Abstract
Mesenchymal stem cells (MSCs) promote therapeutic angiogenesis to cure serious vascular disorders. However, their survival period and cytokine-secretory capacity are limited. Although hepatocyte growth factor (HGF) can accelerate the rate of angiogenesis, recombinant HGF is limited because of its very short half-life (<3–5 minutes). Thus, continuous treatment with HGF is required to obtain an effective therapeutic response. To overcome these limitations, we produced genome-edited MSCs that secreted HGF upon drug-specific induction. The inducible HGF expression cassette was integrated into a safe harbor site in an MSC chromosome using the TALEN system, resulting in the production of TetOn-HGF/human umbilical cord blood-derived (hUCB)-MSCs. Functional assessment of the TetOn-HGF/hUCB-MSCs showed that they had enhanced mobility upon the induction of HGF expression. Moreover, long-term exposure by doxycycline (Dox)-treated TetOn-HGF/hUCB-MSCs enhanced the anti-apoptotic responses of genome-edited MSCs subjected to oxidative stress and improved the tube-formation ability. Furthermore, TetOn-HGF/hUCB-MSCs encapsulated by arginine-glycine-aspartic acid (RGD)-alginate microgel induced to express HGF improved in vivo angiogenesis in a mouse hindlimb ischemia model. This study showed that the inducible HGF-expressing hUCB-MSCs are competent to continuously express and secrete HGF in a controlled manner. Thus, the MSCs that express HGF in an inducible manner are a useful therapeutic modality for the treatment of vascular diseases requiring angiogenesis.
Introduction
Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) can regenerate organs1 and enhance angiogenesis.2 These cells can differentiate into endothelial and smooth muscle cells that participate in angiogenesis and neo-vasculogenesis.3 Additionally, these cells exhibit a low level of immunogenicity upon allogenic transplantation. These properties make hUCB-MSCs ideal for angiogenesis therapy. Generally, the therapeutic efficacy of these MSCs is due to the paracrine effects of the growth factors and cytokines that they secrete.4,5 Therefore, growth factor secretion by MSCs is therapeutically important. However, the amounts of growth factors that these cells secrete are often insufficient for a therapeutic effect, and it is difficult to control their levels of expression/secretion to achieve physiologically adequate concentrations. The level of growth factor secretion varies depending on the state of the cells and their passage number.6,7 Furthermore, the in vitro methodologies used to harvest, cultivate, and maintain MSCs so that therapeutic doses of the cells are obtained are challenges that require solution before these cells can be applied in the clinic. These challenges must be overcome and a better approach to stem-cell therapies must be developed.
To control the amount of a secreted growth factor in a system, recombinant protein is widely applied. Depending on the specific concentration of a recombinant growth factor, it has an effect similar to that produced by MSC treatment.8 However, some growth factors have a short half-life and a very low level of therapeutic efficacy. Hepatocyte growth factor (HGF), which is also known as scatter factor and has been identified as a superb factor for therapeutic angiogenesis, has a very short half-life of only <3–5 minutes.9 Although recombinant HGF showed promise in in vitro assays, its in vivo utility is negligible due to its short half-life.
HGF, a growth factor that is secreted by MSCs, binds to the c-Met receptor on endothelial cells. HGF not only stimulates endothelial cell growth without inducing vascular smooth muscle cell proliferation but also accelerates re-endothelialization while causing a low level of intimal hyperplasia.10,11 HGF also prevents the death of endothelial cells through its anti-apoptotic activities.12,13,14 Moreover, HGF is one of the major determinants of whether the epithelium remains in a quiescent state or switches to a proliferative state during development and tissue repair.15 However, the level of HGF in normal liver, kidney, and spleen cells is very low, and HGF expression is restricted to cells of mesenchymal origin.16 Although the endogenous HGF level increases after injury, the level reached is not sufficient for repair due to a very short half-life of <3 to 5 minutes in vivo.9 Thus, more new stable and controllable expression system is required for the improved angiogenic therapy. One of many strategies to resolve HGF problem in clinical field is genome editing technique capable of expressing therapeutic gene into the PPP1R12C site on human chromosome 19 (called the safe harbor site).
With respect to these, we integrated a Dox-inducible HGF-expression system into the safe harbor site of hUCB-MSCs via transcription activator-like effector nucleases (TALEN)-mediated genome editing to allow long-term and controllable HGF secretion. Herein, we report that the HGF secreted by these hUCB-MSCs had long-term and controllable therapeutic effects on endothelialization and angiogenesis. We also showed that treatment with the genome-edited stem cells had an improved therapeutic effect on the mouse hindlimb ischemia model.
Results
Generation of the inducible TetOn-HGF-expression construct and induction of HGF expression in hUCB-MSCs
For consistent delivery of HGF in vivo, we designed a human HGF cDNA construct for integration into human stem cells. However, because consistent HGF-Met signaling is known to trigger tumor growth,17 the level of HGF expression must be controlled. Thus, we created a construct in which HGF expression was under the control of a TetOn inducible system. In this system, tetracycline/ Dox treatment activated the expression of the target HGF cDNA. After cloning the HGF cDNA, we tested whether HGF expression was controlled by Dox.
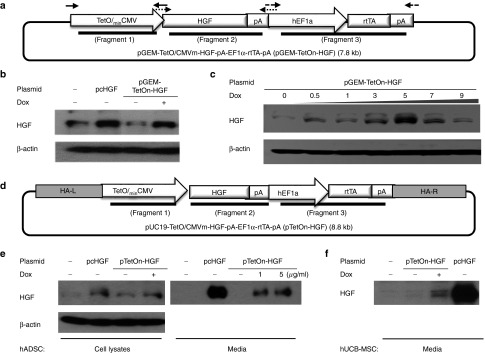
We first cloned the inducible HGF-expression construct into the interim pGEM vector, resulting in the production of pGEM-TetOn/CMVm-HGF-EF1a-rtTA, as explained in the Materials and Methods section (Figure 1a). This plasmid was transiently transfected into 293T cells, and their expression of HGF via this vector was evaluated by Western blotting (Figure 1b). HGF expression was strongly induced by Dox treatment. The expression level was similar to that of a positive control in which HGF expression was under the control of the CMV promoter in the pcDNA3.1 vector. The level of HGF expression was dependent on the Dox concentration at lower concentrations <5 µg/ml (Figure 1c). The level of expression was decreased when the Dox dosage was >10 µg/ml (see Supplementary Figure S3C), most likely due to cellular damage caused by the toxic effect of Dox because we observed cell death above that concentration. Indeed, it has been reported that 10 µg/ml of Dox suppresses cell proliferation.18 Based on our results and the cited report, we decided that ideal Dox dose would be 5 µg/ml to maximize HGF expression and minimize the side effects of Dox treatment.
Figure 1.
Transient expression of HGF was controlled by the Dox concentration. (a) Diagram of the inducible HGF-expression system. The In-fusion system was used to combine the TetOn and HGF systems in the vector. (b) Western blotting (WB) analysis of HGF expression by 293T cells at 48 hours post-Dox treatment. (c) The level of HGF expression by 293T cells was dependent on the Dox concentration. (d) Diagram of the vector constructed for integration into the PPPR12C site of chromosome 19 using the In-fusion system. (e) WB analysis of HGF expression via the integration vector in ADSCs cultured for 48 hours and the level of HGF in the medium that they conditioned as well as that (f) in the hUCB-MSC conditioned medium.
To safely integrate the inducible TetOn-HGF-expression construct into human stem cells, we then cloned this construct into a pUC19 vector that has two homologous recombination arms (HA-L and HA-R) for targeting the safe harbor PPPR12C site on chromosome 19 (Figure 1d). The pTetOn-HGF plasmid was evaluated by restriction mapping, colony PCR, and DNA sequencing (see Supplementary Figure S1A,B). The induction of HGF expression via the pUC-TetOn-HGF vector and the secretion of HGF were confirmed by Western blotting analysis of ADSCs and the medium conditioned by these ADSCs, respectively (Figure 1e), and of transfected hUCB-MSCs (Figure 1f) treated with Dox. Transfected hUCB-MSCs secreted more HGF than transfected ADSCs. When we tested the transfection efficiency of these two cell types using a GFP-expression plasmid, hUCB-MSCs were found to be transfected at >50% efficiency, whereas ADSCs were transfected at ~10% efficiency (see Supplementary Figure S2A,B). The high transfection efficiency of hUCB-MSCs will be beneficial for later genome editing. It is also known that hUCB-MSCs have a low level of immunogenicity. For these reasons, we mainly used hUCB-MSCs for the subsequent studies that we performed. Taken together, the results obtained at this stage showed that HGF expression and secretion could be controlled by Dox via the pUC19 TetOn system in which rtTA expression was driven by the EF1α promoter.
TALEN-mediated generation of hUCB-MSCs with a safe-harbored inducible HGF expression system
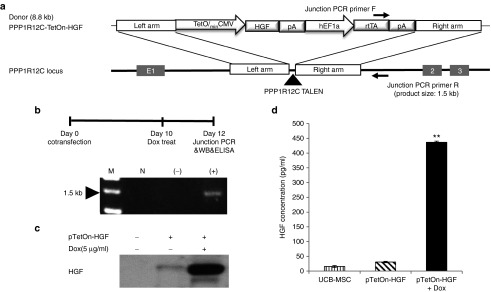
For the consistent and safe expression of HGF, the inducible pUC19-TetOn-HGF expression cassette was integrated into the safe-harbor PPPR12C site on chromosome 19 via TALEN-mediated genome editing (Figure 2a). The pUC19 vector has two arms that are coordinated with the TALEN-L/R sequences for homologous recombination. The original TALEN-L/R sequences and the commercially available HA-L/R sequences did not result in efficient gene integration. Thus, we designed several different TALEN-L/R and HA-L/R sequences and selected very effective (˃10-fold increase in efficiency compared with those of the original L/R sequences) sequences that had 50 bp spacers between the HA-L/R sequences of each TALEN-L/R sequences (Cho et al., manuscript submitted). With the newly designed TALEN system and new HA-L/R sequences in the pUC19 vector, we produced potent HGF-secreting hUCB-MSCs. Ten days after co-transfecting hUCB-MSCs with the TALEN and TetOn-HGF systems, they were treated with Dox for 2 days. Integration of the pTetOn-HGF-expression cassette into the safe harbor site was confirmed using a junction-PCR assay (Figure 2b) and by sequencing the PCR product. The results showed the expression cassette and the rtTA construct had been integrated correctly into the PPP1R12C site (see Supplementary Figure S3). HGF secretion was also demonstrated 12 days after co-transfection when the transiently present genes had disappeared (Figure 2c). ELISA analysis showed the concentration of secreted HGF was ~15 times higher in medium conditioned by Dox-treated HGF/hUCB-MSC cells than in that conditioned by non-Dox-treated controls (Figure 2d). After generation of TetOn-HGF/hUCB-MSC, MSC was characterized with stem cell markers, to test it still has stemness property with or without dox (Supplementary Figure S6). Integration of the expression system in human ADSCs was also observed 12 days after their transfection (see Supplementary Figure S3B). The level of HGF secretion by hADSCs was also optimal when they were treated with 5–7 µg/ml of Dox (see Supplementary Figure S3B). The results showed that using the newly designed TALEN system and new HA-L/R sequences in the pUC19 vector, we produced potent HGF-secreting hUCB-MSCs.
Figure 2.
Integration of the inducible HGF-expression cassette into the safe harbor site of an MSC chromosome via a TALEN system. (a) Schematic representation of integration into the PPP1R12C site of chromosome 19 via the junction polymerase chain reaction (PCR) primers. (b) Schematic showing junction-PCR sample preparation and the results obtained by PCR of UCB-MSCs. M, marker; N, negative control; UCB-MSC only genomic DNA; (−), GFP-gene transfected UCB-MSCs; (+), pTetOn-HGF vector-transfected UCB-MSCs. (c) Western blotting analysis of HGF expression at 12 days posttransfection of cells grown with and without Dox at 5 µg/ml. (d) Results of the ELISA-based analysis (*P < 0.05).
HGF secreted by HGF/hUCB-MSCs promoted cell migration
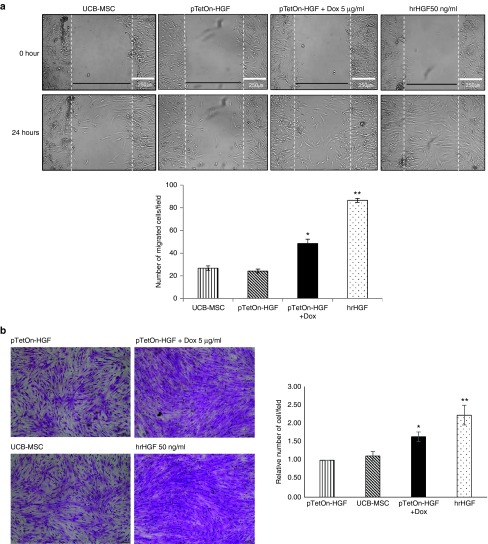
The HGF/Met pathway plays an important role in vascular remodeling after tissue damage.19,20 We evaluated the migration-enhancing effect of HGF on HGF/hUCB-MSCs, which is one of its most noted effects.21 Stem-cell migration is crucial for tissue regeneration.22,23 In the wound-healing assay, hUCB-MSCs induced to express HGF by Dox treatment migrated at a significantly higher rate than did untreated controls, as did hUCB-MSCs treated with hrHGF (at 50 ng/ml) (Figure 3a). The results of the trans-well cell-migration assay also showed that hUCB-MSCs induced to express HGF by Dox treatment migrated at a significantly higher rate than did their untreated counterparts (Figure 3b). These results were expected because HGF, which is also known as scatter factor, causes cells to migrate. When we counted the cells 1- and 2-day after seeding, the cell number in TetOn-HGF/hUCB-MSCs (Dox+) group was not significantly different from the TetOn-HGF/hUCB-MSCs (Dox−) and hUCB-MSCs control groups (data not shown), suggesting that the cell migration was not due to cell proliferation in short-term culture. Thus, our results showed that HGF secreting hUCB-MSCs had an increased migration (scattering effect).
Figure 3.
Functional effects of conditionally expressed HGF. (a) Wound healing at 24 hours after scratching monolayers of UCB-MSCs, pTetOn-HGF-transfected UCB-MSCs grown with and without Dox or treated with human-recombinant HGF (hrHGF) at 50 ng/ml. The graph shows the number of cells within the area between the lines (*P < 0.05, **P < 0.01). (b) Trans-well migration analysis at 24 hours after treatment with Dox or hrHGF. The graph shows the relative number of stained cells per field (*P < 0.05, **P < 0.01).
Long-term biological effects of HGF secreted by HGF/hUCB-MSCs
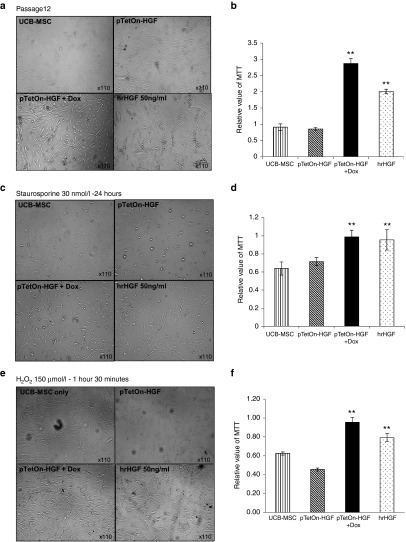
Although TetOn-HGF/hUCB-MSCs (Dox+) did not show changes in their short-term proliferation rate, long-term cultures of these cells showed a high rate of cell viability even at late passages (Figure 4a,b). The viability rate of TetOn-HGF/hUCB-MSCs (Dox+) group was slightly higher than that of the hrHGF-treated group. Previous studies showed that HGF has anti-apoptotic and antioxidative activities.19 To test whether TetOn-HGF/hUCB-MSCs (Dox+) were more resistant to apoptosis under pro-apoptotic conditions, TetOn-HGF/hUCB-MSCs were treated with STS, a pro-apoptosis reagent, or with H2O2, an oxidative reagent, at 2-day intervals for 10 days. TetOn-HGF/hUCB-MSCs (Dox+) treated with staurosporine had a better viability rate than that of staurosporine-treated control cells (Figure 4c,d), suggesting that HGF secreted by the former cells protected them against apoptosis. A similar protective effect was also observed in TetOn-HGF/hUCB-MSCs (Dox+) treated with 150 μmol/l H2O2 (Figure 4e,f), indicating that long-term HGF treatment had an antioxidative effect. This cell-protective effect was not affected by Dox itself because the morphology and viability rate of UCB-MSCs treated or not treated with Dox did not differ (see Supplementary Figure S4A,B). These data showed that the long-term secretion of HGF by TetOn-HGF/hUCB-MSCs (Dox+) enhanced their survival of pro-apoptotic and oxidative-stress conditions.
Figure 4.
Biological effects of the long-term secretion of HGF by engineered MSCs. (a) Images demonstrating the long-term natural anti-senescence effect of the HGF secreted by passage-12 cells that were not exposed to Dox or were exposed to Dox at 5 µg/ml for ~10 days. (b) MT-based viability analysis of the passage-12 cells. (c) Images of cells treated with staurosporine (STS) at 30 nmol/l for 24 hours. (d) MT-based viability analysis of STS-treated cells. (e) Images of cells treated with H2O2 at 150 µmol/l for 1 hour 30 minutes. (f) MT-based viability analysis of the H2O2-treated cells (*P < 0.05, **P < 0.01).
Angiogenesis is enhanced by medium conditioned by TetOn-HGF/hUCB-MSCs (Dox+)
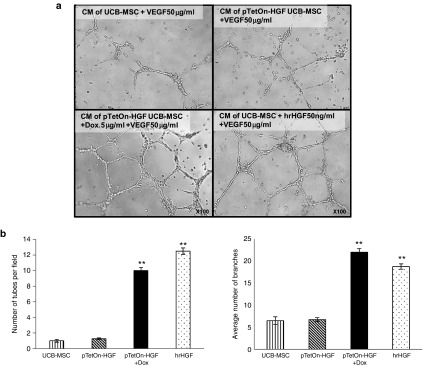
HGF is an essential factor for re-endothelialization without vascular smooth muscle cell hyperplasia.11 To evaluate the pro-angiogenesis effect of medium conditioned by TetOn-HGF/hUCB-MSCs (Dox−), a tube formation assay was performed. Media conditioned by cells grown under different conditions were collected and were used for a tube formation assay of human umbilical vein endothelial cells grown with a minimal concentration of vascular endothelial growth factor in medium. Conditioned by UCB-MSCs or by TetOn-HGF/hUCB-MSCs (Dox−) demonstrated a weak tube-formation capacity.
However, their tube formation was dramatically enhanced by medium conditioned by HGF-secreting TetOn-HGF/hUCB-MSCs (Dox+) and medium to which hrHGF was added (Figure 5a,b). The effect of the former medium was comparable with that of medium containing 50 ng/ml of hrHGF.
Figure 5.
Secreted HGF enhanced angiogenesis. (a) Images of HUVECs subjected to the matrigel assay. HUVECs were seeded at 5 × 104 cells/well and 200 µl of matrigel was used per well. After 48 hours of cultivation in Dulbecco's Modified Eagle's Medium containing 50 ng/ml of vascular endothelial growth factor (VEGF) and 5% FBS, the conditioned media (CM) were collected. The media were conditioned by only MSCs, by HGF-transfected MSCs, or by 1 × 106 HGF-transfected MSCs treated with Dox at 5 µg/ml. After the CMs were filtered using a 30K filter, HUVECs were incubated in each CM for 12 hours. (b) Analysis of endothelial tube formation according to the numbers of tubes and branches present (*P < 0.05, **P < 0.01).
TetOn-HGF/hUCB-MSCs (Dox+) enhanced blood flow in the mouse hindlimb ischemia model
HGF secreted by TetOn-HGF/hUCB-MSCs (Dox+) enhanced angiogenesis in vitro. To test the effect of TetOn-HGF/hUCB-MSCs (Dox+) in vivo, we used the mouse hindlimb ischemia model. One of the hurdles that must be overcome when applying stem-cell treatments to in vivo animal models is the loss of the injected cells. Before modified stem cells can have an effect, they can be lost due to an immune response or a biological activity. For this reason, we encapsulated the engineered cells in a biodegradable RGD-alginate material. In a previous study, encapsulating cells in RGD-alginate microgels not only decreased the rate of cell loss but also increased the survival rate of the cells and the period over which the growth factor was secreted into the tissues surrounding the injection site.24 Before and after microgel formation, the level of HGF secretion by TetOn-HGF/hUCB-MSCs (Dox+) was determined using a Western blotting assay and an ELISA (see Supplementary Figure S5A,C,D). The concentration of HGF secreted by TetOn-HGF/hUCB-MSCs (Dox+) was slightly but not significantly changed after bead formation.
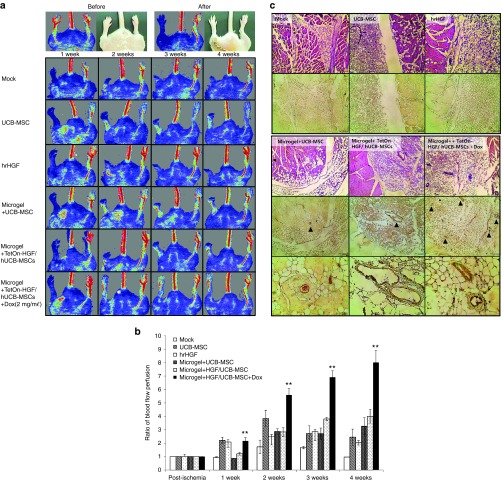
The blood flow in both hindlimbs of the model mice was measured before and after inducing ischemia in one hindlimb. One week after inducing hindlimb ischemia, the mice were treated with PBS, UCB-MSC, hrHGF, an RGD-alginate microgel containing UCB-MSCs, an RGD-alginate microgel containing TetOn-HGF/hUCB-MSCs (Dox−) or an RGD-alginate microgel containing TetOn-HGF/hUCB-MSCs (Dox+). The blood perfusion rates were measured using a Doppler flowmeter weekly for 4 weeks. As shown in Figure 6a, the blood flow to the affected hindlimb was greatly decreased after surgery had been performed. At the early postsurgery point, the non-microgel-treated groups (treated with MSCs or hrHGF) showed increased blood flow that gradually decreased by the later weeks postsurgery. In contrast, the microgel bead-treated groups showed a consistently increased level of blood flow in the ischemic limb. The TetOn-HGF/hUCB-MSCs (Dox+)-treated group showed a high level of continuous improvement in blood flow during the entire experimental period (Figure 6a,b). As expected, despite treatment with a high concentration of hrHGF (2 µg), the blood flow in the ischemic limb was not restored in the hrHGF group and this limb was lost within 1 week postsurgery (Figure 6a,b). The greatly improved effect of TetOn-HGF/hUCB-MSCs (Dox+) could be attributed not only to the proangiogenic paracrine effect of MSCs but also to their constant secretion of HGF.
Figure 6.
HGF enhanced angiogenesis in the mouse hindlimb ischemia model. A 1 week of induced hindlimb ischemia, mice were treated with phosphate buffered saline, UCB-MSCs only, hrHGF only, an RGD-alginate microgel containing UCB-MSCs, an RGD-alginate microgel containing HGF integrated UCB-MSC, an RGD-alginate microgel containing HGF-secreting UCB-MSCs treated with Dox. After treatment, the levels of blood perfusion of the hindlimbs were measured using a laser-Doppler flowmeter weekly for 4 weeks. (a) Images showing the blood flow in mice given each treatment. The ischemic limbs are on the left and the normal limbs are on the right. (b) Ratio of the blood flow perfusion rate of the ischemic limbs versus those of the normal limbs. (c) Image of hematoxylin and eosin stained sections and of sections stained with an anti-vonWillebrand factor antibody (immunohistochemistry).
Enhanced angiogenesis was also observed under the skin of the hindlimb ischemic models treated with TetOn-HGF/hUCB-MSCs (Dox+) encapsulated in microgels (data not shown). Using hematoxylin and eosin staining and immunohistochemistry analysis of vonWillebrand factor expression, we demonstrated an increase in the number of capillaries and microvessels in the group treated with microgel-encapsulated TetOn-HGF/hUCB-MSCs (Dox+) but not in the groups treated with nonencapsulated cells (Figure 6c). The thickness of the endothelial cells in the former group was also increased. These data showed that TetOn-HGF/hUCB-MSCs (Dox+) encapsulated in an RGD-alginate microgel significantly improved the level of in vivo angiogenesis through the combined effects of the hUCB-MSCs and the HGF secreted by these cells.
Discussion
It is no doubt that stem cells are strong regenerative material and potent therapeutic source. The paracrine effects of cytokines or proteins secreted by MSCs greatly facilitate regeneration and therapeutic outcomes.5,25 However, the therapeutic effects of cytokines secreted by MSCs are inadequate due to their short half-life and the rapid degradation of various types of MSCs. Therefore, new strategy to improve the therapeutic efficacy and paracrine effect of stem cell is required. For this, we developed the improved functional MSCs that secrete a growth factor using TALEN-mediated genomic editing. Genetically modified MSCs are able to controllably express and induce HGF under special condition using a drug-responsive promoter cassette. TALENs are promising tools for editing genomes. A TALEN is an artificial restriction enzyme that is generated by fusing a TAL effector DNA-binding domain to a DNA-cleavage domain. TALENs exhibit strong and specific protein-to-nucleotide recognition.26
Although TALENs can target any site, few sites are safe for exogenous-gene integration and are in an open chromosomal state. The AAVS1, which is in the PPP1R12C gene on human chromosome 19, is one of the safe harbor sites. When a gene is inserted into the AAVS1 on chromosome 19, it is efficiently expressed.27 The engineered MSCs produced using our safe harbor site system secreted HGF protein continuously in an inducible manner that could be controlled by Dox. Thus, the TALEN-mediated integration of the HGF-expression system into a chromosome of the stem cells not only provided stem cells for therapy but also solved the problem of the short half-life of a therapeutic protein.
An HGF ELISA showed that the concentration of HGF secreted by the TetOn-HGF/hUCB-MSCs reached 0.5–0.8 ng/ml (Figure 2d and see Supplementary Figure S5A,D). Although this concentration is ~1% that of the hrHGF used as a positive control, the effects of the HGF-secreting TetOn-HGF/hUCB-MSCs in most of the in vitro experiments were similar to those of the control (Figures 3, 4, and 5). These comparable effects might be due to the consistent release of HGF by the TetOn-HGF/hUCB-MSCs with Dox. We postulated that due to the short half-life of HGF (~5 minutes), spike treatment of a high concentration of hrHGF was not sufficient and only temporarily effective. Then, continuous exposure of HGF at physiological concentration produced by the engineered cells had effects comparable with those of a high concentration of hrHGF. Thus, our results showed the importance of the continuous secretion of a short half-life cytokine such as HGF.
One of major hurdle of stem-cell based cell therapy for clinical application is cell survival under harsh condition at treated site. Stem cells of superior ability are difficult to survive in the presence of oxidative environment condition. However, HGF has the function to overcome anti-apoptotic and antioxidative stress mediated cellular damage. For that reason, the maintenance of proper and constant concentration of HGF is particularly important in vivo. The long-term therapeutic effect of a low concentration of HGF secreted by the engineered stem cells was proven in the in vitro cell-viability assay, which showed that even the viability rate of these cells was higher than that of the positive control group treated with a high dose of HGF (50 ng/ml) (Figure 4). To evaluate whether the long-term secreted HGF from genetically engineered stem cells has more significant effect in vivo, immune competent mice were induced hindlimb ischemia. To improve the therapeutic efficacy and overcome low cell survival, engineered stem cells were encapsulated in injectable RGD-alginate microgel by electrospinning. In Figure 6, the hrHGF-treated group showed the least improvement in the ischemic hindlimb among all of the treated groups. The mice in the hrHGF-treated group lost their injured hindlimb within the first week following femoral-artery ligation and hrHGF treatment. The degree of limb deterioration was similar to that of the negative control group treated with PBS. On the other hand, HGF secreting MSCs in microgel significantly improved therapeutic effects among all groups. These results strongly indicate that the persistent secretion of HGF greatly enhanced angiogenesis.
Notably, we did not administer an immune suppressor to the mice even though human stem cells were implanted in them. Reportedly, immune modulation can be accomplished not only by protecting stem cells by encapsulating them in a microgel but also through the effects of the MSCs and HGF. We speculated that due to the combined effect of RGF-alginate microgel-encapsulated hUCB-MSCs and the HGF that these cells secreted, our therapeutic system provided even better immune modulation so that treatment with an immune-system suppressor was not required for the therapeutic efficacy of this system.28,29,30
Although Dox-treated TetOn-HGF/hUCB-MSCs greatly enhanced the rates of tube formation and in vivo angiogenesis, further improvement is required before they can be used as therapeutic agents. Because the hUCB-MSCs are primary cells, their transfection efficiency rate and the rate of integration of the TetOn-HGF transgene differ depending on their status. Different rates of gene integration via TALEN may result in different concentrations of the secreted protein. Late passages of MSCs are an important concern. By the time that the expression system has become integrated into the hUCB-MSCs, they begin to reach senescence. Therefore, it is difficult to produce sufficient numbers of TetOn-HGF/hUCB-MSCs for use as therapeutic agents. Further studies are needed to standardize the procedure we utilized and to overcome the problem of senescence.
Safety is always an important issue for stem-cell therapy. A major problem for stem-cell-mediated gene therapy is controlling the level of gene expression. However, our system provided an example of how to control the side effects of stem-cell and gene therapies. Regarding the side effects of gene therapy, HGF transgene expression by our system can be controlled by a drug, Dox (tetracycline) because it is under the control of a tetracycline-on system, which was also integrated into the safe harbor site using TALEN-mediated gene delivery. Regarding the side effects of cell therapy, hUCB-MSCs are tolerated by the immune system, and these cells are further protected from immune-system attack by being encapsulated in RGD-alginate. Taken all together, our inducible HGF secreting MSCs are an effective tool as a stem-cell-based therapeutic agent.
Conclusion
HGF is a pleiotropic cytokine that has long been known to be involved in cell and tissue regeneration. However, the available tools that can produce its therapeutic effects are clinically insufficient. In this study, we generated inducible TetOn-HGF/hUCB-MSCs via TALEN-based genome editing. Notably, these cells in RGD-alginate microgel secreted HGF and enhanced cell mobility, protected cells against apoptosis, and improved the level of angiogenesis in a mouse hindlimb ischemia model. Our study clearly demonstrated that gene editing allowed HGF secretion by hUCB-MSCs and overcame the limitations of other HGF-based pro-angiogenesis therapies for vascular diseases such as ischemia.
Materials and Methods
Cell cultures. hUCB-MSCs isolated from hUCB were collected as previously described.31 The hUCB-MSC isolation procedure was approved by the Borame Institutional Review Board and Seoul National University (IRB No. 0603/001-002-07C1). The hUCB-MSCs were maintained in keratinocyte-serum-free medium supplemented with human-recombinant epidermal growth factor and bovine pituitary extract (Gibco–Life Technologies, Grand Island, NY) at 37 °C in 5% CO2. Adipose-derived stem cells (ADSCs) isolated from human adipose tissue were kindly provided by EHL Bio and were maintained in MesenPro medium containing the supplement-kit reagents (Gibco–Life Technologies) under the same conditions. HEK293T cells, a human embryonic kidney cell line, were purchased from the American Type Culture Collection (ATCC, Manassas, VA). HEK293T cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (HyClone-GE, South Logan, UT) supplemented with 10% fetal bovine serum (FBS) (HyClone-GE) and penicillin/streptomycin (Gibco–Life Technologies).
Preparation of a plasmid containing human HGF cDNA and the TetOn system. To clone the human HGF cDNA into a specific homologous recombination vector for inducible expression, primers that overlapped the end of each DNA fragment were designed, and polymerase chain reaction (PCR) was performed. We first separately cloned TetOn/CMVmin promoter, HGF-pA, and hEF1a-rtTA-pA constructs into a pGEM vector. Then, these three DNA fragments were PCR-amplified and were ligated into the basic plasmid pGEM using an In-fusion kit (Clontech Lab, Mountin View, CA) (Figure 1a). Then, the human HGF cDNA and TetOn construct were transferred into the pUC19-adeno-associated virus integration site 1 (AAVS1) vector, which contains two homologous recombination sites (HA-L and HA-R) for targeting AAVS1 on human chromosome 19. In this plasmid, the expression of human-recombinant HGF (hrHGF) cDNA is regulated by the TetOn/minimal promoter, which is activated by the tetracycline-rtTA protein that is expressed via the EF1α promoter (Figure 1a). Correct ligation into the vector was confirmed using restriction mapping (using NotI and AgeI) and colony PCR (see Supplementary Figure S1A). The final plasmid was also evaluated by DNA sequencing and was found to be correct (see Supplementary Figure S1B). The purified pUC19-TetOn-HGF plasmid was transfected into HEK 293-T cells using Lipofectamine. The expression of HGF was confirmed by Western blotting using an anti-HGF antibody (ab83760; Abcam, Cambridge, UK) after Trichloroacetic acid (TCA)-based precipitation of the proteins in the medium conditioned by the transfected cells.
Western blotting and Enzyme-linked Immunosorbent Assay. After the HGF-expression plasmid was transfected into cells using a Neon electroporation device (Invitrogen, Carlsbad, CA), 2 × 105 transfected cells/well were seeded in a six-well culture dish (Thermo-Nunc, Jiangsu, China). The cells were treated with Dox (at 5 µg/ml) for 2 days at 37 °C. Then, the cells in each well were washed with phosphate buffered saline (PBS) and were lysed using 100 µl of lysis buffer (RIPA buffer; Thermo Fisher, Bellefonte, PA) containing a protease-inhibitor cocktail (PIC, Roche, Mannheim, Germany). The cells were collected using a scraper, and the cell lysates were incubated on ice for 20 minutes. The cell extracts were centrifuged for 10 minutes at 13,000 rpm to remove debris. The supernatants were collected, and the protein concentrations were determined using a Bradford assay kit (Bio-Rad, Berkeley, CA). To produce conditioned media, the cells were incubated in serum-free media for 48 hours under different conditions. Then, the proteins in the conditioned media were precipitated using TCA, as previously described. Western blotting assays were performed as previously described.32 Briefly, 20 µg of each protein sample were separated in a 10% gel by SDS-PAGE. After blocking the blot for 1 hour using 5% skim milk, the blot was incubated with an antihuman HGF antibody for 16 hours at 4 °C and then was incubated with an anti-rabbit secondary antibody for 1 hour at room temperature. Then, the labeled bands were detected using ECL + reagents (Thermo Fisher). For the ELISA assay, the media were filtered using a 30K cut-off filter (Merck Millipore, Billerica, MA) and the proteins were assayed using an antihuman HGF ELISA kit (ab100534; Abcam), as previously described.33
Junction PCR. To confirm that the human HGF expression cassette was integrated into the AAVS1 on chromosome 19, a forward primer (5ʹ-ACTAAGTAAGGATCCA GACATGATAAGA-3ʹ) was designed to detect the human HGF portion of the cassette and a reverse primer (5ʹ-CCCACCCCAATGCTCCAGGC-3ʹ) was designed to detect part of the PPPR12C genomic locus. PCR was performed using LA taq polymerase (TaKaRa) according to the following protocol: one cycle of 92 °C for 3 minutes, then 35 cycles of 92 °C for 1 minute 30 seconds, 60.7 °C for 3 minutes, and 72 °C for 2 minutes, and a final cycle of 72 °C for 3 minutes. The PCR product was evaluated using a 1% agarose gel. The PCR product was also sequenced using the primers described above.
Cell migration assay. For the wound healing/cell migration assay, TetOn-HGF/hUCB-MSCs (5 × 104 cells) were seeded in 24-well plates. The cells were cultured until reaching confluence and then were starved for 24 hours. A linear wound was created in each monolayer using a pipette tip. Cell motility in terms of wound closure was evaluated by photographing three random fields 24 hours after wounding the monolayer. For the trans-well migration assay, the bottom of the upper chamber of the trans-well was coated with 0.2% gelatin (Sigma, St. Louis, Missouri). Each group of cells was starved for 16 hours, then same number of the cells (2.5 × 104) were resuspended in each conditioned medium, and then were seeded in the upper chamber. The plate was then incubated in 5% CO2 at 37 °C for 24 hours. Then, the cells on the membrane were stained with 0.1% crystal violet. The migration rate was determined by counting the number of migrated cells in three random fields under a light microscope.
Cell viability assay. TetOn-HGF/hUCB-MSC cells were seeded in 48-well plates at 1 × 104 cells/well. The MTT assay (Amresco, Cleveland, OH) was used to determine the relative rate of cell growth at 1, 3, 5, and 7 days of cultivation. MTT reagent (100 μl of a 0.2 mg/ml solution) was added to the media and the plates were incubated for 5 hours at 37 °C. After removing the culture media, the crystals remaining were dissolved in 500 μl of DMSO (Duksan, Seoul Korea) and the absorbance at 470 nm was measured.
Anti-apoptosis and antioxidative stress assay. After TetOn-HGF/hUCB-MSC cells (5 × 105) had been grown under each condition (with Dox or without Dox), they were seeded in 60 ø culture plates (Thermo Fisher) and were treated with STC (30 nmol/l) for 24 hours or with H2O2 (150 mmol/l) for 3 hours. Then, the cell morphology was observed under a light microscope (Juli; NanoEndTech, Seoul, Korea) and the cell viability rate was measured using the MTT assay.
Tube formation. Human umbilical vein endothelial cells (5 × 104 cells/well) were seeded on a layer of BD Matrigel (BD Biosciences, San Jose, CA) in 24-well plates. The cells were then exposed to vascular endothelial growth factor at 50 ng/ml (R&D) and conditioned media that had been collected from hUCB-MSCs, TetOn-HGF/hUCB-MSCs that were grown with Dox and without Dox (2 × 106 cells/100 ø dish) in high-glucose DMEM (Thermo Fisher) containing 5% FBS (Thermo) and antibiotics/antimycotics (Gibco) for 48 hours. DMEM media containing 5% FBS was used as a control. The protein concentrations in the conditioned media were enriched 10-fold using a 30K cut-off filter, and the filtrates were added to human umbilical vein endothelial cells, after which the cells were incubated for 12 hours to allow them to form tube-like structures. Tube formation was analyzed by counting the number of branches per high-power field.
Cell-microgel transplantation in the hindlimb ischemia model. RGD-alginate/cell microgels were prepared as described in our previous publication.24 To assess the level of HGF secretion by the cells in the microgels, media conditioned by different cells were collected after 48 hours of incubation in high-glucose DMEM containing 10% FBS. Then, the HGF levels in the conditioned media were determined using a Western blotting assay and an ELISA. The shapes of the cells in the microgels were examined using a JuLI microscope (NanoEnTek). The hindlimb ischemia model was generated as described in our previous publication.24 One week after surgery, the mice were injected with either PBS, hUCB-MSCs only, hrHGF (2 µg), a microgel containing hUCB-MSCs (2 × 107 cells in 1 ml of RGD-alginate per mouse), a microgel containing TetOn-HGF/hUCB-MSCs or a microgel containing TetOn-HGF/hUCB-MSCs treated with Dox. Mircogels containing cells were injected into three or four gracilis muscles at the medial thigh in the ischemic limbs. About 0.5 ml of mixture were injected into the gracilis muscles using insulin syringes. The blood perfusion of the hindlimb before occlusion and the complete lack of blood perfusion immediately after occlusion were evaluated using a Laser-Doppler Flowmeter (Moor LDI, Moor Instruments, Devon, UK); the perfusion of both hindlimbs was then evaluated weekly for 4 weeks. The data were analyzed using Moor LDI-PC-software. All of the animal studies were performed according to the Seoul National University Animal Care Committee guidelines after acquiring permission from the committee (Protocol #SNU-1).
Immunohistochemistry. Tissues were harvested, fixed in 4% paraformaldehyde (Wako), embedded in paraffin, and cut into 5 μm sections (Leica, Buffalo Grove, IL). Immunohistochemistry was performed using an anti-vonWillebrand factor antibody at a dilution of 1:100 (Merck Millipore) and the appropriate secondary antibody. Sections were also stained with hematoxylin and eosin. The number of blood vessels was counted as previously described.31,32 The data presented are the mean values ± SE of three hindlimbs per group.
Statistical analysis. The data were expressed as the mean values ± standard error. The data were analyzed using Excel software, and a t-test was used to compare the data pertaining to the different experimental groups. Differences were considered significant when the P values were <0.05.
SUPPLEMENTARY MATERIAL Figure S1. HGF vector construction. Figure S2. Comparison of the transfection efficiency of ADSCs and UCB-MSCs. Figure S3. The integration of the HGF-expression system was confirmed by sequencing junction-PCR fragments and WB analysis of the expression and secretion of HGF by ADSCs. Figure S4. Doxycycline treatment did not affect cell proliferation. Figure S5. HGF expression by engineered UCB-MSCs encapsulated in a microgel. Figure S6. The expression of UCB-MSC makers after transfection and doxycycline treatment.
Acknowledgments
This research investigation was supported by grants from the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning (grants No. 2012M3A9C6049716 and 2014M3A9D5A01073598).
Supplementary Material
References
- Caplan, AI (2007). Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341–347. [DOI] [PubMed] [Google Scholar]
- Wu, Y, Chen, L, Scott, PG and Tredget, EE (2007). Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25: 2648–2659. [DOI] [PubMed] [Google Scholar]
- Lin, CS, Xin, ZC, Deng, CH, Ning, H, Lin, G and Lue, TF (2010). Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol 25: 807–815. [DOI] [PubMed] [Google Scholar]
- Gnecchi, M, Zhang, Z, Ni, A and Dzau, VJ (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, YL, Zhao, Q, Qin, X, Shen, L, Cheng, L, Ge, J et al. (2005). Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 80: 229–36; discussion 236. [DOI] [PubMed] [Google Scholar]
- Smith, S, Neaves, W and Teitelbaum, S (2007). Adult versus embryonic stem cells: treatments. Science 316: 1422–3; author reply 1422. [DOI] [PubMed] [Google Scholar]
- Raff, M (2003). Adult stem cell plasticity: fact or artifact? Annu Rev Cell Dev Biol 19: 1–22. [DOI] [PubMed] [Google Scholar]
- Ferrara, N (2004). Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 9 (suppl. 1): 2–10. [DOI] [PubMed] [Google Scholar]
- Kawaida, K, Matsumoto, K, Shimazu, H and Nakamura, T (1994). Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA 91: 4357–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y, Morishita, R, Higaki, J, Kida, I, Aoki, M, Moriguchi, A et al. (1996). Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. J Hypertens 14: 1067–1072. [DOI] [PubMed] [Google Scholar]
- Hayashi, K, Nakamura, S, Morishita, R, Moriguchi, A, Aoki, M, Matsumoto, K et al. (2000). In vivo transfer of human hepatocyte growth factor gene accelerates re-endothelialization and inhibits neointimal formation after balloon injury in rat model. Gene Ther 7: 1664–1671. [DOI] [PubMed] [Google Scholar]
- Morishita, R, Nakamura, S, Nakamura, Y, Aoki, M, Moriguchi, A, Kida, I et al. (1997). Potential role of an endothelium-specific growth factor, hepatocyte growth factor, on endothelial damage in diabetes. Diabetes 46: 138–142. [DOI] [PubMed] [Google Scholar]
- Nakagami, H, Morishita, R, Yamamoto, K, Yoshimura, SI, Taniyama, Y, Aoki, M et al. (2001). Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes 50: 1472–1481. [DOI] [PubMed] [Google Scholar]
- Ponzetto, C, Bardelli, A, Zhen, Z, Maina, F, dalla Zonca, P, Giordano, S et al. (1994). A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77: 261–271. [DOI] [PubMed] [Google Scholar]
- Kopp, JB (1998). Hepatocyte growth factor: mesenchymal signal for epithelial homeostasis. Kidney Int 54: 1392–1393. [DOI] [PubMed] [Google Scholar]
- Bottaro, DP, Rubin, JS, Faletto, DL, Chan, AM, Kmiecik, TE, Vande Woude, GF et al. (1991). Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251: 802–804. [DOI] [PubMed] [Google Scholar]
- Gao, CF and Vande Woude, GF (2005). HGF/SF-Met signaling in tumor progression. Cell Res 15: 49–51. [DOI] [PubMed] [Google Scholar]
- Fife, RS, Rougraff, BT, Proctor, C and Sledge, GW Jr (1997). Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J Lab Clin Med 130: 530–534. [DOI] [PubMed] [Google Scholar]
- Gallo, S, Sala, V, Gatti, S and Crepaldi, T (2014). HGF/Met axis in heart function and cardioprotection. Biomedicines 2: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, S, Sala, V, Gatti, S and Crepaldi, T (2015). Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci (Lond) 129: 1173–1193. [DOI] [PubMed] [Google Scholar]
- Naldini, L, Weidner, KM, Vigna, E, Gaudino, G, Bardelli, A, Ponzetto, C et al. (1991). Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J 10: 2867–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, T, Shiojima, I, Matsuura, K and Komuro, I (2005). Promotion of cardiac regeneration by cardiac stem cells. Circ Res 97: 615–617. [DOI] [PubMed] [Google Scholar]
- Matsumoto, K, Funakoshi, H, Takahashi, H and Sakai, K (2014). HGF–Met pathway in regeneration and drug discovery. Biomedicines 2: 275–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, PH, Yim, HG, Choi, YJ, Kang, BJ, Kim, J, Kwon, SM et al. (2014). Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J Control Release 187: 1–13. [DOI] [PubMed] [Google Scholar]
- Gnecchi, M, Zhang, Z, Ni, A and Dzau, VJ (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell, VM, Wang, Y, Campbell, JM, Poshusta, TL, Starker, CG, Krug, RG 2nd et al. (2012). In vivo genome editing using a high-efficiency TALEN system. Nature 491: 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Y, Liu, C, Cerbini, T, San, H, Lin, Y, Chen, G et al. (2014). Stable enhanced green fluorescent protein expression after differentiation and transplantation of reporter human induced pluripotent stem cells generated by AAVS1 transcription activator-like effector nucleases. Stem Cells Transl Med 3: 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunishi, K, Dohi, M, Nakagome, K, Tanaka, R, Mizuno, S, Matsumoto, K et al. (2005). A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol 175: 4745–4753. [DOI] [PubMed] [Google Scholar]
- Hoogduijn, MJ, Popp, F, Verbeek, R, Masoodi, M, Nicolaou, A, Baan, C et al. (2010). The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol 10: 1496–1500. [DOI] [PubMed] [Google Scholar]
- Abdi, R, Fiorina, P, Adra, CN, Atkinson, M and Sayegh, MH (2008). Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 57: 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, BJ, Kim, H, Lee, SK, Kim, J, Shen, Y, Jung, S et al. (2014). Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater 10: 3007–3017. [DOI] [PubMed] [Google Scholar]
- Ahn, JM, Sung, HJ, Yoon, YH, Kim, BG, Yang, WS, Lee, C et al. (2014). Integrated glycoproteomics demonstrates fucosylated serum paraoxonase 1 alterations in small cell lung cancer. Mol Cell Proteomics 13: 30–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, S, Hirohashi, Y, Torigoe, T, Inoue, R, Kitamura, H, Tanaka, T et al. (2013). Prostate cancer stem-like cells/cancer-initiating cells have an autocrine system of hepatocyte growth factor. Cancer Sci 104: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.