Abstract
Antimicrobial peptides (AMPs) are a class of host-defense molecules that neutralize a broad range of pathogens. Their membrane-permeabilizing behavior has been commonly attributed to the formation of pores; however, with the continuing discovery of AMPs, many are uncharacterized and their exact mechanism remains unknown. Using atomic force microscopy, we previously characterized the disruption of model membranes by protegrin-1 (PG-1), a cationic AMP from pig leukocytes. When incubated with zwitterionic membranes of dimyristoylphosphocholine, PG-1 first induced edge instability at low concentrations, then porous defects at intermediate concentrations, and finally worm-like micelle structures at high concentrations. These rich structural changes suggested that pore formation constitutes only an intermediate state along the route of PG-1’s membrane disruption process. The formation of these structures could be best understood by using a mesophase framework of a binary mixture of lipids and peptides, where PG-1 acts as a line-active agent in lowering interfacial bilayer tensions. We have proposed that rather than being static pore formers, AMPs share a common ability to lower interfacial tensions that promote membrane transformations. In a study of 13 different AMPs, we found that peptide line-active behavior was not driven by the overall charge, and instead was correlated with their adoption of imperfect secondary structures. These peptide structures commonly positioned charged residues near the membrane interface to promote deformation favorable for their incorporation into the membrane. Uniquely, the data showed that barrel-stave-forming peptides such as alamethicin are not line-active, and that the seemingly disparate models of toroidal pores and carpet activity are actually related. We speculate that this interplay between peptide structure and the distribution of polar residues in relation to the membrane governs AMP line activity in general and represents a novel, to our knowledge, avenue for the rational design of new drugs.
Introduction
With the overuse and misuse of antibiotics, pathogens that were once highly susceptible to treatment have acquired multidrug resistance, leading to escalating hospitalizations and healthcare costs (1). Host-defense peptides, commonly known as antimicrobial peptides (AMPs), are a potential solution to this crisis. Serving as a first line of defense against infections, AMPs are small, predominantly cationic, amphipathic peptides that are found ubiquitously in the innate (i.e., nonspecific) immune response of living organisms (2, 3). Their localization to vulnerable tissues ensures that they can rapidly neutralize a broad range of microorganisms, including bacteria, fungi, and even viruses. Unlike conventional antibiotics, which interfere with easily mutable targets (e.g., cell wall construction, protein biosynthesis, and genetic material replication), AMPs are believed to target the more evolutionarily stable cell membranes of invading pathogens by disrupting their barrier function to cause eventual cell death. The membrane-permeabilizing activity of AMPs is an advantageous characteristic in the design of new drugs; however, the general mechanism by which this activity occurs remains poorly understood and contested, limiting their potential clinical application.
It has long been suggested that membrane-active AMPs induce membrane pores (4, 5, 6, 7), with the pore structure conforming to either the barrel-stave or toroidal type (8, 9). For example, alamethicin (ALM) (4, 9, 10) and pardaxin (6, 11) are thought to form barrel-stave pores in which the peptide monomers form tight cylindrical bundles, whereas AMPs such as protegrin-1 (12, 13), magainin (7, 12), human defensins (14), and frog caerins (15) form toroidal pores with curved peptide-lipid edges. However, direct observation to differentiate between these pore types for an AMP is difficult and largely limited to intensive x-ray and neutron scattering methods (8, 9, 12). Unlike AMPs that form pores of a well-defined size, AMPs that disrupt cell membranes through a nonpore mechanism, known as the carpet model, accumulate on the membrane surface and remain interfacially in contact with the lipid headgroups throughout the process of membrane destabilization, never inserting to span the bilayer in an organized fashion. Although it was originally proposed to describe the action of dermaseptin (16), an amphipathic α-helix rich in Lys that resembles magainin, the carpet model has been referenced to explain the activity of AMPs that are too short to span the entire bilayer (e.g., aurein and citropin) and thus are needed in higher concentrations to elicit membrane permeation (15, 17, 18).
With more than 1000 AMPs discovered to date (19), it is evident that these peptides vary widely in their primary sequences and adopt all kinds of secondary structures, including random linear, α-helical, and β-sheet motifs. This variety has prompted the development of a number of models to explain observations from the many techniques that have been used to investigate AMP interactions (20, 21). Relatively few AMPs have been thoroughly investigated; however, it would be a daunting task to categorize the activity of each AMP into a prevailing pore or nonpore model. Although sequence homology has been implicated in eliciting AMP membrane activity (22), the diversity of AMPs across species challenges our ability to predict key structure-function relationships when designing novel drugs. For this reason, attempts to achieve rational peptide design remain rare and have principally identified positive charge and amphiphilicity as key properties for AMP activity. These properties have encouraged the development of novel polymeric mimics that display selective antimicrobial activity across a variety of ordered and nonordered structures (3). Evolutionarily speaking, it would be costly (and thus unlikely) for each peptide to adopt its own membrane-disruption mechanism. Peptide plasticity would offer a clear evolutionary advantage in terms of both sequence and structure, allowing AMPs to utilize a single underlying mechanism in interacting with the membranes of various pathogens. If such a universal mechanism exists, we could improve the rational design of new antimicrobial agents by gaining a better understanding of the underlying physiochemical properties that govern a shared AMP mechanism.
We recently showed that the disruption of supported lipid bilayers by protegrin-1 (PG-1), an 18-residue, cationic, β-sheet AMP isolated from pig leukocytes, proceeded in a concentration-dependent manner (23, 24). The insertion of PG-1 into zwitterionic 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) bilayers resulted in membrane structural transformations that extended beyond simple pores and included instabilities at the bilayer edge and the presence of worm-like micelles at higher peptide concentrations. The spectrum of self-assembled structures showed that PG-1 acts to lower the interfacial line energy of the bilayer in a manner similar to that exhibited by amphiphilic detergents, which are capable of generating and stabilizing normally unfavorable line geometries (i.e., curved edges) in membranes. Classifying PG-1’s mechanism as a static pore former does not account for the full structural transformations we observed. Instead, PG-1’s activity should be reframed as a dynamic transition between different self-assembled structures that depends on the relative ratio between peptide and lipid. To this end, we have proposed that AMPs in general share this line-active behavior and act universally in a detergent-like manner.
In this work, we used atomic force microscopy (AFM) to image the structural transformations of supported DMPC bilayer patches induced by different AMPs (listed in Table S1 of the Supporting Material). The investigated AMPs are broadly active against many pathogens (see Table S2 for representative minimum inhibitory concentrations (MICs) against common pathogenic bacteria) and vary considerably in their secondary structures (Figs. S1–S4). By deliberately using a zwitterionic lipid rather than one with a charged headgroup, we were able to better identify other membrane attributes that dictate AMP interactions. We show that with the exception of ALM, the activity of the investigated peptides is driven by a common physical principle that reduces membrane line tension. Our findings indicate that line-active behavior is not dependent on the peptide charge, and we suggest instead that the activity originates from imperfect amphipathic structures, which usually position charged Lys and Arg residues at the bilayer interfaces to promote the creation of line geometries within the membrane. These line geometries facilitate binding of the peptides to the edge, which raises the peptide/lipid (P/L) ratio and structurally transforms the lamellar bilayer into micelles. Our results show that any barrel-stave-forming peptide would not be line-active, and that seemingly disparate peptides categorized into either the toroidal-pore or carpet model are actually related through this line-active model of AMP interaction.
Materials and Methods
Materials
Chemical reagents (certified ACS or BioReagent grade, purity ≥ 99%) and high-performance liquid chromatography-grade organic solvents were purchased from Fisher Scientific (Waltham, MA). Powder DMPC was purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. ALM was obtained as a 5 mg/mL stock solution in DMSO from Sigma-Aldrich (St. Louis, MO). Custom synthesis of aurein-1.1, dermaseptin-1, human β-defensin-1, histatin-2, indolicidin, and magainin-1 was done by Anaspec (Fremont, CA), and synthesis of caerin-1.3, citropin-1.1, kalata-B3, and pardaxin-1 was performed by bioWORLD (Dublin, OH). PG-1 synthesis was conducted in house and has been thoroughly described elsewhere (13). Peptide purity was assessed by high-performance liquid chromatography and determined to be ≥95% by peak area analysis. All aqueous solutions were prepared with ultrapure water (resistivity >18 MΩ⋅cm) from a Milli-Q Advantage A-10 purification system (EMD Millipore, Billerica, MA). Peptide stocks were prepared and quantified as described in Supporting Materials and Methods.
Membrane-disruption assay
Details concerning the preparation of large unilamellar vesicles (LUVs) and solid-supported phospholipid bilayers (SPBs) on mica are provided in Supporting Materials and Methods along with descriptions of the image acquisition, processing, and analysis. Briefly, DMPC SPB patches were imaged under buffer by tapping-mode AFM at 30°C before and after the addition of peptide. SPB patches were used instead of a contiguous membrane because the latter does not permit membrane expansion upon peptide insertion, and only increased membrane roughness has been reported for pore-forming AMPs such as PG-1 (25). The peptide was equilibrated with the bilayers for at least 15 min, after which structural changes of the same SPB patch were monitored. This protocol was repeated by sequentially adding increasing bulk peptide concentrations. During equilibration, the free peptides in the bulk adsorbed to the membranes until a surface partition equilibrium was achieved, resulting in the P/L being directly proportional to the bulk peptide concentration.
Results and Discussion
Peptide line activity induces membrane structural transformations beyond pores
We recently demonstrated a detergent-like behavior of PG-1 (23, 24) that induces membrane disruption through a range of structural transformations beyond pore formation. The structural transformations induced by PG-1 serve as a reference in the study presented here, which focuses on AMP line activity in general. We performed new image analysis in this study to calculate a bilayer shape factor (S) that is used to illustrate contrasting behavior between line-active and nonline-active peptides. In the absence of peptide (Fig. 1 A), the SPB patches were nearly round, with an average shape factor of S = 1.33 ± 0.17 (standard deviation (SD), n = 100) and displayed smooth edges from a high interfacial line tension. The average membrane thickness was 4.7 ± 0.7 nm (SD, n = 45), which is within error of our previous value (23, 24) and comparable to neutron scattering results obtained for DMPC SPBs and LUVs (26, 27). To prevent hydrophobic core exposure to the aqueous environment, lipids self-assemble at the edge to form a seamlessly curved cap (28). As lipids have an innate curvature, edge alignment causes clashing of the acyl chains and induces steric stress within this confining geometry. A penalty is exacted to maintain lipids in this less favorable state and manifests as a line energy, a one-dimensional (1D) equivalent of the familiar two-dimensional (2D) surface energy. The line tension then acts to minimize the overall energy of the system by reducing the ratio of bilayer perimeter length to surface area. The equilibrium size of an SPB patch is therefore determined by the balance of two forces: the line tension, which favors compact shapes, and a repulsive interaction from the phospholipid dipoles, which favors extended boundaries (29). Line-active agents (e.g., amphiphilic detergents) can adsorb to an exposed edge, reducing the line tension and stabilizing extended line geometries (e.g., curved edges like those of pores) (30, 31, 32, 33, 34). The presence of a line geometry provides a metric for assessing AMP line activity, although a bilayer edge is initially absent in vivo when an AMP encounters a target membrane.
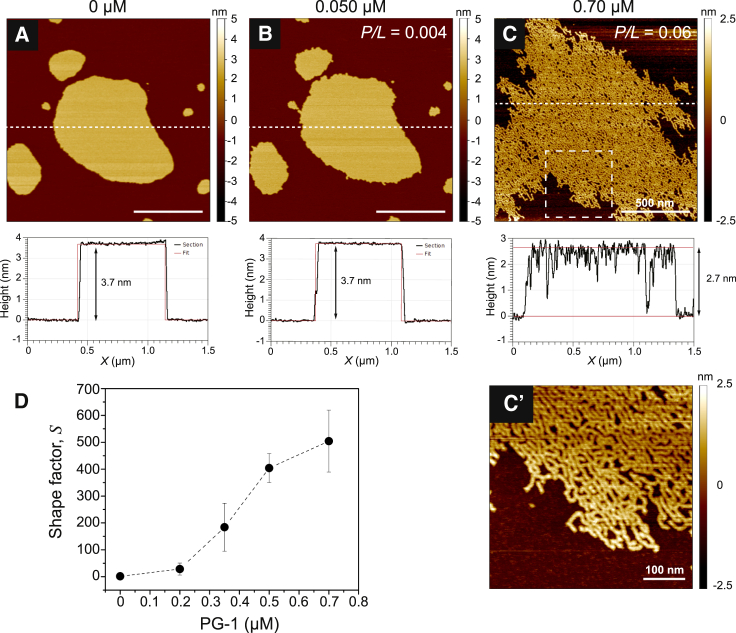
Figure 1.
DMPC membrane structural transformations induced by PG-1’s line-active behavior. (A) In the absence of PG-1, the bilayer was compact and nearly circular with a smooth and minimized edge. (B) Introduction of 0.050 μM PG-1 caused the bilayer to extend and become roughened, and this remained stable over time. Line sections (dashed white lines) of the bilayer before and after the introduction of 0.050 μM PG-1 showed that the lamellar core remained unaffected, and that PG-1 adsorption at the curved edge resulted in a reduction in the bilayer line tension. (C) With increasing bulk PG-1 concentrations, the lamellar organization of the bilayer was compromised by 0.70 μM PG-1, with the formation of worm-like micelles. Peptide insertion thinned the membrane considerably (by ∼1 nm). To better reveal the bilayer’s transformation in (C), the height data were rescaled to a 5 nm range. The dashed box in (C) indicates a zoomed-in region (500 × 500 nm2) that is shown in (C′). (D) A shape factor analysis of the bilayer morphology (S) as a function of the bulk PG-1 concentration showed that PG-1 had a considerable effect in promoting extended morphologies with increased perimeter lengths beyond initial values near S = 1. Error bars in (D) are SDs from at least three replicate bilayer monitoring experiments. All images were obtained at 30°C. White scale bars are 500 nm unless otherwise indicated. To see this figure in color, go online.
As shown in Fig. 1 B, a low concentration of PG-1 (0.050 μM) caused the bilayer boundary to extend and become destabilized from its once smooth contour, whereas the bilayer core remained undisturbed with no discernible pore formation. Line sections confirmed that the bilayer center remained flat and did not alter in thickness with the addition of PG-1. The calculated shape factor increased from an initial, nearly circular value (S ≈ 1) to S = 1.94, confirming that an extended boundary had resulted from the presence of peptides. This suggested that the curved bilayer edge was more susceptible to initial PG-1 binding as compared with the lamellar region of the bilayer patch, as the line sections revealed no discernible membrane height change. The stability of the extended morphology resulted from an apparent reduction in the line tension caused by PG-1’s line-active behavior. Indeed, recent molecular dynamics (MD) simulations have shown that PG-1 readily adsorbs to curved edges (35) and lowers line tensions in a concentration-dependent manner (24).
PG-1 interaction within the bilayer core also caused remodeling of the membrane structure. New edges were created within the patch, presumably from the initial formation of toroidal pores, which facilitated favorable binding of more PG-1, further lowering the line tension and allowing porous defects to grow to an observable size (Fig. S5 A). This edge extension allowed for yet more binding of PG-1 and ever-expanding porous defects (Fig. S5, B and C), culminating in the complete structural transformation of the lamellar bilayer into a network of worm-like micelles by 0.70 μM PG-1 (Fig. 1 C). The rise of S as a function of bulk PG-1 concentration (Fig. 1 D) revealed a considerable expansion of the bilayer boundary and demonstrated the accelerating loss of membrane integrity that coincided with PG-1’s line-active behavior. A relative height change between the data in Fig. 1, A and C, showed a thinning of nearly 27%. This agrees with the work by Huang and coworkers in which they established a relationship between the insertion state of AMPs and the degree of elastic membrane deformation by showing that various AMPs, including ALM (36), magainin-2 (37), and PG-1 (38), caused membrane thinning in direct proportion to the peptide concentration. The lipid-peptide self-assembled structures that formed were identical to those that formed between bilayer-forming lipids and amphiphilic detergents such as the short-chain lipid dihexanoylphosphatidylcholine (39) and sodium cholate (40). The variety of structural transformations observed in these studies shows that PG-1’s antimicrobial activity is similar to the behavior of a detergent adsorbing to an interface, in the manner of a Gibbs adsorption isotherm (32). Therefore, we propose that membrane-active AMPs act universally within a detergent-like framework in which pore formation is not the defining mechanism but instead represents a small part of a complex process that encompasses lipid-peptide aggregates in addition to lamellar bilayers.
Barrel-stave-forming peptides exhibit nonline-active behavior
For comparison with PG-1’s line-active behavior, we assessed the interaction of another common pore-forming AMP, ALM, whose barrel-stave pore has been well characterized. Fig. S1 contrasts the secondary structure of ALM with that of PG-1, showing that ALM achieves an ideal, amphipathic helix, whereas PG-1 lacks discrete, separated faces of hydrophilic and hydrophobic residues. The concentration-dependent interaction of ALM with DMPC bilayers is shown in Fig. 2. As the ALM concentration increased, the bilayer patches in Fig. 2 A expanded and fused upon contact (Fig. 2 B) to result in a single bilayer patch by 25 μM (Fig. 2 C) that continued to grow laterally (Fig. 2 D). A control injection mimicking that of 50 μM ALM (Fig. S6) showed no large-scale membrane expansion and ruled out possible effects from the traces of DMSO and methanol present in the ALM stock. Assuming lipid conservation between injections, membrane expansion coincides with peptide insertion as additional molecules are progressively added. When a peptide partitions to the bilayer, the lipid acyl chain volume remains constant; therefore, the expansion coincides with a decrease in the membrane thickness (41). From a comparison of the micrographs in Fig. 2, it is evident that the membrane thinned in a concentration-dependent manner, and a line section analysis showed that by 50 μM (Fig. 2 D) the membrane was relatively thinner (by 25%). As with PG-1, membrane thinning is an indication of peptide insertion and is a documented trait of ALM’s interaction with membranes (36).
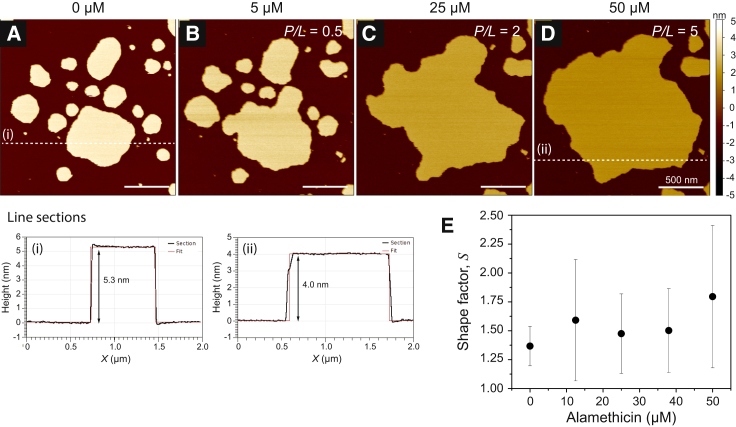
Figure 2.
Concentration-dependent interaction of ALM with DMPC bilayer patches. (A) The unperturbed bilayer patches in the absence of ALM displayed smooth edges and compact shapes, as shown in Fig. 1A. (B–D) Through the course of successive injections, the concentration of ALM was raised from 5 μM (B) to 25 μM (C) and finally to 50 μM (D). In contrast to the structural transformations induced by PG-1, ALM had a negligible effect on the bilayer morphology and instead only caused gross lateral expansion of the bilayers. Line sections (dashed white lines) revealed progressive membrane thinning, presumably from peptide insertion, although pores were not observed. (E) Shape factor analysis as a function of increasing ALM concentration. Error bars in (E) are SDs from five replicate bilayer monitoring experiments to confirm repeatability. All images were obtained at 30°C. White scale bars are 500 nm. To see this figure in color, go online.
Pores were not detectable via AFM imaging, as the diameter of the ALM pore was smaller than the quoted tip radius of 8 nm and thus too small to resolve. Using a calcein-leakage assay (see Supporting Materials and Methods), we confirmed ALM-induced pore formation in our DMPC bilayer system, which contributed to the bilayer expansion observed in Fig. 2. The degree of ALM-induced leakage (shown in Fig. S7 A) was greater than the background leakage of dye-loaded DMPC vesicles alone. The fraction of released dye and the apparent rate, kapp, of the efflux (Fig. S7 B) increased in a concentration-dependent manner, eventually reaching complete leakage. The leakage profiles fit well to a double-exponential decay function, from which we extract the rates of contributing processes, including pore formation. In a double-exponential model, the overall leakage profile must be a combination of at least two simultaneous kinetic processes. As previously described for several membrane-active peptides and polymers, the leakage mechanism is assumed to involve one kinetic process at a rate of k1, which causes only transient leakage and approaches a finite value over time, and a second process at a rate of k2, which causes unlimited leakage through stable pore formation (42, 43, 44). At the low concentration of 0.05 μM ALM, the leakage profile (Fig. S7 A) reached a maximum leakage near 70% and thus was dominated more by the transient leakage process. As the ALM concentration increased, the second process began to dominate and ultimately led to complete vesicle leakage. Fig. S7 C shows the amplitude of the second leakage process as a function of the rate constant k2. ALM-induced pore formation dominated the leakage profile as the concentration increased, since the probability of pore formation must increase in a concentration-dependent manner. Although ALM pores were too small to visualize by AFM, they were present at the higher concentrations shown in Fig. 2 and thus contributed to the observed bilayer expansion. The interaction was devoid of the membrane structural transformation seen with PG-1 (Figs. 1 and S5). Dynamic light scattering (Fig. S8) showed that the addition of ALM did not disrupt the dye-loaded LUVs, as only single peaks were obtained, indicating intact vesicles with a homogeneous size distribution. An increase in the LUV diameter from 122 to 173 nm occurred at a P/L ratio of ∼0.3 (50 μM ALM) and corroborates the onset of membrane expansion observed in the AFM measurements at a similar P/L ratio (Fig. 2 B).
Fig. 2 E shows the bilayer shape factor as a function of ALM concentration, averaged across several experiments. The shape factor remained invariant with increasing concentrations, indicating that ALM’s insertion had no pronounced effect on the promotion and stabilization of extended bilayer morphologies. This trend is contrary to the case with PG-1 (Fig. 1 D), where a persistent change in bilayer morphology indicated a considerable expansion of the bilayer boundary, supporting the line-active behavior of PG-1. We conclude that ALM interacts with the membranes in a nonline-active way. This conclusion is supported by the different pore structure formed by ALM compared with PG-1. In the barrel-stave configuration, ALM monomers tightly associate to form a cylindrical pore without a curved edge as a result of their high hydrophobicity and lack of charged residues along the helix. The hydrophobic face of the ALM helix, to a first approximation, assumes the shape of a wedge, and thus only a finite number of peptide monomers can favorably associate to create such a tight circular bundle. This constraint on the aggregate size of the pore places an upper limit on the size to which an ALM pore can grow. Consequently, ALM does not favorably permit bilayer edge growth, which explains the invariant shape factor trend (Fig. 2 E) and the retention of bilayer integrity seen in both AFM (see Fig. 2) and dynamic light scattering (see Fig. S8) measurements across a large concentration range. In contrast, the greater polarity of PG-1 necessitates peptide spacing along an edge with lipid headgroups dispersed in between. These lipids in turn must curve from one leaflet to the other to accommodate the polar and apolar regions of the peptide. Toroidal pores can vary in size and grow in diameter as more peptides bind to the edge. In the case of PG-1, this behavior resulted in the growth of defects to the extent that the bilayer was structurally transformed into worm-like micelles.
Recent simulations have shown that many AMPs bind more strongly to curved membrane edges (24, 35, 45, 46). Using nanoscopic secondary ion mass spectrometry imaging, Rakowska et al. (47) showed that the peptide amhelin was enriched at the membrane edge and could induce the growth of unusually large defects, several microns in diameter. The contrasting results for PG-1 and ALM suggest that line activity may depend on the overall charge of the peptide and an interplay between hydrophilic and hydrophobic residues. To address this issue, we tested 11 other peptides (listed in Table S1) that differed in charge and amphipathic secondary structures to ascertain their line-active ability.
The concentration response of line activity is invariant with peptide charge
Within a line-active model for AMP behavior, we assume that peptides with increasing charge, either positive or negative, should be more disruptive than neutral peptides. The size of a bilayer patch is determined by a balance between the opposing forces of line tension and electrostatic repulsion. Peptide adsorption charges the membrane, intensifying the electrostatic repulsion, and when this is coupled with line-active behavior, the compact shape of the bilayer becomes unstable and displays extended morphologies. In effect, the instabilities observed in these 2D membranes are related to the more common 3D case of the Rayleigh effect, which describes shape instabilities in spherical drops of increasing charge.
Figs. 3, 4, and 5 show the concentration-dependent interactions of charged and neutral AMPs. Most AMPs are predominantly cationic, and this characteristic has long been used as a simple electrostatic argument for AMP selectivity toward pathogens, as prokaryotic membranes are largely negatively charged and mammalian membranes are largely zwitterionic. Fig. 3 shows that the positively charged peptides aurein-1.1 (Fig. 3 A), citropin-1.1 (Fig. 3 B), magainin-1 (Fig. 3 C), dermaseptin-1 (Fig. 3 D), indolicidin (Fig. 3 E), and human β-defensin-1 (HBD-1) (Fig. 3 F) clearly display line activity that causes the membrane to undergo structural transformations similar to those observed with PG-1. The line-active behavior of the cationic peptides is most notable in Fig. 3, A2, B2, C2, D2, E2, and F2, which show edge instability at low peptide concentrations. We observe that AMPs that are thought to interact only according to the carpet model (e.g., aurein-1.1, citropin-1.1, and dermaseptin-1) result in the same final micellized state as peptides that insert into and span the membrane, forming a transmembrane pore (e.g., PG-1, magainin-1, indolicidin, and HBD-1). This suggests that these two models share an underlying ability to lower line tension that drives peptide-induced membrane transformations. Moreover, we show that this commonality extends to negatively charged (Fig. 4) and neutral (Fig. 5) AMPs.
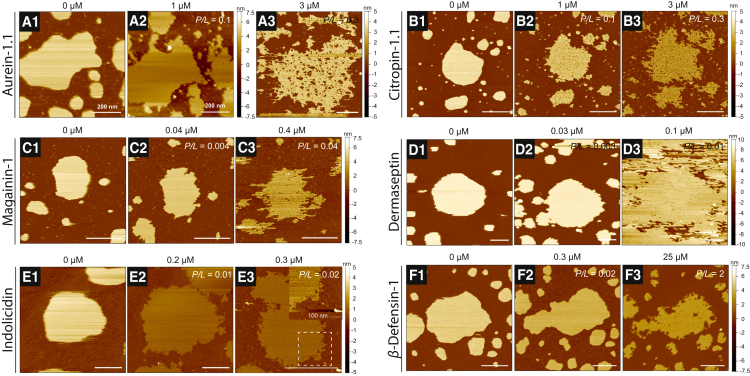
Figure 3.
(A–F) Membrane structural transformations of DMPC bilayer patches induced by the positively charged AMPs aurein-1.1 (A), citropin-1.1 (B), magainin-1 (C), dermaseptin-1 (D), indolicidin (E), and HBD-1 (F). Bilayer patches were first imaged in the absence of peptide (A1, B1, C1, etc.) and then monitored through the course of successive peptide concentrations (increasing from left to right). (A) Increasing concentrations of aurein-1.1 revealed edge instability and a porated membrane (A2). Micrograph (A3) is from a separate experiment showcasing worm-like micelle formation by 3 μM aurein-1.1. (B) Citropin-1.1 exhibited a similar response to aurein-1.1, displaying edge instability and a porated membrane (B2), with worm-like micelle formation (B3) occurring by 3 μM. The concentration responses of magainin-1, dermaseptin-1, and indolicidin were similar, as the onset of micellization (C3, D3, and E3) occurred in the same range below 0.5 μM. The bilayer patch monitored through images (E1) and (E2) was inadvertently smeared, and a nearby patch (E3) on the substrate was chosen to obtain a high-resolution image of worm-like micelles induced by indolicidin. To better reveal the self-assembled structures, the dashed box in (E3) indicates a zoomed-in region shown in the inset (data scaled to a 5 nm range). (F) Although bilayer edge instability was observed at 0.3 μM HBD-1 (F2), a significantly higher amount of peptide was needed to observe detergent behavior (F3). All images were obtained at 30°C. White scale bars are 500 nm unless otherwise indicated. To see this figure in color, go online.
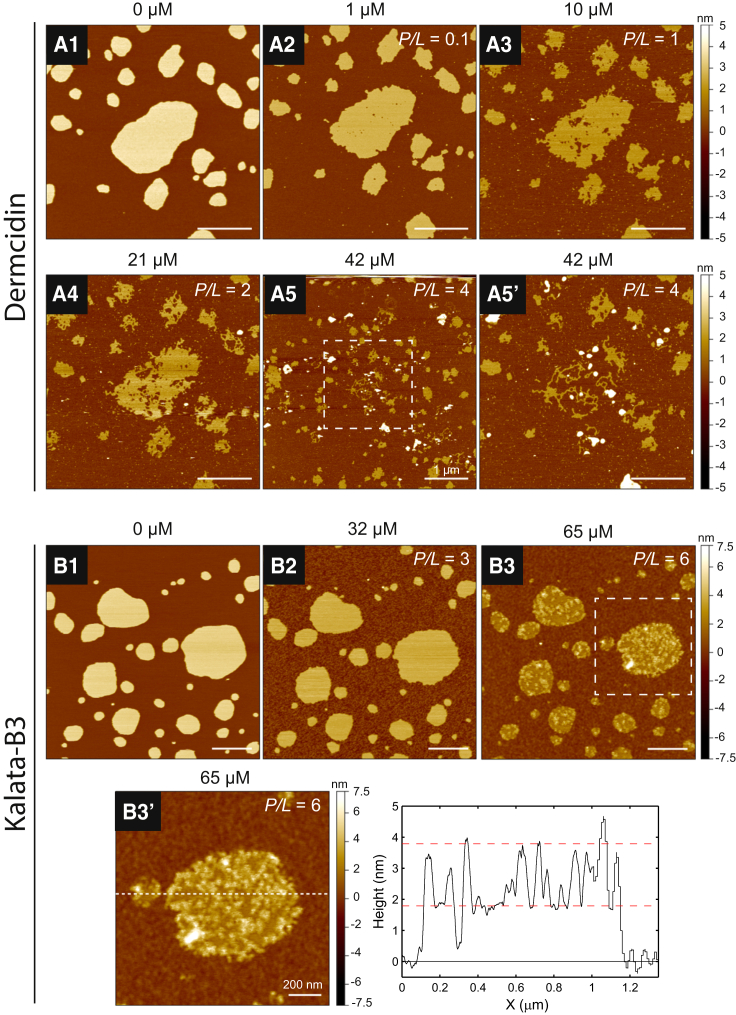
Figure 4.
(A and B) Membrane structural transformations of DMPC bilayer patches induced by the negatively charged AMPs DCD (A) and KB3 (B). Bilayer patches in the absence of peptide (A1 and B1) were monitored through the course of increasing peptide concentrations. (A) DCD exhibited a noticeable line-active behavior from an observed expanded bilayer edge (A2). The number and size of porous defects within the bilayer core grew (A3 and A4), and eventually the integrity of the membrane was fully compromised as micellization resulted (A5). (B) KB3 exhibited less activity than DCD. Bilayer edge instability was not observed until 32 μM KB3 (B2). Detergent behavior was less evident and the membrane was decorated with peripherally bound peptide (B3). A line section in (B3′) shows that the features are ∼2 nm high from the surrounding surface. The dashed boxes in (A5) and (B3) indicate zoomed-in regions that are shown in (A5′) and (B3′). All images were obtained at 30°C. White scale bars are 500 nm unless otherwise indicated. To see this figure in color, go online.
Figure 5.
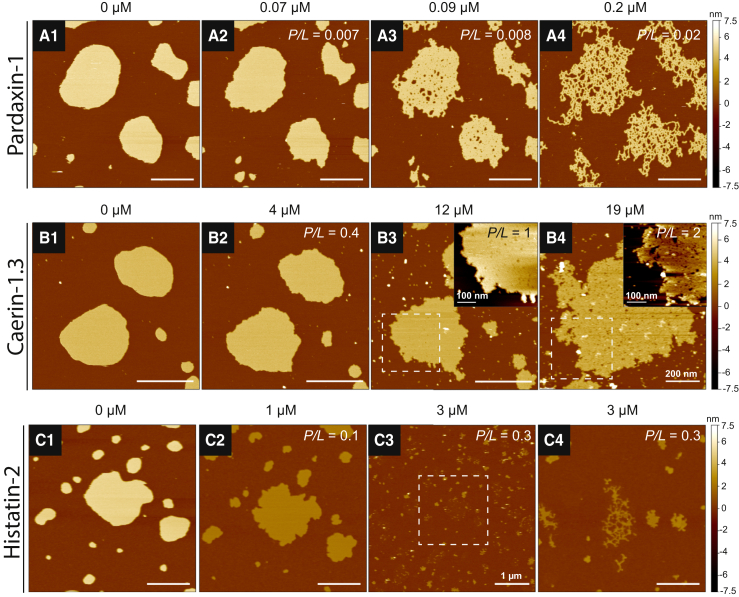
(A–C) Membrane structural transformations of DMPC bilayer patches induced by the neutral AMPs pardaxin-1 (A), caerin-1.3 (B), and histatin-2 (C). Bilayer patches were first imaged in the absence of peptide (A1, B1, and C1) and then monitored through the course of successive peptide concentrations (increasing from left to right). (A) With increasing pardaxin-1, the bilayer underwent structural transformations exhibiting edge instability (A2), porous defects (A3), and finally worm-like micelles (A4) within a low concentration regime similar to that of PG-1, magainin-1, dermaseptin-1, and indolicidin. (B) The line activity of caerin-1.3 was evident from the bilayer edge instability observed at 4 μM (B2) that caused porous defects to form within the core of the bilayer (B3) and led to micellization (B4). The bilayer patch monitored through images (B1)–(B3) was damaged from its softened state, and a nearby patch (B4) on the substrate was chosen to obtain a high-resolution image of the micellized state at 19 μM caerin-1.3. To better reveal caerin-induced membrane transformations, the dashed boxes in (B3) and (B4) indicate zoomed-in regions shown in the inset (data scaled to a 5 nm range). (C) Edge instability and membrane thinning were observed upon introduction of 1 μM histatin-2 (C2). (C3) Zoomed-out image showing bilayer solubilization in the surrounding area. A dashed box is included to indicate the scanning area corresponding to images (C1) and (C2). The sample was translated laterally to a new area (C4) to capture the presence of worm-like micelle structures. All images were obtained at 30°C. White scale bars are 500 nm unless otherwise indicated. To see this figure in color, go online.
Distinct from the majority of cationic AMPs, dermcidin (DCD), a flexible peptide from human sweat that bears a helix-hinge-helix motif (48, 49), and kalata-B3 (KB3), a plant cyclotide that displays a knotted topology of three disulfide bridges (50), are AMPs with net negative charges at physiological pH, and represent important exceptions to the prevailing electrostatic model of AMP selectivity. Both DCD and KB3 are broadly active against many prokaryotic pathogens despite having noncanonical negative charges (48, 51), and are speculated to form multimeric pores when permeabilizing pathogens (52, 53, 54, 55). Paulmann et al. (52) showed that DCD preferentially forms a helical structure with negatively charged phosphoglycerol over zwitterionic phosphocholine membranes. High ionic strength and the presence of divalent cations, specifically Zn2+, were shown to enhance DCD’s channel-like behavior in membranes, to support a pore-forming model. Recently, Song et al. (53) posited that DCD forms a hexameric, barrel-stave channel in POPE/POPG membranes.
Our experiments suggest that a barrel-stave pore-forming model does not account for DCD’s behavior. Fig. 4 A shows the membrane interaction of DCD as the bulk concentration was increased. Upon injection of 1 μM DCD (Fig. 4 A2), a distinctive line-active behavior and several defects were observed within the bilayer core at this low concentration. Insertion of DCD into the DMPC bilayer was evidenced by a relative thinning of the membrane as the bulk concentration was increased (Fig. 4, A1–A5). The bilayer edge continued to expand and the number and size of porous defects within the lamellar core increased, providing more edges to which DCD could adsorb. Ultimately, the detergent behavior of DCD resulted in micellization of the membrane into the bulk superphase at a final concentration of 42 μM (Fig. 4 A5), where remaining lipid/peptide material on the mica surface assembled into worm-like micelles (Fig. 4 A5′). Clearly, DCD’s action required a line geometry for favorable binding to cause such degradation of membrane integrity. Using AFM, Paulmann et al. (52) observed defect formation in supported POPC membranes when 2 μM DCD was present. However, their study was limited to a single concentration and no detergent behavior was observed, precluding a line-active explanation for DCD’s behavior. It is possible that the contiguous membrane used in their study prevented peptide insertion, since room for bilayer expansion was constrained. As ALM did not show line activity, we concluded that a barrel-stave pore is unlikely for DCD, given the observed membrane disruption in our study.
In contrast, the onset of KB3 activity with DMPC membranes (Fig. 4 B) was shifted to a higher dosage regime as compared with DCD, as no significant bilayer changes were observed below 32 μM. The membrane interaction of kalata cyclotides has a lipid headgroup preference, and a higher binding affinity has been observed with phosphoethanolamine compared with phosphocholine lipids (55, 56). This helps to explain why higher amounts of KB3 were needed for us to see detergent-like behavior in our experimental system. Upon incubation with 32 μM KB3 (Fig. 4 B2), membrane thinning and disruption at the bilayer edge were observed. Additionally, the surrounding bare mica surface was covered by the apparent, nonspecific adsorption of the peptides. The line activity we observed for KB3 does not agree with the AFM results of Hall et al. (56), who reported that no bilayer structural transformations were seen in DMPC membranes upon addition of 10 μM kalata-B1. At a concentration of 65 μM KB3 (Fig. 4 B3), the surface of the bilayer became decorated with aggregates, presumably made of peptide, that were ∼2 nm high from the undecorated portion of the bilayer. Kalata peptides can self-associate to form tetramers in solution, suggesting that an oligomerized state may be important for their membrane binding and possible pore formation (57). Using coarse-grained MD simulations, Nawae et al. (58) showed that kalata-B1 remained peripherally bound and did not penetrate deeply into the membrane to form well-organized pores. Instead, the cyclotides clustered on the surface, resulting in curvature deformation of the membrane. The low penetration depth is likely a consequence of the facial organization of the hydrophobes (Fig. S3 B) that creates a shallow surface for membrane insertion, a trait of kalata cyclotides (50). Moreover, Nawae et al. (58) reported that the center of mass of kalata-B1 on the membrane was on average 1.9 nm from the center of mass of the lipid headgroups. Our line section analysis resulted in a nearly identical value and supports the conclusion that the aggregates on the membrane (Fig. 4 B3) are interfacially bound KB3 peptides. Subsequent time-lapse imaging (Fig. S9) revealed slow membrane solubilization into the superphase.
We have shown that both positively and negatively charged AMPs share a line-active behavior in their mechanism for inducing membrane disruption. Curiously, lower-charge peptides and higher-charge peptides were observed to disrupt membranes at similar concentrations. This was especially clear when the interactions of magainin-1 (+3), dermaseptin-1 (+3), and indolicidin (+4) were juxtaposed with those of PG-1 (+7), as all reached a final micellized state at a maximum concentration of 1 μM. Although HBD-1 has a +4 charge, much higher peptide concentrations were required to completely disrupt the membrane, and its response even differed by two orders of magnitude when compared with indolicidin (+4).
The apparent lack of a charge-dependent effect on the concentration response of the peptides was further reinforced when we examined the interaction of neutral AMPs (Fig. 5). Pardaxin-1 and caerin-1.3 share similar secondary structures characterized by helix-hinge-helix motifs (59, 60) and are thought to form barrel-stave pores (pardaxin) and toroidal pores (caerin) (11, 15). The exact structure of histatin-2 when membrane bound remains unknown; however, work on the related histatin-5 indicates that C-terminal α-helical content is important for its bactericidal activity (61). A putative zinc-binding motif has been implicated in the stabilization of secondary structure in histatins in the presence of negatively charged membranes (62). Mechanistically, the membrane interaction of histatins remains largely unknown, and our AFM results represent a unique investigation of these poorly studied AMPs. Fig. 5, A2, B2, and C2, show that pardaxin-1, caerin-1.3, and histatin-2 display characteristic line-active behavior, as evidenced by the morphological changes that occurred at the bilayer edge. Increasing concentrations led to the formation of porous defects (Fig. 5, A3 and B3) and eventually to complete disruption of the bilayer patches. Zemel et al. (63) showed computationally that neutral to weakly charged peptides form barrel-stave pores and that higher peptide charges are needed to favor toroidal-pore formation, suggesting that line activity is only present with charged peptides. Our investigations reveal that, with the exception of ALM, all of the AMPs we studied exhibited line activity despite having different charges and concentration responses.
Molecular origins that drive line-active behavior
Given the ability of AMPs to lower line tension independently of charge, it is energetically favorable for the peptides to bind at the edge, increasing the P/L ratio in the membrane that drives structural transformations. A potential molecular origin of line activity is the adoption of amphipathic secondary structures. Segregation of hydrophobic and hydrophilic amino acid residues in spatially arranged faces affords AMPs the ability to favorably partition and insert into membranes. For instance, the disulfide bonds present in β-sheet peptides, such as PG-1 and human defensins, create stable amphiphilic structures, as a reduction of these covalent linkages was shown to diminish their membrane lytic activity (64, 65). The interfacial activity model proposed by Wimley (66) emphasizes the altered packing and organization of lipids that results from imperfect amphipathic secondary structures. These imperfect structures can be consequences of poor segregation of hydrophilic/hydrophobic residues or deviations of the peptide from ideal secondary structures. They distort the membrane in a way that accommodates the apolar, polar, and charged groups of the peptide distributed along the structure. The emphasis on altered packing and organization of lipids upon peptide partitioning accords with the work of Huang and co-workers (36, 37, 38), which showed that surface-bound AMPs elastically thin membranes, and with studies by Bechinger (67) and Zemel et al. (68) that correlated peptide shape with the induction of membrane curvature strain. In essence, AMPs can be assumed to have intrinsic curvatures (e.g., a wedge), and their incorporation into the membrane can transform it into different structures limited by the molecular dimensions of the peptide. This is related in part to the behavior of proteins in the Bin/Amphiphysin/Rvs-domain superfamily, variants of which display positive or negative spontaneous curvatures that are needed to stabilize common cell membrane topologies found in endo- and exocytotic events and in membrane protrusions (69).
Using MD simulations, Lazaridis and coworkers (35, 45, 70) convincingly showed the effects that imperfect amphipathic structures have on the generation of barrel-stave or toroidal pores. For instance, when they investigated K7Q or K7A in silico mutants of melittin, they found that preformed barrel-stave pores did not evolve into toroidal pores. In contrast, when Gln7 was mutated to Lys in ALM, barrel-stave pores were not stable and toroidal pores were formed. These results indicated that a nonuniform charge distribution along the peptide structure is a critical factor in organizing phospholipids into the curved configuration found in toroidal pores (70). Moreover, when melittin’s structure was made to be a more ideal, linear helix through Leu mutation of two charged residues found along its hydrophobic face, the initial cylindrical pore remained stable. This finding suggested that the more-ideal amphiphilic structure deterred the induction of membrane curvature. Combined, these results implicate the overall peptide structure and the distribution of charged residues in line-active behavior.
Many of the peptides we investigated displayed an imperfect amphiphilic structure (Figs. S1–S4) resulting from poor segregation of apolar and polar residues, or exhibited deviations from ideal secondary structural motifs, such as the breaking of an ideal helix into more flexible helix-hinge-helix constructs. A comparison of indolicidin and PG-1 demonstrates the effect that an imperfect amphiphilic secondary structure has on line activity. Although they differ in charge and structure, they both transform the membrane into worm-like micelles within almost identical concentration regimes. Whereas indolicidin assumes a random, linear conformation, PG-1 has a well-defined β-hairpin; however, both share a similar planar amphiphilicity with a central hydrophobic zone bounded by clusters of Arg residues (Figs. S1 B and S2 E). Tang et al. (71) showed that for an inserted state, the Arg residues of PG-1 make proximal guanidinium-phosphate connections that may underlie the lipid orientational changes necessary for the formation of toroidal pores. Hence, the line-active behavior we observe for both indolicidin and PG-1 may depend on the terminal placement of these residues so that they can interact simultaneously with both membrane leaflets.
The line activity of the α-helical peptides studied here seems to be governed by their ability to achieve imperfect helical arrangements that optimize polar and apolar interactions across the membrane. A flexible hinge region bounded by Pro15 and Pro19 in caerin-1.3 (60) and the subtle bend in magainin-1 from a Gly(X4)Gly motif, which is known to arc related piscidin peptides (72), cause both caerin-1.3 and magainin-1 to adopt curved architectures necessary for their biological activity. For example, Fernandez et al. (73) showed that in the caerin-related peptide maculatin-1.1, mutation of the arc-inducing Pro15 residue lowered its membrane-disrupting capabilities. The Gly(X4)Gly motif is also seen between Gly18 and Gly23 in the primary sequence of dermaseptin-1, where four Lys residues span the length of the structure. Additionally, Mihajlovic and Lazaridis (70) noted that Lys4 in an analog of magainin is a more important residue for toroidal-pore formation than the other lysines positioned in the middle of the helix (Lys11 and Lys14), and implicates the N-terminal Lys in the line activity of both magainin-1 and dermaseptin-1. We observed that magainin-1 and dermaseptin-1 caused membrane micellization at nearly the same concentration, whereas aurein-1.1 and citropin-1.1 interacted at slightly higher concentrations. This suggests that peptides that can easily insert into and span the length of a membrane are more effective at forming line geometries, as key residues are positioned to facilitate distortion of the membrane into a curved edge. The helix-bend-helix motifs present in pardaxin and DCD ensure that a C-terminal portion of the peptide can span a membrane and an N-terminal helix can lie along the membrane surface (52, 59). As can be seen in the secondary-structure representation for both DCD and pardaxin-1 (Figs. S3 A and S4 A), this break in the ideal helical structure primes the N-terminal Lys residue at the headgroup interface through surface-lying helices. Although the exact structure of histatin-2 remains to be determined in a membrane-mimetic environment, it is suggestive that both Lys and Arg residues (Arg1, Lys2, Lys6, and Arg11) are clustered within the N-terminal half of the peptide.
The guanidinium group of Arg has been proposed to be a greater inducer of membrane curvature than the primary amine group of Lys, given its bidentate hydrogen binding (14). β-sheet-containing peptides have a higher Arg content as compared with α-helical peptides, and when we examined the interaction of HBD-1 and KB3, we found that both were less line-active than PG-1 and indolicidin, as higher concentrations were needed to elicit an interaction with DMPC bilayers. The lower activity of HBD-1 and KB3 may result from a more diffuse distribution of charged residues and the presence of a flat, hydrophobic face, which, as noted in the case of KB3, presumably keeps the peptides peripherally bound. Schmidt et al. (14) suggested a condition in which peptides with lower Arg contents can still induce curved membrane topologies by offsetting to higher contents of Lys and hydrophobic residues. Fig. S10, A and B, show the primary sequence analysis of our line-active peptides, which we performed in a manner similar to that described by Schmidt et al. (14) and Mishra et al. (74). Although our sample size is small, we are in agreement that the α-helical AMPs examined tend to have higher average hydrophobicities and greater Lys contents as compared with their β-sheet counterparts (Fig. S10 A). Moreover, peptides that are line-active at lower concentrations have higher fractions of total Lys and Arg residues in their primary sequence (see Fig. S10 B). However, there are exceptions to the rule: caerin-1.3 has no positive residues within its primary sequence but can still impart its line activity within a moderate concentration regime, and HBD-1 has a fairly high fraction of total positive charges and requires higher concentrations as compared with other positively charged AMPs to cause complete membrane disruption. Theoretically, peptides with nearly identical Kyte-Doolittle hydropathy values would partition similarly to zwitterionic DMPC bilayers; however, the line activities of PG-1 (−0.25), KB3 (−0.20), and HBD-1 (−0.27) display drastically different concentration responses. Our examination suggests that a simple categorization of AMPs based on their overall Arg and Lys contents may not be a direct indicator of their eventual membrane line activity. Instead, our data show that membrane distortion results from a more complex interplay between peptide structure and the distribution of these key residues.
Conclusions
Collectively, our results show that AMPs share a common ability to reduce membrane line tension in a concentration-dependent manner that is physically similar to the action of simple detergents. Reduction of the line tension from the edge adsorption of these peptides (referred to as linactants due to their line activities) causes the edge contour to expand, and in turn enables further binding events at the edge that increase the P/L ratio in the membrane. With further increases in the bulk peptide concentration, the accumulated surface density is able to reach a critical stage for peptide insertion into the bilayer. The critical concentration necessary for AMP insertion would therefore be dependent on factors that modulate peptide partitioning to the membrane, such as lipid headgroup specificity (75, 76), packing defects (77, 78), and line interfaces at phase boundaries (79, 80, 81). The creation of new edges within the bilayer facilitates the binding of more AMPs, further reducing the bilayer line tension. Ultimately, the lamellar organization of the membrane is transformed into new peptide-lipid self-assembled structures that are limited in scale by the molecular dimensions of the peptide itself. Although an exposed edge does not initially exist in a cell membrane, this positively reinforcing condition will become evident once an initial edge geometry is created within the membrane from peptide insertion. The self-assembled structures that we observed through the course of AMP-induced membrane disruption, culminating in the formation of worm-like micelles, could not be explained by a pore-only model. Rather, a common reduction of line tension may underlie AMP activity and appears to be biologically relevant, as membrane protrusions resembling worm-like micelles have been observed to emanate from AMP-exposed bacteria (82, 83, 84).
Careful selection of a variety of peptides that differ in both charge and secondary structure revealed important physical parameters that affect the observed line-active behavior. With the exception of the most hydrophobic peptide, ALM, which exhibited no line activity, more polar peptides that exhibited line activity were surprisingly invariant in their concentration response with respect to their overall charge. The ability of positive, negative, and neutral AMPs to utilize a common line-active mechanism in their interaction with membranes challenges the prevailing electrostatic model of AMP selectivity and its general application to all membrane-active AMPs. Instead, factors such as membrane fluidity and lipid acyl chain length are relevant parameters that modulate the ability of AMPs to distort the membrane into the geometries necessary for their favorable incorporation into the membrane.
The line-active behavior of the AMPs correlated with their adoption of imperfect secondary structures resulting from either poor amphiphilic segregation of residues or breaks in the symmetry of ideal secondary motifs, such as flexible kinks introduced into linear helices. As a consequence of these structural imperfections, polar residues (typically Lys and Arg) were strategically placed at the periphery of the membrane for subsequent reorganization of the phospholipids. Moreover, the type of secondary motif employed by an AMP did not ultimately determine its line activity, as peptides with α-helical, β-sheet, and even random, linear backbone configurations (e.g., indolicidin) exhibited similar detergent-like behavior. We speculate that this interplay of peptide structure and the manner in which polar residues are distributed in relation to the membrane governs the line activity of AMPs. Future experiments to investigate the positional placement of Lys and Arg residues along a given AMP would clarify the role that residue distribution has in modulating line activity. Additionally, by systematically altering the degree of rotational amphiphilicity present in antimicrobial polypeptides, as recently developed by Xiong et al. (85), the role of imperfect amphipathicity in peptide structures can be more definitively correlated with AMP line activity. Future work should also examine the role of negatively charged residues in line-active behavior, given that caerin-1.3, lacking both Lys and Arg residues, exhibited line activity within a moderate concentration regime.
Author Contributions
J.M.H. and K.Y.C.L. conceived the study and designed the experiments. J.M.H. performed the research and wrote the manuscript with contributions from A.J.W., F.S., and K.Y.C.L. All authors discussed the results and commented on the manuscript.
Acknowledgments
We thank Drs. Justin Jureller and Qiti Guo (The University of Chicago Materials Research Science and Engineering Center) for their training and help in using the Asylum Cypher ES atomic force microscope, and their general expertise in scanning probe microscopy throughout the course of this research. We also thank Dr. Elena Solomaha (Biophysics Core, The University of Chicago) for her assistance in data collection in the calcein leakage experiments.
This research was supported by the National Science Foundation (NSF; MCB-1413613) and the NSF-supported MRSEC program at the University of Chicago (DMR-1420709). The Asylum Cypher ES atomic force microscope was made possible by an NSF Materials Research Instrumentation Grant (DMR-1429550).
Editor: Paulo Almeida.
Footnotes
Supporting Materials and Methods, ten figures, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30888-8.
Supporting Material
References
- 1.Hughes J.M. Preserving the lifesaving power of antimicrobial agents. JAMA. 2011;305:1027–1028. doi: 10.1001/jama.2011.279. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 3.Henderson J.M., Lee K.Y.C. Promising antimicrobial agents designed from natural peptide templates. Curr. Opin. Solid State Mater. Sci. 2013;17:175–192. [Google Scholar]
- 4.Baumann G., Mueller P. A molecular model of membrane excitability. J. Supramol. Struct. 1974;2:538–557. doi: 10.1002/jss.400020504. [DOI] [PubMed] [Google Scholar]
- 5.Christensen B., Fink J., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc. Natl. Acad. Sci. USA. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapaport D., Shai Y. Interaction of fluorescently labeled pardaxin and its analogues with lipid bilayers. J. Biol. Chem. 1991;266:23769–23775. [PubMed] [Google Scholar]
- 7.Matsuzaki K., Murase O., Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 8.Yang L., Harroun T.A., Huang H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian S., Wang W., Huang H.W. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc. Natl. Acad. Sci. USA. 2008;105:17379–17383. doi: 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pieta P., Mirza J., Lipkowski J. Direct visualization of the alamethicin pore formed in a planar phospholipid matrix. Proc. Natl. Acad. Sci. USA. 2012;109:21223–21227. doi: 10.1073/pnas.1201559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallock K.J., Lee D.-K., Ramamoorthy A. Membrane composition determines pardaxin’s mechanism of lipid bilayer disruption. Biophys. J. 2002;83:1004–1013. doi: 10.1016/S0006-3495(02)75226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L., Weiss T.M., Huang H.W. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys. J. 2000;79:2002–2009. doi: 10.1016/S0006-3495(00)76448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi S., Hong T., Hong M. Solid-state NMR investigations of peptide-lipid interaction and orientation of a β-sheet antimicrobial peptide, protegrin. Biochemistry. 2002;41:9852–9862. doi: 10.1021/bi0257991. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt N.W., Mishra A., Wong G.C.L. Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J. Am. Chem. Soc. 2011;133:6720–6727. doi: 10.1021/ja200079a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R., Mark A.E. The effect of membrane curvature on the conformation of antimicrobial peptides: implications for binding and the mechanism of action. Eur. Biophys. J. 2011;40:545–553. doi: 10.1007/s00249-011-0677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez D.I., Le Brun A.P., Separovic F. The antimicrobial peptide aurein 1.2 disrupts model membranes via the carpet mechanism. Phys. Chem. Chem. Phys. 2012;14:15739–15751. doi: 10.1039/c2cp43099a. [DOI] [PubMed] [Google Scholar]
- 18.Ambroggio E.E., Separovic F., Bagatolli L.A. Direct visualization of membrane leakage induced by the antibiotic peptides: maculatin, citropin, and aurein. Biophys. J. 2005;89:1874–1881. doi: 10.1529/biophysj.105.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., Wang G. APD: the Antimicrobial Peptide Database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wimley W.C., Hristova K. Antimicrobial peptides: successes, challenges and unanswered questions. J. Membr. Biol. 2011;239:27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen L.T., Haney E.F., Vogel H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Yount N.Y., Yeaman M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA. 2004;101:7363–7368. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam K.L.H., Ishitsuka Y., Lee K.Y.C. Mechanism of supported membrane disruption by antimicrobial peptide protegrin-1. J. Phys. Chem. B. 2006;110:21282–21286. doi: 10.1021/jp0630065. [DOI] [PubMed] [Google Scholar]
- 24.Lam K.L.H., Wang H., Lee K.Y.C. Mechanism of structural transformations induced by antimicrobial peptides in lipid membranes. Biochim. Biophys. Acta. 2012;1818:194–204. doi: 10.1016/j.bbamem.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Vidu R., Stroeve P. Electrochemical and surface properties of solid-supported, mobile phospholipid bilayers on a polyion/alkylthiol layer pair used for detection of antimicrobial peptide insertion. Langmuir. 2002;18:1318–1331. [Google Scholar]
- 26.Johnson S.J., Bayerl T.M., Sackmann E. Structure of an adsorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys. J. 1991;59:289–294. doi: 10.1016/S0006-3495(91)82222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kučerka N., Kiselev M.A., Balgavý P. Determination of bilayer thickness and lipid surface area in unilamellar dimyristoylphosphatidylcholine vesicles from small-angle neutron scattering curves: a comparison of evaluation methods. Eur. Biophys. J. 2004;33:328–334. doi: 10.1007/s00249-003-0349-0. [DOI] [PubMed] [Google Scholar]
- 28.Smith A.M., Vinchurkar M., Parikh A.N. Order at the edge of the bilayer: membrane remodeling at the edge of a planar supported bilayer is accompanied by a localized phase change. J. Am. Chem. Soc. 2010;132:9320–9327. doi: 10.1021/ja100294k. [DOI] [PubMed] [Google Scholar]
- 29.Lee K.Y.C., McConnell H.M. Quantized symmetry of liquid monolayer domains. J. Phys. Chem. 1993;97:9532–9539. [Google Scholar]
- 30.Fošnarič M., Kralj-Iglič V., May S. Stabilization of pores in lipid bilayers by anisotropic inclusions. J. Phys. Chem. B. 2003;107:12519–12526. [Google Scholar]
- 31.Karatekin E., Sandre O., Brochard-Wyart F. Cascades of transient pores in giant vesicles: line tension and transport. Biophys. J. 2003;84:1734–1749. doi: 10.1016/S0006-3495(03)74981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puech P.-H., Borghi N., Brochard-Wyart F. Line thermodynamics: adsorption at a membrane edge. Phys. Rev. Lett. 2003;90:128304. doi: 10.1103/PhysRevLett.90.128304. [DOI] [PubMed] [Google Scholar]
- 33.de Joannis J., Jiang F.Y., Kindt J.T. Coarse-grained model simulations of mixed-lipid systems: composition and line tension of a stabilized bilayer edge. Langmuir. 2006;22:998–1005. doi: 10.1021/la051278d. [DOI] [PubMed] [Google Scholar]
- 34.Moldovan D., Pinisetty D., Devireddy R.V. Molecular dynamics simulation of pore growth in lipid bilayer membranes in the presence of edge-active agents. Appl. Phys. Lett. 2007;91:204104. [Google Scholar]
- 35.Lazaridis T., He Y., Prieto L. Membrane interactions and pore formation by the antimicrobial peptide protegrin. Biophys. J. 2013;104:633–642. doi: 10.1016/j.bpj.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F.-Y., Lee M.-T., Huang H.W. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys. J. 2003;84:3751–3758. doi: 10.1016/S0006-3495(03)75103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludtke S., He K., Huang H. Membrane thinning caused by magainin 2. Biochemistry. 1995;34:16764–16769. doi: 10.1021/bi00051a026. [DOI] [PubMed] [Google Scholar]
- 38.Heller W.T., Waring A.J., Huang H.W. Membrane thinning effect of the β-sheet antimicrobial protegrin. Biochemistry. 2000;39:139–145. doi: 10.1021/bi991892m. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y., Wang H., Kindt J.T. Atomistic simulations of bicelle mixtures. Biophys. J. 2010;98:2895–2903. doi: 10.1016/j.bpj.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haustein M., Wahab M., Schiller P. Vesicle solubilization by bile salts: comparison of macroscopic theory and simulation. Langmuir. 2015;31:4078–4086. doi: 10.1021/acs.langmuir.5b00035. [DOI] [PubMed] [Google Scholar]
- 41.Huang H.W. Elasticity of lipid bilayer interacting with amphiphilic helical peptides. J. Phys. B At. Mol. Opt. Phys. 1995;5:1427–1431. [Google Scholar]
- 42.Liu D., DeGrado W.F. De novo design, synthesis, and characterization of antimicrobial β-peptides. J. Am. Chem. Soc. 2001;123:7553–7559. doi: 10.1021/ja0107475. [DOI] [PubMed] [Google Scholar]
- 43.Gregory S.M., Pokorny A., Almeida P.F. Magainin 2 revisited: a test of the quantitative model for the all-or-none permeabilization of phospholipid vesicles. Biophys. J. 2009;96:116–131. doi: 10.1016/j.bpj.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hovakeemian S.G., Liu R., Heerklotz H. Correlating antimicrobial activity and model membrane leakage induced by nylon-3 polymers and detergents. Soft Matter. 2015;11:6840–6851. doi: 10.1039/c5sm01521a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mihajlovic M., Lazaridis T. Antimicrobial peptides bind more strongly to membrane pores. Biochim. Biophys. Acta. 2010;1798:1494–1502. doi: 10.1016/j.bbamem.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun D., Forsman J., Woodward C.E. Amphipathic membrane-active peptides recognize and stabilize ruptured membrane pores: exploring cause and effect with coarse-grained simulations. Langmuir. 2015;31:752–761. doi: 10.1021/la5038266. [DOI] [PubMed] [Google Scholar]
- 47.Rakowska P.D., Jiang H., Ryadnov M.G. Nanoscale imaging reveals laterally expanding antimicrobial pores in lipid bilayers. Proc. Natl. Acad. Sci. USA. 2013;110:8918–8923. doi: 10.1073/pnas.1222824110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schittek B., Hipfel R., Garbe C. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 49.Jung H.H., Yang S.-T., Kim J.I. Analysis of the solution structure of the human antibiotic peptide dermcidin and its interaction with phospholipid vesicles. BMB Rep. 2010;43:362–368. doi: 10.5483/bmbrep.2010.43.5.362. [DOI] [PubMed] [Google Scholar]
- 50.Shenkarev Z.O., Nadezhdin K.D., Arseniev A.S. Conformation and mode of membrane interaction in cyclotides. Spatial structure of kalata B1 bound to a dodecylphosphocholine micelle. FEBS J. 2006;273:2658–2672. doi: 10.1111/j.1742-4658.2006.05282.x. [DOI] [PubMed] [Google Scholar]
- 51.Tam J.P., Lu Y.-A., Chiu K.-W. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc. Natl. Acad. Sci. USA. 1999;96:8913–8918. doi: 10.1073/pnas.96.16.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paulmann M., Arnold T., Schittek B. Structure-activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat. J. Biol. Chem. 2012;287:8434–8443. doi: 10.1074/jbc.M111.332270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song C., Weichbrodt C., Zeth K. Crystal structure and functional mechanism of a human antimicrobial membrane channel. Proc. Natl. Acad. Sci. USA. 2013;110:4586–4591. doi: 10.1073/pnas.1214739110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y.-H., Colgrave M.L., Craik D.J. The biological activity of the prototypic cyclotide kalata b1 is modulated by the formation of multimeric pores. J. Biol. Chem. 2009;284:20699–20707. doi: 10.1074/jbc.M109.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C.K., Wacklin H.P., Craik D.J. Cyclotides insert into lipid bilayers to form membrane pores and destabilize the membrane through hydrophobic and phosphoethanolamine-specific interactions. J. Biol. Chem. 2012;287:43884–43898. doi: 10.1074/jbc.M112.421198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall K., Lee T.-H., Aguilar M.-I. Gly6 of kalata B1 is critical for the selective binding to phosphatidylethanolamine membranes. Biochim. Biophys. Acta. 2012;1818:2354–2361. doi: 10.1016/j.bbamem.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Nourse A., Trabi M., Craik D.J. A comparison of the self-association behavior of the plant cyclotides kalata B1 and kalata B2 via analytical ultracentrifugation. J. Biol. Chem. 2004;279:562–570. doi: 10.1074/jbc.M306826200. [DOI] [PubMed] [Google Scholar]
- 58.Nawae W., Hannongbua S., Ruengjitchatchawalya M. Defining the membrane disruption mechanism of kalata B1 via coarse-grained molecular dynamics simulations. Sci. Rep. 2014;4:3933. doi: 10.1038/srep03933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhunia A., Domadia P.N., Bhattacharjya S. NMR structure of pardaxin, a pore-forming antimicrobial peptide, in lipopolysaccharide micelles: mechanism of outer membrane permeabilization. J. Biol. Chem. 2010;285:3883–3895. doi: 10.1074/jbc.M109.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pukala T.L., Brinkworth C.S., Bowie J.H. Investigating the importance of the flexible hinge in caerin 1.1: solution structures and activity of two synthetically modified caerin peptides. Biochemistry. 2004;43:937–944. doi: 10.1021/bi035760b. [DOI] [PubMed] [Google Scholar]
- 61.Raj P.A., Edgerton M., Levine M.J. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J. Biol. Chem. 1990;265:3898–3905. [PubMed] [Google Scholar]
- 62.Melino S., Rufini S., Petruzzelli R. Zn(2+) ions selectively induce antimicrobial salivary peptide histatin-5 to fuse negatively charged vesicles. Identification and characterization of a zinc-binding motif present in the functional domain. Biochemistry. 1999;38:9626–9633. doi: 10.1021/bi990212c. [DOI] [PubMed] [Google Scholar]
- 63.Zemel A., Fattal D.R., Ben-Shaul A. Energetics and self-assembly of amphipathic peptide pores in lipid membranes. Biophys. J. 2003;84:2242–2255. doi: 10.1016/S0006-3495(03)75030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harwig S.S.L., Waring A., Lehrer R.I. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur. J. Biochem. 1996;240:352–357. doi: 10.1111/j.1432-1033.1996.0352h.x. [DOI] [PubMed] [Google Scholar]
- 65.Wanniarachchi Y.A., Kaczmarek P., Nolan E.M. Human defensin 5 disulfide array mutants: disulfide bond deletion attenuates antibacterial activity against Staphylococcus aureus. Biochemistry. 2011;50:8005–8017. doi: 10.1021/bi201043j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wimley W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bechinger B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr. Opin. Colloid Interface Sci. 2009;14:349–355. [Google Scholar]
- 68.Zemel A., Ben-Shaul A., May S. Modulation of the spontaneous curvature and bending rigidity of lipid membranes by interfacially adsorbed amphipathic peptides. J. Phys. Chem. B. 2008;112:6988–6996. doi: 10.1021/jp711107y. [DOI] [PubMed] [Google Scholar]
- 69.Simunovic M., Voth G.A., Bassereau P. When physics takes over: BAR proteins and membrane curvature. Trends Cell Biol. 2015;25:780–792. doi: 10.1016/j.tcb.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mihajlovic M., Lazaridis T. Charge distribution and imperfect amphipathicity affect pore formation by antimicrobial peptides. Biochim. Biophys. Acta. 2012;1818:1274–1283. doi: 10.1016/j.bbamem.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang M., Waring A.J., Hong M. Phosphate-mediated arginine insertion into lipid membranes and pore formation by a cationic membrane peptide from solid-state NMR. J. Am. Chem. Soc. 2007;129:11438–11446. doi: 10.1021/ja072511s. [DOI] [PubMed] [Google Scholar]
- 72.Perrin B.S., Jr., Tian Y., Cotten M.L. High-resolution structures and orientations of antimicrobial peptides piscidin 1 and piscidin 3 in fluid bilayers reveal tilting, kinking, and bilayer immersion. J. Am. Chem. Soc. 2014;136:3491–3504. doi: 10.1021/ja411119m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez D.I., Lee T.-H., Separovic F. Proline facilitates membrane insertion of the antimicrobial peptide maculatin 1.1 via surface indentation and subsequent lipid disordering. Biophys. J. 2013;104:1495–1507. doi: 10.1016/j.bpj.2013.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mishra A., Lai G.H., Wong G.C.L. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc. Natl. Acad. Sci. USA. 2011;108:16883–16888. doi: 10.1073/pnas.1108795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishitsuka Y., Pham D.S., Lee K.Y.C. Insertion selectivity of antimicrobial peptide protegrin-1 into lipid monolayers: effect of head group electrostatics and tail group packing. Biochim. Biophys. Acta. 2006;1758:1450–1460. doi: 10.1016/j.bbamem.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Arouri A., Kerth A., Blume A. The binding of an amphipathic peptide to lipid monolayers at the air/water interface is modulated by the lipid headgroup structure. Langmuir. 2011;27:2811–2818. doi: 10.1021/la104887s. [DOI] [PubMed] [Google Scholar]
- 77.Strandberg E., Tiltak D., Ulrich A.S. Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim. Biophys. Acta. 2012;1818:1764–1776. doi: 10.1016/j.bbamem.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 78.Garten M., Prévost C., Vanni S. Methyl-branched lipids promote the membrane adsorption of α-synuclein by enhancing shallow lipid-packing defects. Phys. Chem. Chem. Phys. 2015;17:15589–15597. doi: 10.1039/c5cp00244c. [DOI] [PubMed] [Google Scholar]
- 79.Shaw J.E., Epand R.F., Yip C.M. Cationic peptide-induced remodelling of model membranes: direct visualization by in situ atomic force microscopy. J. Struct. Biol. 2008;162:121–138. doi: 10.1016/j.jsb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Nicolini C., Baranski J., Winter R. Visualizing association of N-ras in lipid microdomains: influence of domain structure and interfacial adsorption. J. Am. Chem. Soc. 2006;128:192–201. doi: 10.1021/ja055779x. [DOI] [PubMed] [Google Scholar]
- 81.Hutchison J.B., Weis R.M., Dinsmore A.D. Change of line tension in phase-separated vesicles upon protein binding. Langmuir. 2012;28:5176–5181. doi: 10.1021/la204225a. [DOI] [PubMed] [Google Scholar]
- 82.Gidalevitz D., Ishitsuka Y., Lee K.Y.C. Interaction of antimicrobial peptide protegrin with biomembranes. Proc. Natl. Acad. Sci. USA. 2003;100:6302–6307. doi: 10.1073/pnas.0934731100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lehrer R.I., Andrew Tincu J., Waring A.J. Natural peptide antibiotics from tunicates: structures, functions and potential uses. Integr. Comp. Biol. 2003;43:313–322. doi: 10.1093/icb/43.2.313. [DOI] [PubMed] [Google Scholar]
- 84.Rabanal F., Grau-Campistany A., Cajal Y. A bioinspired peptide scaffold with high antibiotic activity and low in vivo toxicity. Sci. Rep. 2015;5:10558. doi: 10.1038/srep10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong M., Lee M.W., Cheng J. Helical antimicrobial polypeptides with radial amphiphilicity. Proc. Natl. Acad. Sci. USA. 2015;112:13155–13160. doi: 10.1073/pnas.1507893112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.