Abstract
The complement system is proposed to play an important role in the pathogenesis of rheumatoid arthritis (RA). The complement system mannan-binding lectin associated serine proteases 1 and 3 (MASP-1/3) cleave proDf (inactive) into Df (active), but it is unknown where this cleavage occurs and whether inhibition of MASP-1/3 is a relevant therapeutic strategy for RA. We show herein that the cleavage of proDf into Df by MASP-1/3 can occur in the circulation and that inhibition of MASP-1/3 by gene silencing is sufficient to ameliorate collagen antibody-induced arthritis (CAIA) in mice. Specifically, to examine the cleavage of proDf into Df, MASP-1/3 producing Df−/− liver tissue (donor) was transplanted under the kidney capsule of MASP-1/3−/− (recipient) mice. Five weeks after the liver transplantation, cleaved Df was present in the circulation of MASP-1/3−/− mice. To determine the individual effects of MASP-1/3 and Df gene silencing on CAIA, mice were injected with scrambled, MASP-1/3 targeted, or Df targeted siRNAs. The mRNA levels for MASP-1 and 3 decreased in the liver to 62% and 58%, respectively, in mice injected with MASP-1/3 siRNAs, and Df mRNA decreased to 53% in the adipose tissue of mice injected with Df siRNAs; additionally, circulating MASP-1/3 and Df protein levels were decreased. In mice injected with both siRNAs the clinical disease activity, histopathologic injury scores, C3 deposition, and synovial macrophage/ neutrophil infiltration were significantly decreased. Thus MASP-1/3 is a new therapeutic target for the treatment of RA, likely through both direct effects on the LP and indirect through the AP.
Keywords: complement, inflammation, arthritis, RNA interference
Introduction
Rheumatoid arthritis (RA) is an inflammatory autoimmune disease that affects approximately 1% of the US population (1). Many potential risk factors for the development of RA have been identified, but the precise pathogenesis of RA is unknown (2). Disease-modifying anti-rheumatic drugs (DMARDS) such as tumor necrosis factor inhibitors, CTLA4-Ig, Rituxan, Tocilizumab and Methotrexate are being used for the treatment of RA, but rarely induce remission in patients with longstanding disease (3). These therapeutic strategies were initially focused upon through observations from rodent models of RA pathogenesis. The complement system (CS) is also thought to play an important role in the development of RA, but the optimal target within the system is uncertain (4). We and others have developed complement-based therapeutics which have shown efficacy in mouse models of RA (5). One of the major goals of our current study was to design and develop a RNA interference-based therapy to inhibit mannan-binding lectin (MBL)-associated serine proteases (MASPs) in order to determine whether these proteins would play an important role in the pathogenesis of experimental RA and might be appropriate therapeutic targets for human RA (6).
Complement is a part of the innate immune system and plays an important role in host resistant to infection. However, complement also mediates tissue damage in autoimmune and inflammatory diseases that affect many different organs. The mechanism(s) which regulate the activation of complement on the surface of pathogens or host cells are finely balanced. Any dysregulation of these mechanisms may cause excessive complement activation, which may lead to tissue injury and inflammation. The system is activated by one of these three pathways: classical (CP), alternative (AP) and lectin (LP) pathways (7). The CP is initiated by IgG or IgM engagement of the C1 complex consisting of C1q, C1s, and C1r. This is followed by the local cleavage of C4 and C2 leading to formation of the CP C3 convertase C4b2a. The LP is activated by the binding of a complex of MBL, ficolin (FCN), collectins (CL), and MASPs to carbohydrates and acetylated residues on cell surfaces (8, 9). C2 and C4 are then cleaved, generating the same C3 convertase as the CP. The AP is activated by low-grade spontaneous hydrolysis of C3 in plasma to form C3(H2O), which interacts with factor B to form a C3(H2O)B complex. Complement factor D (Df) then cleaves factor B into fragments Ba and Bb with formation of the AP initiation C3 convertase C3(H2O)Bb (10, 11).
Several studies have documented the role of the complement system in various mouse model of arthritis, where it has been shown that strains deficient in various upstream and downstream complement components and receptors, including as C3−/−, fB−/−, fD−/−, C5−/−, the C6 component of the membrane attack complex (MAC), and C5aR1−/− mice, are resistant to the collagen-induced arthritis (CIA), glucose-6-phosphate isomerase (GPI) antibody-induced arthritis, and/or collagen antibody-induced arthritis (CAIA) models (12–17).
MASP-1/3 is a single gene that generates by alternative splicing three different mRNAs and three functionally different proteins, i.e. MASP-1, MASP-3 and MAp44 (a.k.a. MAP-1) (18, 19). The MASP-2 gene generates by alternative splicing MASP-2 and MAp19 (a.k.a. MAP-2 and sMAp) proteins (20). Unexpectedly, MASP-1/3 was found to mediate cleavage of proDf (inactive) into Df (active); gene-targeted mice lacking MASP-1/3 have only proDf in their circulation as well as exhibit no LP activity and defective AP activity (21). This study established a direct link between the LP and AP and suggested that upstream components of the LP may be therapeutic targets through indirect inhibition of the AP. Consistent with the idea, MASP-1/3−/− mice lacking MASP-1, MASP-3 and MAp44 were found to be resistant to the development of CAIA (6). The relative importance of MASP-1 versus MASP-3 for the cleavage of proDf into Df is not yet fully defined; however, preliminary in vitro studies suggest MASP-3 may be the dominant cleaving enzyme in vivo (22).
The in vivo physical location of the proDf to Df cleavage event is similarly undefined. This is important as the location of the cleavage event can affect the therapeutic strategy by which one might consider inhibiting the MASPs in order to decrease active Df levels. Interestingly, two morphologically, anatomically and functionally different organs, the liver and adipose tissue, primarily generate MASP-1/MASP-3 and proDf, respectively (21, 23).
MASP-3 has also been shown to cleave Factor B (24). Furthermore it has been reported using a reverse translational approach that in one patient lacking both MASP-1 and MASP-3 the LP was non-functional but still the AP was functional (25). Recently it has been reported that in another patient lacking MASP-1 and MASP-3 the AP was 2.5-fold lower compared with healthy subjects (26). The presence of proDf has also been confirmed later on in the serum of this one patient lacking MASP-1and MASP-3 with intact AP (22). With these findings, as well as the potential unrecognized effects of gene-targeting which complicate analyses, the relative value of MASP-1/3 as therapeutic targets has been uncertain.
Small interfering RNAs (siRNAs) are a new class of bio-therapeutics, as these synthetic siRNAs provide an excellent tool to specifically and effectively silent pathogenic genes and the production of their encoded proteins. These are double stranded RNAs duplexes, 20 – 25 bp long, that interfere with the expression of specific genes via the engagement of the RNA-inducing silencing complex (RISC). Various clinical trials with siRNAs have been performed in patients with cancer as well as ocular and infectious diseases (27). In addition, in rats it has been shown that intra-articular injections of TNF-α and IL-1 siRNAs ameliorated CIA (28). Interestingly, PLGA (poly-lactic-co-glycolic acid) TNF-α siRNAs upon intra-articular administration slowly released TNF-α siRNAs into the joint space and effectively inhibited the local expression of TNF-α eleven days post-injection, which resulted in reduced inflammation (29). Additionally, viral gene delivery methods such as lentiviral vectors and adeno-associated virus type 5 expressing short hairpin RNA (shRNA) for the silencing of B-cell activating factor or galectin-3 or TNF-α, respectively, have shown efficacy in mouse models of arthritis (30–32). Recently, we have shown that the conjugates of anti-C5aR1 siRNA-protamine-C5siRNA ameliorated CAIA in mice (5).
In this study we addressed two inter-related questions related to the functions and effects of inhibiting MASP-1/3. The first was the location where the proteolytic function of MASP-1/3 relative to proDf cleavage could be manifest, and the second was the effect on the development of CAIA by inhibiting MASP-1/3 via RNA interference.
Materials and Methods
Mice
Eight week old C57BL/6 WT male mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were used as controls for the liver/kidney transplant experiments and as the strain in which the CAIA studies were performed. For all studies presented herein a total of 85 C57BL/6 mice were used. MASP-1/3−/− mice provided by Drs. Minoru Takahashi and Teizo Fujita, Fukushima Medical University, Japan. Factor D deficient mice were provided by Dr. Kazue Takahashi, Massachusetts General Hospital Boston. For the liver/kidney transplant studies MASP-1/3−/− and fD−/− mice on the C57BL/6 mice were bred and backcrossed for more than 10 generations prior to use in studies. Liver pieces from an fD−/− donor mouse were implanted beneath the kidney capsule of a MASP-1/3−/− recipient. Mice were maintained in filter top cages with 4–5 mice in each cage in a barrier animal facility with a climate-controlled environment having 12 h light/dark cycles. All mice were fed breeder’s chow provided by the Center for Laboratory Animal Care, University of Colorado Denver. All experiments done were approved by the Institutional Animal Care committee (IACUC).
Liver transplantation under the kidney capsule of mice to examine the cleavage of proDf in the circulation
To examine whether the cleavage of inactive, proDf into active Df could be mediated by MASP-1/3 proteases in the circulation, iso-transplantation was performed as previously described (33) using liver from fD−/− mice under the kidney capsule of MASP-1/3−/− mice. Briefly, 10 MASP-1/3−/− mice were anesthetized using 3–4% isoflurane delivered via a precision vaporizer. Hair over the left dorsal-lateral lumber region was shaved and the site prepped with betadine. A 1–1.5 cm incision was made through the skin and then in the retro-peritoneal wall in the dorsal-lateral lumber region to expose the left kidney. Then a 1–2 mm incision was made in the kidney capsule with a 26G needle and the end of PE-50 tubing and the end of a piece of PE-50 tubing (Instech labs) was inserted beneath the kidney capsule. The PE-50 tubing contained 5–6 pieces of fresh liver (< than 1/10 of mm) in serum-free DMEM medium, obtained from six fD−/− mice. Using this technique, 200–400 hepatocytes are present in each 1 mm piece of the liver. One milliliter of warm saline (0.85%) was injected subcutaneously to prevent the dehydration of mice after surgery. Illustrations of the liver transplantation procedure under the kidney capsule are shown in the Supplement Figures 1 and 2.
Western blot analysis for proDf, Df and MASPs before and after liver transplantation under the kidney capsule
To examine the presence of cleaved Df in the circulation of MASP-1/3−/− mice, before and after liver transplantation, serum was analyzed for proDf, Df and MASP-1 or MASP-3 by using western blot analysis. To detect and examine proDf and Df simultaneously on the same blot in the mouse serum, a 12% Bis-Tris SDS-PAGE gel under reducing conditions was used. Mouse serum (25ul) was pre-incubated with sheep anti-mouse Df antibody (2 ul) (R & D Systems, Inc. Minneapolis, MN), and then ProDf and Df were immunoprecipitated with A/G beads (12 ul) (Santa Cruz Biotechnology, Dallas, Texas), followed by the treatment with Peptide -N-Glycosidase F (PNGase F) overnight at 37°C according to the manufacturer’s suggested protocol (New England BioLabs, Ipswich, MA). After transfer, the blots were incubated for 1 hour with biotinylated sheep anti-mouse anti-Df Ab as an alternative Ab which binds to a different a epitope than the primary anti-Df Ab (dilution 1:500) (R & D Systems, Inc.). Finally proDf and Df bands at ~26 kDa were detected using Streptavidin HRP-conjugated (dilution 1:2000) (Dako, Carpinteria, CA). The band of proDf was visually slightly bigger in kDa than the Df band, and these differences were only visible by using 12% Bis-Tris gel (21).
To detect the presence or absence of MASP-1 and MASP-3 proteins in mouse serum non-reducing 10% SDS-PAGE and a 4–12% Tris glycine gels, respectively, were used. MASP-1 and MASP-3 proteins were first pulled-down from the mouse serum using pre-equilibrated D mannose-agarose beads (1:1 ratio) and N-acetyl-D-glucosamine agarose beads, respectively, (Sigma-Aldrich®) with 1xTBS buffer (Tris-buffered saline) containing Ca2+/Mg2+ according to the previously describe methods (34) along with a slight modification for pulling down MASP-3 protein. This procedure detects MASP-1 or MASP-3 bound to MBL/Ficolins. After transfer, the blots were incubated overnight at 4°C with rabbit polyclonal anti-MASP-1 or MASP-3 antibodies (dilution 1:200). To detect MASP-1, anti-rabbit HRP-conjugated Ab was used as the secondary Ab (dilution 1:5000) (Hycult Biotech, Plymouth Meeting, PA). To detect MASP-3, goat anti-rabbit HRP-conjugated Ab was used as the secondary Ab (dilution 1:5000) (Jackson Immune). The blots were washed 3×10 min in 1xPBS 0.5% Tween 20 and developed for 3 min using a 1:1 mixture of SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific). Bands at ~80 kDa and at 94–100 kDa in serum revealed the presence of MASP-1 and MASP-3, respectively. The blots were scanned using Genegnome XRQ Chemiluminescence Imaging System and Gene Tool analysis software from Syngene (Frederick, MD), and the density of each band was quantified using the Quantity One® software (Bio-Rad, Hercules, California).
Measurement of alternative pathway activation in the serum of recipient MASP-1/3 deficient mouse after liver transplantation
Five weeks after the liver transplantation, serum from the recipient MASP-1/3−/− mouse was examined specifically for AP activity. Sera were diluted (1:10) in GBV (MgEGTA) (Complement Technology, Inc. Tyler, Texas) and added to the 96-well Costar ELISA plates (Corning, Lowell, MA) pre-coated with a mixture of anti-collagen antibodies (ArthritoMab™ antibody)(MD Biosciences, St Paul, MN). The levels of C3 deposition as well as C5a generation from the serum on adherent anti-collagen antibodies were measured according to our previously published studies (6). Sera from mice prior to liver transplantation were used as pre-treatment controls. Sera from C3−/− and NOD (naturally deficient in C5) were used as negative controls for the C3 and C5a ELISA assays, respectively. The data were expressed as mean ± SEM OD value.
Collagen antibody-induced arthritis and treatment with siRNAs
CAIA was induced in C57BL/6 WT male mice using a cocktail of 5 mAbs to bovine CII (ArthritoMab™ antibody) suspended in sterile 1xPBS as previously described (5). Briefly, WT mice were injected i.p. with 4 mg/mouse of ArthritoMab on day 0 and 50 µg/mouse of LPS from E. coli strain 0111B4 on day 3 to synchronize the development of arthritis (5). All mice started to develop arthritis at day 4 and were sacrificed at day 10. Clinical disease activity (CDA) was examined daily until day 10 as per our previously published studies (5–7). CDA was examined by a blinded observer. Two separate CAIA studies were performed, and a total of 30 mice were used. In these two separate studies two different injection routes of the commercially available siRNAs were utilized to assess efficacy. For the first study, CAIA was induced in three cohorts of WT mice. Mice were injected intraperitoneally (i.p.) three times, at day −5, at day 0 and at day 3 with commercially available Accell siRNAs (Thermo Fisher Scientific, Inc. Lafayette). These siRNAs were duplexes of four scrambled control siRNAs, siRNAs targeting proDf and siRNAs targeting MASP-1/3, which were designed specifically to knock down both MASP-1 and MASP-3 simultaneously from the MASP-1 gene. Here mice were injected i.p. with a mixture of four siRNAs (2 ug of each duplex) i.e. 8 ug/mouse of 4 duplexes of siRNAs in 200 ul. Similarly, in the second CAIA study, arthritis in all three cohorts of WT mice was induced as noted above. However, mice were injected intraarticularly (i.a.) in the right knee joint near the patella three times, i.e. at -day 5, at day 0 and at day 3, with a dose of the respective siRNAs (2 ug of each i.e. 8 ug/mouse of 4 duplexes of siRNAs in 50 ul). In parallel, mice injected with scrambled siRNAs (8 ug/mouse i.p. or i.a.) in 1x PBS served as negative controls. All mice were weighed before, during and after the induction of CAIA. All mice were sacrificed at day 10.
Histopathology and immunohistochemical analysis
At day 10, joints from both fore limbs and the right hind limb with knee joint, ankle and paw, from siRNA-treated mice were fixed in a 10% Neutral buffered formalin (NBF) and processed according to our previously published studies (5, 12) for histopathology and immunohistochemical analysis. Due to technical issues mice injected with siRNAs in the right knee joint were not processed for histology. Toluidine-blue (T-blue) stain was used to assess histopathology scores for inflammation, pannus formation, cartilage and bone damage according to published criteria (35). Histology sections were also processed for C3 immunohistochemistry staining on the synovium and on the surface of the cartilage (5, 12). Finally, histology sections obtained at day 10 from the knee joints of all i.p. siRNA injected mice with CAIA were also examined for the infiltration of monocytes/macrophages and neutrophils according to our published methods (5).
Determining the levels of proDf, Df, MASP-1 and MASP-3 proteins in the circulation of CAIA mice treated with MASP-1/3 and Df siRNAs
Western blot analysis was used to examine for the presence of proDf, Df, MASP-1 and MASP-3 proteins in the circulation before and after the i.p. injection of CAIA mice with siRNAs. For scrambled, MASP-1/3 or Df siRNAs, sera were obtained prior (day −5), day 3 and after the induction of disease (day 10). All sera from mice injected three times i.p. with the respective scrambled, MASP-1/3 and Df siRNAs were analyzed. Serum samples from the mice injected with siRNAs, before (day 3) and after (day 10) inflammation, were analyzed for the presence proDf, Df, MASP-1 and MASP-3 proteins as outlined above.
Examination using qRT-PCR of mRNAs to assess specific gene silencing in the knee joints
To examine the tissue specific silencing of the mRNAs by specific siRNAs the technique of quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used according to our previously published studies (36). The mRNAs for MASP-1, MASP-3, MAp44, C1q, C4, Df, fB, C3, IL-1β and TNF-α were measured from the knee joints of mice injected i.p. with siRNAs scramble or Df or MASP-1/3. The mRNA for MASP-1, MASP-3, MAp44 and Df were also measured from the knee joints of WT mice injected with respective siRNAs but with no CAIA to obtain the baseline levels. The standard curve for Df was made using adipose tissue from mice. The standard curves for MASP-1, MASP-3, fB and C3, were made using liver from WT mice and the standard curves for TNF-α, and IL-1β mRNAs were made using RAW cells stimulated with LPS (5 ug/ml) for 24 hrs. All data were expressed in pg/ng 18S ribosomal RNA (rRNA). All mRNA samples from the knee joint of each mouse were analyzed in duplicate.
Examination of tissue specific mRNA gene silencing in CAIA mice injected subcutaneously with siRNAs
To examine the tissue specific silencing of the mRNAs in the liver as well as in the adipose tissue under inflammatory conditions by MASP-1/3 siRNAs or by Df siRNAs, the mRNA levels were measured from these organs. Mice with CAIA were injected three times (day −5, day 0 and at day 3) subcutaneously with scrambled siRNAs or MASP-1/3 siRNAs or Df siRNAs. Liver and visceral adipose tissue was removed at day 10, followed by RNA extraction (36). The mRNAs for MASP-1, MASP-3, MAp44, and Df were measured from the liver and from the adipose tissue. All data were expressed in pg/ng 18S ribosomal RNA as mentioned above. A total of 15 mice were used for this study.
ELISA to measure MASP-1 and Df protein levels
To quantify MASP-1 and Df proteins in the serum samples from mice with CAIA mice before (day 3) and the after i.p. treatments (day 10) with siRNA scramble, siRNA MASP-1/3 and siRNA Df enzyme-linked immunosorbent assay (ELISA) was used. The level of mouse MASP-1 protein was measured using an assay kit (MyBiosource.com) (San Diego, CA). Serum samples were diluted in a standard diluent (1:10) provided with the kit and added to the 96-well ELISA plate. MASP-1 levels were detected according to the manufacturer’s instructions. All measurements were conducted at 450nm. Serum from MASP-1/3−/− mice and WT mice were used a negative and positive controls, respectively. The Df proteins levels were measured from the serum samples from all siRNA treatment groups according to our previously published studies (17). This Df ELISA method does not distinguish between proDf and Df levels. The serum from fD−/− mice and WT mice were used as a negative and positive controls respectively. All OD values have been expressed for consistency and comparison as mean + SEM.
Statistical analysis
The normality of the CDA and of the histological data was examined by using w-statistics. One way analysis of variance (ANOVA) was used to compare all three treated groups and these p-values have been calculated separately for each experiment. The p - values indicated by stars in all graphs and histograms were calculated using Student’s t test with GraphPad Prism® 4. Each treatment group was compared with siRNA scrambled treatment. The differences in the levels of MASP-1 and Df before and after the siRNA treatments were also confirmed by paired t-test. The data in graphs and histograms are shown as the mean + SEM, with p < 0.05 considered significant.
Results
Morphological and histological analysis of the transplanted liver under the kidney capsule of MASP-1/3−/− mice
Transplants were performed as described in Materials and Methods. The kidneys of MASP-1/3−/− recipient mice were collected at week 5 to determine the presence of hepatocytes and their regeneration under the kidney capsule (Fig. 1A). All mice survived the liver transplantation surgery, and there was no weight loss. The morphology of the hepatocytes was confirmed by the presence of healthy and dividing cuboidal and hexagonal cells (Fig. 1B). The boundary between the hepatocytes and kidney was confirmed by the presence of large round renal corpuscles with glomerulus in the cortex of kidney (Fig. 1A, 1B). Hematoxylin and eosin (H & E) staining shows the presence of an intact nucleus in each cell (Fig. 1B). Non-transplanted second kidneys from the transplanted MASP-1/3−/− mouse group were also stained in parallel with H & E and used as negative controls (data not shown). H & E histological data confirmed the presence of healthy hepatocytes derived from the liver from fD−/− (donor) mouse under the kidney capsule of MASP-1/3−/− mouse even after 5 weeks of the transplantation. The success rate of reconstitution after the liver transplantation under the kidney capsule was 75% as assessed by the presence of cleaved Df and visible transplanted tissue.
Figure 1.
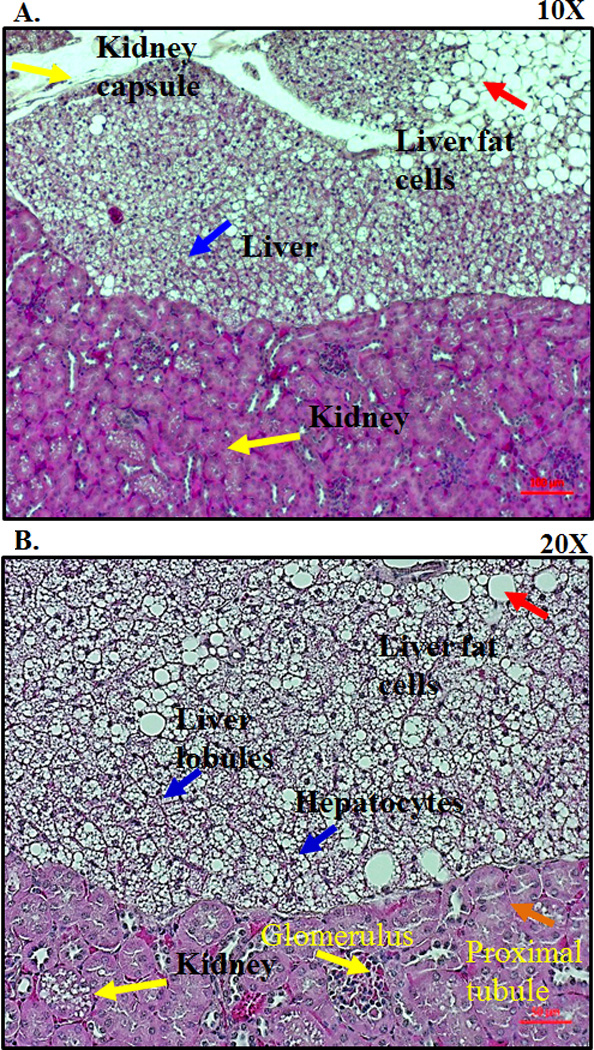
Histological analysis of the transplanted liver tissue under the kidney capsule. MASP-1/3−/−(recipient) mouse was transplanted with the fD−/− (donor) mouse liver tissue under the kidney capsule. All mice were sacrificed at 5–14 weeks (n = 5). Kidneys, both with (left organ) and without (right organ) transplanted liver fragments, were removed and fixed in a 10% neutral buffered formalin. Histology sections from the kidneys were obtained from all mice to assess for viable liver tissue. A. Hematoxylin and eosin staining from one transplanted mouse at 5-weeks showing the presence of healthy hepatocytes (blue arrow) under the kidney capsule (yellow arrow) at a 10X magnification. Presence of adipose tissue or fat cells (red arrow) in the liver tissue as well as under the kidney capsule at a 10X magnification. B. Presence of hexagonal type hepatocytes (blue arrow) with dividing nucleus under the kidney capsule at a 20X magnification. Presence of kidney capsule showing glomerulus (yellow arrow) and proximal renal tubule (orange arrow) at a 20X magnification. Red scale bars in A and B equals 0.1 mm (100 µm).
Functional effect of liver transplantation under the kidney capsule on proDf in the circulation of MASP-1/3−/− mice
Sera from transplanted MASP-1/3−/− mice obtained before (day 0) and after week 5 of transplantation were examined using Western blot analysis for the cleavage of proDf into Df (Fig. 2A) as well as the presence of MASP-1 and MASP-3 proteins (Fig. 2B). As expected fD−/− mice expressed MASP-1 but did not express Df (Fig. 2A lane 1), while WT mice expressed Df (Fig. 2A lane 2) and both MASP-1and MASP-3 (Fig 2B, lane 2, upper & lower panels). MASP1/3−/− mice did not express MASP-1 or MASP-3 (Fig. 2B, lane 3, upper & lower panels), and as expected expressed proDf but not mature Df (Fig 2A, lane 3). Sera from transplant mice on day 0 contained only proDf (Fig. 2A, lane 4) and no MASP-1 or MASP-3 (Fig. 2B, lane 4, upper & lower panels), while sera from transplanted mice after 5 weeks revealed the new expression of MASP1 and MASP-3 (Fig. 2B, lane 5, upper & lower panels) as well as cleavage of proDf into mature Df (Fig. 2A, lane 5).
Figure 2.
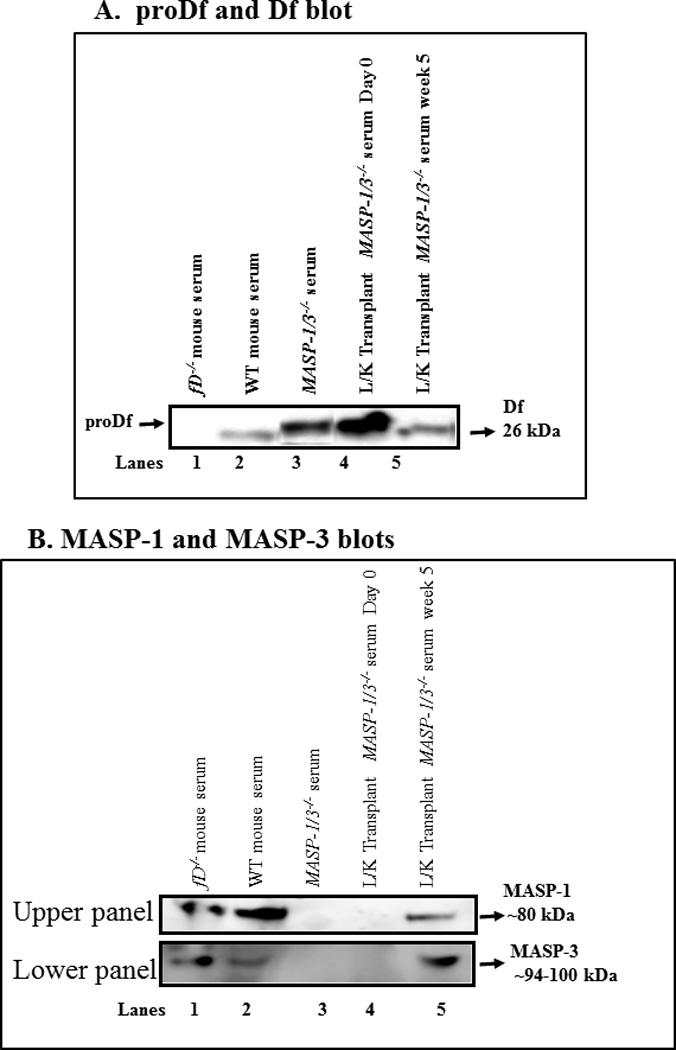
Western blot analysis of the cleavage of the proDf into Df and hepatocyte-specific MASP-1 and MASP-3 proteins generated in the circulation. MASP-1/3 cleavage of proDf results in a loss of 5 amino acids which can be visualized as a slight increase in mobility in SDS-PAGE. Sera from MASP-1/3−/− (receipt) mice were obtained before day 0 and 5 weeks following liver transplantation. Serum from fD−/− (donor) mouse mice were also obtained. A. To examine the cleavage of proDf into Df protein in the circulation of MASP-1/3−/− mice, before and after liver transplantation, sera were immunoprecipatated for Df and then digested with PNGase F overnight at 37°C, according to Material and Methods. A 12% Bis-Tris reducing SDS-PAGE gel was used to distinguish between proDf and Df proteins. Sera from fD−/− and WT mice were used as negative and positive controls (lanes 1, 2). A band of proDf was still intact in the serum from a MASP-1/3−/− mouse (lane 3) and also in MASP-1/3−/−(recipient) mice prior to liver transplantation (lane 4). A clear Df band of 26 kDa was present in the serum of a MASP-1/3−/− mouse 5 weeks after liver transplantation (lane 5), indicating the cleavage of proDf into Df. The presence of MASP-1 and MASP-3 proteins in the circulation of MASP-1/3−/− mice transplanted with the liver under the kidney capsule was confirmed by using mannose and N-acetyl-D-glucosamine, respectively, pull-down assays followed by Western blot analysis as described in the Methods section. B (upper panel) Absence of MASP-1 before liver transplantation in the serum from a MASP-1/3−/− mouse as expected (lane 4) but its presence 5 weeks after liver transplantation in a MASP-1/3−/− mouse (lane 5). Sera from fD−/− and WT mice were used as positive controls (lanes 1, 2), and serum from a MASP-1/3−/− mouse was used as a negative control (lane 3). B (lower panel) Absence of MASP-3 before liver transplantation in the serum from a MASP-1/3−/− mouse as expected (lane 4) but its presence 5 weeks after the liver transplantation in a MASP-1/3−/− mouse (lane 5). Sera from fD−/− and WT mice were used as positive controls (lanes 1, 2), and serum from a MASP-1/3−/− mouse was used as a negative control (lane 3).
Presence of a functional alternative pathway in recipient MASP-1/3−/− mice after liver transplantation
We next investigated the ability of these transplanted mice to activate complement via the AP (Fig. 3). Normally MASP-1/3−/− mice exhibit a defective AP (21). C3 deposition and C5a generation through the AP were assessed using adherent anti-collagen antibodies in Mg++/EGTA buffer as described in Materials and Methods (Fig. 3A & 3B). As expected, the AP was fully functional in WT mice and inactivated in C3−/− and fD−/− mice (Fig 3A). The mixing of serum from MASP-1/3−/− and fD−/− mice also restored the AP (Fig. 3A). Following liver transplantation, adherent anti-collagen antibodies in vitro significantly activated C3 deposition (p < 0.05) and generated C5a (p < 0.05) in the serum from transplanted MASP-1/3−/− mouse (Fig. 3A & 3B). Overall, AP activity at week 5 was restored in the serum from recipient MASP-1/3−/− mouse and was almost equivalent to WT mice (Fig. 3A & 3B).
Figure 3.
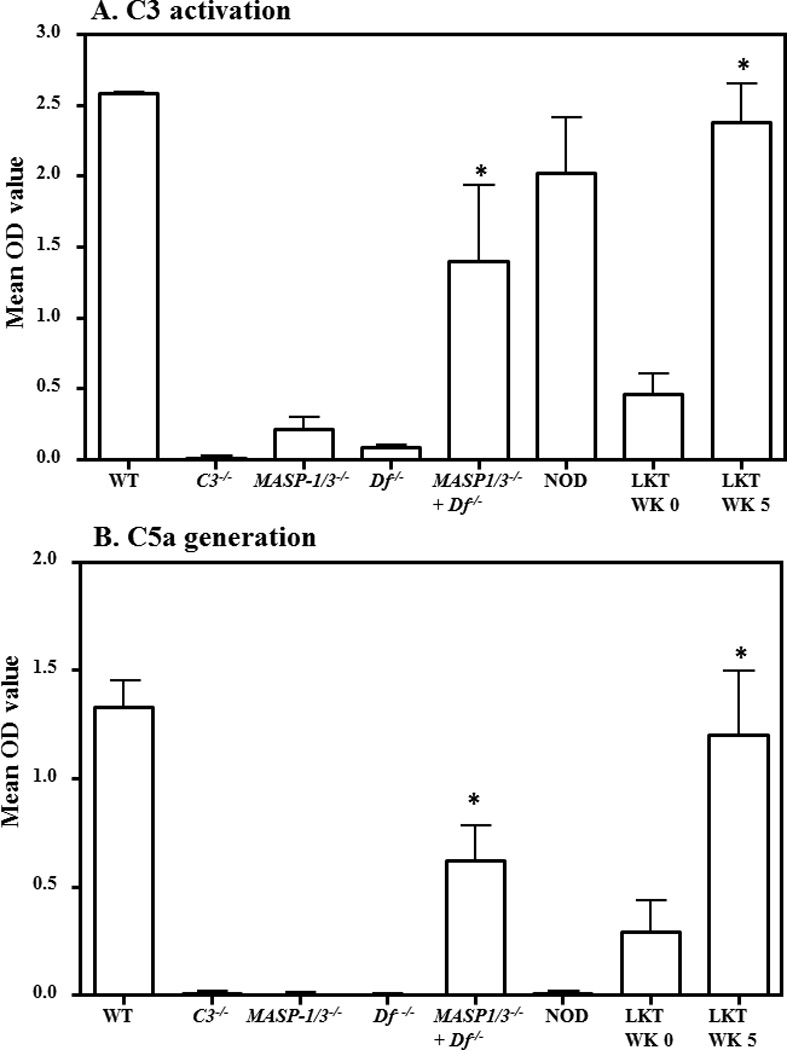
ELISA demonstrating the restoration of defective AP activity in liver transplanted MASP-1/3−/− mice. The AP activity was examined by ELISA after incubation of serum on adherent anti-collagen antibodies followed by measurement of C3 deposition and C5a generation in a Ca++ deficient buffer (Mg++/EGTA). A. C3 deposition using sera from transplanted MASP-1/3−/− mice before (WK 0) and after transplantation (WK 5). Sera from WT and C3−/− mice were used a positive and negative controls, respectively. Sera from non-transplanted MASP-1/3−/− and fD−/− mice were also used to show the defective AP followed by a full restoration of the defective AP in MASP-1/3−/− mice when sera were mixed (1:1 ratio). B. C5a generated after incubation of sera from transplanted MASP-1/3−/− mice before (WK 0) and after transplantation (WK 5). Sera from WT and NOD mice were used as positive and negative controls, respectively. Sera mixed in vitro from MASP-1/3−/− and fD−/− (1:1 ratio) mice also generated C5a. All data represent the mean + SEM OD values based on n = 4 WT mice; n = 4 C3−/− mice; n = 3 MASP-1/3−/− mice; n = 3 fD−/− mice; n = 3 NOD mice, n = 4 WK 0 transplanted MASP-1/3−/− mice and n = 4 WK 5 transplanted MASP-1/3−/− mice. *p < 0.05 in comparison to the MASP-1/3−/− mice or WK 0. WK = Week
Effect of subcutaneously injected MASP-1/3 and Df siRNAs on MASPs and Df mRNAs in the liver and adipose tissue of mice with CAIA
Given our previous work demonstrating the necessity of complement for the development of arthritis in the CAIA model, the above data strongly suggest that MASP-1/3 and Df might serve as therapeutic targets. To test this, we generated siRNAs for MASP1/3 and Df. We validated the efficacy and specific ability of the SQ injected siRNAs for MASP-1/3 or Df to inhibit the mRNAs for MASP-1, MASP-3, and Df in the liver as well as in the visceral adipose tissue in mice with CAIA (Table 1). Adipose and liver tissue was harvested on day 10 post initiation of the CAIA model and RNA prepared. The expression levels of MASP-1, MASP-3, MAp44 and Df mRNAs were determined at day 10 from the liver and from the visceral adipose tissue. In CAIA mice, injected three times SQ with MASP-1/3 siRNAs, there was a specific decrease in the mRNAs for both MASP-1 and MASP-3 (Table 1). These mRNAs were downregulated significantly to 62% and 58%, respectively, in the liver compared with the mRNAs from the liver of mice treated with scrambled siRNAs (Table 1) (p < 0.05). In contrast, when CAIA mice were treated three times SQ with Df siRNAs, there was a statistically significant (p < 0.008) 53% decrease in mRNA expression levels for Df only in the adipose tissue, but no significant change was seen in the mRNA levels for MASP-1 and MASP-3 respectively (Table 1). Surprisingly the expression of MAp44 in contrast increased significantly in the liver when mice were treated with MASP-1/3 (p < 0.01) and Df (p < 0.02) siRNAs (Table 1). No effect of siRNAs treatment was seen on the expression of MAp44 expression in the adipose tissue (Table 1). These data confirm that siRNAs for MAPS-1/3 or Df injected through the SQ route were still functional in vivo and decreased their specific mRNAs in respective target tissues.
Table 1.
mRNA for MASPs, MAp44 and Df in the liver and in adipose tissue in WT mice with CAIA1
| mRNA | Tissue | siRNAs scramble2 | siRNAs MASP-1/32 | siRNAs Df2 |
|---|---|---|---|---|
| MASP-1 | Liver | 555.9 ± 92.4 | 213.5 ± 99.3 | 359.4 ± 25.4 |
| P | 0.034* | 0.04 | 0.07 | |
| Decrease | 62% | 35% | ||
| MASP-1 | Adipose | 2.7 ± 0.94 | 2.12 ± 0.37 | 2.75 ± 0.47 |
| p | 0.53* | |||
| MASP-3 | Liver | 99.97 ± 17.06 | 41.62 ± 12.4 | 65.81 ± 6.74 |
| p | 0.042* | 0.04 | 0.09 | |
| Decrease | 58% | 34% | ||
| MASP-3 | Adipose | 2.11 ± 0.64 | 1.70 ± 0.31 | 1.53 ± 0.18 |
| p | 0.64* | |||
| MAp44 | Liver | 23.9 ± 3.1 | 49.41 ± 8.95 | 40.5 ± 4.0 |
| p | 0.019* | 0.01 | 0.02 | |
| Increase | 52% | 41% | ||
| Map44 | Adipose | 0.42 ± 0.29 | 0.95 ± 0.28 | 0.49 ± 0.07 |
| p | 0.36* | 0.068 | 0.28 | |
| Df | Liver | 0.85 ± 0.23 | 0.47 ± 0.18 | 0.97 ± 0.34 |
| p | 0.44* | |||
| Df | Adipose | 8632.2 ± 824.3 | 6290.2 ± 508.3 | 4076.0 ± 1042.1 |
| p | 0.009* | 0.06 | 0.008 | |
| Decrease | 27% | 53% |
Data are expressed in pg/ng 18s rRNA with mean ± SEM based on the indicated number of mice (n).
Mice with injected three times i.e. at day −5, day 0 and at day 3 subcutaneously (SQ) with scrambled siRNAs (n = 5), MASP-1/3 siRNAs (n = 5) and Df siRNAs (n =5). CAIA was also induced in these mice as mentioned in the Methods section. At day 10, liver and adipose tissue (visceral fat) from all CAIA was removed and analyzed for mRNAs for MASP-1, MASP-3 and Df. All p-values for different mRNAs in mice treated SQ either with siRNAs MASP-1/3 or siRNAs Df were compared with the corresponding values of WT mice treated with SQ treated scrambled siRNAs. The percent (%) decrease in mRNA levels in mice treated SQ with siRNAs MASP-1/3 or siRNAs Df in each column has been shown compared with mice treated SQ with scrambled siRNAs. The mRNA levels for MASP-1 or MASP-3 were below the detection limits in the adipose tissue and similarly the mRNA levels for Df were below the detection limits in the liver of mice with CAIA injected SQ with siRNAs for scramble, MASP-1/3 or Df. p values were compared between CAIA mice treated either with scrambles siRNA and siRNAs MASP-1/3 or siRNA Df. p < 0.05 were considered statistically significant.
p value among all siRNA treatment groups using ANOVA
In vivo effect of MASP-1/3 and Df siRNAs on CAIA
To determine the effect of MASP-1/3 or Df siRNAs on arthritis, two different CAIA experiments were performed using different routes of injections (Fig. 4). Mice were either injected three times, i.p. or i.a. at days −5, day 0 and at day 3 with 8 µg of scrambled siRNAs or MASP-1/3 siRNAs or Df siRNAs. Overall we found that mice injected either i.p. or i.a. were significantly (p < 0.05) protected from the CAIA (Fig. 4A & 4D). The differences in the CDA among three siRNA treatment groups were significant (p < 0.030) as confirmed by ANOVA. The CDA in mice, at day 10, injected i.p. with scrambled siRNAs, MASP-1/3 siRNAs and Df siRNAs, at day 10, was 12.0 ± 0.0, 3.6 ± 2.68, and 3.2 ± 2.60, respectively (Fig. 4A). The prevalence of disease in CAIA mice injected i.p. with siRNAs was 100%, 40%, and 40%, respectively (Fig. 4B). There was no major change in weight in mice treated with siRNAs for MASP-1/3 or Df, but there was a significant (p < 0.05) loss of weight in mice injected with scrambled siRNAs (Fig. 4C). However one way ANOVA test showed that the differences among the weights of i.p. siRNA treated mice were not significant (p < 0.13). The CDA in mice at day 10 injected i.a. with scrambled siRNA, MASP-1/3 siRNAs and Df siRNAs at day 10 was 10.2 ± 1.47, 6.25 ± 2.17 and 4.2 ± 1.59, respectively (Fig. 4D). The prevalence of disease in CAIA mice injected i.a. was 100%, 75% and 80%, respectively (Fig. 4E). There was no major change in weight in all three groups of mice treated with siRNAs (Fig. 4F). These data show that mice injected using two different routes (i.p. or i.a.) either with MASP-1/3 siRNAs or with Df siRNAs were protected.
Figure 4.
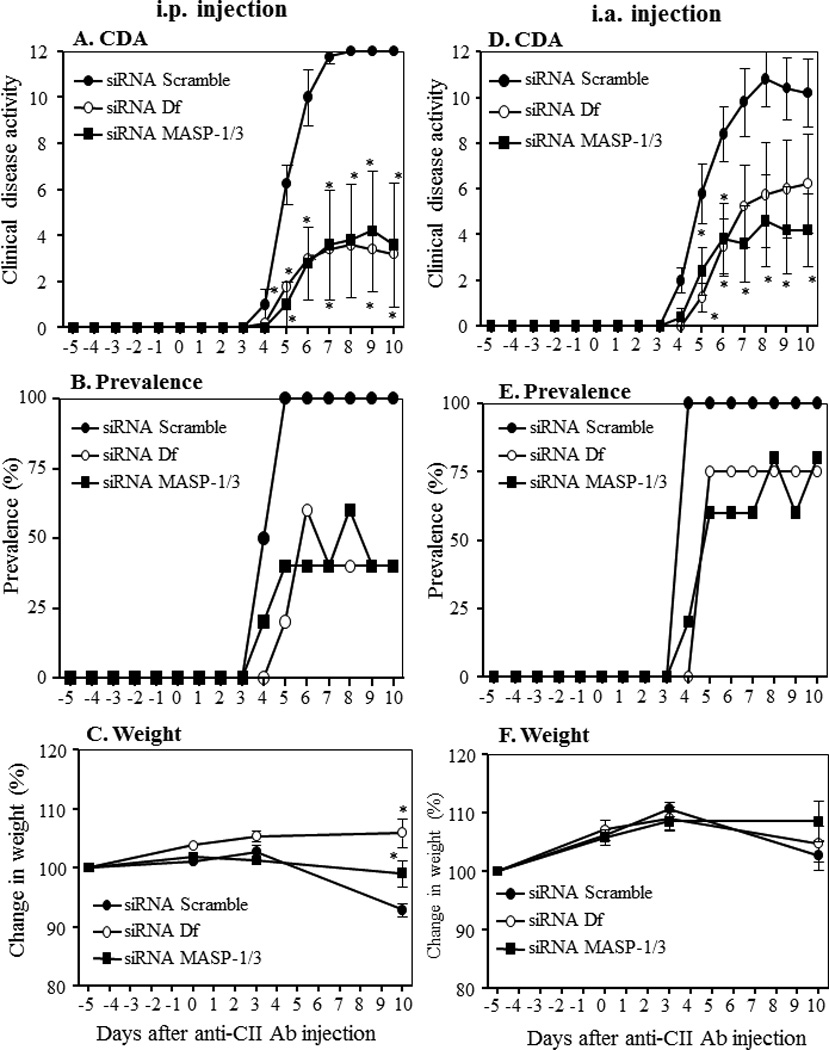
Decrease in the clinical disease activity in mice treated with siRNAs as scrambled control compared to siRNA specific for MASP-1/3 or Df. CAIA was induced in C57BL/6 WT mice with a mixture of anti-collagen monoclonal antibodies. A-C WT mice were injected i.p with a mixture of siRNAs duplexes as scrambled, MASP-1/3 specific or Df specific at day −5, day 3 and at day 10. A. CDA in mice treated with siRNAs as scrambled or specific for MASP-1/3 and Df. B. Prevalence (%) at day 10 in mice treated with siRNAs as scrambled or specific for MASP-1/3 and Df. C. Change in weight (%) in mice treated with siRNAs as shown. D-F In a separate cohort mice were injected i.a. (only in the right knee joint) with a mixture of siRNAs duplexes as scrambled or specific for MASP-1/3 and Df at day −5, day 3 and at day 10. D. CDA in mice treated with siRNAs indicated. E. prevalence (%) in mice treated with siRNAs as indicated. F. change in weight (%) in mice treated with siRNAs as indicated. Data shown represent the mean + SEM based on WT mice injected i.p. with siRNAs scrambled, n = 5, siRNAs for MASP-1/3, n = 5 and siRNAs for Df, n = 5 and also based on WT mice injected i.a. in the right knee joint with siRNAs scrambled, n = 5, siRNAs for MASP-1/3, n = 5 and siRNAs for Df, n = 5. *p<0.05 in comparison to mice to scrambled siRNAs injected mice.
Effect of treatment with siRNAs for MASP-1/3 and Df on joint histopathology
All CAIA mice treated i.p. or i.a. with siRNAs with scrambled as well as MASP-1/3 or Df were sacrificed at day 10. All joints from i.p. treated mice were processed for histopathology and immunohistochemical analysis (Fig. 5). Both forelimbs and the right hind limb (five joints) were processed for histopathology using T-blue and for the measurement of local C3 deposition (Fig. 5A, 5B). Five joints from scrambled siRNA or MASP-1/3 siRNA or Df siRNA treated mice were examined for inflammation, pannus formation (multi-layered synovium), cartilage damage, and bone damage (Fig. 5A). The ANOVA showed that overall there were significant (p < 0.04) differences in the histopathology scores among all the groups of mice treated with siRNAs. The all joint mean inflammation scores for the scrambled siRNAs, MASP-1/3 siRNAs and Df siRNAs treatment groups were 3.04 ± 0.59, 1.12 ± 0.47 and 0.88 ± 0.49, respectively (Fig. 5A). Individual scores for pannus formation, cartilage and bone damage were also significantly (p < 0.05) decreased in mice treated with MASP-1/3 siRNAs or Df siRNAs compared with the scrambled siRNAs (Fig. 5A). Overall, all joint mean (AJM) scores for histopathology were significantly (p < 0.001) reduced by 72% and 74% in mice treated with MASP-1/3 siRNAs and Df siRNAs as compared with scrambled siRNAs (Fig. 5A). Representative histopathology pictures of the knee joints these mice are shown in Fig 6 (A, B and C).
Figure 5.
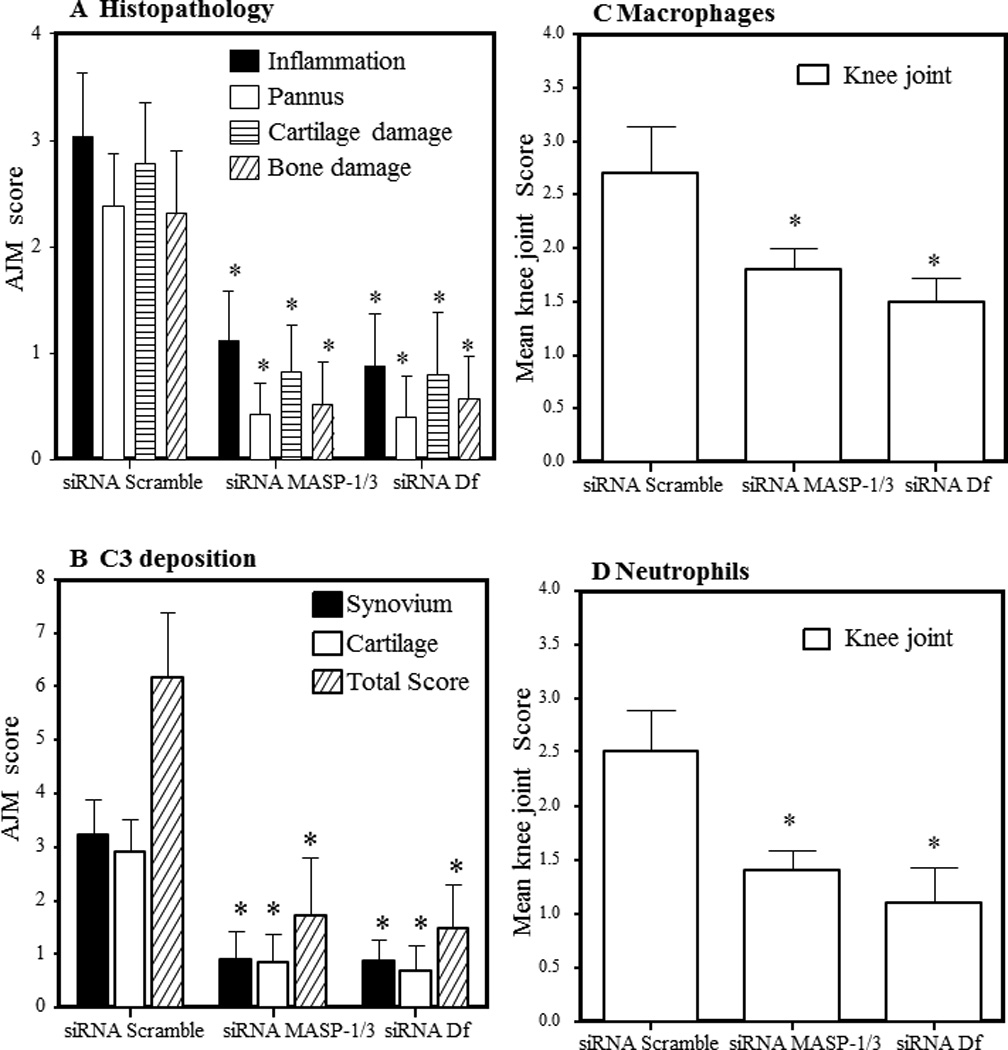
Decrease in histopathology scores, C3 deposition, monocyte/macrophage infiltration and neutrophil infiltration in CAIA mice injected i.p. with scrambled siRNAs as compared to siRNAs for MASP-1/3 and Df. WT mice with CAIA were treated at day −5, day 0, and at day 3 with the indicated siRNAs. All mice were sacrificed at day 10 and histopathology using T-blue measured in all joints mean score (AJM) at day 10. A. Histopathology for inflammation, pannus formation, cartilage damage and bone damage. B. All joint mean (AJM) of C3 deposition from all joints in the synovium, on the surface of cartilage and total scores (synovium plus cartilage). C) Knee joint monocyte/macrophage infiltration. D. Knee joint neutrophil infiltration. Mean score of macrophages and neutrophils was obtained from the knee joints of mice in all siRNA treatment groups at day 10. All data represent the mean + SEM based on n = 15 for all groups. *p < 0.05 in comparison to the scrambled siRNAs.
Figure 6.
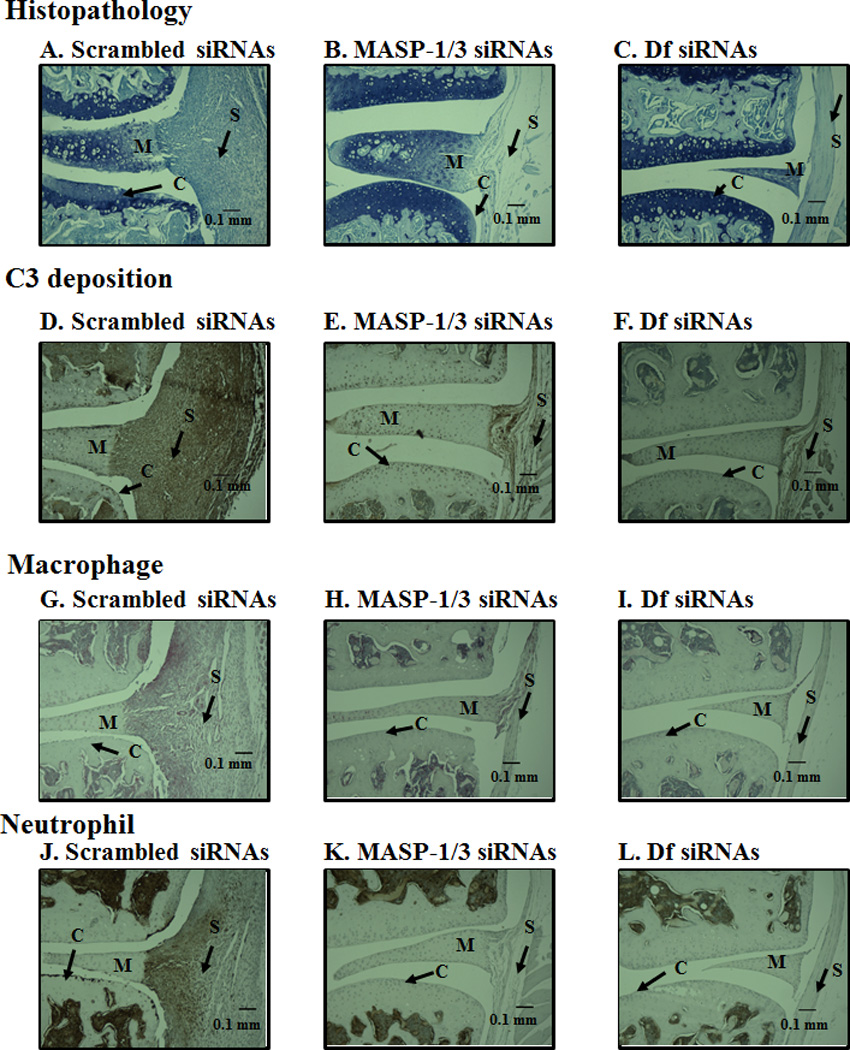
Representative histopathology, C3 deposition, macrophage and neutrophil images from the knee joints of CAIA mice injected i.p. with scrambled siRNAs or for MASP-1/3 or Df. All joints were fixed with 10% neutral buffered formalin, paraffin embedded, and sectioned at a thickness of 5 µm. The top three panels from left to right (A, B & C) show staining with toluidine-blue (blue color) from the knee joints of CAIA mice treated with scrambled siRNA (left panel) or MASP-1/3 siRNAs (center panel) or Df siRNAs (right panel). The second set of three panels from left to right (D, E & F) show staining with anti-C3 Ab (dark brown color) from the ankle joints of CAIA mice treated with scramble siRNAs (left panel) or MASP-1/3 siRNAs (center panel) or Df siRNAs (right panel). The third set of three panels from left to right (G, H, & I) show staining with F4/80 for macrophages (red color) from the knee joints of CAIA mice treated with scrambled siRNA (left panel) or MASP-1/3 siRNAs (center panel) or Df siRNAs (right panel). The fourth set of three panels from left to right (J, K & L) show staining for neutrophils (brown color) from the knee joints of CAIA mice treated with scramble siRNA (left panel) or MASP-1/3 siRNAs (center panel) or Df siRNAs (right panel). Areas of synovium (S-black arrow), cartilage (C-black arrow), bone (B-black arrow) and meniscus (M-black arrow) are identified. The sections were counterstained with hematoxylin & eosin and photographed under the 10x objective using Zeiss Observer. D1 (AXIO) microscope. Red scale bar in A-L equals 0.1 mm (100 µm).
Immunohistochemical evaluation of C3 deposition in the joints of CAIA mice treated with MASP-1/3 and Df siRNAs
C3 deposition scores at day 10 in the synovium as well as on the surface of the cartilage from the knee joints of CAIA mice treated i.p. with scrambled siRNAs, MASP-1/3 siRNAs and Df siRNAs were 6.16 ± 1.23, 1.72 ± 1.08, and 1.48 ± 0.81, respectively (Fig. 5B). There were significant (p < 0.003) among three siRNA treatment groups related to the deposition of C3 in all joint in all joints. Thus C3 deposition in the synovium (p < 0.023) and on the cartilage surface (p < 0.0013) was significantly reduced in mice treated with MASP-1/3 siRNAs or Df siRNAs as compared with mice treated with the scrambled siRNAs (Fig. 5B). Overall, all joint mean score (AJM) (synovium and cartilage) for C3 deposition were reduced by 72% and 76% in mice treated with MASP-1/3 siRNAs or Df siRNAs, respectively, as compared to scrambled siRNAs. Individually, C3 deposition in the synovium of mice with CAIA treated with the MASP-1/3 siRNAs and Df siRNAs as compared with mice treated with scrambled siRNA was decreased by 72% and 73%, respectively (Fig. 5B). Knee joints from WT with or without CAIA and C3−/− mice with no disease were used as positive and negative controls respectively for C3 staining (data not shown). Representative C3 deposition from the knee joints from mice treated with scrambled siRNAs or MASP-1/3 siRNAs or Df siRNAs are shown in Fig 6 (D, E and F).
Effect of siRNA for MASP-1/3 and Df on macrophage and neutrophils infiltration in the synovium from the knee joints in mice with CAIA
Knee joint synovial infiltrates were assessed, by immunohistochemistry for macrophages and neutrophils on day 10 from mice treated i.p. with scrambled siRNAs or MASP-1/3 siRNAs or Df siRNAs using methods as described in our previously published studies (5, 36). The percentage of macrophages and neutrophils was decreased significantly (p < 0.05) in the synovium of CAIA mice treated with MASP-1/3 siRNAs or Df siRNAs in comparison with the mice treated with scrambled siRNA (Fig. 5C, 5D). Macrophage counts in the knee joints of three groups were 2.70 ± 0.44, 1.80 ± 0.20, and 1.50 ± 0.22, respectively, in mice treated scrambled siRNAs, MASP-1/3 siRNAs and Df siRNAs, respectively (Fig. 5C). The overall decrease in the knee joint synovial macrophages in MASP-1/3 siRNA or Df siRNA treated mice was 33% (p < 0.033) and 44% (p < 0.025), respectively as compared with the scrambled siRNA treated mice (Fig. 5D). Neutrophil counts for the three groups were 2.50 ± 0.39, 1.40 ± 0.19, and 1.10 ± 0.33, respectively, treated for mice treated with scrambled siRNAs, MASP-1/3 siRNAs and Df siRNAs (Fig. 5D). The decrease in the percentages of synovial neutrophils was 44% (p < 0.045) and 56% (p < 0.039), respectively, in CAIA mice treated with MASP-1/3 siRNAs and Df siRNAs as compared to scrambled siRNA treated mice (Fig. 5D). Representative pictures of macrophage and neutrophil IHS from the knee joints of mice treated with scrambled siRNAs or MASP-1/3 siRNAs or Df siRNAs are shown in Fig. 6 (G, H and I) and Fig. 6 (J, K and L), respectively. The immunohistochemical data show that the treatment of CAIA mice with either siRNAs for MASP-1/3 or Df decreased the percentages of macrophages and neutrophils in the knee joint synovium as one relevant potential mechanism of effect.
Effect of the MASP-1/3 and Df siRNAs on mRNA levels for MASPs, complement components and cytokines in the knee joints of mice with CAIA
We next determined the extent to which i.p. injected siRNAs for MASP-1/3 or Df decreased mRNAs encoding MASP-1, MASP-3 or various complement components and cytokines in the knee joints in mice with CAIA (Table 2). Knee joints from the mice with CAIA and injected i.p. with scrambled siRNAs were used as controls (Table 2). RNA from left knee joints was extracted from siRNA injected CAIA mice and analyzed by qRT-PCR (Table 2). The expression levels of MASP-1, MASP-3, MAp44, C1q, C4, Df, fB, C3, IL-1β and TNF-α mRNAs obtained at day 10 were examined from the left knee joints from all mice. Both MASP-1 and MASP-3 mRNAs were downregulated significantly to 53% and 61%, respectively, (p <0.05) in the knee joints of mice treated with MASP-1/3 siRNAs as compared to scrambled siRNAs treated mice (Table 2). No significant (p < 0.07) change was observed in the expression of MAp44 in the knee joints when mice were treated MASP-1/3 or Df siRNAs (Table 2). Interestingly, when mice were treated with Df siRNAs there was not only a 71% statistically significant (p < 0.031) decrease in mRNA expression levels for Df but also a significant (p < 0.05) decrease of 37% and 44% in the mRNA levels for MASP-1 and MASP-3, respectively (Table 2). The mRNAs for C1q and C4 decreased significantly to 54% (p < 0.012) and 63% (p < 0.006) in the knee joints of mice treated with siRNAs for MASP-1/3. The mRNAs for fB and C3 also were decreased significantly (p < 0.05) in mice treated with siRNAs for MASP-1/3 or Df (Table 1). The mRNA levels for IL-1β, in the knee joints of CAIA mice treated with siRNAs for MASP-1/3 or Df were decreased significantly (p < 0.034 or p < 0.012) to 83% and 79%, respectively (Table 2). However, no significant change in the mRNA levels for TNF-α was seen in the knee joints of CAIA mice treated with siRNAs for either MASP-1/3 or Df (Table 2). We found no change in the expression of MASP-1, MASP-3, MAp44 and Df in the knee joints of mice without CAIA treated with respective siRNAs (Table 3). These data confirm that siRNAs for MASP-1/3 or Df were functional in vivo and specifically decreased within the synovium their respective mRNAs as well as those encoding specific pro-inflammatory cytokines.
Table 2.
mRNA for MASPs, complement components and cytokines in the knee joints of WT mice with CAIA1
| mRNA | siRNAs scramble2 | siRNAs MASP-1/32 | siRNAs Df2 |
|---|---|---|---|
| MASP-1 | 67.15 ± 7.54 | 31.45 ± 8.64 | 42.09 ± 3.25 |
| p | 0.009* | 0.014 | 0.015 |
| Decrease | 53% | 37% | |
| MASP-3 | 26.89 ± 3.65 | 10.48 ± 1.60 | 15.12 ± 1.82 |
| p | 0.002* | 0.0032 | 0.019 |
| Decrease | 61% | 44% | |
| MAp44 | 7.30 ± 1.55 | 7.11 ± 1.13 | 11.34 ± 1.27 |
| p | 0.07* | 0.92 | 0.08 |
| Decrease/increase | 3% | 36% | |
| Df | 650.2 ± 169.7 | 474.5 ± 92.08 | 190.22 ± 45.1 |
| p | 0.043* | 0.38 | 0.031 |
| Decrease | 27% | 71% | |
| C1q | 597.2 ± 82.1 | 274.8 ± 58.37 | 398.64 ± 43.96 |
| p | 0.012* | 0.012 | 0.065 |
| Decrease | 54% | 33% | |
| C4 | 142.7 ± 22.7 | 53.13 ± 9.34 | 103.6 ± 13.9 |
| p | 0.009* | 0.006 | 0.179 |
| Decrease | 63% | 27% | |
| fB | 280.3 ± 68.4 | 79.7 ± 30.1 | 108.4 ± 18.97 |
| p | 0.014* | 0.027 | 0.032 |
| Decrease | 72% | 61% | |
| C3 | 1123.1 ± 229 | 184.2 ± 68.5 | 337.9 ± 93.5 |
| p | 0.003* | 0.006 | 0.019 |
| Decrease | 83% | 70% | |
| TNF-α | 19.58 ± 3.73 | 10.85 ± 1.39 | 17.80 ± 4.83 |
| p | 0.22* | 0.122 | 0.77 |
| Decrease | 44% | 9% | |
| IL-1β | 102.3 ± 25.5 | 17.03 ± 3.65 | 21.59 ± 5.31 |
| p | 0.002* | 0.034 | 0.012 |
| Decrease | 83% | 79% | |
Data are expressed in pg/ng 18s rRNA with mean ± SEM.
Mice were injected i.p. at day −5, day 0 and at day 3 with scrambled siRNA (n = 5), MASP-1/3 siRNA (n = 5) and Df siRNA (n =5). Total RNA was prepared from the knee joints containing synovium and mRNA levels were measured for MASPs, complement components and cytokines. p values were compared between CAIA mice treated either with scrambled siRNA and siRNAs MASP-1/3 or siRNA Df. p < 0.05 = significant.
p value among all siRNA treatment groups using ANOVA
Table 3.
mRNA for MASP-1, MASP-3 and Df in the knee joints of WT mice without CAIA1
| mRNA | siRNAs scramble2 | siRNAs MASP-1/32 | siRNAs Df2 |
|---|---|---|---|
| MASP-1 | 3.71 ± 0.85 | 2.98 ± 0.59 | 2.28 ± 0.38 |
| p | 0.39* | 0.35 | 0.23 |
| MASP-3 | 1.79 ± 0.29 | 1.24 ± 0.15 | 1.0 ± 0.11 |
| p | 0.44* | 0.71 | 0.32 |
| MAp44 | 5.11 ± 1.1 | 9.0 ± 1.5 | 7.2 ± 1.73 |
| p | 0.21* | 0.07 | 0.33 |
| Df | 60.5 ± 11.5 | 48.7 ± 13.2 | 44.9 ± 7.29 |
| p | 0.47* | 0.42 | 0.28 |
Data are expressed in pg/ng 18s rRNA with mean ± SEM based on the indicated number of mice (n).
Mice were injected i.p. at day −5, day 0 and at day 3 with scrambled siRNA (n = 5), MASP-1/3 siRNA (n = 5) and Df siRNA (n =5). Total RNA was prepared from the knee joints containing synovium and the baseline mRNA levels were measured for MASP-1, MASP-3 and Df. Liver and adipose tissue from WT mice with no CAIA were used as a positive control to measuring the mRNA levels for MASP-1 or MASP-3 and Df respectively (data not shown). All p-values for three different mRNAs in mice treated either with siRNAs MASP-1/3 or siRNAs Df were compared with the corresponding values of WT mice treated with scrambled siRNAs. p values were compared between WT mice treated either with scrambles siRNA and siRNAs MASP-1/3 or siRNA Df. p < 0.05 were considered statistically significant.
p value among all siRNA treatment groups using ANOVA
MASP-1 and MASP-3 protein levels in the circulation of CAIA mice treated with MASP-1/3 or Df siRNAs
To determine the effect of mice injected i.p. with scrambled, MASP-1/3 or Df siRNAs on the levels of MASP-1 and MASP-3 proteins in the circulation, sera from mice before and after the induction of CAIA were analyzed using Western blot analysis (Fig. 7). MASP-1 and MASP-3 were pulled-down from serum using mannose agarose and N-Acetyl-D-glucosamine agarose beads, respectively. Sera from mice before (at day 3) and after (at day 10) the induction of CAIA, treated i.p. with scrambled siRNAs, MASP-1/3 siRNAs or Df siRNAs, were analyzed for the presence of MASP-1 (Fig. 7A) or MASP3 (Fig. 7B). A band of ~80 kDa of MASP-1 was present in the sera from all mice injected i.p. with scrambled siRNAs or MASP-1/3 siRNAs or Df siRNAs but the band was less dense at day 10 vs day 3 in the serum from mice treated with MASP-1/3 siRNAs (Fig. 7A lanes 6, 7). By measuring the density of bands there was a 25% decrease at day 10 vs at day 3 in the density of MASP-1 bands in sera from mice treated with siRNAs for MASP-1/3 (Fig. 7A lanes 6, 7). No effect of Df siRNA was seen on MASP-1 protein (Fig. 7A lanes 8, 9). In contrast, there was an increase (37%) in the density of the MASP-1 protein band at day 10 vs day 3 in the serum from mice treated with scrambled siRNAs after the induction of disease (Fig. 7A lane 4 and 5). Serum from a fD−/− mouse and a WT mouse with no CAIA were used as positive controls (Fig. 7A lanes 1, 3). Serum from a MASP-1/3−/− mouse with no CAIA was used as a negative control for MASP-1 or MASP-3 proteins (Fig. 7A lane 2). These data show that the treatment of CAIA mice with MASP-1/3 or Df siRNAs specifically decreased the levels of MASP-1 and MASP-3 proteins in the circulation, in contrast to the mice treated with scrambled siRNAs.
Figure 7.
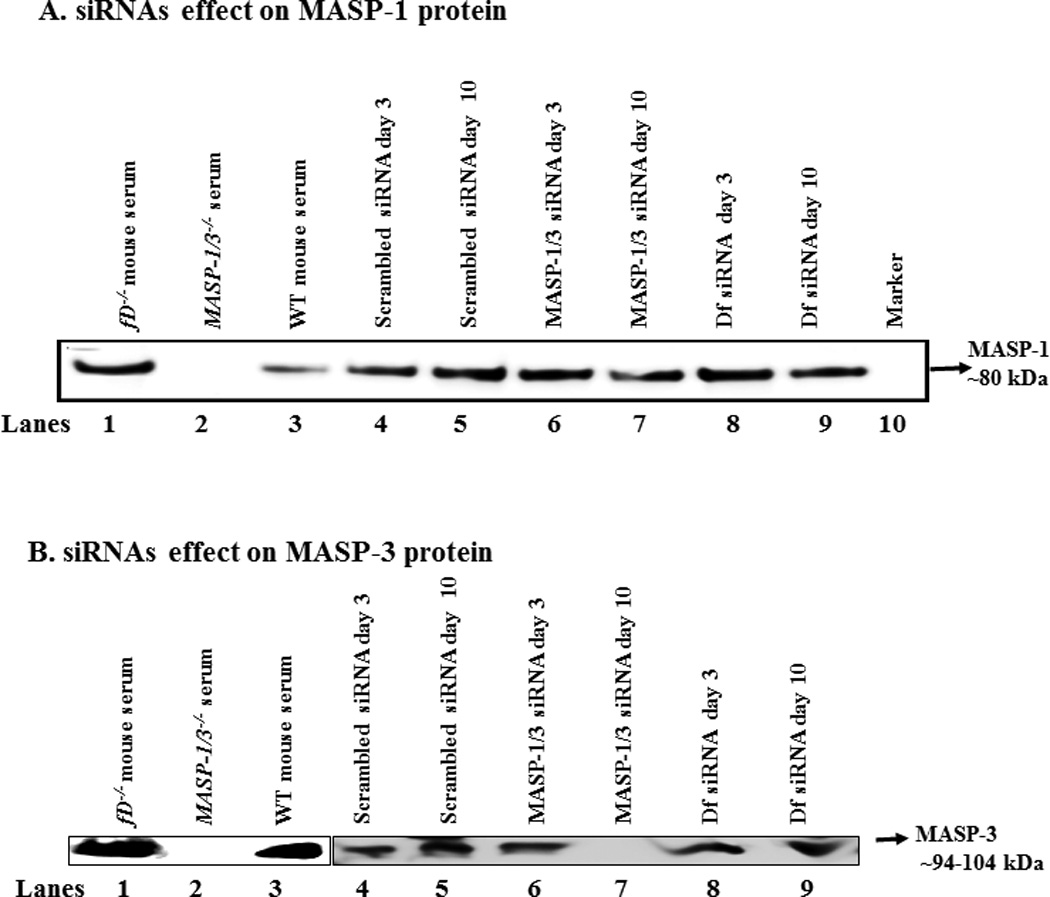
In vivo efficiency of the siRNAs for MASP-1/3 was assessed by using Western blot analysis for MASP-1 and MASP-3 proteins in the sera of WT mice before and after the treatments of mice with CAIA. Mice were injected i.p. with scrambled siRNAs, MASP-1/3 siRNAs or Df siRNA. To examine the efficiency of MASP-1/3 siRNAs on MASP-1 or MASP-3, a 10% SDS-PAGE (non-reducing) gel was used. After SDS-PAGE and transfer to nitrocellulose, the blots were probed separately with anti-MASP-1 or MASP-3 antibodies. The presence of no band or a less dense band of MASP-1 protein (~80 kDa) or of MASP-3 protein (~94–100 kDa) in serum indicates the successful in vivo transfection of cells in mice treated with their respective siRNAs. A. For the MASP-1 Western blots a mannose agarose pull-down assay was used. No effect of the scrambled siRNAs was seen on the levels of MASP-1 protein at day 3 (lane 4) vs. at day 10 (lane 5) after mice were injected i.p. at day 3 and at day 10. A dense MASP-1 protein band was present, at day 3 (lane 6) vs. a faint MASP-1 protein band at day 10 (lane 7), after mice were injected i.p. with MASP-1/3 siRNAs. Presence of a dense MASP-1 protein band at day 3 (lane 8) vs. a light protein band at day 10 (lane 9) after mice were injected i.p. with Df siRNAs. Serum from a fD−/− mouse was also used as a positive control (lane 2) for the presence of MASP-1 protein. Sera from MASP-1/3−/− and WT mice, with no injections of siRNAs, were used as a negative (lane 2) and positive controls (lane 3) respectively. B. For the MASP-3 Western blots N-Acetyle-D-glucosamine agarose pull-down assay was used. No effect of the scrambled siRNAs was seen on the levels of MASP-3 protein at day 3 (lane 4) vs. at day 10 (lane 5) after mice were injected i.p. at day 3 and at day 10. MASP-3 protein band was present, at day 3 (lane 6) vs. no band of MASP-3 protein at day 10 (lane 7), after mice were injected i.p. with MASP-1/3 siRNAs. No differences were seen in the density of MASP-3 protein band at day 3 (lane 8) vs. at day 10 (lane 9) after mice were injected i.p. with Df siRNAs. Serum from a fD−/− mouse was also used as a positive control (lane 1) for the presence of MASP-1 protein. Sera from WT and MASP-1/3−/− and mice, with no injections of siRNAs, were used as a positive (lane 2) and negative controls (lane 3), respectively.
Interestingly there was a complete inhibition of the MASP-3 protein in the sera from mice treated with MASP-1/3 siRNAs (Fig. 7B lane 7). This decrease in MASP-3 protein ranged from 61%-98% in various mice treated with MASP-1/3 siRNAs. There was no effect of Df siRNA treatment on MASP-3 protein in mice with CAIA (Fig. 7 lane 9). Serum from a fD−/− mouse and a WT mouse with no CAIA were used as positive controls (Fig. 7B lanes 1, 3). Serum from a MASP-1/3−/− mouse with no CAIA was used as a negative control for MASP-1 or MASP-3 proteins (Fig. 7B lane 2). These data suggest that the inhibition of CAIA in mice treated with MASP-1/3 siRNAs was predominately due to the inhibition of MASP-3 protein.
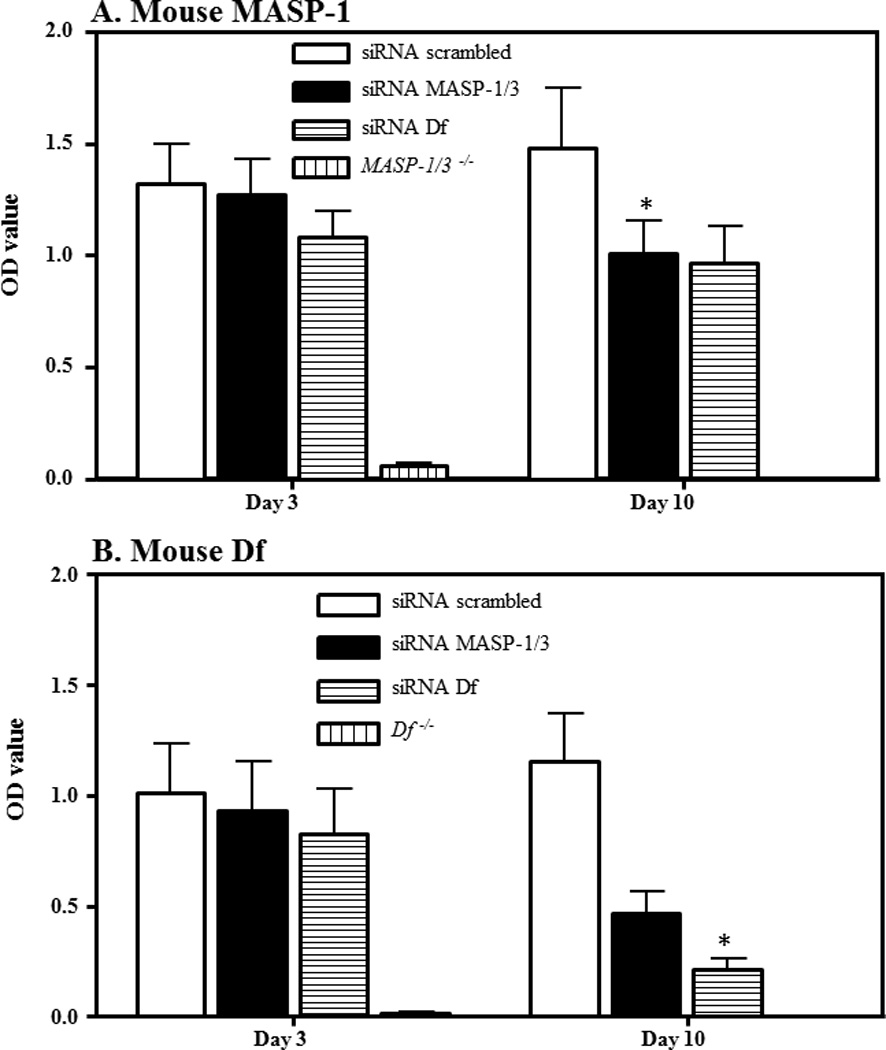
ProDf and Df protein levels in the circulation of CAIA mice treated with MASP-1/3 or Df siRNAs
To qualitatively determine the effect of i.p. injected scrambled, MASP-1/3 or Df siRNAs on the levels of proDf and Df proteins in the circulation, sera from mice before (at day 3) and after (at day 10) the induction of CAIA, treated i.p. with scrambled siRNAs, MASP-1/3 siRNAs and Df siRNAs, were analyzed for the presence of proDf or Df (Fig. 8). A specific mouse anti-factor D antibody was used which recognized both proDf and Df simultaneously. A band of ~26 kDa of Df was present in the serum of all mouse injected i.p. with scrambled siRNAs or MASP-1/3 siRNAs or Df siRNAs, but the band was less dense at day 10 vs at day 3 in the serum from mice treated with either MASP-1/3 or Df siRNAs (Fig. 8 lanes 3–9). By measuring the density of bands there was an impressive 80% decrease at day 10 vs at day 3 in the density of Df bands using sera from mice treated with siRNAs for MASP-1/3 or Df (Fig. 8 lanes 7, 9). No proDf protein band was visible in the serum from mouse treated with MASP-1/3 siRNAs, but a less dense band of Df protein, at day 10, was present (Fig. 8 lane 7). The ProDf protein band visually is slightly bigger than the Df 26 kDa protein band (Fig. 8 lane 2). In contrast, there was no change in the density of Df protein band at day 10 vs day 3 in the serum from mice treated with scrambled siRNAs (Fig. 8 lanes 4, 5). Sera from a fD−/− mouse and from a WT mouse with no CAIA were used as negative and positive controls, respectively (Fig. 8 lanes 1, 3). Serum from a MASP-1/3−/− mouse with no CAIA was used as a positive control for proDf protein (Fig. 8 lane 2). As expected no major changes in the proDf and Df proteins levels were seen using sera from three groups of mice at day −5 (before disease and before any treatment) (data not shown). These data show that the treatment of the mice with CAIA with MASP-1/3 or Df siRNAs specifically decreased the levels of Df protein in the circulation in contrast to mice treated with scrambled siRNAs.
Figure 8.
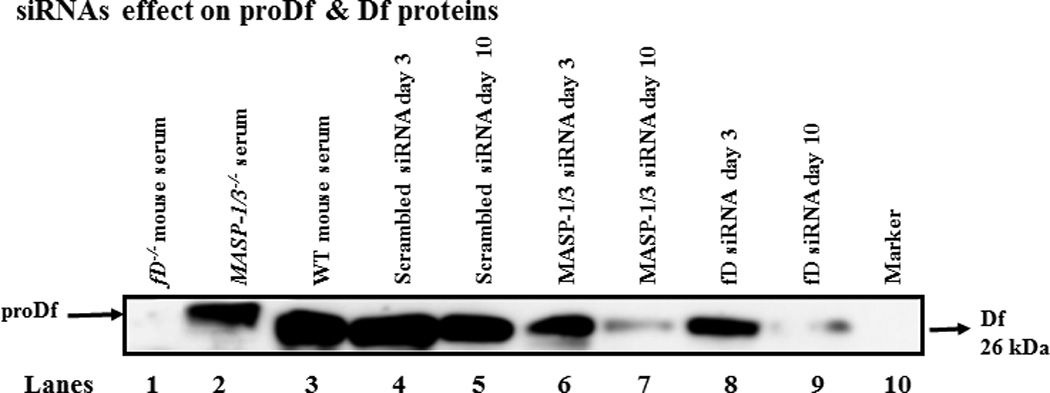
In vivo efficiency of the siRNAs for Df was assessed by using Western blot analysis for proDf and Df proteins in the sera of WT mice before and after the treatments and induction of CAIA. Mice were injected i.p. with scrambled siRNAs, MASP-1/3 siRNAs or Df siRNAs. For the Df Western blots analysis the immunoprecipation method with anti-Df antibody was used. To examine the efficiency of Df siRNAs on Df, a 12% Bis-Tris reducing SDS-PAGE gel was used. After SDS-PAGE and transfer to nitrocellulose, the blots were probed separately with a different anti-factor D antibody. The presence of less dense band of Df protein (~26 kDa) in serum indicated the successful in vivo transfection of cells in mice treated with Df siRNAs. A. No effect of the scrambled siRNAs was seen on the levels of Df protein at day 10 (lane 5) vs at day 3 (lane 4) after mice were injected i.p. Mice injected with MASP-1/3 siRNAs have decreased levels of Df protein at day 10 (lane 7) vs. at day 3 (lane 6). The presence of a dense Df protein band at day 3 (lane 8) vs a faint Df protein band at day 10 (lane 9) after mice were injected i.p. with Df siRNAs. Sera from fD−/− and WT mice, with no injections of siRNAs, were used as a negative (lane 1) and positive controls (lane 3) respectively. Serum from a MASP-1/3−/− mouse was also used as a positive control (lane 2) for the presence of proDf protein (slightly larger than 26 kDa than the Df 26 kDa protein band).
MASP-1 and factor D levels in levels in the circulation of CAIA mice treated with MASP-1/3 or Df siRNAs
To quantitatively determine the effect of i.p. injected scrambled, MASP-1/3 or Df siRNAs on the levels of MASP-1 and Df proteins in the circulation, sera from mice before (at day 3) and after (at day 10) the induction of CAIA, treated i.p. with scrambled siRNAs, MASP-1/3 siRNAs and Df siRNAs, were analyzed for the presence of mouse MASP-1 or Df (Fig. 9). Sera from MASP-1−/− and Df−/− mice were used as negative controls (Fig 9). There were 20% decrease at day 10 vs. at day 3 in the levels of MASP-1 protein in the serum from CAIA mice treated with siRNA MASP-1/3 and this decrease was nonsignificant (p < 0.055) but when compared with the siRNA scramble it was 30% decrease (Fig. 9A). These results were consistent with the western blot data (Fig. 8A). There was a 74% decrease at day 10 vs. at day 3 in the levels of Df protein in the serum from CAIA mice treated with siRNA Df and this decrease was significant (p < 0.0003) but when compared with the siRNA scramble it was decreased 81% (Fig. 9B). The treatment of CAIA with MASP-1/3 siRNA also effected the levels of Df by 50% again consistent with the mRNA expression data (Fig. 9B). A slight nonsignificant increase at day 10 vs. at day 3 in the levels of MASP-1 and Df were seen in mice treated with scrambled siRNAs possibly indicating the initiation of an immune or inflammatory response to the siRNA. Overall these data show that the treatment of mice with CAIA with MASP-1/3 or Df siRNAs specifically decreased the levels of MASP-1 and Df protein, in the circulation as compared to scrambled siRNA treatment.
Figure 9.
Levels of MASP-1 and Df proteins in the sera from WT mice before and after the treatments and induction of CAIA. Mice were injected i.p. with scrambled siRNAs, MASP-1/3 siRNAs or Df siRNAs. ELISA were used to measure the absolute levels of MASP-1 and Df in the serum samples as mentioned in the Methods. A. Levels of MASP-1 before (day 3) and after (day 10) treatments. B. Levels of Df before (day 3) and after (day 10) treatments. Each treatment group n = 5. All data represent the OD values mean + SEM based on n = 15 for all groups. Each sample was run in duplicate *p < 0.05 in comparison to the scrambled siRNAs or day 3 vs. day 10.
Discussion
In this study our purpose was first to address the question of where MASP-1/3 could mediate proDf cleavage to active Df in vivo and second to determine if siRNA-mediated targeting MASP-1/3 would be effective in the treatment of the CAIA model of human RA. The major findings of this study are as follows: First, five weeks after liver transplantation normal hepatocytes were clearly visible under the kidney capsule of MASP-1/3−/− mice, and these hepatocytes also generated functionally competent MASP-1 and MASP-3 proteins in the circulation. Second, MASP-1 and/or MASP-3 proteins generated by the hepatocytes in vivo cleaved proDf into Df in the circulation of transplanted MASP-1/3−/− mice and also allowed in vitro activation of the AP on adherent anti-collagen antibodies. Third, duplexes of the siRNAs for MASP-1/3 and Df not only silenced the mRNAs for MASP-1, MASP-3 and Df their respective mRNAs in the liver and adipose tissue but also decreased the generation of these proteins in the circulation and synovium under acute inflammatory conditions. And fourth, MASP-1/3 siRNAs when injected systemically or locally in the knee joints attenuated the development of CAIA in mice. A parallel decrease in the histopathology scores for inflammation, pannus formation, cartilage and bone C3 deposition, and infiltration of macrophage and neutrophil in the joints of mice was consistent with the decrease in the CDA in mice treated with the these siRNAs.
Previously, we demonstrated that active Df is required for the development of CAIA, as both MASP-1/3−/− and fD−/− mice are resistant to arthritis (6, 17). Factor D is essential for the AP as its only known substrate is fB, leading to the formation of the AP C3 initiation (C3(H2O)Bb) and amplification (C3bBb) convertases. Realization that Df (a critical component of the AP) is cleaved by members of the MASP family of proteases has been instrumental in our current understanding of the close interaction between these two pathways of complement activation. The questions which remain open concern which MASP family member is primarily responsible for Df cleavage and whether other proteases might function in vivo, for example during an acute inflammatory response, to cleave proDf. We assessed serum cleavage using a transplant model taking advantage of the observation that MASP-1/3 is primarily expressed in the liver while Df is primarily expressed in adipose tissue (21). Hepatocytes from fD−/− mice were transplanted under the kidney capsule of MASP1/3−/− mice. We would predict that if MASP-1/3 is capable of cleaving proDf in the blood one would see evidence of activated Df in these mice. Indeed, secreted MASP-1 and MASP-3 was found in the blood and correlated with cleavage of proDf and the reconstitution of the AP. Using CAIA as our acute inflammatory condition we found no evidence of cleavage of proDf in MASP1/3−/− mice and these data are consistent with our previous observations (6). This would argue that, at least in mice, the MASP-1 and MASP-3 are the only relevant proteases for proDf cleavage because these mice have MASP-2 in the circulation and have the capacity to activate other pro-inflammatory and clotting pathways. Interestingly, proDf was intact in MASP-1/3−/−/MRLlpr mice with no AP activity and these mice were protected from the lupus-like glomerunephritis (37). These results regarding the presence of proDf and the resistance of mice to CAIA were consistent with studies due to the dependency of this mouse model of RA on the AP (6). In MASP-1/3−/−/fH−/− mice proDf was also intact even in the presence of cobra venom factor (CVF) - dependent AP activity, and these mice were not protected in the C3 glomerulopathy model (38). Conceptually there are not much differences between MASP-1/3−/− and fD−/− mice due to the presence of proDf (inactive) and absence of Df (active or mature) respectively. A previous study has also shown that the AP of the complement system can get activated in the absence of Df after an injection of the CVF due to the formation of a CVF-fB convertase, which then cleaved C3 without the cleavage of fB (39). Nonetheless this study also showed that there was no AP activity in the absence of Df using traditional AP activators such as rabbit erythrocytes and zymosan. Therefore the AP activation seen in mice MASP-1/3−/−/fH−/− could be due to the formation of CVF-fB convertase in the presence of proDf and if so then these observations will be consistent regarding the activation of AP in fD−/− mice (39). Orozslan and colleagues recently published data in support of MASP-3 being the primary family member involved in the cleavage of proDf (40). This study has also provided evidence as to why only MASP-3 cleaves proDf as it is resistant to C1inhibitor and antithrombin, in contrast to MASP-1 or MASP-2. In our siRNA experiments we found that MASP-1 protein was minimally affected while MASP-3 protein was greatly diminished in abundance after 10 days and therefore the decrease in the CDA must be due to the decrease in MASP-3 protein. This lends further support to the contention that MASP-3 is the dominant protease regulating proDf cleavage and subsequent AP activation.
Our current results using MASP-1/3 or Df targeting siRNAs are consistent with our earlier findings in regards to the development of CAIA (6, 17). Mice injected either systemically or locally in one knee joint with MASP-1/3 or Df siRNAs showed a significant decrease in CDA bilaterally. These observations are also consistent with our previous study in which an adenovirus expressing in inhibitor of the LP, MAp44/MAP-1, when injected locally provided systemic treatment of disease (36). Interestingly the expression of the inhibitory protein, MAp44 in the liver was increased under acute inflammatory conditions in mice treated with siRNAs for MASP-1/3 or Df, suggesting that the increase of MAp44 levels may serve as a protective effects under inflammatory conditions (36) in a manner consistent with the cardioprotective and antitherothrombotic effect of MAp44 in two mouse models (41) and in our prior studies over-expressing MAp44 using an adenoviral expression system (36). These mRNA data also confirmed that treatment with MASP-1/3 siRNAs not only target specific respective proteins but also these effects were tissue specific. The mRNAs for pro-inflammatory cytokines such as IL-1β and complement components of the AP were also affected in mice treated i.p. with MASP-1/3 or Df siRNAs. Thus by using duplexes of siRNAs for MASP-1/3 or Df a therapeutic effect similar to the MASP-1/3−/− or fD−/− mice can be achieved to ameliorate the development of CAIA, presumably through the inhibition of C5a and other mediator generation (6).
In MASP1/3−/− mouse serum one can find large amounts of proDf and no active Df. However, in MASP-1/3 siRNAs treated WT mice we see a greatly diminished active Df and no proDf. The reason for this is unknown. We note that MASP-1/3 siRNA treatment decreases mRNAs for MASP-1, MASP-3, and Df (Table 1) suggesting a transcriptional mechanism. Alternatively, it may be the case that proDf expression in MASP-1/3−/− mice is upregulated as a compensatory response. More experiments are necessary to answer this question.
Our data from SQ injected MASP-1/3 or Df siRNAs in CAIA show that the liver and the adipose tissue predominately produce mRNAs for MASP-1/MASP-3/MAp44 and proDf, respectively. However, MASP-3 can be produced by other tissues (40). Nonetheless the cleavage of proDf into Df by MASP-1/3 takes place in the extracellular space and circulation, as it is evident from our studies related to transplantation of liver under the kidney capsule. Of note, it is interesting that, in vitro, the mixing and incubation for 6 hrs at 37°C of serum from MASP-1/3−/− mouse with fD−/− mouse have not yielded the same results as seen with liver transplantation, as one would expect, indicating that cleavage of proDf into Df by MASP-1/3 in vivo is a complex and highly regulated process. It may require some cell surface for the cleavage of proDf because in vitro mixing and adding sera from MASP-1/3−/− and fD−/− mice on adherent anti-collagen antibodies activated the AP and also generated a faint band of Bb only in the mixed sera as confirmed by using western blot analysis (data not shown). We have shown that MASP-1/3 proteases generated by the fibroblast-like synoviocytes can cleave proDf generated by adipocytes on the surface of cartilage microparticles (23). Recently it was shown the levels of M-ficolin, MASP-1, MASP-2 and MASP-3 were significantly higher in human plasma than in the synovial fluid, except for MASP-3 indicating MASP-3 may be produced locally in the joint from patients with juvenile idiopathic arthritis (JIA)(42). There are reports that MASP-3 could cleave proDf into Df and also to fB (22, 24, 40); therefore, MASP-3 itself can form the AP convertase. MASP-3 can also by produced at different sites and then transported locally (42, 43) such as in the knee joints. These findings in aggregate are consistent with our hypothesis that the pathogenic activation of the AP can occur by MASP-1/MASP-3 locally in the joint through immune complexes adherent to cartilage and the local production of necessary AP proteins by adipocytes and synovium.
One of the limitations of our study was that we have not used separate duplexes of siRNAs to silence MASP-1 and MASP-3 mRNAs separately. This is a technical challenge for the MASP-1 gene as alternative splicing generates three different mRNAs, i.e. MASP-1, MASP-2 and MAp44. No separate siRNAs for MASP-1 or MASP-3 are commercially available. So far attempts to synthesize specifically the MASP-1 and MASP-3 siRNAs which individually target the relevant mRNAs in vivo have not been successful. Therefore, for the current study we choose to use siRNAs to silence both MASP-1 and MASP-3. We concentrated on in vivo effects of our siRNAs in this study given the therapeutic focus of this work. One of the most interesting observations was that when mice with CAIA were treated with Df siRNAs there was a decrease in the mRNA for MASP-1 or MASP-3 in the knee joints, but no such effect was discernable on MAp44 mRNA. This observation was also supported by the fact that there was a decrease in the levels of MASP-1/3 proteins when mice were treated i.p. with Df siRNAs. At present, the reason for this finding is not known, but the question of whether MASP-1 or MASP-3 controls the transcription of Df is unknown and will be pursued in the future. We could not measure mouse MASP-3 protein in the serum due to the non-availability of a specific ELISA method.
We then asked one of the intriguing questions whether MASPs are more important in mouse than in man because it has been reported using a reverse translational approach that in one patient lacking both MASP-1 and MASP-3 the LP was non-functional but still the AP was functional (25). Interestingly the presence of proDf was confirmed later on in the serum of this same patient lacking MASP-1 and MASP-3 with intact AP (44). This important observation regarding the presence of proDf in the serum of this patient was identical to the presence of proDf in the circulation of MASP-1/3−/−, MASP-1/3−/−/MRLlpr and MASP-1/3−/−/fH−/− mice (6, 37, 38) again confirming that MASP-1/3 are the predominant and important proteases both in mouse and man cleaving proDf into Df. Recently it was reported that mutations in the SP domain of MASP-3 with normal levels of MASP-1 and MAp44 is sufficient to cause 3MC syndrome (26). This study also showed that there was a 2.5 fold decrease in AP activation, using rabbit erythrocyte lysis, in this one patient compared with healthy control and completely lacking the LP (26). One can draw inferences that this patient has the depressed or the defective AP of the CS because even at a low dilution it sub-optimally lysed rabbit erythrocytes. Previously, similarly it has been reported that MASP-1/3−/− mice have defective AP and completely lacking LP of the CS (21). Overall it appears that MASPs are very important both in mouse and man because the AP is defective and proDf is present in both lacking MASP-1/3 consistent with the original observations (21) but some species-specific differences and disease stage can’t be rules out. It has also been shown in vitro that human thrombin, kallikrein, and plasmin could activate the recombinant proDf but the hemolytic activity of the activated proDf was one-third of the native Df (45). We emphasize that more studies with large number of subjects deficient in MASP-1/3, MASP-1, MASP-2 and MASP-3 are needed to find out the real functional differences and importance of MASPs between mouse and man. Furthermore studies are in progress not only to generate inhibitory anti-MASP-1 or anti-MASP-3 antibodies for both mouse and man but also to develop, target and test separately MASP-1 or MASP-3 using N-acetylgalactosamine (GalNAc) to attain maximum gene silencing of MASP-1 or 3 in the liver and Df in the adipose tissue for the treatment of arthritis. The conjugate of GalNAc-siRNA-MASP-1 or GalNAc-siRNA-MASP-3 will separately target liver cells in arthritic mice.
Finally, the liver transplantation method under the kidney capsule that we used could be a new in vivo tool to reconstitute not only MASPs but also various other complement proteins for the study of various questions related to complement deficiencies.
Supplementary Material
Acknowledgments
The authors thank Ms. Umarani Pugazhenthi, University of Colorado qRT-PCR core for performing quantitative RT-PCR from the knee joints mRNA of mice with or without CAIA. We are also grateful to Mr. Scott Beard, Colorado Center for Transplantation Care, Research and Education (CCTCARE), Barbara Davis Center, University of Colorado for helping us in the liver/kidney transplant surgeries. We are also thankful to Dr. Pat Skavlen, DVM Office of Laboratory Animal Resources, University of Colorado for all help related to the post-surgery recovery and valuable suggestions from time to time.
This work was supported by NIH grants AR051749 to PI, VMH and COI, NKB
Abbreviations used in this paper
- CS
Complement system
- CP
classical pathway
- LP
lectin pathway
- AP
alternative pathway
- TP
Terminal pathway
- CAIA
collagen antibody-induced arthritis
- CIA
collagen-induced arthritis
- CII
type II collagen
- CDA
clinical disease activity
- AJM
all joint mean
- MBL
Mannan-binding lectin
- WT
wild type
- proDf
pro-factor D
- siRNAs
Small interfering RNAs
- MASP-1/3
Mannan-binding lectin-associated serine protease 1 and 3
- Df
factor D
- TNF-α
Tumor necrosis factor alpha
- IL-1β
Interleukin 1 beta
References
- 1.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis research & therapy. 2009;11:229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nature reviews. Rheumatology. 2012;8:573–586. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- 3.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 4.Ballanti E, Perricone C, Greco E, Ballanti M, Di Muzio G, Chimenti MS, Perricone R. Complement and autoimmunity. Immunologic research. 2013;56:477–491. doi: 10.1007/s12026-013-8422-y. [DOI] [PubMed] [Google Scholar]
- 5.Mehta G, Scheinman RI, Holers VM, Banda NK. A New Approach for the Treatment of Arthritis in Mice with a Novel Conjugate of an Anti-C5aR1 Antibody and C5 Small Interfering RNA. Journal of Immunology. 2015;194:5446–5454. doi: 10.4049/jimmunol.1403012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banda NK, Takahashi M, Levitt B, Glogowska M, Nicholas J, Takahashi K, Stahl GL, Fujita T, Arend WP, Holers VM. Essential role of complement mannose-binding lectin-associated serine proteases-1/3 in the murine collagen antibody-induced model of inflammatory arthritis. Journal of Immunology. 2010;185:5598–5606. doi: 10.4049/jimmunol.1001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banda NK, Takahashi K, Wood AK, Holers VM, Arend WP. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J Immunol. 2007;179:4101–4109. doi: 10.4049/jimmunol.179.6.4101. [DOI] [PubMed] [Google Scholar]
- 8.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway--its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 9.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–3888. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell Mol Med. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b–like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981;154:856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, Merkel TJ, Stahl GL, Mueller-Ortiz S, Wetsel R, Arend WP, Holers VM. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J Immunol. 2012;188:1469–1478. doi: 10.4049/jimmunol.1102310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hietala MA, Jonsson IM, Tarkowski A, Kleinau S, Pekna M. Complement deficiency ameliorates collagen-induced arthritis in mice. Journal of Immunology. 2002;169:454–459. doi: 10.4049/jimmunol.169.1.454. [DOI] [PubMed] [Google Scholar]
- 14.Hietala MA, Nandakumar KS, Persson L, Fahlen S, Holmdahl R, Pekna M. Complement activation by both classical and alternative pathways is critical for the effector phase of arthritis. Eur J Immunol. 2004;34:1208–1216. doi: 10.1002/eji.200424895. [DOI] [PubMed] [Google Scholar]
- 15.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Kristan J, Hao L, Lenkoski CS, Shen Y, Matis LA. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. Journal of Immunology. 2000;164:4340–4347. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 17.Banda NK, Levitt B, Wood AK, Takahashi K, Stahl GL, Holers VM, Arend WP. Complement activation pathways in murine immune complex-induced arthritis and in C3a and C5a generation in vitro. Clinical and experimental immunology. 2010;159:100–108. doi: 10.1111/j.1365-2249.2009.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degn SE, Hansen AG, Steffensen R, Jacobsen C, Jensenius JC, Thiel S. MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. Journal of Immunology. 2009;183:7371–7378. doi: 10.4049/jimmunol.0902388. [DOI] [PubMed] [Google Scholar]
- 19.Skjoedt MO, Hummelshoj T, Palarasah Y, Honore C, Koch C, Skjodt K, Garred P. A novel mannose-binding lectin/ficolin-associated protein is highly expressed in heart and skeletal muscle tissues and inhibits complement activation. The Journal of biological chemistry. 2010;285:8234–8243. doi: 10.1074/jbc.M109.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi M, Endo Y, Fujita T, Matsushita M. A truncated form of mannose-binding lectin-associated serine protease (MASP)-2 expressed by alternative polyadenylation is a component of the lectin complement pathway. International immunology. 1999;11:859–863. doi: 10.1093/intimm/11.5.859. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Ishida Y, Iwaki D, Kanno K, Suzuki T, Endo Y, Homma Y, Fujita T. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi M, Hideharu S, Endo Y, Schwaeble W, Fujita T. MASP-3 is the main converting enzyme for complement factor D. Molecular Immunology. 2014;61:280–281. [Google Scholar]
- 23.Arend WP, Mehta G, Antonioli AH, Takahashi M, Takahashi K, Stahl GL, Holers VM, Banda NK. Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. Journal of Immunology. 2013;190:6423–6433. doi: 10.4049/jimmunol.1300580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwaki D, Kanno K, Takahashi M, Endo Y, Matsushita M, Fujita T. The role of mannose-binding lectin-associated serine protease-3 in activation of the alternative complement pathway. J Immunol. 2011;187:3751–3758. doi: 10.4049/jimmunol.1100280. [DOI] [PubMed] [Google Scholar]
- 25.Degn SE, Jensen L, Hansen AG, Duman D, Tekin M, Jensenius JC, Thiel S. Mannan-binding lectin-associated serine protease (MASP)-1 is crucial for lectin pathway activation in human serum, whereas neither MASP-1 nor MASP-3 is required for alternative pathway function. Journal of Immunology. 2012;189:3957–3969. doi: 10.4049/jimmunol.1201736. [DOI] [PubMed] [Google Scholar]
- 26.Atik T, Koparir A, Bademci G, Foster J, 2nd, Altunoglu U, Mutlu GY, Bowdin S, Elcioglu N, Tayfun GA, Atik SS, Ozen M, Ozkinay F, Alanay Y, Kayserili H, Thiel S, Tekin M. Novel MASP1 mutations are associated with an expanded phenotype in 3MC1 syndrome. Orphanet J Rare Dis. 2015;10:128. doi: 10.1186/s13023-015-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apparailly F, Jorgensen C. siRNA-based therapeutic approaches for rheumatic diseases. Nature reviews. Rheumatology. 2013;9:56–62. doi: 10.1038/nrrheum.2012.176. [DOI] [PubMed] [Google Scholar]
- 28.Inoue A, Takahashi KA, Mazda O, Terauchi R, Arai Y, Kishida T, Shin-Ya M, Asada H, Morihara T, Tonomura H, Ohashi S, Kajikawa Y, Kawahito Y, Imanishi J, Kawata M, Kubo T. Electro-transfer of small interfering RNA ameliorated arthritis in rats. Biochemical and biophysical research communications. 2005;336:903–908. doi: 10.1016/j.bbrc.2005.08.198. [DOI] [PubMed] [Google Scholar]
- 29.Presumey J, Salzano G, Courties G, Shires M, Ponchel F, Jorgensen C, Apparailly F, De Rosa G. PLGA microspheres encapsulating siRNA anti-TNFalpha: efficient RNAi-mediated treatment of arthritic joints. Eur J Pharm Biopharm. 2012;82:457–464. doi: 10.1016/j.ejpb.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Khoury M, Courties G, Fabre S, Bouffi C, Seemayer CA, Vervoordeldonk MJ, Tak PP, Jorgensen C, Apparailly F. Adeno-associated virus type 5-mediated intraarticular administration of tumor necrosis factor small interfering RNA improves collagen-induced arthritis. Arthritis and rheumatism. 2010;62:765–770. doi: 10.1002/art.27302. [DOI] [PubMed] [Google Scholar]
- 31.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang CR, Shiau AL, Chen SY, Cheng ZS, Li YT, Lee CH, Yo YT, Lo CW, Lin YS, Juan HY, Chen YL, Wu CL. Intra-articular lentivirus-mediated delivery of galectin-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritis. Gene therapy. 2010;17:1225–1233. doi: 10.1038/gt.2010.78. [DOI] [PubMed] [Google Scholar]
- 33.Banda NK, Akkina RK, Terrell K, Shpall EJ, Tomczak J, Campain J, Claman H, Cagle L, Harrison GS. Diphtheria toxin A gene-mediated HIV-1 protection of cord blood-derived T cells in the SCID-hu mouse model. Journal of hematotherapy. 1998;7:319–331. doi: 10.1089/scd.1.1998.7.319. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi M, Iwaki D, Kanno K, Ishida Y, Xiong J, Matsushita M, Endo Y, Miura S, Ishii N, Sugamura K, Fujita T. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. Journal of Immunology. 2008;180:6132–6138. doi: 10.4049/jimmunol.180.9.6132. [DOI] [PubMed] [Google Scholar]
- 35.Banda NK, Levitt B, Glogowska MJ, Thurman JM, Takahashi K, Stahl GL, Tomlinson S, Arend WP, Holers VM. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. Journal of Immunology. 2009;183:5928–5937. doi: 10.4049/jimmunol.0901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banda NK, Mehta G, Kjaer TR, Takahashi M, Schaack J, Morrison TE, Thiel S, Arend WP, Holers VM. Essential role for the lectin pathway in collagen antibody-induced arthritis revealed through use of adenovirus programming complement inhibitor MAp44 expression. Journal of Immunology. 2014;193:2455–2468. doi: 10.4049/jimmunol.1400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeshi Machida, Takeshi Natsumi, Teizo Fujita MT, Sekine aH. Masp-1/3 Deficient MRL/Lpr Mice Lack The Alternative Complement Pathway Activation and Are Protected From Development Of Lupus-Like Glomerulonephritis. Arthritis & Rheumatism. 2013;65:2892. [Google Scholar]
- 38.Ruseva MM, Takahashi M, Fujita T, Pickering MC. C3 dysregulation due to factor H deficiency is MASP-1 and MASP-3 independent in vivo. Clinical and experimental immunology. 2014;176:84–92. doi: 10.1111/cei.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Ma M, Ippolito GC, Schroeder HW, Jr, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14577–14582. doi: 10.1073/pnas.261428398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oroszlan G, Kortvely E, Szakacs D, Kocsis A, Dammeier S, Zeck A, Ueffing M, Zavodszky P, Pal G, Gal P, Dobo J. MASP-1 and MASP-2 Do Not Activate Pro-Factor D in Resting Human Blood, whereas MASP-3 Is a Potential Activator: Kinetic Analysis Involving Specific MASP-1 and MASP-2 Inhibitors. Journal of Immunology. 2016;196:857–865. doi: 10.4049/jimmunol.1501717. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov VI, Skjoedt MO, Siow Tan Y, Rosbjerg A, Garred P, Stahl GL. Endogenous and natural complement inhibitor attenuates myocardial injury and arterial thrombogenesis. Circulation. 2012;126:2227–2235. doi: 10.1161/CIRCULATIONAHA.112.123968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petri C, Thiel S, Jensenius JC, Herlin T. Investigation of Complement-activating Pattern Recognition Molecules and Associated Enzymes as Possible Inflammatory Markers in Oligoarticular and Systemic Juvenile Idiopathic Arthritis. The Journal of rheumatology. 2015;42:1252–1258. doi: 10.3899/jrheum.141449. [DOI] [PubMed] [Google Scholar]
- 43.Dahl MR, Thiel S, Matsushita M, Fujita T, Willis AC, Christensen T, Vorup-Jensen T, Jensenius JC. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15:127–135. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi M, Sekine H, Fujita T. Comment on “the pro-factor D cleaving activity of MASP-1/−3 is not required for alternative pathway function”. Journal of Immunology. 2014;192:5448–5449. doi: 10.4049/jimmunol.1400766. [DOI] [PubMed] [Google Scholar]
- 45.Yamauchi Y, Stevens JW, Macon KJ, Volanakis JE. Recombinant and native zymogen forms of human complement factor D. Journal of Immunology. 1994;152:3645–3653. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


