Abstract
Selective depletion (SD) of alloreactive T cells from allogeneic hematopoeitic stem cell transplants to prevent graft-versus-host disease (GVHD) without compromising immune reconstitution and antitumor responses remains a challenge. Here, we demonstrate a novel SD strategy whereby alloreacting T cells are efficiently deleted ex vivo with adenosine. SD was achieved in human leukocyte antigen (HLA) mismatched cocultures by multiple exposures to 2 mmol/l adenosine over 7 days. Adenosine depleted greater than to 90% of alloproliferating T cells in mismatched, haploidentical, and matched sibling pairs while conserving response to third-party antigens. Alloreactive CD4 and CD8 T cells were targeted for depletion while NK and B cells were preserved. Our novel approach also preserved nonalloreactive naive, central, and effector memory T-cell subsets, Tregs, and notably preserved T-cell responses against DNA viruses that contribute to transplant related mortality after allogeneic hematopoeitic stem cell transplants. Additionally, T cells recognizing leukemia-associated antigens were efficiently generated in vitro from the cell product post-SD. This study is the first to demonstrate that adenosine depletion of alloactivated T cells maintains a complete immune cell profile and recall viral responses. Expansion of tumor antigen-specific subsets postdepletion opens the possibility of generating T-cell products capable of graft-versus-tumor responses without causing GVHD.
Introduction
The focus of any strategy to optimize the outcome of allogeneic stem cell transplant (SCT) is to manipulate the incoming donor T-cell repertoire so as to prevent graft-versus-host disease (GVHD) provoked by major and minor recipient histocompatibility antigens (HLA) while preserving cells capable of restoring cellular immunity, especially against microbial infections, common viruses, and tumors in the case of transplants for malignant disease. Current haploidentical transplant approaches to prevent GVHD, which preserve at least some protective immunity against viral reactivation and disease recurrence, range from T-cell depletion,1 intensive post-transplant immunosuppression,2 or in vivo elimination of alloreacting T cells early postgraft using high-dose cyclophosphamide.3,4 An important contribution to the successful suppression of GVHD is the preservation of regulatory T cells (Tregs), which have a selective advantage in suppressing GVHD while permitting immune recovery.5,6,7 An alternative approach is the ex vivo selective depletion (SD) of T cells responsible for the donor's alloresponse against recipient mononuclear cells.8 In this approach, the donor lymphocytes are cocultured with either irradiated lymphocytes from the recipient in a mixed lymphocyte reaction (MLR) or other irradiated recipient mononuclear cells in a modified MLR (mMLR). Alloactivated donor T cells can then be eliminated or rendered nonfunctional by antibodies or immunotoxins targeting activation antigens (CD25, CD69), or by photodepletion of activated T cells.9 Off-target depletion of the crucial CD25+ Treg population along with poor depletion efficacy, possibly due to downregulation of targeted antigens, has hampered the success of immune-magnetic and immunotoxin approaches. Photodepletion with a TH9402-based dye failed to improve outcomes in HLA-matched sibling transplants but has shown promise in haploidentical transplants.10,11,12 An effective, immune modulating strategy for SD which conserves Tregs and can be consistently and easily reproduced in clinical scale has not yet been achieved.
Here, we present a novel strategy for allodepletion employing ex vivo treatment of alloreacting donor T cells with adenosine. Adenosine is a purine nucleoside approved by the United States Food and Drug Administration to assist in treatment of supraventricular tachycardia by intravenous injection. In physiological conditions, adenosine plays an integral role in thymic function and peripheral tissue homeostasis. In hypoxic and inflammatory environments, such as the tumor microenvironment, adenosine regulates inflammation, inducing tolerance and immune suppression.13,14,15 Here, we exploited the immune-regulatory effects of adenosine for prevention of GVHD and demonstrate that pharmacological doses of adenosine efficiently deplete activated alloreactive T cells from mismatched or haplo identical donor lymphocyte populations while retaining desirable cell subsets essential for normal immune reconstitution.
Results
Adenosine selectively depletes allo-activated T cells
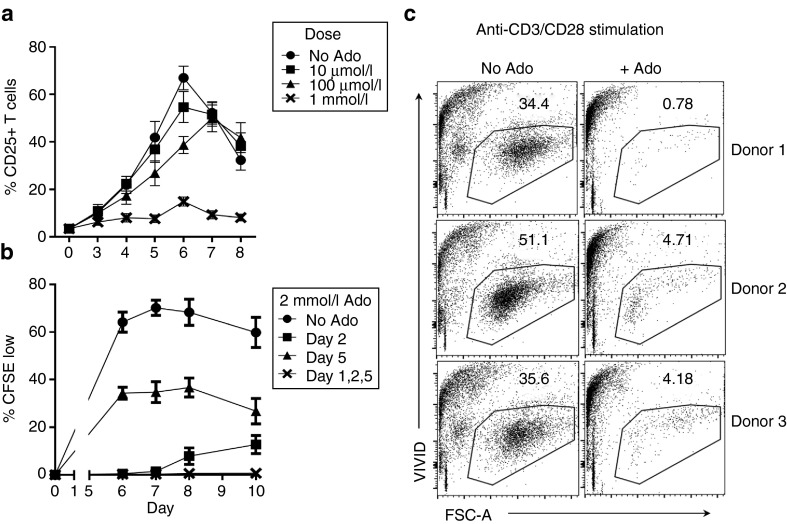
Healthy donor lymphocytes were stimulated with randomly mismatched dendritic cells (DC) in mMLR assay. T cells were analyzed for CD25 expression to measure activation and dilution of carboxyfluorescein succinimidyl ester (CFSE) dye to measure proliferation. Adenosine, a short-lived biological agent, was added at 48-hour intervals (days 1, 3, and 5) in concentrations varying from 10 µmol/l to 1 mmol/l. Suppression of CD25 expression was observed at the highest 1 mmol/l concentration of adenosine (Figure 1a). In subsequent experiments, 2 mmol/l adenosine was used to ensure suppression of activation and proliferation. Multiple treatments of adenosine over a 5-day period were necessary to achieve sustained suppression of T-cell proliferation (Figure 1b). A single early treatment on the second day of culture suppressed activation, but T-cell proliferation was noticed on days 8–10 indicating the presence of a residual group of late-responding cells. A single late treatment (day 5) only eliminated about half of proliferating cells. Combining the early and late treatments by administering adenosine on days 1, 2, and 5 after establishing mMLR successfully suppressed proliferation up to 5 days after the final treatment.
Figure 1.
Millimolar adenosine suppresses T-cell alloactivation and proliferation. (a) FACS analysis showing percent CD25+ T cells in culture after stimulation of healthy donor lymphocytes with HLA-mismatched recipient DC over 8 days in the presence or absence of various doses of adenosine on days 1, 2, and 5 after establishing cocultures. (b) CFSE-labeled donor lymphocytes were cocultured with HLA-mismatched recipient DCs for 10 days. Cultures were treated with 2 mmol/l adenosine on day 2 or 5 only, or days 1, 2, and 5 after establishing mMLR. Two days after the final treatment, CFSE dye dilution in T cells was determined as a measure of cell proliferation by flow cytometry. Results shown in Figure 1a and 1b are the mean of 6 HLA-mismatched donor–recipient pairs. (c) Lymphocytes from three healthy donors were stimulated with anti-CD3/CD28 beads and treated with 2 mmol/l adenosine on days 1, 2, and 5 (+Ado) or not treated (No Ado). Two days after the final treatment cultures were stained with vivid and viability was determined by flow cytometry. Results shown are representative of three donors.
To explore cytotoxic effects of adenosine on activated T cells, lymphocytes were stimulated with anti-CD3/CD28 beads and treated with adenosine (Figure 1c). Vivid viability staining revealed major reduction in viable lymphocytes and increased cell debris in adenosine-treated samples. Direct induction of apoptosis was assessed by culturing lymphocytes from four healthy donors either alone, with a mix of DC from three mismatched donors, or anti-CD3/CD28 beads. After 72 hours, half of the cultures were incubated with 2 mmol/l adenosine for 5 minutes before staining with Annexin V and PI. Paired T-test statistical analysis showed a significant increase in PI+AnnexinV+ cells in both allo-stimulated and bead-stimulated cultures (P = 0.04, P = 0.02), but no significant change observed in this population for unstimulated lymphocytes (P = 0.6, Supplementary Table S1). These results suggest that adenosine is toxic toward and capable of depleting T cells activated by allo- or anti-CD3/CD28 stimulation. The lack of significant induction of apoptosis in unstimulated T cells suggests that this depletion is selective to activated cells, but does not definitively discount that some nonactivated, nonproliferating population of cells may also be affected.
From these experiments, a standard SD protocol was devised in which cultures established on day 0 were treated with 2 mmol/l adenosine as a 2× solution on days 1, 2, and 5. In 12 consecutive experiments, the allodepleted product, collected on day 7, comprised 54 ± 0.09% of initial lymphocytes with a viability of 91.32 ± 2.4%. In comparison, lymphocytes cultured without manipulation for 7 days comprised 42.3 ± 11.2% of initial cells with a viability of 89.3 ± 3.7% (N = 6).
Adenosine inhibits T-cell response against allostimulating DCs while retaining third-party responses and proliferative capacity
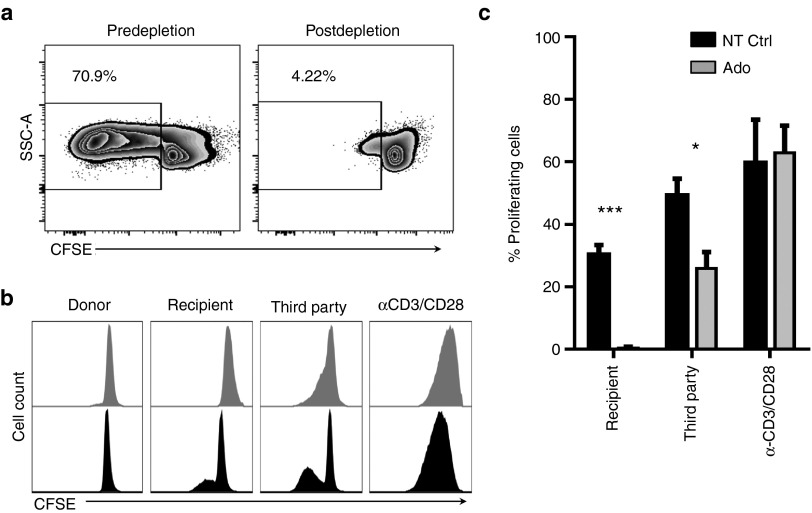
The ability of adenosine to functionally deplete “donor” alloreactive T cells was assessed by restimulation of adenosine allodepleted lymphocytes against DC stimulators from the original HLA-mismatched “recipient.” Elimination of alloreactive cells was identified as the absence of proliferation on rechallenge with the original “recipient” cells. A comparison of the proliferation of freshly thawed lymphocytes and adenosine allodepleted lymphocytes stimulated by DC in a representative donor–recipient pair is shown in Figure 2a. The frequency of proliferating donor-derived responder T cells fell from 70.9 to 4.2% after depletion.
Figure 2.
Adenosine selectively depletes alloreactive T cells in HLA-mismatch mMLR while retaining activity against third-party APCs. Adenosine allodepleted lymphocytes (Ado) and unmanipulated lymphocytes cultured for 7 days (NT Ctrl) were stained with CFSE and tested for proliferative response against a panel of stimulators in secondary mMLR. CFSE dye dilution in T cells was determined by flow cytometry as a measure of proliferation. (a) Representative data from one donor depicting proliferation of freshly thawed lymphocytes (predepletion) and Ado-depleted lymphocytes (postdepletion) challenged against stimulator DCs for 5 days. (b) Overlayed histograms of CFSE dilution profile for representative donor shows abrogation of response against stimulator DCs. (c) Pooled data from five responder–recipient pairs, background proliferation against autologous DCs subtracted from presented values.
Selectivity of allodepletion was assessed in side-by-side proliferation challenge of responder T lymphocytes from adenosine-depleted cocultures and unmanipulated control cultures against a panel of stimulators. To account for possible confounding effects of prolonged culture, unstimulated lymphocytes cultured alone for 7 days were used as unmanipulated controls. Stimulators employed in this assay were autologous DC to measure background proliferation, recipient DC to measure depletion, a mix of DCs from three unrelated donors to measure third-party mMLR response, and anti-CD3/CD28 beads to measure maximum proliferative capacity. Proliferation of responder cells was assessed by dilution of CFSE dye measured by flow cytometry after 5-day coculture period with stimulators.
Pooled data from five experiments (Figure 2b,c) show that adenosine treatment achieved significant depletion of proliferating alloreactive cells in mMLR. As shown in Figure 2c, 0.4 ± 0.4% T cells from allodepleted mMLR proliferated in secondary response against recipient DC, compared to the 31.3 ± 3.1% primary response of unmanipulated control cultures and indicates a significant depletion of 98.7% of alloproliferative response (P = 0.000015). Donor T cells from both adenosine treated and control cultures elicited a strong allo-response against third-party DCs (25.9 ± 5.3% and 49.3 ± 5.3%, respectively), but this response was significantly decreased in the depleted cultures (P = 0.014, *). Notably, both cultures proliferated robustly in response to anti-CD3/CD28 stimulation, 62.9 ± 8.7% for adenosine depleted and 59.6 ± 14.0% for the untreated control, suggesting that remaining T cells were viable and able to proliferate after adenosine treatment.
Impact of adenosine on viability and individual lymphocyte subsets
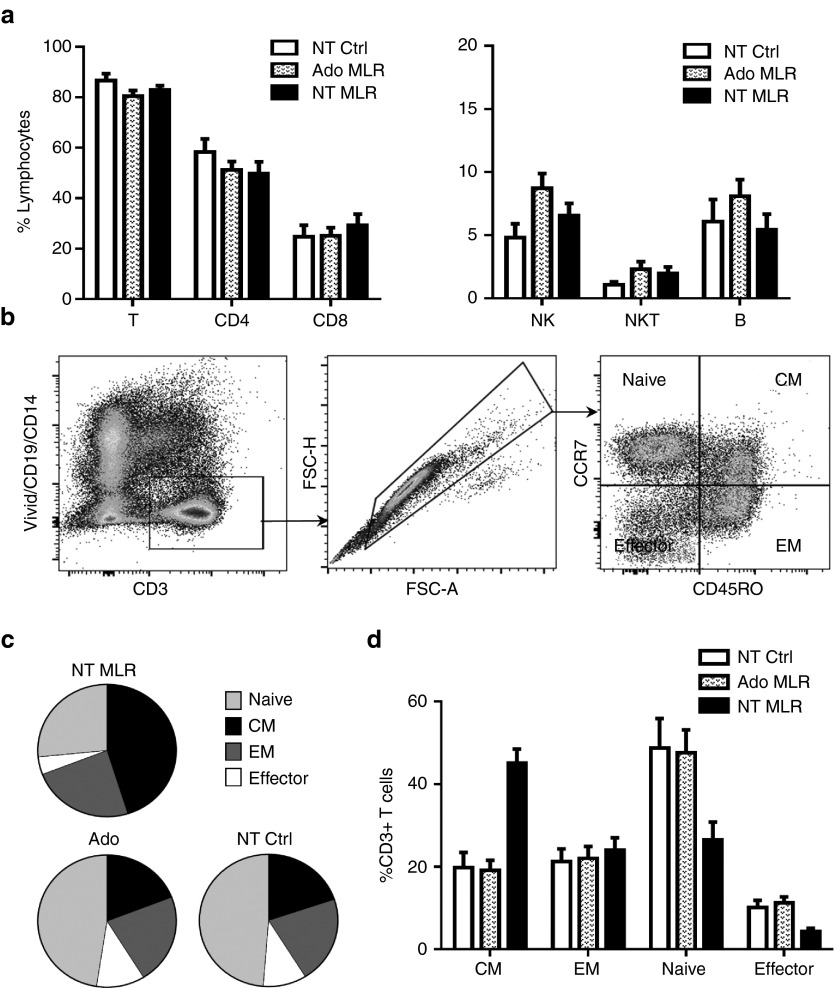
Figure 3a shows that adenosine-mediated cellular depletion was restricted to the T lymphocyte compartment; the frequency of B- and NK-cell populations increased in adenosine treated cultures (NK: 8.72 ± 1.17%, B: 8.08 ± 1.33%, N = 12) compared to untreated control cultures (NK: 4.82 ± 1.09%, B: 6.06 ± 1.78%, N = 6). Although adenosine appeared to primarily affect the CD4+ Th subset (Figure 3a), as evidenced by the slight decrease (51.23 ± 3.35%, N = 18) compared to unstimulated control cultures (58.33 ± 5.15%, N = 6), this effect was not statistically significant.
Figure 3.
Allodepletion with adenosine preserves lymphocyte and T-cell subsets. Primary mMLR cultures were established using 12 distinct donor lymphocyte and mismatched recipient DC pairs. Cultures were treated with adenosine on day 1, 2, and 5 (Ado MLR, N = 12) or left untreated (NT MLR, N = 12). Responder lymphocytes were cultured alone (NT Ctrl, N = 6). After 7 days, the samples were harvested, washed, and phenotype was analyzed by flow cytometry. Lymphocyte subsets were identified as B cells (CD19+CD3-), total NK cells (CD56+CD19-), NKT cells (CD3+ CD56+), total T cells (CD3+CD19-CD56-), CD4+ T cells (CD3+CD4+), or CD8+ T cells (CD3+CD8+). (a, left) Total T, CD4+ Th, and CD8+ CTL frequencies. (a, right) Total NK, NKT, and B cell frequencies. (b) Gating strategy used to identify various T-cell subset denominations. CD3+ T cells were classified as naive (CCR7+CD45RO-), central memory (CM, CCR7+CD45RO+), effector memory (EM, CCR7-CD45RO+), or terminal effector (EMRA, CCR7-CD45RO-). (c, d) Pooled data from one experiment showing the frequency and distribution of various T-cell subsets in adenosine-treated or untreated mMLR cultures (N = 12) or responder lymphocyte alone control cultures (N = 6).
Phenotypic analysis of the T-cell subsets revealed no significant changes in the distribution of T cells among naive, effector memory (EM), central memory (CM), and effector compartments in adenosine treated mMLR cocultures compared to the phenotype of unstimulated T cells (Figure 3c,d). However, we found a significant reduction in the frequency of naive T cells with a concurrent increase in CM and to a lesser extent EM compartments in untreated, alloreacting mMLR cultures (Figure 3c,d). These results corroborate previous findings that alloreactive T cells are mainly recruited from the naive subset.16
Regulatory T cells are preserved in adenosine-treated cultures
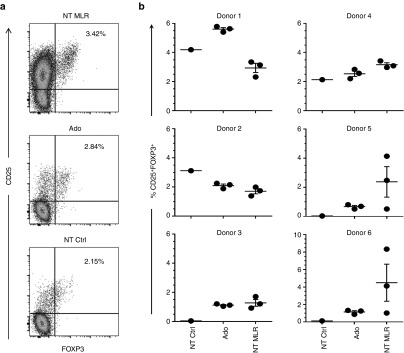
Donor-regulatory lymphocytes, in particular Tregs, are essential for protection against GVHD in patients following cell transfer. In six out of six donors, CD4+CD25+FOXP3+ Tregs were identified after SD with adenosine (Figure 4a,b) and five out of six donors showed an increased frequency over unstimulated lymphocyte controls. Although the frequency of Tregs varied in untreated mMLR according to the individual stimulator DC, the frequency of Tregs persisting in cultures treated with adenosine was mostly dependent on the responder T cells. Data shown in Figure 4b indicate that the relative frequency of Treg populations within CD3+CD4+ subset in mMLR cultures treated with adenosine (2.20 ± 0.40%, N = 18) was comparable to that of untreated mMLR cultures (2.66 ± 0.42%, N = 18) and cultures containing unstimulated lymphocytes alone (1.60 ± 0.74%, N = 6).
Figure 4.
Tregs are not depleted by adenosine. Responder lymphocytes were cultured with no stimulation (NT Ctrl), in mMLR against mismatch stimulator DCs with adenosine treatment (Ado), or in mMLR without treatment (NT MLR). After 7 days, the cultures were analyzed to determine the frequency of CD25+FOXP3+ Tregs within CD3+CD4+ T by flow cytometry. (a) Representative dot plot analysis of sample from one donor showing the gating strategy used to determine the CD3+CD4+CD25+FOXP3+ Tregs. (b) Frequency of CD3+CD4+ CD25+FOXP3+ Tregs in NT Ctrl (N = 1), Ado (N = 3), and NT mMLR (N = 3) cultures for six donors. Each donor was depleted or cultured in mMLR against three distinct recipients.
Allodepleted T cells recognizing CMV, EBV, and adenovirus antigens and viral-specific cells are enriched by stimulation
Donor T-cell responses to viral pathogens can protect the immunocompromised patient from early viral reactivation after SCT and support graft survival, engraftment, and immune reconstitution. Therefore, successful SD should preserve donor-derived viral-specific memory T cells.
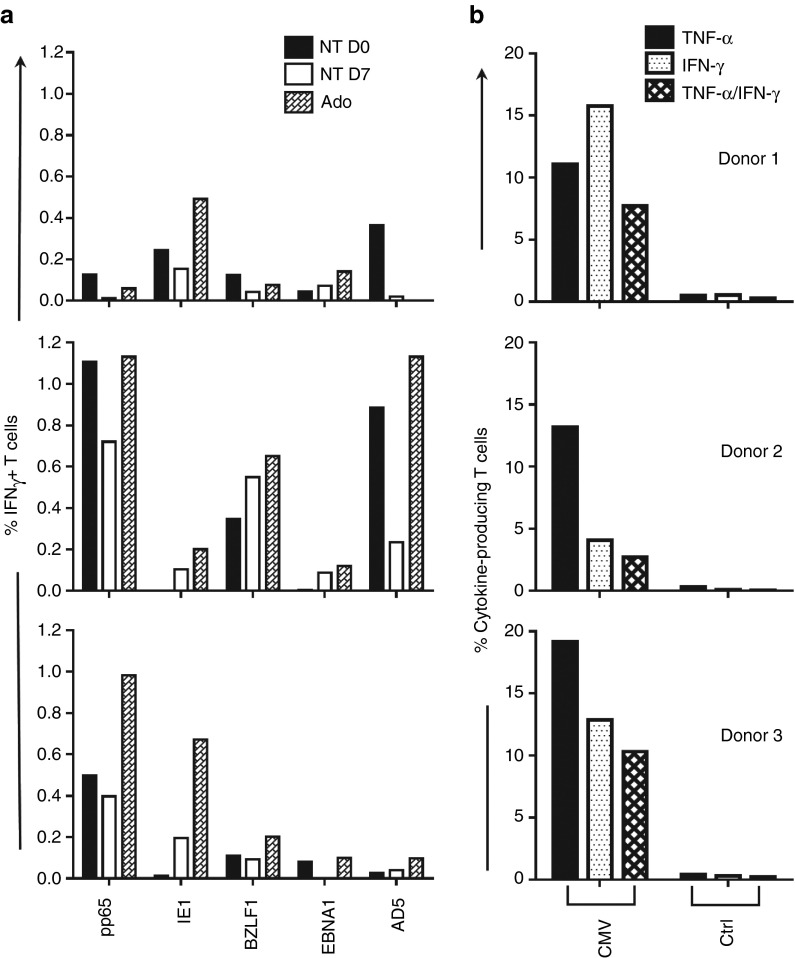
Allodepleted cultures generated from three donors were tested for recognition of peptide libraries for cytomegalovirus (CMV) antigens pp65 and IE-1, Epstein-Barr virus antigens BZLF1 and EBNA1, and adenovirus antigen AD5 (Figure 5a). Viral peptide recognition measured by IFN-γ production varied between donors. Antigen-specific T-cell responses to all viruses were retained, and in 11 out of 15 instances, antigen-specific T-cell frequencies were increased after adenosine-mediated allodepletion.
Figure 5.
T cells recognizing viral peptides persist after depletion and can be expanded through stimulation with peptide-pulsed APCs. Recognition of CMV (pp65, IE-1), EBV (EBNA1, BZLF1), and adenovirus (AD5) antigens tested by 6-hour peptide stimulation; intracellular expression of IFN-γ and TNF-α were measured by flow cytometry. (a) Lymphocytes from three CMV seropositive donors were depleted against allogeneic DCs with adenosine (Ado), cultured for an equal period with no stimulation (NT Day 7), or thawed fresh (NT Day 0) and tested against panel of viral peptide libraries. The background of IFN-γ+ cells against irrelevant peptide is subtracted from raw data in the presented values. (b) CMV-specific cells expanded from adenosine depleted cultures through two rounds of peptide stimulation show polyfunctional response against pp65 and IE-1 peptides.
Transfer of ex vivo expanded viral-specific T cells is a promising strategy to improve immune reconstitution after stem cell transplant made increasingly accessible by recent innovations in cell culture expansion.17 Since viral-specific T cells were preserved after adenosine treatment, our approach serves as a useful platform for expanding viral-specific T cells free of the risk of GVHD for adoptive transfer. To test this, cultures depleted with adenosine were washed and stimulated twice at weekly intervals with autologous DC pulsed with pp65 and IE-1 peptides. One week after the second stimulation, products were rested overnight and tested for reactivity against pp65, IE-1, or control WT1 as determined by the expression of TNF-α and IFN-γ 6-hour post-peptide stimulation by flow cytometry. Enrichment of CMV-reacting cells, particularly polyfunctional T cells producing both TNF-α and IFN-γ, was achieved in three donors (Figure 5b).
T cells targeting leukemia-associated antigens (LAA), WT1, and PRAME can be generated from allodepleted products
Graft lymphocyte-mediated antitumor immune responses are an essential function of the allograft in preventing relapse.18 T cells in particular can exhibit powerful toxicity against malignant cells if targeted to recognize tumor-identifying antigens. Tumor antigens such as PRAME and WT1 are highly expressed in some leukemias, but precursor memory T cells recognizing these antigens are infrequent to nonexistent in healthy donors.
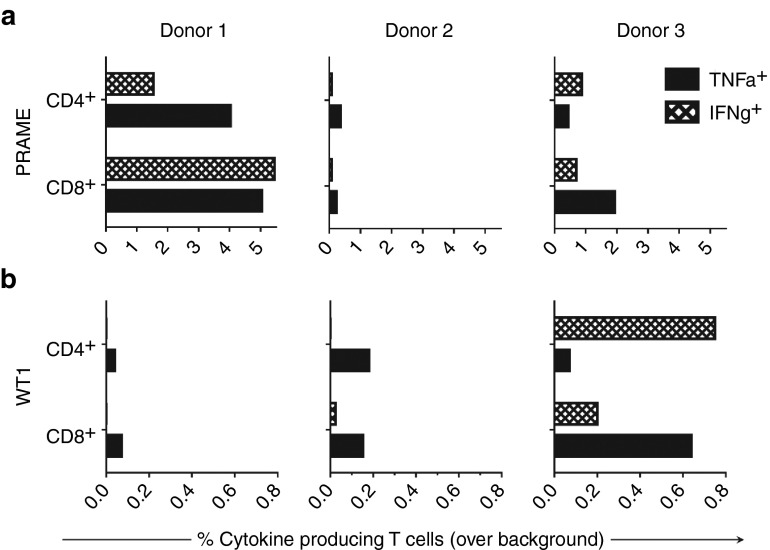
To generate LAA-specific cells, lymphocytes allodepleted against mismatched DCs with adenosine were stimulated twice at weekly intervals with autologous DCs transduced to express PRAME or WT1 peptide. Polyfunctional recognition of PRAME and WT1 antigens was achieved in CD4+ and CD8+ T cell subsets of three donors (Figure 6a,b), establishing the feasibility of generating tumor-targeted T cells from allodepleted cultures for adoptive immunotherapy where appropriate.
Figure 6.
Leukemia associated antigen-specific T cells were generated from allodepleted cultures. Adenosine-depleted lymphocytes from six mismatched donor–recipient pairs were stimulated twice at weekly interval with autologous DCs transduced with a lentiviral vector expressing PRAME or WT1 to generate and enrich antigen-recognizing T cells. Antigen specificity was tested against DCs transduced with the cognate antigen PRAME or WT1, or irrelevant control antigen in a 6-hour coculture assay followed by intracellular flow cytometry for cytokine production analysis. Production of IFN-γ and TNF-α in recognition of cognate antigen over background control in CD4+ and CD8+ T cell subsets is presented for (a) PRAME-specific cultures and (b) WT1-specific cultures from three donor–recipient pairs.
Adenosine-mediated selective depletion is successful in haploidentical and matched sibling donor–recipient pairs
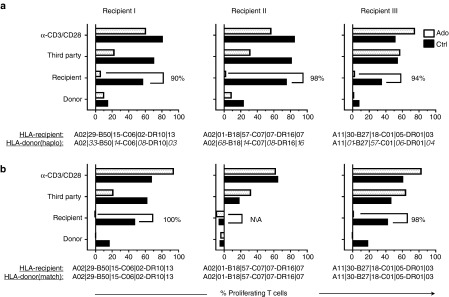
SD was performed in haploidentical and matched sibling mMLR to verify the feasibility of this method in clinically relevant pairings. Recipient DC stimulators were generated from pretransplant patient peripheral blood mononuclear cells (PBMCs) and cocultured separately with haploidentical and matched sibling donor PBMC responder cells. Adenosine treatments were the same for matched and mismatched donors. On day 7 post-mMLR, cells persisting in the cultures were labeled with CFSE and the selectivity of allodepletion was assessed by challenging cultures against a panel of stimulators in a 5-day proliferation assay as previously described. To reveal possible minor alloreactive cell populations, adenosine-depleted cultures were compared to control cultures of unmanipulated mMLR. In three experiments, SD with adenosine reduced alloreactivity below that of untreated controls in both haploidentical and matched sibling mMLR (Figure 7). Statistical analysis revealed that in the haploidentical setting, proliferation of the “donor” allodepleted product against “recipient” antigen presenting cells (APCs) was significantly decreased compared to undepleted controls (*, P = 0.011) while proliferation against third-party APCs was not significantly affected (ns, P = 0.073). In the matched sibling setting, statistics revealed no significant differences between allodepleted cultures and controls, which are likely due to the low initial alloreactivity of Donor 2 against the matched sibling recipient. Further studies and in vivo models may provide a clearer case for employing this strategy in matched sibling transplants.
Figure 7.
Adenosine-mediated allodepletion was highly effective in haploidentical and matched sibling mMLR. SD with adenosine was performed in mMLR coculture of “donor” haploidentical (a) and matched sibling (b) PBMCs with “recipient” DCs in three experiments. HLA serology of donors and recipients for each experiment are reported. Reactivity of allodepleted product (Ado) and untreated mMLR control (Ctrl) against donor DCs, recipient DCs, a mix of unrelated third-party DCs, and anti-CD3/CD28 beads is presented. The reported % reactivity values reflect percentage of CFSE low T cells minus background proliferation of unstimulated cultures.
Discussion
In this study, we show that pharmacological concentrations of adenosine can selectively deplete alloreactive T cells responding to HLA-matched or mismatched APCs. This method exploits naturally tolerogenic functions of purine signaling to selectively eliminate activated T cells. Adenosine is produced physiologically by the metabolism of extracellular ATP released in response to cell injury, lysis, inflammation, or hypoxia.13 Conversion of ATP to AMP and then adenosine by the sequential action of ectonucleotidases CD39 and CD73 results in accumulation of adenosine in the extracellular space where the purine interacts with a family of cell surface G-protein coupled adenosine receptors (A1, A2A, A2B, A3) inducing a concentration-dependent response13,15,19. Endogenous adenosine accumulation is implicated in the promotion of tumor metastasis and neoplastic expansion by local immunosuppression, reduction of inflammation, and protection against immune-mediated damage.13,14 In particular, adenosine signaling through the A2A receptor has been shown to disable NK and CTL cytotoxic functions and suppress the proliferation of activated T cells while additionally promoting tolerogenic DC differentiation.14,15,20 The mechanism of adenosine-mediated toxicity is thought to result from substantial accumulation of cytosolic cyclic AMP (cAMP), leading to DNA fragmentation and apoptosis.21,22 Our results confirm that at pharmacological doses, adenosine is cytotoxic to activated T cells. The specific effects of pharmacological doses of adenosine on activated lymphocytes are not well understood. Extracellular adenosine is regulated by adenosine deaminase (ADA, CD26), which converts adenosine into inosine. ADA deficiency results in lymphopenia and severe combined immunodeficiency (SCID) highlighting the integral role of adenosine signaling in regulation of lymphocyte differentiation, survival and immune function, and homeostasis.20 It is worth noting that several artificial adenosine receptor ligands which might further boost adenosine-mediated SD have been synthesized and are available commercially.23
We hypothesized that the immunosuppressive properties of adenosine might be harnessed to selectively deplete alloreacting cells from SCT in order to create GVHD-free cell products, particularly in haplo SCT where robust methods to prevent GVHD are needed. Here, we demonstrated that adenosine blocked both the initiation of the alloresponse and also prevented subsequent proliferation of alloreactive T cells (Figure 1a,b) in dose- and time-dependent manner. Effective depletion of alloreactive cells was achieved by treating the cultures with 2 mmol/l adenosine on days 1, 2, and 5 of coculture. This method also depleted lymphocytes stimulated with anti-CD3/CD28 beads, demonstrating that adenosine is toxic to activated T cells at millimolar concentrations (Figure 1c). The abrogation of alloresponse was maintained upon rechallenge of depleted cultures against the original stimulator DCs (Figure 2). The finding that the allodepleted lymphocytes do not proliferate upon secondary stimulation with the same stimulators in the absence of adenosine suggests that the alloreactive T cells are truly depleted from the culture. Interestingly, the response against third-party stimulators, though still present and robust, was significantly decreased in adenosine depleted cultures. This result may either reflect depletion of T cells reactive against shared minor antigens or depletion of some nonalloreactive cells. Adenosine-treated cell cultures displayed unchanged proportions of naive, CM, EM, and effector T cells, indicating that only actively triggered memory (CM and EM) cells were targeted while in untreated allo-MLR cultures accumulation of memory T cells was observed. To ensure that cell populations desirable for transplant were retained, we further investigated the lymphocyte and T-cell subsets persisting after depletion, particularly Tregs, virus recognizing T cells, and leukemia-recognizing T cells.
Phenotypic analysis revealed that while removing alloreactive T cells, adenosine preserved B and NK cells as well as naive and memory T-cell repertoires (Figure 3). Memory T cells provide important immune competence against a variety of infectious agents including DNA viruses such as CMV, which complicate post-transplant outcome. Effective SD must retain these cells to prevent reactivation and opportunistic infection. We therefore tested the SD product for CMV, EBV, and AD5 reactivity. In three independent experiments, we observed that allodepleted cultures maintained or sometimes enriched these viral-specific T cells. Using CMV as a model, we also showed that these peptide-recognizing cells can be expanded ex vivo through antigen-specific stimulation and exhibit the properties of adoptively transferred T cells used to control CMV after stem cell transplantation (Figure 4).24
It has been shown that donor T cells recognizing leukemia antigens are implicated in the GVL response, which is in part responsible for relapse-free survival after SCT.18 Particular antigens of interest are WT1 and PRAME, both of which are overexpressed in acute leukemias.25 We therefore explored the ability of SD cultures to generate T-cell responses to these antigens. Using autologous DCs transduced with lentiviral vectors to express and present naturally processed epitopes of the target antigens, we showed in three experiments that CD4+ and CD8+ T cells specifically recognizing WT1 and PRAME can be elicited from SD cell products (Figure 6). This result suggests that cultures depleted of alloreactive cells with adenosine could safely be used for adoptive transfer contributing to immune surveillance and protection against relapse while avoiding GVHD.
Our findings indicate that adenosine SD preserves important immune responses, which can determine SCT outcome. Furthermore, technologies are being developed to generate antigen-specific T cells to treat or prevent viral reactivation24,26 and leukemia relapse after SCT.27,28 Such cell products may still retain harmful alloreactivity, particularly in the haplo setting. Our data suggest that pre-emptive use of adenosine to deplete alloreactive cells could improve the quality and purity of such products.
In this study, we employed mature DC as stimulator APCs for their high expression of MHC and costimulatory molecules. DC proved highly effective stimulators, to the point of inducing background proliferation in autologous controls (Figures 2 and 7). In designing selectively depleted SCT for hematopoietic malignancy, the choice of stimulator cell would determine the degree of antigenic overlap between the stimulator and the malignancy. In both HLA-matched and HLA-mismatched settings, T-cell recognition of minor histocompatibility antigens contributes to both GVHD and GVL.29 Thus, employing an APC sharing lineage with a patient's-specific tumor may unfavorably deplete donor T cells recognizing minor antigens identifying the tumor, reducing the GVL response. Selective depletion has been achieved with a variety of stimulator types such as unmanipulated PBMCs,30 ex vivo expanded CD8+ T lymphocytes,31 and EBV-transformed B lymphoblastoid cell lines (EBV-LCL).32 Further work is required to identify the best APC for specific situations.
The concept of using SD to reduce GVHD and maintain GVL is not new. Clinical studies employing anti-CD25 conjugated immunotoxin to eliminate CD25-expressing alloactivated T cells in ex vivo mMLR have been conducted previously in both haploidentical and matched sibling transplants.32,33 The studies in haploidentical transplants found that infusion of SD donor lymphocytes significantly aided immune reconstitution,32 but persisting GVHD was observed in matched sibling transplants.33 Further studies revealed that donor CD4+CD25+FOXP3+ Tregs significantly contributed to protection against acute GVHD in these transplants.6 Our experiments show that SD with adenosine spares these critical Tregs, highlighting the potential for GVHD protection by this approach. Subsequent clinical studies adopted a photo depletion technique, which targeted alloactivated cells in mMLR by their reduced ability to efflux a photosensitizing TH9402-based dye.31,34 While photo depletion exhibited positive results in haploidentical transplant,34,35 off-target depletion of CD4+ CM T cells in matched sibling transplants was associated with increased rate of infection and transplant-related mortality.10 We have shown that adenosine specifically targets alloactivated T cells in mMLR and does not significantly deplete resting memory T cells. Importantly, using adenosine we achieved SD in both haploidentical and matched sibling mMLR (Figure 7). In the matched sibling setting, we observed significant reduction in allo-responses between some two out of three donor/recipient pairs, while the initial alloreactivity was very low in one case. Further studies and in vivo models may provide a clearer case for employing this strategy in matched sibling transplants.
In conclusion, adenosine-based SD represents a readily applicable, inexpensive and robust approach to the persisting problem of refining the T-cell content of the allograft to optimize SCT outcome for both HLA matched and mismatched donor–recipient pairs. The protocol is scalable and all reagents are GMP compliable.
Materials and Methods
Cell culture. Elutriated monocytes and lymphocytes were prepared from healthy donors, aged 20–80 years, from the Department of Transfusion Medicine at the National Institutes of Health. Recipient leukapheresis products were obtained from patients with acute myeloblastic leukemia in remission 20–30 days prior to transplantation. Responder (donor) leukapheresis products were obtained from HLA-mismatched donors, matched sibling donors, and haploidentical parents or siblings of the corresponding recipient patients. Monocytes were immunomagnetically purified from PBMC by CD14-positive selection (Stemcell Technologies, Vancouver, BC, Canada). Dendritic cells (DC) were generated from elutriated or magnetically purified monocytes as described in the Supplementary Methods. HLA typing of the matched and haplodentical donor–recipients is indicated in corresponding data figures. All participants in this study provided written informed consent. Ethical approval was granted by the NHLBI Institutional Review Board for all blood–product acquisitions in accordance with the declaration of Helsinki. Culture media composed of a 50:50 mix of AIM V and RPMI 1640 media supplemented with 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA), 5% heat-inactivated normal AB serum (Gemini Bio-Product, Woodland, CA) was used for culturing the cells.
Ex vivo depletion of alloreactive donor cells. GMP-grade adenosine injection was obtained from Fresenius Kabi USA formulated as an 11 mmol/l solution (3 mg/ml) in normal saline. Recipient DC stimulators were cocultured with or without mismatched, matched, or haploidentical sibling donor lymphocytes at a 1:10 ratio in 24-well plates for 7–10 days. Half of each culture was treated with different concentrations of adenosine on days 1, 2, and 5 of coculture (or as indicated in the figure legends) to deplete alloreactive cells. Control cultures were fed concurrently with an equal volume of normal saline. Two days after the final adenosine treatment, cells from allodepleted and untreated control cultures were sampled for phenotypic analysis. Remaining cells were rested for 16–24 hours and subjected to proliferation assay in secondary mMLR, after which, viability and phenotype were assessed by flow cytometry. Detailed methods and reagents used for flow cytometry analysis are provided in the Supplementary Methods.
Proliferation assay. Adenosine treated and control cell cultures were harvested and labeled with CFSE as described in the Supplementary Methods. Cells were then cocultured with a panel of targets in 96-well plates for 5 days after which proliferation by CFSE dye dilution was measured by flow cytometry. The targets tested were: DC autologous to responder T cells to measure background proliferation, stimulator DC to measure alloreacting cells, and a mix of DC from three unrelated donors to measure response to de novo antigens. Stimulation of T cells with anti-CD3/CD28 Beads (Dynabeads Human T-Activator CD3/CD28; Invitrogen) was used to measure maximum proliferative capacity. Responder-DC cocultures were established at a 10:1 ratio and responder-bead cocultures were established at a 3:1 ratio. Cultured cells were assessed for viability and phenotype by flow cytometry. In some experiments, lymphocytes from three healthy donors were stimulated with anti-CD3/CD28 beads and treated with 2 mmol/l adenosine on days 1, 2, and 5 or left untreated. Two days after the final treatment, cells in culture were assessed for viability by flow cytometry to determine the cytotoxicity of adenosine to lymphocytes. Detailed methods and reagents used to analyze the cell viability and phenotype by flow cytometry were provided in the Supplementary Methods.
Testing for pre-existing antiviral activity. Pepmix peptide pools composed of a 15-mer peptides spanning the entire protein sequence and overlapping by 11 amino acids for pp65, IE-1, EBNA1, BZLF1, and AD5 antigens were acquired from JPT (Berlin, Germany). Cultures to be tested were rested overnight and then individually stimulated with the indicated peptides in 96-well plates according to manufacturer's instructions. One hour post-peptide stimulation at 37 °C, protein transport inhibitors Brefeldin A and Monensin (BD Biosciences, San Jose, CA) were added. Cultures were incubated for additional 5 hours and then stained with fluorescent labeled antibodies against T-cell surface markers and intracellular cytokines and analyzed by flow cytometry to determine their reactivity and antigen specificity.
Ex vivo expansion of CMV-specific T cells. To expand effector T cells with specificity against CMV antigens, lymphocytes allodepleted with adenosine were primed twice at weekly interval with mature autologous DC pulsed with CMV pp65 and IE-1 pepmix (JPT) at a responder to stimulator ratio of 5:1. Cultures received IL-7 (10 ng/ml) and IL-15 (10 ng/ml) during first priming and IL-2 (10 IU) in addition to IL-7 and IL-15 during second priming step. Resulting effector T-cell cultures were tested for reactivity against CMV pp65 and IE-1 antigens as described above.
Ex vivo generation of leukemia-associated antigen-specific T cells. To generate and expand leukemia-associated antigen PRAME and WT1-specific effector T cells, we used autologous DC as APC engineered with lentivirus to express these antigens as described in the Supplementary Methods. Cells were harvested from adenosine-treated mMLR cultures on day 7 and primed twice at weekly interval with indicated target antigen-transduced mature autologous DC at a responder to stimulator ratio of 5:1. Cultures were expanded in the presence of cytokines as described for CMV-specific T cells. Resulting effector T-cell cultures were tested for reactivity against autologous DC pulsed with peptide libraries derived from corresponding target antigen or irrelevant control antigen.
Statistical analysis. Graphs were plotted using Graphpad Software (La Jolla, CA). Data are presented as mean, with standard error bars where appropriate. Statistical analysis was performed either using paired or unpaired Student t-test where P ≤ 0.05 was considered statistically significant (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
SUPPLEMENTARY MATERIAL Table S1. Induction of apoptosis following 2mM adenosine treatment of allostimulated, anti-CD3/CD28 bead stimulated, or unstimulated lymphocytes from 4 healthy donors measured by %PI+AnnexinV+ cells in culture.
Supplementary Methods.
Author Contributions
D.C. conceived, designed, and supervised the study; D.C. and G.D.W. planned and executed the experiments, interpreted the data, and wrote the manuscript; S.A. and B.M. assisted with experimental design and commented on the manuscript; K.K. assisted with flow cytometry; P.M. commented on of the manuscript; A.J.B. supervised the study and assisted in the manuscript writing. All authors approved the final manuscript.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health at the NHLBI. The authors thank Nancy Hensel and Fariba Chinian (Hematology Branch, NHLBI, NIH, Bethesda, MD) for their technical assistance. The authors declare no competing financial interests.
Supplementary Material
References
- Martelli, MF, Di Ianni, M, Ruggeri, L, Falzetti, F, Carotti, A, Terenzi, A et al. (2014). HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 124: 638–644. [DOI] [PubMed] [Google Scholar]
- Huang, XJ, Liu, DH, Liu, KY, Xu, LP, Chen, H, Han, W et al. (2009). Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 15: 257–265. [DOI] [PubMed] [Google Scholar]
- Kanakry, CG, Tsai, HL, Bolaños-Meade, J, Smith, BD, Gojo, I, Kanakry, JA et al. (2014). Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 124: 3817–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luznik, L, O'Donnell, PV, Symons, HJ, Chen, AR, Leffell, MS, Zahurak, M et al. (2008). HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly, S, Ross, DB, Panoskaltsis-Mortari, A, Kanakry, CG, Blazar, BR, Levy, RB et al. (2014). Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 124: 2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, S, Rezvani, K, Savani, BN, Nunes, R, Yong, AS, Schindler, J et al. (2007). Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood 110: 1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani, K, Mielke, S, Ahmadzadeh, M, Kilical, Y, Savani, BN, Zeilah, J et al. (2006). High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood 108: 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, S, Solomon, SR and Barrett, AJ (2005). Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy 7: 109–115. [DOI] [PubMed] [Google Scholar]
- Bastien, JP, Roy, J and Roy, DC (2012). Selective T-cell depletion for haplotype-mismatched allogeneic stem cell transplantation. Semin Oncol 39: 674–682. [DOI] [PubMed] [Google Scholar]
- McIver, ZA, Melenhorst, JJ, Grim, A, Naguib, N, Weber, G, Fellowes, V et al. (2011). Immune reconstitution in recipients of photodepleted HLA-identical sibling donor stem cell transplantations: T cell subset frequencies predict outcome. Biol Blood Marrow Transplant 17: 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, S, McIver, ZA, Shenoy, A, Fellowes, V, Khuu, H, Stroncek, DF et al. (2011). Selectively T cell-depleted allografts from HLA-matched sibling donors followed by low-dose posttransplantation immunosuppression to improve transplantation outcome in patients with hematologic malignancies. Biol Blood Marrow Transplant 17: 1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, D-C, Lachance, S, Roy, J, Walker, I, Maertens, J, Cohen, S, et al. (2015). Donor lymphocytes depleted of alloreactive T-cells (ATIR101) reduce transplant related mortality and improve overall survival in haploidentical HSCT for patients with AML and ALL, using an immunosuppressant-free transplant regimen. Blood 126: 4391. [Google Scholar]
- Antonioli, L, Blandizzi, C, Pacher, P and Haskó, G (2013). Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13: 842–857. [DOI] [PubMed] [Google Scholar]
- Young, A, Mittal, D, Stagg, J and Smyth, MJ (2014). Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov 4: 879–888. [DOI] [PubMed] [Google Scholar]
- Zarek, PE and Powell, JD (2007). Adenosine and anergy. Autoimmunity 40: 425–432. [DOI] [PubMed] [Google Scholar]
- Melenhorst, JJ, Scheinberg, P, Williams, A, Ambrozak, DR, Keyvanfar, K, Smith, M et al. (2011). Alloreactivity across HLA barriers is mediated by both naïve and antigen-experienced T cells. Biol Blood Marrow Transplant 17: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, AJ and Bollard, CM (2015). The coming of age of adoptive T-cell therapy for viral infection after stem cell transplantation. Ann Transl Med 3: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, AJ (2008). Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol 142: 877–888. [DOI] [PubMed] [Google Scholar]
- Haskó, G, Linden, J, Cronstein, B and Pacher, P (2008). Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S, Apasov, S, Koshiba, M and Sitkovsky, M (1997). Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 90: 1600–1610. [PubMed] [Google Scholar]
- Kizaki, H, Suzuki, K, Tadakuma, T and Ishimura, Y (1990). Adenosine receptor-mediated accumulation of cyclic AMP-induced T-lymphocyte death through internucleosomal DNA cleavage. J Biol Chem 265: 5280–5284. [PubMed] [Google Scholar]
- McConkey, DJ, Orrenius, S and Jondal, M (1990). Agents that elevate cAMP stimulate DNA fragmentation in thymocytes. J Immunol 145: 1227–1230. [PubMed] [Google Scholar]
- Klotz, KN (2000). Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol 362: 382–391. [DOI] [PubMed] [Google Scholar]
- Leen, AM, Bollard, CM, Mendizabal, AM, Shpall, EJ, Szabolcs, P, Antin, JH et al. (2013). Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121: 5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, M, Hensel, N, Smith, BD, Prince, GT, Qin, L, Levitsky, HI et al. (2014). Expression of putative targets of immunotherapy in acute myeloid leukemia and healthy tissues. Leukemia 28: 1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou, A, Gerdemann, U, Katari, UL, Tzannou, I, Liu, H, Martinez, C et al. (2014). Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 6: 242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, G, Caruana, I, Rouce, RH, Barrett, AJ, Gerdemann, U, Leen, AM et al. (2013). Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia–implications for immunotherapy. Clin Cancer Res 19: 5079–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, G, Gerdemann, U, Caruana, I, Savoldo, B, Hensel, NF, Rabin, KR et al. (2013). Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia 27: 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierings, E, Kim, YH, Hendriks, M, Borst, E, Sergeant, R, Canossi, A et al. (2013). Multicenter analyses demonstrate significant clinical effects of minor histocompatibility antigens on GvHD and GvL after HLA-matched related and unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 19: 1244–1253. [DOI] [PubMed] [Google Scholar]
- André-Schmutz, I, Le Deist, F, Hacein-Bey-Abina, S, Vitetta, E, Schindler, J, Chedeville, G et al. (2002). Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase ½ study. Lancet 360: 130–137. [DOI] [PubMed] [Google Scholar]
- Mielke, S, Nunes, R, Rezvani, K, Fellowes, VS, Venne, A, Solomon, SR et al. (2008). A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood 111: 4392–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrolia, PJ, Muccioli-Casadei, G, Huls, H, Adams, S, Durett, A, Gee, A et al. (2006). Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood 108: 1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, SR, Mielke, S, Savani, BN, Montero, A, Wisch, L, Childs, R et al. (2005). Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood 106: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, DC, Lachance, S, Kiss, T, Cohen, S, Busque, L, Fish, D, et al. (2009). Haploidentical stem cell transplantation: high doses of alloreactive-T cell depleted donor lymphocytes administered post-transplant decrease infections and improve survival without causing severe Gvhd. Blood 114: 212. [Google Scholar]
- Roy, DC, Guerin, M, Boumedine, RS, Lachance, S, Cohen, S, Sauvageau, G, et al. (2011). Reduction in incidence of severe infections by transplantation of high doses of haploidentical T cells selectively depleted of alloreactive units. Blood 118: 1301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.