Abstract
Many future therapeutic applications of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 and related RNA-guided nucleases are likely to require their use to promote gene targeting, thus necessitating development of methods that provide for delivery of three components—Cas9, guide RNAs and recombination templates—to primary cells rendered proficient for homology-directed repair. Here, we demonstrate an electroporation/transduction codelivery method that utilizes mRNA to express both Cas9 and mutant adenoviral E4orf6 and E1b55k helper proteins in association with adeno-associated virus (AAV) vectors expressing guide RNAs and recombination templates. By transiently enhancing target cell permissiveness to AAV transduction and gene editing efficiency, this novel approach promotes efficient gene disruption and/or gene targeting at multiple loci in primary human T-cells, illustrating its broad potential for application in translational gene editing.
Introduction
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 and related programmable endonuclease systems have rapidly evolved into the workhorse gene editing tools of the biomedical research laboratory, with their application for gene disruption and gene targeting demonstrated in a variety of cultured cell and model organism systems (e.g., see various reviews on CRISPR applications1,2,3,4,5,6). Although the flexibility with which the Cas9 nuclease can be reprogrammed to target new sites is a major advantage for genome engineering in the research setting, several practical barriers limit the direct extension of research-based gene editing methods to editing of primary human cells for therapeutic purposes. Examples of some of these practical barriers include limited opportunities to identify and enrich for cells that have incurred a desired editing event; the requirement for transient (e.g., a few days) nuclease delivery due to safety and immunogenicity issues associated with longer term and/or in vivo nuclease expression; and limitations in vector systems for nuclease or recombination template delivery posed by primary cells' robust capacity to detect the presence of cytosolic DNA and consequent generation of antiviral or proapoptotic signals.7,8,9,10
Driven by the practical barriers delineated above, therapeutic gene editing strategies utilizing zinc finger nucleases, TAL effector nucleases, and meganucleases, have gravitated toward delivery approaches that ensure transient nuclease expression, most notably mRNA transfection, and the use of viral vectors for recombination template delivery.11,12,13,14,15,16,17,18 For these same reasons, mRNA-based CRISPR component expression has recently been extended to human primary cells for the purpose of gene disruption through the use of electroporation to deliver Cas9 mRNA or protein in conjunction with either native or degradation-resistant guide RNAs.19,20
While RNA or protein/RNA-based nuclease delivery are straightforward methods for disrupting individual genes, applications of CRISPR-based gene editing that involve gene targeting require efficient delivery of three components: Cas9, guide RNA, and a recombination template. Here we demonstrate an electroporation/transduction codelivery method for CRISPR/Cas9 gene editing that utilizes mRNA electroporation-mediated expression of Cas9 in conjunction with variants of two adenoviral serotype five proteins, E4orf6 and E1b55k,21,22,23,24,25,26 that transiently enhance both primary cells' capacity for transduction by adeno-associated virus (AAV) and gene editing efficiency. Using a cell culture/manufacturing protocol compatible with clinical translation, we demonstrate the application of this method for efficient gene disruption and homology-directed gene targeting in primary human T-cells.
Results
An mRNA/AAV delivery approach effects Cas9-mediated gene disruption in primary human T-cells
We have recently shown that AAV6 capsid-based AAV vectors are able to achieve sufficient transduction efficiencies of human primary T-cells and CD34+ cells to serve as templates for TALEN and megaTAL nuclease-catalyzed homologous recombination.18 Thus, we hypothesized that AAV vectors might serve as safe and effective vectors for transient expression of guide RNAs as well as delivery of recombination templates for Cas9-induced gene targeting.
To evaluate the potential of an mRNA/AAV delivery method in which spCas9 was expressed through mRNA electroporation, and an AAV vector was used to provide guide RNA expression, we generated an AAV construct which included both a U6 promoter driven guide RNA cassette and an MND promoter driven Green Fluorescent Protein (GFP) cassette—the latter provides for tracking of AAV transduction efficiency (Supplementary Figure S1a). We tested mRNA electroporation of Cas9 (as a Cas9-T2A-mCherry fusion) both before and after AAV transduction for guide delivery, and were able to achieve moderately efficient Cas9 cleavage within the constant region of the TCRα gene using several protocols with two different guides. Cas9 cleavage was detected as indel formation demonstrated by T7 assay of amplicons surrounding the predicted target site in TCRα (Supplementary Figure S1b), and as loss of surface TCR/CD3 complex expression by flow cytometry (TCR/CD3 complex expression requires expression of a functional TCRα chain, Supplementary Figure S1d). Through this series of experiments and our previous experience with other nuclease platforms,18 we observed that performing the mRNA electroporation step first appeared to work most reliably, and thus mRNA electroporation followed by AAV transduction was adopted as our standard approach. Using the mRNA/AAV transduction protocol, we further evaluated a range of Cas9 mRNA and AAV-guide doses (Supplementary Figure S1c,d) to determine ranges that maximize Cas9 cleavage efficiency and minimize toxicity—while mRNA dose appeared to saturate (1 μg in our standard electroporation conditions), we observed a dose-dependent increase in knockout with AAV up to the maximum tolerated mode of infection (MOI). We also compared both single stranded and self-complementary AAV vectors (Supplementary Figure S1e), and observed no significant differences between self-complementary and single stranded AAV in the efficiency of Cas9 target cleavage as assessed by loss of surface CD3.
Adenoviral serotype 5 E4orf6 and E1b55k helper proteins enhance permissiveness of primary human T-cells to AAV transduction
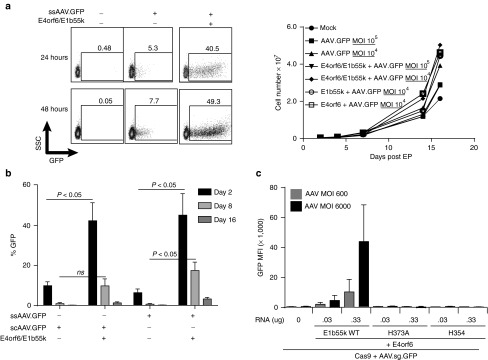
The dependence of Cas9 cleavage efficiency on AAV dose observed in our initial analyses suggested to us that efficiency of AAV transduction is a key limiting factor for application of the mRNA/AAV method in T-cells. AAV transduction in many human cell types is known to be subject to restriction at the cell entry stage by surface receptor expression binding properties of the capsid,27,28,29 and postentry based on multiple mechanisms.21,22,23,24,25 In cultured transformed cells, it has been previously shown that plasmid-based expression of E4orf6 and E1b55k proteins from multiple adenoviral serotypes is effective at relieving postentry restrictions on AAV expression, among them genome concatamerization by DNA damage response proteins, activation of cell cycle DNA damage checkpoints, and proapoptotic DNA damage signaling.21,22,23,24,25 As DNA plasmid-based expression is toxic to primary T-cells, we evaluated whether electroporation of adenoviral serotype 5 E4orf6/E1b55k mRNAs could achieve a transient relief of postentry restriction of AAV-based expression in primary T-cells (Figure 1). Following mRNA-based coexpression of E4orf6/E1b55k in primary T-cells (but not either protein alone; data not shown), we observed a four-log increase in GFP mean fluorescence intensity (MFI), and an 8-fold increase in GFP expression, driven from an AAV vector encoding a promoter/GFP cassette (Figure 1a, left panel), without compromising the rate of cell expansion (Figure 1a, right panel). The same enhancing effect was observed irrespective of whether a self complementary or single stranded AAV was used (Figure 1b), suggesting that E4orf6/E1b55k complexes possess a robust capacity to relieve postentry restrictions on AAV expression in primary human T-cells that is unrelated to initiation of second strand DNA synthesis.
Figure 1.
Enhanced AAV-mediated gene expression in primary human T-cells using adenoviral E4orf6/E1b55k proteins to relieve postentry AAV restriction mechanisms. (a) Relief of postentry restriction of AAV-mediated gene expression. Left panel: AAV-mediated GFP expression following relief of postentry AAV restriction by E4orf6/E1b55k. Primary CD4+ T-cells were electroporated with mRNA encoding adenoviral serotype 5 E4orf6/E1b55k (0.33 μg each), rested for 2–4 hours, then transduced with AAV driving GFP expression. Cells were placed in culture for the indicated periods of time, following which the cells were collected and analyzed for GFP expression by flow cytometry. Right panel: Expansion of cell populations following the indicated exposure to E4orf6/E1b55k mRNA transfection and AAV transduction, following the same protocol as described in the left panel. (b) Comparison of effect of E4orf6/E1b55k proteins on self-complementary and single-stranded AAV6-mediated gene expression. Primary CD4+ T-cells were electroporated with mRNA encoding E4orf6/E1b55k proteins (0.33 μg each), rested for 2–4 hours, and transduced with either single-stranded or self-complementary AAV6 driving GFP expression (MOI of both viruses: 2 × 104). Cells were placed in culture for the indicated periods of time, following which the cells were collected and analyzed for GFP expression by flow cytometry. (c) E1b55k Mre11/Rad51/NBS1 (MRN) degradation mutants demonstrate requirement for MRN inactivation for complete relief of postentry restriction on AAV-mediated expression. Primary CD4+ T-cells were electroporated with mRNA encoding Cas9-2A-mCherry (1 µg) along with E4orf6/E1b55k (Wild type) proteins or the indicated E1b55k mutants at the indicated RNA doses, rested for 2–4 hours, and transduced with ssAAV driving both TCRα guide and GFP expression. E4orf6 mRNA dose was the same as each E1b55k RNA dose for each indicated point. Cells were placed in culture for two days, following which the cells were collected and analyzed for GFP MFI by flow cytometry. See also Supplementary Figure S2 demonstrating a 2–3 fold increase in GFP expression catalyzed by the E4orf6/E1b55k H373A mutant complex that is not visible on the same scale as GFP expression catalyzed by the WT E4orf6/E1b55k complex.
The MRN complex is a dominant postentry restriction mechanism on AAV transduction of primary human T-cells
The ability of E4orf6/E1b55k proteins to enhance AAV expression in cultured cell models has been reported to depend on their capacity to target the Mre11/Rad51/NBS1 DNA repair complex (MRN) for degradation, thus allowing incoming AAV genomes to escape intranuclear detection and silencing.23,24,26,30 To determine if this same mechanism applied to primary human T-cells, for which as a substrate for AAV transduction, there is little published data, we compared AAV-driven gene expression alone or following transient expression of wild type E4orf6/E1b55k proteins or E4orf6/E1b55k-H373A and E4orf6/E1b55k-H354 mutants (Figure 1c). Both of these mutants have been shown to have largely lost the capacity for inducing degradation of MRN, while fully preserving other functions of wild type E4orf6/E1b55k.30 As assessed by GFP MFI, these E1b55k mutants were markedly less efficient at relieving postentry restrictions on AAV expression than WT E4orf6/E1b55k, in a dose-dependent manner. However, replicate experiments using the E1b55k-H373A mutant, analyzed at a smaller scale, demonstrated that E4orf6/E1b55k-H373A expression was able to support a level of GFP expression significantly greater (1.58 ± 0.09-fold at 96 hours) than that observed in the absence of E4orf6/E1b55k expression, consistent with its possessing a residual capacity to relieve postentry transduction or expression restrictions (Supplementary Figure S2a,b). We also evaluated the role of other known E4orf6/E1b55k functional capabilities in relieving postentry restriction of AAV transduction in T-cells using two additional mutants—E1b55k-R240A, which disrupts the ability of E1b55k to catalyze degradation of p53, and E4orf6 AXA, which binds inefficiently to E1b55k and results in generally hypofunctional E4orf6/E1b55k complexes.23,26,30,31 These mutants exhibit full (R240A) or partial (AXA) capacities to degrade the MRN complex,30 and consistent with a key role for MRN in postentry restriction of AAV expression in T-cells, these mutants retained full (R240A) or partial (AXA; data not shown) capacities to enhance GFP expression following AAV transduction of T-cells (Supplementary Figure S2c,d). Expression of AXA/E1b55k was only able to enhance GFP expression from AAV vectors at high doses of mRNA electroporation (0.33 µg), and not 10-fold lower, as shown in Supplementary Figure S2.
H373A and H354 mutant E4orf6/E1b55k expression enhance CRISPR-mediated knockout in primary human T-cells
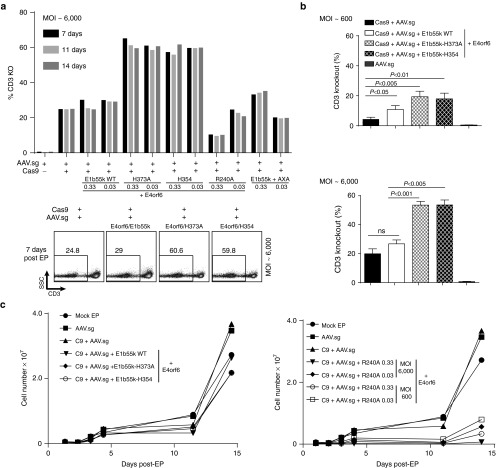
Based on the capacity of E4orf6/E1b55k proteins to enhance AAV-driven GFP expression in the analyses above, we hypothesized that their transient expression would similarly enhance the level and/or duration of guide RNA expression from a polIII-driven U6 promoter/guide RNA cassette incorporated into an AAV genome, and thus potentially enhance Cas9-mediated gene disruption efficiency by the mRNA/AAV system. To test this hypothesis, we expressed wild type E4orf6/E1b55k proteins using the mRNA/AAV codelivery protocol by including their respective mRNAs with Cas9 in the electroporation step, followed by transduction of the cells with an AAV vector encoding both a TCRα guide RNA and a promoter/GFP expression cassette. To gain information on the influence of specific biochemical activities attributed to E4orf6/E1b55k complexes on gene editing outcome, we also expressed the H373A, H354, R240A, and AXA mutants, and compared the TCRα knockout efficiency among the various contexts (Figure 2).
Figure 2.
Enhanced CRISPR-mediated gene knockout in primary human T-cells through use of adenoviral E4orf6/E1b55k proteins. (a) E1b55k mutants enhance gene knockout achieved using mRNA/AAV delivery of CRISPR components. Representative experiment indicating primary CD4+ T-cells electroporated with mRNA encoding Cas9-T2A-mCherry, (1 μg), E4orf6/E1b55k (Wild Type), or the indicated mutants (at 0.33 μg or 0.03 μg each), rested for 2–4 hours, and transduced with AAV driving TCRα guide expression. Cells were placed in culture, following which the cells were collected and analyzed for CD3 expression by flow cytometry at the indicated time-points following EP/transduction. Upper panel—quantification of CD3 knockout. Bottom panel—representative flow plots from a subset of the experiment at 7 days post EP/transduction. (b) E4orf6/E1b55k MRN-inactivation deficient mutants enhance CRISPR-mediated knockout. Quantification of n = 3–4 independent experiments at two different AAV MOIs indicating that both E1b55k H373A and H354 mutants significantly increase CRISPR-mediated TCRα knockout, quantified by CD3 staining at 7 days post EP/transduction. Primary human CD4+ or CD3+ T-cells were used. (c) T-cells edited using mRNA/AAV delivery exhibit normal expansion kinetics. Primary CD4+ T-cells were electroporated with mRNA encoding Cas9-T2A-mCherry (1 μg) proteins along with Wild Type E4orf6/E1b55k proteins or the indicated E1b55k mutants, rested for 2–4 hours, and transduced with AAV driving TCRα guide expression. Cells were placed in culture for the indicated periods of time, during which aliquots of the cells were collected and counted for quantification of cell expansion. Left panel: low dose (0.03 μg) of E4orf6/E1b55k mutants were electroporated with high dose of AAV (MOI ~ 6000). Right panel: both low and high dose (0.33 μg) E4orf6/E1b55k-R240A RNA, and low and high dose AAV, were electroporated and transduced.
As expected from the analyses in Figure 1, we observed that expression of wild type E4orf6/E1b55k proteins markedly increased GFP expression from the TCRα guide/GFP AAV. Despite the marked increase in AAV-based expression, we observed only a moderately increased level of TCRα knock-out (Figure 2a), and only at lower AAV vector doses (Figure 2b, top panel). We also observed apparent toxicity that had not been observed in the absence of Cas9 expression (manifesting as a loss, rather than stable level, of CD3− cells over time—Supplementary Figure S3a). While expression of E4orf6 with E1b55k-H373A and H354 mutants produced the expected much smaller effects on GFP expression (i.e., Figure 1c), unexpectedly, substantial increases in TCRα knockout efficiency were also observed that were not accompanied by toxicity or loss of CD3− cells (Figure 2a, Supplementary Figure S3a). This suggests that despite their inability to fully relieve posttransduction restrictions on AAV gene expression, these E1b55k mutants retained residual activities that were sufficient to markedly enhance the overall efficiency of gene knockout achievable with the mRNA/AAV delivery approach. This conclusion is supported by the results obtained when the E4orf6/E1b55k R240A mutant was expressed—in this case, we observed potentiated toxicity relative to what was observed with the wild type E4orf6/E1b55k, along with marked reductions in efficiency of CD3 knockout (Figure 2a,c). This indicates that p53 inactivation, for which R240A is deficient but which is preserved in the H373A and H354 mutants, contributes to the knockout potentiating properties of H373A and H354. The H373A and H354 mutants exhibited considerable potency in their effects on Cas9-mediated gene disruption, with dose/response testing of the H373A mutant demonstrating that it maintained a nearly full effect at enhancing TCRα knockout at mRNA doses down to < 0.04 μg (Supplementary Figure S3b). Additionally, amplicon sequencing of the indel spectra generated by Cas9-mediated gene disruption in the context of both H373A and H354 mutant E4orf6/E1b55k complexes demonstrated the expected higher rate of mutations as well as an increased proportion of larger deletions, up to 150 bp, spanning the predicted Cas9 cleavage site (Supplementary Figure S3c).
To evaluate whether expression of the E4orf6/E1b55k H373A mutant was detrimentally influencing T-cell phenotype or signaling properties over the course of the editing process, we assessed expression of a panel of surface markers and measured phytohemagglutinin (PHA)-induced calcium signaling at 2 weeks postediting. Flow cytometric assessment of cell surface markers that define naive and memory T-cell populations showed no differences between edited versus unedited populations (Supplementary Figure S4a). Similarly, the signaling properties of edited T-cells were as expected, with those cells exhibiting a loss of surface TCR following TCRα gene editing showing a loss of capacity to mobilize Ca2+ in response to stimulation with PHA relative to cells retaining surface TCR (Supplementary Figure S4b).
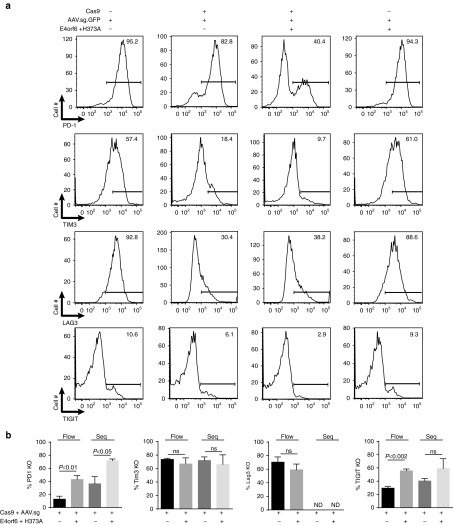
To evaluate the influence of E4orf6/E1b55k H373A and H354 mutant complexes on the efficiency of CRISPR/Cas9 gene disruption at other genomic targets, we generated and validated guide RNAs targeting four translationally relevant human surface protein targets: the T-cell inhibitory checkpoint proteins PD-1, TIGIT, LAG-3, and Tim3. These guide RNAs were incorporated into U6-guide expression cassettes in AAV vector backbones upstream of the MND-GFP cassette to provide for tracking of transduction/expression, and packaged into AAV vectors. Use of these AAV vectors with the E4orf6/E1b55k H373A mutant in a scaled up expansion/manufacturing protocol based on the MaxCyte GT electroporation system resulted in generation of human T-cell populations with approximate respective targeted gene disruption efficiencies of 71.6 ± 2.7%, 59.1 ± 14.8%, 59.2 ± 8.5%, and 66.1 ± 14.3% of indels at the intended cleavage site of PD-1, TIGIT, LAG-3, and Tim3, respectively, as derived from sequence analysis (PD-1, TIGIT, Tim3) or flow cytometry (LAG-3). The representative flow cytometry data is presented in Figure 3a, and quantitated summary data in Figure 3b. An important secondary observation that emerged from these experiments was that the influence of E4orf6/E1b55k H373A mutant complexes on gene disruption efficiency was particularly prominent when the activity of a particular guide RNA was relatively low—e.g., approximately doubling the knockout rates at the PD-1 and TIGIT loci, for which knockout rates with the chosen guides in the absence of E4orf6/E1b55k expression were only 36.4 ± 10.9 and 40.4 ± 3.6%, respectively (rates assessed by sequencing). This is a particularly salient feature of E4orf6/E1b55k H373A expression, in that it can “rescue” poor guide activity, while guides that are already highly active are not as effectively potentiated by E4orf6/E1b55k protein expression.
Figure 3.
Implementation of CRISPR/Cas9 with mRNA/AAV delivery is able to achieve efficient knockout at multiple genomic sites. (a) Primary human CD3+ T-cells were electroporated using the MaxCyte GT system with mRNA encoding Cas9-2A-mCherry proteins (1 µg) along with E4orf6/E1b55k-H373A proteins (0.03 μg each), rested for 2–4 hours, and transduced with AAV driving guide expression against the indicated surface proteins. Cells were placed in culture and allowed to expand; 9–12 days following initial stimulation, cells were restimulated using Dynal CD3/CD28 beads for 48 hours, following which the cells were collected and analyzed for expression of the indicated surface proteins by flow cytometry and assessed for knockout by amplicon sequencing. Representative flow cytometry plots indicating that T-cells edited using mRNA/AAV codelivery exhibit loss of targeted surface checkpoint proteins. Data represent n = 3–5 independent editing experiments. (b) Quantification and summary data from flow cytometry analysis of gene knockout and sequencing analysis of amplicons from genomic target sites. Analysis of knockout by flow cytometry is assessed by percent loss of induction of surface protein expression. Data represent n = 3–5 independent editing experiments. ND, not determined.
H373A mutant E4orf6/E1b55k expression enhance CRISPR-mediated multiplex knockout in primary human T-cells
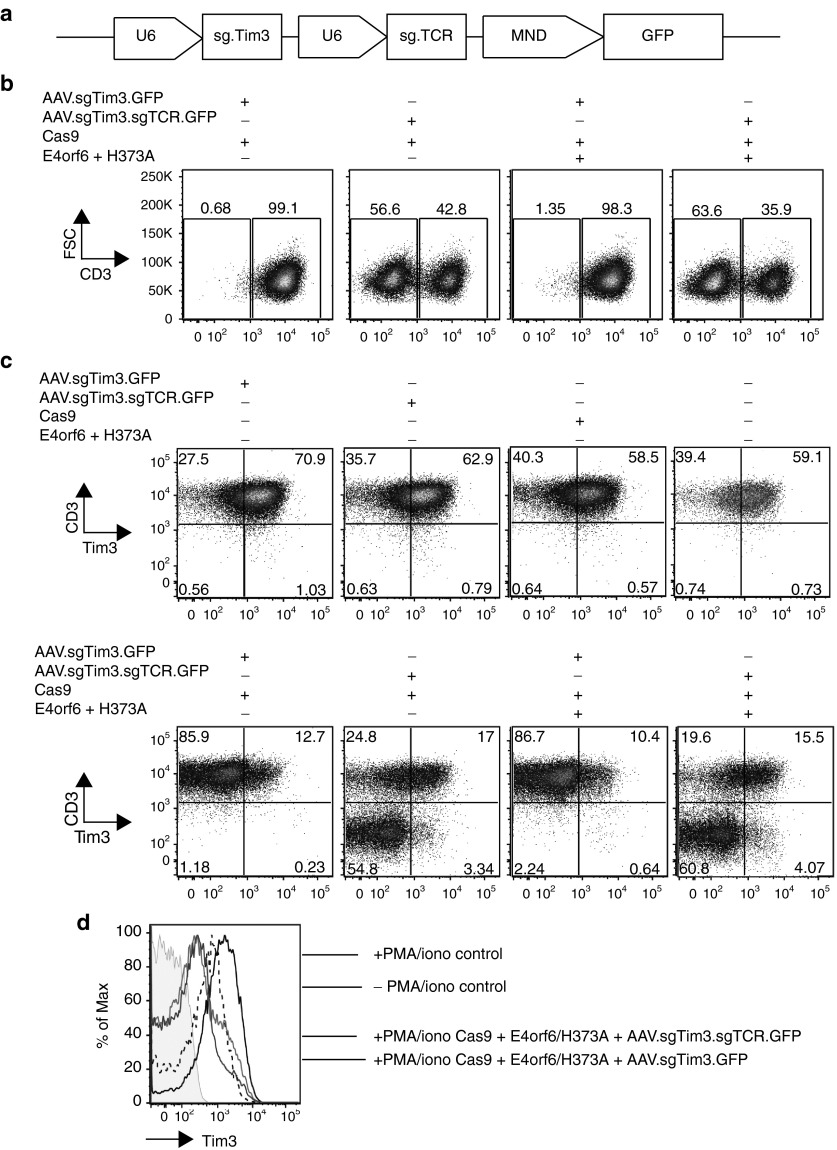
Based on our analyses above, we hypothesized we could successfully achieve efficient levels of multiplex knockout, using a single AAV vector to deliver multiple guides. Using the same architecture as our single AAV guide vectors, we built a dual-guide Tim3/TCRα vector, with both guides driven by individual U6 promoters (Figure 4a). Following MaxCyte electroporation with Cas9 or E4orf6/E1b55k-H373A and AAV transduction with both the single Tim3 guide AAV or the AAV containing dual Tim3/ TCRα guides, TCRα and Tim3 knockout was analyzed (Figure 4b–d). We observed successful knockout of both targeted genes using a single AAV, along with an increased efficiency of knockout when E4orf6/E1b55k-H373A were cotransfected with Cas9. Notably, we did not observe significant differences in knockout efficiency between the single Tim3 guide AAV and the dual Tim3 guide/TCRα guide AAV. In addition, the majority of Tim3− cells were also CD3− cells (thus TCRα−), consistent with the expected outcome that any cell sufficiently well-transduced to drive guide expression to cleave one target gene also experienced a high level of guide expression for the other target gene. As translocations are a potential consequence of simultaneous induction of multiple double strand breaks, we performed karyotyping as an unbiased approach to detecting cell engineering-associated translocations, and did not observe any gross abnormalities in any of twenty metaphase spreads from each condition (Supplementary Figure S5).
Figure 4.
Cas9 mRNA/AAV guide delivery is able to achieve efficient CRISPR-mediated multiplex knockout in primary human T-cells with E4orf6/E1b55k H373A expression. Primary human CD3+ T-cells were electroporated using the MaxCyte GT system with mRNA encoding Cas9-2A-mCherry proteins (1 µg) along with E4orf6/E1b55k-H373A proteins (0.03 μg each), rested for 2–4 hours, and transduced with AAVs driving guide expression against Tim3 and TCRα, as well as GFP expression to track transduction efficiency. Cells were placed in culture and allowed to expand; 7 days following EP/transduction, cells were assessed for TCRα knockout by CD3 stain. Three weeks following initial stimulation, cells were restimulated using PMA/ionomycin for 3–4 hours and allowed to recover for 48 hours, following which the cells were collected and analyzed for expression of Tim3 by flow cytometry. (a) Schematic of the multiplex AAV vector expressing guide RNAs against Tim3 and TCRα, with individual U6 promoters. (b) Representative flow cytometry analysis of TCRα knockout by CD3 staining, 7 days after EP/transduction. (c) Representative flow cytometry analysis of TCRα knockout and Tim3 knockout, 3 weeks after initial stimulation. To upregulate Tim3 surface expression independently of TCR, cells were stimulated using PMA/ionomycin (10 ng/ml and 1 µg/ml, respectively), for 3–4 hours and rested for 48 hours. (d) Upregulation of Tim3 with and without PMA/ionomycin stimulation. Shown are control cells (AAV treatment only) with and without PMA/ionomycin treatment, and stimulated cells with Tim3 knockout compared with stimulated cells missing both Tim3 and TCRα. There were no differences between cells proficient and deficient in TCRα signaling, suggesting that PMA/ionomycinn stimulation is independent of this pathway. In shaded gray are unstained cells.
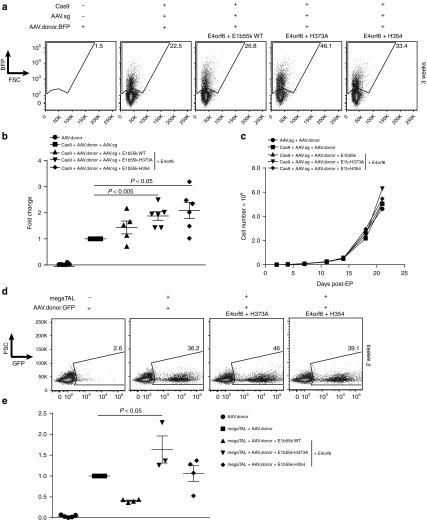
E4orf6/E1b55k H373A and H354 mutants enhance nuclease-mediated homologous recombination in primary human T-cells
Given their biochemical activities and capacity to enhance gene disruption efficiency achievable with the mRNA/AAV codelivery method, we hypothesized that the E4orf6/E1b55k H373A and H354 mutants would have similarly enhancing effects on homology-directed gene targeting rates. To test this hypothesis, we electroporated Cas9 mRNA along with E4orf6 and wild type E1b55k, H373A or H354 mutant mRNAs into primary human T-cells, followed by transduction of the cells with separate AAV vectors to provide guide RNA expression and recombination template delivery, respectively (Figure 5). In these experiments, Cas9 alone, and in addition, the two AAVs allowed for integration of a promoter-BFP cassette into the CCR5 locus at an efficiency of 17.6 ± 4.0%. Consistent with the results observed above with the various E4orf6/E1b55k complexes, we observed substantial potentiation of early BFP expression by the wild type E4orf6/E1b55k complexes relative to either the H373A or H354 mutants or without any Ad5 protein (Supplementary Figure S6, top panel). Similar to the effects of each of these mutant complexes on gene knockout, expression of either mutant complex generated significantly higher efficiency of recombination-based genome editing (Figure 5a, b), increasing homology-directed repair (HDR) by 1.8-fold (31.2 ± 5.7% BFP+ with H373A, and 30.8 ± 1.8 with H354). Importantly, expression of E4orf6/E1b55k complexes (whether wild type or mutant) did not affect the rates of expansion of T-cells that had undergone homology-directed gene targeting (Figure 5c) or the rates nonhomologous AAV integration, as assessed by the rate of long term fluorophore expression following codelivery of Cas9 with AAVs expressing the same guide and a promoter/fluorophore cassette lacking CCR5 homology arms (Supplementary Figure S6, top panel). Furthermore, we assessed the molecular nature of the HDR events, sequencing through the junctions to confirm seamless HDR (Supplementary Figure S7). Finally, given the broad interest in genome editing tools, we were curious to know whether the effect of E4orf6/E1b55k mutant expression was specific to CRISPR-mediated gene editing, as might be expected if their sole activity were to potentiate AAV-driven guide RNA expression. To answer this question, we evaluated their effect on homologous recombination induced by a CCR5 megaTAL nuclease (Figure 5d, e). We observed that the E4orf6/E1b55k-H373A mutant consistently and significantly increased HDR, following megaTAL mRNA/AAV transduction, while E4orf6/E1b55k-H354 expression, in contrast to its effect on CRISPR-Cas9 breaks, did not exhibit a detectable effect in several replicate experiments (Figure 5e).
Figure 5.
Enhanced targeted knock-in using E1b55k mutant proteins. E4orf6/E1b55k H373A and H354 mutants enhance CRISPR- and megaTAL-mediated recombination achieved with mRNA/AAV codelivery. (a) Primary CD4+ or CD3+ T-cells were electroporated using the MaxCyte GT or the Neon systems with mRNA encoding Cas9-2A-mCherry proteins (1 μg) along with Wild Type E4orf6/E1b55k proteins or the indicated E1b55k mutants (0.03 μg each), rested for 2–4 hours, and transduced with separate AAVs driving CCR5 guide expression and a targeting template for the CCR5 locus (See Supplementary Figure S7). Cells were placed in culture, following which the cells were collected and analyzed for BFP expression by flow cytometry. Shown are representative flow plots from the indicated manipulations at 3 weeks post EP/transduction, from n = 5–6 independent experiments. (b) Quantification of n = 5–6 independent experiments of the fold change in knock-in frequency (BFP+ cells) over baseline (Cas9+ guide + donor) at 3 weeks post EP/transduction. (c) T-cells edited via mRNA/AAV CRISPR-mediated recombination exhibit normal expansion kinetics. Primary T-cells were electroporated with mRNA encoding control or Cas9-2A-mCherry proteins (1 µg) along with Wild Type E4orf6/E1b55k and the E1b55k mutants (0.03 μg each), rested for 2–4 hours, and transduced with AAV driving CCR5 guide expression as well as AAV CCR5 BFP template. Cells were placed in culture for the indicated periods of time, following which the cells were collected and counted for quantification of cell expansion. (d) Primary CD4+ or CD3+ T-cells were electroporated using the MaxCyte GT or the Neon systems with mRNA encoding CCR5 megaTAL (0.5–1 μg) along with Wild Type E4 or f6/E1b55k proteins or the indicated E1b55k mutants (0.03 μg each), rested for 2–4 hours, and transduced with a GFP targeting template for the CCR5 locus. Cells were placed in culture, following which the cells were collected and analyzed for GFP expression by flow cytometry. Shown are representative flow plots from the indicated manipulations, at 2 weeks post EP/transduction, from n = 3–5 independent experiments. (e) Quantification of n = 3–5 independent experiments of the fold change in knock-in frequency (GFP+ cells) over baseline (megaTAL + donor) at 2 weeks post EP/transduction. We found the fold change in HDR was significantly increased using E4orf6/E1b55k-H373A only.
Discussion
Here we demonstrate implementation of CRISPR/Cas9 nuclease technology in primary human T-cells using an mRNA/AAV codelivery method in which mRNA is used for Cas9 expression and an AAV vector is used for guide RNA expression and/or recombination template delivery. As AAV transduction manifested as an important limitation on efficacy during initial development of the mRNA/AAV codelivery method, we evaluated mRNA-based coexpression of E4orf6/E1b55k adenoviral helper proteins and a panel of mutants to determine whether a combination of biochemical activities could be identified that would disable postentry restrictions on AAV-mediated gene expression while maintaining a cellular DNA repair environment conducive to efficient gene editing. We identified two of the mutants, E4orf6/E1b55k H373A and H354, as uniquely capable of supporting substantially enhanced efficiencies of both CRISPR-Cas9-mediated gene disruption and homology-directed gene targeting.
Adenoviral serotype 4 E4orf6/E1b55k complexes have previously been reported to enhance CRISPR-mediated gene targeting (but not gene disruption) in cultured cell models, an effect attributed to inhibition of DNA ligase IV activity and, as a consequence, reduced nonhomologous end joining repair activity.32 Thus, we anticipated that expression of adenoviral serotype 5 E4orf6/E1b55k complexes with mRNA/AAV codelivery might produce a synergistic effect on gene targeting efficiency through both inhibition of DNA ligase IV activity and relief of AAV postentry transduction restrictions. Surprisingly, we observed that although transient expression of wild type E4orf6/E1b55k complexes was highly efficacious at relieving restrictions on AAV-driven gene expression, it produced only modest increases in gene disruption or gene targeting efficiency in the primary human T-cell context. In contrast, E4orf6/E1b55k H373A or H354 mutants, which exhibited only a modest capacity to enhance AAV-driven gene expression, were observed to produce substantial enhancements in both gene disruption and gene targeting efficiency.
The H373A and H354 mutants' modest effect on AAV-driven gene expression (e.g., as in Supplementary Figure S2), which would be predicted to produce a correspondingly modest increase in both the level and duration of guide RNA expression, may in part explain their capacities to potentiate both gene disruption and gene targeting. Their collective residual biochemical activities may also allow an increased proportion of AAV genomes to remain available to participate in homology-directed repair, thus contributing to increased gene targeting efficiency. However, we speculate that two known biochemical activities are central to these mutants' capacity to potentiate both gene disruption and gene targeting efficiency relative to the WT E1b55k: (i) they preserve MRN-dependent DNA damage signaling (which promotes double strand break resolution by various forms of homology-directed DNA repair) while (ii) limiting p53-dependent DNA damage signaling (which arrests cells in G1 in response to DNA double strand breaks). As a consequence, T-cells expressing these complexes would be predicted to permit a high fraction of Cas9-induced double strand breaks to transition into S-phase, where they would be repaired by mutagenic alternative end joining in the absence of a recombination template, or by homology-directed repair in the case that a recombination template is provided. This latter hypothesis is consistent with the observed potentiation of CCR5 megaTAL-mediated HDR by the E1b55k H373A mutant, an effect which would be expected to be independent of any effects on guide expression, although as noted above, we cannot exclude that the H373A mutant may promote AAV genome accessibility for participation in gene targeting.
In summary, we have identified adenoviral serotype 5 E4orf6/E1b55k H373A and H354 mutant “helper” proteins as possessing the capacity to enhance the efficiency of Cas9-mediated gene editing in primary human T-cells. Collectively, our results suggest that E4orf6/E1b55k-H373A might be the best choice for an investigator interested in a general tool to enhance CRISPR/Cas9 gene editing, as it exhibited the most consistent activity across multiple types of gene editing applications. The previously characterized biochemical activities of these proteins suggest that their effects on gene editing efficiency are likely to be attributable to their promotion of S-phase repair of DNA double strand breaks, and thus they are likely to be generally applicable as a means to enhance homology-directed genome modification in primary human cells, including with alternative nuclease platforms and viral template delivery methods other than AAV. Furthermore, as accessory proteins from many human viruses have been shown to disable or modify various components of cellular DNA damage response and repair mechanisms, we propose that such proteins may represent a rich trove of biochemical activities for use as tools in human genome engineering.
Materials and Methods
DNA constructs. Cas9 was obtained from Addgene (plasmid # 41815), PCR amplified and cloned into pWNY backbone (an inhouse modified pUC57), pEVL200 and pEVL300 (linear mRNA vector with a 200 or 300 encoded polyA tail; Grier et al, in submission) with a T7 promoter and two Nuclear Localization Signals – one each at the N-terminus and C-terminus, respectively. Cas9 was modified to remove BsaI sites to clone into the pEVL vector, without changing amino acid sequence. mCherry was linked to Cas9 with a T2A peptide at 3' end of Cas9. The mCherry only control containing a T7 promoter and a single Nuclear Localization Signals at the 5' end of mCherry was also generated in pWNY and pEVL200. E4ORF6 and E1b55k genes were gene synthesized (Integrated DNA Technologies, IDT, Coralville, IA) and cloned into pWNY downstream of a T7 promoter. E1b55k mutants were generated using site-directed mutagenesis (QuikChange II XL Site-Directed Mutagenesis Kit, Agilent, Santa Clara, CA).
sgRNA design and cloning. Guides targeting the constant region of TCRα, PD-1, TIGIT, Lag3, Tim3, and CCR5 were designed using online CRISPR design tools (http://crispr.mit.edu and the Broad Institute's sgRNA designer - http://www.broadinstitute.org/rnai/public/analysis-tools/sgrna-design). Guides were then generated as gblocks by commercial DNA synthesis (IDT). The gblocks were cloned into scAAV6 or ssAAV6-GFP constructs using standard cloning techniques. TCRα guide target: ACAAAACTGTGCTAGACATG. PD1 guide target: GCCCACGACACCAACCACCA. TIGIT guide target: TCTTCCCTAGGAATGATGAC. Lag3 guide target: GCGGTCCCTGAGGTGCACCG Tim3 guide target: AGAAGTGGAATACAGAGCGG.
Production of recombinant AAV6 vectors. AAV stocks were produced by triple transfection of AAV vector, serotype helper and Adenoviral helper (HGT1-Adeno) plasmids in HEK293T-cells. Transfected cells were collected 48 hours later, lysed by freeze-thaw, benzonase treated and purified over iodixanol density gradient as previously described.33 Titers of the viral stocks were determined by qPCR of AAV genomes and ranged from 1010–1012/ml.34
mRNA production. DNA template was linearized with unique restriction enzymes and linearized plasmids were purified using the QiaQuick PCR purification kit (Qiagen, Valencia, CA). mRNA was transcribed invitro using commercial kits (mMessage mMachine T7 Ultra; Ambion or T7 mScript Standard RNA production system; CellScript) with slight modifications from the manufacturer's protocol. Briefly, the IVT reaction incubated for 2.5–3 hours, followed by DNase treatment for 1 hour. Poly(A) tailing, if required, was done for 1 hour and mRNA was purified using RNeasy (Qiagen).
Primary human T-cell electroporation. T-cells were obtained from frozen peripheral blood mononuclear cells (PBMCs), using CD4+ or CD3+ isolation kits (Miltenyi Biotec, Auburn, CA). Briefly, PBMCs were thawed using drop-wise addition of cold DNase I Buffer: phosphate-buffered saline, 5 mmol/l MgCl2, 20 Kunitz Units/ml DNase I (EMD-Millipore, Billerica, MA), followed by centrifugation, and left to rest overnight in T-cell media (Roswell Park Memorial Institute medium, 20% fetal bovine serum, 1% N-2-hydroxyethylpiperazine-N9-2-ethanesufonic acid, 1% L-glutamine) supplemented with low-dose IL-15 (0.1 ng/ml). T-cells were isolated the following day, according to the manufacturer's instructions, and purified T-cells were resuspended at 1 × 106 live cells/ml in T-cell growth media (culture media supplemented with IL-2 and IL-15; 5 and 1 ng/ml, respectively), and stimulated by using CD3/CD28 beads (Dynal Beads, Life Technologies, Carlsbad, CA) for 72 hours at a 1:1 cell-bead ratio. Beads were then removed and cells were allowed to rest in T-cell growth media for 0.5–2 hours. Next, cells were electroporated with mRNA using either a Neon Transfection System (Figures 1, 2 and 4) or MaxCyte GT (Figure 3) as follows: Cells were washed twice with PBS, resuspended in Neon Buffer T or MaxCyte Buffer at a density of 4.5 × 107 cells/ml (Neon, Thermo Fischer Scientific, Waltham, MA) or 1.25 × 108 cells/ml (MaxCyte Gaithersburg, MD). After mixing, cells were electroporated (Neon conditions: 1400 V, 10 milliseconds, 3 pulses, 10 µl tip; MaxCyte conditions determined by the manufacturer for primary T-cells) and immediately dispensed into 200 µl of prewarmed T-cell growth media in a 96-well plate. Cells were immediately incubated at 30°C, and AAV was added to the culture 2–4 hours post electroporation, followed by continued 30°C incubation for 20 additional hours. AAV donor was added as 10% of the final culture volume regardless of titer (~1 × 104–5 MOI), unless specified otherwise. Subsequently, edited cells were cultured using standard conditions: 37°C and expanded in T-cell growth media, replenished as needed to maintain a density of ~1 × 106 cells/ml every 2–3 days.
Flow cytometry and antibodies. Analysis of knock-out (TCRα, PD-1, TIGIT, Lag3, and Tim3) and HDR (BFP) was performed using the LSRII flow cytometer (BD Biosciences) and data was analyzed using FlowJo software (Treestar). All antibodies were from Biolegend, unless otherwise indicated. To assess knockout of surface markers, cells were labeled with fluorophore-conjugated antibodies, as follows: CD3-Alexa 488, CD3-PerCPCy5.5, or CD3-APC clone HIT3a; PD1-APC clone eh12.2H7; TIGIT-Alexa 700 clone 741182 (Novus Biologicals, Littleton, CO); Tim3-APC-Cy7 clone F38-2E2; and Lag3-FITC clone 3DS223H (eBioscience, San Diego, CA). CD4 or CD8 staining was done using CD4-BFP (clone OKT4) or CD8-BFP (RPA-T8). To upregulate surface expression of T-cell exhaustion markers, T-cells were stimulated using CD3/CD28 beads for 48 hours, 9–12 days following initial stimulation, except in the absence of TCRα, in which case PMA/ionomycin stimulation was used (see below).
Stimulation with PMA/ionomycin. Cells were plated at a density of 1 × 106 in a 48 or 24 well plate. 10 ng/ml phorbol myristate acetate (PMA) (Sigma, St. Louis, MO) and 1 µg/ml of ionomycin (Sigma) was added to the media for 3–4 hours, cells were washed 3–4 times with phosphate-buffered saline with 2% fetal bovine serum, and then replated with fresh media. Cells were allowed to recover for 48 hours before flow cytometry, or for 72 hours for karyotype analysis.
Karyotype analysis. Cells were grown in T-25 flasks at a density of 1 × 106–1.5 × 106 per sample. Each sample was stimulated with PMA/ionomycin for 3–4 hours, and then allowed to rest for 72 hours in full media and cytokines. Karyotype analysis was done by the University of Washington Cytogenetics and Genomics Laboratory as Research Testing. Standard G-banding (GTW stain) chromosome analysis was performed, on 20 cells per sample.
T7 Endonuclease I (T7EI) assay. The cleavage efficiency of Cas9 and sgRNA was estimated using the T7EI assay for both TCRα and CCR5 loci. Targeted genomic loci were amplified using either Accuprime Pfx or Hifi Platinum Taq DNA polymerase (Life Technologies, Carlsbad, CA), using the manufacturer's instructions. 400 ng of the purified product (Qiaquick PCR clean-up kit, Qiagen) was denatured, subjected to T7EI (New England Biolabs, Ipswich, MA) digestion for 30 minutes at 37°C and analyzed on a 1–2% agarose gel.
Analysis of calcium signaling. 5 × 106 cells were used per sample. Cells were washed in HBSS (with Ca2+ and Mg2+) (Thermofisher), loaded with 30 µmol/l Indo-1 AM (Molecular Probes, Life Technologies) in HBSS and incubated at 37 °C for 30 minutes, washed twice, and resuspended in buffer. Baseline flow was obtained for 30 seconds, after which cells were stimulated with 200 µg/ml PHA to stimulate T-cells, and data was collected for 5 minutes.
Statistical analysis. Statistical analyses were performed with Prism 6 (GraphPad Software, San Diego, CA). Data is shown as mean ± SEM unless otherwise noted. Tests of statistical significance were performed using an unpaired two-tailed Student's t-test with Welsh's correction for unequal standard deviations when appropriate.
SUPPLEMENTARY MATERIAL Figure S1. Implementation of CRISPR gene editing in primary human T-cells using mRNA/AAV codelivery. Figure S2. Effect of E4orf6/E1b55k mutants on AAV-driven GFP expression. Figure S3. Effect of E4orf6/E1b55k MRN mutants on indel spectra. Figure S4. Edited primary T-cells with E4orf6/E1b55k H373A exhibit normal surface marker phenotype and expected Ca2+ signaling. Figure S5. Cells exhibit normal karyotype following multiplex CRISPR editing. Figure S6. E4orf6/E1b55k proteins do not enhance nonhomologous AAV insertion. Figure S7. Molecular confirmation of precise HDR events following CRISPR-Cas9 breaks with E4orf6/E1b55k H373A or H354.
Acknowledgments
This work was supported by the Program for Cell and Gene Therapy by Seattle Children's Research Institute. A.M.S. holds equity, consults for, and receives compensation from bluebird bio. M.C.J. holds equity, consults, and receives compensation from Juno Therapeutics. K.S.G, A.M.S, D.J.R, A.E.G, and K.J. are inventors on patents being filed for the Ad5 E1b55k/E4orf6 proteins. The other authors declare no conflicts of interest.
Supplementary Material
References
- Gori, JL, Hsu, PD, Maeder, ML, Shen, S, Welstead, GG and Bumcrot, D (2015). Delivery and specificity of CRISPR-Cas9 genome editing technologies for human gene therapy. Hum Gene Ther 26: 443–451. [DOI] [PubMed] [Google Scholar]
- Jiang, W and Marraffini, LA (2015). CRISPR-Cas: New tools for genetic manipulations from bacterial immunity systems. Annu Rev Microbiol 69: 209–228. [DOI] [PubMed] [Google Scholar]
- Xu, J, Ren, X, Sun, J, Wang, X, Qiao, HH, Xu, BW et al. (2015). A toolkit of CRISPR-based genome editing systems in Drosophila. J Genet Genomics 42: 141–149. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rivera, FJ and Jacks, T (2015). Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev Cancer 15: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva, EA, Shuvalov, OU, Garabadgiu, AV, Melino, G and Barlev, NA (2015). Genome-editing tools for stem cell biology. Cell Death Dis 6: e1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem, O, Sanjana, NE and Zhang, F (2015). High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet 16: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner, BA and Cristea, IM (2015). Blowing off steam: Virus inhibition of cGAS DNA sensing. Cell Host Microbe 18: 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q, Liu, X, Zhou, Q and Wang, C (2015). Cytosolic sensing of aberrant DNA: arming STING on the endoplasmic reticulum. Expert Opin Ther Targets 19: 1397–1409. [DOI] [PubMed] [Google Scholar]
- Trigg, BJ and Ferguson, BJ (2015). Functions of DNA damage machinery in the innate immune response to DNA virus infection. Curr Opin Virol 15: 56–62. [DOI] [PubMed] [Google Scholar]
- Sparrer, KM and Gack, MU (2015). Intracellular detection of viral nucleic acids. Curr Opin Microbiol 26: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, DA, Brennan, AL, Jiang, S, Binder-Scholl, GK, Lee, G, Plesa, G et al. (2013). Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5. Hum Gene Ther 24: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas, P, Stein, D, Tang, WW, Frank, I, Wang, SQ, Lee, G et al. (2014). Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock, U, Machowicz, R, Hauber, I, Horn, S, Abramowski, P, Berdien, B et al. (2015). mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Res 43: 5560–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot, L, Philip, B, Schiffer-Mannioui, C, Le Clerre, D, Chion-Sotinel, I, Derniame, S et al. (2015). Multiplex genome-edited T-cell manufacturing platform for “Off-the-shelf” adoptive T-cell immunotherapies. Cancer Res 75: 3853–3864. [DOI] [PubMed] [Google Scholar]
- Didigu, CA, Wilen, CB, Wang, J, Duong, J, Secreto, AJ, Danet-Desnoyers, GA et al. (2014). Simultaneous zinc-finger nuclease editing of the HIV coreceptors ccr5 and cxcr4 protects CD4+ T cells from HIV-1 infection. Blood 123: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino, C, Alzubi, J, Fine, EJ, Morbitzer, R, Cradick, TJ, Lahaye, T et al. (2014). TALENs facilitate targeted genome editing in human cells with high specificity and low cytotoxicity. Nucleic Acids Res 42: 6762–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel, S, Jarjour, J, Astrakhan, A, Adey, A, Gouble, A, Duchateau, P et al. (2014). megaTALs: a rare-cleaving nuclease architecture for therapeutic genome engineering. Nucleic Acids Res 42: 2591–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather, BD, Romano Ibarra, GS, Sommer, K, Curinga, G, Hale, M, Khan, IF et al. (2015). Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci Transl Med 7: 307ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel, A, Bak, RO, Clark, JT, Kennedy, AB, Ryan, DE, Roy, S et al. (2015). Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol 33: 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann, K, Lin, S, Boyer, E, Simeonov, DR, Subramaniam, M, Gate, RE et al. (2015). Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci USA 112: 10437–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, A, Rohleder, KJ, Hanakahi, LA and Ketner, G (2007). Adenovirus E4 34k and E1b 55k oncoproteins target host DNA ligase IV for proteasomal degradation. J Virol 81: 7034–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, J, Rohleder, K and Ketner, G (1999). Adenovirus E4 34k and E4 11k inhibit double strand break repair and are physically associated with the cellular DNA-dependent protein kinase. Virology 263: 307–312. [DOI] [PubMed] [Google Scholar]
- Stracker, TH, Carson, CT and Weitzman, MD (2002). Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418: 348–352. [DOI] [PubMed] [Google Scholar]
- Lentz, TB and Samulski, RJ (2015). Insight into the mechanism of inhibition of adeno-associated virus by the Mre11/Rad50/Nbs1 complex. J Virol 89: 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlioglu, S, Duan, D and Engelhardt, JF (1999). Two independent molecular pathways for recombinant adeno-associated virus genome conversion occur after UV-C and E4orf6 augmentation of transduction. Hum Gene Ther 10: 591–602. [DOI] [PubMed] [Google Scholar]
- Schwartz, RA, Palacios, JA, Cassell, GD, Adam, S, Giacca, M and Weitzman, MD (2007). The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J Virol 81: 12936–12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowski, L, Tay, SS and Alexander, IE (2015). Adeno-associated virus serotypes for gene therapeutics. Curr Opin Pharmacol 24: 59–67. [DOI] [PubMed] [Google Scholar]
- Kotterman, MA and Schaffer, DV (2014). Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet 15: 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn, E and Vandenberghe, LH (2014). Adeno-associated virus: fit to serve. Curr Opin Virol 8: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, RA, Lakdawala, SS, Eshleman, HD, Russell, MR, Carson, CT and Weitzman, MD (2008). Distinct requirements of adenovirus E1b55K protein for degradation of cellular substrates. J Virol 82: 9043–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orazio, NI, Naeger, CM, Karlseder, J and Weitzman, MD (2011). The adenovirus E1b55K/E4orf6 complex induces degradation of the Bloom helicase during infection. J Virol 85: 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, VT, Weber, T, Wefers, B, Wurst, W, Sander, S, Rajewsky, K et al. (2015). Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol 33: 543–548. [DOI] [PubMed] [Google Scholar]
- Khan, IF, Hirata, RK and Russell, DW (2011). AAV-mediated gene targeting methods for human cells. Nat Protoc 6: 482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurnhammer, C, Haase, M, Muether, N, Hausl, M, Rauschhuber, C, Huber, I et al. (2012). Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods 23: 18–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.