Abstract
Objective
Thrombomodulin (TM) is highly expressed on the lumenal surface of vascular endothelial cells (ECs) and possesses potent anticoagulant, anti-fibrinolytic, and anti-inflammatory activities in the vessel wall. However, the regulation of TM expression in ECs remains largely unknown.
Approaches and Results
In the present study, we characterized nuclear receptor 4A (NR4A) family as a novel regulator of TM expression in vascular ECs. We demonstrated that both NR4A receptor Nur77 and Nor1 robustly increase TM mRNA and protein levels in human vascular ECs and in mouse liver tissues after adenovirus-mediated transduction of Nur77 and Nor1 cDNAs. Moreover, Nur77 deficiency and knockdown of Nur77 and Nor1 expression markedly attenuated the basal and VEGF165-stimulated TM expression. Mechanistically, we found that Nur77 and Nor1 increase TM expression by acting through two different mechanisms. We show that Nur77 barely affects TM promoter activity, but significantly increases TM mRNA stability, while Nor1 enhances TM expression mainly through induction of Kruppel-like factor 2 and 4 in vascular ECs. Further, we demonstrate that both Nur77 and Nor1 significantly increase protein C activity and inhibit tumor necrosis factor alpha (TNF-α)-induced prothrombotic effects in human ECs. Deficiency of Nur77 increases susceptibility to arterial thrombosis, while enhanced expression of Nur77 and Nor1 protects mice from arterial thrombus formation.
Conclusions
Our results identified NR4A receptors as novel regulators of TM expression and function in vascular ECs and provided a proof-of-concept demonstration that targeted increasing expression of Nur77 and Nor1 in the vascular endothelium might represent a novel therapeutic approach for the treatment of thrombotic disorders.
Keywords: Orphan Nuclear Receptor 4A family, Thrombomodulin, TNF-α, Thrombosis, Endothelial cells
Introduction
The vascular endothelium plays pivotal roles in maintaining the health and homeostatic states of the mammalian circulatory system via the complementary actions of endothelial cell-derived vasoactive factors1. Dysregulation of these vasoactive factors has serious clinical outcomes ranging from endothelial dysfunction to atherosclerosis, stroke, and thrombosis2. One of these factors is namely thrombomodulin (TM), which is highly expressed on the surface of all vascular endothelial cells and mediates the anticoagulant and anti-inflammatory pathways in vascular endothelium3. For instance, TM has been shown to bind to thrombin to potentiate the protein C (PC) activation cascade to generate activated protein C (APC), which in turn exerts potent anticoagulant effects through the proteolytic inactivation of factor Va and factor VIIIa4. In addition, through binding to thrombin, TM has been shown to inhibit inflammation through reducing circulating levels of thrombin as well as switching its substrate specificity toward of thrombin activatable fibrinolysis inhibitor (TAFI) to yield TAFIa, hence exerting anti-inflammatory and antifibrinolytic activities5,6,7. Recently, much attention has been devoted to the therapeutic values of recombinant TM in various human diseases. Experimental studies demonstrate that recombinant soluble TM (sTM) is effective in attenuating neointimal hyperplasia following balloon injury and inflammatory disorders, such as heat stroke and radiation toxicity8,9. Moreover, recent clinical trials also demonstrate the potential therapeutic benefits of TM in the treatment of DIC10,11 and transplantation-associated sepsis12. Therefore, understanding the molecular mechanisms underlying the regulation of TM expression and function in vasculature is of great scientific and therapeutic interests.
The nuclear orphan NR4A subfamily includes three members, namely Nur77 (also called NR4A1), Nurr1(NR4A2) and Nor1 (NR4A3)13. These receptors have no known ligands and therefore are sometimes referred to as orphan nuclear receptors. As immediate-response genes, the NR4A receptors play essential roles in a variety of critical biological processes, including proliferation, differentiation, cell survival, inflammation, glucose and lipid metabolism, and vascular remodeling14. Recently, much attention has been paid to the functional role of NR4A receptor in cardiovascular system. In vascular endothelial cells, expression of NR4A receptor Nur77 and Nor1 are increased by hypoxia, TNF-α, and vascular endothelial growth factor (VEGF) to modulate EC growth, angiogenesis, and inflammation15,16. In vascular smooth muscle cells, expression of NR4A receptor Nur77 and Nor1 are regulated by multiple mitogens, including PDGF, serum and ox-LDL, thus implicating the functional significance of these receptors in proliferative vascular disorders17,18. In contrast to the functional role of Nur77 and Nor1 in vascular biology, Nurr1 has been shown to play critical roles in the survival of the dopamine neurons and development of schizophrenia19,20.
Although NR4A receptors are critical in EC proliferation, inflammation and angiogenesis, their importance in regulating endothelial thrombotic functions has yet to be explored. In the present study, we identified both NR4A receptor Nur77 and Nor1 as potent positive regulators of TM expression in vascular ECs and demonstrated that overexpression of NR4A receptors substantially attenuates TNF-α-induced downregulation of TM expression in vitro and inhibits arterial thrombus formation in vivo.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
TM expression is increased by Nur77 and Nor1
Since both NR4A receptor Nur77 and Nor1 have been shown to play important roles in regulating endothelial functions, such as angiogenesis, inflammation and vascular barrier function17, we hypothesized that these receptors may regulate endothelial function through affecting the expression of TM, which is a key regulator implicated in EC homeostasis4. To test this hypothesis, human umbilical vein endothelial cells (HUVECs) were transduced with either Ad-Nur77 or Ad-Nor1 at indicated multiplicity of infection (MOI). 48 hr after adenovirus transduction, the expression of TM was determined by both quantitative real-time PCR (qRT-PCR) and western blot. As shown Figure 1A–D, in a dose dependent manner, transduction of Ad-Nur77 or Ad-Nor1 substantially increased TM expression at both mRNA and protein levels. Furthermore, we found that Nu77 overexpression barely affected the Nor1 expression, and vice-versa (data not shown). Together, these data suggest that NR4A receptor Nur77 and Nor1 are potent regulators of TM expression in vascular ECs.
Figure 1.
NR4A receptors Nur77 and Nor1 increases thrombomodulin mRNA and protein expression in HUVECs. A, HUVECs were transduced with Nur77 adenovirus (Ad-Nur77) or LacZ adenovirus (Ad-LacZ) with a total MOI at 100. 48 hours after transduction, expression of TM was determined by qRT-PCR (n=3). *P<0.05 vs MOI at 0. B, HUVECs were infected with Ad-Nur77, Ad-LacZ or both with a total MOI at 100. 48 hours after transduction, the expression of TM was determined by western blot (n=3). *P<0.05 vs Ad-Nur77 at MOI=0. #P<0.05 vs Ad-Nur77 at MOI=0. C, HUVECs were transduced with Nor1 adenovirus (Ad-Nor1), Ad-LacZ or both with a total MOI at 100. 48 hours after transduction, expression of TM was determined by qRT-PCR (n=3). *P<0.05 vs MOI at 0. D, HUVECs were infected with Ad-Nor1, Ad-LacZ or both with a total MOI at 100. 48 hours after transduction, the expression of TM was determined by western blot (n=3). *P<0.05 vs Ad-Nor1 at MOI=0. #P<0.05 vs Ad-Nor1 at MOI=0.
NR4A receptors Nur77 and Nor1 increase TM expression through distinct mechanisms
NR4A receptors have been shown to function as transcriptional factors or co-factors to regulate gene expression14. To determine the molecular mechanism by which Nur77 and Nor1 induce TM expression, we first attempted to determine whether Nur77 and Nor1 affect TM expression at transcriptional levels. To this end, we constructed TM promoter luciferase reporter gene. As shown in Figure 2A–B, Nur77 overexpression barely affected TM promoter activity, but significantly prolonged the TM mRNA half-life (from 2.9 ± 0.6 hr to 5.1 ± 0.8 hr, P< 0.05, n=3), as determined by qRT-PCR in HUVECs. Similar results were obtained in mouse lung microvessel endothelial cells (Figure I in the online-only Data Supplement). To further corroborate the TM-promoter driven luciferase assays, we transduced ECs with adenovirus bearing dominant negative mutant (DN-Nur77), which lacks the transcriptional activation domain of Nur7721. As shown Figure 2C, overexpression of DN-Nur77 also markedly increased TM expression to the extent similar to wild-type Nur77 in HUVECs, further indicating that Nur77 regulates TM expression occurred at the post-transcriptional levels. In contrast to Nur77, overexpression of Nor1 significantly increased TM promoter activity (Figure 3A), but barely affected TM mRNA half-life (Figure 3B). Computer based sequential analysis of TM promoter (Figure II in the online-only Data Supplement) did not reveal any conservative Nor1 binding sites located in the human TM promoter region, suggesting that Nor1 may regulate TM promoter activity indirectly through acting on other transcriptional factors. Since KLFs are critically involved in TM expression at transcriptional levels22,23,24, we attempted to determine whether Nor1 affects expression of KLFs in ECs. As shown in Figure 3C, in a dose dependent manner, overexpression of Nor1 significantly increased KLF2 and KLF4, but not KLF6 expression in HUVECs, as determined by qRT-PCR. Furthermore, siRNA-mediated knockdown of KLF2, KLF4 or both markedly attenuated Nor1-induced TM expression in ECs (Figure 3D), further suggesting that Nor1 increases TM expression through inducing KLF2 and KLF4 transcriptional activities in vascular ECs.
Figure 2.
Nur77 increases Thrombomodulin mRNA stability. A. The effect of Nur77 on the TM promoter activity. EA.Hy926 cells were transduced with Ad-Nur77 at indicated MOIs, and 24 hours after transduction of adenovirus, cells were transfected with TM promoter-Luc reporter plasmid. 24 hours after transfection, cells were harvested to detect the relative TM promoter luciferase activity level (n=5). B. Nur77 prolongs TM mRNA stability. HUVECs were transduced with Ad-Nur77 at MOI=50. 48 hours after transduction, cells were treated with actinomycin D for 0 h, 0.5 h,1 h,2 h,4 h and 6 h. Cells were harvested for the extraction of total RNAs and qRT-PCR was performed to determine the TM mRNA half-life. The data are representative of 3 independent experiments. C. DN-Nur77 increased TM protein levels in a dose dependent manner. HUVECs were transduced with dominant negative Nur77 (DN) adenovirus (Ad-DN-Nur77) at indicated MOIs. 48 hours after transduction, cells were harvested for the determination of TM expression by Western blot. Relative expression of TM was determined by densitometric analysis. *P<0.01 vs Ad-DN-Nur77 at 0 MOI. The data are representative of 3 independent experiments.
Figure 3.
Nor1 upregulates augments thromobodulin promoter activity through Kruppel-like transcription factors (KLFs). A. Nor1 increases TM promoter activity. EA.hy926 cells were infected with Ad-Nor1 at indicated MOIs, and 24 hours after transduction of the adenovirus, cells were transfected with TM promoter-Luc reporter plasmid. 24 hours after transfection, cells were harvested to detect luciferase activity (n=5). *P<0.05 vs MOI at 0. B. Effect of Nor1 on TM mRNA stability. HUVECs were transduced with Ad-Nor1 at MOI=50. 48 hours after virus transduction, cells were treated with actinomycin D. Cells were harvested at 0 h, 0.5 h,1 h,2 h,4 h and 6 h after actinomycin D treatment for the extraction of total RNAs and qRT-PCR then was performed to determine the TM mRNA half-life (n=3). C. Nor1 increases expression of KLF2 and KLF4 in HUVECs. HUVECs were transduced with Ad-Nor1 at indicated MOIs. 48 hours after virus transduction, cells were harvested for the detection of KLF expression by qRT-PCR (n=3). *P<0.05 vs MOI at 0. D, Knockdown of KLF2 and KLF4 attenuates Nor1-induced TM expression. HUVECs were transfected with control siRNA (siCTL), KLF2 siRNA (siKLF2), KLF4 siRNA (siKLF4) and siRNAs for KLF2 and KLF4 (siKLF2/KLF4). 24 hours after transfection, cells were transduced with Ad-LacZ or Ad-Nor1 (MOI=50). 48 hours after virus transduction, the expression of TM was determined by qRT-PCR (n=5). *P<0.05 vs siCTL/Ad-LacZ; #P<0.05 vs siCTL/Ad-Nor1.
NR4A receptors Nur77 and Nor1 are involved in VEGF-induced TM expression in ECs
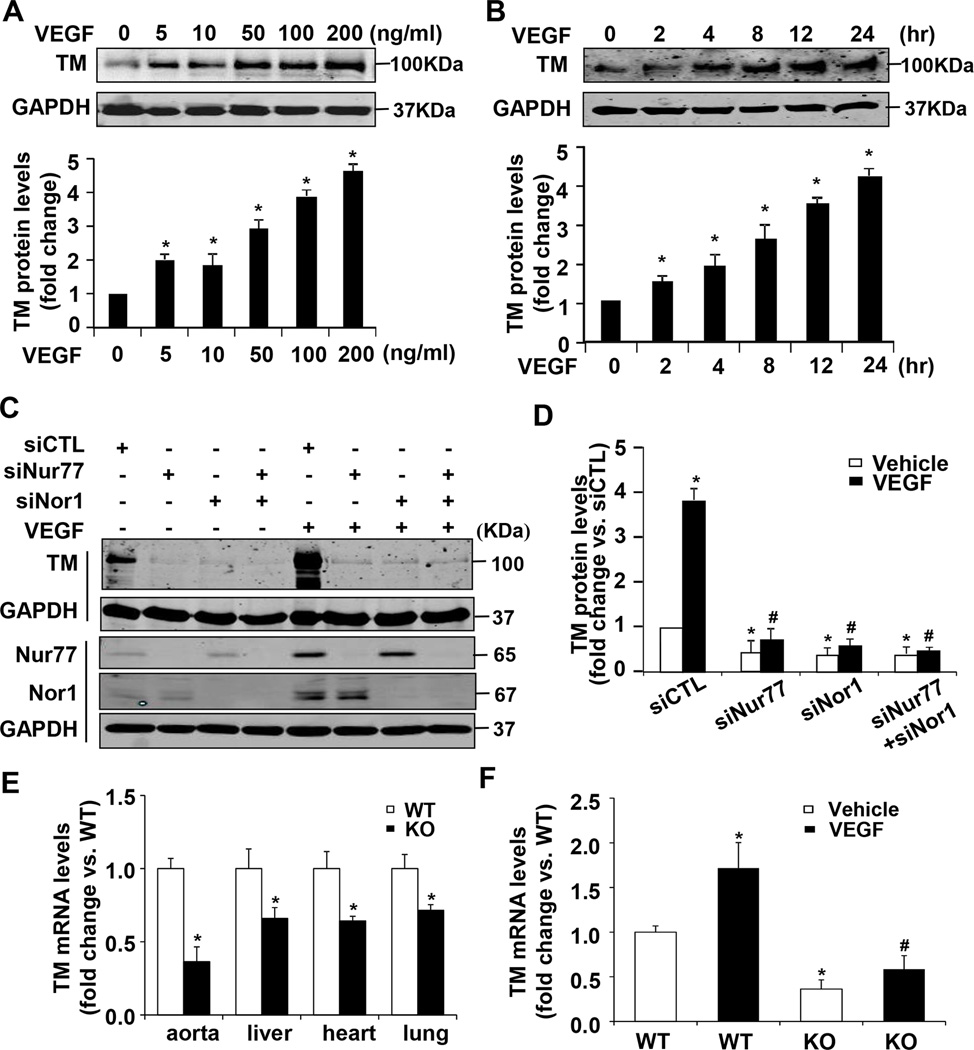
VEGF has been shown to upregulate the expression of both TM and NR4A receptors in vascular ECs15,25, which prompted us to investigate whether up-regulation of NR4A receptors is attributed to the VEGF165-induced TM expression in ECs. Consistent with previous studies25, we demonstrated that in a time and dose dependent manner, VEGF165 significantly increased TM expression in HUVECs (Figure 4A and 4B). To investigate the functional role of NR4A receptors in endothelial TM expression, we performed a loss-of-function study by using a small interference RNA (siRNA) approach. Transfection of Nur77 or Nor1 specific siRNA markedly inhibited both basal and VEGF165-induced expression of Nur77 or Nor1 in HUVECs, as determined by both qRT-PCR (Figure III in the online-only Data Supplement) and western blot (Figure 4C). Accordingly, both basal and VEGF165-induced TM expression was significantly attenuated in Nur77 or Nor1 knockdown cells (Figure 4C and 4D), suggesting that both Nur77 and Nor1 are essentially involved in the basal and VEGF165-induced TM expression in ECs.
Figure 4.
Nur77 and Nor1 are involved in VEGF165-induced TM expression in HUVECs. A. VEGF165 increases TM expression in a dose dependent manner. HUVECs were treated with increasing concentrations of VEGF165 for 24 hours and the expression of TM was determined by Western blot and quantitated by densitometry (n=3). *P<0.05 vs VEGF at 0 ng/ml. B, HUVECs were treated with 100 ng/ml VEGF for indicated time points. Expression of TM was determined by Western blot and quantitated by densitometry (n=3). *P<0.05 vs VEGF at time 0 hr. C, Effect of Nur77 or Nor1 siRNA knockdown on TM expression in HUVECs. HUVECs transfected with control siRNA (siCTL), Nur77 siRNA (siNur77), Nor1 siRNA (siNor1) or both. 72 hours after transfection, cells were treated with VEGF (100ng/ml) or vehicle. Expression of TM was determined at 24 hours after VEGF treatment by western blot (C) and quantitated by densitometry (D) (n=3). Expression of Nur77 and Nor1 was determined by western blot at 3 hours after VEGF treatment. *P<0.05 vs siCTL/vehicle; #P<0.05 vs siCTL/VEGF. E, The expression of TM was determined by qRT-PCR in the aorta, lung, heart and liver tissues of both Nur77 KO mice and their WT littermates (n=5). *P<0.05 vs WT littermates. F, Thoracic aorta from wild-type (WT) and Nur77 knockout mice (KO) was incubated with 100 ng/ml VEGF-165 for 24 hours. The expression of TM was then determined by qRT-PCR. *P<0.05 vs WT/vehicle; #P<0.05 vs WT/VEGF-165.
To further substantiate the functional significance of NR4A receptor in TM expression, we examined the expression of TM in Nur77 knockout mice. As shown in Figure 4E, TM expression was significantly decreased in the heart, liver, lung and aorta of Nur77 knockout (KO) mice, as determined by qRT-PCR. In an ex vivo experiment, VEGF165 stimulation significantly increased TM expression in the aorta of the wild-type (WT) mice, but not in the aorta of Nur77 KO mice (Figure 4F). Together, these results suggest that Nur77 is critically involved in the expression of TM in the vascular wall.
Overexpression of Nur77 and Nor1 promotes protein c activity and inhibits blood clotting in vitro
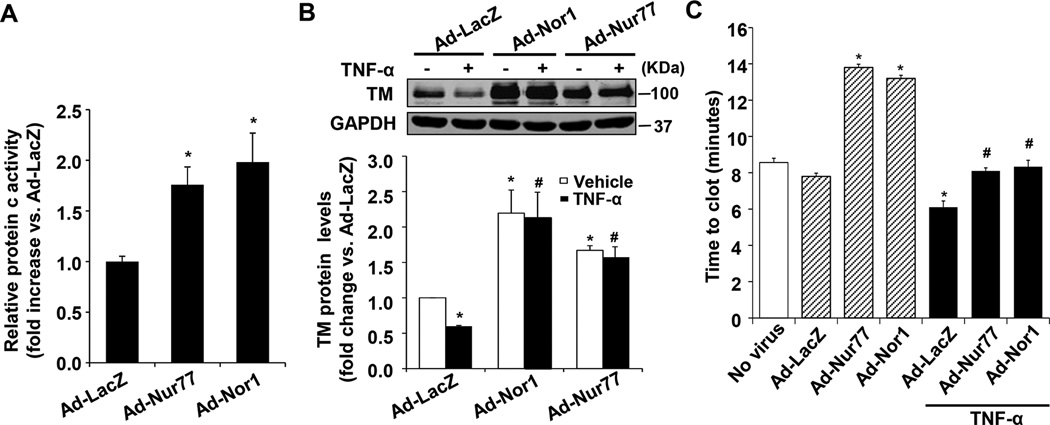
TM is a key regulator of the thrombin-mediated activation of the natural anticoagulant APC4. To investigate whether NR4A receptor Nur77 and Nor1 affect the function of TM in ECs, we determined the enzymatic activity by measuring the production of APC. As shown in Figure 5A, transduction of Ad-Nur77 or Ad-Nor1 significantly increased the activity of APC in HUVECs. Consistent with previous reports26, we found that TNF-α treatment significantly decreased TM expression in HUVECs, which was substantially reversed in ECs overexpressing either Nur77 or Nor1 (Figure 5B). Interestingly, overexpression of Nur77 and Nor1 markedly inhibited the expression of tissue factor under both basal and TNF-α-stimulated conditions (Figure IV in the online-only Data Supplement). To further determine the functional consequence of Nur77 and Nor1-induced TM expression in ECs, we performed an in vitro blood clotting assays. HUVECs were transfected with Ad-LacZ, Ad-Nur77 or Ad-Nor1 for 48 hr, and then the clotting time of recalcified human plasma was determined in the presence or absence of TNF-α stimulation. As shown in Figure 5C, exposure of recalcified human plasma to HUVECs transduced with Ad-Nur77 or Ad-Nor1 significantly prolonged the clotting time, as compared with that in LacZ-infected cells. Moreover, TNF-α stimulation significantly shortened the clotting time, and this pro-thrombotic effect was markedly prevented in HUVECs overexpressing either Nur77 or Nor1. Together, these results suggest that NR4A receptor Nur77 and Nor1 are critically involved in modulating TM function in vascular ECs under both baseline and inflammatory conditions.
Figure 5.
Nur77 and Nor1 overexpression promote protein c activity and inhibit blood clotting in vitro. A, HUVECs were transduced with Ad-LacZ, Ad-Nur77 or Ad-Nor1 (MOI=50) for 48 hours, then incubated with protein C (80 nM), CaCl2 (5mM), thrombin (2 nM). At the end of 2 hr of incubation at 37 °C, hirudin (4 units/ml) was added to neutralize thrombin, and protein c activity was measured using chromogenic substrate S-2366 (0.5 mM). Ad-Nur77 or Ad-Nor1 significantly increase protein c activity as compared with Ad-LacZ control group in HUVECs (n=5). *P<0.05 vs Ad-LacZ. B, HUVECs were transduced with Ad-LacZ, Ad-Nur77 or Ad-Nor1 (MOI=50) for 48 hours, and treated with TNF-α (20 ng/ml) for 6 hours, the expression of TM was determined by Western blot analysis (n=3). *P<0.05 vs Ad-LacZ/vehicle; #P<0.05 vs Ad-LacZ/TNF-α. C, Overexpression of Nur77 and Nor1 inhibits blood clotting in vitro. HUVECs were transduced with Ad-LacZ, Ad-Nur77 or Ad-Nor1 (MOI=50) for 48 hours, and treated with vehicle or TNF-α (20 ng/ml) for 5 hours. Cells were then washed, and recalcified plasma was added to wells. Clotting kinetics was measured by monitoring the absorbance at 405 nm. Analysis of kinetic profiles was performed to determine time to reach half-maximal absorbance (T1/2 max) (n=4). *P < 0.05 vs Ad-LacZ; #P < 0.05 vs Ad-LacZ/TNF-α.
Deficiency of Nur77 Enhances Susceptibility to Arterial Thrombosis
We next examined the effect of Nur77 deficiency on susceptibility to arterial thrombosis by using the Rose Bengal laser injury method27. Baseline white blood cell (WBC), hemoglobin (HB), and platelet (PLT) counts were similar in Nur77 KO mice and their WT littermates (Figure 6A). After photochemical injury of the carotid artery, the time to occlusive thrombus formation was significantly faster in Nur77 KO mice than in WT mice (22.3±4.9 versus 34.0±5.8 minutes, respectively; P<0.05; Figure 6B). Tail bleeding time was similar in Nur77 KO mice and their WT littermates (Figure 6C). To further determine whether Nur77 and Nor1 could be potential therapeutic targets for thrombotic orders, the mice were transduced with either Ad-Nur77 or Ad-Nor1 via a tail vein injection. The expression of TM in the liver was determined at 6 days after virus injection. Similar to the in vitro studies, the in vivo transduction of either Ad-Nur77 or Ad-Nor1 markedly increased the expression of TM in mouse liver tissues, as determined by Western blot (Figure 6D). Importantly, mice transduced with Ad-Nur77 and Ad-Nor1 displayed a significantly prolonged time to occlusive arterial thrombus formation as compared with Ad-LacZ group (43.2±6.5, 45.3±7.2 versus 32.8±6.1 minutes, respectively; P<0.05; Figure 6E). Together, these data demonstrate that Nur77 deficiency increases susceptibility to arterial thrombosis, while targeted overexpression of Nur77 and Nor1 in vivo prevents thrombus formation.
Figure 6.
Nur77 gene deficiency increases susceptibility to thrombus formation in vivo. A, WBC count, hemoglobin (HB), and platelet (PLT) counts in Nur77 KO mice and their WT littermates (n=7 per group). B, Nur77 gene deficiency (Nur77 KO) enhances susceptibility of the mice to carotid artery thrombus formation (n = 7–9 per group). *P <0.05 vs WT mice. C, Tail bleeding time was not changed in Nur77 KO mice and their wild-type (WT) littermates (n=7 per group). D, Mice were administrated with 100 µl Nur77 and Nor1 adenoviruses (Ad-Nur77 and Ad-Nor1, 1×1011 pfu/ml) by a tail vein injection. 6 days after virus transduction, mice were sacrificed and liver tissues were collected. TM protein expression in mouse liver tissues was determined by western blot analysis (n=8 per group). *P<0.05 vs Ad-LacZ. E, Mice were administrated with either 100 µl Ad-LacZ or Ad-Nur77 or Ad-Nor1 (1×1011 pfu/ml) by a tail vein injection. 6 days after adenovirus transduction, carotid artery thrombosis was performed (n=7–9 per group). *P<0.05 vs Ad-LacZ group.
Discussion
NR4A receptors are immediate-early genes that are regulated by various physiological stimuli including growth factors, hormones, and inflammatory signals in cardiovascular system17. An increasing number of studies have demonstrated that NR4A receptors play important roles in the development of various cardiovascular diseases, including atherosclerosis, restenosis, angiogenesis and heart failure28,29,30,15,21. Previously, our studies identified Nur77 as a potent inhibitor of vascular inflammation through inhibiting NF-κB transcriptional pathway16. Here, we provide further evidence highlighting the critical importance of NR4A receptors Nur77 and Nor1 in regulating TM expression in vascular ECs as well as arterial thrombus formation in vivo.
TM is one of the important anticoagulant substances produced by the vascular endothelium to maintain blood fluidity. The binding of TM to thrombin has shown to potentiate the generation of activate protein C and facilitate the formation of TAFIa, thus exerting potent anticoagulant, anti-inflammatory, and antifibrinolytic benefits31,4. Indeed, homozygous mice with TM mutant (Glu404Pro) exhibit reduced ability to generate activated protein C (APC), hence resulting in severe hypercoagulable state and massive thrombosis32. Despite its importance in the regulation of vascular homeostasis, the molecular mechanisms regulating TM expression in vasculature remain largely obscured. In this study, our data provide compelling evidence implicating the functional significance of NR4A receptors in regulating TM expression in vascular ECs and thrombotic function in vivo. Importantly, we found that overexpression of Nur77 and Nor1 robustly increase the TM expression, attenuates the TNF-α-induced pro-thrombotic states in ECs, and inhibits arterial thrombus formation in vivo. Furthermore, we demonstrate that Nur77 deficiency significantly increases susceptibility to arterial thrombus formation, further implicating importance of NR4A receptors in maintaining vascular homeostasis in endothelium.
Several molecular mechanisms at transcriptional, post-transcriptional, and posttranslational levels have been implicated in the regulation of TM expression and function in the vascular endothelium. At transcriptional levels, hypoxia33, oxidized LDL34, phorbolesters (PMA)35, cyclic adenosine monophosphate (cAMP)35, and TNF-α26 have been shown to downregulate endothelial TM expression, while statins36, VEGF25, retinoic acid37, and heat shock38 have been shown to upregulate TM expression. Particularly, Kruppel-like factors including KLF2 and KLF4 have been shown to play essential roles in the regulation of TM expression at transcriptional levels22,24, 39. For example, laminar shear stress, statins, proteasome and inhibitors have been shown to enhance endothelial TM expression via induction of KLF transcriptional activities22,24, 39. Indeed, KLF2 and 4 have multiple endothelial protective effects, which may collectively contribute to the anti-thrombotic effects of Nor1 in ECs. Nevertheless, identification of Nor1 as a novel regulator for KLF2 and KLF4 expression in vascular ECs is of great importance. Although the TM promoter does not contain a classic NF-κB consensus motif, the activation of NF-κB has been implicated in the cytokine-induced repression of TM expression in ECs, possibly through competing p300 for its binding to the TM promoter26. Furthermore, miRNAs, such as miR-92a, have been recently reported to regulate TM expression through post-transcriptional mechanisms40. In the present study, we demonstrate that NR4A receptors Nur77 and Nor1 are critically involved in the regulation of basal and VEGF-stimulated TM expression in ECs. Although the members of the NR4A subgroup are well conserved in the DNA binding domain (~91–95%), these receptors normally exert different or even opposite biological effects through interacting with other co-factors17. Interestingly, in this study, we found that Nur77 and Nor1 regulate TM expression through distinct molecular mechanisms. Nur77 increases TM expression predominantly through increasing TM mRNA stability, while Nor1 involves an induction of KLF2 and KLF4 in ECs. Indeed, depending on the nature of the stimuli and their cellular localization, NR4A receptors can exert biological effects through both genomic and non-genomic actions21. For instance, in the nucleus, NR4A receptors can function as transcription factors to regulate the expression of the target genes by binding to NGFI-B response element (NBRE) (NBRE) (5'-AAAAGGTCA-3') or Nur-responsive element (NurRE)(5’-TGATATTTX6AAAGTCCA-3’)14. In this study, our data suggest that such a genomic action is not likely involved in the stimulatory effects of Nur77 on TM expression, since DN-Nur77, which lacks the functional N-terminal AF-1 domain of Nur77, induces TM expression to a similar extent as did wild-type Nur77. In addition, the sequential analysis of the TM promoter did not reveal any NR4A receptor binding consensus sites, further suggesting that the nongenomic action of Nur77 may contribute to its stimulatory effect on TM expression. In contrast to Nur77, Nor1 augments the TM promoter activity indirectly through induction of both KLF2 and KLF4 in ECs. Future studies will be aimed at elucidating the molecular mechanisms underlying increasing TM mRNA stability by Nur77 and induction of KLFs by Nor1 in vascular ECs.
Inflammation has been shown to induce prothrombotic effects in vascular endothelium1. Inflammatory cytokines, including TNF-α and IL-1β, have been shown to attenuate the expression of TM through activating NF-κB pathways26. Previous studies from our laboratory identified Nur77 as a potent negative regulator of NF-κB activation in ECs through transcriptionally up-regulating the expression of IκBα16. Indeed, our results demonstrate that overexpression of Nur77 and Nor1 markedly prevented TNF-α-induced repression of TM in vascular ECs. Accordingly, we show that overexpression of Nur77 and Nor1 also significantly increases the clotting time under both basal and TNF-α-stimulated conditions. To further define the mechanism by which Nor1 prevents TNF-α-induced downregulation of TM in ECs, we examined the effect of Nor1 on NF-κB activation in TNF-α-stimulated cells. We found that overexpression of Nor1 barely affected TNF-α-stimulated NF-κB activation as determined by EMSA (Figure IV in the online-only Data Supplement). Based on these observations, it is attempted to speculate that in unstimulated ECs, Nur77 increases TM expression mainly through increasing TM mRNA stability, while in TNF-α-stimulated cells, Nur77 increases TM expression most likely through inhibiting NF-κB activation or through both inhibiting NF-κB activation and increasing TM mRNA stability. The molecular mechanism underlying induction of TM expression by Nor1, however, is different, and it may involve the upregulation of the KLF2 and KLF4 transcriptional activities under both basal and TNF-α-stimulated conditions, which merits further investigation. Furthermore, VEGF has been shown to potently increase basal TM expression and block IL-1β-induced suppression of TM expression in ECs, which is believed to be essential for maintaining local hemostasis during angiogenesis and inflammatory processes25. The molecular mechanism underlying VEGF-induced TM expression, however, has never been explored thus far. Since VEGF is a potent stimulus for the expression of NR4A receptors in ECs15, we attempted to speculate that NR4A receptors may also mediate VEGF-induced TM expression in vascular ECs. Indeed, we show that both basal and VEGF-induced TM expression were substantially attenuated in Nur77 and Nor1 knockdown cells as well as in the aorta of Nur77 deficient mice. Collectively, these data clearly demonstrate that NR4A receptors Nur77 and Nor1 are essential regulators of endothelial TM expression under various pathophysiological circumstances.
In conclusion, we identified NR4A receptors Nur77 and Nor1 as critical regulators of endothelial TM expression and arterial thrombosis in vivo and further implicate the essential roles of NR4A receptors in the pathological processes of the inflammatory and thrombotic diseases. Collectively, our results provide a proof-of-concept demonstration that targeting NR4A receptor Nur77 and Nor1 in the vascular wall might have a therapeutic role in ameliorating the onset and/or progression of thrombotic disorders.
Supplementary Material
Significance.
Thrombomodulin (TM) is highly expressed on the lumenal surface of vascular endothelial cells (ECs) and elicits potent anticoagulant, anti-fibrinolytic, and anti-inflammatory activities in the vessel wall. Understanding the molecular mechanism regulating TM expression and activity is essential for developing effective therapies to combat inflammatory and thrombotic disorders. In the present study, we unveiled a fundamental mechanism governing TM expression at multiple levels in ECs. We identified the NR4A receptors Nur77 and Nor1 as critical regulators for inducing TM expression in vascular ECs. Overexpression of Nur77 and Nor1 significantly increases TM expression and prevents inflammation-induced pro-thrombotic states in ECs, while Nur77 gene deficiency predisposes the mice to the thrombus formation. Our results indicate that specific activation of NR4A receptors such as Nur77 in vascular endothelium may represent a novel therapeutic approach for prevention and treatment of thrombotic disorders.
Acknowledgments
None
Sources of Funding
This study was supported by the Chinese Natural Science Foundation grants 81541003 (G.-X. Zhang), 381170114 and 81370418 (J. Sun); the Shanghai Natural Science Foundation 15ZR1413400 (G.-X. Zhang); and the National Institutes of Health HL103869 (J. Sun).
Nonstandard Abbreviations and Acronyms
- TM
thrombomodulin
- TNF-α
tumor necrosis factor alpha
- APC
activated protein C
- NR4A
nuclear receptor 4A family
- qRT-PCR
quantitative real-time PCR
- HUVECs
human umbilical vein endothelial cells
- VEGF
vascular endothelial growth factor
- siRNA
small interference RNA
- KLF
Kruppel-like factors
- MOI
multiplicity of infection
Footnotes
Disclosures
None
References
- 1.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 2.Michiels C. Endothelial cell functions. Journal of cellular physiology. 2003;196:430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 3.Esmon CT. The protein c anticoagulant pathway. Arterioscler Thromb. 1992;12:135–145. doi: 10.1161/01.atv.12.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Conway EM. Thrombomodulin and its role in inflammation. Seminars in immunopathology. 2012;34:107–125. doi: 10.1007/s00281-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 5.Bajzar L, Jain N, Wang P, Walker JB. Thrombin activatable fibrinolysis inhibitor: Not just an inhibitor of fibrinolysis. Critical care medicine. 2004;32:S320–S324. doi: 10.1097/01.ccm.0000126361.00450.b1. [DOI] [PubMed] [Google Scholar]
- 6.Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ, Pearl RG, Leung LL. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. The Journal of biological chemistry. 2003;278:51059–51067. doi: 10.1074/jbc.M306977200. [DOI] [PubMed] [Google Scholar]
- 7.Adams TE, Huntington JA. Thrombin-cofactor interactions: Structural insights into regulatory mechanisms. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1738–1745. doi: 10.1161/01.ATV.0000228844.65168.d1. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Garnette CS, Cahn M, Claytor RB, Rohrer MJ, Dobson JG, Jr, Gerlitz B, Cutler BS. Recombinant thrombomodulin inhibits arterial smooth muscle cell proliferation induced by thrombin. Journal of vascular surgery. 2000;32:804–813. doi: 10.1067/mva.2000.107992. [DOI] [PubMed] [Google Scholar]
- 9.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, Fernandez JA, Cancelas JA, Ryan MA, Kustikova O, Schambach A, Fu Q, Wang J, Fink LM, Petersen KU, Zhou D, Griffin JH, Baum C, Weiler H, Hauer-Jensen M. Pharmacological targeting of the thrombomodulin-activated protein c pathway mitigates radiation toxicity. Nature medicine. 2012;18:1123–1129. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iba T, Nagaoka I, Boulat M. The anticoagulant therapy for sepsis-associated disseminated intravascular coagulation. Thrombosis research. 2013;131:383–389. doi: 10.1016/j.thromres.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Ikezoe T. Thrombomodulin/activated protein c system in septic disseminated intravascular coagulation. Journal of intensive care. 2015;3:1. doi: 10.1186/s40560-014-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai M, Ikezoe T, Bandobashi K, Yokoyama A. Successful treatment of thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus with recombinant human soluble thrombomodulin. Thrombosis research. 2010;126:e392–e393. doi: 10.1016/j.thromres.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Hsu HC, Zhou T, Mountz JD. Nur77 family of nuclear hormone receptors. Curr Drug Targets Inflamm Allergy. 2004;3:413–423. doi: 10.2174/1568010042634523. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Gonzalez J, Badimon L. The nr4a subfamily of nuclear receptors: New early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65:609–618. doi: 10.1016/j.cardiores.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor tr3/nur77 regulates vegf-a-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203:719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor nur77 suppresses endothelial cell activation through induction of ikappabalpha expression. Circ Res. 2009;104:742–749. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 17.van Tiel CM, de Vries CJ. Nr4all in the vessel wall. The Journal of steroid biochemistry and molecular biology. 2012;130:186–193. doi: 10.1016/j.jsbmb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Liu Y, Yi B, Wang G, You X, Zhao X, Summer R, Qin Y, Sun J. Microrna-638 is highly expressed in human vascular smooth muscle cells and inhibits pdgf-bb-induced cell proliferation and migration through targeting orphan nuclear receptor nor1. Cardiovasc Res. 2013;99:185–193. doi: 10.1093/cvr/cvt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buervenich S, Carmine A, Arvidsson M, Xiang F, Zhang Z, Sydow O, Jonsson EG, Sedvall GC, Leonard S, Ross RG, Freedman R, Chowdari KV, Nimgaonkar VL, Perlmann T, Anvret M, Olson L. Nurr1 mutations in cases of schizophrenia and manic-depressive disorder. American journal of medical genetics. 2000;96:808–813. doi: 10.1002/1096-8628(20001204)96:6<808::aid-ajmg23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Xing G, Zhang L, Russell S, Post R. Reduction of dopamine-related transcription factors nurr1 and ngfi-b in the prefrontal cortex in schizophrenia and bipolar disorders. Schizophrenia research. 2006;84:36–56. doi: 10.1016/j.schres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Yan G, Zhu N, Huang S, Yi B, Shang X, Chen M, Wang N, Zhang GX, Talarico JA, Tilley DG, Gao E, Sun J. Orphan nuclear receptor nur77 inhibits cardiac hypertrophic response to beta-adrenergic stimulation. Molecular and cellular biology. 2015 doi: 10.1128/MCB.00229-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathak R, Shao L, Chafekar SM, Feng W, Ponnappan U, Fink LM, Zhou D, Hauer-Jensen M. Ikkbeta regulates endothelial thrombomodulin in a klf2-dependent manner. Journal of thrombosis and haemostasis : JTH. 2014;12:1533–1544. doi: 10.1111/jth.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: Bit by bit, a slow case with perspective. Circ Res. 2008;103:334–336. doi: 10.1161/CIRCRESAHA.108.182220. [DOI] [PubMed] [Google Scholar]
- 24.Hiroi T, Deming CB, Zhao H, Hansen BS, Arkenbout EK, Myers TJ, McDevitt MA, Rade JJ. Proteasome inhibitors enhance endothelial thrombomodulin expression via induction of kruppel-like transcription factors. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1587–1593. doi: 10.1161/ATVBAHA.109.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calnek DS, Grinnell BW. Thrombomodulin-dependent anticoagulant activity is regulated by vascular endothelial growth factor. Exp Cell Res. 1998;238:294–298. doi: 10.1006/excr.1997.3812. [DOI] [PubMed] [Google Scholar]
- 26.Sohn RH, Deming CB, Johns DC, Champion HC, Bian C, Gardner K, Rade JJ. Regulation of endothelial thrombomodulin expression by inflammatory cytokines is mediated by activation of nuclear factor-kappa b. Blood. 2005;105:3910–3917. doi: 10.1182/blood-2004-03-0928. [DOI] [PubMed] [Google Scholar]
- 27.Nieman MT, Warnock M, Hasan AA, Mahdi F, Lucchesi BR, Brown NJ, Murphey LJ, Schmaier AH. The preparation and characterization of novel peptide antagonists to thrombin and factor viia and activation of protease-activated receptor 1. The Journal of pharmacology and experimental therapeutics. 2004;311:492–501. doi: 10.1124/jpet.104.069229. [DOI] [PubMed] [Google Scholar]
- 28.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. Nr4a1 (nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamers AA, Vos M, Rassam F, Marinkovic G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, de Vries CJ. Bone marrow-specific deficiency of nuclear receptor nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 30.Pires NM, Pols TW, de Vries MR, van Tiel CM, Bonta PI, Vos M, Arkenbout EK, Pannekoek H, Jukema JW, Quax PH, de Vries CJ. Activation of nuclear receptor nur77 by 6-mercaptopurine protects against neointima formation. Circulation. 2007;115:493–500. doi: 10.1161/CIRCULATIONAHA.106.626838. [DOI] [PubMed] [Google Scholar]
- 31.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein c receptor augments protein c activation by the thrombin-thrombomodulin complex. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rijneveld AW, Weijer S, Florquin S, Esmon CT, Meijers JC, Speelman P, Reitsma PH, Ten Cate H, van der Poll T. Thrombomodulin mutant mice with a strongly reduced capacity to generate activated protein c have an unaltered pulmonary immune response to respiratory pathogens and lipopolysaccharide. Blood. 2004;103:1702–1709. doi: 10.1182/blood-2002-05-1380. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. The Journal of clinical investigation. 1990;85:1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii H, Tezuka T, Ishikawa H, Takada K, Oida K, Horie S. Oxidized phospholipids in oxidized low-density lipoprotein down-regulate thrombomodulin transcription in vascular endothelial cells through a decrease in the binding of rarbeta-rxralpha heterodimers and sp1 and sp3 to their binding sequences in the tm promoter. Blood. 2003;101:4765–4774. doi: 10.1182/blood-2002-08-2428. [DOI] [PubMed] [Google Scholar]
- 35.Grey ST, Csizmadia V, Hancock WW. Differential effect of tumor necrosis factor-alpha on thrombomodulin gene expression by human monocytoid (thp-1) cell versus endothelial cells. International journal of hematology. 1998;67:53–62. doi: 10.1016/s0925-5710(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 36.Masamura K, Oida K, Kanehara H, Suzuki J, Horie S, Ishii H, Miyamori I. Pitavastatin-induced thrombomodulin expression by endothelial cells acts via inhibition of small g proteins of the rho family. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:512–517. doi: 10.1161/01.ATV.0000060461.64771.F0. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti M, Vignoli A, Bani MR, Balducci D, Barbui T, Falanga A. All-trans retinoic acid modulates microvascular endothelial cell hemostatic properties. Haematologica. 2003;88:895–905. [PubMed] [Google Scholar]
- 38.Fu Q, Wang J, Boerma M, Berbee M, Qiu X, Fink LM, Hauer-Jensen M. Involvement of heat shock factor 1 in statin-induced transcriptional upregulation of endothelial thrombomodulin. Circ Res. 2008;103:369–377. doi: 10.1161/CIRCRESAHA.108.174607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr, Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (klf2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 40.Irani K. Crippling of kruppel (-like factor 2) by bad flow portends a mirky day for endothelial function. Circulation. 2011;124:541–543. doi: 10.1161/CIRCULATIONAHA.111.043299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.