Abstract
Background and objectives
Although morning stiffness has long been recognized as a characteristic feature of rheumatoid arthritis (RA), it is no more included in the 2010 ACR/EULAR Classification Criteria or in the current major instruments for evaluating disease activity of RA. In this cross-sectional study, we aimed to determine the independent value and the optimal measurement of morning stiffness by clarifying the associations between morning stiffness and synovial inflammation.
Patients and methods
We enrolled 76 consecutive RA patients who underwent musculoskeletal ultrasound examination and agreed to participate in the study. In addition to asking the duration of morning stiffness, we asked patients to complete a diagram which represents the time course of their morning stiffness in the dominant hand. Based on this diagram, we calculated the severity and the diurnal improvement of morning stiffness. We also determined the activity of intra-articular synovitis in 11 joints and tenosynovitis in 8 tendons/tendon compartments in the same hand by using power Doppler (PD) ultrasound with a semiquantitative score (0–3).
Results
For intra-articular synovitis, swollen/tender joint counts more strongly correlated with total PD scores (ρ = 0.379–0.561, p ≤ 0.001) than did any parameters of morning stiffness (ρ = 0.217–0.314, p = 0.006–0.021). For tenosynovitis, however, the severity on awakening and the improvement of morning stiffness more strongly correlated with total PD scores (ρ = 0.503–0.561, p < 0.001) than did swollen/tender joint counts (ρ = 0.276–0.388, p = 0.001–0.016). Multivariate analyses identified the severity on awakening and the improvement but not the duration of morning stiffness as factors that independently associate with the total tenosynovial PD score.
Conclusions
Our data demonstrate a pathophysiological link between morning stiffness and tenosynovitis and also give an insight into the optimal measurement of morning stiffness. Our data support an independent value of evaluating morning stiffness in the management of RA.
Introduction
Morning stiffness has long been recognized by both patients and rheumatologists as a characteristic feature of rheumatoid arthritis (RA) [1] and was thus included in ACR 1981 remission criteria [2] and 1987 diagnostic criteria for RA [3]. Thereafter, morning stiffness has been widely used in decision-making on changing medication in daily practice [4, 5] and in clinical trials [6, 7]. Studies have also shown that stiffness has considerable impact on patients’ daily life and work [8–11].
Nevertheless, morning stiffness is no more included in 2010 ACR/EULAR Classification Criteria for RA [12, 13] or in the current major instruments for evaluating disease activity of RA such as Disease Activity Score (DAS) [14–16], ACR Core Set [17], Simplified Disease Activity Index (SDAI) [18], and 2011 ACR/EULAR Provisional Definition of Remission [19]. Some studies which these instruments/definitions are based on failed to demonstrate an independent value of assessing morning stiffness [14, 20].
Most of the previous studies measured the duration of morning stiffness [6, 21], while there is conflicting evidence on the optimal measurement of morning stiffness (e.g. duration vs. severity) [22]. In addition, although the typical morning stiffness with inflammatory disorders is considered to improve with activities [23], this possibly important feature of morning stiffness is captured neither by duration nor severity. Furthermore, the lack of an objective, external comparator that represents disease activity, such as imaging findings, was also a major limitation of the previous studies.
In this study, we captured the time course of morning stiffness by employing a patient-reported diagram. We also determined the activity of intra-articular synovitis and tenosynovitis by using musculoskeletal ultrasound, which has been reported to be more accurate than clinical information [24–26], aiming to elucidate the associations between morning stiffness and synovial inflammation.
Methods
Patients
We recruited consecutive patients who fulfilled the 2010 ACR/EULAR Classification Criteria for RA [12, 13] and underwent musculoskeletal ultrasound in our hospitals from April 2014 to March 2015. Patients receiving corticosteroids ≥ 10 mg/day were excluded.
The study was approved by Ethics Committee of Chiba University and patients’ written informed consent was obtained according to Declaration of Helsinki.
Clinical and laboratory assessment
We collected information on patients’ characteristics and current treatment from medical records. We performed clinical evaluation of disease activity in the morning (8:30–12:00) and calculated DAS28 (C-reactive protein-based) [15] and SDAI [18].
Assessment of morning stiffness
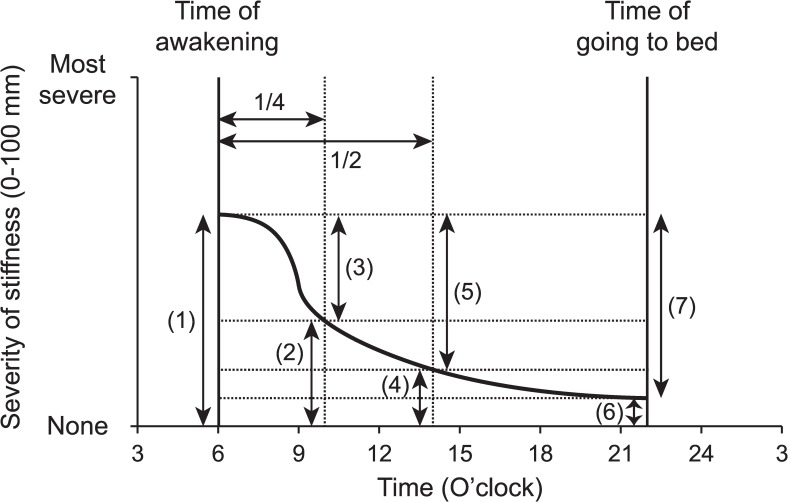
We asked patients about their representative morning stiffness during the past week in their hand with stronger arthritic symptoms. In addition to the duration (i.e. time until maximum improvement [22]), we asked patients to report the severity and time course of their morning stiffness using a diagram (Fig 1). Patients were first asked to place vertical lines which indicated their representative times of awakening and going to bed, respectively. Patients were next asked to indicate the severity of stiffness on wakening based on the visual analogue scale (VAS) (0–100 mm) and draw a free curved line which demonstrates the time course of stiffness during awake time.
Fig 1. An example diagram for time course of morning stiffness in dominant hand.
(1) Severity of stiffness on awakening, (2) Severity of stiffness at the first 1/4 of total awake time, (3) Improvement of stiffness at the first 1/4 of total awake time, (4) Severity of stiffness at the first 1/2 of total awake time, (5) Improvement of stiffness at the first 1/2 of total awake time, (6) Severity of stiffness at bed time, (7) Improvement of stiffness at bed time.
Based on this diagram, we determined the severity of stiffness on awakening, at the first 1/4 of total awake time, at the first 1/2 of total awake time, and at bed time. We also determined the diurnal improvement of morning stiffness at these time points by subtraction (Fig 1).
Musculoskeletal ultrasound
Ultrasound was performed within an hour after clinical and laboratory evaluation in an air-conditioned room (temperature 24–26°C) by rheumatologists trained for musculoskeletal ultrasound, who were blinded to the clinical/laboratory information. Gray-scale (GS) and power Doppler (PD) ultrasound examination of dominant hand was performed using either a HI VISION Avius, a HI VISION Ascendus (Hitachi Medical Corporation, Tokyo, Japan), or an Aplio XG (Toshiba Medical Systems Corporation, Tochigi, Japan), depending on availability. For intra-articular synovitis, 11 joints (1st interphalangeal and 2nd-5th proximal interphalangeal joints, 1st-5th metacarpophalangeal joints, and wrist [radiocarpal/midcarpal/distal radioulnar joints]) were scanned from the dorsal aspect. For tenosynovitis, 8 tendons/tendon compartments (1st-5th flexor digitorum and extensor compartment II/IV/VI of the wrist) were scanned. Machine setting for PD ultrasound was optimized as previously described [25–27].
The severity of ultrasound findings was graded semi-quantitatively on a scale of 0–3 as previously described [25–27]. Each patient’s intra-articular synovitis and tenosynovitis scores (i.e. total GS score and total PD score) were calculated by summing the corresponding GS and PD scores of 11 joints and 8 tendons/tendon compartments, respectively.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 22 (IBM Japan, Tokyo, Japan). Normally distributed continuous data were summarized with mean and standard deviation (SD). Non-normally distributed data were summarized with median and interquartile range (IQR) and were analyzed using Spearman’s correlation coefficient. Categorical data were summarized with number and percentage. Multivariate linear regression analysis was performed using forced entry method. Two-sided P values less than 0.05 were considered significant.
Results
Patients’ characteristics and disease activity
A total of 76 patients fulfilling the inclusion/exclusion criteria were enrolled. These patients were predominated by women (78.9%) with a mean age of 58.4 (SD 14.6) years and a median disease duration of 24 (IQR 8–63.75) months. Rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) had been positive in 84.2% and 77.6%, respectively (Table 1).
Table 1. Patients’ characteristics, current treatment, and disease activity.
| Patients’ characteristics | ||
| Age, mean ± SD years | 58.4 ± 14.6 | |
| Female, n (%) | 60 (78.9) | |
| Disease duration, median (IQR) months | 24 (8–63.75) | |
| RF positive, n (%) | 64 (84.2) | |
| ACPA positive, n (%) | 59 (77.6) | |
| Concomitant autoimmune disease, n (%) | 11 (14.5) | |
| Current treatment | ||
| Treatment naïve, n (%) | 17 (22.4) | |
| Methotrexate, n (%) | 39 (51.3) | |
| Dose, median (IQR) mg/week | 10 (8–12) | |
| Salazosulfapyridine (Sulfasalazine), n (%) | 19 (25.0) | |
| Bucillamine, n (%) | 4 (5.3) | |
| Tacrolimus, n (%) | 3 (3.9) | |
| Auranofin, n (%) | 1 (1.3) | |
| TNF antagonist, n (%) | 8 (10.5) | |
| Tocilizumab, n (%) | 4 (5.3) | |
| Abatacept, n (%) | 3 (3.9) | |
| Corticosteroid, n (%) | 22 (28.9) | |
| Dose, median (IQR) mg/day (prednisolone equivalent) | 4.5 (2.375–5) | |
| NSAID, n (%) | 42 (55.3) | |
| Disease activity | ||
| Swollen joint count/ 28, median (IQR) | 2.5 (1–4) | |
| Tender joint count/ 28, median (IQR) | 2 (0–4.75) | |
| Patient’s global assessment VAS, mean ± SD mm | 39.4 ± 23.1 | |
| Physician’s global assessment VAS, mean ± SD mm | 36.3 ± 22.8 | |
| Serum C-reactive protein (CRP) level, median (IQR) mg/dL | 0.3 (0.0–1.1) | |
| DAS28-CRP, median (IQR) | 3.1 (2.1–4.2) | |
| SDAI, median (IQR) | 12.8 (7–20.675) | |
SD, standard deviation; IQR, interquartile range; RF, rheumatoid factor; ACPA, anti-citrullinated protein antibody; DMARD, disease modifying anti-rheumatic drug; TNF, tumor necrosis factor; VAS, visual analogue scale; DAS28, Disease Activity Score 28; SDAI, Simplified Disease Activity Index
Nineteen patients (25.0%) underwent ultrasonography for diagnosis and 17 (22.4%) were treatment-naïve. Fifty-seven patients (75.0%) underwent ultrasonography for the evaluation of disease activity. Thirty-nine patients (51.3%) were receiving methotrexate with a median dose of 10 mg/week, 19 (25.0%) salazosulfapyridine, and 15 (19.7%) biologics (TNF antagonists 8, tocilizumab 4, and abatacept 3). Twenty-two patients (28.9%) were receiving corticosteroids with a median dose of prednisolone 4.5 mg/day and 42 (55.3%) non-steroidal anti-inflammatory drugs (NSAIDs) (Table 2).
Table 2. Local clinical manifestations and ultrasound scores in dominant hand.
| Joint count | ||
| Swollen joint count/ 11, median (IQR) | 1 (1–3) | |
| Tender joint count/ 11, median (IQR) | 1 (0–2) | |
| Morning stiffness | ||
| Duration, median (IQR) minutes | 30 (5–83.75) | |
| Severity VAS on wakening, median (IQR) mm | 43 (5–60) | |
| Severity VAS at 1/4, median (IQR) mm | 5 (0–26.75) | |
| Severity VAS at 1/2, median (IQR) mm | 0 (0–13.75) | |
| Severity VAS at bedtime, median (IQR) mm | 0 (0–5) | |
| Ultrasound | ||
| Intra-articular synovitis | ||
| Gray-scale score, median (IQR) | 3 (1–6) | |
| Power Doppler score, median (IQR) | 1 (0–4) | |
| Tenosynovitis | ||
| Gray-scale score, median (IQR) | 1 (0–4) | |
| Power Doppler score, median (IQR) | 0 (0–2) | |
IQR, interquartile range; VAS, visual analogue scale
Median DAS28 and median SDAI were 3.1 (IQR 2.1–4.2) and 12.8 (7–20.675), respectively (Table 1).
Clinical and sonographic findings in dominant hand
Clinical and sonographic findings in the dominant hand are summarized in Table 2. Both swollen/tender joint counts and sonographic scores were low, which was consistent with median DAS28 and SDAI values. Median duration of morning stiffness in the same hand was 30 (IQR 5–83.75) minutes. While median severity VAS of hand stiffness on wakening was 43 (IQR 5–60) mm, the majority of patients report disappearance of morning stiffness within the first half of total awake time (Table 2).
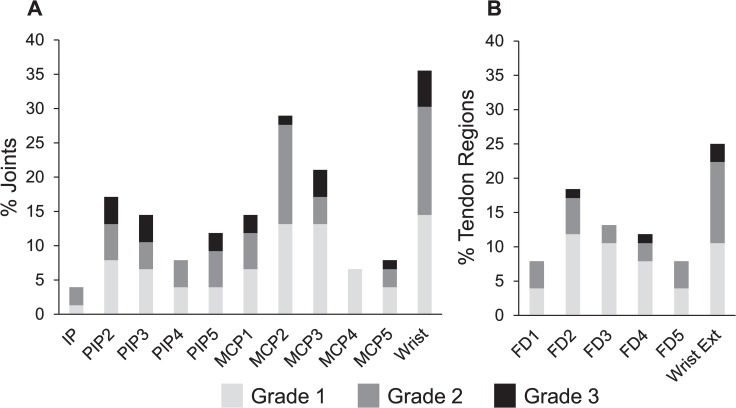
Fig 2 shows the prevalence of each PD grade > 0 in each joint and each tendon region. Although there was some similarity in the pattern of distribution between intra-articular synovitis (Fig 2A) and tenosynovitis (Fig 2B), these two types of synovial inflammation did not always coexist in the same anatomical area (agreement 50–63%, κ value 0.200–0.388 [data not shown]).
Fig 2. Prevalence of power Doppler signals in each joint/tendon region.
Prevalence of each power Doppler (PD) grade > 0 in each joint (A) and each tendon region (B). IP, interphalangeal joint; PIP, proximal interphalangeal joint; MCP, metacarpo-phalangeal joint; FD, flexor digitorum; Wrist Ext, wrist extensor tendons.
Associations between clinical and sonographic findings
We first examined the associations between clinical findings in the dominant hand and total PD scores in the same hand by univariate analysis (Table 3). For intra-articular synovitis, swollen/tender joint counts more strongly correlated with total PD scores (ρ = 0.379–0.561, p ≤ 0.001) than did any parameters of morning stiffness (ρ = 0.217–0.314, p = 0.006–0.021). For tenosynovitis, however, the severity VAS on awakening and the improvement afterwards more strongly correlated with total PD scores (ρ = 0.503–0.561, p < 0.001) than did swollen/tender joint counts (ρ = 0.276–0.388, p = 0.001–0.016).
Table 3. Correlations between clinical manifestations and ultrasound scores in dominant hand.
| Joint count | Morning stiffness | |||||||
|---|---|---|---|---|---|---|---|---|
| Swollen joint | Tender joint | Duration | Severity VAS on awakening | Improvement of VAS at 1/4 | Improvement of VAS at 1/2 | Improvement of VAS at bedtime | ||
| Intra-articular synovial power Doppler score | ||||||||
| ρ* | 0.561 | 0.379 | 0.265 | 0.314 | 0.217 | 0.266 | 0.306 | |
| p value * | < 0.001 | 0.001 | 0.021 | 0.006 | 0.060 | 0.020 | 0.007 | |
| Tenosynovial power Doppler score | ||||||||
| ρ* | 0.388 | 0.276 | 0.280 | 0.503 | 0.505 | 0.561 | 0.538 | |
| p value * | 0.001 | 0.016 | 0.014 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
* Spearman’s correlation coefficient
VAS, visual analogue scale
We next examined whether the parameters of morning stiffness independently associate with total PD scores by multivariate analysis (Table 4). We tested linear regression models which incorporated swollen/tender joint counts and either one of the 5 parameters on morning stiffness as explanatory variables. For intra-articular synovitis, none of the 5 models retained morning stiffness as a variable which independently associated with total PD score. For tenosynovitis, however, 4 out of the 5 models retained morning stiffness as a single independent variable that significantly associated with total PD score. The duration was the only parameter on morning stiffness that was not identified as a significant independent factor in multivariate analysis.
Table 4. Multivariate linear regression models to identify factors which independently associate with power Doppler scores.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS: duration | MS: severity VAS on awakening | MS: VAS improvement at first 1/4 of awake time | MS: VAS improvement at first 1/2 of awake time | MS: VAS improvement at bedtime | |||||||||||
| SJC | TJC | MS | SJC | TJC | MS | SJC | TJC | MS | SJC | TJC | MS | SJC | TJC | MS | |
| Intra-articular synovial power Doppler score | |||||||||||||||
| β | 0.506 | 0.243 | -0.039 | 0.469 | 0.240 | 0.069 | 0.465 | 0.250 | 0.083 | 0.453 | 0.247 | 0.119 | 0.446 | 0.244 | 0.126 |
| p value | < 0.001 | 0.025 | 0.659 | < 0.001 | 0.027 | 0.499 | < 0.001 | 0.021 | 0.388 | < 0.001 | 0.022 | 0.213 | < 0.001 | 0.023 | 0.197 |
| Tenosynovial power Doppler score | |||||||||||||||
| β | 0.218 | 0.210 | -0.067 | 0.018 | 0.200 | 0.384 | 0.057 | 0.243 | 0.332 | 0.028 | 0.228 | 0.429 | 0.013 | 0.216 | 0.436 |
| p value | 0.105 | 0.119 | 0.541 | 0.896 | 0.112 | 0.002 | 0.680 | 0.059 | 0.005 | 0.832 | 0.063 | < 0.001 | 0.919 | 0.078 | < 0.001 |
MS, morning stiffness; VAS, visual analogue scale; SJC, swollen joint count; TJC, tender joint count; β, standardized coefficient
Discussion
The major strength of this study is that we determined the activity of intra-articular synovitis and tenosynovitis with PD ultrasound, which has been reported to be more accurate than clinical information [24–26]. Our data indicate that morning stiffness is more closely related with tenosynovitis than it is with intra-articular synovitis. Although the association of morning stiffness with tenosynovitis was investigated decades ago [28, 29], the current study is the first to compare its associations between tenosynovitis and intra-articular synovitis using modern ultrasound equipment.
Tenosynovitis has been recognized as a significant and important feature of RA in studies using modern imaging modalities. Tenosynovitis has been shown to be prevalent and responsive to anti-rheumatic treatment [30, 31], associates with functional disability [32], and predicts erosive disease [33]. Because data from these studies and ours show that joint counts are insufficient to reflect tenosynovitis, evaluating morning stiffness, which can better reflect tenosynovitis, is indispensable in the assessment of disease activity and prognosis of RA.
Another advantage of this study is that we devised a method using a patient-reported diagram to capture the time course of morning stiffness. Our data demonstrate that the severity and the diurnal improvement of morning stiffness are more closely related to the activity of synovial inflammation than is its duration. This does not only apply to tenosynovitis but seems to also apply to intra-articular synovitis (Tables 1 and 2). These data are concordant with the long-standing notion that typical inflammatory joint symptoms are the worst on wakening and improve with activities [23]. Our data indicate that the severity and diurnal improvement are more important than the duration when we ask patients about morning stiffness in order to estimate the activity of synovial inflammation. However, the other aspects of stiffness may have direct impact on patients’ experience and independent meaning as a patient-reported outcome [34].
This study has some limitations. Firstly, relatively small sample size did not allow for stratification by background information such as disease duration and activity. Results may have been different in patients with longer disease duration or higher disease activity with a larger number of involved joints. In addition, we cannot exclude the possibility of selection bias although patients’ characteristics were representative of RA patients in our daily practice. Secondly, our data may not be applicable to joints other than hands. However, hands are the region that is most frequently involved in RA and where patients most frequently reports morning stiffness [1], and are likely to reflect pathophysiology typical of RA. Thirdly, we did not assess patients with other diseases that cause morning stiffness. The presence of a disease control group could have clarified the specificity of our findings in RA patients.
In conclusion, our data demonstrate a pathophysiological link between morning stiffness and tenosynovitis and give an insight into the optimal measurement of morning stiffness. Moreover, our data add to the evidence supporting an independent value of evaluating morning stiffness in the management of RA.
Supporting Information
(XLSX)
Acknowledgments
We thank all hospital staff who gave care to the patients.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Scott JT. Morning stiffness in rheumatoid arthritis. Ann Rheum Dis. 1960;19:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinals RS, Masi AT, Larsen RA. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum. 1981;24:1308–1315. [DOI] [PubMed] [Google Scholar]
- 3.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 4.Kirwan JR, Chaput de Saintonge DM, Joyce CR, Currey HL. Clinical judgment in rheumatoid arthritis. III. British rheumatologists' judgments of 'change in response to therapy'. Ann Rheum Dis. 1984;43:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soubrier M, Zerkak D, Gossec L, Ayral X, Roux C, Dougados M. Which variables best predict change in rheumatoid arthritis therapy in daily clinical practice? J Rheumatol. 2006;33:1243–1246. [PubMed] [Google Scholar]
- 6.Kalyoncu U, Dougados M, Daures JP, Gossec L. Reporting of patient-reported outcomes in recent trials in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2009;68:183–190. 10.1136/ard.2007.084848 [DOI] [PubMed] [Google Scholar]
- 7.Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet. 2008;371:205–214. 10.1016/S0140-6736(08)60132-4 [DOI] [PubMed] [Google Scholar]
- 8.Yazici Y, Pincus T, Kautiainen H, Sokka T. Morning stiffness in patients with early rheumatoid arthritis is associated more strongly with functional disability than with joint swelling and erythrocyte sedimentation rate. J Rheumatol. 2004;31:1723–1726. [PubMed] [Google Scholar]
- 9.Westhoff G, Buttgereit F, Gromnica-Ihle E, Zink A. Morning stiffness and its influence on early retirement in patients with recent onset rheumatoid arthritis. Rheumatology (Oxford). 2008;47:980–984. [DOI] [PubMed] [Google Scholar]
- 10.Orbai AM, Smith KC, Bartlett SJ, De Leon E, Bingham CO 3rd. "Stiffness has different meanings, I think, to everyone": examining stiffness from the perspective of people living with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66:1662–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva JA, Phillips S, Buttgereit F. Impact of impaired morning function on the lives and well-being of patients with rheumatoid arthritis. Scand J Rheumatol Suppl. 2011;125:6–11. 10.3109/03009742.2011.566434 [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 14.van der Heijde DM, van 't Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49:916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–960. 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. [DOI] [PubMed] [Google Scholar]
- 17.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729–740. [DOI] [PubMed] [Google Scholar]
- 18.Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 2003;42:244–257. [DOI] [PubMed] [Google Scholar]
- 19.Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–413. 10.1136/ard.2011.149765 [DOI] [PubMed] [Google Scholar]
- 20.Funovits J, Aletaha D, Bykerk V, Combe B, Dougados M, Emery P, et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: methodological report phase I. Ann Rheum Dis. 2010;69:1589–1595. 10.1136/ard.2010.130310 [DOI] [PubMed] [Google Scholar]
- 21.Cutolo M. How should morning function in rheumatoid arthritis be assessed? Bibliographic study of current assessment. Scand J Rheumatol Suppl. 2011;125:17–22. 10.3109/03009742.2011.566436 [DOI] [PubMed] [Google Scholar]
- 22.Hazes JM, Hayton R, Silman AJ. A reevaluation of the symptom of morning stiffness. J Rheumatol. 1993;20:1138–1142. [PubMed] [Google Scholar]
- 23.Cash J. Approach to Articular and Musculoskeletal Disorders In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 19th ed. New York: McGraw-Hill; 2015. p. 2216–2225. [Google Scholar]
- 24.Colebatch AN, Edwards CJ, Ostergaard M, van der Heijde D, Balint PV, D'Agostino MA, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–814. 10.1136/annrheumdis-2012-203158 [DOI] [PubMed] [Google Scholar]
- 25.Ikeda K, Nakagomi D, Sanayama Y, Yamagata M, Okubo A, Iwamoto T, et al. Correlation of radiographic progression with the cumulative activity of synovitis estimated by power Doppler ultrasound in rheumatoid arthritis: difference between patients treated with methotrexate and those treated with biological agents. J Rheumatol. 2013;40:1967–1976. 10.3899/jrheum.130556 [DOI] [PubMed] [Google Scholar]
- 26.Nakagomi D, Ikeda K, Okubo A, Iwamoto T, Sanayama Y, Takahashi K, et al. Ultrasound can improve the accuracy of the 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis to predict the requirement for methotrexate treatment. Arthritis Rheum. 2013;65:890–898. 10.1002/art.37848 [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto T, Ikeda K, Hosokawa J, Yamagata M, Tanaka S, Norimoto A, et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission. Arthritis Care Res (Hoboken). 2014;66:1576–1581. [DOI] [PubMed] [Google Scholar]
- 28.Hug C, Huber H, Terrier F, Hauselmann HJ, Aue W, Vock P, et al. Detection of flexor tenosynovitis by magnetic resonance imaging: its relationship to diurnal variation of symptoms. J Rheumatol. 1991;18:1055–1059. [PubMed] [Google Scholar]
- 29.Millis MB, Millender LH, Nalebuff EA. Stiffness of the proximal interphalangeal joints in rheumatoid arthritis. The role of flexor tenosynovitis. J Bone Joint Surg Am. 1976;58:801–805. [PubMed] [Google Scholar]
- 30.Hammer HB, Kvien TK. Ultrasonography shows significant improvement in wrist and ankle tenosynovitis in rheumatoid arthritis patients treated with adalimumab. Scand J Rheumatol. 2011;40:178–182. 10.3109/03009742.2010.517549 [DOI] [PubMed] [Google Scholar]
- 31.Haavardsholm EA, Ostergaard M, Hammer HB, Boyesen P, Boonen A, van der Heijde D, et al. Monitoring anti-TNFalpha treatment in rheumatoid arthritis: responsiveness of magnetic resonance imaging and ultrasonography of the dominant wrist joint compared with conventional measures of disease activity and structural damage. Ann Rheum Dis. 2009;68:1572–1579. 10.1136/ard.2008.091801 [DOI] [PubMed] [Google Scholar]
- 32.Burgers LE, Nieuwenhuis WP, van Steenbergen HW, Newsum EC, Huizinga TW, Reijnierse M, et al. Magnetic resonance imaging-detected inflammation is associated with functional disability in early arthritis-results of a cross-sectional study. Rheumatology (Oxford). 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.Lillegraven S, Boyesen P, Hammer HB, Ostergaard M, Uhlig T, Sesseng S, et al. Tenosynovitis of the extensor carpi ulnaris tendon predicts erosive progression in early rheumatoid arthritis. Ann Rheum Dis. 2011;70:2049–2050. 10.1136/ard.2011.151316 [DOI] [PubMed] [Google Scholar]
- 34.Orbai AM, Halls S, Hewlett S, Bartlett SJ, Leong AL, Bingham CO 3rd, et al. More than Just Minutes of Stiffness in the Morning: Report from the OMERACT Rheumatoid Arthritis Flare Group Stiffness Breakout Sessions. J Rheumatol. 2015;42:2182–2184. 10.3899/jrheum.141172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.