Abstract
The results of recent clinical trials using mesenchymal stem cells (MSCs) have been unsatisfactory, indicating that current MSC-based therapies need to be improved. We and others have previously demonstrated that MSCs activate complement by unknown mechanisms after infusion, leading to damaged MSCs. In the study reported here, we found that incorporation of N-glycolylneuraminic acid onto MSCs during in vitro culture was a factor in the activation of complement by MSCs. In addition, we developed a way to “paint” heparin onto MSCs. This novel method improved the viability of MSCs and enhanced their function after infusion by directly inhibiting complement and by recruiting factor H, another potent complement inhibitor in serum, onto the surface of the MSCs. These data suggest that cell-surface engineering of MSCs with heparin to locally inhibit complement activation on MSCs might be a straightforward and effective method for improving the outcome of current MSC-based therapies.
Introduction
Mesenchymal stem cells (MSCs) are adult stem cells that not only have the ability to differentiate into different type of cells, including osteoblasts, adipocytes, and chondrocytes, but also possess strong immunosuppressive activity on both the innate and adaptive immune systems through multiple mechanisms.1 Importantly, it is widely believed that MSCs are able to escape host immune surveillance because of this immunosuppressive activity and other features.2 It has therefore been assumed that MSCs expanded from one donor could be used to treat other patients without being rejected.3,4 Because of the convenience of preparation, timing, cost effectiveness, and lack of need for human leukocyte antigen (HLA) matching, many clinical trials on MSCs have used allo-MSCs.5 Infusion of MSCs by intravenous injection is the most common delivery route in both humans and animals. Infusion of allo- or xeno-MSCs has been found to be effective in ameliorating pathological conditions in many animal models of disease, including multiple sclerosis,6 rheumatoid arthritis,7 myasthenia gravis,8 diabetes,9 inflammatory bowel disease,10 and allograft rejection,11 thus providing the rationale for testing the use of MSCs in clinical trials. However, in both human and animal studies,5 it has been observed that most of the infused cells are first trapped in the lung, then some migrate out to other tissues and mysteriously disappear within a few days. The extremely low survival rate of administered MSCs suggests that they work in a “hit and run” fashion,12 and therefore the initial survival and health status of the MSCs after administration are critical for their therapeutic efficacy.
Complement is an important part of the innate immune system, the primary role of which is to serve as the first defense against foreign pathogens.13 Activated complement can directly attack invading pathogens by forming membrane attack complexes (MACs) to damage/lyse the foreign cells. To avoid pathogenic bystander attack on self-tissues, complement activation is tightly controlled by native complement regulators, both in the fluid phase and on self-cell surfaces. However, when the naturally existing complement regulatory mechanisms fail to control excessive activation of complement, tissue damage and disease can occur. Because of the important pathological roles of complement in many diseases, pharmaceutical companies are developing complement inhibitors as potential therapeutics. One of these, a humanized anti-C5 monoclonal antibody (mAb), is in clinical use for treating paroxysmal nocturnal hemoglobinuria, atypical hemolytic-uremic syndrome, and myasthenia gravis.14
We recently reported that, immediately after infusion, MSCs activate complement in the blood and are injured by MACs, leading to reduced viability and decreased functionality of the MSCs, a process involving naturally existing antibodies in the blood.15 We also demonstrated two means to protect the MSCs from serum-mediated damage15: (i) systemic inhibition of complement using anti-C5 antibodies or (ii) transfection of MSCs with a recombinant adenovirus to upregulate levels of a cell-surface complement inhibitor, CD55. These studies provide proof of concept that either systemic inhibition of complement in the blood or local inhibition of complement activation on the MSC surface effectively protects MSCs. However, transfecting MSCs with a recombinant virus is costly and involves many safety concerns for clinical use, whereas long-term systemic inhibition of complement by administration of an anti-C5 mAb has practical issues, including cost (current anti-C5 mAb therapy costs ~$400,000/year/patient) and side-effects (e.g., opportunistic infections). It is therefore clear that a new, safe, and economical yet effective approach needs to be developed. Here we report promising results from our study: (i) we have identified an antigen on MSCs propagated in vitro that is recognized by the naturally occurring antibodies to activate complement, and (ii) we have developed a simple and economical method to locally inhibit complement on MSCs and protect them after administration.
Results
N-Glycolylneuraminic acid is present on in vitro propagated MSCs
Although we and others have demonstrated that in vitro propagated MSCs spontaneously activate complement upon contact with blood,15,16 a process in which pre-existing antibodies are integrally involved, the underlying mechanism remains unclear. N-Glycolylneuraminic acid (Neu5GC) is a sialic acid molecule found on the cells of most mammals (except humans), and almost all humans develop anti-Neu5GC antibodies due to exposure to Neu5GC from different sources, including animal meat consumption.17 It is therefore possible that these antibodies may have been responsible for the observed complement activation by in vitro propagated human MSCs, if the cells took up Neu5GC during culture. To examine whether Neu5GC was present on in vitro propagated human MSCs, we incubated MSCs from five donors with fish gelatin (blocking), then stained them with chicken anti-Neu5GC IgY antibodies and fluorescein isothiocyanate (FITC)-labeled anti-IgY antibodies and examined the staining by flow cytometry. As shown in Figure 1a, Neu5GC was found to be present on all tested in vitro propagated MSCs.
Figure 1.
Sialic acid Neu5GC on in vitro propagated mesenchymal stem cells (MSCs) triggers complement-mediated damage. (a) MSCs from different donors (640, 678, 741, 742, and 784) were evaluated for the presence of Neu5GC by flow cytometry after staining with anti-Neu5GC IgY (solid lines) or control IgY (dotted lines). (b) Left panel: Levels of Neu5GC on MSCs cultured for 7 days in the absence or presence of Neu5AC. Dotted line and solid line: MSCs incubated with Neu5AC and stained with control IgY (dotted line) or with anti-Neu5GC IgY (solid line). Dashed lines and shaded areas, MSCs cultured in the absence of Neu5AC and stained with control IgY (dashed line) or anti-Neu5GC IgY (shaded area). middle panels: Levels of IgM and IgGs on these MSCs after incubation with 30% serum for 30 minutes. Right panel, levels of C3b deposition on MSCs cultured in the absence or presence of Neu5AC after incubating them with 30% serum for 30 minutes in GVB++. (c) Complement-mediated cell damage when MSCs cultured in the presence (black bars) or absence (gray bars) of Neu5AC were incubated with 20 or 30% normal human serum in GVB++. The data are the combined results for three individual experiments ± SEM. *P < 0.05.
Neu5GC contributes to MSC-initiated complement activation
To test the role of Neu5GC on the surface of MSCs in MSC-initiated complement activation, we cultured MSCs in complete medium in the absence or presence of N-acetylneuraminic acid (Neu5AC) for 7 days. Consistent with previous reports,18 the addition of Neu5AC to the culture medium significantly reduced Neu5GC levels on the MSC surface (Figure 1b, left panel). We then incubated these cells with pooled normal human serum and, using flow cytometry, examined whether human immunoglobulins (IgGs and IgMs) were present on the MSCs. As shown in Figure 1b, we found that both IgGs and IgMs were indeed bound to the MSCs and that reducing Neu5GC levels on MSCs led to a decrease in the amount of bound IgM and IgG (middle panels). We also tested these MSCs for levels of complement activation in a cell-surface C3b deposition assay and in complement-mediated cell-damage assays. We found that MSCs with lower Neu5GC levels showed C3b deposition (Figure 1b, right panel) and less complement-mediated injury than those with higher levels (Figure 1c), suggesting that Neu5GC on MSCs contributes to MSC-initiated complement activation, and, consequently, complement-mediated MSC damage.
Systemic administration of heparin protects MSCs from serum-mediated attack
Previous studies have shown that heparin is a potent complement inhibitor.19,20 To test whether it could protect MSCs after their contact with serum, we first injected wild-type (WT) C57BL/6 mice subcutaneously (s.c.) with either saline or heparin at a dose of 20 U per mouse. We then bled them 30 minutes later and measured complement activity in the plasma using a standard zymosan C3 uptake assay (alternative pathway) and a C3b deposition assay employing antibody-sensitized sheep erythrocytes (EshA) (classical pathway) in the presence or absence of 1 mmol/l ethylenediaminetetraacetic acid (EDTA), which completely inhibits complement activation). As shown in Figure 2a, these ex vivo assays demonstrated that administration of heparin significantly inhibited complement activity in vivo, as shown by markedly reduced C3 deposition on the surface of both EshA and zymosan.
Figure 2.
Systemic administration of heparin inhibits complement activation and protects mesenchymal stem cells (MSCs) from complement-mediated damage. (a) Ex vivo evaluation of complement activation in heparin-injected or control mice. Mice were injected s.c. with heparin (500 U/mouse) or the same volume of saline and plasma was collected 30 minutes later and incubated with EshA in GVB++ (classical pathway) or zymosan in GVB EGTA Mg++ (alternative pathway) in the presence or absence of 1 mmol/l ethylenediaminetetraacetic acid (EDTA), then C3b/iC3b on the surface was evaluated after fluorescein isothiocyanate-labeled anti-mouse C3 IgG staining followed by flow cytometric analyses. Dotted lines, EDTA-treated samples; solid lines, samples from saline-treated mice; shades areas, samples from heparin-treated mice. (b) In vivo MSC damage after infusion into heparin- or saline-injected mice. Mice were injected s.c. with heparin (500 U/mouse) or saline, then, 10 minutes later, BCECF-labeled MSCs (1 × 106/mouse) were infused via the tail vein and in vivo MSC damage at different time points assessed by measuring released BCECF in the blood. n = 2 in each group; *P < 0.05.
After confirming that heparin inhibited complement activity in vivo, we repeated the experiment, but, this time, infused MSCs labeled with 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) into the heparin-treated and control mice via the tail vein 10 minutes after administration of heparin or saline and examined in vivo MSC cell damage by measuring the amount of released BCECF in the plasma at different time points after infusion. As shown in Figure 2b, these results showed that, consistent with the results of the ex vivo complement assay, systemic administration of heparin protected MSCs from serum (complement)-mediated damage in vivo.
Heparin can be painted onto the MSC surface
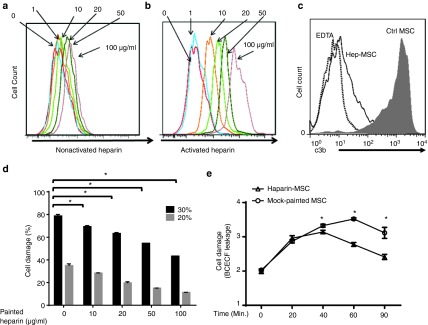
The above studies showed that systemic administration of heparin inhibited complement activation and protected MSCs after infusion. However, this approach could raise problems, as heparin not only inhibits complement, but also has strong anticoagulation activity; thus, systemic administration of large doses of heparin might lead to many side effects, including hemorrhage. In addition, systemic inhibition of complement increases the risk of opportunistic infections. Thus, it would be much better if heparin could be targeted onto the MSC surface to locally inhibit complement on MSCs, rather than inhibit complement systematically. To achieve this, we first labeled heparin with FITC, then activated the labeled heparin using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS). To paint the MSCs, we incubated MSCs for 30 minutes at 37 °C with 1, 10, 20, 50, or 100 μg/ml of activated or nonactivated labeled heparin, then, after washing, examined the cells for cell-surface-bound heparin by flow cytometry. As shown in Figure 3a, at high concentrations (50 and 100 µg/ml), some binding of nonactivated heparin to MSCs was observed; however, as shown in Figure 3b, activation of heparin resulted in a marked increase in binding of heparin to the MSC surface, and the effect was dose dependent.
Figure 3.
Heparin can be painted onto mesenchymal stem cells (MSCs) to locally inhibit complement and protect the cells. (a and b) MSCs were incubated with different concentrations of fluorescein isothiocyanate (FITC)-labeled nonactivated heparin (a) or activated heparin (b), then, after washing, MSC-bound heparin was assessed by flow cytometry. (c) MSCs painted with 100 µg/ml of heparin or mock-painted MSCs were incubated with 30% normal human serum in GVB++ for 30 minutes at 37 °C, then were washed and stained with FITC-labeled anti-human C3 IgGs to evaluate local complement activation (C3b/iC3b deposition). (d) MSCs painted with different concentrations of activated heparin were labeled with BCECF, then incubated with either 20% normal human serum (gray bars) or 30% normal human serum (black bars) in GVB++ for 30 minutes at 37 °C, and cell damage was assessed by the BCECF release assay. The data are the combined results from three independent experiments ± SEM. *P < 0.05. (e) Heparin-painted or mock-painted MSCs (0.5 × 106/mouse) were labeled with BCECF-AM and infused into WT C57BL/6 mice via the tail vein, then in vivo MSC damage was assessed by measuring released BCECF in the blood at different time points. n = 6 in each group; *P < 0.05
Heparin-painted MSCs are protected from serum-mediated attack
To examine whether painted heparin locally inhibited complement activation on MSCs and protected the cells from complement-mediated attack, we painted one set of MSCs with 100 µg/ml of heparin and mock-painted a second set, then incubated the cells for 30 minutes at 37 °C with 30% normal human serum in gelatin veronal buffer with 0.5 mmol/l Mg2+ and 0.15 mmol/l Ca2+ (GVB++) in the presence or absence of 1 mmol/l EDTA. We then assessed C3b/iC3b deposition on the MSCs by flow cytometry and found that heparin-painted MSCs showed markedly less C3b/iC3b deposition on the cell surface than control MSCs (Figure 3c). We also used the BCECF release assay to examine the extent of serum-mediated damage to MSCs painted with different concentrations of heparin (0–100 μg/ml) using 20 or 30% normal human serum and found that heparin painting resulted in less damage (Figure 3d). Finally, we adoptively transferred BCECF-loaded MSCs with or without painting with 100 μg/ml of heparin into WT C57BL/6 mice via the tail vein and evaluated in vivo MSC injury by measuring levels of released BCECF in the plasma at time points from 0 to 90 minutes and found that heparin-painted MSCs showed significantly less damage in vivo (Figure 3e). These data therefore suggest that painting heparin onto the MSC surface might be a valid strategy for locally inhibiting complement activation on MSCs and protecting them from complement-mediated attack.
Heparin painted on MSCs recruits factor H from serum onto the surface of MSCs
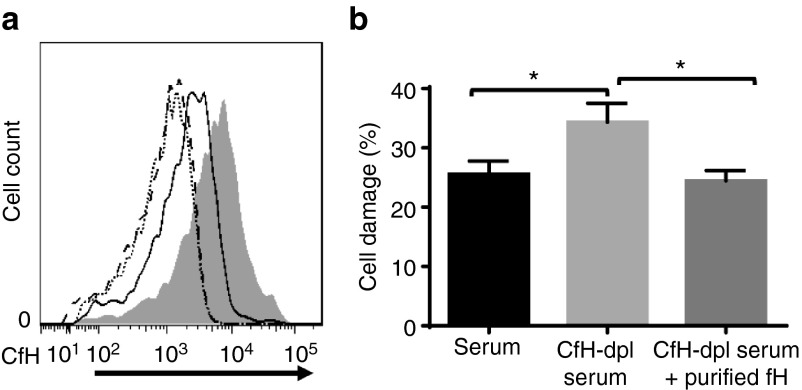
Heparin not only directly inhibits complement, but can also bind factor H,21 the most potent native complement inhibitor in serum. To explore the potential mechanisms by which painted heparin inhibited complement activation on MSCs, we incubated MSCs with or without 100 µg of EDC/NHS-activated heparin, then incubated the mock-painted and heparin-painted MSCs for 30 minutes with 30% normal human serum or 30% factor H-depleted serum (control) in the presence of 1 mmol/l EDTA (to avoid activation of complement). After washing and staining the cells with anti-factor H antibodies, we examined levels of cell-surface-bound factor H on the MSCs by flow cytometry. As shown in Figure 4a, compared with MSCs incubated with factor H-depleted serum (dotted and dashed lines), both heparin-painted and mock-painted MSCs had factor H bound on their cell surface. However, the heparin-painted MSCs registered significantly more factor H bound to their surface after incubation with normal human serum (shaded area) than control MSCs after similar incubation (solid line), showing that the heparin that had been painted on MSCs had also recruited factor H onto the surface of the MSCs.
Figure 4.
Painted heparin recruits complement factor H (CfH) onto mesenchymal stem cells (MSCs), and the heparin-recruited CfH is integrally involved in protecting MSCs from complement-mediated attack. (a) Heparin-painted MSCs were incubated with either 30% factor H-depleted human serum (solid line) or 30% normal human serum (shaded area) in GVB- ethylenediaminetetraacetic acid (EDTA) buffer for 30 minutes. After washing, MSC surface-bound CfH was evaluated by flow cytometry after staining with a goat anti-human CfH IgG. Representative results from two independent experiments. (b) Heparin-painted MSCs were incubated with 30% normal human serum (black bar), 30% CfH-depleted serum (light gray bar), or 30% CfH-depleted serum supplemented with 200 µg/ml of purified CfH in GVB-EDTA for 30 minutes (EDTA was used in this step to allow factor H recruitment without the occurrence of complement activation). After washes, the cells were labeled with BCECF, then incubated with 30% CfH-depleted serum in GVB++ for another 30 minutes. Cell damage was assessed by BCECF release (Factor H-depleted serum had to be used in this step as the source of complement to avoid the interference of factor H presented in the normal serum). The data are the combined results of two independent experiments ± SEM. *P < 0.05.
Factor H recruited from serum is involved in protecting MSCs painted with heparin
To test whether the heparin-recruited factor H on the surface of the MSCs was involved in protecting MSCs from serum-mediated attack, we incubated heparin-painted (100 μg/ml) MSCs for 30 minutes with (i) 30% normal human serum in phosphate-buffered saline (PBS), (ii) 30% factor H-depleted human serum in PBS, or (iii) 30% factor H-depleted human serum in PBS supplemented with 250 µg/ml of purified human factor H. To ensure that complement activation did not occur during this incubation, 1 mmol/l EDTA was included in the PBS. After washing, the cells were labeled with BCECF, and then were incubated for 30 minutes with 30% factor H-depleted serum in GVB++ to allow complement activation to occur without recruitment of any additional factor H from the serum, then MSC injury was measured using the BCECF release assay. As shown in Figure 4b, heparin-painted MSCs that recruited factor H from normal human serum (left column) or from factor H-depleted serum supplemented with factor H (right column) showed less cell damage than those incubated in factor H-depleted serum (center column), demonstrating that heparin-recruited factor H plays an important role in protecting MSCs from complement-mediated damage.
Painting with heparin does not impair the inhibitory activity of MSCs on T cells before contact with serum and preserves it after contact with serum
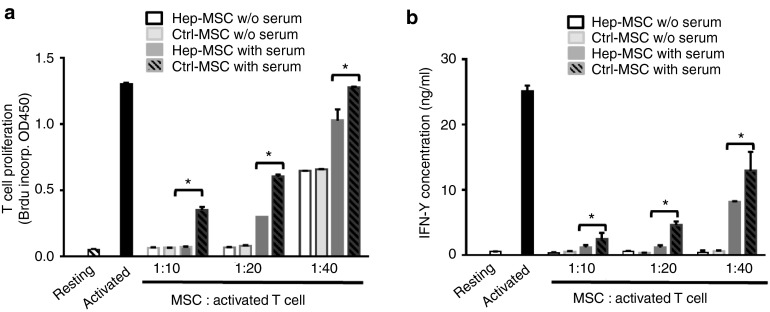
The above studies demonstrated that heparin-painted MSCs underwent less cellular damage after contact with serum because of the direct complement inhibitory activity of heparin and its ability to recruit complement inhibitor factor H from serum onto the MSCs. We next examined whether, after contact with serum, heparin-painted MSCs showed greater preservation of function than mock-painted MSCs. We first incubated heparin-painted or mock-painted MSCs (both treated with mitomycin C to prevent proliferation) with 30% normal human serum at 37 °C for 30 minutes in GVB++. After washes, we then incubated these serum-incubated MSCs with activated mouse T cells at different MSC/activated T-cell ratios for 72 hours and compared the ability of the MSCs to inhibit the proliferation of the activated T cells using a 5-bromo-2'-deoxyuridine (BrdU) incorporation assay and to inhibit interferon-γ (IFNγ) production by the T cells using enzyme-linked immunosorbent assay (ELISA). As seen in Figure 5, these experiments demonstrated that, without serum incubation, heparin-painted and mock-painted MSCs showed similar efficacy in inhibiting the proliferation of activated T cells (panel a) and IFNγ production by the activated T cells (panel b). This step also showed that painted heparin did not interfere with the T-cell inhibitory activity of MSCs. However, after serum incubation, the heparin-painted MSCs had significantly higher T-cell inhibitory activity than the mock-painted MSCs, as demonstrated by the markedly reduced proliferation of activated T cells and IFNγ production.
Figure 5.
Painted heparin does not interfere with the T-cell inhibitory activity of mesenchymal stem cells (MSCs) before contact with serum and preserves MSC function after contact with serum. (a). Heparin-painted MSCs (Hep-MSCs) or mock-painted MSCs (Ctrl-MSCs) (both treated with mitomycin C to prevent proliferation) were incubated for 30 minutes at 37 °C with 30% normal human serum in GVB++ or with GVB++ alone. After washes, the MSCs were then cocultured with 4 × 105 anti-CD3/CD28-activated T cells at different ratios for 72 hours, then the proliferation of the activated T cells was measured by BrdU incorporation. (b) Culture supernatants from these cocultures were assayed for IFNγ released by the activated T cells by IFNγ enzyme-linked immunosorbent assay. The data are the combined results for two independent experiments ± SEM. *P < 0.05.
Heparin-painted MSCs are more effective in suppressing T-cell function in vivo and survive better than mock-painted MSCs
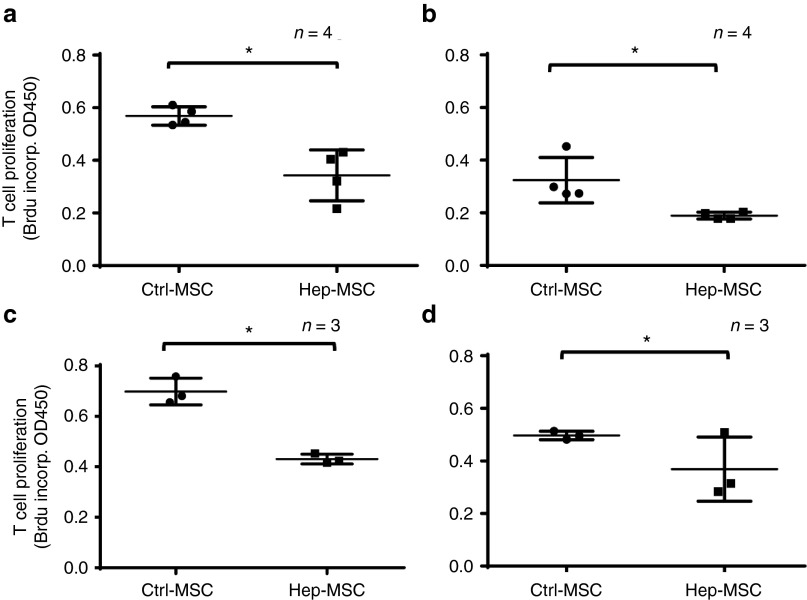
The above studies suggest that heparin-painted MSCs should be more effective than mock-painted MSCs in inhibiting T cells in vivo. To test this, we compared the efficacy of heparin-painted and mock-painted MSCs in inhibiting the proliferation of activated antigen-specific T cells in vivo. In brief, we first injected WT C57BL/6 mice with ovalbumin (OVA)-pulsed dendritic cells (DCs) and OVA323–339-specific T cells (T cells from OT-II mice (Jackson Laboratory, Bar Harbor, ME)), then treated them with the same number of heparin-painted or mock-painted MSCs, and measured the efficacy of MSC-mediated T-cell inhibition by assessing in vivo OT-II T-cell proliferation in the draining lymph nodes (popliteal lymph nodes) using a BrdU incorporation assay. Figure 6 shows the results from four independent experiments (panels a–d) using the indicated number of mice per group. The results showed that, as suggested by the in vitro studies, heparin-painted MSCs were significantly more effective in suppressing antigen-specific T-cell responses in vivo, as indicated by markedly reduced OT-II T-cell proliferation in the mice treated with heparin-painted MSCs than those treated with mock-painted MSCs.
Figure 6.
Heparin-painted mesenchymal stem cells (MSCs) (Hep-MSCs) are more effective in inhibiting the proliferation of antigen-specific T cells in vivo than mock-painted MSCs (Ctrl-MSCs). Naive C57BL/6 mice were infused with 1 × 106 purified T cells from OT-II mice via the tail vein, then, 24 hours later, OVA-loaded DCs were injected into the hind footpads. The mice were then randomly divided into two groups, one of which was injected with 1 × 106 heparin-painted MSCs and the other with the same number of mock-painted MSCs via the tail vein. After 24 hours, 1 mg of BrdU was injected intraperitoneally into each mouse, then, 24 hours later, the mice were euthanized and the draining lymph nodes collected to assess the proliferation of activated T cells by 5-bromo-2'-deoxyuridine (BrdU) incorporation using a conventional BrdU enzyme-linked immunosorbent assay. Panels a–d are the results from four independent experiments with a total of 14 mice in each group. Each dot represents one mouse ± SD, *P < 0.05.
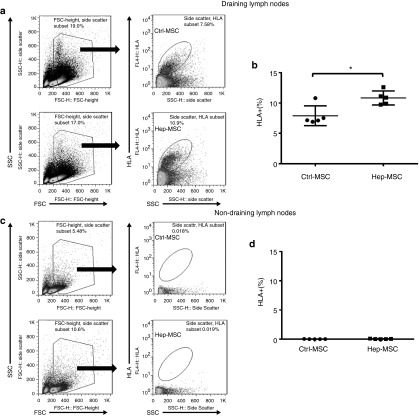
We also compared MSC survival in these mice by staining for HLA+ cells in the draining lymph nodes. As shown in Figure 7a,b, the percentage of HLA+ cells in the draining lymph nodes was 7.9 ± 1.6% in the mock-painted MSC-injected mice, but 10.8 ± 1.1% in those from heparin-painted MSC-injected mice. As expected, we did not detect any HLA+ MSCs in the distal, nondraining cervical lymph nodes (Figure 7c,d). These results show that systemically infused MSCs selectively migrated into the sites of inflammation (draining lymph nodes) and that heparin-painted MSCs survived better in vivo than the control MSCs.
Figure 7.
Heparin-painted MSCs (Hep-MSCs) survive better in vivo than mock-painted mesenchymal stem cells (MSCs) (Ctrl-MSCs). Using the same experimental setup as above, one side of the draining (popliteal) lymph nodes were collected, and the percentage of live MSCs evaluated by staining the single cell suspension with an anti-human HLA-ABC IgG, followed by flow cytometric analysis (a,b); the distal nondraining (cervical) lymph nodes were collected and processed at the same time as controls (c,d). n = 5 in each group; *P < 0.05.
Discussion
In this study, we demonstrated that Neu5GC incorporated onto bone marrow-derived MSCs during in vitro propagation is recognized by natural IgGs and IgMs in the blood, suggesting that this recognition process could be an important mechanism in provoking complement attack on MSCs after administration. We found that heparin, a drug with potent complement-inhibitory activity, could be painted onto MSCs to locally inhibit complement activation. Painted heparin did not interfere with the T-cell inhibitory activity of MSCs and helped to protect them from complement-mediated attack by directly inhibiting complement on MSCs and by recruiting serum complement inhibitor factor H onto MSCs to indirectly inhibit complement activation. Consequently, both in vitro and in vivo, the heparin-painted MSCs showed better survival and function after contact with complement than mock-painted MSCs.
Due to their strong immunosuppressive activity and their ability to differentiate into different types of cells, MSCs are under intensive development as a potential new therapy for many inflammatory conditions and for applications in regenerative medicine. Currently, more than 500 registered MSC-related clinical trials (www.clinicaltrials.gov) are under way. Unfortunately, despite promising preclinical study results and excellent safety data from the clinical studies, no MSC-based therapy has yet been approved in the US or Europe. Clearly, current MSC-based therapies need to be improved to be successful.
In both animal and human studies, it is widely known that, after administration, MSCs mysteriously disappear within a short period of time, suggesting that these cells are recognized and attacked by the host. Contrary to the long-held belief that MSCs are immunoprivileged and can escape host immune surveillance, clinical studies have shown increased levels of the complement activation product C3a in patients' blood immediately after MSC infusion,22,23 indicating complement activation. We have reported that MSCs propagated in vitro activate complement through all three complement-activating pathways and are attacked by MACs, resulting in cell damage and impaired function.15 Although we found that depleting immunoglobulins in the serum reduces MSC damage after serum incubation,15 the mechanism by which complement is activated to attack MSCs was unclear.
In this study, we found that Neu5GC, a sialic acid molecule that exists on the cell surface in almost all mammals (but not humans) in vivo, was present on human MSCs that had been expanded in vitro. We have also linked the levels of Neu5GC with IgG/IgM binding onto the surface of MSCs and with serum (complement)-mediated MSC injury, suggesting that antibody binding to Neu5GC on the surface of MSCs is one mechanism by which complement is activated. Humans cannot make Neu5GC because of an inactivating mutation in the gene encoding CMP-N-acetylneuraminic acid hydroxylase, the rate-limiting enzyme in the synthesis of Neu5GC in mammalian cells.24 But because of exposure to Neu5GC from different sources, including animal meat consumption, most humans develop antibodies against Neu5GC. Thus, our two major findings from this study—the identification of Neu5GC on MSCs that had been expanded in vitro and the observation that MSCs with reduced levels of Neu5GC showed correspondingly decreased levels of IgG/IgM binding and reduced cell damage after contact with serum—both suggest that the Neu5GC incorporated onto MSCs during in vitro propagation, potentially from xenoproteins in the culture medium, is integrally involved in the mechanism by which complement recognizes and attacks MSCs after their administration. However, the presence of Neu5GC on in vitro propagated MSCs is not the only factor involved in triggering complement activation by MSCs, because we found that markedly reducing Neu5GC levels on MSCs by the addition of Neu5AC to the culture medium resulted in only a moderate reduction in the complement-mediated attack. These data are consistent with our previous findings15 that antibodies and the classical pathway of complement activation contribute only partially to the mechanism by which MSCs are attacked by complement after serum contact. Other mechanisms may exist, a likelihood that warrants further investigation.
Heparin is a potent complement inhibitor, and this effect has been implicated in its clinical benefit in preventing obstetrical complications in pregnant patients with antiphospholipid syndrome.25 Consistent with previous discoveries, we found that systemic administration of heparin inhibited activation of both the classical and alternative complement pathways. But this approach might result in many side effects, including severe hemorrhage due to the strong anticoagulation activity of heparin. Our novel method for painting heparin locally onto MSCs should significantly reduce the amount of heparin required to protect MSCs and result in only local heparin activity on MSCs. In addition, we found that the painted heparin not only directly inhibited complement on MSCs, but also recruited factor H from serum onto the MSCs to locally inhibit complement. This heparin-recruited factor H seems to play a small but important role in protecting the MSCs together with the painted heparin, as suggested by the limited difference shown between the cells with or without heparin-recruited factor H. Factor H is present in serum at a high concentration (~0.2–0.5 mg/ml), and it is the most potent complement regulator in serum.26 These combined direct and indirect complement-inhibitory mechanisms might explain the effectiveness of heparin-painting in protecting the MSCs.
In our study reported here, we used EDC/NHS to activate the heparin to paint the MSCs. EDC/NHS has been used in bioconjugation, crosslinking, labeling, and immobilization applications.27 Carboxylate groups on heparin can react with EDC and NHS, resulting in an active NHS ester, which can then react with amine groups on the MSC surface to covalently coat the heparin onto MSCs, a process that we call “painting.” Painting heparin onto MSCs, at least at the doses used in this study, did not interfere with the cells' immunosuppressive activity, as heparin-painted and mock-painted MSCs showed similar potency in inhibiting the proliferation of, and production of IFNγ by, activated T cells. Although the half-life of the painted heparin is relatively short (data not shown), given the hypothesis that MSCs work in a “hit and run” fashion in treating inflammatory diseases,12 even a modest extension of their “hit” period before they “run” should have a significant impact on their function in vivo. Indeed, our in vivo studies showed that heparin-painted MSCs survived better in the draining lymph nodes and that they were more potent in suppressing the proliferation of activated antigen-specific T-cell responses.
In addition, it has been suggested that, after systemic administration, MSCs tend to migrate into sites of inflammation to exercise their functions.28 This claim is supported by our observation that relatively large numbers of MSCs that were systemically administered (by intravenous injection) were found in the draining lymph nodes (popliteal lymph nodes) after footpad injection of antigen-pulsed dendritic cells (DCs), but not in the distal nondraining lymph nodes (cervical lymph nodes). We also want to point out that, in these in vivo studies, because mice produce Neu5GC, they do not normally have anti-Neu5GC antibodies, and it is unlikely that the complement-mediated MSC damage we saw in these studies was initiated by the anti-Neu5GC IgMs and IgGs shown in our in vitro studies using human sera. The infused MSCs must be damaged by complement initiated through other similar mechanisms, e.g., natural antibodies against xeno-antigens on the infused MSCs.
In summary, we have found that the sialic acid Neu5GC contributes to the mechanism by which MSCs activate complement after administration. We have developed a simple method for painting heparin onto MSCs to locally inhibit complement activation. The painted heparin not only directly inhibits the detrimental action of complement on MSCs, but also recruits factor H from serum to indirectly inhibit complement activation. We also showed, both in vitro and in vivo, that heparin painting does not interfere with the immunosuppressive activity of MSCs and that heparin-painted MSCs maintain better viability and function after administration. These results suggest that painting heparin onto MSCs could be a simple and effective approach to improve the outcome of current MSC-based therapies.
Materials and Methods
Animals. OT II mice (C57BL/6 background) and C57BL/6 mice, purchased from the Jackson Laboratory, were maintained in the animal facility at Cleveland Clinic. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees at the Cleveland Clinic.
MSCs and other reagents. Primary bone-marrow-derived human MSCs from healthy donors, provided by the MSC Core Facility at the National Center for Regenerative Medicine of Case Western Reserve University (Cleveland, OH), were cultured in complete medium (α-minimal essential medium containing 16% fetal bovine serum; both from Thermo Fisher Scientific, Grand Island, NY) following previously described protocols.15 Pooled normal human serum and factor H-depleted serum were purchased from CompTech (Tyler, TX) and N-acetylneuraminic acid (Neu5AC) was purchased from Nacalai USA (San Diego, CA). The production of the anti-Neu5GC IgY antibodies and control IgY for Neu5GC detection has been described previously.18
Neu5GC detection on MSCs. Detection of Neu5GC was performed as described previously.18 In brief, after blocking with a 0.5% solution of fish skin gelatin (Sigma-Aldrich, St. Louis, MO), MSCs were incubated for 30 minutes on ice with 5 µg/ml of chicken anti-Neu5GC IgY or control IgY, then were washed, stained with 5 µg/ml of FITC-labeled anti-IgY antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), and analyzed on a flow cytometer (BD FACSCalibur, BD Biosciences, San Jose, CA).
MSC serum immunoglobulin binding assay. MSCs (0.5 × 106) cultured in the presence or absence of 3 mmol/l of Neu5AC for 1 week were incubated for 30 minutes on ice with 30% pooled normal human serum in PBS. After washing, the cells were stained for cell-surface-bound human IgGs and IgMs with 2 µg/ml of anti-human IgG or anti-human IgM antibodies (Jackson ImmunoResearch Laboratories) and examined by flow cytometry.
Complement alternative pathway and classical pathway activation ex vivo assays. Mice were injected s.c. with 20 U of heparin (MP Biomedicals, Solon, OH) in saline or with saline alone, then blood samples were collected 30 minutes later and immediately centrifuged for 5 minutes at 4 °C to collect the plasma. The activity of the alternative complement pathway was assessed using a zymosan C3b deposition assay and that of the classical complement pathway using a C3b deposition assay employing EshA, following previously established protocols.18
Heparin painting. Heparin was first activated by incubation for 15 minutes at room temperature with 2 mmol/l EDC and 5 mmol/l NHS in a 2-(N-morpholino)-ethanesulfonic acid/NaCl buffer (all from Thermo Fisher Scientific), then 2-mercaptoethanol (final concentration 20 mmol/l) was added to quench the reaction and excess EDC, EDC byproducts, NHS, and 2-mercaptoethanol removed on a PBS-equilibrated desalting column (Thermo Fisher Scientific). The activated heparin was then incubated for 30 minutes at room temperature with MSCs (100 μg of heparin/106 cells) in 1 ml of Hanks' balanced salt solution, and the cells were washed three times with PBS to remove excess free heparin.
To confirm that the activated heparin was painted onto MSCs, in some experiments, the heparin was first labeled with FITC using an FITC labeling kit (Thermo Fisher Scientific) following the manufacturer's protocol, then the FITC-labeled heparin was activated with EDC/NHS and used to paint MSCs as described above. The amount of heparin painted onto MSCs was quantified in a flow cytometer (BD FACSCalibur, BD Biosciences). Mock-painted cells went through the same process, but without heparin.
Measurement of C3b deposited on MSCs after contact with serum. Heparin-painted or mock-painted MSCs (0.5 × 106) were incubated for 30 minutes at 37 °C with 30% of normal human serum in GVB++, without or with 1 mmol/l EDTA (an agent that completely inhibits complement activation). After washes with PBS plus 1% BSA, the cells were incubated with 2 μg/ml of FITC-labeled rat anti-human C3 IgG (Cedarlane, Burlington, Ontario, Canada), washed again and analyzed by a flow cytometer (BD FACSCalibur, BD Biosciences). In some experiments, MSCs cultured in media in the absence or presence of 3 mmol/l of Neu5AC for 1 week were used in the same C3b deposition assays.
Measurement of in vitro damage of MSCs. An established fluorescence dye release assay was used to assess the extent of MSC injury after contact with serum.15 In brief, 2 × 105 heparin-painted or mock-painted MSCs were first loaded by incubation for 30 minutes at 37 °C with 5 µmol/l BCECF-AM (Thermo Fisher Scientific). After three washes, the BCECF-loaded MSCs were incubated for 30 minutes at 37 °C with 20 or 30% normal human serum in 100 μl of GVB++, then the cells were removed by centrifugation. The released BCECF in the supernatant was measured on a SpectraMax Gemini XS (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths of 485 and 538 nm, respectively. Damage to MSCs (percentage of BCECF release) was calculated as ((A – B)/(C – B)) × 100%, where A represents the mean experimental BCECF release, B the mean spontaneous BCECF release, and C the mean maximum BCECF release induced by incubating the cells with 0.1% Triton-X100.
Measurement of in vivo damage of MSCs. In vivo MSC damage after infusion was also examined using a BCECF release assay. In brief, each mouse was injected s.c. with 500 U of heparin in saline or saline alone, then 1 × 106 MSCs labeled with BCECF were injected by the tail vein. Blood samples were collected after 0, 20, 40, and 60 minutes, and the amount of released BCECF in the plasma was measured on a SpectraMax Gemini XS (Molecular Devices) as above.
To evaluate in vivo damage to MSCs with or without heparin painting, 0.5 x 106 heparin-painted or mock-painted MSCs were labeled with BCECF, then injected into the tail vein. Blood samples were collected and the plasma assayed for levels of released BCECF as above.
Heparin-recruited factor H assays. Heparin-painted MSCs (0.5 × 106) were incubated for 30 minutes on ice with 30% normal human serum or factor H-depleted serum in PBS containing 1 mmol/l EDTA to prevent complement activation. After washing, the cells were incubated with 5 µg/ml of goat anti-factor H IgG (Complement Technology, Tyler, TX) followed by 2 µg/ml of FITC-labeled donkey anti-goat IgG (Jackson ImmunoResearch Laboratories). The cells were then washed and the amount of MSC-bound factor H was assessed by flow cytometry.
To examine the function of heparin-recruited factor H, heparin-painted MSCs were first incubated for 30 minutes on ice with 30% normal human serum, 30% factor H-depleted serum, or 30% factor H-depleted serum supplemented with 250 µg/ml of purified human factor H (CompTech) in PBS containing 1 mmol/l EDTA. In this step, different sera were used to provide factor H, and EDTA was used to allow factor H recruitment without complement activation. The MSCs were then washed with GVB++, labeled with BCECF, incubated with 30% factor H-depleted serum in GVB++ for 30 minutes at 37 °C, and BCECF release measured as above. Factor H-depleted serum was used in this step as the source of complement to avoid the interference of factor H presented in the normal serum.
MSC in vitro function assay. After treatment of MSCs for 2 hours with 10 µg/ml mitomycin C (Sigma-Aldrich) to inhibit their proliferation, MSC immunosuppressive activity was examined in vitro by measuring their ability to inhibit the proliferation of anti-CD3/CD28 mAb-activated mouse T cells using a BrdU incorporation assay with a BrdU ELISA kit (Roche, Chicago, IL). In addition, the ability of these MSCs to inhibit inflammatory cytokine production by the activated mouse T cells was evaluated by measuring IFNγ levels in the culture supernatants using an IFNγ ELISA kit (BioLegend, San Diego, CA), following the manufacturer's protocol.
MSC in vivo function and survival assays. The in vivo immunosuppressive activity of MSCs was assessed by measuring their ability to inhibit the proliferation of OVA-specific T cells in the draining lymph nodes. In brief, T cells were enriched with splenocytes from OT II mice using nylon wool and injected via the tail vein into naive WT C57BL/6 mice (4 × 106 cells/mouse). After 24 h, syngeneic bone marrow-derived DCs loaded with OVA were injected s.c. into the hind footpads (0.4 × 106/mouse) and heparin- or mock-painted MSCs (1 × 106/mouse) were injected via the tail vein. After a further 24 hours, 1 mg of BrdU was injected intraperitoneally into each mouse, then, 24 hours later, the draining lymph nodes (popliteal lymph nodes) were harvested and the proliferation of the T cells quantified by measuring BrdU incorporation using a BrdU ELISA kit (Roche).
To evaluate in vivo survival of MSC, in some of the above experiments, draining lymph nodes (popliteal lymph nodes) were collected 48 hours after injection of the mock-painted or heparin-painted MSCs and the survival of the injected MSCs assessed by staining the cells with 3 μg/ml of mAb against human HLA-ABC antigen (Clone W6/32; BioLegend, San Diego, CA), then measuring numbers of living human cells in a flow cytometer (BD FACSCalibur, BD Biosciences). Cells from distal non-draining lymph nodes (cervical lymph nodes) were included as controls.
Author Contributions
Y.L. performed experiments, analyzed data, and helped manuscript preparation. J.F. contributed to data analysis, discussion and edited manuscript. F.L. conceived the study, analyzed data, and wrote the manuscript.
SUPPLEMENTARY MATERIAL Figure S1. 0.5 × 106 of MSCs or HUVECs were stained with 5 µg/ml of antibodies against CD46, CD55, CD59, factor H or CD35 (blue lines), or their respective isotype controls (red lines), then analyzed by a flow cytometer.
Acknowledgments
We thank Ajit Varki, Glycobiology Research and Training Center, University of California, San Diego (La Jolla, CA, USA) for providing the anti-Neu5GC antibodies and other related reagents and helpful discussions. We thank David Wald, Director of the MSC Core Facility at the National Center for Regenerative Medicine, Case Western Reserve University (Cleveland, OH, USA) for providing MSCs. This work was supported in part by NIH grants DK103581 (FL) and AR061564 (FL). The authors declare no conflict of interest.
Supplementary Material
References
- Singer, NG and Caplan, AI (2011). Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 6: 457–478. [DOI] [PubMed] [Google Scholar]
- Tsuji, H, Miyoshi, S, Ikegami, Y, Hida, N, Asada, H, Togashi, I et al. (2010). Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res 106: 1613–1623. [DOI] [PubMed] [Google Scholar]
- English, K and Mahon, BP (2011). Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem 112: 1963–1968. [DOI] [PubMed] [Google Scholar]
- Caplan, AI (2009). Why are MSCs therapeutic? New data: new insight. J Pathol 217: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum, J and Karp, JM (2010). Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med 16: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia, E, Casazza, S, Pedemonte, E, Benvenuto, F, Bonanni, I, Gerdoni, E et al. (2005). Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755–1761. [DOI] [PubMed] [Google Scholar]
- Augello, A, Tasso, R, Negrini, SM, Cancedda, R and Pennesi, G (2007). Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 56: 1175–1186. [DOI] [PubMed] [Google Scholar]
- Yu, J, Zheng, C, Ren, X, Li, J, Liu, M, Zhang, L et al. (2010). Intravenous administration of bone marrow mesenchymal stem cells benefits experimental autoimmune myasthenia gravis mice through an immunomodulatory action. Scand J Immunol 72: 242–249. [DOI] [PubMed] [Google Scholar]
- Fiorina, P, Jurewicz, M, Augello, A, Vergani, A, Dada, S, La Rosa, S et al. (2009). Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, IK, Kim, BG, Awadallah, A, Mikulan, J, Lin, P, Letterio, JJ et al. (2010). Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther 18: 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinovis, M, Casiraghi, F, Remuzzi, G and Perico, N (2015). Mesenchymal stromal cells to control donor-specific memory T cells in solid organ transplantation. Curr Opin Organ Transplant 20: 79–85. [DOI] [PubMed] [Google Scholar]
- von Bahr, L, Batsis, I, Moll, G, Hägg, M, Szakos, A, Sundberg, B et al. (2012). Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30: 1575–1578. [DOI] [PubMed] [Google Scholar]
- Ricklin, D, Hajishengallis, G, Yang, K and Lambris, JD (2010). Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester, CM and Brophy, PD (2013). Eculizumab in the treatment of atypical haemolytic uraemic syndrome and other complement-mediated renal diseases. Curr Opin Pediatr 25: 225–231. [DOI] [PubMed] [Google Scholar]
- Li, Y and Lin, F (2012). Mesenchymal stem cells are injured by complement after their contact with serum. Blood 120: 3436–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll, G, Jitschin, R, von Bahr, L, Rasmusson-Duprez, I, Sundberg, B, Lönnies, L et al. (2011). Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS One 6: e21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki, A (2001). Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol Suppl 33: 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi, D, Taylor, RE, Padler-Karavani, V, Diaz, S and Varki, A (2010). Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol 28: 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazatchkine, MD, Fearon, DT, Silbert, JE and Austen, KF (1979). Surface-associated heparin inhibits zymosan-induced activation of the human alternative complement pathway by augmenting the regulatory action of the control proteins on particle-bound C3b. J Exp Med 150: 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler, JM, Yurt, RW, Fearon, DT and Austen, KF (1978). Modulation of the formation of the amplification convertase of complement, C3b, Bb, by native and commercial heparin. J Exp Med 147: 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn, MK, Atkinson, MA and Meri, S (1991). Localization of the heparin-binding site on complement factor H. J Biol Chem 266: 16847–16853. [PubMed] [Google Scholar]
- Moll, G, Rasmusson-Duprez, I, von Bahr, L, Connolly-Andersen, AM, Elgue, G, Funke, L et al. (2012). Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 30: 1565–1574. [DOI] [PubMed] [Google Scholar]
- Le Blanc, K and Davies, LC (2015). Mesenchymal stromal cells and the innate immune response. Immunol Lett 168: 140–146. [DOI] [PubMed] [Google Scholar]
- Chou, HH, Takematsu, H, Diaz, S, Iber, J, Nickerson, E, Wright, KL et al. (1998). A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA 95: 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi, G, Redecha, P and Salmon, JE (2004). Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med 10: 1222–1226. [DOI] [PubMed] [Google Scholar]
- Zipfel, PF (2001). Complement factor H: physiology and pathophysiology. Semin Thromb Hemost 27: 191–199. [DOI] [PubMed] [Google Scholar]
- Fischer, MJ (2010). Amine coupling through EDC/NHS: a practical approach. Methods Mol Biol 627: 55–73. [DOI] [PubMed] [Google Scholar]
- Karp, JM and Leng Teo, GS (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4: 206–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.