoS One. 2015;10:e0125839. doi: 10.1371/journal.pone.0125839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis CW, Carron JD, Perkins JA, et al. Tracheotomy in pediatric patients: a national perspective. Arch Otolaryngol Head Neck Surg. 2003;129:523–529. doi: 10.1001/archotol.129.5.523. [DOI] [PubMed] [Google Scholar]
- 12.Wood D, McShane P, Davis P. Tracheostomy in children admitted to paediatric intensive care. Arch Dis Child. 2012;97:866–869. doi: 10.1136/archdischild-2011-301494. [DOI] [PubMed] [Google Scholar]
- 13.Wakeham MK, Kuhn EM, Lee KJ, et al. Use of tracheostomy in the PICU among patients requiring prolonged mechanical ventilation. Intensive Care Med. 2014;40:863–870. doi: 10.1007/s00134-014-3298-4. [DOI] [PubMed] [Google Scholar]
- 14.Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124:563–572. doi: 10.1542/peds.2008-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wetzel RC, Sachedeva R, Rice TB. Are all ICUs the same? Paediatr Anaesth. 2011;21:787–793. doi: 10.1111/j.1460-9592.2011.03595.x. [DOI] [PubMed] [Google Scholar]
- 16.Keens TG, Kun SS, Ward SLD. Chronic respiratory failure. In: Nichols DG, editor. Rogers’ Textbook of Pediatric Intensive Care. 4. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 753–766. [Google Scholar]
- 17.Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Multi-institutional profile of adults admitted to pediatric intensive care units. JAMA Pediatr. 2013;167:436–443. doi: 10.1001/jamapediatrics.2013.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: A population-based study of Washington State, 1980–1997. Pediatrics. 2000;106:205–209. [PubMed] [Google Scholar]
- 19.Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med. 2012;40:2196–2203. doi: 10.1097/CCM.0b013e31824e68cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 21.Slater A, Shann F, Pearson G. A revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 22.Rul B, Carnevale F, Estournet B, et al. Tracheotomy and children with spinal muscular atrophy type 1: ethical considerations in the French context. Nurs Ethics. 2012;19:408–418. doi: 10.1177/0969733011429014. [DOI] [PubMed] [Google Scholar]
- 23.Ryan MM. The use of invasive ventilation is appropriate in children with genetically proven spinal muscular atrophy type 1: the motion against. Paediatr Respir Rev. 2008;9:51–54. doi: 10.1016/j.prrv.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Bach JR. The use of mechanical ventilation is appropriate in children with genetically proven spinal muscular atrophy type 1: the motion for. Paediatr Respir Rev. 2008;9:45–50. doi: 10.1016/j.prrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Gowans M, Keenan HT, Bratton SL. The population prevalence of children receiving invasive home ventilation in Utah. Pediatr Pulmonol. 2007;42:231–236. doi: 10.1002/ppul.20558. [DOI] [PubMed] [Google Scholar]
- 26.Graham RJ, Fleegler EW, Robinson WM. Chronic ventilator need in the community: a 2005 pediatric census of Massachusetts. Pediatrics. 2007;119:e1280–1287. doi: 10.1542/peds.2006-2471. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez TE. Are we exchanging morbidity for mortality in pediatric intensive care? Pediatr Crit Care Med. 2014;15:898–899. doi: 10.1097/PCC.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 28.Mallory GB, Jr, Stillwell PC. The ventilator-dependent child: issues in diagnosis and management. Arch Phys Med Rehabi. 1991;72:43–55. [PubMed] [Google Scholar]
- 29.Edwards JD, Kun SS, Keens TG. Outcomes and causes of death in children on home mechanical ventilation via tracheostomy: an institutional and literature review. J Pediatr. 2010;157:955–959. doi: 10.1016/j.jpeds.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Boroughs D, Dougherty JA. Decreasing accidental mortality of ventilator-dependent children at home: a call to action. Home Healthc Nurse. 2012;30:103–111. doi: 10.1097/NHH.0b013e3182429243. [DOI] [PubMed] [Google Scholar]
- 31.Glass KC, Carnevale FA. Decisional challenges for children requiring assisted ventilation at home. HEC Forum. 2006;18:207–221. doi: 10.1007/s10730-006-9008-z. [DOI] [PubMed] [Google Scholar]
- 32.Perkin RM, Orr R, Ashwal S, et al. Long-term ventilation in children with severe central nervous system impairment. Semin Neurol. 1997;17:239–248. doi: 10.1055/s-2008-1040935. [DOI] [PubMed] [Google Scholar]
- 33.van Gestel JP, Robroch AH, Bollen CW, et al. Mechanical ventilation for respiratory failure in children with severe neurological impairment: is it futile medical treatment? Dev Med Child Neurol. 2010;52:483–488. doi: 10.1111/j.1469-8749.2009.03582.x. [DOI] [PubMed] [Google Scholar]
- 34.Overman AE, Liu M, Kurachek SC, et al. Tracheostomy for Infants Requiring Prolonged Mechanical Ventilation: 10 Years' Experience. Pediatrics. 2013;131:e1491–1496. doi: 10.1542/peds.2012-1943. [DOI] [PubMed] [Google Scholar]
- 35.Murthy K, Savani RC, Lagatta JM, et al. Predicting death or tracheostomy placement in infants with severe bronchopulmonary dysplasia. J Perinatol. 2014;34:543–548. doi: 10.1038/jp.2014.35. [DOI] [PubMed] [Google Scholar]
- 36.Lemoine TJ, Swoboda KJ, Bratton SL, et al. Spinal muscular atrophy type 1: are proactive respiratory interventions associated with longer survival? Pediatr Crit Care Med. 2012;13:e161–165. doi: 10.1097/PCC.0b013e3182388ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Box plots of time from PICU admission to tracheotomy by PICU sites. A) Tracheostomy alone group. B) Chronic invasive ventilation group.
Figure 2. Box plots of number of planned and unplanned extubations prior to tracheotomy among patients initiated on chronic invasive ventilation.
Figure 3. Bar graphs of the prevalence of baseline disabilities upon PICU admission among patients receiving tracheostomies or initiated on chronic ventilation by PICU sites. Overall disability (ie, POPC) is on the left; cerebral disability (ie, PCPC) is on the right. Sites (ie, bars) are sorted by proportion of initiated patients with no disability for each graph. A) Tracheostomy alone group. Includes 372 patients from 17 sites and only patients discharged alive from the PICU. B) Chronic invasive ventilation group. Includes 260 patients from 17 sites. C) Chronic noninvasive ventilation group. Includes 165 patients from 9 sites.
Figure 4. Bar graphs of the prevalence of disabilities upon PICU discharge among the same patients receiving tracheostomies or initiated on chronic invasive or noninvasive ventilation by PICU sites. Overall disability (ie, POPC) is on the left; cerebral disability (ie, PCPC) is on the right. Sites (ie, bars) are sorted by proportion of initiated patients with no disability for each graph. A) Tracheostomy alone group. B) Chronic invasive ventilation group. C) Chronic noninvasive ventilation group.
Figure 5. Bar graphs of the number of patients initiated on chronic invasive ventilation versus chronic noninvasive ventilation by PICU sites between July 2009 and December 2011. A) All patients. B) Patients who were <3 years with neuromuscular disease and no craniofacial/airway abnormalities.
Supplemental Digital Content Table 1 Hospital and unit characteristics of participating sites