Abstract
The prerequisite for an “undetectable” HIV viral load has restricted access to transplantation for HIV-infected kidney recipients. However, HCV-infected recipients, due the historic limitations of HCV therapy in patients with renal disease, are commonly viremic at transplant and have universal access. In order to compare the effect of HIV, HCV and HIV/HCV co-infection on kidney transplant patient and allograft outcomes, we performed a retrospective study of kidney recipients transplanted from January 1996 through December 2013. In multivariable analysis, patient (hazard ratio 0.90, 95% confidence interval 0.66–1.24) and allograft survival (0.60, 40–0.88) in 492 HIV patients did not differ significantly from the 117,791 patient uninfected reference group. This was superior to outcomes in both the 5605 patient HCV group for death (1.44, 1.33–1.56) and graft loss (1.43, 1.31–1.56) as well as the 147 patient HIV/HCV co-infected group for death (2.26, 1.45–3.52) and graft loss (2.59, 1.60–4.19). HIV infection did not adversely affect recipient or allograft survival and was associated with superior outcomes compared to both HCV infection and HIV/HCV co-infection in this population. Thus, pre-transplant viral eradication and/ or immediate post-transplant eradication should be studied as potential strategies to improve post-transplant outcomes in HCV-infected kidney recipients.
Introduction
Until recently, Human Immunodeficiency Virus (HIV) infection was a contraindication to kidney transplantation. Transplants performed in this population before the availability of potent antiretroviral therapy (ART) were associated with poor outcomes (1). Since adopting potent ART into practice in 1996, coupled with recommendations that HIV-infected transplant candidates be rendered aviremic and maintained on ART, transplant outcomes have improved (2–5). A recent US multicenter observational trial (2) reported 3-year patient and allograft survival of 88.2% and 73.7%, respectively, comparable to outcomes for older recipients and better than that of waitlisted HIV-infected transplant candidates (6,7). In contrast, the few HIV/hepatitis C virus (HCV) co-infected recipients in both US (2) and European studies (4) have fared poorly.
The prevalence of HCV in the US end-stage renal disease (ESRD) population is about 7–10% (8). Guidelines (9) recommend transplantation over dialysis for HCV-infected ESRD patients based on studies demonstrating a survival advantage for this treatment approach (10,11). Transplantation of viremic HCV-infected patients is widespread even though a) outcomes are worse than in uninfected recipients (12,13) and b) available antiviral treatment options have been largely ineffective in this population. In a meta-analysis (14) of maintenance hemodialysis patients, the overall summary estimate for a sustained virological response to HCV therapy was 30% in patients with HCV genotype 1 infection, the most prevalent genotype in the US.
The benefit of kidney transplantation in both HIV-infected and HCV-infected patients is therefore established. However, fundamental differences remain concerning the approach to transplantation in these populations. Less than 25% of US centers currently offer kidney transplantation to HIV-infected patients, whereas HCV-infected candidates without advanced liver disease have unlimited access. To qualify for transplantation, HIV-infected patients must have an undetectable viral load while HCV-infected candidates can be, and usually are, viremic. Despite these discrepant approaches, no study has directly compared outcomes between HIV and HCV-infected recipients. We hypothesized that stringent control of viral replication required for transplantation in HIV mono-infected kidney candidates would be associated with superior outcomes compared to HCV mono-infected or HIV/HCV co-infected candidates in whom HCV infection is seldom eradicated. The goal of this study was to evaluate the effect of HIV, HCV or HIV/HCV co-infection on kidney recipient and allograft outcomes.
Results
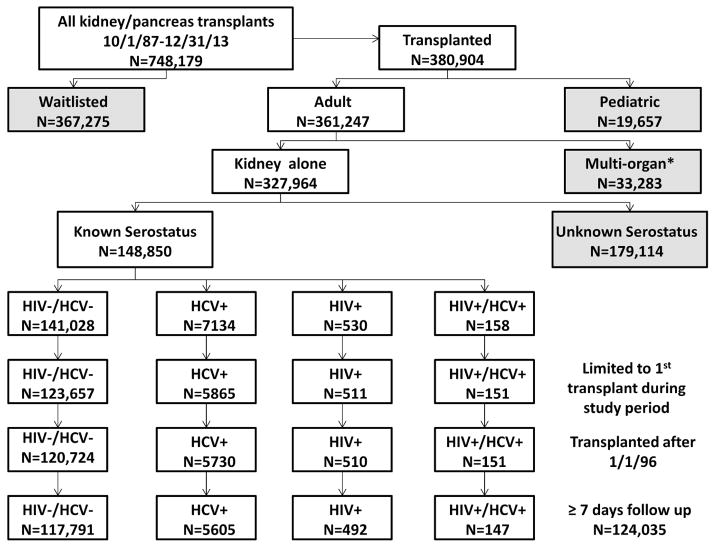
Cohort Assembly (Figure 1)
Figure 1.
Creation of the patient cohort.
Overall, 748,179 transplant and wait list registrations were recorded in the dataset from October 1, 1987 through December 31, 2013. We excluded 367,275 patients who remained waitlisted at the time of analysis, 19,657 pediatric patients, as well as 33,283 recipients of pancreas alone, simultaneous kidney-pancreas or multi-organ transplants. Of 327,964 kidney alone recipients, only the 148,850 with both HCV and HIV serostatus reported were retained. After limiting our cohort to first kidney transplants performed on or after January 1, 1996 with at least 7 days of reported follow up, 124,035 evaluable patients remained. Stratification according to HCV/HIV serostatus yielded groups of 117,791 uninfected, 5,605 HCV mono-infected, 492 HIV mono-infected and 147 HIV/HCV co-infected patients. For patients with missing HIV and/or HCV serostatus (n=179,114), we applied the same restrictions to create a cohort of 93,483 patients for inclusion in the secondary/sensitivity analysis.
The four groups differed significantly with respect to demographic and clinical characteristics (Table 1). African Americans represented about one-fourth of uninfected patients, yet accounted for over half of the HCV mono-infected cohort and over three-quarters of the HIV mono-infected and HIV/HCV co-infected groups (P<0.001). HIV mono-infected patients were younger, while the prevalence of diabetes was higher in the HCV mono-infected cohort (P<0.001). A higher proportion of HIV and/or HCV-infected recipients were male (P<0.001), with longer exposure to dialysis by the time of transplantation (P=0.001) compared to uninfected patients. The median duration of dialysis for HIV mono-infected patients was approximately double that of uninfected patients (P<0.001). Compared to uninfected patients, deceased donor transplantation was more common in the cohorts with HIV and/or HCV infection (P<0.001): among the HCV mono-infected and co-infected groups, most deceased donor kidneys were from non-standard criteria donors. Kidneys from HCV mono-infected donors were transplanted into 28% of HCV mono-infected and 48% of HIV/HCV co-infected recipients.
Table 1.
Clinical and demographic characteristics of the cohort.
| HIV−/HCV− n=117,791 |
HCV+ n=5605 |
HIV+ n=492 |
HIV+/HCV+ n=147 |
p value | |
|---|---|---|---|---|---|
|
| |||||
| Patient Characteristics | |||||
|
| |||||
| Median age in years (IQR) | 52 (41–61) | 54 (48–59) | 46 (41–52) | 50 (45–56) | <0.001 |
|
| |||||
| Male (%) | 70,921 (60.2) | 4105 (73.2) | 381 (77.4) | 120 (81.6) | <0.001 |
|
| |||||
| Race (%) | <0.001 | ||||
| African American | 29,718 (25.2) | 2870 (51.2) | 358 (72.8) | 117 (79.6) | |
| Caucasian | 63,756 (54.4) | 1858 (33.1) | 89 (18.1) | 20 (13.6) | |
| Latino | 16,777 (14.1) | 625 (11.2) | 3 (6.7) | 7 (5.4) | |
| Asian | 5384 (4.5) | 156 (2.8) | 6 (1.2) | 1 (0.8) | |
| Other | 2156 (1.8) | 93 (1.7) | 6 (1.1) | 2 (1.6) | |
|
| |||||
| Cause of ESRD | <0.001 | ||||
| Diabetes | 31,426 (26.7) | 1674 (29.9) | 52 (10.6) | 20 (13.6) | |
| Hypertension | 29,228 (24.8) | 2063 (36.8) | 185 (37.6) | 62 (42.2) | |
| Glomerular disease | 22,027 (18.7) | 615 (11) | 42 (8.5) | 8 (5.4) | |
| Cystic disease | 12,463 (10.6) | 287 (5.1) | 10 (2.0) | 5 (3.4) | |
| HIVAN | 0 (0) | 0 (0) | 39 (7.9) | 7 (4.8) | |
| Other | 7071(6) | 281(5) | 47 (9.5) | 11 (7.5) | |
| Missing data | 15,579 (13.2) | 685 (12.2) | 117 (23.8) | 34 (23.1) | |
|
| |||||
| Pretransplant dialysis (%) | 97,059 (82.4) | 5075 (90.5) | 473 (96.1) | 142 (96.6) | <0.001 |
|
| |||||
| Median years on dialysis (IQR) | 2.7 (1.3–4.6) | 3.2 (1.6–5.4) | 5.5 (2.9–8) | 5 (3.0–7.7) | 0.001 |
|
| |||||
| Median total days on wait list (IQR) | 469 (169–996) | 424 (147–932) | 517 (177–1218) | 404 (109–875) | <0.001 |
|
| |||||
| HBV surface Ag+ | 2191 (1.9) | 200 (3.6) | 25 (5.1) | 7 (4.8) | <0.001 |
|
| |||||
| PRA ≥30% | 17,056 (14.5) | 810 (14.4) | 82 (16.7) | 16 (10.9) | 0.22 |
|
| |||||
| Pretransplant diabetes | 38,660 (32.8) | 2235 (39.9) | 71 (14.4) | 26 (17.7) | <0.001 |
|
| |||||
| Donor Characteristics | |||||
|
| |||||
| Deceased donor | 71,008 (60.3) | 4300 (76.7) | 349 (70.9) | 127 (86.4) | <0.001 |
|
| |||||
| Expanded criteria donor | 13,098 (18.4) | 626 (14.5) | 38 (10.8) | 13 (10.2) | <0.001 |
|
| |||||
| CDC high risk donor | 5067 (8.5) | 707 (20.0) | 64 (19.0) | 48 (44.4) | <0.001 |
|
| |||||
| HCV+ donor | 295 (0.29) | 1586 (28.3) | 3 (0.6) | 71 (48.3) | <0.001 |
|
| |||||
| Diabetic donor | 5060 (4.3) | 262 (4.7) | 27 (5.5) | 5 (3.4) | 0.49 |
|
| |||||
| Median donor age in years (IQR) | 41 (28–51) | 42 (29–50) | 39 (26–49) | 39 (25–48) | 0.006 |
|
| |||||
| Median CIT (hours) | 12 (2–20) | 16 (8–23) | 13 (2–22) | 17 (11–24) | 0.001 |
|
| |||||
| Delayed graft function (%) | 18,948 (16.1) | 1317 (23.5) | 154 (31.3) | 57 (38.8) | <0.001 |
|
| |||||
| Immunosuppression | |||||
|
| |||||
| Tacrolimus maintenance | 83,089 (78.2) | 3948 (76.7) | 316 (73.8) | 72 (55.8) | <0.001 |
|
| |||||
| Induction Type | <0.001 | ||||
| Lymphodepleting | 62,153 (52.8) | 2862 (51) | 184 (37.4) | 42 (28.6) | |
| Non-lymphodepleting | 28,271 (24) | 1367 (24.4) | 173 (35.2) | 40 (27.2) | |
From an immunosuppression standpoint, induction therapy was uniformly used in the uninfected, HCV mono-infected and HIV mono-infected cohorts. Tacrolimus was the predominant calcineurin inhibitor in each group, although it was used 20–25% less frequently in co-infected patients than in the other groups (P<0.001).
Patient and allograft outcomes
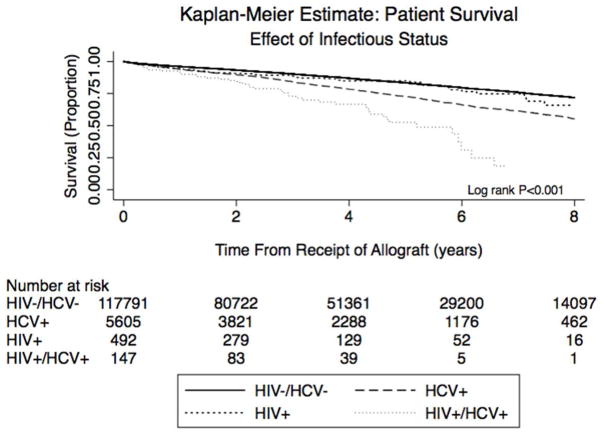
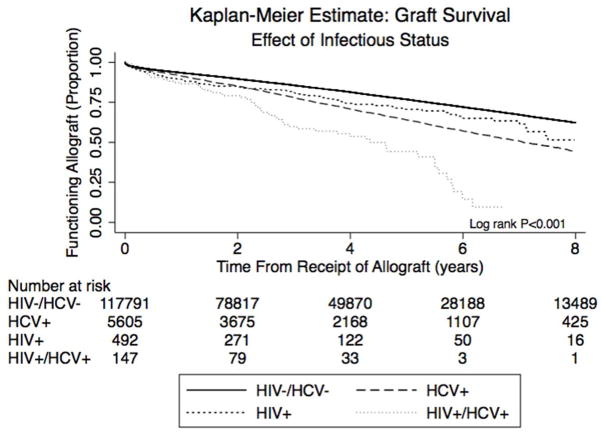
Three-year patient survival was highest in the HIV mono-infected (89%) and uninfected groups (90%), and lowest in the HCV mono-infected (84%) and HIV/HCV co-infected groups (73%), (Figure 2a, p<0.001; Kaplan Meier patient survival estimates in Supplemental Table 1a). Among patients with a reported cause of death, cardiovascular (12–27%) and infectious (14–36%) causes predominated across all groups. Three-year allograft survival (Figure 2b) was similarly higher in the HIV mono-infected (81%) and uninfected groups (86%) than in the HCV mono-infected (78%) and HIV/HCV co-infected patients (60%; p<0.001; Kaplan Meier allograft survival estimates in Supplemental Table 1b). The most common reason for all-cause allograft loss in all groups was rejection, although no cause was recorded in nearly 40% of patients. Acute rejection occurred most frequently in the HIV mono-infected (19.4%) and HIV/HCV co-infected (18.7%) patients.
Figure 2.
Figure 2a. Kaplan Meier curves illustrating time to patient death, stratified by viral status.
Figure 2b. Kaplan Meier curves illustrating time to allograft loss, non-death censored.
Multivariable models
Univariable and multivariable cox regression models were fit for mortality and graft loss (Tables 2, 3). Compared to uninfected patients, HCV mono-infection (HR 1.44, 95% CI 1.33–1.56) and HIV/HCV co-infection (HR 2.26, 95% CI 1.45–3.52) were associated with an increased risk of death, while HIV mono-infection was not (HR 0.90, 95% CI 0.66–1.24). Age, pre-transplant diabetes, pre-transplant dialysis, deceased donor transplant, and HCV+ donor were significantly associated with mortality, though male gender, African American race, and lymphodepleting induction immunosuppression were protective.
Table 2.
Univariable and Multivariable Cox Regression for recipient death.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| variable | HR | p value | 95% CI | HR* | p value | 95% CI |
| Viral Factors | ||||||
| HIV−/HCV− | REF | REF | ||||
| HCV+ | 1.75 | <0.001 | 1.66–1.85 | 1.44 | <0.001 | 1.33–1.56 |
| HIV+ | 1.22 | 0.11 | 0.95–1.55 | 0.90 | 0.53 | 0.66–1.24 |
| HIV+/HCV+ | 3.38 | <0.001 | 2.53–4.51 | 2.26 | <0.001 | 1.45–3.52 |
| Recipient Age | ||||||
| Age <40 years | REF | REF | ||||
| Age 40–60 years | 2.25 | <0.001 | 2.14–2.36 | 1.91 | <0.001 | 1.79–2.03 |
| Age >60 years | 4.69 | <0.001 | 4.47–4.92 | 3.49 | <0.001 | 3.27–3.73 |
| Other recipient factors | ||||||
| Male | 0.84 | <0.001 | 0.81–0.86 | 0.93 | 0.001 | 0.90–0.97 |
| African American | 1.18 | <0.001 | 1.15–1.22 | 1.00 | 0.99 | 0.96–1.04 |
| Pre-transplant diabetes | 2.23 | <0.001 | 2.17–2.30 | 1.66 | <0.001 | 1.61–1.72 |
| Pre-transplant dialysis | 2.21 | <0.001 | 2.10–2.31 | 1.62 | <0.001 | 1.52–1.73 |
| Years on dialysis | 1.05 | <0.001 | 1.05–1.06 | 1.04 | <0.001 | 1.03–1.04 |
| Transplant Factors | ||||||
| Deceased donor | 1.94 | <0.001 | 1.88–2.00 | 1.33 | <0.001 | 1.27–1.39 |
| Expanded criteria donor | 2.26 | <0.001 | 2.18–2.34 | - | - | - |
| CDC high risk donor | 0.98 | 0.64 | 0.91–1.06 | - | - | - |
| HCV+ donor | 2.24 | <0.001 | 2.06–2.43 | 1.97 | <0.001 | 1.54–2.53 |
| Acute rejection in 1st year | 1.22 | <0.001 | 1.17–1.27 | - | - | - |
| HCV donor* HCV recipient | 0.59 | 0.006 | 0.48–0.74 | 0.55 | <0.001 | 0.41–0.73 |
| Antibody induction use | ||||||
| Lymphodepleting | REF | REF | ||||
| Non-lymphodepleting | 1.00 | 0.82 | 0.96–1.03 | 1.13 | <0.001 | 1.09–1.17 |
HR is adjusted to reflect tacrolimus based immunosuppression and an interaction term for transplant year and tacrolimus use.
Table 3.
Univariable and Multivariable Cox Regression for allograft loss.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| variable | HR | p value | 95% CI | HR* | p value | 95% CI |
| Viral Factors | ||||||
| HIV−/HCV− | REF | REF | ||||
| HCV+ | 1.66 | <0.001 | 1.58–1.74 | 1.43 | <0.001 | 1.31–1.56 |
| HIV+ | 1.42 | <0.001 | 1.18–1.71 | 0.60 | 0.01 | 0.40–0.88 |
| HIV+/HCV+ | 3.18 | <0.001 | 2.48–4.08 | 2.59 | <0.001 | 1.60–4.19 |
| Recipient Age | ||||||
| Age <40 years | REF | REF | ||||
| Age 40–60 years | 1.01 | 0.53 | 0.98–1.04 | 0.82 | <0.001 | 0.78–0.88 |
| Age >60 years | 1.58 | <0.001 | 1.53–1.63 | 1.09 | 0.002 | 1.03–1.16 |
| Other recipient factors | ||||||
| Male | 0.91 | <0.001 | 0.89–0.93 | 0.94 | 0.006 | 0.91–0.98 |
| African American | 1.50 | <0.001 | 1.47–1.54 | 1.27 | <0.001 | 1.22–1.32 |
| Pre-transplant diabetes | 1.50 | <0.001 | 1.46–1.53 | 1.34 | <0.001 | 1.28–1.39 |
| PRA ≥30% | 1.14 | <0.001 | 1.10–1.18 | 0.99 | 0.78 | 0.94–1.05 |
| Pre-transplant dialysis | 1.14 | <0.001 | 1.13–1.16 | 1.60 | <0.001 | 1.48–1.72 |
| Transplant Factors | ||||||
| Deceased donor | 1.74 | <0.001 | 1.70–1.79 | 1.42 | <0.001 | 1.34–1.50 |
| Expanded criteria donor | 2.05 | <0.001 | 1.99–2.11 | 1.41 | <0.001 | 1.34–1.48 |
| CDC high risk donor | 0.96 | <0.001 | 0.96–0.96 | - | - | - |
| Diabetic donor | 1.81 | <0.001 | 1.72–1.90 | 1.19 | <0.001 | 1.10–1.28 |
| HCV+ donor | 2.11 | <0.001 | 1.97–2.27 | 1.70 | 0.001 | 1.25–2.32 |
| HCV donor* HCV recipient | 0.66 | <0.001 | 0.55–0.80 | 0.64 | 0.01 | 0.46–0.91 |
| Acute rejection in 1st year | 1.81 | <0.001 | 1.75–1.88 | 1.86 | <0.001 | 1.77–1.95 |
| Antibody induction use | ||||||
| Lymphodepleting | REF | REF | ||||
| Non-lymphodepleting | 1.06 | <0.001 | 1.03–1.09 | 1.16 | <0.001 | 1.11–1.21 |
HR is adjusted to reflect tacrolimus based immunosuppression and an interaction term for transplant year and tacrolimus use.
In the multivariable analysis for all-cause graft loss, HIV mono-infection was not associated with graft loss (HR 0.60, 95% CI 0.40–0.88), while HCV mono-infection (HR 1.43, 95% CI 1.31–1.56) and HIV/HCV co-infection (HR 2.59, 95% CI 1.60–4.19) were. Other variables associated with graft loss in this model included age >60 years, African American race, pre-transplant diabetes, pre-transplant dialysis, acute rejection, use of a deceased donor kidney, expanded criteria donor kidney, HCV+ donor kidney, and use of non-lymphodepleting induction therapy. Male gender and lymphodepleting induction were inversely associated with graft loss. We did not observe any significant differences in hazard ratios for death or graft loss when models were stratified by donor HCV status (data not shown). An analysis taking in account transplant era also yielded similar results and is presented in Supplemental Table 2.
Patients with incomplete serostatus (Supplemental Tables 3–4 and Figures 1–2)
After applying the same exclusion criteria, there were 89,780 patients with missing HIV and/or HCV serologies that were compared to the uninfected patients; 3680 HIV unknown/HCV+ patients were compared to the HCV mono-infected group and 23 HIV+/HCV unknown patients were compared to the HIV infected group. While all 3 unknown groups differed from their respective reference groups, similar clinical and demographic trends were observed.
Patient survival was approximately 20% lower in the HIV/HCV unknown (log rank test p <0.001; HR 1.21, 95% CI 1.19–1.24) and HIV unknown/HCV+ groups (log rank p<0.001; HR 1.19 95% CI 1.11–1.28), when compared to the uninfected and HCVmono-infected groups respectively. HIV+/HCV unknown patient survival (log rank p=0.17; HR 1.79, 95% CI 0.77–4.14) did not differ significantly from the HIV mono-infected group. Allograft survival was 9% lower for HIV/HCV unknown patients compared to uninfected patients (log rank p <0.001; HR 1.09, 95% 1.07–1.10) and 7% lower for the HIV unknown/HCV+ patients compared to the HCV mono-infected (log rank p=0.027, HR 1.07, 95% CI 1.00–1.14). There was no difference in allograft survival between the HIV+/HCV unknown and HIV mono-infected cohorts (log rank p=0.11, HR 1.71, 95% CI 0.89–3.26).
Finally we combined the unknown and known groups and examined the effect on hazard ratio estimates from our models. For risk of mortality compared to uninfected patients, there was no significant difference in the HIV mono-infected group (HR 0.77, 95% CI 0.57–1.06), while it was higher in the HCV mono-infected (HR 1.44, 95% CI 1.36–1.53) and HIV/HCV co-infected (HR 1.87, 95% CI 1.20–2.90) groups. In the combined model for allograft loss, HIV mono-infected patients did not differ significantly from uninfected patients (HR 0.55, 95% CI 0.37–0.81) whereas the HCV mono-infected (HR 1.42, 95% CI 1.33–1.52) and HIV/HCV co-infected (HR 2.22, 95% CI 1.38–3.58) cohorts did. These findings are similar to those observed in the models incorporating only the known serostatus patients.
Discussion
This study of kidney recipients transplanted from 1996–2013 is the largest to date examining outcomes in HIV-infected patients and includes 492 with HIV mono-infection and 147 with HIV/HCV co-infection. The major findings are that a) HIV mono-infected recipients have patient and allograft survival comparable to uninfected recipients, b) patient and allograft outcomes are superior in HIV mono-infected than in HCV mono-infected recipients, c) HIV/HCV co-infected patients have worse outcomes than their mono-infected counterparts.
Our observation that HIV mono-infected recipient outcomes are similar to uninfected kidney transplant patients in the US contrasts with a recent multicenter observational trial (2). In this trial, survival rates for HIV mono-infected patients fell between those reported for older recipients and for all uninfected transplant recipients. The most likely explanation for this discrepancy is that 20% of the multicenter trial cohort had HIV/HCV co-infection whereas we separated HIV mono-infected and HIV/HCV co-infected patients into different cohorts on the basis of distinctive viral comorbidity profiles.
That outcomes for HIV mono-infected recipients are similar to uninfected patients should not be unexpected. HIV-infected patients selected for transplantation must meet rigorous screening criteria, including a minimum CD4 count, an undetectable HIV viral load, and absence of AIDS-defining illness. Besides mandatory ART use, follow up post-transplant care is more intensive than in uninfected patients, due partly to monitoring for drug interactions and higher rejection risk. Many HIV-infected recipients have been transplanted in larger-volume centers in research protocols that entail close scrutiny by multidisciplinary teams with increased frequency of office visits and laboratory testing.
Compared to the HIV mono-infected cohort, the outcomes of HCV infected patients are sobering. Guidelines (9) recommend transplantation as the treatment of choice for HCV-infected ESRD patients compared to remaining on dialysis. Transplantation of patients with HCV viremia has been a widespread practice even though a) outcomes are worse compared to their uninfected counterparts and b) until now, antiviral treatment options have been largely ineffective in this population. There is no prerequisite that potential HCV mono-infected recipients be rendered viral replication-free in order to be transplanted.
Prior to 2012, HCV therapy was limited to interferon and ribavirin, seldom efficacious in the predominantly genotype 1 US ESRD population (14,15), and contraindicated after transplant due to the risk of acute rejection (16,17). The recent approval of direct acting HCV anti-viral agents offers the opportunity to improve our ability to eradicate HCV in this population. Based on our study findings, evaluation of these therapies in chronic kidney disease and chronic immunosuppression settings and an assessment of the effect of pre-transplant or preemptive post-transplant HCV eradication on post-transplant outcomes should be a priority for this population.
A consistent finding by others, and corroborated in our study, is that HIV/HCV co-infected patients experience worse outcomes than HIV mono-infected recipients. Since the standard of care for HIV-infected recipients would be expected to be consistent, this suggests that HCV co-infection exerts a strongly negative effect on post-transplant outcomes. There are several plausible explanations for this including (i) the burden of complications associated with untreated HCV infection dwarfs any protective effect offered by intense monitoring and control of HIV viral replication; (ii) HIV infection is permissive of HCV viral replication and this is enhanced in the setting of chronic immunosuppression; (iii) transplant professionals may be more cautious with co-infected patients, favoring minimized immunosuppression initially: however, once rejection ensues, intensification of the regimen results in loss of control of the net immunosuppression state; (iv) there is greater use of kidneys from HCV-infected donors (49% in our study), a well-established adverse outcome determinant (18). The poor outcomes observed in HIV/HCV co-infected patients suggest a re-evaluation of candidate selection policies while underscoring the need to better understand the behavior of these viruses in the setting of chronic immunosuppression and the urgency of evaluating the new antiviral agents in clinical trials in this population.
Our study has several strengths. It is the largest of its kind to date, including more than 320,000 kidney recipients. Being registry data, center level and regional variation are less prominent and larger trends in patient and allograft outcomes can be studied. The registry provides intermediate term follow up and allows us to ascertain changes in outcomes based on transplant era, of which there were none observed. While missing data can complicate registry analyses, our secondary analysis of the data with the addition of the unknown serostatus patients yielded results that were consistent with our primary analysis, underscoring the robustness of our findings. Our findings are further supported by a very recent smaller study (19) that compared outcomes between recipients of paired mate-kidneys from the same donor, where one recipient was HIV-infected and the other uninfected. In contrast to our study, this prior analysis did not account for unknown viral serostatus, could not compare outcomes across infection groups or provide much granularity regarding recipient characteristics, and was further limited by (i) small sample size, (ii) transplant center bias (because only a small number of experienced centers transplant HIV-infected patients relative to the mate-kidneys) and (iii) failure to adjust for several important adverse outcome determinants such as induction and maintenance immunosuppression, donor type, dialysis vintage and duration on the waiting list.
There are also limitations to our study. First, we are limited by the completeness and level of detail in the data that UNOS collects and centers document; this is an inherent weakness of any study employing registry data. In particular this applies to new onset post-transplant diabetes, an important complication of renal transplant that cannot be accurately ascertained in the UNOS dataset. Second, UNOS has historically collected only HCV serological, rather than viral load, data of recipients at the time of transplant. Consequently, we could not distinguish patients with active HCV viremia from those with immunity from prior infection or those who have previously received treatment. However, as traditional HCV therapies are poorly tolerated in patients with advanced kidney disease and response rates are low, even treated patients would likely be viremic at the time of transplant. Since a percentage of HCV-infected ESRD patients never mount an antibody response, a screening strategy based on antibody results alone would assign some viremic but seronegative patients to the reference group, biasing our results towards the null. Third, UNOS data neither contains information regarding liver histology nor permits assessment of liver disease progression in kidney transplant recipients, information that might elucidate the reason for inferior outcomes observed for HCV infected transplant recipients. Additionally as HIV-infected patients were traditionally transplanted under more rigorous conditions associated with research protocols, this population is highly selected for adherence and enjoys more comprehensive follow-up. This is an inherent selection bias that cannot be quantified in our study.
In conclusion, under current US kidney transplant practice, HIV mono-infection does not adversely affect recipient or allograft survival and is associated with superior outcomes compared to both HCV mono-infection and HIV/HCV co-infection in this population. Investigation of pre-transplant or immediate post-transplant viral eradication with contemporary therapies should be prioritized as a strategy to improve post-transplant outcomes in HCV-infected kidney recipients.
Methods
Data source
A retrospective cohort study was performed using national registry data provided from the United Network for Organ Sharing (UNOS) for all kidney transplants and registrations from October 1, 1987 through December 31, 2013. The Institutional Review Board at the University of Pennsylvania approved this study (protocol # 819058) under exempt status.
Subjects
We assembled a cohort of all adult, first-kidney transplant patients, age ≥ 18 years, transplanted between January 1, 1996 and December 31, 2013 (Figure 1). The first HIV-infected kidney transplant was recorded in the database on October 3, 1987. January 1, 1996 was chosen as the start date to reflect availability of potent triple ART for HIV care and only 6 HIV infected recipients were recorded in the UNOS dataset prior to that date. Recipients of prior transplants or dual organs were excluded. We required that all study subjects have at least seven days of follow up reported after transplantation. Patients with a positive or negative value for HIV and HCV serostatus were included in the primary analysis. Patients missing one or both serostatuses were evaluated in a secondary/sensitivity analysis (see below). Patients were divided into four groups based upon serostatus: HIV/HCV uninfected (the reference group), HCV mono-infected, HIV mono-infected, and HIV/HCV co-infected. The serostatus groups were considered the exposure of interest for the primary and secondary analyses.
Variables
Recipient HCV serostatus was reported in the database as “HCV_SEROSTATUS” and deceased donor HCV serostatus was reported in “HEP_C_ANTI_DON”, which did not allow for differentiation of active viremia versus antibody positivity alone. Recipient HIV status was reported in the variables “HIV_SEROSTATUS” provided in a separate file that was linked to the UNOS kidney dataset using the variable “PTCODE” and “TRR_ID” to identify a unique patient and transplant episode; it was only provided for transplanted patients. Pre-transplant dialysis exposure and time on dialysis were calculated using the earliest dialysis date provided in the dataset. Additional variables of interest included in the analytic dataset were: 1) recipient variables such as age (categorized as <40, 40–60, >60 years), gender, race (African American versus other), diabetes mellitus, hypertension, and pre-transplant dialysis exposure and duration; 2) donor characteristics including but not limited to age, gender, race, diabetes mellitus, HCV serostatus, extended criteria donor, center for disease control (CDC) high risk donor, and 3) transplant variables such as delayed graft function, immunosuppression regimen at discharge from the index hospitalization, and occurrence of acute rejection. Patient and allograft outcomes were assessed using data provided on survival and allograft function in the UNOS dataset. Data on patient survival was supplemented by linkage to the Social Security Master Death File.
Study design
Primary Statistical Analysis
Analysis of variance (ANOVA) was used to compare normally distributed continuous variables by serostatus groups; Kruskal Wallis test was used for non-normally distributed variables. Dichotomous variables were compared using Chi square or Fisher’s exact testing as appropriate. Kaplan Meier curves were generated and compared using log rank statistics (20). Hazard ratios (HRs) with 95% CIs for the study outcomes, time to death and time to all-cause allograft loss, were estimated using Cox proportional hazards regression (21). Any variable known to be a risk factor for patient or allograft loss as well as those associated with the exposure and outcome were included in the multivariable analysis. Confounders were retained in adjusted models if their inclusion changed the unadjusted HR of the outcome of interest in our exposure categories by more than 10% or were proposed a priori (22) including recipient age, race and sex.
Model checking procedures, inclusive of examination of the proportional hazards assumption of the primary exposure and other covariates in multivariable adjusted models and visualization of log-log plots, were conducted for multivariable models generated for each outcome. If a variable was found to violate the proportional hazards assumption, i.e. a p<0.05 was found, but appeared to have a parallel appearance on visual inspection of the log-log plot, it was retained in the model without adjustment as this discrepancy was attributed to the large size of the dataset (23). This scenario occurred for the following variables: recipient age, recipient diabetes mellitus, donor type (deceased versus live) and induction. Variables not meeting the proportional hazards assumption, i.e. a p<0.05 was found, as well as not meeting visual inspection of log-log plots, were included with an interaction term with time to address changes in the effect size over time. This strategy was undertaken for one variable, discharge immunosuppression, for which the patterns of use appeared to change over the study period.
Interaction terms were created to explore notable clinical differences between groups, including the high proportion of African Americans in the HIV-infected cohort, the high proportion of HIV-infected patients on pretransplant dialysis, and the high proportion of pretransplant diabetes in the HCV-infected and HIV/HCV co-infected cohorts. None of these were statistically significant and were not retained in the final models. An interaction term (“HCV donor*HCV recipient”) created to account for the high proportion of HCV+ donor kidneys allocated to HCV or HIV/HCV co-infected recipients was significant and employed in the final model.
Secondary Statistical Analysis
To account for patients with missing HIV and/ or HCV serostatus, a sensitivity analysis was performed by creating groups that encompassed all combinations of incomplete viral serologies (HIV unknown/HCV unknown, HIV unknown/HCV+ and HIV+/HCV unknown). We then fit a model that included these, as well as the complete viral serostatus groups. The hazard ratios obtained for each incomplete serostatus group was compared statistically to the HIV/HCV uninfected group and secondarily to the group to which they could have been assigned, assuming the missing serostatus was in fact negative (e.g., HIV unknown/HCV+ were compared to HIV−/HCV+ patients and then to HIV+/HCV+ patients), based on the low prevalence of HIV in the general US population (24). We next fit models of the entire cohort assuming that all missing serologies were negative (e.g., HIV unknown/HCV+ were combined with HIV-uninfected/HCV+ to form one “HCV-infected” group). The hazard ratios in this model were then compared to those in the primary analysis.
Handling of Covariate Missingness
The majority of covariates used for model building were less than 5% incomplete. Given the large size of the dataset, no specific procedures were employed to deal with this missingness and a complete case analysis was conducted. In the case of treated rejection at one year and induction, missing in 28% and 23% of subjects respectively, a complete case analysis was conducted and followed by analyses in which missing values were assumed to be the presence or absence of exposure to determine if there were changes in the association of interest. No significant changes in the associations of interest were noted in these models, therefore treated acute rejection and induction were also used in a complete case fashion. CDC high risk donor was missing in 41% of subjects and was not included in any of the multivariable models.
Statistical analyses were performed using Stata 12.1 (Statacorp LP, College Station, TX). All p-values reported are two-sided and a p<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
Funding/Support
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the view or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. KF is supported by the NIH/NIDDK DK090209. DS is supported by the Penn Center for AIDS Research P 30 AI 045008
Footnotes
Disclosures
The authors declare no financial or intellectual conflicts of interest relevant to this article.
References
- 1.Swanson SJ, Kirk AD, Ko CW, Jones CA, Agodoa LY, Abbott KC. Impact of HIV seropositivity on graft and patient survival after cadaveric renal transplantation in the United States in the pre highly active antiretroviral therapy (HAART) era: an historical cohort analysis of the United States Renal Data System. Transpl Infect Dis. 2002;4(3):144–147. doi: 10.1034/j.1399-3062.2002.01009.x. [DOI] [PubMed] [Google Scholar]
- 2.Stock PG, Barin B, Murphy B, et al. Outcomes of Kidney Transplantation in HIV-Infected Recipients. N Engl J Med. 2010;363(21):2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landin L, Rodriguez-Perez JC, Garcia-Bello MA, et al. Kidney transplants in HIV-positive recipients under HAART. A comprehensive review and meta-analysis of 12 series. Nephrol Dial Transplant. 2010;25:3106–3115. doi: 10.1093/ndt/gfq125. [DOI] [PubMed] [Google Scholar]
- 4.Mazuecos A, Fernandez A, Andres A, et al. HIV infection and renal transplantation. Nephrol Dial Transplant. 2011;26:1401–1407. doi: 10.1093/ndt/gfq592. [DOI] [PubMed] [Google Scholar]
- 5.Touzot M, Pillebout E, Matignon M, et al. Renal Transplantation in HIV-Infected Patients: The Paris Experience. Am J Transplant. 2010;10:2263–2269. doi: 10.1111/j.1600-6143.2010.03258.x. [DOI] [PubMed] [Google Scholar]
- 6.Trullas JC, Cofan F, Barril G, et al. Spanish HIV Infection in Dialysis Study Group. Outcome and prognostic factors in HIV-1 infected patients on dialysis in the cART era: a GESIDA/SEN cohort study. J Acquir Immune Defic Syndr. 2011;57(4):276–283. doi: 10.1097/QAI.0b013e318221fbda. [DOI] [PubMed] [Google Scholar]
- 7.Kumar MS, Khan SM, Ranganna K, et al. In HIV+ patients with end stage renal disease (ESRD) kidney transplantation significantly prolongs long-term patient survival compared to chronic dialysis treatment. Abstract presented at American Transplant Congress; 2008 May 31; Toronto, Canada. [Google Scholar]
- 8.Fissell RB, Bragg-Gresham JL, Woods JD, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65(6):2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 9.KDIGO Guideline 4: Management of HCV-infected patients before and after kidney transplantation. Kidney Int. 2008;73(S109):S53–68. [Google Scholar]
- 10.Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy KR. Outcome of Hepatitis C virus Infected Kidney Transplant Candidates who Remain on the Waiting List. Am J Transplant. 2005;5:139–44. doi: 10.1111/j.1600-6143.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 11.Ingsathit A, Kamanamool N, Thakkinstian A, Sumethkul V. Survival Advantage of Kidney Transplantation Over Dialysis in Patients with Hepatitis C: A Systematic Review and Meta-Analysis. Transplantation. 2013;95(7):943–948. doi: 10.1097/TP.0b013e3182848de2. [DOI] [PubMed] [Google Scholar]
- 12.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Martin P. Hepatitis C Virus Antibody Status and Survival After Renal Transplantation: Meta Analysis of Observational Studies. Am J Transplant. 2005;5:1452–61. doi: 10.1111/j.1600-6143.2005.00864.x. [DOI] [PubMed] [Google Scholar]
- 13.Batty DS, Swanson SJ, Kirk AD, Ko CW, Agodoa LY, Abbott KC. Hepatitis C Seropositivity at the Time of Renal Transplantation in the United States: Associated Factors and Patient Survival. Am J Transplant. 2001;1:179–184. [PubMed] [Google Scholar]
- 14.Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Meta Analysis: Interferon for the treatment of chronic hepatitis C in dialysis patients. Aliment Pharmacol Ther. 2003;18(11–12):1071–81. doi: 10.1046/j.1365-2036.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 15.Russo MW, Goldsweig CD, Jacobsen IM, Brown RS. Interferon monotherapy for dialysis patient with chronic hepatitis C: An analysis of the literature on efficacy and safety. Am J Gastroenterol. 2003;98(7):1610–15. doi: 10.1111/j.1572-0241.2003.07526.x. [DOI] [PubMed] [Google Scholar]
- 16.Ozgur O, Boyacioglu S, Telatar H, Haberal M. Recombinant alpha interferon in renal allograft recipients with chronic hepatitis C. Nephrol Dial Transplant. 1995;10(11):2104–6. [PubMed] [Google Scholar]
- 17.Rostaing l, Izopet J, Baron E, Duffault M, Puel J, Durrand D. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation. 1995;59 (10):1426–31. doi: 10.1097/00007890-199505270-00012. [DOI] [PubMed] [Google Scholar]
- 18.Abbott KC, Bucci JR, Matsumoto CS, et al. Hepatitis C and Renal Transplantation in the Era of Modern Immunosuppression. J Am Soc Nephrol. 2003;14:2908–2918. doi: 10.1097/01.asn.0000090743.43034.72. [DOI] [PubMed] [Google Scholar]
- 19.Xia Y, Friedmann P, Yaffe H, Phair J, Gupta A, Kayler LK. Effect of HCV, HIV and Coinfection in Kidney Transplant Recipients: Mate Kidney Analyses. Am J Transplant. 2014;14:2037–47. doi: 10.1111/ajt.12847. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–481. [Google Scholar]
- 21.Cox D. Regression models and life tables. J R Stat Soc Ser. 1972;34:187–220. [Google Scholar]
- 22.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia: Lippincott Williams and Wilkins; 1998. [Google Scholar]
- 23.Thereau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health) New York: Singer Verlag; 2001. [Google Scholar]
- 24. [Accessed on October 11, 2014];HIV Surveillance Report: Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas. 2011 23 http://www.cdc.gov/hiv/statistics/basics. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.