Abstract
Cryptococcal meningitis is an important fungal infection among systemic lupus erythematosus patients. We conducted a pooled analysis and systematic review to describe the epidemiological and clinical profile of cryptococcal meningitis in systemic lupus erythematosus patients. From two hospitals in China and nine literature databases, cases and prevalence data were collected for pooled analysis and meta-analysis, respectively. Categorical variables of cases were compared using a χ2-test on the statistical program of SAS. A multiple regression analysis was performed to ascertain independent predictors significantly correlated with prognosis. Meta-analysis was conducted by the statistical program of R. The prevalence of cryptococcal meningitis in systemic lupus erythematosus patients was 0.5%. Patients were predominantly females and adults. A prednisone equivalent of more than 30 mg/day before infection was associated with higher mortality (odds ratio (OR)=9.69 (1.54, 60.73)). In all, 36.8–38.9% patients showed low lupus activity when they developed the crytococcal infection. Moreover, 38.2% of the patients were misdiagnosed. The estimated case-fatality rate was 23.6%. Our results suggest that more emphasis should be placed to further understand lupus-related cryptococcal meningitis and to develop better prophylaxis and management strategies to combat this condition.
Keywords: cryptococcal meningitis, opportunistic infections, systemic lupus erythematosus
INTRODUCTION
Microbial infections rank as one of the most important causes of morbidity and mortality in patients with systemic lupus erythematosus (SLE), leading to the death of 20%–40% of SLE patients.1 The most common risk factors for infection in SLE patients are the use of high-dose corticosteroids, antibiotics exposure,2 high SLE activity and intrinsic disorders of cell-mediated immunity.3 Moreover, as SLE is a systemic disease, ~20%–70% of SLE patients develop central nervous system (CNS) damage due to autoimmune-mediated attack,4 making the CNS susceptible to infection through impairment of the blood–brain barrier.
Cryptococcal meningitis (CM) is a deadly systemic opportunistic fungal infection caused by members of the Cryptococcus neoformans/C. gattii species complex, and it primarily occurs in immunosuppressed patients.5 Globally, ~1 000 000 HIV-infected patients per year develop CM, and nearly one-third of them die within three months of infection.6 The global epidemiological characteristics of CM among non-HIV population remain poorly understood, including the patients with SLE. A recent study suggests that CM is the most frequent mycosis in SLE patients, accounting for 25.8% of invasive fungal infections (IFIs) in these patients7 and is ranked as the number one cause of death (85.7%) due to IFIs in SLE patients.1 Moreover, Cryptococcus ranks as the first or second most important causative agent among the microbial pathogens (including bacteria, fungi and viruses) causing 30.4–58.8% CNS infections in SLE patients.8, 9, 10, 11, 12
Because of the unfamiliarity with this infection, the nonspecific clinical presentations at the early stage of the disease of the disease, and the limited information available in the literature, rheumatologists may underestimate the risk of CM and misdiagnose CM as psychosis triggered by steroid treatment,13 CNS lupus disease activity9, 14, 15 or infection caused by non-fungal pathogens.16, 17 The resulting wrong therapy aggravates the infection and delays the administration of the appropriate intervention using antifungal agents.16, 18, 19, 20
Dozens of cases and prevalence studies have been reported about CM in SLE patients. The epidemiological and clinical profiles, however, have not been critically reviewed and summarized so far. Thus, we conducted a pooled analysis and systematic review of original and published data. Our objective is to describe the epidemiological and clinical profiles of CM in SLE patients to foster the development of improved prophylaxis and management strategies for this mycosis in SLE patients.
MATERIALS AND METHODS
SEARCH STRATEGY AND SELECTION CRITERIA
This study was approved by the Ethics Committee of the Second Military Medical University, Shanghai, China. Given the retrospective nature of the study, the need for informed consent was waived. Our study strictly followed the PRISMA guidelines for reporting systematic reviews, and our protocol was previously registered in PROSPERO (the international database of prospectively registered systematic reviews in health and social care) with registration number NO CRD42015016552. We searched and collected the medical records of all patients who had a confirmed diagnosis of either CM or SLE from January 2001 to July 2015 and were hospitalized at one of in two hospitals affiliated to our university (Shanghai Changzheng Hospital (1280 beds) and Shanghai Changhai Hospital (2000 beds)). This information was used to identify cases of CM among SLE patients. In addition, nine literature databases, namely PubMed, Embase, Web of Science, Wiley Online Library, Springer Link, Science Direct, Cochrane database, Chinese Biomedical Literature Service System (SinoMed) and China National Knowledge Infrastructure, were searched for cases and prevalence studies. The search included all language and publication dates as long as they were archived in the above nine databases. The major search terms used were ‘systemic lupus erythematosus' and ‘cryptococcal meningitis': (‘Lupus Erythematosus, Systemic' [Mesh] OR ‘Lupus' [tiab] OR ‘LE' [tiab] OR ‘Libman Sacks' [tiab]) AND (Cryptococcosis [Mesh] OR ‘Meningitis, Cryptococcal' [Mesh] OR Cryptococcus [Mesh] OR ‘Cryptococcus neoformans' [Mesh] OR ‘Cryptococcus gattii' [Mesh] OR Cryptococc* OR neoformans OR grubii OR gatti* OR Torul*).
This strategy was an example of our retrieval in the database of PubMed, and similar searches have been conducted in the other databases. Articles not written in English or Chinese were professionally translated for further review. The reference lists of the relevant articles were also manually searched to supplement the searches of the computerized databases. All potentially relevant papers were obtained and evaluated in detail. The pooled analysis required extractable information for each case to allow a quantitative analysis. Hence, the authors of related studies were contacted for necessary information if necessary. Cases or case series without detailed medical history and lacking extractable clinical and laboratory data were excluded. Publications were included in the pooled analysis if the criteria for the diagnosis of SLE were followed according to the American College of Rheumatology guidelines,21, 22, 23 and the diagnosis of CM was based on the presence of positive findings in at least one of the following tests in cerebral spinal fluid and/or brain tissue samples, including positive Cryptococcus culture, India ink staining and cryptococcal antigen (CrAg) test.24 Cases without clinical and laboratory data supporting the diagnosis of SLE or CM, even if the authors declared those patients were positively diagnosed, were excluded.
DATA COLLECTION AND STATISTICAL ANALYSIS
Data from original and published cases were loaded into the EpiData software (version 3.1, The EpiData Association, Odense, Denmark) using a pre-designed form containing the following information: date of inpatient admission, gender, geographic information, age at SLE and CM diagnoses, time from SLE diagnosis to the onset of infection, time from the onset of CM to infection diagnosis, drugs used for SLE control before infection, clinical manifestations and laboratory findings of infection, Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score at infection diagnosis, information on misdiagnoses, details on antifungal therapies used, and outcome. Corticosteroid doses were converted into prednisone equivalent doses via the online tool available at http://www.globalrph.com/steroid.cgi. For original and published prevalence data, a pre-designed Microsoft Excel form (Microsoft Office, 2010 version, Microsoft Corporation, Redmond, Washington, USA) with the following information was used: continent, country, study base, study design, diagnostic criteria for SLE and CM, study period, number of SLE cases and number of CM–SLE cases. Disagreements were resolved and consensuses were reached through discussion.
SAS (version 9.00, SAS Institute, Cary, NC, USA) was used for the pooled statistical analysis. Results were presented as mean±s.d. for normal data or median with interquartile ranges (IQRs) for non-normal data. The categorical variables were compared using the χ2-test. A P-value less than 0.05 was considered to indicate a statistically significant difference. A multiple regression analysis was performed to ascertain independent predictors significantly correlated with outcome.
The meta-analysis of disease prevalence was conducted using the R (version 3.2.1, The R Foundation for Statistical Computing, Vienna, Austria). Heterogeneity was evaluated using the Q statistic and inconsistency index (I2) statistic (I2 values of ~25%, 50% and 75% correspond to low, medium and high heterogeneity, respectively). The pooled data values were generated using the random-effects model. Publication bias was examined by Egger's test (if >10 studies were included in the analysis).
RESULTS
STUDIES INCLUDED
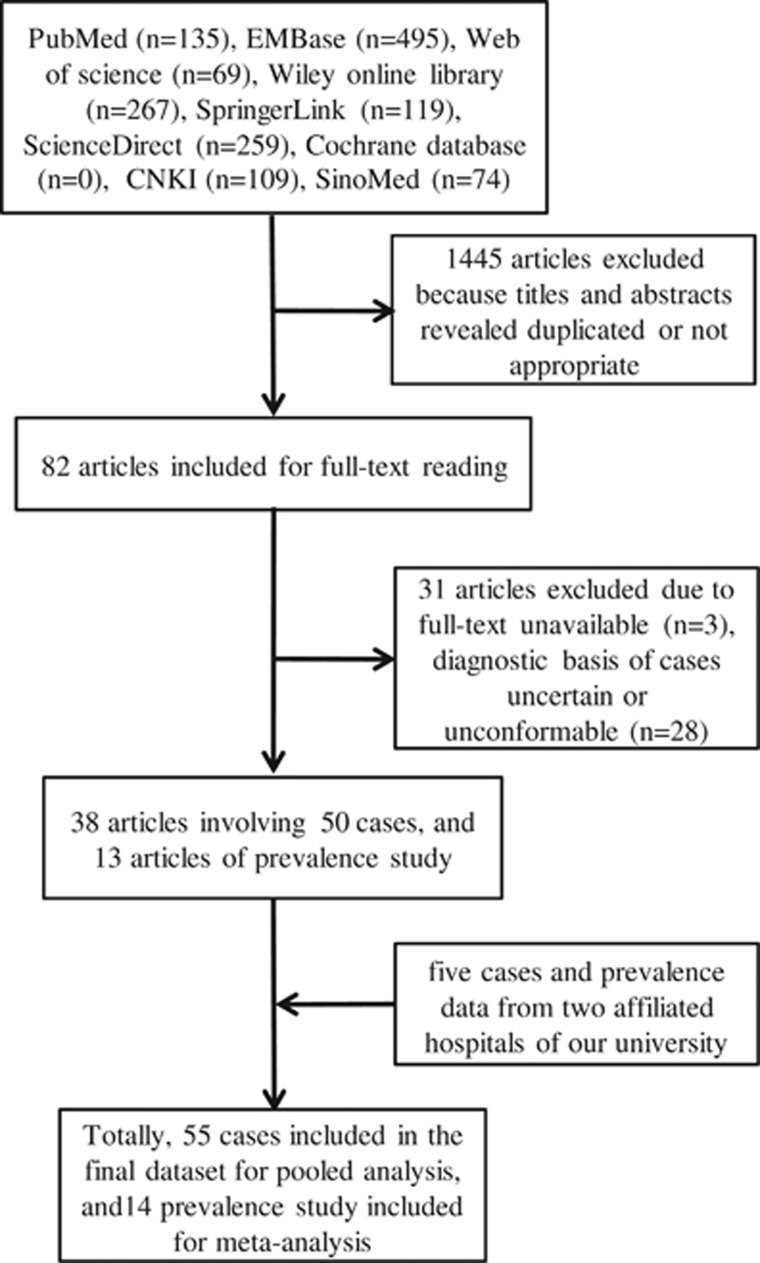
For case analysis, a total of 55 cases that met our criteria were identified and extracted. These included five cases from our hospitals and 50 cases from 38 published articles (Supplementary Table S1).
For the prevalence meta-analysis, data from 13 studies (Supplementary Table S2) were combined with the original data from two hospitals affiliated to university, resulting in the inclusion of a total of 19 840 SLE cases and 87 CM–SLE cases from 1966 to 2015. Among the 14 studies, five were conducted in the Americas, including Argentina (n=1), Colombia (n=1), Mexico (n=2) and the United States of America (n=1), and nine were conducted in Asia, including mainland China (n=4), Korea (n=1), the Philippines (n=1) and Taiwan (n=3). Prevalence data reported from Europe, Oceania and Africa were not retrievable. More details on the search strategy used are given in Figure 1.
Figure 1.
Flow diagram of study selection. China National Knowledge Infrastructure, CNKI; Chinese Biomedical Literature Service System, SinoMed.
EPIDEMIOLOGIC AND DEMOGRAPHIC INFORMATIOn
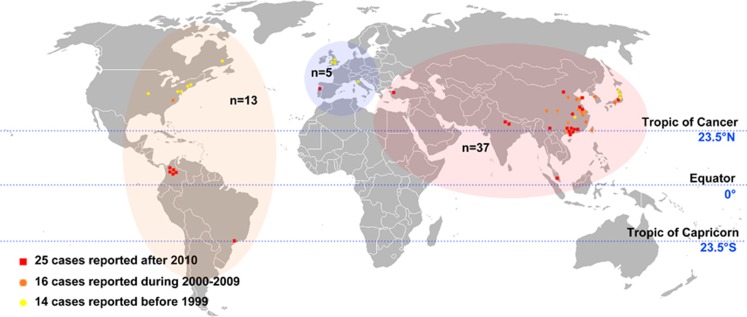
Figure 2 shows the global distribution of the 55 cases of CM–SLE included in the pooled analysis. The patient demographic details are available in Supplementary Table S1. Fourteen cases were reported before 1999, 16 from 2000 to 2009 and 25 after 2010. The majority of cases were collected from Asia (n=37; 67.27%), followed by North America (n=7; 12.73%), South America (n=6; 10.91%) and Europe (n=5; 9.09%). No case was reported from Oceania or Africa. More cases were reported from developing countries (n=36; 65.5% that is, Brazil, Columbia, China, India, Malaysia and Turkey) than in developed countries (n=19; 34.5% that is, the United States of America, Canada, Italy, Japan, Korea, Portugal and United Kingdom).
Figure 2.
Global distribution of cases. Thirty-seven cases were collected from Asia, 13 from Americas and five from Europe. Fourteen cases were reported before 1999, 16 from 2000 to 2009 and 25 after 2010.
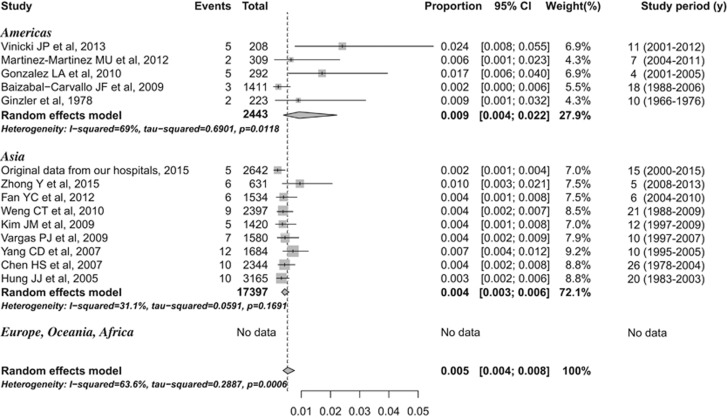
Data from 13 published studies on the prevalence of CM–SLE as well as those unpublished from our hospitals were included in our analyses. The overall pooled prevalence of CM among SLE patients was 0.5% (95% confidence interval (CI), 0.4%–0.8%), and there was a high degree of heterogeneity between studies (I2=63.6%, P=0.001; Figure 3). From the subgroup analysis based on geography, the estimated prevalence was 0.9% (95% CI, 0.4%–2.2%) in the Americas and 0.4% (95% CI, 0.3%–0.6%) in Asia, and this difference was not statistically significant. The levels of heterogeneity in the prevalence of CM among SLE patients in studies conducted in the Americas and Asia were high (I2=69.0%, P=0.012) and low (I2=31.1%, P=0.169), respectively. There was no evidence of publication bias or other small study effects (linear regression test of funnel plot asymmetry, P=0.64, Egger's test P=0.636). The details on the study design and literature quality assessment are presented in Supplementary Table S2.
Figure 3.
Prevalence of cryptococcal meningitis in systemic lupus erythematosus.
The CM–SLE patients were predominantly female (female/male ratio: 8:17). The percentage of cases diagnosed with CM within 1 year of SLE diagnosis was 50.1% (28/55). The median age at definitive CM diagnosis was 32 years (IQR: 21–42 years). The median age at CM diagnosis in developing countries (27 years (IQR: 19–39 years)) was much lower than that in developed countries (40 years (IQR: 25–47 years); P=0.03). In contrast, there was no difference in the age at SLE diagnosis between developing and developed countries (P=0.10). No patients were found to be HIV-seropositive. Two patients declared being exposed to pigeon droppings, and one patient traveled to a cave region 20 days before experiencing neurological symptoms that constituted an emergency. Before infection, the administration of prednisone equivalents of ≥30 mg/day was associated with higher mortality (P=0.02). In addition, more patients received a prednisone equivalent of ≥30 mg/day in developing countries than in developed countries (P=0.03), and no patients received a prednisone equivalent of ≥60 mg/day in developed countries. A multiple regression analysis revealed that prednisone equivalent >30 mg (odds ratio (OR)=9.69 (1.54, 60.73)) predicted prognosis of death. However, age of <18 years (OR=0.49 (0.09, 2.67)) and living in developing countries (OR=5.304 (0.86, 32.68)) were not significantly associated with prognosis.
CLINICAL MANIFESTATIONS AND LABORATORY EXAMINATIONS
The detailed clinical and laboratory findings are available in Supplementary Table S3 (clinical manifestations and laboratory examinations of each individual case) and are partially summarized in Tables 1 and 2. The SLEDAI or SLEDAI-2K score at infection was available in 19 cases. The scores in seven cases (36.8%) were <4 points, and the mean score was 7.58±6.91 points (95% CI, 4.52–10.91). After reviewing the medical charts of the remaining 36 patients whose SLEDAI/SLEDAI-SK scores were not evaluated, 14 (~38.9%) were found to have no indication of SLE activity.
Table 1. Clinical manifestations.
| Number | Percentage | |
|---|---|---|
| Fever | 40 | 72.7 |
| Headache | 45 | 81.8 |
| Nausea | 10 | 18.2 |
| Vomiting | 22 | 40.0 |
| Neck rigidity | 17 | 30.9 |
| Impairment of consciousness | 16 | 29.1 |
| Vision impaired | 7 | 12.7 |
| Papilledema | 4 | 7.3 |
| Seizure | 5 | 9.1 |
| Psychiatric disorder | 0 | 0.0 |
| Twitching | 3 | 5.5 |
DIAGNOSTIC PROFILE
To be included in our study, CM infection had to be diagnosed by India ink staining, culture and/or the detection of CrAg. Among the 55 CM cases, India ink staining was positive in 43 patients, negative in seven patients and unknown in the remaining five cases. Cerebral spinal fluid from 49 patients were cultured for evidence of Cryptococcus strains; 47 had a positive culture, 39 of which were identified as C. neoformans and one of which was identified as C. laurentii. CrAg tests were only performed in 20 cases, and one of them was negative. In 11 cases, India ink staining, fungal culture and CrAg tests were positive.
Twenty-one of the fifty-five cases of CM (~38.2%) were initially misdiagnosed. The most common diseases mimicking CM in SLE patients were non-fungal infections, with nine patients being misdiagnosed with bacterial infection (including two with tuberculosis) and two with viral infection. In addition, nine cases were misdiagnosed as CNS lupus and five as lupus activity. Regarding the causes of misdiagnosis, 16 patients did not undergo a diagnostic lumbar puncture (LP) in a timely manner, and the diagnosis of CM in those patients was initially only based on physical examination. Four patients underwent LP but not the CrAg test at initial presentation. The details of the diagnostic profiles are presented in Supplementary Table S4 (diagnostic details of each individual case).
MANAGEMENT AND OUTCOMES
The estimated case-fatality rate was 23.64% (13/55). Among the 13 patients who died, four (30.8%) died because they abandoned treatment owing to an unaffordable financial burden for antifungal drugs, and one died before receiving treatment. Approximately 38% (21/55) patients received corticosteroids to control active lupus or as an adjunctive therapy to antifungal treatment. The details on the management of CM are listed in Supplementary Table S5 (Therapeutic strategy of each individual case).
DISCUSSION
CM has a high prevalence and mortality among CNS infections in SLE patients, and is ranked as the most important CNS infection. The prevalence of this disease seems underestimated, and its characteristics have not been adequately described in the literature, causing delay in treatment and control of this lethal invasive infection. Therefore, we conducted a pooled analysis and systematic review of both original and published data to generate a systematic profile of CM in SLE.
In the present study, 55 individual cases were included in the pooled analysis. Despite the fact that CM is normally a male-predominant disease, the patients were mainly females (89.1%), likely because SLE occurs more frequently in females.25 This finding indicates that CM may also have a high prevalence in women with SLE, especially in cases with certain female-predominant underlying diseases. More than half (50.1%) of the cases were diagnosed with CM within one year of obtaining a confirmed SLE diagnosis, with a median age at infection of 32 years. However, Wang et al.7 reported that only 39% of IFIs (including CM) occurred within the first year of SLE diagnosis and that the average age of IFIs was ~35.8±13.5 years. This result indicated that CM tends to emerge relatively early in SLE disease progression compared with other IFIs. Most of the cases were collected from Asia (67.27%), followed by the Americas (23.64%) and Europe (9.09%). The case distribution characteristics were similar to those in a recent systematic review that summarized IFIs in SLE (57.3% cases in Asia, followed by 32.8% in the Americas and 8.7% in Europe, with no data from Oceania or Africa).7 However, according to the findings of the current meta-analysis, the CM prevalence in SLE was not higher in Asia than in the Americas. Moreover, there was no difference in SLE prevalence between various continents; for example, the prevalence of SLE ranged from 31.1 to 70.1 cases per 100 000 persons in mainland China, 58.6 cases per 100 000 persons (95% CI, 46.1–73.5) in Argentina and ranged from 14.6–68 cases per 100 000 persons in the United States of America.26, 27 This noteworthy inconsistency between the distribution and prevalence of CM–SLE cases could be explained by the fact that the Asian population base (~3.8 billion) is four- to fivefold greater than those in Europe and the Americas (~0.73 and 0.87 billion, respectively). However, the pooled prevalence with high heterogeneity should be interpreted with caution, as it could not be addressed by statistical method. The observed heterogeneity could have come from two main sources. In the first, the included prevalence studies are mostly hospital-based, and the diagnosis of cryptococcosis can vary significantly among hospitals and medical centers. Second, the different epidemiologic characteristics, such as geographically related difference in virulence among strains, the variable genetic susceptibilities to CM–SLE among geographic populations and the different methods of lupus controls among regions, may also have a role. Additional efforts should be made to determine the cause of this prevalence heterogeneity among regions and address the epidemiological profile differences of CM in SLE patients among Africa, Europe and Oceania.
This study revealed that patients taking a prednisone equivalent of ≥30 mg/day before infection had a higher mortality rate. In SLE patients, the CNS was commonly affected (20%–70%).4 Damage to the blood–brain barrier accelerated the permeability of corticosteroids, resulting in an increased risk of infection or a worse clinical outcome.28 In an SLE population, Staples et al.29 found that higher prednisone doses were associated with a higher infection rate, however, they did not adequately study the association between prednisone dosage and mortality. One study aimed to explore the relationship between prednisone dosage and mortality from IFIs in SLE.1 However, that investigation had a small sample size (n=15), and no statistically significant results were obtained. Our study is the first to reveal the relationship between prednisone dosage and mortality from CM in SLE, and this finding may help in directing the research on other IFIs in SLE patients. Our results suggested the importance of effective control of SLE with low dosages of corticosteroids. Further multicenter investigations among cryptococcosis patients with various autoimmune disease conditions need to be designed to address the problem of steroids as an independent risk factor for CM.
The current study highlighted the gap in SLE control between developing and developed countries. Patients in developing countries received higher doses of prednisone and were diagnosed with CM at an earlier stage of SLE than patients in developed countries. SLE patients were more susceptible to infection in developing countries where CM was usually ranked as the leading cause of death.30, 31 In developing countries, some medications (even for the basic management of SLE) are not always affordable or available, which has led to the overuse of steroids in place of antimalarial and immunosuppressant drugs and, consequently, a high risk of treatment-related infections. Low therapy adherence is an important risk factor for comorbidities. Low income and education level are associated with poor compliance to therapeutic regimens in lupus.32, 33 This phenomenon can be partially explained by the genetic characteristics of the SLE populations in developing countries.34, 35, 36 For example, low levels of mannose-binding lectin in Chinese patients with SLE increase the risk of bacterial infection. The management of SLE and prophylaxis for SLE-related infections remains a challenge, and rheumatologists and policymakers should make efforts to establish new management strategies based on the available resources to improve the current situation in developing countries.37
The SLEDAI score, a widely used tool for evaluating SLE activity based on clinical manifestation and laboratory tests, was <four points in 36.8% of CM patients. Among the remaining patients in whom the SLEDAI score was not evaluated, 39% exhibited no SLE activity. This percentage is relatively high compared with those reported in previous studies. Among SLE patients with IFIs, only 14% had a SLEDAI<4;7 among SLE patients with meningitis 10% had a SLEDAI<4;10 and among patients with CNS infection, 4.3%–6%8 had a SLEDAI<4.9 These results suggested that, even for patients with low SLE activity, the possibility of CM should not be underestimated.
More than one-third (38.2%) of CM cases among SLE patients was misdiagnosed. The most important cause of misdiagnosis is that most physicians (76.19%, 16/21) did not consider CM as a possible diagnosis initially. They made their initial diagnoses based on clinical manifestations or laboratory tests instead of LP followed by cryptococcal tests. CM was generally misdiagnosed as a non-fungal infection, CNS lupus and lupus disease activity. Rheumatologists are commonly unfamiliar with CM and tend to underestimate the prevalence of CM infection among SLE patients. The definitive diagnosis of CM is based on cerebral spinal fluid tests using microscopy (for example, India ink staining), culture and/or CrAg tests after the diagnosis of LP has been made. Although microscopic examination is rapid and inexpensive, it has limited clinical value because of its low sensitivity and specificity. Culture-based methods, as the reference standard for diagnosis, are slow and have a low sensitivity. In contrast, CrAg tests are simple to perform, are less time-consuming, and have a high sensitivity and specificity. In particular, a newly developed CrAg test, namely, the lateral flow immunoassay, was demonstrated to be affordable, sensitive, specific and rapid.38, 39 In the reviewed cases, two physicians performed cryptococcal tests other than the CrAg test once the infection emerged, and the CM diagnosis was hampered by the low sensitivity of India ink staining and the time necessary for culture. Therefore, we recommend CrAg tests (especially lateral flow immunoassay) as the routine tests for SLE patients with CNS symptoms and signs.
The management of IFIs, especially CM, is hampered by the unaffordability or unavailability of antifungal agents in resource-limited settings. Notably, our study found that 33% of deaths were attributed to the refusal to take antifungal agents after the establishment of CM diagnosis because the patient could not afford it. In addition, some basic antifungal agents were unavailable in some resource-limited countries. For example, flucytosine is not available in Malaysia. The revised guideline of the Infectious Diseases Society of America40 divides CM patients into three treatment categories, namely, HIV-infected individuals, organ transplant recipients and ‘non-HIV-infected, non-transplant' hosts (including SLE patients). The ‘non-HIV-infected, non-transplant hosts' category of CM patients includes both otherwise healthy patients and patients with underlying diseases (for example, autoimmune diseases and cancer). At present, only one set of therapeutic strategy is recommended by this guideline for this category of CM patients despite the heterogeneity within this patient group. In our study, 38.2% patients received corticosteroids to control lupus or as adjunctive therapy to antifungal treatment. However, a recent multicenter randomized controlled trial recommended against the use of corticosteroids with antifungal treatment because of the fact that dexamethasone failed to lower the death rate among HIV-associated CM patients while increasing adverse events (for example, infection, gastrointestinal disorders, renal disorders and cardiac events) compared with a placebo group.41 Because SLE control is essential to infection treatment, corticosteroids cannot be simply discontinued. Instead, we believe that the appropriate dosage and timing of corticosteroid use should be further explored. Indeed, more clinical trials (especially randomized controlled trials) should be conducted in the future to facilitate the development of different therapeutic strategies for various populations within the ‘non-HIV-infected, non-transplant hosts' category.
CM is one of the most important CNS infections in SLE patients, with an estimated prevalence of 0.5%. However, CM maybe easily underestimated and misdiagnosed because of the lack of SLE activity and because of the non-standard diagnostic strategy for CM. Emphasis should be placed on prophylaxis for SLE-related infections and management of CM, especially in developing countries where basic agents are unavailable or unaffordable. More efforts, including prevalence studies and clinical trials, are necessary to increase our understanding and improve our control of this disease.
Table 2. Laboratory examinations.
| Median | Interquartile range | |
|---|---|---|
| Cerebrospinal fluid | ||
| Intracranial pressure (mmH2O)* | 260 | 200, 360 |
| Glucose (mg/L) | 37.8 | 20.16, 50 |
| Protein (mg/L) | 1001 | 500, 1610 |
| Chlorine (mmol/L) | 119 | 112.7, 126 |
| WBC (/μL) | 32 | 4, 85 |
| Blood | ||
| WBC (106/μL) | 6500 | 5300, 9800 |
| Lymphocyte (106/μL) | 725.9 | 490, 1280 |
| C reactive protein (mg/dL) | 17.95 | 3.5, 99.3 |
| Erythrocyte sedimentation rate (mm/h) | 63 | 34, 102 |
| Complement component 3 (mg/dL) | 78.15 | 58.5, 112.5 |
| Complement component 4 (mg/dL) | 11.2 | 9, 31.6 |
Abbreviation: White blood cell, WBC.
Acknowledgments
This work was supported by the Ministry of Science and Technology of China (grant number 2013CB531601, 2013CB531606 and 2013ZX10004612); the National Natural Science Foundation of China (grant number 81201269 and 31270180); Shanghai Science and Technology Committee (grant number 14DZ2272900 and 14495800500); and Qatar National Research Fund (grant number NPRP 5-298-3-086). The authors are solely responsible for the content of this report.
Footnotes
Supplementary Information for this article can be found on the Emerging Microbes & Infections website (http://www.nature.com/emi)
Supplementary Material
References
- Chen HS, Tsai WP, Leu HS, Ho HH, Liou LB. Invasive fungal infection in systemic lupus erythematosus: an analysis of 15 cases and a literature review. Rheumatology (Oxford) 2007; 46: 539–544. [DOI] [PubMed] [Google Scholar]
- Chen GL, Chen Y, Zhu CQ, Yang CD, Ye S. Invasive fungal infection in Chinese patients with systemic lupus erythematosus. Clin Rheumatol 2012; 31: 1087–1091. [DOI] [PubMed] [Google Scholar]
- Gonzalez LA, Vasquez G, Restrepo JP, Velasquez M, Ramirez LA. Cryptococcosis in systemic lupus erythematosus: a series of six cases. Lupus 2010; 19: 639–645. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Mendonca LL, Dolman DE. The blood-brain barrier in systemic lupus erythematosus. Lupus 2003; 12: 908–915. [DOI] [PubMed] [Google Scholar]
- Hagen F, Khayhan K, Theelen B et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol 2015; 78: 16–48. [DOI] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23: 525–530. [DOI] [PubMed] [Google Scholar]
- Wang LR, Barber CE, Johnson AS, Barnabe C. Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum 2014; 44: 325–330. [DOI] [PubMed] [Google Scholar]
- Vargas PJ, King G, Navarra SV. Central nervous system infections in Filipino patients with systemic lupus erythematosus. Int J Rheum Dis 2009; 12: 234–248. [DOI] [PubMed] [Google Scholar]
- Hung JJ, Ou LS, Lee WI, Huang JL. Central nervous system infections in patients with systemic lupus erythematosus. J Rheumatol 2005; 32: 40–43. [PubMed] [Google Scholar]
- Kim JM, Kim KJ, Yoon HS et al. Meningitis in Korean patients with systemic lupus erythematosus: analysis of demographics, clinical features and outcomes; experience from affiliated hospitals of the Catholic University of Korea. Lupus 2011; 20: 531–536. [DOI] [PubMed] [Google Scholar]
- Lu XY, Zhu CQ, Qian J et al. Intrathecal cytokine and chemokine profiling in neuropsychiatric lupus or lupus complicated with central nervous system infection. Lupus 2010; 19: 689–695. [DOI] [PubMed] [Google Scholar]
- Yang CD, Wang XD, Ye S et al. Clinical features, prognostic and risk factors of central nervous system infections in patients with systemic lupus erythematosus. Clin Rheumatol 2007; 26: 895–901. [DOI] [PubMed] [Google Scholar]
- Akcaglar S, Sevgican E, Akalin H, Ener B, Tore O. Two cases of cryptococcal meningitis in immunocompromised patients not infected with HIV. Mycoses 2007; 50: 235–238. [DOI] [PubMed] [Google Scholar]
- Mok CC, Lau CS, Yuen KY. Cryptococcal meningitis presenting concurrently with systemic lupus erythematosus. Clin Exp Rheumatol 1998; 16: 169–171. [PubMed] [Google Scholar]
- Diaz Coto JF, Alpizar Campos R. [Central nervous system cryptococcosis in 10 patients with systemic lupus erythematosus]. Rev Clin Esp 1995; 195: 12–15. [PubMed] [Google Scholar]
- Khairullah S, Sulaiman H, Yahya F et al. Cryptococcal meningitis and SLE: a diagnostic and therapeutic challenge. Acta Reumatol Port 2014; 39: 254–258. [PubMed] [Google Scholar]
- Chen YJ, Hu P, Xu SZ. [Treating cryptococcal meningitis with combination of fluconazole and itraconazole: report of one case]. Chin J Dermatol 1999; 32: 216–217. [Google Scholar]
- Ventura Aguiar P, Lopes V, Martins LS et al. Cryptococcal infection in non-HIV immunosuppressed patients – three case reports in a nephrology setting. Med Mycol Case Rep 2014; 3: 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Singh YP, Agarwal V et al. Cryptococcal meningitis in systemic lupus erythematosus—Magnetic resonance imaging is not good. Indian J Rheum 2011; 6: 50–52. [Google Scholar]
- Suzuki K, Nakase K, Ino K et al. Breakthrough cryptococcosis in a patient with systemic lupus erythematosus (SLE) receiving micafungin. J Infect Chemother 2008; 14: 311–314. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Reynolds WE, Franklin EC et al. Preliminary criteria for the classification of systemic lupus erythematosus. Bull Rheum Dis 1971; 21: 643–648. [Google Scholar]
- Yang Y, Sang J, Pan W et al. Cryptococcal meningitis in patients with autoimmune hemolytic anemia. Mycopathologia 2014; 178: 63–70. [DOI] [PubMed] [Google Scholar]
- Mitchell DH, Sorrell TC, Allworth AM et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis 1995; 20: 611–616. [DOI] [PubMed] [Google Scholar]
- Zeng QY, Chen R, Darmawan J et al. Rheumatic diseases in China. Arthritis Res Ther 2008; 10: R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik M, Marin J, Valeiras SM et al. Incidence and prevalence of lupus in Buenos Aires, Argentina: a 11-year health management organisation-based study. Lupus Sci Med 2014; 1: e000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Harigai M, Omori M, Sato E, Hara M. Blood-brain barrier damage as a risk factor for corticosteroid-induced psychiatric disorders in systemic lupus erythematosus. Psychoneuroendocrinology 2008; 33: 395–403. [DOI] [PubMed] [Google Scholar]
- Staples PJ, Gerding DN, Decker JL, Gordon RS Jr. Incidence of infection in systemic lupus erythematosus. Arthritis Rheum 1974; 17: 1–10. [DOI] [PubMed] [Google Scholar]
- Shayakul C, Ong-aj-yooth L, Chirawong P et al. Lupus nephritis in Thailand: clinicopathologic findings and outcome in 569 patients. Am J Kidney Dis 1995; 26: 300–307. [DOI] [PubMed] [Google Scholar]
- Wadee S, Tikly M, Hopley M. Causes and predictors of death in South Africans with systemic lupus erythematosus. Rheumatology 2007; 46: 1487–1491. [DOI] [PubMed] [Google Scholar]
- Garcia Popa-Lisseanu MG, Greisinger A, Richardson M et al. Determinants of treatment adherence in ethnically diverse, economically disadvantaged patients with rheumatic disease. J Rheumatol 2005; 32: 913–919. [PubMed] [Google Scholar]
- Garcia-Gonzalez A, Richardson M, Garcia Popa-Lisseanu M et al. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol 2008; 27: 883–889. [DOI] [PubMed] [Google Scholar]
- Lau YL, Lau CS, Chan SY, Karlberg J, Turner MW. Mannose-binding protein in Chinese patients with systemic lupus erythematosus. Arthritis Rheum 1996; 39: 706–708. [DOI] [PubMed] [Google Scholar]
- Navarra SV, Villamin CA, Baes RP et al. Increased frequency of mannose-binding lectin promoter LX haplotype among Filipinos with systemic lupus erythematosus. Lupus 2007; 16: 147–148. [DOI] [PubMed] [Google Scholar]
- Mok MY, Ip WK, Lau CS et al. Mannose-binding lectin and susceptibility to infection in Chinese patients with systemic lupus erythematosus. J Rheumatol 2007; 34: 1270–1276. [PubMed] [Google Scholar]
- Tikly M, Navarra SV. Lupus in the developing world—is it any different? Best Pract Res Clin Rheumatol 2008; 22: 643–655. [DOI] [PubMed] [Google Scholar]
- Chen M, Zhou J, Li J et al. Evaluation of five conventional and molecular approaches for diagnosis of cryptococcal meningitis in non-HIV-infected patients. Mycoses 2016; 59: 494–502. [DOI] [PubMed] [Google Scholar]
- Lindsley MD, Mekha N, Baggett HC et al. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis 2011; 53: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR, Dismukes WE, Dromer F et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50: 291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley J, Wolbers M, Kibengo FM et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016; 11: 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.