Abstract
Recovery from ischemic tissue injury can be promoted by cell proliferation and neovascularization. Transient expression of four pluripotency factors (Pou5f1, Sox2, Myc, and Klf4) has been used to convert cell types but never been tested as a means to promote functional recovery from ischemic injury. Here we aimed to determine whether transient in situ pluripotency factor expression can improve neurobehavioral function. Cerebral ischemia was induced by transient bilateral common carotid artery occlusion, after which the four pluripotency factors were expressed through either doxycycline administration into the lateral ventricle in transgenic mice in which the four factors are expressed in a doxycycline-inducible manner. Histologic evaluation showed that this transient expression induced the proliferative generation of astrocytes and/or neural progenitors, but not neurons or glial scar, and increased neovascularization with upregulation of angiogenic factors. Furthermore, in vivo pluripotency factor expression caused neuroprotective effects such as increased numbers of mature neurons and levels of synaptic markers in the striatum. Dysplasia or tumor development was not observed. Importantly, neurobehavioral evaluations such as rotarod and ladder walking tests showed that the expression of the four factors dramatically promoted functional restoration from ischemic injury. These results provide a basis for novel therapeutic modality development for cerebral ischemia.
Introduction
Restoration or regeneration of damaged tissue is important for functional recovery from injuries or diseases. Compared to other mammalian organs and tissues, the brain lacks significant regenerative capacity: An injury in brain tissue often leads to semipermanent functional impairment. Because proliferative generation of the brain cells required for functional recovery is not sufficiently provided by endogenous cell sources, supplementation with other cell types may aid recovery. Transplantation of exogenous cells such as bone marrow mononuclear cells,1 bone marrow stromal cells,2 and neural stem cells3 has been reported to enhance brain tissue regeneration, leading to functional improvement. However, the engraftment of transplanted cells is often limited4 and the generation of neural-lineage cells from transplanted cells may not be sufficient for functional restoration.5 Furthermore, cell transplantation into the brain can have adverse effects: Allogenic or xenogenic cells can be rejected by the immune system or tumor development can occur after cell injections.6
Previous studies reported enhancement of functional recovery by augmented proliferative generation, activation, and mobilization of brain cells from endogenous cell sources using exogenously delivered humoral factors such as epidermal growth factor and brain-derived neurotrophic factor. Intraventricular delivery of brain-derived neurotrophic factor, noggin, or epidermal growth factor into the lateral ventricle via an adenoviral vector or an osmotic pump has been reported to activate endogenous neural stem/progenitor cells in the subventricular zone, leading to striatal regeneration and consequent functional improvement in mouse models of chronic hypoxic-ischemic brain injury7 and Huntington's disease.8,9 However, the endogenous cell types directly affected by these humoral factors are restricted to those with receptors for these factors, which might limit more robust functional recovery.
Yamanaka's group showed that exogenous expression of four pluripotency-associated transcription factors, i.e., Pou5f1 (octamer-binding protein, Oct4), Sox2 (SRY-box containing gene 2), Myc (c-myelocytomatosis oncogene, c-myc), and Klf4 (Kruppel-like factor 4), can convert fibroblasts into pluripotent stem cells.10 Subsequent studies have shown that these pluripotency factors can also be used to convert one cell type to another directly: Transient expression of pluripotency factors renders cells into an activated plastic status, poising them to undergo direct cell type conversion into other lineages, such as neural progenitors,11 angioblasts,12 hepatocytes,13 or cardiomyocytes,11 depending on the culture environment. Recently, it has been shown that these pluripotency factor–induced lineage conversions are achieved via transient acquisition of pluripotent status.14
Direct conversion of cell types without mediation of pluripotent status has also been studied for possible practical applications. For example, fibroblasts were converted into neurons15 and neural progenitor cells16 by in vitro expression of defined transcription factors. Important potential uses of cell type conversion include disease modeling and regeneration of damaged tissue.17 In vitro central nervous system disease models using direct conversion have been shown to recapitulate the disease in question.18,19 In vivo direct conversion has also been studied as a therapeutic modality for functional recovery from damage or injury in brain tissue: Glial cells and transplanted fibroblasts were converted into neuronal lineage cells in vivo.20,21,22 However, neurobehavioral functional improvement has never been reported using these in vivo direct conversion strategies.
One of the earliest noticeable responses of cells that are exposed to forced expression of the four pluripotency factors is enhanced cell proliferation, which can be observed as early as 24 hours after induction of this expression.23 This enhanced proliferation is associated molecularly with the induction of proliferative genes.24 Based on this observation, we postulated that in situ transient expression of pluripotency factors would facilitate in vivo cell proliferation, a critical and essential process for recovery of injured tissue. Furthermore, Myc induces neovascularization,25 an essential process for recovery from ischemic injury.
In this study, we, for the first time, investigated whether in situ transient expression of pluripotency factors can enhance functional recovery from ischemic injury. To address this issue, we used a mouse model of cerebral ischemia, in which spontaneous regeneration or recovery is limited. Here we show that in situ transient expression of pluripotency factors after an ischemic injury facilitates functional recovery, which is associated with proliferative generation of astrocytes and/or neural progenitors coupled with enhanced neovascularization in brain tissue.
Results
In situ pluripotency factor expression increases new neural progenitors and astrocytes in the subventricular zone
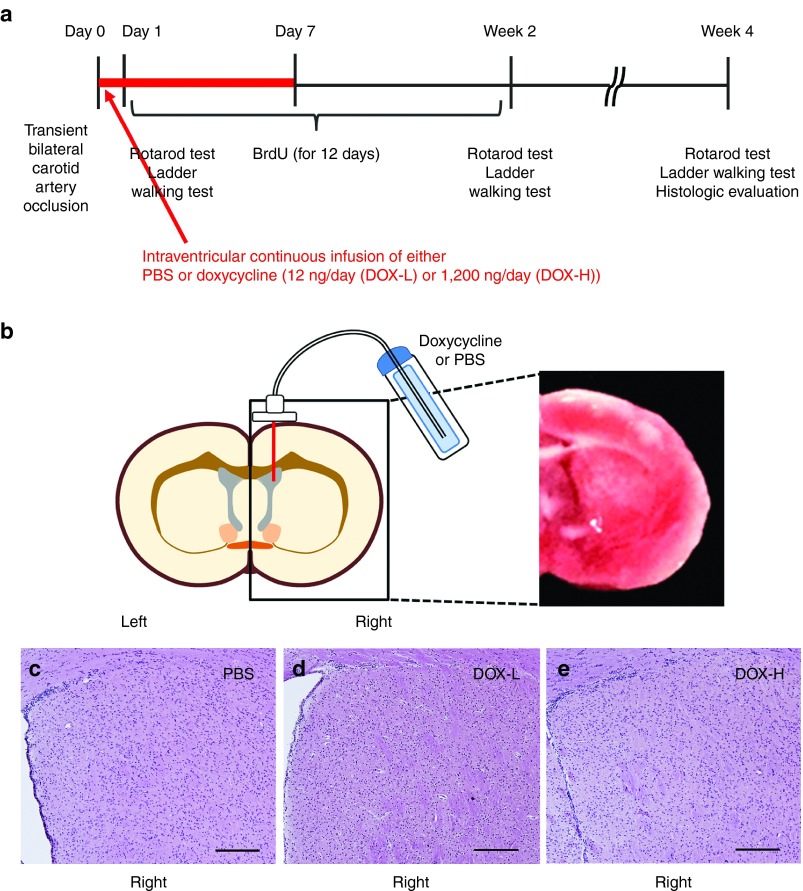
To elicit in situ expression of pluripotency factors in vivo, we first used transgenic mice (hereafter, referred to as reprogrammable mice) in which the four factors, i.e., Pou5f1 (Oct4), Sox2, Myc, and Klf4, are expressed in a doxycycline-inducible manner.26 We validated doxycycline's effects using embryonic fibroblasts isolated from reprogrammable mice. Reverse transcription polymerase chain reaction (RT-PCR) showed that the four pluripotency factors were expressed in the presence of 10~1,000 ng/ml doxycycline (Supplementary Figure S1a). To express the factors in damaged brain tissues in situ, we used an osmotic pump to infuse low (12 ng/day, DOX-L group) or high (1,200 ng/day, DOX-H group) amounts of doxycycline or control buffer (phosphate buffered saline (PBS)) into the lateral ventricle for 7 days starting immediately after 20-minute bilateral common carotid artery occlusion27 in the reprogrammable mice (Figure 1a,b). To determine whether doxycycline induced expression of pluripotency factors in vivo, we measured expression of Sox2 using quantitative real-time polymerase chain reaction (qRT-PCR) 3 days after the infusion of doxycycline. We found that Sox2 expression in the striatum significantly increased in mice treated with DOX-H compared with the other groups in a dose-dependent manner (Supplementary Figure S1b; P < 0.05). Although in vivo doxycycline-induced expression of pluripotency factors has been reported to cause dysplasia or tumor formation in epithelial tissues,28,29 we did not observe either of these effects 4 weeks after the initiation of this 1-week low-dose doxycycline infusion (Figure 1c–e).
Figure 1.
Study design and doxycycline injection. (a) Study design. Transient cerebral ischemia was induced by 20-minute bilateral carotid artery occlusion (two vessel occlusion) in the doxycycline (DOX)-inducible reprogrammable mice. Thereafter, DOX or phosphate buffered saline (PBS, control buffer) was continuously infused into the right lateral ventricle via an osmotic pump over 7 days and BrdU was daily injected for 12 days. Behavioral tests such as the rotarod and ladder walking tests were performed. Four weeks after the cerebral ischemia induction, the mice were sacrificed and histologically analyzed. (b) DOX injection into the lateral ventricle via an osmotic pump (left side) to infuse low (12 ng/day, DOX-L) or high (1,200 ng/day, DOX-H). Brain damage is shown using TTC strain at 1 day after ischemic injury (right side). (c–e) Absence of dysplasia or tumor development in brain tissue. Four weeks after the initiation of DOX-H, DOX-L, or PBS infusion, brain sections were stained with hematoxylin and eosin and observed using a microscope. Neither dysplasia nor tumors were observed in any groups. Scale bars = 200 μm. BrdU, 5-bromo-2-deoxyuridine; TTC, 2,3,5-triphenyltetrazolium chloride.
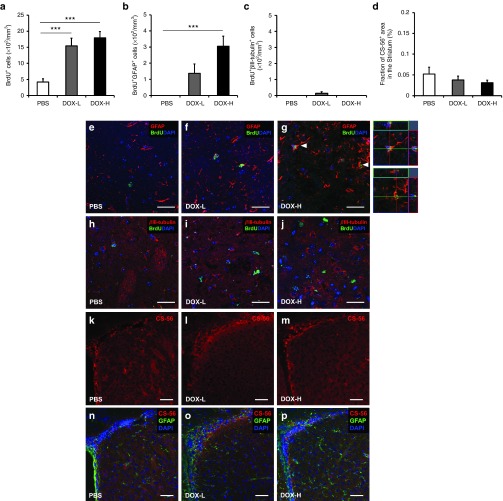
To determine whether this in situ expression enhances cell proliferation in the damaged tissue, we first determined neurogenesis and gliogenesis levels in the subventricular zone, the site of the highest expected concentration of doxycycline after diffusion from the lateral ventricle. This is also the site where neural stem/progenitor cells, which contribute to regeneration, exist in the adult mammalian brain.30,31 To label proliferating cells, 5-bromo-2-deoxyuridine (BrdU) was given to the mice every day for 12 days after the stereotaxic surgery. The numbers of BrdU+ cells in the subventricular zone in mice in the DOX-H and DOX-L groups were 7.7- and 4.5-fold, respectively, significantly higher than in the PBS control (Supplementary Table S1; Figure 2a; P < 0.001 and P < 0.01, respectively) and the number of BrdU+ cells in the DOX-H group was 1.7-fold significantly higher than in the DOX-L group (Figure 2a; P < 0.01), indicating that pluripotency factors enhance cell proliferation in the subventricular zone in a dose-dependent manner.
Figure 2.
Increased number of newly generated neural progenitors and astrocytes induced by pluripotency factor expression in the subventricular zone. After the induction of cerebral ischemia, the doxycycline (DOX)-inducible reprogrammable mice were infused with two dose of doxycycline (12 ng/day, DOX-L; 1,200 ng/day, DOX-H) or phosphate buffered saline (PBS, control buffer) into the right lateral ventricle for 7 days. To identify newly generated cells, the mice were daily injected daily with 5-bromo-2-deoxyuridine (BrdU) for 12 days. Four weeks after ischemia induction, histologic evaluations were performed. (a) The density of BrdU+ cells in the subventricular zone was significantly higher in both the DOX-H and DOX-L groups than in the PBS controls. n = 5/group; *P < 0.05, **P < 0.01, and ***P < 0.001 (one-way analysis of variance (ANOVA) followed by a post-hoc Bonferroni comparison). (b–d) The density of newly generated neural progenitors, astrocytes, and neurons was determined through confocal microscopy by calculating the density of cells triple positive for 4ʹ,6-diamidino-2-phenylindole (DAPI) (blue, nuclei), BrdU (green), and cell type–specific markers such as Nestin, GFAP, and βIII-tubulin, respectively. The densities of BrdU+Nestin+ (b) and BrdU+GFAP+ cells (c) but not BrdU+βIII-tubulin+ cells (d) in both the DOX-H and DOX-L groups were significantly higher than in the PBS controls. n = 5/group; *P < 0.05, **P < 0.01, and ***P < 0.001 (one-way ANOVA followed by a post-hoc Bonferroni comparison). (e–m) Representative confocal microscopic images. (g,j) Cells triple positive for DAPI, BrdU, and cell type–specific markers are indicated with white arrow heads in the right panel. Scale bars = 25 μm. In panels a–d, bars, mean + SEM.
We next attempted to identify the proliferating cells by double-staining with BrdU and cell type–specific markers such as βIII-tubulin (Tuj1, neuronal marker), GFAP (astrocyte marker), or Nestin (neural progenitor cell marker) in the subventricular zone. The numbers of BrdU+Nestin+ cells in the DOX-H and DOX-L groups were 26.5- and 20.4-fold, respectively, significantly higher than in the PBS control group (Figure 2be–g; P < 0.01 and P < 0.05, respectively), indicating that in situ expression of pluripotency factors enhances the proliferative generation of neural progenitor cells. The numbers of BrdU+GFAP+ cells in the DOX-H and DOX-L groups were 15.2- and 10.8-fold, respectively, significantly higher than in the PBS control group (Figure 2c,h–j; P < 0.001 and P < 0.01, respectively), suggesting that pluripotency factor expression causes a robust proliferative generation of astrocytes. However, the numbers of BrdU+βIII-tubulin+ cells were similar among all groups (Figure 2d,k–m), suggesting that neurogenesis in the subventricular zone is not affected by the pluripotency factors.
Pluripotency factor expression increases newly generated astrocytes but not neurons or glial scar in the striatum
Motor performances evaluated by rotarod and forelimb slip tests are largely affected by the striatal function. Moreover, the striatum is the second closest site to the lateral ventricle where doxycycline is infused. Furthermore, the activation of neural stem/progenitor cells in the subventricular zone through the intraventricular administration of growth factors including brain-derived neurotrophic factor can also turn the striatum into a regenerative environment.7,8,32 Thus, we next evaluated neurogenesis and gliogenesis in the right striatum to determine whether the in vivo pluripotency factor expression can similarly turn the damaged striatum into a restorative environment.
The numbers of BrdU+ cells in both the DOX-H and DOX-L groups were 4.3- and 3.7-fold, respectively, significantly higher than in the PBS control group (Figure 3a; P < 0.001), suggesting that doxycycline-induced pluripotency factor expression increases the number of proliferating cells in the striatum. As in the subventricular zone, the number of BrdU+GFAP+ cells in the DOX-H group was 2.2-fold significantly higher than in the PBS control group, in which BrdU+GFAP+ cells were barely observed (Figure 3b,e–g; P < 0.001), suggesting that pluripotency factor expression robustly increases the number of newly generated astrocytes in the striatum. The numbers of BrdU+βIII-tubulin+ cells in the striatum were much lower than in the subventricular zone and were similar among all groups (Figure 3c,h–j), suggesting that neurogenesis in the striatum is not altered by the expression of pluripotency factors. In addition, the levels of the glial scar marker CS-56 did not differ among the groups (Figure 3d,k–p), suggesting that the astrocytes that were newly generated by in vivo pluripotency factor expression do not increase the detrimental glial scar formation that inhibits neuroregeneration after ischemic brain damage.
Figure 3.
Pluripotency factor expression increases the number of newly generated astrocytes in the striatum. (a) The densities of BrdU+ cells in the striatum of both DOX-H and DOX-L groups were significantly higher than in the PBS controls. n = 5/group; **P < 0.01; ***P < 0.001 (one-way analysis of variance (ANOVA) followed by a post-hoc Bonferroni comparison). (b,c) The density of newly generated astrocytes and neurons was determined through confocal microscopy by calculating the density of cells triple positive for 4ʹ,6-diamidino-2-phenylindole (DAPI) (blue, nuclei), BrdU (green), and cell type–specific markers such as GFAP and βIII-tubulin, respectively. The density of BrdU+GFAP+ cells (b), but not BrdU+βIII-tubulin+ cells (c), was significantly higher in the DOX-H group than in the DOX-L and PBS groups. n = 5/group; ***P < 0.001 (one-way ANOVA followed by a post-hoc Bonferroni comparison). (d) The density of area positive for CS-56, a marker of glial scar. The area of CS-56 staining did not differ among the groups (n = 5/group). (e–j) Representative confocal microscopic images of BrdU+GFAP+ cells (e–g) and BrdU+βIII-tubulin+ cells (h–j) in the striatum. (g) Three-dimensional images of cells triple positive for DAPI, BrdU, and GFAP are indicated with arrow heads in the right panel. Scale bars = 25 μm. (k–p) Representative fluorescent microscopic images of staining for CS-56. Scale bars = 50 μm. In panels a–d, bars, mean + SEM. CS-56, chondroitin sulfate-56.
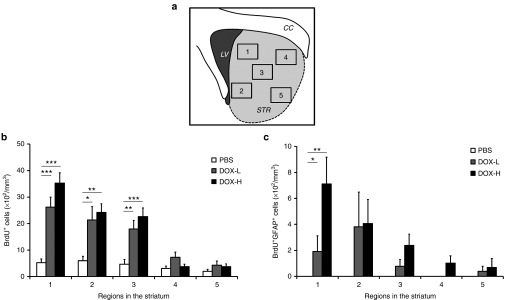
We next determined whether the density of the astrocytes generated through proliferation varies in regions within the striatum. The striatum was divided into five regions (Figure 4a) and the densities of BrdU+ and BrdU+GFAP+ cells in each region were evaluated. In the PBS group, the densities of BrdU+ cells were comparable between regions and the density of BrdU+GFAP+ cells was below the detection range in all regions (Supplementary Figure S2a,b and Figure 4b,c). In both DOX-L and DOX-H groups, the densities of BrdU+ and BrdU+GFAP+ cells showed a tendency to be high as the analyzed region is closer to the lateral ventricle where doxycycline was infused. This regional tendency showed that the density of BrdU+ cells was significantly higher in region 1, where is closest to the lateral ventricle, in the DOX-H and DOX-L groups (Supplementary Figure S2a). Furthermore, the density of BrdU+GFAP+ cells in region 1 in the DOX-H group was significantly higher than in regions 4 and 5 (P < 0.05) (Supplementary Figure S2b). Likewise, in regions 1, 2, and 3, the densities of BrdU+ cells in the DOX-H and DOX-L groups were significantly higher than PBS group (Figure 4b). The densities of BrdU+GFAP+ cells in region 1 in the DOX-H and DOX-L groups were significantly higher than PBS group (P < 0.01 and P < 0.05, respectively) (Figure 4c). Taken together, these data indicate that the densities of all proliferated cells and proliferated astrocytes were significantly higher in the striatum regions closer to the lateral ventricle.
Figure 4.
Regional density of BrdU+ and BrdU+GFAP+ cells in the striatum. (a) The striatum was divided into five regions and each region was subjected to analysis. (b,c) Regional analysis. The density of BrdU+ and BrdU+GFAP+ cells in the right striatum from mice in the DOX group showed a tendency to decrease as the distance between the lateral ventricle and analyzed regions increased. The density of BrdU+ cells was comparable between the regions in the PBS group. (b) The densities of BrdU+ cells in regions 1, 2, and 3 of the DOX-H and DOX-L groups were significantly higher than in the PBS group (n = 5/group; *P < 0.05, **P < 0.01, and ***P < 0.001, one-way analysis of variance followed by a post-hoc Bonferroni comparison). (c) The density of BrdU+GFAP+ cells in region 1 in the DOX-H and DOX-L groups were significantly higher than in the PBS group. (n = 5/group; **P < 0.01 and *P < 0.05, respectively). In panels b,c, bars, mean + SEM.
In vivo pluripotency factor expression enhances neovascularization
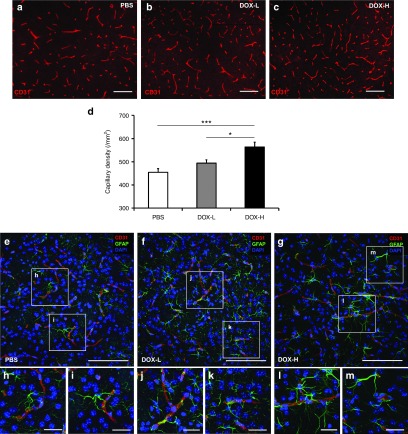
Neovascularization is a critical process for the recovery of ischemic tissue. We evaluated blood vessel density in the striatum by staining the tissue with an anti-CD31 antibody, which recognizes a representative endothelial cell marker (Figure 5a,c). The number of CD31+ capillaries in the DOX-H group was 1.2- and 1.1-fold significantly higher than in the PBS control and DOX-L groups (Figure 5d; P < 0.001 and P < 0.05, respectively), indicating that the neovascularization level is the highest in the DOX-H group. We next attempted to identify the potential underlying mechanism of the increased neovascularization in the ischemic brain. Given that astrocytes play crucial roles in brain neovascularization,33,34 the increased neovascularization induced by pluripotency factor expression might be, at least in part, attributable to the enhanced proliferative generation of astrocytes. As supportive data for this point, Western blotting showed that astrocytes expressed 1.5-, 2.2-, and 1.6-fold higher levels of VEGFA, ANGPT1, and FGF2, respectively, than neuroblasts in vitro (Supplementary Figure S3a–c; P < 0.05). Furthermore, using confocal microscopy, we also observed that CD31+ blood vessels were frequently surrounded by astrocytes (Figure 5e–m).
Figure 5.
Enhanced neovascularization by pluripotency factor expression in the striatum. Four weeks after ischemia induction, the blood vessel density was determined by immunofluorescent staining against CD31. (a–c) Representative fluorescent microscopic images. Scale bar = 100 μm. (d) The density of CD31+ capillaries in the striatum was significantly higher in DOX-H group than in both the DOX-L and PBS controls (n = 6/group; ***P < 0.001 and *P < 0.05 respectively, one-way analysis of variance followed by a post-hoc Bonferroni comparison). (e–m) Confocal microscopic pictures showing blood vessels close to astrocytes. CD31+ endothelial cells lining blood vessels are surrounded with GFAP+ astrocytes in PBS (e–i), DOX-L (f–k), and DOX-H (g–m) groups. Scale bar = 25 μm. In panel d, bars, mean + SEM. CD31, cluster of differentiation 31.
In vivo pluripotency factor expression enhances neuronal survival and synaptic plasticity
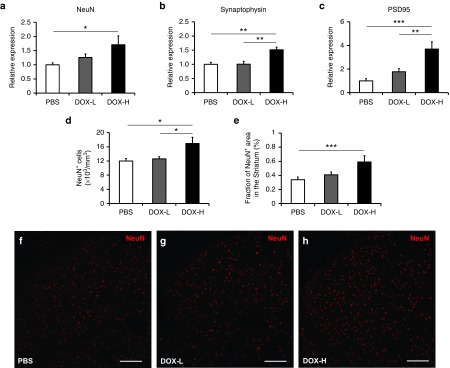
Astrocytes play a crucial role in neuroprotection by releasing several growth factors and cytokines.35,36 To test whether newly generated astrocytes and neovascularization induced by in vivo pluripotency factor expression can increase neuronal survival and synaptic plasticity, we evaluated the expression of the mature neuronal marker NeuN and synaptic markers such as synaptophysin and PSD95 using qRT-PCR. We found that expression of striatal NeuN significantly increased in mice treated with DOX-H compared to the PBS control group (Figure 6a; P < 0.05). Similarly, expression of synaptophysin and PSD 95 in the striatum significantly increased in the DOX-H group compared to the DOX-L and PBS groups (Figure 6b,c; P < 0.01 and P < 0.001). We also confirmed that the number of NeuN+ cells (/mm3) and the fraction of area in the striatum that was NeuN+ (%) were significantly higher in mice treated with DOX-H (Figure 6d–h).
Figure 6.
In vivo pluripotency factor expression enhances neural survival and synaptic plasticity. Gene expression of markers of neural survival and synaptic plasticity was evaluated by quantitative real-time polymerase chain reaction. (a) Expression of striatal NeuN significantly increased in mice treated with DOX-H compared to the phosphate buffered saline (PBS) control group (n = 4/group; *P < 0.05). (b,c) Similarly, expression of synaptic markers such as synaptophysin and PSD 95 significantly increased in the striatum in the DOX-H group compared to the DOX-L and PBS groups (n = 4/group; **P < 0.01, ***P < 0.001). (d,e) The number of NeuN+ cells (/mm3) and the fraction of area that was NeuN+ in the striatum (%) were significantly higher in mice treated with DOX-H (n = 5/group; *P < 0.05, ***P < 0.001). (f–h) Representative microscopic images of NeuN+ cells in the striatum. Scale bar = 100 μm. In panels a–e, bars, mean + SEM.
In situ pluripotency factor expression facilitates behavioral functional restoration after ischemic brain injury
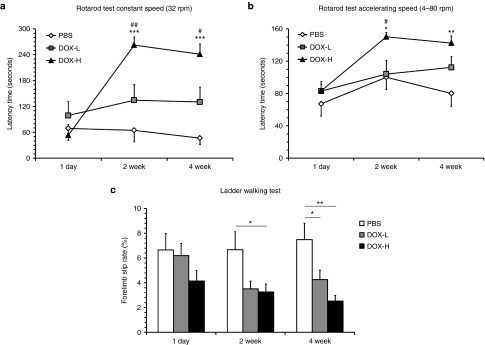
To determine whether this in situ pluripotency factor expression enhances functional recovery in the ischemic brain damage model, we evaluated neurobehavioral functions using rotarod tests and ladder walking tests 2 and 4 weeks after the doxycycline infusion. Rotarod tests with constant (32 rpm) speed showed that the latency periods before falling in the DOX-H group were 262.4 ± 18.8 and 241.1 ± 23.7 seconds at post-treatment 2 and 4 weeks, respectively, which are 4.0- and 5.1-fold, significantly higher than those in the PBS control group (64.9 ± 26.9 and 46.6 ± 15.1 seconds, respectively) (Figure 7a and Supplementary Video S1; P < 0.001). Rotarod tests with accelerating speed (4–80 rpm) also revealed that the latency periods before falling in the DOX-H group were 150.4 ± 5.0 and 140.7 ± 7.9 seconds at post-treatment 2 and 4 weeks, 1.5- and 1.8-fold significantly higher than those in the PBS control group (100.4 ± 14.7 and 80.1 ± 15.4 seconds, respectively) (Figure 7b; P < 0.05 and P < 0.01), corroborating that pluripotency factor expression enhances the improvement of motor function. Ladder walking tests, which evaluate fine motor coordination, showed that the forelimb slip rates among total steps in the DOX-H group at post-treatment 2 and 4 weeks were 3.3 ± 0.6% and 2.5 ± 0.4%, respectively, which are 2.0- and 3.0-fold, significantly lower than those in the PBS group (6.7 ± 1.5% and 7.5 ± 1.3%, respectively; Figure 7c; P < 0.05 and P < 0.01, respectively). The forelimb slip rate in the DOX-L group was 4.3 ± 0.8%, which is also 1.8-fold, significantly lower than that in the PBS group at post-treatment 4 weeks (Figure 7c; P < 0.05), indicating that in situ pluripotency factor expression can enhance the recovery of coordinated motor function in this cerebral ischemia model.
Figure 7.
In situ pluripotency factor expression enhances recovery of neurobehavioral functions in mice with cerebral ischemia. Neurobehavioral functions were evaluated by rotarod tests at constant (a) and accelerating (b) speed and by ladder walking tests (c). Neurobehavioral functions in the DOX-H group were significantly better than in the phosphate buffered saline (PBS) controls at 2 and 4 weeks after the ischemic injury (n = 7/group; *P < 0.05, **P < 0.01, and ***P < 0.001 versus PBS, #P < 0.05, ##P < 0.01 versus DOX-L, one-way analysis of variance followed by a post-hoc Bonferroni comparison). In all panels, mean + SEM.
To provide insight into the effects of pluripotency factor expression in mice in the absence of brain ischemia, we performed that sham-operated reprogrammable mice (such that occlusion of the carotid arteries did not occur) and then treated with PBS, DOX-L, or DOX-H via an osmotic pump. Up to 4 weeks after surgery, there were no differences in rotarod performances among three groups (Supplementary Figure S4a,b). This result suggests that in vivo pluripotency factor expression has no beneficial effect on sham-operated reprogrammable mice in the absence of brain ischemia and indicates that the protective effects of pluripotency factors are ischemia-dependent. We also treated C57BL/6J wild-type mice with bilateral common carotid artery occlusion with PBS, DOX-L, or DOX-H. Although doxycycline has been shown to have a neuroprotective effect in a previously published paper,37 the rotarod performances of these mice were not different among the groups up to 4 weeks after surgery in this study (Supplementary Figure S4c,d).
Discussion
Our study is the first to report that in situ expression of pluripotency factors facilitates functional recovery from injury in vivo. Although in vivo expression of pluripotency factors has been studied with respect to tumor development28 and reprogramming into pluripotency,29 it had not yet been investigated whether these factors can augment functional restoration of injured tissues.
This pluripotency factor–induced functional recovery was associated with proliferative generation of Nestin+ or GFAP+ cells in the subventricular zone and striatum. Given that the early responses to pluripotency factor expression include cell proliferation,38 the source of these cells could be endogenous Nestin+ cells (neural progenitors) and GFAP+ cells (astrocytes), respectively. However, it cannot be ruled out that these cells could be also derived from other cell types because transient overexpression of pluripotency factors can epigenetically activate the cells into an intermediate plastic state that allows cells to take on alternate fates.11,12,13,39,40 Elucidating the source of these newly generated neural progenitors and astrocytes would require complex lineage tracing experiments,41,42 which are beyond the scope of the current study. Because functional improvement was associated with selective expansion of neural progenitors and astrocytes, it might be speculated that these cell types are required for recovery from brain injury and the prevention of additional tissue damage. This idea is in line with previous results showing that neural stem/progenitor cells, but not mature neurons, can lead to functional recovery when transplanted into damaged brain tissue43,44 and that astrocytes are responsible for the prevention of tissue damage and neuronal loss in several mouse models of brain injury.45 Our data are also compatible with results from a previous interesting report showing that gliogenic, but not neurogenic, neural stem/progenitor cells promoted axonal growth, remyelination, angiogenesis, and locomotor functional recovery when transplanted into a mouse model of spinal cord injury.46
Here in the acute brain ischemia model, pluripotency factor–induced astrogliosis was associated with functional restoration and neovascularization, which is compatible with previous studies showing that astrogliosis has beneficial effects in the acute phase of injury.45,47 However, the long-term consequence of astrogliosis could be detrimental45,47: It can produce glial scar and prevent neurogenesis from the endogenous progenitors or transplanted stem/progenitor cells and axonal regeneration. In this study, gliogenesis induced by in vivo pluripotency factor expression did not increase the detrimental glial scar formation in the striatum of the damaged brain. Thus, the GFAP+ cells generated by pluripotency factor expression might be a novel cell source with stem cell potential in the injured brain.47
Angiogenesis is essential for recovery from ischemic injury. The role of astrocytes in angiogenesis in the retina, a part of the central nervous system like the brain, has been relatively well characterized48,49; here, astrocytes form a template that guides retinal angiogenesis in both normal development and injury-associated regeneration. Although the role of astrocytes in brain angiogenesis has yet to be elucidated, astrocytes have been proposed to be the cell type that forms the trophic microenvironment that supports angiogenesis and enables the survival of transplanted striatal tissue in the human brain.50 In this study, key angiogenic factors robustly expressed in astrocytes might contribute to this trophic environment that favors angiogenesis and tissue regeneration. Furthermore, it is well established that astrocytes contribute to the formation of the blood–brain barrier, which is compatible with our results showing that CD31+ blood vessels are surrounded by GFAP+ cells. Previous studies showed that astrocytes, which produce the proangiogenic and neurogenic factors such as FGF2 and VEGFA, can facilitate angiogenesis and neurogenesis and enhance functional recovery after cerebral ischemia and hypoxic-ischemic brain injury.51,52,53 Taken together, these results suggest that the pluripotency factor–induced functional recovery would be mediated by the proliferative generation of astrocytes, which express factors that are both angiogenic and neurogenic.
Astrocytes are the main neural-lineage cell type for the maintenance of brain homeostasis and contribute to the neuroprotection and survival of neuron as secretion of neurotransmitters, cytokines, and growth factors.35,36 This study showed that in vivo expression of pluripotency factors induced an increase in the number of newly generated astrocytes without effects on neurogenesis, further suggesting motor function recovery. Expression of NeuN (a mature neuronal marker), synaptophysin (a presynaptic marker), and PSD95 (a postsynaptic marker) also increased in a doxycycline-dependent manner, suggesting increased neuronal survival and synaptic plasticity. These results might provide an explanation for the relation between newly generated astrocytes induced by in vivo pluripotency factor expression and functional recovery. Additional experiments revealed that sham-operated reprogrammable mice, lacking occlusion of the carotid artery, that received either PBS, DOX-L, or DOX-H showed no beneficial effect in the absence of brain ischemia in the rotarod test up to 4 weeks after surgery (Supplementary Figure S4a,b), suggesting that the protective effects of pluripotency factors are ischemia-dependent.
It has been recently reported that doxycycline-induced in vivo expression of pluripotency factors can lead to the development of dysplasia or tumors including teratoma.29 Similar dysplasia has been previously observed in mice in which Pou5f1 expression was induced by doxycycline in vivo.28 However, we did not observe any dysplasia or tumor development in our study. This discrepancy may be attributable to differences in both delivery methods and analyzed tissues between our study and the others. In each of the other studies, doxycycline was systemically applied to the mice through drinking water,28,29 whereas doxycycline was given to the lateral ventricle in our study, where systemic effects of doxycycline would be minimal or negligible. Among the three studies, one study,29 but not the other two, showed the expression of pluripotency factors in brain tissue. However, dysplasia or tumor development in the brain was not described even in this study. In all three studies, this dysplasia or tumor development was observed predominantly in epithelial tissues from the stomach, intestine, pancreas, kidney, and skin, but not brain.
Many established studies have shown that gender-specific hormones such as estrogen and progesterone have neuroprotective effects on the ischemic brain.54,55 In this respect, this study is limited in that we have not performed experiments according to gender. Therefore, gender-specific responses to in vivo pluripotency factor expression in a model of brain ischemia should be examined in the future.
In conclusion, here we showed that in situ transient expression of the four pluripotency factors promoted functional recovery in a mouse model of cerebral ischemia. This facilitated functional recovery was associated with enhanced neovascularization and proliferative generation of astrocytes and/or neural progenitors. Our in situ pluripotency factor–mediated functional restoration provides insight into the recovery from tissue damage and potentially enables the development of novel therapeutic modalities for restoration from tissue injury including cerebral ischemia.
Materials and Methods
Mice. The reprogrammable mice (Gt(ROSA)26Sortm1(rtTA*M2)Jae Col1a1tm3(tetO-Pou5f1,-Sox2,-Klf4,-Myc)Jae/J), in which the four pluripotency factors Pou5f1, Sox2, Klf4, and Myc are expressed in the presence of doxycycline,26 were purchased from the Jackson laboratory (Bar Harbor, ME). Control C57BL/6J mice were also purchased from the Jackson laboratory. Mice were housed under climate controlled conditions with a 12-hour light/dark cycle and provided standard food and water ad libitum. Mice of both sexes, aged 8–16 weeks, were used. All procedures were reviewed and approved by the Animal Care and Use Committee of the Yonsei University College of Medicine (2013–0220).
Experimental grouping. A total of 62 reprogrammable mice were used in this study. For behavioral assessment, 21 mice were randomly assigned to either PBS, DOX-L, or DOX-H (n = 7 each). Of this group, 15 mice were used for histological assessment at 4 weeks post-treatment (n = 5 each). An additional 12 mice were used for qRT-PCR experiments to evaluate the expression of the mature neuronal marker NeuN and synaptic markers such as synaptophysin and PSD95 at 4 weeks post-treatment (n = 4 each). To evaluate the expression of in vivo pluripotency factors, 12 mice were also sacrificed 3 days after the infusion of doxycycline via an osmotic pump (n = 4 each). An additional 17 reprogrammable mice that received sham operations were used to provide insight into the effects of pluripotency factor expression in the absence of brain ischemia. (n = 5, PBS; n = 6, DOX-L; n = 6, DOX-H). A total of 14 C57BL6/J wild-type mice were also used for behavioral assessment to examine whether doxycycline has a neuroprotective effect (n = 5, PBS; n = 4, DOX-L; n = 5, DOX-H).
Ischemic brain injury induction and doxycycline infusion. Mice were anesthetized via i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Transient cerebral ischemia was induced by bilateral common carotid artery occlusion for 20 minutes as previously described,27,56 after which mice were continuously injected with low (1 μg/ml; DOX-L) or high (100 μg/ml; DOX-H) concentrations of doxycycline (No. 631311; Clontech, Mountain View, CA) in PBS, or PBS (solvent control) into the lateral ventricle via a micro-osmotic pump (100 μl volume; Alzet 1007D, Cupertino, CA) at a speed of 0.5 μl/hour (12 ng or 1,200 ng doxycycline/day). The infusion cannula (Brain Infusion Kit 3; Durect, Cupertino, CA) was inserted using stereotaxic coordinates (AP +0.3 mm from Bregma; ML −0.7 mm from Bregma; DV −2.0 mm from Dura) into the lateral ventricle (Figure 1b), and the connected osmotic pump was placed in the dorsal subcutaneous tissue. Mice were daily given an i.p. injection of BrdU (50 mg/kg, Sigma-Aldrich, St. Louis, MO) for 12 days, beginning after stereotaxic surgery. Histologic evaluation was performed 4 weeks after the surgery (Figure 1a). For the sham-operated control group, reprogrammable mice were first deeply anesthetized and then received the same surgical procedure as above without actual occlusion of carotid arteries. The mice were randomly treated with either low (1 μg/ml; DOX-L) or high (100 μg/ml; DOX-H) concentrations of doxycycline or PBS (solvent control), which were infused into the lateral ventricle via a micro-osmotic pump.
Neurobehavioral assessment. Neurobehavioral function was evaluated at 1, 14, and 28 days after the surgery. The rotarod (No. 47600; UGO Basile, Comerio, VA, Italy) test was used to assess motor coordination and balance using constant speed (32 rpm) and accelerating speed (4–80 rpm) paradigms. For this assessment, mice were placed on a rotarod treadmill, and the latency to fall, which is the length of time that the animals remained on the rolling rod, was measured. The latency period before mice fell from the rod was measured twice during each test, and individual tests were terminated at a maximum latency of 300 seconds.7,52 For the ladder walking test, the mice were required to walk a distance of 1 m three times on a horizontal ladder with unevenly spaced metal rungs (Jeung Do B&P, Seoul, South Korea). The number of slips from the transverse rungs with each forelimb was measured by videotape analysis. The percentage of slips on the transverse rungs of the ladder relative to the total number of steps taken was calculated.7,52
Cell culture. Mouse embryonic fibroblasts were isolated from the reprogrammable mice as previously described.26 Mouse embryonic fibroblasts seeded at a density of 1 × 104 cells/well in six-well plates were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gibco Invitrogen) and 1% antibiotics (penicillin streptomycin; Gibco Invitrogen). Mouse embryonic fibroblasts were daily treated with various doses of doxycycline (10, 100, 500, 1,000 ng/ml) for 7 days and subjected to RT-PCR.
The mouse astrocyte cell line (C8-D1A; Astrocyte Type 1 clone, ATCC, CRL-2541) and neuroblast cell line (N1E-115, ATCC, CRL-2263) were cultured in DMEM and DMEM without sodium pyruvate (No. 11965-092; Gibco Invitrogen), respectively, both supplemented with 10% FBS and 1% antibiotics (penicillin, streptomycin). Each cell line was seeded at a density of 8 × 105 cells/100-mm culture dish; 1 day after seeding, the cells were washed six times using DMEM without FBS. This washing procedure efficiently eliminated all serum proteins, because we did not detect any trace of bovine albumin, the major serum protein. The cells were incubated in this FBS-free media for 48 hours and then subjected to Western blotting.
Immunohistochemistry. Immunohistochemistry was performed as previously described.7 Briefly, the animals were sacrificed and perfused with 4% paraformaldehyde. The harvested brain tissues were cryosectioned at 16-μm thickness along the coronal plane and immunohistochemistry staining was performed on four sections over a range of >128 µm (AP + 1.0 mm to AP − 0.2 mm from Bregma). Sections were stained with primary antibodies against BrdU (1:200; Abcam, Cambridge, UK), βIII-tubulin (1:400; Covance, Princeton, NJ), GFAP (1:400; Abcam), Nestin (1:400; Abcam), NeuN (1:200; Abcam), CS-56 (1:200; Abcam), and CD31 (1:200; BD Bioscience, San Jose, CA) and secondary antibodies such as Alexa Fluor 488 goat anti-Rat (1:400; Invitrogen), Alexa Fluor 568 goat anti-Rabbit (1:400; Invitrogen), and Alexa Fluor 594 goat anti-Mouse (1:400; Invitrogen) and mounted on glass slides with fluorescent mounting medium containing 4ʹ,6-diamidino-2-phenylindole (Vectorshield, Vector, Burlingame, CA). The stained sections were analyzed using confocal microscopy (LSM700; Zeiss, Gottingen, Germany) and the MetaMorph Imaging System (Molecular Device, Sunnyvale, CA). The densities of blood vessels and mature neurons were evaluated using a fluorescent microscope (BS51; Olympus, Tokyo, Japan) and the MetaMorph Imaging System. Images of glial scarring were taken using a fluorescent microscopy (Axio Imager M2; Zeiss) and the density was evaluated using ZEN Imaging Software (Blue edition; Zeiss).
2,3,5-triphenyltetrazolium chloride staining. Infarct region were confirmed by 2,3,5-triphenyltetrazolium chloride (Sigma) staining. At 1 day after bilateral common carotid artery occlusion, brains were sectioned into 2.0 mm coronal sections using a mouse brain matrix. The sections were incubated in 2% 2,3,5-triphenyltetrazolium chloride at 37 °C for 20 minutes. The brain sections were washed in PBS and then stored in 4% paraformaldehyde at 4 °C refrigerator. The sections were photographed with digital camera (Figure 1b).
RT-PCR. RNA was isolated from the mouse embryonic fibroblasts using Trizol (Invitrogen Life Technologies). cDNAs, which was synthesized from 1 μg total RNA using the cDNA kit (Invitrogen Life Technologies), were subjected to PCR using a RT-PCR analysis kit (Bioneer, Daejeon, South Korea) according to the manufacturer's instructions and a Gene Amp PCR system 9700 (Applied Biosystems/Life Technologies, Carlsbad, CA). The primer sequences are described in Supplementary Table S2. The thermocycler conditions were as follows: denaturation for 1 minute at 95 °C, followed by 35 cycles of 95 °C for 30 seconds, 58 °C for 30 seconds, and 72 °C 30 seconds. PCR products were subjected to 1.5% agarose gel electrophoresis.
qRT-PCR. One microgram of purified total RNA was used as a template to generate the cDNA using a ReverTra Ace qPCR TR master Mix with gDNA remover (TOYOBO). Then, 2 μl of cDNA in a total volume of 20 μl was used in the following reaction. The qRT-PCR was performed in triplicate on a LightCycler 480 (Roche Applied Science, Mannheim, Germany) using the LightCycler 480 SYBR Green master mix (Roche Applied Science). The thermocycler conditions were as follows: amplifications were performed starting with a 300-second template preincubation step at 95 °C, followed by 40 cycles of 95 °C for 5 seconds, 60 °C for 20 seconds, and 95 °C for 15 seconds. The melting curve analysis began at 95 °C for 15 seconds, followed by 1 minute at 60 °C. The specificity of the amplification product was confirmed by an examination of the melting curve, which showed a distinct single sharp peak with the expected melting temperature (Tm) for all samples. A distinct single peak indicates that a single DNA sequence was amplified during qRT-PCR. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. The expression of each gene of interest was obtained using the 2−ΔΔCt method. The primer sequences are described in Supplementary Table S2.
Western blot analysis. Harvested cells were lysed in 50 μl cold lysis buffer (50 mmol/l Tris-HCl, pH 7.5, 1% Triton X-100, 150 mmol/l NaCl, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate) with a protease inhibitor cocktail (Sigma-Aldrich). Cell lysates were then centrifuged at 13,000g for 15 minutes at 4 °C. The supernatant was harvested, and the protein concentration was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Proteins (30 μg) dissolved in sample buffer (60 mmol/l Tris-HCl, pH 6.8, 14.4 mmol/l β-mercaptoethanol, 25% glycerol, 2% sodium dodecyl sulfate, and 0.1% bromophenol blue) were denatured for 10 minutes at 70 °C and subjected to electrophoresis on a 10% sodium dodecyl sulfate polyacrylamide gel. Separated proteins were transferred onto 0.45 mm invitrolon polyvinylidene difluoride (Invitrogen) membrane using an XCell IITM Blot Module (Invitrogen). The membranes were blocked for 1 hour in TBST (Tris-buffered saline (10 mmol/l Tris-HCl, pH 7.5, 150 mmol/l NaCl) containing 0.05% Tween 20) supplemented with 5% nonfat dry milk (Bio-Rad Laboratories) at room temperature, washed three times with TBST, and incubated at 4 °C overnight in TBST supplemented with 5% nonfat dry milk and the antibodies against ANGPT1 (1:500; Abcam), VEGFA (1:1,000; Abcam), FGF2 (1:500; Abcam), or glyceraldehyde 3-phosphate dehydrogenase (1:1,000; Cell Signaling Technology, Danvers, MA). The blots were washed three times with TBST and incubated for 1 hour with horseradish peroxidase–conjugated secondary antibodies (1:3,000; Santa Cruz Biotechnology, Dallas, TX) at room temperature. After washing three times with TBST, the protein was visualized with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Little Chalfont, UK).
Statistical analysis. All data were expressed as means ± SEM. The variables between among groups were analyzed using one-way analysis of variance followed by a post-hoc Bonferroni comparison using the SPSS statistics (IBM, Armonk, NY; version 20.0). The comparison of variables between two groups was used using Student's t-test. A P value <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Doxycycline-induced expression of the pluripotency factors. Figure S2. Regional density of BrdU+ and BrdU+GFAP+ cells in the striatum. Figure S3. Expression of angiogenic factors by astrocytes. Figure S4. Neurobehavioral evaluation in both sham-operated reprogrammable mice and C57BL6/J mice with bilateral common carotid artery occlusion. Table S1. Cell type analysis of BrdU+ cells in the subventricular zone and the striatum Table S2. The sequences of primers used for RT-PCR and qRT-PCR. Video S1. In situ pluripotency factor expression improves rotarod performance in mice with cerebral ischemia.
Acknowledgments
This study was supported by grants from the National Research Foundation (S.-R.C., NRF-2014R1A2A1A11052042; 2015M3A9B4067068; H.K., 2015R1A2A1A15052668), the Ministry of Science and Technology, Republic of Korea, the Korean Health Technology R&D Project (H.K., HI14C2019; S.-R.C., HI16C1013; HI16C1012), Ministry of Health & Welfare, Republic of Korea, and the Yonsei University College of Medicine (6-2016-0126). We thank to Ahreum Baek for qRT-PCR and MinGi Kim for English editing. The authors indicate no potential conflicts of interest. J.H.S. performed most of the experiments, wrote the manuscript, and contributed to the study concept and design; M.-Y.L. and M.-S.K. performed in vitro experiments; J.H.Y. and M.S. performed animal experiments; C.H.S. contributed to the study concept and design; H.K. and S.-R.C. analyzed data, wrote the manuscript, developed the study concept, and supervised the project. All authors read and approved the manuscript.
Supplementary Material
References
- Iihoshi, S, Honmou, O, Houkin, K, Hashi, K and Kocsis, JD (2004). A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res 1007: 1–9. [DOI] [PubMed] [Google Scholar]
- Chen, J, Li, Y, Wang, L, Lu, M, Zhang, X and Chopp, M (2001). Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci 189: 49–57. [DOI] [PubMed] [Google Scholar]
- Park, KI, Teng, YD and Snyder, EY (2002). The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol 20: 1111–1117. [DOI] [PubMed] [Google Scholar]
- Delcroix, GJ, Schiller, PC, Benoit, JP and Montero-Menei, CN (2010). Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials 31: 2105–2120. [DOI] [PubMed] [Google Scholar]
- Roitberg, B (2004). Transplantation for stroke. Neurol Res 26: 256–264. [DOI] [PubMed] [Google Scholar]
- Fong, SP, Tsang, KS, Chan, AB, Lu, G, Poon, WS, Li, K et al. (2007). Trophism of neural progenitor cells to embryonic stem cells: neural induction and transplantation in a mouse ischemic stroke model. J Neurosci Res 85: 1851–1862. [DOI] [PubMed] [Google Scholar]
- Im, SH, Yu, JH, Park, ES, Lee, JE, Kim, HO, Park, KI et al. (2010). Induction of striatal neurogenesis enhances functional recovery in an adult animal model of neonatal hypoxic-ischemic brain injury. Neuroscience 169: 259–268. [DOI] [PubMed] [Google Scholar]
- Cho, SR, Benraiss, A, Chmielnicki, E, Samdani, A, Economides, A and Goldman, SA (2007). Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest 117: 2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss, A, Toner, MJ, Xu, Q, Bruel-Jungerman, E, Rogers, EH, Wang, F et al. (2013). Sustained mobilization of endogenous neural progenitors delays disease progression in a transgenic model of Huntington's disease. Cell Stem Cell 12: 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K and Yamanaka, S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. [DOI] [PubMed] [Google Scholar]
- Efe, JA, Hilcove, S, Kim, J, Zhou, H, Ouyang, K, Wang, G et al. (2011). Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol 13: 215–222. [DOI] [PubMed] [Google Scholar]
- Kurian, L, Sancho-Martinez, I, Nivet, E, Aguirre, A, Moon, K, Pendaries, C et al. (2013). Conversion of human fibroblasts to angioblast-like progenitor cells. Nat Methods 10: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S, Rezvani, M, Harbell, J, Mattis, AN, Wolfe, AR, Benet, LZ et al. (2014). Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 508: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maza, I, Caspi, I, Zviran, A, Chomsky, E, Rais, Y, Viukov, S et al. (2015). Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat Biotechnol 33: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen, T, Ostermeier, A, Pang, ZP, Kokubu, Y, Südhof, TC and Wernig, M (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan, E, Chanda, S, Ahlenius, H, Südhof, TC and Wernig, M (2012). Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA 109: 2527–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen, T and Wernig, M (2011). Direct lineage conversions: unnatural but useful? Nat Biotechnol 29: 892–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, K, Ferraiuolo, L, Miranda, CJ, Likhite, S, McElroy, S, Renusch, S et al. (2014). Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci USA 111: 829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda, S, Marro, S, Wernig, M and Südhof, TC (2013). Neurons generated by direct conversion of fibroblasts reproduce synaptic phenotype caused by autism-associated neuroligin-3 mutation. Proc Natl Acad Sci USA 110: 16622–16627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Z, Zhang, L, Wu, Z, Chen, Y, Wang, F and Chen, G (2014). In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell 14: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, W, Zang, T, Zou, Y, Fang, S, Smith, DK, Bachoo, R et al. (2013). In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol 15: 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torper, O, Pfisterer, U, Wolf, DA, Pereira, M, Lau, S, Jakobsson, J et al. (2013). Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci USA 110: 7038–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, ZD, Nachman, I, Regev, A and Meissner, A (2010). Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat Biotechnol 28: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen, TS, Hanna, J, Zhang, X, Ku, M, Wernig, M, Schorderet, P et al. (2008). Dissecting direct reprogramming through integrative genomic analysis. Nature 454: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudino, TA, McKay, C, Pendeville-Samain, H, Nilsson, JA, Maclean, KH, White, EL et al. (2002). c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev 16: 2530–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, BW, Markoulaki, S, Beard, C, Hanna, J and Jaenisch, R (2010). Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nat Methods 7: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C, Zhan, RZ, Qi, S, Fujihara, H, Taga, K and Shimoji, K (2001). A forebrain ischemic preconditioning model established in C57Black/Crj6 mice. J Neurosci Methods 107: 101–106. [DOI] [PubMed] [Google Scholar]
- Hochedlinger, K, Yamada, Y, Beard, C and Jaenisch, R (2005). Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121: 465–477. [DOI] [PubMed] [Google Scholar]
- Abad, M, Mosteiro, L, Pantoja, C, Cañamero, M, Rayon, T, Ors, I et al. (2013). Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502: 340–345. [DOI] [PubMed] [Google Scholar]
- Morshead, CM, Reynolds, BA, Craig, CG, McBurney, MW, Staines, WA, Morassutti, D et al. (1994). Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13: 1071–1082. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla, A and Garcia-Verdugo, JM (2002). Neurogenesis in adult subventricular zone. J Neurosci 22: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea, V, Bingaman, KD, Wiegand, SJ and Luskin, MB (2001). Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 21: 6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai, K, Jin, G, Navaratna, D and Lo, EH (2009). Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J 276: 4644–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, HS, Han, J, Bai, HJ and Kim, KW (2009). Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J 276: 4622–4635. [DOI] [PubMed] [Google Scholar]
- Bélanger, M and Magistretti, PJ (2009). The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma, K, Baba, A and Matsuda, T (2004). Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol 72: 111–127. [DOI] [PubMed] [Google Scholar]
- Wang, Z, Xue, Y, Jiao, H, Liu, Y and Wang, P (2012). Doxycycline-mediated protective effect against focal cerebral ischemia-reperfusion injury through the modulation of tight junctions and PKCδ signaling in rats. J Mol Neurosci 47: 89–100. [DOI] [PubMed] [Google Scholar]
- Plath, K and Lowry, WE (2011). Progress in understanding reprogramming to the induced pluripotent state. Nat Rev Genet 12: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladewig, J, Koch, P and Brüstle, O (2013). Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat Rev Mol Cell Biol 14: 225–236. [DOI] [PubMed] [Google Scholar]
- Kim, J, Ambasudhan, R and Ding, S (2012). Direct lineage reprogramming to neural cells. Curr Opin Neurobiol 22: 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, PC, Segers, VF, Davis, ME, MacGillivray, C, Gannon, J, Molkentin, JD et al. (2007). Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13: 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyo, SE, Steinhauser, ML, Pizzimenti, CL, Yang, VK, Cai, L, Wang, M et al. (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493: 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, YH, Ko, JY, Chang, MY, Yi, SH, Kim, D, Kim, CH et al. (2011). Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest 121: 2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, SU and de Vellis, J (2009). Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res 87: 2183–2200. [DOI] [PubMed] [Google Scholar]
- Sofroniew, MV (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, G, Okada, Y, Yamane, J, Nagoshi, N, Kitamura, K, Mukaino, M et al. (2009). Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS One 4: e7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel, S, Berninger, B and Götz, M (2011). The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12: 88–104. [DOI] [PubMed] [Google Scholar]
- Otani, A, Kinder, K, Ewalt, K, Otero, FJ, Schimmel, P and Friedlander, M (2002). Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 8: 1004–1010. [DOI] [PubMed] [Google Scholar]
- Lee, SW, Kim, WJ, Choi, YK, Song, HS, Son, MJ, Gelman, IH et al. (2003). SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med 9: 900–906. [DOI] [PubMed] [Google Scholar]
- Cisbani, G, Freeman, TB, Soulet, D, Saint-Pierre, M, Gagnon, D, Parent, M et al. (2013). Striatal allografts in patients with Huntington's disease: impact of diminished astrocytes and vascularization on graft viability. Brain 136(Pt 2): 433–443. [DOI] [PubMed] [Google Scholar]
- Briones, TL, Woods, J, Wadowska, M, Rogozinska, M and Nguyen, M (2006). Astrocytic changes in the hippocampus and functional recovery after cerebral ischemia are facilitated by rehabilitation training. Behav Brain Res 171: 17–25. [DOI] [PubMed] [Google Scholar]
- Seo, JH, Kim, H, Park, ES, Lee, JE, Kim, DW, Kim, HO et al. (2013). Environmental enrichment synergistically improves functional recovery by transplanted adipose stem cells in chronic hypoxic-ischemic brain injury. Cell Transplant 22: 1553–1568. [DOI] [PubMed] [Google Scholar]
- Krum, JM, Mani, N and Rosenstein, JM (2008). Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp Neurol 212: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed, NJ, Harukuni, I, Kimes, AS, London, ED, Traystman, RJ, and Hurn, PD (1998). Gender-linked brain injury in experimental stroke. Stroke 29: 159–165; discussion 166. [DOI] [PubMed] [Google Scholar]
- Gibson, CL (2013). Cerebral ischemic stroke: is gender important? J Cereb Blood Flow Metab 33: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S, McCulloch, J and Horsburgh, K (2001). Minimal ischaemic neuronal damage and HSP70 expression in MF1 strain mice following bilateral common carotid artery occlusion. Brain Res 914: 185–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.