Abstract
Purpose of review
Antimicrobials are a leading cause of severe T-cell-mediated adverse drug reactions (ADRs). The purpose of this review is to address the current understanding of antimicrobial cross-reactivity and the ready availability of and evidence for in vitro, in vivo and ex vivo diagnostics for T-cell-mediated ADRs.
Recent findings
Recent literature has evaluated the efficacy of traditional antibiotic allergy management including patch testing, skin prick testing, intradermal testing and oral challenge. While patch and intradermal testing are specific for the diagnosis of immune-mediated (IM) ADRs, they suffer from drug-specific limitations in sensitivity. The use of ex vivo diagnostics, especially ELISpot has been highlighted as a promising new approach to assigning causality. Knowledge of true rates of antimicrobial cross-reactivity aids empirical antibiotic choice in the setting of previous IM-ADRs.
Summary
In an era of increasing antimicrobial resistance and use of broad-spectrum antimicrobial therapy, ensuring patients are assigned the correct “allergy label” is essential. Re-exposure to implicated antimicrobials, especially in the setting of severe adverse cutaneous reaction is associated with significant morbidity and mortality. The process through which an antibiotic label gets assigned, acted on and maintained is still imprecise. Predicting T-cell-mediated ADRs via personalised approaches, including HLA-typing may pave future pathways to safer antimicrobial prescribing guidelines.
Keywords: ELISpot, antibiotic allergy, severe cutaneous adverse reactions, patch testing, hypersensitivity, lymphocyte transformation test
Introduction
T-cell-mediated drug hypersensitivities are a group of immune-mediated (IM) adverse drug reactions (ADRs) of varying phenotype and severity. Descriptions of antimicrobial associated T-cell-mediated ADRs date back to the use of the first sulfa antimicrobials [1] and then almost a decade later to early preparations of penicillins [2,3]. These IM-ADRs result in antimicrobial allergy “labels” that impact patient outcomes and antimicrobial usage [4–6]. For the diagnosis of antimicrobial allergy, the use of skin prick and intradermal testing (SPT/IDT) remain the mainstay of first-stage diagnosis for immediate reactions suspected to be IgE-mediated. This should be followed by an ingestion challenge which, in combination with SPT/IDT, is still considered to be the gold standard [7]. However, in the setting of serious T-cell-mediated ADRs, both patch testing, a more established test for the diagnosis of delayed reactions, and SPT/IDT lack the 100% negative predictive value necessary to re-challenge patients to drugs either orally or systemically following negative testing [8]. In this review, we will address the current understanding of antimicrobial cross-reactivity and the ready availability of and evidence for IM-ADR in vitro, in vivo and ex vivo diagnostics.
The epidemiology of serious T-cell-mediated reactions varies according to the region studied and is driven by genetic predisposition to these reactions. In general, given the high prevalence of antibiotic use, 50% or more of severe cutaneous adverse reactions (SCAR) globally are associated with antimicrobials, commonly penicillins, glycopeptides and sulphonamide antibiotics and antiretrovirals [5,9,10]. The most serious of these reactions include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) and acute generalised exanthematous pustolosis (AGEP). Additionally, abacavir, a guanosine analogue nucleoside reverse transcriptase inhibitor (NRTI), is associated with a severe HLA-B*57:01-restricted, CD8+ T-cell-mediated hypersensitivity reaction (AHS) which is characterized clinically by fever, malaise, gastrointestinal symptoms and late onset of rash (70%) a median of 8 days after initiation of dosing. In the setting of multiple implicated antimicrobials, the cause of SCAR and other IM-ADRs is often unclear despite application of published causality assessments [11,12].
Effector immunology of T-cell-mediated ADRs
IM-ADRs can be classified by the revised Gell and Coombs classification (Table 1)[13]. This review focuses on Type IV, T-cell-dependent IM-ADRs. The pathogenesis of T-cell-mediated immune responses has been long debated, yet the presence of allergen-specific T lymphocytes is an observation in most drug-allergy reactions. White et al. reviewed the current mechanistic hypotheses of T-cell-dependent IM-ADRs namely (i) pharmacological interaction of drugs with immune receptors (the p-i concept), (ii) the hapten/pro-hapten model and (iii) the altered peptide repertoire model (Figure 1)[4]. The cellular and cytokine response within IM-ADRs vary (Table 1).
Table 1.
T-cell-mediated ADR classification, pathogenesis and phenotype guide
| Type IV ADR | Cellular mediators | Cytokine mediators | Phenotype | Specific immunological parameters for phenotype |
|---|---|---|---|---|
| Type IVa |
Primary: Th1 Secondary: Macrophages |
IFN-y TNF-a IL-18 |
Contact dermatitis Tuberculin reactions |
Contact dermatitis – Primarily CD8+ T cell infiltrate.
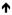 IFN-y, TNF-a, IL-18. Also noted IFN-y, TNF-a, IL-18. Also noted
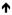 lL-31, IL-6 in serum and IL-33 IL-9, IL-4 in skin [14–18]. lL-31, IL-6 in serum and IL-33 IL-9, IL-4 in skin [14–18]. |
| Type IVb |
Primary: Th2 Secondary: B-cells, IgE, IgG4, mast cells, Eosinophils |
IL-4 IL-5 IL-13 |
MPEa
HSS DRESS |
MPE – CD4 > CD8+ T cells. Acute episodes Th1 predominate,
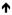 IL-12, IFN-y/TNF-b in blood, CXCL9/CXCL10 skin. IL-12, IFN-y/TNF-b in blood, CXCL9/CXCL10 skin.
 IL-17 compared with SJS/TEN. IL-17 compared with SJS/TEN.
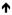 Th2/IL-5 later explains pruritis [19–24]. Th2/IL-5 later explains pruritis [19–24].DRESS -  TNF-a, IFN-y and IL-2 production, production correlates with disease severity. Activation-regulated chemokine (TARC/CCL17) drive Th2 responses, higher than observed in SJS/TEN. Skin biopsies noted eosinophils in 20%; whilst CD8+ T cells and granzyme B(+)lymphocytes TNF-a, IFN-y and IL-2 production, production correlates with disease severity. Activation-regulated chemokine (TARC/CCL17) drive Th2 responses, higher than observed in SJS/TEN. Skin biopsies noted eosinophils in 20%; whilst CD8+ T cells and granzyme B(+)lymphocytes
 in severe disease [25–27]. in severe disease [25–27]. |
| Type IVc | Primary: Cytotoxic T cells | Granzyme B Perforin Fas ligand Granulysin |
SJS TEN Linear IgA disease DILIb * FDE * EM |
SJS/TEN – CD8+ T-cells and NK cells lead to keratinocyte apoptosis. Granulysin specific to SJS/TEN.
 IL-10 and Treg associated with resolution of TEN/SJS. Treg function often impaired. IL-10 and Treg associated with resolution of TEN/SJS. Treg function often impaired.
 IL-2, IL5, IL6, IL-17 and CCL27 in plasma/blister fluid. Th17 cells also have a role [23,28–35]. IL-2, IL5, IL6, IL-17 and CCL27 in plasma/blister fluid. Th17 cells also have a role [23,28–35].Linear IgA disease – Often mistaken for TEN, however characteristic linear IgA deposits are evident on direct immunofluorescence studies.  CD4+ T-cell, neutrophils and eosinophilis. Mixed Th1/Th2 cytokine response. CD4+ T-cell, neutrophils and eosinophilis. Mixed Th1/Th2 cytokine response.
 IL-2, IL-4, IL-5 and IL-8 noted [36–41]. IL-2, IL-4, IL-5 and IL-8 noted [36–41]. FDE –  Intraepidermal CD8+ T-cells, Intraepidermal CD8+ T-cells,
 IFN-y, cytotoxic granules, granzyme B and perforin. IFN-y, cytotoxic granules, granzyme B and perforin.
 CD8+ T-cells, CD4+ T-cells and neutrophils cause tissue damage. Late - CD8+ T-cells, CD4+ T-cells and neutrophils cause tissue damage. Late -
 IL-10 & Treg(CD4+CD25+Foxp3+) control immune reaction, however IL-15 secreted by keratinocytes continue to propagate CD8+ T-cell mediated injury [42,43]. IL-10 & Treg(CD4+CD25+Foxp3+) control immune reaction, however IL-15 secreted by keratinocytes continue to propagate CD8+ T-cell mediated injury [42,43]. EM –  IL2, IL6, IL8, IL17A, IFN-y. IL2, IL6, IL8, IL17A, IFN-y.
 Th1/CD4+ T-cell infiltrate with IL-17 expression. Th1/CD4+ T-cell infiltrate with IL-17 expression.
 IL10, noted. At skin level, IL10, noted. At skin level,
 CD4+ T cell with IL-17 (Th2) expressing cells. CD8+ T cells noted within epidermis, and CD4+ T cells are noted in dermis. Variations in T-cell/cytokine expression if the stimulant is HSV or drug induced (e.g. higher CD8+ T cells and TNF-a in drug-induced EM) [44–46]. CD4+ T cell with IL-17 (Th2) expressing cells. CD8+ T cells noted within epidermis, and CD4+ T cells are noted in dermis. Variations in T-cell/cytokine expression if the stimulant is HSV or drug induced (e.g. higher CD8+ T cells and TNF-a in drug-induced EM) [44–46]. |
| Type IVd |
Primary: Th1/Th17 Secondary: Neutrophils |
GM-CSF IL-8 CXCL8 |
AGEP |
AGEP –
 CD4+ T cells infiltrate, CD8+ T cells and CD4+ T cells infiltrate, CD8+ T cells and
 CXCL8 and GM-CSF. CXCL8 is involved in the chemotaxis of neutrophils; Th17 cells involved [47–50]. CXCL8 and GM-CSF. CXCL8 is involved in the chemotaxis of neutrophils; Th17 cells involved [47–50]. |
References:[13]
Abbreviations: Th1, Type 1 T helper cells, Th2, Type 2 T helper cells; Th17, Type 17 T helper cells; IL, interleukin; DHR, Drug hypersensitivity reaction; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; MPE, maculopapular exanthema; DRESS, drug reaction with eosinophilia and systemic symptoms; HSS, hypersensitivity syndrome; FDE, fixed drug eruption; EM, erythema multiforme; DILI, drug induced liver injury; AGEP, acute generalised exanthematous pustolosis; GM-CSF, granulocyte monocyte colony-stimulating factor; PMN, polymorphonuclear cell.
Not classically described by Gell and Coombs criteria of T cell-mediated hypersensitivity
MPE, otherwise known as ‘morbilliform’ drug eruption, is the most commonly reported antibiotic-associated T-cell-mediated ADR.
DILI - DILI will not be covered in detail in this review, as the mechanism can be dose dependent/predictable or unpredictable. The unpredictable reactions may in fact be IM or metabolic in origin. T lymphocytes secreting granzyme B have been noted on liver biopsy. CD4+/CD8+ T cells secreting IL-13 and IFN-y have been detected in serum from in patients with DILI.. The most commonly implicated antimicrobials are amoxicillin-clavulanate and flucloxacillin, in particular in those with HLA-B*57:01
Figure 1. Heading: Schematic of proposed T-cell-mediated ADR pathogenesis theories.
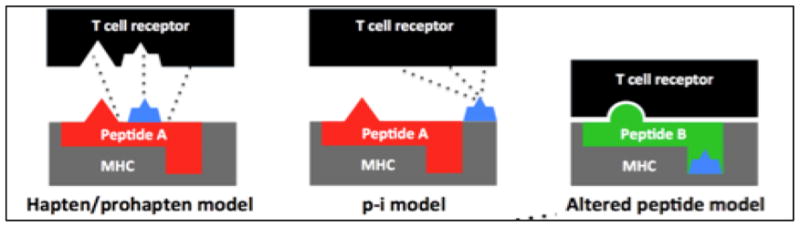
(i) The hapten/prohapten model is where an antigen (e.g. antibiotic) covalently binds to a self-peptide, is intracellularly processed and then presented with MHC to T cells as a ‘foreign antigen’ [51,52]. An example of the hapten/prohapten model is when penicillin G derivatives bind lysine residues on serum albumin [53–55].
(ii) The p-i concept (the pharmacological interaction with immune receptor) is based upon non-covalent binding of antigens to HLA or TCR without immune processing, explaining how reactions can occur upon first presentation [51,56].
(iii) The ‘altered self-repertoire model’ is based upon drug models (e.g. abacavir) that demonstrated that drugs can occupy positions in the peptide binding groove of the MHC, altering the binding cleft and subsequently the specificity of MHC binding [57–59]. Source: [51,52], [53–55], [51,56], [57–59].
Many of the SCAR reactions are known to rely on drug specific T-cell responses that can persist in the circulation for >20 years after drug exposure [60]. Blistering and severe IM-ADRs (SJS/TEN or AHS) are thought to correlate with CD8+ T-cell infiltration, while simple exanthema and DRESS are largely associated with CD4+ T cells or mixtures of CD4+ and CD8+ T cells [61,62]. In general, cytokines upregulated in IM-ADRs are IL-2, IL-5, IL-13 and IFN-y [63]. The key immune mediators differ slightly for each IM-ADR phenotype, summarised in Table 1. An understanding of immune mediators is vital for future works measuring cytokines in ex vivo T-cell diagnostics.
Historical approaches to T-cell-mediated hypersensitivities
Testing for IM-ADRs remains problematic due to both lack of widespread availability and low sensitivity of conventional methods. Many patients with non-specific rashes or those that occur during the course of an acute infection will not demonstrate reproducible symptoms on future rechallenge. Caubet et al. demonstrated that only 6.8% of patients with a history of antibiotic associated “rash” had a reproducible phenotype on oral challenge. In recent studies, IDT has been suggested to be more sensitive than patch testing (PT) for T-cell-mediated ADRs [64]. However, in the setting of serious T-cell-mediated ADRs, PT is still considered “safer” than delayed-SPT/IDT [65,66]. The details of PT and IDT for T-cell-mediated ADRs are described below and a summary of T-cell-mediated ADRs is provided in Table 2.
Table 2.
Summary of antimicrobial associated T-cell-mediated ADRs
| Characteristics | SJS/TEN | DRESS | AGEP | EM | FDE | Drug-induced linear IgA | MPE |
|---|---|---|---|---|---|---|---|
| Drug latency (days) | 4–28a | 14–42 | 1–18b | <1–10 | <1 to 14c | 1–18d | 4–9 |
| Prodrome | Common | Common | Uncommon – Fever with acute phase | Uncommon – Unless severe | Uncommon | Uncommon | Uncommon |
| Distinguishing cutaneous features | Starts face
 thorax. thorax.Palms, soles and scalp rarely involved. Nikolsky signe |
Morbilliform +/− follicular accentuation. Usually >50% BSA involvement and >2 of (i) facial oedema (50% cases) (ii) infiltrated lesions, (iii) scaling or (iv) purpura. |
Starts face
 thorax. Dozens to hundreds non-follicular, sterile, pin-sized pustules, generally with background erythema. Flexural accentuation. thorax. Dozens to hundreds non-follicular, sterile, pin-sized pustules, generally with background erythema. Flexural accentuation. |
Can involve all regions. Symmetrical target lesions, spreading in centripetal fashion. Oral involvement can be isolated finding. |
Can involve all regions. Commonly lips, genitalia, perianal area, hands, feet. Well demarcated +/− vesiculation or blistering. |
Sub-epidermal blisters on trunk, extensor surfaces, buttocks and face (especially perioral region). | Morbilliform eruption –macules, papules or rarely pustules/bullae. Desquamation often follows resolution. |
| Mucosal involvement | Yes (very common - 90%) | Yes (infrequent) | Yes (uncommon, only lips) | Yes (common, 70%) | Yes (infrequent) | Yes (common – 80%) | No |
| Commonly implicated antibiotics | Beta-lactams (penicillins > cephalosporins), vancomycin, sulphonamides, macrolides, quinolones, tetracycline, clindamycin | Sulphonamides, vancomycin, minocycline, dapsone ≫ beta-lactams, pristinamycin nevirapine, telaprevir, acyclovir | Vancomcyinf, amoxycillin, ciprofloxacin, gentamicin, carbapenemsg | Sulphonamides, penicillins, quinolonesh | Sulphonamides, tetracyclines, penicillins, quinolones, macrolides, metronidazole, | Vancomycin ≫ amoxycillin, ADF, quinolones, sulphonamides | Beta-lactams, (especially penicillin, amoxicillin/amoxicillin- clavulanate), sulphonamides, cephalosporins, lincosamides |
| Scoring Algorithmsi | ALDEN[11] | RegiSCAR[67] | EuroSCAR[68] | Nil | Nil | Nil | Nil |
| Preferred diagnostics (in vitro) | PT | PT> Delayed-IDT | PT | PT | PT> Delayed-IDTj | PT | Delayed-DT |
| Research diagnostics (ex vivo) | LTT ELISpot |
LTT ELISpot |
LTT ELISpot |
LTT ELISpot |
LTT ELISpot |
LTT ELISpot |
LTT ELISpot |
Abbreviations: T-cell-mediated ADRs, delayed hypersensitivity reactions; SJS, Stevens-Johnson Syndrome; TEN, toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; AGEP, acute generalised exanthematous pustolosis; EM, erythema multiforme; FDE, fixed drug eruption; Linear IgA, linear immunoglobulin IgA disease; MPE, maculopapular examthem; TMP-SMX, trimethoprim-sulfamethoxazole; ADF, amoxicillin-clavulanate; LTT, lymphocyte transformation test; ELISpot, enzyme-linked immunospot assay; BSA, body surface area.
Much shorter duration for antibiotics than other drugs (1 vs. 11)
Can be as early as 48 hours on drug re-exposure, median time 14 days
Can be as short as 30 mins to 8 hours post drug administration [69]
Latency periods are rarely up to 30 days.
Nikolsky sign – The ability to extend the area of sloughing with the application of gentle lateral pressure on seemingly unaffected skin. Asboe- Hansen sign (“bullae spread”) – Lateral extension of bullae with gentle pressure
Vancomycin most commonly implicated antibiotic
Infective causes are more common in EM than SJS (e.g. HSV1 and Mycoplasma)
In cases where a specific scoring system has not been developed, ‘Naranjo score’ can be employed as a guide [72]
At the site of previously described reaction
Patch testing (PT)
The specificity of PT for SCAR has been high in settings where drug concentrations have been validated against negative controls. The sensitivity of PT varies, however, and is highest for DRESS (32–80%) [112,113] and AGEP (58–64%) [112,114], and lowest for SJS/TEN (9–24%) [112,114] and MPE (10–40%) [65,113]. Patch testing lacks an appropriate positive control and results may be difficult to interpret in patients who are on immunosuppressants that impact T-cell-mediated immunity. For antibiotics, PT to the upper back is generally recommended 6 weeks – 6 months post skin healing [115]. In a multicentre study of PT in SCAR, Barbaud et al. demonstrated that PTs were most frequently positive for beta-lactams (primarily amoxicillin) and pristinamycin [112]. Buonomo et al. demonstrated PT’s utility in IM-ADRs, predominately cephalosporin-associated MPE, in a retrospective cohort [116]. Barbaud et al. utilized PT in 29 cases of pristinamycin-associated IM-ADRs, with a higher that expected sensitivity noted (69%) [66]. In 27 patients with oral challenge confirmed FDE to TMP-SMX, a 93% sensitivity for PT was demonstrated [117]. However, in a recent study by Andrade et al. 0% (0/15) of FDE were positive on PT [118]. The utility of PT in IM-ADRs caused by quinolones and trimethoprim-sulfamethoxazole (TMP-SMX) is notoriously poor [112,119,120]. PT has been demonstrated to be effective in a small number of antibiotic-associated SJS/TEN [114,121–123], AGEP [70,112,120,124,125], FDE [126–128], DRESS [112,129], MPE [130] and EM [131] case series. To date, success with PT in cases of suspected antiretroviral hypersensitivity has been limited to abacavir. PT for abacavir showed 100% specificity and 87% diagnostic sensitivity when used as an adjunctive test to define true AHS [8,132].
Summary & Recommendations
A positive PT has high specificity for a specific antibiotic-associated IM-ADRs and appears most useful for DRESS >AGEP and of lessor utility for FDE, MPE and SJS/TEN.
A negative PT does not exclude a drug-specific IM-ADR and should never be used as the sole basis for rechallenge of the implicated antibiotic(s).
Delayed intradermal testing (Delayed-IDT)
The use of delayed-IDT (0.02–0.05 mLs of highest non-irritating concentration of antimicrobial applied to volar forearm skin, then read at 48–72 hours [133]) is recommended in the investigation of T-cell-mediated ADRs [134,135]. Similar to patch testing, delayed-IDT is limited by the significantly less than 100% sensitivity and lack of a suitable positive control [136]. Recommendations for IDT vary regionally and there is a lack of evidence-based volumes and reagents (beta-lactam versus non-beta-lactam) [121,133–135]. IDT has predominately been utilized for beta-lactam antimicrobials, especially penicillins > cephalosporins, in patients with a history of non-SJS/TEN T-cell-mediated ADR [122,123,137]. A positive result involves dermal induration/erythema at injection site, which will significantly exceed 5 mm from baseline, 24–72 hours post-testing. Although extension of the local dermal response at the skin testing site is uncommon, IDT is generally not recommended for the assessment of SJS/TEN [123,138], due to risk of systemic events. Adverse reactions following delayed IDT for non-SJS/TEN ADRs are rarely reported [139–141], primarily occur in the setting of immediate testing [142–144] and are often related to errors in concentrations and/or volumes used.
Alternative guidelines do not specify the same ‘contraindications’ to IDT, however suggest performing IDT only after a negative PT [145]. Whilst it appears PT is preferred over IDT for FDE [118], the sensitivity of IDT for other T-cell-mediated ADRs appears higher than that observed with PT [64,130,141,146,147]. In a study of patients with suspected reactions to beta-lactams (n = 235 MPE), 7% (18/235) had a positive delayed-IDT, while 8.5% (20/235) with negative IDT demonstrated a positive result with OC [147]. IDT has also been used less frequently for other antimicrobials associated with IM-ADRs, such as metronidazole [148]. Limitations include (i) only antimicrobials in a commercially available and sterile injectable form can be utilized, (ii) short-lived local histamine release (e.g. ciprofloxacin and vancomycin) and irritation (e.g. flucloxacillin) of some products and (iii) overall low NPV. The sensitivity of delayed-IDT from a mixture of small studies has been reported as 6.6–36.3% for MPE (higher with penicillins > cephalosporins) [149–151] and 64%–100% for DRESS [113,137].
Summary & Recommendations
Delayed-IDT can be employed as a first line investigation for non-SJS/TEN IM-ADRs, although the highest non-irritating concentrations for DELAYED testing have not been validated for most drugs.
A positive delayed-IDT result is highly suggestive of an IM-ADR, but a negative delayed-IDT does not exclude an IM-ADR and should never be used as the sole basis for rechallenge.
Direct oral challenge
Since first-stage tests such as PT and IDT do not have 100% negative predictive values, oral challenge is contraindicated in certain SCAR (e.g. SJS/TEN/DRESS) [8,152] and AHS. Oral challenge is required to confirm IM-ADRs following negative delayed-IDT or PT in the remaining phenotypes [150,153]. For the investigation of delayed reactions, a prolonged oral challenge (5–7 versus 3 days) increases sensitivity [150,154]. Due to the low rate of positives obtained from isolated delayed-IDT or PT [153,155–157], and high rate of Type A ADRs clouding “labels” [6], a move toward direct oral challenge has been proposed, especially for ‘low-risk’ phenotypes [6,158]. This is particularly true in children where viral infections or drug-infection interactions are prevalent. Direct oral rechallenge in a cohort of patients with a history of MPE demonstrated only a 6.9% adverse event rate (compared with 3.5% prior) [159]. A direct 5-day oral rechallenge in 119 pediatric patients with mild antibiotic-associated MPE elicited a 5.4% positive response rate, none of which were serious [80]. The safety of oral rechallenge for antiretroviral IM-ADRs has not been established, but guidelines advise that patients with mild to moderate rash without constitutional symptoms can continue antiretrovirals with close clinical monitoring. In these cases, symptoms should be managed with anti-histamines and topical corticosteroids. Physicians commonly “treat through” mild ADRs to non-nucleoside reverse transcriptase inhibitors (NNRTIs) such as nevirapine or efavirenz, hepatitis C drugs such as telaprevir and antibiotics such as beta-lactams and sulfa antimicrobials [160,161]. Desensitization protocols exist for hypersensitivity reactions to the antiretrovirals tipranavir [162], amprenavir [163], darunavir [164], efavirenz [165] and have been tried with nevirapine [166].
Recommendations
Direct oral challenge for 5–7 days should be employed after a negative PT or delayed-IDT in the setting of mild to moderate antibiotic skin rashes without evidence of fever, mucosal involvement, malaise or internal organ involvement.
Oral challenge with a suspected drug should never be employed in the setting of SJS/TEN or DRESS.
Ideally, an observed oral or ingestion challenge in the setting of required antibiotic therapy should be employed following negative IDT/PT and knowledge of antibiotic cross-reactivity (Box 1).
In acute settings, of mild to moderate rash without fever, mucosal or internal organ involvement, antimicrobials can be continued with close monitoring.
Box 1. Heading: Empirical antimicrobial therapy recommendations in the setting of T-cell-mediated ADR (non-SCAR) where in vivo and ex vivo testing is not available.
| Antimicrobial allergy “label” | Antimicrobials to avoid in the setting of known T-cell-mediated ADR history |
|---|---|
| Penicillin V/G | Cephalothin Cefoxitin |
| Aminopenicillins | Ampicillin/amoxicillin Cefaclora Cephalexina |
| Anti-staphylococcal penicillin | Penicillin V/G Flucloxacillin/dicloxacillin/oxacillin Piperacillin-tazobactam Ticarcillin-clavulanate |
| 1st Generation cephalosporinsb | Amoxicillinc
Cefaclord |
| 2nd Generation cephalosporins | Ceftriaxonee
Cefotaximee Cefepimee Cephalexinf |
| 3rd Generation cephalosporins | Cefepimeg
Cephalothinh Cefuroximeg Cefotaximeg |
| 4th Generation cephalosporins | Aztreonami
Cefitraxonej Cefuroximej Cefotaximej |
| Carbapenems | Carbapenems |
| Monobactams | Ceftazadimek |
| Antibiotic sulphonamides | Nil |
Abbreviations:
SCAR, severe cutaneous adverse reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis; drug reaction with eosinophilia and systemic symptoms; acute generalised exanthematous pustolosis.
ADR, adverse drug reaction.
Avoid if amoxicillin/ampicillin delayed IM-ADR due to shared/similar R1 side chain
If cefazolin is the implicated antimicrobial, this is generally an isolated reaction due to the absence of shared side chains and therefore other beta-lactams could be employed for non-SCAR phenotypes.
If cephalexin allergy then avoid amoxicillin/ampicillin due to shared/similar R1 side chain
Avoid if cephalexin allergy due to shared/similar R1 side chain
Avoid if cefuroxime allergy due to shared/similar R1 side chain
Avoid if cefaclor allergy, due to shared/similar R1 side chain
Avoid if ceftriaxone allergy due to shared/similar R1 side chain.
Avoid if cefoxitin allergy due to shared/similar R1 side chain.
Avoid if ceftazadime allergy due to shared/similar R1 side chain
Avoid if cefepime allergy due to shared/similar R1 side chain
Avoid if aztreonam allergy due to shared/similar R1 side chain
T-Cell Diagnostics
Lymphocyte transformation test
Ex vivo investigations have been explored for T-cell-mediated ADRs, including the lymphocyte transformation test (LTT). LTT has a reported sensitivity of 27–70% and specificity of 72.7–100%, however remains hindered by testing time, requirement for radioactive materials and potential dependence on B-cell proliferation [8,175–177]. LTT has been used for causality assessments in ceftriaxone, ampicillin/sulbactam and metronidazole-associated linear IgA disease, ceftriaxone-associated MPE, penicillin/amoxicillin-induced MPE and ceftazidine-induced DRESS [178–181]. In a small study of amoxicillin-induced IM-ADR, correlation between positive in vivo IDT and LTT was not demonstrated [182]. LTT has also been used in a small number of other case reports/series for IM-ADRs secondary to anti-tuberculosis therapies [129], aminopenicillins [122,123,177], cephalosporins [183] and anti-staphylococcal penicillins [137].
Recommendation
Antibiotic LTT is an unvalidated test that has been associated with both false positive and false negative results and currently remains a research tool used in specialized centres for the investigation of T-cell-mediated ADRs.
Enzyme-Linked ImmunoSpot (ELISpot) Assay
ELISpot is an ex vivo technique used to analyse low-frequency antigen-specific, cytokine-producing (e.g. IFN-γ) cells in peripheral blood following exposure to pharmacological drug concentrations [8]. ELISpot can be employed for a range of cytokine responses depending on the underlying drug hypersensitivity immunopathogenesis. For example, AGEP can have high IL-13 and IFN-γ, FDE raises IL-10, while DRESS can have high IL-5 or IFN-γ [60,184]. ELISpots measuring granzyme have also been employed [175]. ELISpot studies have demonstrated that 1:150 to 1:5000 T cells remain ‘reactive’ in patients post ADR for up to 12–20 years [60,185]. ELISpot has also been shown to have better sensitivity than LTT in detecting drug-specific T-cell responses [185,186]. Nonetheless, ELISpot has only been employed in research settings for the investigation of antimicrobial allergy. Estimations of sensitivity and specificity are flawed due to the absence of a reference gold standard. However, increasing the drug concentration used to stimulate the patients’ cells and increasing incubation periods (48 hours vs. overnight) have been shown to increase assay sensitivity without decreasing specificity. An examination of ELISpot use in antimicrobial T-cell-mediated ADRs is outlined below:
ELISpot & Antiviral IM-ADRs
ELISpot is described in studies examining antiretroviral hypersensitivity reactions, notably abacavir and nevirapine. ELISpot has been used to detect abacavir hypersensitivity in patients that are HLA-B*57:01 negative [187]. IFN-γ ELISpot has also been used to demonstrate that abacavir unexposed HLA-B*57:01 positive patients have a ‘resting’ abacavir reactive CD8+ T-cell population [188]. In nevirapine hypersensitivity reactions, IFN-γ ELISpot has been utilised to demonstrate that specific combinations of CD4 class II-restricted and CD8 class I-restricted T cells contribute to the hypersensitivity immunopathogenesis [189].
ELISpot & Antibiotic IM-ADRs
Penicilins
Earlier studies demonstrate that ELISpot IFN-γ testing was positive in patients with a history of amoxicillin IM-ADRs [185,190]. No positive ELISpot results were identified in control patients or those with a history of IgE-mediated disease, highlighting the specificity of the test. The intensity of response was, however, proportional to time after diagnosis. The overall sensitivity and specificity was 91% and 95% respectively. Khalil et al. demonstrated a sensitivity and specificity of 80% and 100% respectively for ELISpot measuring IL-2, IL-5 and IFN-y in patients with amoxicillin IM-ADR. Rozieres et al. demonstrated ex vivo effectiveness for other beta-lactams, including ticarcillin [185,191]. ELISpot has also been used in models using antigen-specific T-cell clones to confirm patients with a history to piperacillin hypersensitivity [192].
Cephalosporins
Tanvarasethee et al. examined the use of ELISpot to diagnose cephalosporin-induced MPE and compare against SPT, delayed-IDT and PT [193]. From the 25 patients, 40% had a positive IFN-γ and IL-5 response compared with 8% who had a positive delayed-IDT or PT (p= 0.008). There was a higher probability of positive ELISpot if performed within 2 years of reaction (p=0.046) [193].
Other antimicrobials
The use of ELISpot for quinolones, glycopeptides, trimethoprim-sulfamethoxazole and other commonly used antibacterial therapy is absent. Aminoglycosides are an infrequent cause of SCAR, yet a case of amikacin-induced DRESS was confirmed on patch testing and ELISpot [194]. A case of sulfasalazine hypersensitivity syndrome was also confirmed with ELISpot [195]. The use in other antimicrobials is also ill-defined. Further research is required to evaluate this testing in a range of antimicrobial therapies.
Recommendation
ELISpot remains a test available only in specialized centres for the investigation of T-cell-mediated ADRs.
Predicting T-cell Responses – HLA typing
Recently, an increasing number of antimicrobial IM-ADRs have been associated with various HLA alleles (Table 3). In general, due to varying HLA allele frequencies, different ethnic populations have different genetic associations. To date, the best characterized antimicrobial-induced, HLA-associated IM-ADRs that appear to generalize across populations include AHS and nevirapine SCAR. The association between AHS and HLA-B*57:01 resulted in the implementation of a routine screening test that is widely employed in the developed world before abacavir treatment. Before widespread acceptance, the HLA-B*57:01 genetic association with abacavir was established in a large population with a diverse genetic background. This screening test has a positive predictive value (PPV) of 55% and a negative predictive value (NPV) of 100%, which is crucial for drug safety [218–220]. Less than 100% NPVs and very low PPVs of other antimicrobial drug hypersensitivity HLA associations have limited their translation into routine clinical practice as screening tests. For example, although only 13 individuals would need to be screened for HLA-B*57:01 to prevent a single case of AHS, over 14,000 individuals would have to be tested for this same allele to prevent a single case of flucloxacillin-associated hepatitis.
Table 3.
Human leukocyte antigen associations for antimicrobial associated T-cell-mediated hypersensitivity syndromes
| Antimicrobial | Clinical Presentation | Associated HLA allele(s) | Population | NPV | PPV | NNT |
|---|---|---|---|---|---|---|
| Abacavir | Hypersensitivity syndrome (fever, rash, GI distress, malaise) | HLA-B*57:01 | European, African | 100% for patch test confirmed | 55% | 13 |
| Efavirenz | Rash | HLA-DRB1*01 | French | |||
| Nevirapine | Rash | HLA-B*35:05 HLA*Cw4 |
Thai African, Asian, European, Thai |
97% | 16% | |
| DRESS | HLA-B*14/Cw8 HLA-Cw8 HLA-Cw*4 and HLA- DRB1*15 HLA-B*3505 HLA-B*3501 and HLA- B*15/DRB1*15 |
Italian Japanese Han Chinese Asian Australian |
||||
| Hepatitis | HLA-DRB1*01:01 HLA-DRB1*01:02 |
Australian, European South African |
96% | 18% | ||
| SJS/TEN | HLA-C*04:01 | Malawian | ||||
| Dapsone | Rash, hepatitis | HLA-B*13:01 | Chinese | 99.8% | 7.8% | 84 |
| Flucloxacillin | Hepatitis (DILI) | HLA-B*57:01 HLA-DRB1*0107- DQB1*0103 |
European | 99.99% | 0.12% | 13.819 |
| Amoxicillin- clavulanate; coamoxiclav | Hepatitis (cholestatic) | HLA*02:01 HLA-DQB1*0602 and rs3135388, a tag SNP of HLA-DRB*15:01- DQB1*06:02 |
European | |||
| Sulfamethoxazole | SJS/TEN | HLA-B*38 | European | |||
| FDE | HLA-A*30-B*14-Cw*6 haplotype | Turkish | ||||
| Aminopenicillins | Rash | HLA-A*2 HLA-DR*52 |
Italian | |||
| Sulphonamides | SJS/TEN | HLA-A*29 HLA-B*12 HLA-DR7 |
European | |||
| Isoniazid | DILI | NAT2 slow acetylator, CYP2E1*5 and *1B |
European | |||
| Drug-induced lupus erythematous | HLA-DR*4 | Italian | ||||
| Levamisole | Agranulocytosis | HLA-B*27 | South American |
Abbreviations: DILI, drug-induced liver injury; DRESS, drug reaction with eosinophilia and systemic symptoms; GI, gastrointestinal; HLA, human leukocyte antigen; NNT, number needed to treat; NPV, negative predictive value; PPV, positive predictive value; SJS/TEN, Stevens-Johnson Syndrome/Toxic epidermal necrolysis References: [196–221]
The story of nevirapine-induced IM-ADRs is quite complex. Nevirapine-induced IM-ADRs have been associated with different HLA alleles across different ethnic populations. These HLA associations appear to be phenotype specific and involve both Class I and Class II HLA alleles. An association between nevirapine-induced hepatitis and HLA-DRB1*01:01 was first reported in a Western Australian population [217] and has since been reported in other Caucasian populations [216]. The closely related allele HLA-DRB1*01:02 was associated with nevirapine-induced hepatitis in a South African cohort [196]. Nevirapine DRESS has been associated with the HLA-Cw*8 or Cw*8-B*14 haplotype in Japanese and Italian populations and also with HLA-Cw*4 and HLA–DRB1*15 in Han Chinese, HLA-B*35:05 in Asians and HLA-B*35:01 and HLA-B*15/DRB1*15 in an Australian cohort [189,212–215]. Many of these alleles including HLA-DRB*01, HLA-Cw*04 and HLA-B*35:05 are also associated with nevirapine-induced rash [209–211,215,216].
Other HLA associations have been described for IM-ADRs to efavirenz, dapsone, flucloxacillin, amoxicillin-clavulanante, sulfamethoxazole, aminopenicillins, sulphonamides, isoniazid and levamisole (Table 3).
Many of these antimicrobials such as flucoxacillin and amoxicillin-clavulanate are specifically associated with drug-induced liver injury (DILI), which can be associated with fulminant hepatic failure [220]. Although few HLA screening tests have advanced to the level of routine clinical practice, HLA associations have significantly advanced our understanding of the immunopathogenesis of IM-ADRs.
Recommendation
Level IA evidence exists to support screening for HLA-B*57:01 prior to initiation of abacavir therapy. This screening test has a 100% negative predictive value and is widely recommended as part of guideline-based practice.
Cross Reactivity in T-Cell-Mediated Reactions
In settings where in vivo and ex vivo diagnostics are unavailable, understanding cross-reactivity based on shared chemical structure amongst antimicrobials is essential (Box 1). Most of the rates of cross-reactivity for delayed IM-ADRs are extrapolated from data that exists for cross-reactivity in the setting of immediate hypersensitivities. Earlier reports of high rates of penicillin/cephalosporin cross-reactivity were confounded by penicillin contamination of cephalosporin manufacturing [2,3,222]. Current literature supports that most cross-reactivity that occurs in the beta-lactam class occurs on the basis of shared R1 and/or R2 side-chains [85,149,150). Recent reports suggest patients with a history of delayed hypersensitivity to aminopenicillins most commonly cross react with aminocephalosporins sharing an R1 group such as cephalexin, cefaclor and cephadroxil and generally tolerate all other cephalosporins [223,224]. Challenging patients with a penicillin/amoxicillin allergy history with a cephalosporin not sharing the same side chain (e.g. cefuroxime or ceftriaxone) proved successful in a study of 41 patients by Novalbas et al. [225]. The rate of cross-reactivity between penicillin and 3rd generation cephalosporins now approaches 1%, a far cry from the 10–25% initially quoted in very early studies [226] Romano et al. demonstrated that patients with cephalosporin immediate hypersensitivity can still be safely treated with compounds that have side-chain determinants different from those of the responsible cephalosporin [169].
Cross-reactivity between carbapenems has been infrequently reported [227]; a shared T-cell epitope remains unknown [227]. Cross-reactivity between macrolides also appears rare, with infrequent reports of immediate cross-reactivity noted particularly between those with 14-membered ring such as erythromycin, clarithromycin and roxithromycin and the 15-membered azalide, azithromycin [228]. T-cell-mediated cross-reactivity between tetracyclines [229], in particular doxycycline and minocycline has been reported [229]. Cross-reactivity [230] and tolerance [231] have been reported for aminoglycoside antibiotics in which ADRs are more common for topical than systemic agents due to contact sensitization [194,232]. For nitroimidazoles (e.g. metronidazole, tindazole) T-cell-mediated ADRs have been reported, with cross-reactivity noted [94–96,233].
Delayed IM-ADRs are less frequent than immediate ADRs in regards to quinolones [234], with cross-reactivity more commonly occurring between 1st and 2nd generation quinolones than 3rd and 4th generation [234–237]. Glycopeptide (vancomycin and teicoplanin) cross-reactivity is also reported [238–240], however remains controversial, with many reports extrapolated from reoccurrence of haematological disturbances. Patients with isolated vancomycin hypersensitivity have also been known to tolerate teicoplanin [97,238,241–243].
An estimated 3–6% of the population are considered “allergic” to sulphonamides, with trimethoprim-sulfamethoxazole (TMP/SMX) the most commonly implicated example [244]. Whilst belief in overall sulphonamide cross-reactivity persists [245], recent reviews do not support cross-reactivity between antibacterial and non-antibacterial sulphonamides [244,246–249]. There is cross-reactivity between antibiotic sulfonamides, especially sulfasalazine and sulfamethoxazole [250]. The non-antibacterial sulphonamides (e.g. azetazolamide, forusemide, celecoxib, thiazide diuretics, sumatriptan, sotalol, probenacid) do not contain the structural region known to cause the allergic response (i.e., N1 heterocyclic ring; an N-containing ring attached to the N1 nitrogen of the sulfonamide group and arylamine group at the N4 position). Although early reports questioned the potential for cross-reactivity between TMP-SMX and darunavir [249,251,252], authors have noted an absence of TMP-SMX allergy history in those with darunavir hypersensitivity [253–255]. Notably patients with a history of sulfa antimicrobial allergy were not excluded from darunavir clinical trials.
The potential for cross-reactivity between dapsone and TMP-SMX is now somewhat controversial with most reports occurring in HIV-infected individuals without evidence of positive rechallenge. The current estimated rate of cross-reactivity is less than previously reported (9–11% vs. 20–45%) [256,257]. In those requiring TMP-SMX therapy with a history of non-SCAR adverse drug reaction to antibacterial sulfonamide, we recommend a supervised oral rechallenge, rather than drug avoidance [258,259].
Antiretroviral
Cross-reactivity between most antiretroviral classes is likely very low due to the lack of structural similarities. However, patients with prior severe hypersensitivity to an NNRTI should be monitored if new NNRTI therapy is initiated. Mehta and Maartens reported recurrent reactions in 12.6% of patients with reported rash who were switched from nevirapine to efavirenz, compared with 50% of patients switched from efavirenz to nevirapine [260]. Cross-reactivity is reported to be higher between nevirapine and delavirdine which have a similar structure, but delavirdine is not currently used because of its difficult dosing, pill burden, drug interactions and lower efficacy compared to contemporary NNRTIs [261].
Recommendations for antimicrobial use, in relation to likely cross-reactivity, in patients with delayed hypersensitivities to isolate antimicrobials are given in Box 1.
Conclusions
In an era of increasing antimicrobial resistance and use of broad-spectrum antimicrobial therapy, ensuring patients are correctly “labelled” in respect to antimicrobial-associated IM-ADRs is essential. Re-exposure to the implicated antimicrobial, especially in the setting of SCAR and AHS is associated with significant morbidity and mortality. The key messages from this review are:
Antimicrobials are a leading cause of T-cell-mediated ADRs.
The antimicrobials primarily associated with T-cell-mediated ADRs include glycopeptides, sulphonamides, beta-lactams, antiretrovirals and hepatitis C antivirals.
An understanding of drug latency and allergy ‘phenotypes’ can aid drug causality assessment.
Whilst PT and IDT are specific in the diagnosis of T-cell-mediated ADRs, they suffer from drug-specific limitations in sensitivity and when negative they can never be used as the sole basis for rechallenge.
A knowledge of side chain cross-reactivity aids empirical antibiotic choice in the setting of IM-ADRs.
-
The use of ex vivo diagnostics, especially ELISpot are promising new approaches to assigning causality in antimicrobial associated T-cell-mediated ADRs.
An understanding of cytokine outputs specific to each phenotype will aid the development of these tools in the future.
Predicting T-cell-mediated ADRs via personalised approaches, including HLA-typing may pave future pathways to safer antimicrobial prescribing.
Acknowledgments
None
Footnotes
Conflicts of interest: None
Financial support and sponsorship: JAT is supported by an NHMRC postgraduate scholarship This work was also supported by NIH 1P50GM115305-01, 1R01AI103348-01, 1P30AI110527-01A1, 2T32GM7347-36, NHMRC APP1123499 and ACH2.
References
Papers of particular interest, published within the annual period of review, (2015–2016) have been highlighted as:
* of special interest
** of outstanding interest
- 1.Goodman MH, Levy CS. The development of a cutaneous eruption (toxicodermatosis): During administration of sulfanilamide; report of two cases. Journal of the American Medical Association. 1937;109:1009–1011. [Google Scholar]
- 2.Jaslowitz H. Reaction to penicillin. Br Med J. 1945;2:767. [PubMed] [Google Scholar]
- 3.Kolodny MH, Denhoff E. Reactions in penicillin therapy. J Am Med Assoc. 1946;130:1058–1061. doi: 10.1001/jama.1946.02870160004002. [DOI] [PubMed] [Google Scholar]
- 4**.White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: The role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136:219–234. doi: 10.1016/j.jaci.2015.05.050. quiz 235. In this review, the authors summarize the role of host genetics, microbes, and drugs in immune-mediated adverse drug reaction development and expand on the existing models of immune-mediated adverse drug reaction pathogenesis by proposing the heterologous immunity model to address multiple unexplained observations such as the high negative predictive value for HLA associations. They also discuss the implications of this work in clinical practice today including HLA genotyping to prevent abacavir, carbamazepine and allopurinol hypersensitivity reactions and describe future applications for preclinical drug toxicity screening, drug design, and development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin YF, Yang CH, Sindy H, Lin JY, Rosaline Hui CY, Tsai YC, Wu TS, Huang CT, Kao KC, Hu HC, et al. Severe cutaneous adverse reactions related to systemic antibiotics. Clin Infect Dis. 2014;58:1377–1385. doi: 10.1093/cid/ciu126. [DOI] [PubMed] [Google Scholar]
- 6**.Trubiano JA, Cairns KA, Evans JA, Ding A, Nguyen T, Dooley MJ, Cheng AC. The prevalence and impact of antimicrobial allergies and adverse drug reactions at an Australian tertiary centre. BMC Infect Dis. 2015;15:572. doi: 10.1186/s12879-015-1303-3. In this study of 509 patients treated in an Australian tertiary care center, an antimicrobial allergy label was found to significantly impact the rate of oral antimicrobial administration, beta-lactam usage, antimicrobial duration and antimicrobial appropriateness. The median antimicrobial duration was longer in patients with an antimicrobial allergy label and that same cohort was less likely to receive a beta-lactam, be prescribed an oral antibiotic and unfortunately, also less likely to be prescribed an appropriate antimicrobial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger AJ, Eisen B. Feasibility of skin testing for penicillin sensitivity; a study of one thousand cases. J Am Med Assoc. 1955;159:191–193. doi: 10.1001/jama.1955.02960200037007a. [DOI] [PubMed] [Google Scholar]
- 8.Rive CM, Bourke J, Phillips EJ. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. 2013;34:15–38. [PMC free article] [PubMed] [Google Scholar]
- 9**.Blumenthal KG, Wickner PG, Lau JJ, Zhou L. Stevens-Johnson syndrome and toxic epidermal necrolysis: a cross-sectional analysis of patients in an integrated allergy repository of a large health care system. J Allergy Clin Immunol Pract. 2015;3:277–280. e271. doi: 10.1016/j.jaip.2014.10.002. By examining a large repository of patients' electronic allergy records, the authors identified a prevalence of 375 patients per million for Stevens-Johnson syndrome or toxic epidermal necrolysis. They also identified new drugs that may be emerging as causative agents of severe cutaneous adverse reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Trubiano JA, Aung AK, Nguyen M, Fehily SR, Graudins L, Cleland H, Padiglione A, Peleg AY. A Comparative Analysis Between Antibiotic- and Nonantibiotic-Associated Delayed Cutaneous Adverse Drug Reactions. J Allergy Clin Immunol Pract. 2016 doi: 10.1016/j.jaip.2016.04.026. In this restrospective observational inpatient cohort sudy of 84 patients, the authors evaluated the difference in clinical presentation, causality assessments and outcomes of patients with delayed antibiotic-associated and nonantibiotic-associated cutaneous adverse drug reactions. Antibiotics were the casue of cutaneous adverse drug reaction requiring hospital admission in 48% of cases, and were assoicated with longer length of stay, higher age-adjusted Charlson comorbidiity index, shorter drug latency and high mortality. In antibiotic-associated reactions, glycopeptide and sulfonamide antibiotic exposure predominated. [DOI] [PubMed] [Google Scholar]
- 11.Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, Haustein UF, Vieluf D, Roujeau JC, Le Louet H. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88:60–68. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 12.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, Sidoroff A, Naldi L, Mockenhaupt M, Roujeau JC. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 13.Pichler WJ. Drug Hypersensitivity. Karger Publishers; 2007. [Google Scholar]
- 14.Lee JH, Cho DH, Park HJ. IL-18 and Cutaneous Inflammatory Diseases. Int J Mol Sci. 2015;16:29357–29369. doi: 10.3390/ijms161226172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarneri F, Minciullo PL, Mannucci C, Calapai F, Saitta S, Cannavo SP, Gangemi S. IL-31 and IL-33 circulating levels in allergic contact dermatitis. Eur Ann Allergy Clin Immunol. 2015;47:156–158. [PubMed] [Google Scholar]
- 16.Liu J, Harberts E, Tammaro A, Girardi N, Filler RB, Fishelevich R, Temann A, Licona-Limon P, Girardi M, Flavell RA, et al. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J Invest Dermatol. 2014;134:1903–1911. doi: 10.1038/jid.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taniguchi K, Yamamoto S, Hitomi E, Inada Y, Suyama Y, Sugioka T, Hamasaki Y. Interleukin 33 is induced by tumor necrosis factor alpha and interferon gamma in keratinocytes and contributes to allergic contact dermatitis. J Investig Allergol Clin Immunol. 2013;23:428–434. [PubMed] [Google Scholar]
- 18.Lee HY, Stieger M, Yawalkar N, Kakeda M. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013;2013:916497. doi: 10.1155/2013/916497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Chem Immunol Allergy. 2012;97:1–17. doi: 10.1159/000335612. [DOI] [PubMed] [Google Scholar]
- 20.Gomez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012;4:251–263. doi: 10.4168/aair.2012.4.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez TD, Mayorga C, Torres MJ, Cornejo-Garcia JA, Lopez S, Chaves P, Rondon C, Blanca M. Cytokine and chemokine expression in the skin from patients with maculopapular exanthema to drugs. Allergy. 2008;63:712–719. doi: 10.1111/j.1398-9995.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 22.Tapia B, Morel E, Martin-Diaz MA, Diaz R, Alves-Ferreira J, Rubio P, Padial A, Bellon T. Up-regulation of CCL17, CCL22 and CCR4 in drug-induced maculopapular exanthema. Clin Exp Allergy. 2007;37:704–713. doi: 10.1111/j.1365-2222.2007.02699.x. [DOI] [PubMed] [Google Scholar]
- 23.Fujiyama T, Kawakami C, Sugita K, Kubo-Kabashima R, Sawada Y, Hino R, Nakamura M, Shimauchi T, Ito T, Kabashima K, et al. Increased frequencies of Th17 cells in drug eruptions. J Dermatol Sci. 2014;73:85–88. doi: 10.1016/j.jdermsci.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, He D, Tang X, Zhang X. Chemokine expression in diverse nonimmediate drug hypersensitivity reactions: focus on thymus activation-regulated chemokine, cutaneous T-cell-attracting chemokine, and interleukin-10. Ann Allergy Asthma Immunol. 2014;113:204–208. doi: 10.1016/j.anai.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 25*.Niu J, Jia Q, Ni Q, Yang Y, Chen G, Yang X, Zhai Z, Yu H, Guan P, Lin R, et al. Association of CD8(+) T lymphocyte repertoire spreading with the severity of DRESS syndrome. Sci Rep. 2015;5:9913. doi: 10.1038/srep09913. The authors isolated CD4+ and CD8+ T cells from the peripheral blood of eight patients with drug reaction with eosinophilia and systemic symptoms (DRESS) at 10-day intervals and sequenced the CDR3 regions of the TCRbeta chain to analyze the T-cell repertoire. In this study, the extent of fluctuation of dominanat CD8+ T-cell clones correlated positively with clinical severity. Additionally, the anti-herpesvirus response was higher in this "fluctuant" group supporting the notion that hervesviruses contribute to the pathogenesis of DRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa K, Morito H, Hasegawa A, Miyagawa F, Kobayashi N, Watanabe H, Sueki H, Tohyama M, Hashimoto K, Kano Y, et al. Elevated serum thymus and activation-regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS) Br J Dermatol. 2014;171:425–427. doi: 10.1111/bjd.12948. [DOI] [PubMed] [Google Scholar]
- 27.Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, Wechsler J, de Feraudy S, Duong TA, Delfau-Larue MH, Chosidow O, Wolkenstein P, Roujeau JC. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50–58. doi: 10.1111/bjd.13683. [DOI] [PubMed] [Google Scholar]
- 28.Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012;129:1562–1569. e1565. doi: 10.1016/j.jaci.2011.12.990. [DOI] [PubMed] [Google Scholar]
- 29.Teraki Y, Kawabe M, Izaki S. Possible role of TH17 cells in the pathogenesis of Stevens-Johnson syndrome and toxic epidermal necrolysis. J Allergy Clin Immunol. 2013;131:907–909. doi: 10.1016/j.jaci.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009;182:8071–8079. doi: 10.4049/jimmunol.0804002. [DOI] [PubMed] [Google Scholar]
- 31.Lee HY, Chung WH. Toxic epidermal necrolysis: the year in review. Curr Opin Allergy Clin Immunol. 2013;13:330–336. doi: 10.1097/ACI.0b013e3283630cc2. [DOI] [PubMed] [Google Scholar]
- 32.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–1350. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 33.Murata J, Abe R, Shimizu H. Increased soluble Fas ligand levels in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis preceding skin detachment. J Allergy Clin Immunol. 2008;122:992–1000. doi: 10.1016/j.jaci.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66:190–196. doi: 10.1016/j.jdermsci.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Hakuta A, Fujita H, Kanaoka M, Watanabe M, Izumi K, Watanabe T, Komitsu N, Itoh M, Tanito K, Takahashi Y, et al. Reduction of interleukin–10 production by B cells in intractable toxic epidermal necrolysis. J Dermatol. 2015;42:804–808. doi: 10.1111/1346-8138.12909. [DOI] [PubMed] [Google Scholar]
- 36.Kakar R, Paugh H, Jaworsky C. Linear IgA bullous disease presenting as toxic epidermal necrolysis: a case report and review of the literature. Dermatology. 2013;227:209–213. doi: 10.1159/000353584. [DOI] [PubMed] [Google Scholar]
- 37.Khan I, Hughes R, Curran S, Marren P. Drug-associated linear IgA disease mimicking toxic epidermal necrolysis. Clin Exp Dermatol. 2009;34:715–717. doi: 10.1111/j.1365-2230.2008.03011.x. [DOI] [PubMed] [Google Scholar]
- 38.Cummings JE, Snyder RR, Kelly EB, Raimer SS. Drug-induced linear immunoglobulin A bullous dermatosis mimicking Stevens-Johnson syndrome: a case report. Cutis. 2007;79:203–207. [PubMed] [Google Scholar]
- 39.Coelho S, Tellechea O, Reis JP, Mariano A, Figueiredo A. Vancomycin-associated linear IgA bullous dermatosis mimicking toxic epidermal necrolysis. Int J Dermatol. 2006;45:995–996. doi: 10.1111/j.1365-4632.2006.02752.x. [DOI] [PubMed] [Google Scholar]
- 40.Tranvan A, Pezen DS, Medenica M, Michelson GC, Vogelzang N, Soltani KM. Interleukin–2 associated linear IgA bullous dermatosis. J Am Acad Dermatol. 1996;35:865–867. doi: 10.1016/s0190-9622(96)90106-1. [DOI] [PubMed] [Google Scholar]
- 41.Lin MS, Fu CL, Olague-Marchan M, Hacker MK, Zillikens D, Giudice GJ, Fairley JA. Autoimmune responses in patients with linear IgA bullous dermatosis: both autoantibodies and T lymphocytes recognize the NC16A domain of the BP180 molecule. Clin Immunol. 2002;102:310–319. doi: 10.1006/clim.2001.5177. [DOI] [PubMed] [Google Scholar]
- 42.Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230–1238. doi: 10.1111/j.1365-2133.2008.08516.x. [DOI] [PubMed] [Google Scholar]
- 43.Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316–321. doi: 10.1097/ACI.0b013e32832cda4c. [DOI] [PubMed] [Google Scholar]
- 44.Akkurt ZM, Ucmak D, Turkcu G, Yuksel H, Yildiz K, Arica M. Expression of interleukin-17 in lesions of erythema multiforme may indicate a role for T helper 17 cells. Cent Eur J Immunol. 2014;39:370–376. doi: 10.5114/ceji.2014.45950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chodorowska G, Czelej D, Niewiedziol M. Interleukin-2 and its soluble receptor in selected drug-induced cutaneous reactions. Ann Univ Mariae Curie Sklodowska Med. 2003;58:7–13. [PubMed] [Google Scholar]
- 46.Kokuba H, Aurelian L, Burnett J. Herpes simplex virus associated erythema multiforme (HAEM) is mechanistically distinct from drug-induced erythema multiforme: interferon-gamma is expressed in HAEM lesions and tumor necrosis factor-alpha in drug-induced erythema multiforme lesions. J Invest Dermatol. 1999;113:808–815. doi: 10.1046/j.1523-1747.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 47.Ueda T, Abe M, Okiyama R, Oyama S, Satoh K, Aiba S, Kaneko S, Katsuoka K. Acute generalized exanthematous pustulosis due to allylisopropylacetylurea: role of IL-17-producing T cells. Eur J Dermatol. 2011;21:140–141. doi: 10.1684/ejd.2010.1205. [DOI] [PubMed] [Google Scholar]
- 48.Smith K, Norwood C, Skelton H. Do the physical and histologic features and time course in acute generalized exanthematous pustulosis reflect a pattern of cytokine dysregulation? J Cutan Med Surg. 2003;7:7–12. doi: 10.1007/s10227-002-1151-9. [DOI] [PubMed] [Google Scholar]
- 49.Britschgi M, Pichler WJ. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol. 2002;2:325–331. doi: 10.1097/00130832-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322–328. doi: 10.1097/ACI.0b013e32832cf64e. [DOI] [PubMed] [Google Scholar]
- 51.Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:683–693. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- 52.Pichler W, Yawalkar N, Schmid S, Helbling A. Pathogenesis of drug-induced exanthems. Allergy. 2002;57:884–893. doi: 10.1034/j.1398-9995.2002.02161.x. [DOI] [PubMed] [Google Scholar]
- 53.Padovan E, Mauri-Hellweg D, Pichler WJ, Weltzien HU. T cell recognition of penicillin G: structural features determining antigenic specificity. Eur J Immunol. 1996;26:42–48. doi: 10.1002/eji.1830260107. [DOI] [PubMed] [Google Scholar]
- 54.Yawalkar N, Pichler WJ. Pathogenesis of drug-induced exanthema. Int Arch Allergy Immunol. 2001;124:336–338. doi: 10.1159/000053750. [DOI] [PubMed] [Google Scholar]
- 55.Park BK, Naisbitt DJ, Gordon SF, Kitteringham NR, Pirmohamed M. Metabolic activation in drug allergies. Toxicology. 2001;158:11–23. doi: 10.1016/s0300-483x(00)00397-8. [DOI] [PubMed] [Google Scholar]
- 56.Pichler WJ, Beeler A, Keller M, Lerch M, Posadas S, Schmid D, Spanou Z, Zawodniak A, Gerber B. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006;55:17–25. doi: 10.2332/allergolint.55.17. [DOI] [PubMed] [Google Scholar]
- 57.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, Miles JJ, Kjer-Nielsen L, Gras S, Williamson NA, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–558. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- 58.Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, Oseroff C, Lu S, Jakoncic J, de Oliveira CA, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012;109:9959–9964. doi: 10.1073/pnas.1207934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norcross MA, Luo S, Lu L, Boyne MT, Gomarteli M, Rennels AD, Woodcock J, Margulies DH, McMurtrey C, Vernon S, et al. Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. 2012;26:F21–29. doi: 10.1097/QAD.0b013e328355fe8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. 2006;117:455–462. doi: 10.1016/j.jaci.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 61.Depta JP, Altznauer F, Gamerdinger K, Burkhart C, Weltzien HU, Pichler WJ. Drug interaction with T-cell receptors: T-cell receptor density determines degree of cross-reactivity. J Allergy Clin Immunol. 2004;113:519–527. doi: 10.1016/j.jaci.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Hari Y, Frutig-Schnyder K, Hurni M, Yawalkar N, Zanni MP, Schnyder B, Kappeler A, von Greyerz S, Braathen LR, Pichler WJ. T cell involvement in cutaneous drug eruptions. Clin Exp Allergy. 2001;31:1398–1408. doi: 10.1046/j.1365-2222.2001.01164.x. [DOI] [PubMed] [Google Scholar]
- 63.Lochmatter P, Beeler A, Kawabata TT, Gerber BO, Pichler WJ. Drug-specific in vitro release of IL-2, IL-5, IL-13 and IFN-gamma in patients with delayed-type drug hypersensitivity. Allergy. 2009;64:1269–1278. doi: 10.1111/j.1398-9995.2009.01985.x. [DOI] [PubMed] [Google Scholar]
- 64.Osawa J, Naito S, Aihara M, Kitamura K, Ikezawa Z, Nakajima H. Evaluation of skin test reactions in patients with non-immediate type drug eruptions. J Dermatol. 1990;17:235–239. doi: 10.1111/j.1346-8138.1990.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 65.Pereira N, Canelas MM, Santiago F, Brites MM, Goncalo M. Value of patch tests in clindamycin-related drug eruptions. Contact Dermatitis. 2011;65:202–207. doi: 10.1111/j.1600-0536.2011.01942.x. [DOI] [PubMed] [Google Scholar]
- 66.Barbaud A, Trechot P, Weber-Muller F, Ulrich G, Commun N, Schmutz JL. Drug skin tests in cutaneous adverse drug reactions to pristinamycin: 29 cases with a study of cross-reactions between synergistins. Contact Dermatitis. 2004;50:22–26. doi: 10.1111/j.0105-1873.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y-C, Cho Y-T, Chang C-Y, Chu C-Y. Drug reaction with eosinophilia and systemic symptoms: A drug-induced hypersensitivity syndrome with variable clinical features. Dermatologica Sinica. 2013;31:196–204. [Google Scholar]
- 68.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001;28:113–119. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 69.Brahimi N, Routier E, Raison-Peyron N, Tronquoy AF, Pouget-Jasson C, Amarger S, Machet L, Amsler E, Claeys A, Sassolas B, et al. A three-year-analysis of fixed drug eruptions in hospital settings in France. Eur J Dermatol. 2010;20:461–464. doi: 10.1684/ejd.2010.0980. [DOI] [PubMed] [Google Scholar]
- 70.Fernando SL. Ertapenem-induced acute generalized exanthematous pustulosis with cross-reactivity to other beta-lactam antibiotics on patch testing. Ann Allergy Asthma Immunol. 2013;111:139–140. doi: 10.1016/j.anai.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 71.Sawada Y, Sugita K, Fukamachi S, Bito T, Nakamura M, Tokura Y. Doripenem-induced intertriginous drug eruption as a mild form of AGEP. J Eur Acad Dermatol Venereol. 2009;23:974–976. doi: 10.1111/j.1468-3083.2008.03079.x. [DOI] [PubMed] [Google Scholar]
- 72.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 73.Chanal J, Ingen-Housz-Oro S, Ortonne N, Duong TA, Thomas M, Valeyrie-Allanore L, Lebrun-Vignes B, Andre C, Roujeau JC, Chosidow O, et al. Linear IgA bullous dermatosis: comparison between the drug-induced and spontaneous forms. Br J Dermatol. 2013;169:1041–1048. doi: 10.1111/bjd.12488. [DOI] [PubMed] [Google Scholar]
- 74.Shimanovich I, Rose C, Sitaru C, Brocker EB, Zillikens D. Localized linear IgA disease induced by ampicillin/sulbactam. J Am Acad Dermatol. 2004;51:95–98. doi: 10.1016/j.jaad.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 75.Ho JC, Ng PL, Tan SH, Giam YC. Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatr Dermatol. 2007;24:E40–43. doi: 10.1111/j.1525-1470.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 76.Santos-Juanes J, Coto Hernandez R, Trapiella L, Caminal L, Sanchez del Rio J, Soto J. Amoxicillin-associated linear IgA bullous dermatosis. J Eur Acad Dermatol Venereol. 2007;21:992–993. doi: 10.1111/j.1468-3083.2006.02066.x. [DOI] [PubMed] [Google Scholar]
- 77.Bernstein EF, Schuster M. Linear IgA bullous dermatosis associated with vancomycin. Ann Intern Med. 1998;129:508–509. doi: 10.7326/0003-4819-129-6-199809150-00022. [DOI] [PubMed] [Google Scholar]
- 78.Nousari HC, Costarangos C, Anhalt GJ. Vancomycin-associated linear IgA bullous dermatosis. Ann Intern Med. 1998;129:507–508. doi: 10.7326/0003-4819-129-6-199809150-00021. [DOI] [PubMed] [Google Scholar]
- 79.Buonomo A, Nucera E, De Pasquale T, Pecora V, Lombardo C, Sabato V, Colagiovanni A, Rizzi A, Aruanno A, Pascolini L, et al. Tolerability of aztreonam in patients with cell-mediated allergy to beta-lactams. Int Arch Allergy Immunol. 2011;155:155–159. doi: 10.1159/000318844. [DOI] [PubMed] [Google Scholar]
- 80.Vezir E, Dibek Misirlioglu E, Civelek E, Capanoglu M, Guvenir H, Ginis T, Toyran M, Kocabas CN. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol. 2015 doi: 10.1111/pai.12493. [DOI] [PubMed] [Google Scholar]
- 81.Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol. 2001;137:765–770. [PubMed] [Google Scholar]
- 82.Mockenhaupt M. Adverse Cutaneous Drug Eruptions. 1. Vol. 97. Karger; 2012. Epidemiology of cutaneous adverse drug reactions (cADR) [Google Scholar]
- 83.Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, Mockenhaupt M, Fagot JP, Roujeau JC. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157:989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 84.Navi D, Michael DJ, Fazel N. Drug-induced linear IgA bullous dermatosis. Dermatol Online J. 2006;12:12. [PubMed] [Google Scholar]
- 85.Fortuna G, Aria M, Marasca F, Salas-Alanis JC. Linear immunoglobulin A disease and vancomycin: two real ancestral enemies? Br J Dermatol. 2014;171:1248–1253. doi: 10.1111/bjd.13014. [DOI] [PubMed] [Google Scholar]
- 86.Forman R, Koren G, Shear NH. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a review of 10 years' experience. Drug Saf. 2002;25:965–972. doi: 10.2165/00002018-200225130-00006. [DOI] [PubMed] [Google Scholar]
- 87.Roujeau JC. Immune mechanisms in drug allergy. Allergol Int. 2006;55:27–33. doi: 10.2332/allergolint.55.27. [DOI] [PubMed] [Google Scholar]
- 88.Hallgren J, Tengvall-Linder M, Persson M, Wahlgren CF. Stevens-Johnson syndrome associated with ciprofloxacin: a review of adverse cutaneous events reported in Sweden as associated with this drug. J Am Acad Dermatol. 2003;49:S267–269. doi: 10.1016/s0190-9622(03)00478-x. [DOI] [PubMed] [Google Scholar]
- 89.Gonul M, Kulcu Cakmak S, Yayla D, Unal T. Linear IgA bullous dermatosis induced by moxifloxacin. Clin Exp Dermatol. 2014;39:78–80. doi: 10.1111/ced.12167. [DOI] [PubMed] [Google Scholar]
- 90.Jimenez I, Anton E, Picans I, Sanchez I, Quinones MD, Jerez J. Fixed drug eruption from amoxycillin. Allergol Immunopathol (Madr) 1997;25:247–248. [PubMed] [Google Scholar]
- 91.Nantel-Battista M, Al Dhaybi R, Hatami A, Marcoux D, Desroches A, Kokta V. Childhood linear IgA bullous disease induced by trimethoprim-sulfamethoxazole. J Dermatol Case Rep. 2010;4:33–35. doi: 10.3315/jdcr.2010.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nair PA. Ciprofloxacin induced bullous fixed drug reaction: three case reports. J Family Med Prim Care. 2015;4:269–272. doi: 10.4103/2249-4863.154673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.San Pedro de Saenz B, Gomez A, Quiralte J, Florido JF, Martin E, Hinojosa B. FDE to macrolides. Allergy. 2002;57:55–56. doi: 10.1034/j.1398-9995.2002.13439.x. [DOI] [PubMed] [Google Scholar]
- 94.Thami GP, Kanwar AJ. Fixed drug eruption due to metronidazole and tinidazole without cross-sensitivity to secnidazole. Dermatology. 1998;196:368. [PubMed] [Google Scholar]
- 95.Kanwar AJ, Sharma R, Rajagopalan M, Kaur S. Fixed drug eruption due to tinidazole with cross-reactivity with metronidazole. Dermatologica. 1990;180:277. doi: 10.1159/000248048. [DOI] [PubMed] [Google Scholar]
- 96.Mishra D, Mobashir M, Zaheer MS. Fixed drug eruption and cross-reactivity between tinidazole and metronidazole. Int J Dermatol. 1990;29:740. doi: 10.1111/j.1365-4362.1990.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 97.Yang LP, Zhang AL, Wang DD, Ke HX, Cheng Q, Wang C. Stevens-Johnson syndrome induced by the cross-reactivity between teicoplanin and vancomycin. J Clin Pharm Ther. 2014;39:442–445. doi: 10.1111/jcpt.12159. [DOI] [PubMed] [Google Scholar]
- 98.O'Meara P, Borici-Mazi R, Morton AR, Ellis AK. DRESS with delayed onset acute interstitial nephritis and profound refractory eosinophilia secondary to Vancomycin. Allergy Asthma Clin Immunol. 2011;7:16. doi: 10.1186/1710-1492-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.An SY, Hwang EK, Kim JH, Kim JE, Jin HJ, Jin SM, Kyun JO, Lee YH, Park HS, Choi YW, et al. Vancomycin-associated spontaneous cutaneous adverse drug reactions. Allergy Asthma Immunol Res. 2011;3:194–198. doi: 10.4168/aair.2011.3.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roujeau JC, Chosidow O, Saiag P, Guillaume JC. Toxic epidermal necrolysis (Lyell syndrome) J Am Acad Dermatol. 1990;23:1039–1058. doi: 10.1016/0190-9622(90)70333-d. [DOI] [PubMed] [Google Scholar]
- 101.Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355–361. doi: 10.1111/j.1365-2133.1956.tb12766.x. [DOI] [PubMed] [Google Scholar]
- 102.Letko E, Papaliodis DN, Papaliodis GN, Daoud YJ, Ahmed AR, Foster CS. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005;94:419–436. doi: 10.1016/S1081-1206(10)61112-X. quiz 436–418, 456. [DOI] [PubMed] [Google Scholar]
- 103.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, Sidoroff A, Naldi L, Mockenhaupt M, Roujeau JC, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 104.Prange B, Marini A, Kalke A, Hodzic-Avdagic N, Ruzicka T, Hengge UR. Acute localized exanthematous pustulosis (ALEP) J Dtsch Dermatol Ges. 2005;3:210–212. [PubMed] [Google Scholar]
- 105.Betto P, Germi L, Bonoldi E, Bertazzoni M. Acute localized exanthematous pustulosis (ALEP) caused by amoxicillin-clavulanic acid. Int J Dermatol. 2008;47:295–296. doi: 10.1111/j.1365-4632.2008.03477.x. [DOI] [PubMed] [Google Scholar]
- 106.Kostopoulos TC, Krishna SM, Brinster NK, Ortega-Loayza AG. Acute generalized exanthematous pustulosis: atypical presentations and outcomes. J Eur Acad Dermatol Venereol. 2015;29:209–214. doi: 10.1111/jdv.12721. [DOI] [PubMed] [Google Scholar]
- 107.Wetter DA, Davis MD. Recurrent erythema multiforme: clinical characteristics, etiologic associations, and treatment in a series of 48 patients at Mayo Clinic, 2000 to 2007. J Am Acad Dermatol. 2010;62:45–53. doi: 10.1016/j.jaad.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 108.Bean SF, Quezada RK. Recurrent oral erythema multiforme. Clinical experience with 11 patients. JAMA. 1983;249:2810–2812. [PubMed] [Google Scholar]
- 109.Korkij W, Soltani K. Fixed drug eruption. A brief review. Arch Dermatol. 1984;120:520–524. [PubMed] [Google Scholar]
- 110.Venning VA. Linear IgA disease: clinical presentation, diagnosis, and pathogenesis. Dermatol Clin. 2011;29:453–458. ix. doi: 10.1016/j.det.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 111.Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38–50. doi: 10.1016/j.clindermatol.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, Grange A, Amarger S, Girardin P, Guinnepain MT, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–562. doi: 10.1111/bjd.12125. [DOI] [PubMed] [Google Scholar]
- 113.Barbaud A. Skin testing and patch testing in non-IgE-mediated drug allergy. Curr Allergy Asthma Rep. 2014;14:442. doi: 10.1007/s11882-014-0442-8. [DOI] [PubMed] [Google Scholar]
- 114.Wolkenstein P, Chosidow O, Flechet ML, Robbiola O, Paul M, Dume L, Revuz J, Roujeau JC. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234–236. doi: 10.1111/j.1600-0536.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 115.Romano A, Viola M, Gaeta F, Rumi G, Maggioletti M. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol. 2008;4:66–74. doi: 10.1186/1710-1492-4-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buonomo A, Nucera E, Pecora V, Rizzi A, Aruanno A, Pascolini L, Ricci AG, Colagiovanni A, Schiavino D. Cross-reactivity and tolerability of cephalosporins in patients with cell-mediated allergy to penicillins. J Investig Allergol Clin Immunol. 2014;24:331–337. [PubMed] [Google Scholar]
- 117.Ozkaya-Bayazit E, Bayazit H, Ozarmagan G. Topical provocation in 27 cases of cotrimoxazole-induced fixed drug eruption. Contact Dermatitis. 1999;41:185–189. doi: 10.1111/j.1600-0536.1999.tb06127.x. [DOI] [PubMed] [Google Scholar]
- 118.Andrade P, Brinca A, Goncalo M. Patch testing in fixed drug eruptions--a 20-year review. Contact Dermatitis. 2011;65:195–201. doi: 10.1111/j.1600-0536.2011.01946.x. [DOI] [PubMed] [Google Scholar]
- 119.Seitz CS, Brocker EB, Trautmann A. Diagnostic testing in suspected fluoroquinolone hypersensitivity. Clin Exp Allergy. 2009;39:1738–1745. doi: 10.1111/j.1365-2222.2009.03338.x. [DOI] [PubMed] [Google Scholar]
- 120.Hausermann P, Scherer K, Weber M, Bircher AJ. Ciprofloxacin-induced acute generalized exanthematous pustulosis mimicking bullous drug eruption confirmed by a positive patch test. Dermatology. 2005;211:277–280. doi: 10.1159/000087024. [DOI] [PubMed] [Google Scholar]
- 121.Barbaud A. Skin testing in delayed reactions to drugs. Immunol Allergy Clin North Am. 2009;29:517–535. doi: 10.1016/j.iac.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 122.Romano A, Di Fonso M, Pocobelli D, Giannarini L, Venuti A, Garcovich A. Two cases of toxic epidermal necrolysis caused by delayed hypersensitivity to beta-lactam antibiotics. J Investig Allergol Clin Immunol. 1993;3:53–55. [PubMed] [Google Scholar]
- 123.Tagami H, Tatsuta K, Iwatski K, Yamada M. Delayed hypersensitivity in ampicillin-induced toxic epidermal necrolysis. Arch Dermatol. 1983;119:910–913. [PubMed] [Google Scholar]
- 124.Bomarrito L, Zisa G, Delrosso G, Farinelli P, Galimberti M. A case of acute generalized exanthematous pustulosis due to amoxicillin-clavulanate with multiple positivity to beta-lactam patch testing. Eur Ann Allergy Clin Immunol. 2013;45:178–180. [PubMed] [Google Scholar]
- 125.Chaabane A, Aouam K, Gassab L, Njim L, Boughattas NA. Acute generalized exanthematous pustulosis (AGEP) induced by cefotaxime. Fundam Clin Pharmacol. 2010;24:429–432. doi: 10.1111/j.1472-8206.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 126.Chaabane A, Fredj NB, Chadly Z, Boughattas NA, Aouam K. Fixed drug eruption: a selective reaction to amoxicillin. Therapie. 2013;68:183–185. doi: 10.2515/therapie/2013027. [DOI] [PubMed] [Google Scholar]
- 127.Prieto A, De Barrio M, Infante S, Torres A, Rubio M, Olalde S. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. Contact Dermatitis. 2005;53:169–170. doi: 10.1111/j.0105-1873.2005.0407a.x. [DOI] [PubMed] [Google Scholar]
- 128.Alonso JC, Melgosa AC, Gonzalo MJ, Garcia CM. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermatitis. 2005;53:121–122. doi: 10.1111/j.0105-1873.2005.0650h.x. [DOI] [PubMed] [Google Scholar]
- 129.Kim JY, Sohn KH, Song WJ, Kang HR. A case of drug reaction with eosinophilia and systemic symptoms induced by ethambutol with early features resembling Stevens-Johnson syndrome. Acta Derm Venereol. 2013;93:753–754. doi: 10.2340/00015555-1600. [DOI] [PubMed] [Google Scholar]
- 130.Romano A, Viola M, Mondino C, Pettinato R, Di Fonso M, Papa G, Venuti A, Montuschi P. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol. 2002;129:169–174. doi: 10.1159/000065876. [DOI] [PubMed] [Google Scholar]
- 131.Gonzalo-Garijo MA, Rodriguez-Nevado I, de Argila D. Patch tests for diagnosis of delayed hypersensitivity to cephalosporins. Allergol Immunopathol (Madr) 2006;34:39–41. doi: 10.1157/13084227. [DOI] [PubMed] [Google Scholar]
- 132.Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–2225. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 133.Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, Bircher A, Blanca M, Bonadonna B, Campi P, et al. Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68:702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 134.Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, Khan DA, Lang DM, Park HS, Pichler W, et al. International Consensus on drug allergy. Allergy. 2014;69:420–437. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 135.Joint Task Force on Practice P, American Academy of Allergy A, Immunology, American College of Allergy A, Immunology, Joint Council of Allergy A, Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 136.Rodriguez-Alvarez M, Santos-Magadan S, Rodriguez-Jimenez B, Reig-Rincon de Arellano I, Vazquez-Cortes S, Martinez-Cocera C. Reproducibility of delayed-type reactions to betalactams. Allergol Immunopathol (Madr) 2008;36:201–204. [PubMed] [Google Scholar]
- 137.Cabanas R, Calderon O, Ramirez E, Fiandor A, Prior N, Caballero T, Herranz P, Bobolea I, Lopez-Serrano MC, Quirce S, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. 2014;24:425–430. [PubMed] [Google Scholar]
- 138.Barbaud A, Goncalo M, Bruynzeel D, Bircher A European Society of Contact D. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45:321–328. doi: 10.1034/j.1600-0536.2001.450601.x. [DOI] [PubMed] [Google Scholar]
- 139.Makris MP, Koulouris S, Kalogeromitros D. Nonimmediate systemic hypersensitivity reaction to beta–lactam intradermal tests. J Investig Allergol Clin Immunol. 2010;20:630–631. [PubMed] [Google Scholar]
- 140.Sala Cunill A, Labrador-Horrillo M, Guilarte M, Luengo O, Cardona V. Generalised delayed desquamative exanthema after intradermal testing with betalactam antibiotics. Allergy. 2011;66:702–703. doi: 10.1111/j.1398-9995.2010.02495.x. [DOI] [PubMed] [Google Scholar]
- 141.Torres MJ, Sanchez-Sabate E, Alvarez J, Mayorga C, Fernandez J, Padial A, Cornejo-Garcia JA, Bellon T, Blanca M. Skin test evaluation in nonimmediate allergic reactions to penicillins. Allergy. 2004;59:219–224. doi: 10.1046/j.1398-9995.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 142.Koshak EA. Could a routine skin test to penicillin lead to fatal anaphylaxis? East Mediterr Health J. 2000;6:526–531. [PubMed] [Google Scholar]
- 143.Weber-Mani U, Pichler WJ. Anaphylactic shock after intradermal testing with betalactam antibiotics. Allergy. 2008;63:785. doi: 10.1111/j.1398-9995.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- 144.Hausermann P, Bircher AJ. Immediate and delayed hypersensitivity to ceftriaxone, and anaphylaxis due to intradermal testing with other beta-lactam antibiotics, in a previously amoxicillin-sensitized patient. Contact Dermatitis. 2002;47:311–312. doi: 10.1034/j.1600-0536.2002.4705103.x. [DOI] [PubMed] [Google Scholar]
- 145.Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002;57:45–51. [PubMed] [Google Scholar]
- 146.Padial A, Antunez C, Blanca-Lopez N, Fernandez TD, Cornejo-Garcia JA, Mayorga C, Torres MJ, Blanca M. Non-immediate reactions to beta-lactams: diagnostic value of skin testing and drug provocation test. Clin Exp Allergy. 2008;38:822–828. doi: 10.1111/j.1365-2222.2008.02961.x. [DOI] [PubMed] [Google Scholar]
- 147.Hjortlund J, Mortz CG, Skov PS, Bindslev-Jensen C. Diagnosis of penicillin allergy revisited: the value of case history, skin testing, specific IgE and prolonged challenge. Allergy. 2013;68:1057–1064. doi: 10.1111/all.12195. [DOI] [PubMed] [Google Scholar]
- 148.Garcia-Rubio I, Martinez-Cocera C, Santos Magadan S, Rodriguez-Jimenez B, Vazquez-Cortes S. Hypersensitivity reactions to metronidazole. Allergol Immunopathol (Madr) 2006;34:70–72. doi: 10.1157/13086750. [DOI] [PubMed] [Google Scholar]
- 149.Torres MJ, Romano A, Mayorga C, Moya MC, Guzman AE, Reche M, Juarez C, Blanca M. Diagnostic evaluation of a large group of patients with immediate allergy to penicillins: the role of skin testing. Allergy. 2001;56:850–856. doi: 10.1034/j.1398-9995.2001.00089.x. [DOI] [PubMed] [Google Scholar]
- 150.Bousquet PJ, Pipet A, Bousquet-Rouanet L, Demoly P. Oral challenges are needed in the diagnosis of beta-lactam hypersensitivity. Clin Exp Allergy. 2008;38:185–190. doi: 10.1111/j.1365-2222.2007.02867.x. [DOI] [PubMed] [Google Scholar]
- 151.Romano A, Gaeta F, Valluzzi RL, Caruso C, Alonzi C, Viola M, Bousquet PJ. Diagnosing nonimmediate reactions to cephalosporins. J Allergy Clin Immunol. 2012;129:1166–1169. doi: 10.1016/j.jaci.2011.12.995. [DOI] [PubMed] [Google Scholar]
- 152.Trubiano J, Phillips E. Antimicrobial stewardship's new weapon? A review of antibiotic allergy and pathways to 'de-labeling'. Curr Opin Infect Dis. 2013;26:526–537. doi: 10.1097/QCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153**.Bourke J, Pavlos R, James I, Phillips E. Improving the Effectiveness of Penicillin Allergy De-labeling. J Allergy Clin Immunol Pract. 2015 doi: 10.1016/j.jaip.2014.11.002. Approximately 10–20% of hospitalized patients are labeled, often incorrectly, as penicillin allergic and this is assoicated with signfiicant healthy and economic costs. In this study of >400 patients in Western Australia, the authors found that skin prick testing, intradermal testing and oral challenge safely de-labels the majority of patients and identifies selective beta-lactam allergies in others. [DOI] [PubMed] [Google Scholar]
- 154.Hjortlund J, Mortz CG, Skov PS, Eller E, Poulsen JM, Borch JE, Bindslev-Jensen C. One-week oral challenge with penicillin in diagnosis of penicillin allergy. Acta Derm Venereol. 2012;92:307–312. doi: 10.2340/00015555-1254. [DOI] [PubMed] [Google Scholar]
- 155.Caubet JC, Kaiser L, Lemaitre B, Fellay B, Gervaix A, Eigenmann PA. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218–222. doi: 10.1016/j.jaci.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Caubet JC, Frossard C, Fellay B, Eigenmann PA. Skin tests and in vitro allergy tests have a poor diagnostic value for benign skin rashes due to beta-lactams in children. Pediatr Allergy Immunol. 2015;26:80–82. doi: 10.1111/pai.12314. [DOI] [PubMed] [Google Scholar]
- 157.Rosenfield L, Kalicinsky C, Warrington R. A retrospective comparison of false negative skin test rates in penicillin allergy, using pencilloyl-poly-lysine and minor determinants or Penicillin G, followed by open challenge. Allergy Asthma Clin Immunol. 2015;11:34. doi: 10.1186/s13223-015-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Blanca-Lopez N, Zapatero L, Alonso E, Torres MJ, Fuentes V, Martinez-Molero MI, Blanca M. Skin testing and drug provocation in the diagnosis of nonimmediate reactions to aminopenicillins in children. Allergy. 2009;64:229–233. doi: 10.1111/j.1398-9995.2008.01903.x. [DOI] [PubMed] [Google Scholar]
- 159*.Blumenthal KG, Shenoy ES, Varughese CA, Hurwitz S, Hooper DC, Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol. 2015;115:294–300. e292. doi: 10.1016/j.anai.2015.05.011. The authors of this article implemented an inpatient antibiotic prescribing guidelines for patients with self-reported penicillin or cephalosporin allergy. The implementation of the guidelines was associated with an almost 7-fold increase in the number of test doses to beta-lactams without an increase in adverse drug reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carr A, Cooper DA. Pathogenesis and management of HIV-associated drug hypersensitivity. AIDS Clin Rev. 1995:65–97. [PubMed] [Google Scholar]
- 161.Zolopa AR. The evolution of HIV treatment guidelines: current state-of-the-art of ART. Antiviral Res. 2010;85:241–244. doi: 10.1016/j.antiviral.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 162.Martinez Castro B, Ferrando Piqueres R, Martinez Garcia M, Soler Company E. Desensitization to tipranavir caused by toxicodermia. Farm Hosp. 2009;33:340–342. doi: 10.1016/s1130-6343(09)72981-2. [DOI] [PubMed] [Google Scholar]
- 163.Kohli-Pamnani A, Huynh P, Lobo F. Amprenavir-induced maculopapular exanthem followed by desensitization in a patient with late-stage human immunodeficiency virus. Ann Allergy Asthma Immunol. 2006;96:620–623. doi: 10.1016/S1081-1206(10)63559-4. [DOI] [PubMed] [Google Scholar]
- 164.Marcos Bravo MC, Ocampo Hermida A, Martinez Vilela J, Perez Rodriguez MT, Gavilan Montenegro MJ, Arenas Villarroel LJ, Miralles Alvarez C, Rodriguez Dasilva A, Martinez Vazquez C. Hypersensitivity reaction to darunavir and desensitization protocol. J Investig Allergol Clin Immunol. 2009;19:250–251. [PubMed] [Google Scholar]
- 165.Phillips EJ, Kuriakose B, Knowles SR. Efavirenz-induced skin eruption and successful desensitization. Ann Pharmacother. 2002;36:430–432. doi: 10.1345/aph.1A287. [DOI] [PubMed] [Google Scholar]
- 166.Demoly P, Messaad D, Fabre J, Reynes J, Bousquet J. Nevirapine-induced cutaneous hypersensitivity reactions and successful tolerance induction. J Allergy Clin Immunol. 1999;104:504–505. doi: 10.1016/s0091-6749(99)70403-3. [DOI] [PubMed] [Google Scholar]
- 167.Romano A, Gaeta F, Valluzzi RL, Alonzi C, Maggioletti M, Zaffiro A, Caruso C, Quaratino D. Absence of cross-reactivity to carbapenems in patients with delayed hypersensitivity to penicillins. Allergy. 2013;68:1618–1621. doi: 10.1111/all.12299. [DOI] [PubMed] [Google Scholar]
- 168.Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010;126:994–999. doi: 10.1016/j.jaci.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 169.Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Zaffiro A, Caruso C, Quaratino D. IgE-mediated hypersensitivity to cephalosporins: Cross-reactivity and tolerability of alternative cephalosporins. J Allergy Clin Immunol. 2015;136:685–691. e683. doi: 10.1016/j.jaci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 170.Romano A, Gueant-Rodriguez RM, Viola M, Gaeta F, Caruso C, Gueant JL. Cross-reactivity among drugs: clinical problems. Toxicology. 2005;209:169–179. doi: 10.1016/j.tox.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 171.Lagace-Wiens P, Rubinstein E. Adverse reactions to beta-lactam antimicrobials. Expert Opin Drug Saf. 2012;11:381–399. doi: 10.1517/14740338.2012.643866. [DOI] [PubMed] [Google Scholar]
- 172.Antunez C, Blanca-Lopez N, Torres MJ, Mayorga C, Perez-Inestrosa E, Montanez MI, Fernandez T, Blanca M. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J Allergy Clin Immunol. 2006;117:404–410. doi: 10.1016/j.jaci.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 173.Saxon A, Hassner A, Swabb EA, Wheeler B, Adkinson NF., Jr Lack of cross-reactivity between aztreonam , a monobactam antibiotic, and penicillin in penicillin-allergic subjects. J Infect Dis. 1984;149:16–22. doi: 10.1093/infdis/149.1.16. [DOI] [PubMed] [Google Scholar]
- 174.Vega JM, Blanca M, Garcia JJ, Miranda A, Carmona MJ, Garcia A, Moya MC, Sanchez F, Terrados S. Tolerance to aztreonam in patients allergic to beta-lactam antibiotics. Allergy. 1991;46:196–202. doi: 10.1111/j.1398-9995.1991.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 175.Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013;43:1027–1037. doi: 10.1111/cea.12145. [DOI] [PubMed] [Google Scholar]
- 176.Romano A, Blanca M, Torres MJ, Bircher A, Aberer W, Brockow K, Pichler WJ, Demoly P Enda, Eaaci. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153–1160. doi: 10.1111/j.1398-9995.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- 177.Trautmann A, Seitz CS, Stoevesandt J, Kerstan A. Aminopenicillin-associated exanthem: lymphocyte transformation testing revisited. Clin Exp Allergy. 2014;44:1531–1538. doi: 10.1111/cea.12437. [DOI] [PubMed] [Google Scholar]
- 178.Yawalkar N, Reimers A, Hari Y, Hunziker T, Gerber H, Muller U, Pichler W. Drug-induced linear IgA bullous dermatosis associated with ceftriaxone- and metronidazole-specific T cells. Dermatology. 1999;199:25–30. doi: 10.1159/000018173. [DOI] [PubMed] [Google Scholar]
- 179.Dias de Castro E, Leblanc A, Sarmento A, Cernadas JR. An unusual case of delayed-type hypersensitivity to ceftriaxone and meropenem. Eur Ann Allergy Clin Immunol. 2015;47:225–227. [PubMed] [Google Scholar]
- 180.Schnyder B, Pichler WJ. Skin and laboratory tests in amoxicillin- and penicillin-induced morbilliform skin eruption. Clin Exp Allergy. 2000;30:590–595. doi: 10.1046/j.1365-2222.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 181.Tomida E, Kato Y, Ozawa H, Hasegawa H, Ishii N, Hashimoto T, Akiyama M. Causative drug detection by drug-induced lymphocyte stimulation test in drug-induced linear IgA bullous dermatosis. Br J Dermatol. 2015 doi: 10.1111/bjd.14069. [DOI] [PubMed] [Google Scholar]
- 182.Onodi-Nagy K, Kinyo A, Meszes A, Garaczi E, Kemeny L, Bata-Csorgo Z. Amoxicillin rash in patients with infectious mononucleosis: evidence of true drug sensitization. Allergy Asthma Clin Immunol. 2015;11:1. doi: 10.1186/1710-1492-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Romano A, Torres MJ, Di Fonso M, Leyva L, Andriolo M, Pettinato R, Blanca M. Delayed hypersensitivity to cefazolin: report on a case involving lymphocyte transformation studies with different cephalosporins. Ann Allergy Asthma Immunol. 2001;87:238–242. doi: 10.1016/S1081-1206(10)62233-8. [DOI] [PubMed] [Google Scholar]
- 184.Teraki Y, Shiohara T. IFN-gamma-producing effector CD8+ T cells and IL-10-producing regulatory CD4+ T cells in fixed drug eruption. J Allergy Clin Immunol. 2003;112:609–615. doi: 10.1016/s0091-6749(03)01624-5. [DOI] [PubMed] [Google Scholar]
- 185.Rozieres A, Hennino A, Rodet K, Gutowski MC, Gunera-Saad N, Berard F, Cozon G, Bienvenu J, Nicolas JF. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. 2009;64:534–542. doi: 10.1111/j.1398-9995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 186.Tassignon J, Burny W, Dahmani S, Zhou L, Stordeur P, Byl B, De Groote D. Monitoring of cellular responses after vaccination against tetanus toxoid: comparison of the measurement of IFN-gamma production by ELISA, ELISPOT, flow cytometry and real-time PCR. J Immunol Methods. 2005;305:188–198. doi: 10.1016/j.jim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 187.Esser S, Jablonka R, Heinemann FM, Reuter S, Jaeger H, von Krosigk A, Schenk-Westkamp P, Schadendorf D, Horn PA, Lindemann M. Detection of abacavir hypersensitivity by ELISpot method. Inflamm Allergy Drug Targets. 2012;11:227–234. doi: 10.2174/187152812800392751. [DOI] [PubMed] [Google Scholar]
- 188*.Lucas A, Lucas M, Strhyn A, Keane NM, McKinnon E, Pavlos R, Moran EM, Meyer-Pannwitt V, Gaudieri S, D'Orsogna L, et al. Abacavir-reactive memory T cells are present in drug naive individuals. PLoS One. 2015;10:e0117160. doi: 10.1371/journal.pone.0117160. The goal of this study was to determine whether a pre-existing abacavir reactive memory T-cell population contributes to early abacavir hypersensitivity symptoms. Abacavir reactive CD8+ T-cell responses were detected in vitro in one hundred percent of abacavir unexposed HLA-B*57:01 positive healthy donors. The authors propose that these pre-existing abacavir-reactive memory CD8+ T-cell responses must have been primed by earlier exposure to another foreign antigen and that these T cells cross-react with an abacavir-HLA-B*57:01-endogenous peptide ligand complex, in keeping with the model of heterologous immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keane NM, Pavlos RK, McKinnon E, Lucas A, Rive C, Blyth CC, Dunn D, Lucas M, Mallal S, Phillips E. HLA Class I restricted CD8+ and Class II restricted CD4+ T cells are implicated in the pathogenesis of nevirapine hypersensitivity. AIDS. 2014;28:1891–1901. doi: 10.1097/QAD.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 190.Khalil G, El-Sabban M, Al-Ghadban S, Azzi S, Shamra S, Khalife S, Maroun R. Cytokine expression profile of sensitized human T lymphocytes following in vitro stimulation with amoxicillin. Eur Cytokine Netw. 2008;19:131–141. doi: 10.1684/ecn.2008.0132. [DOI] [PubMed] [Google Scholar]
- 191.Jenkins RE, Yaseen FS, Monshi MM, Whitaker P, Meng X, Farrell J, Hamlett J, Sanderson JP, El-Ghaiesh S, Peckham D, et al. beta-Lactam antibiotics form distinct haptenic structures on albumin and activate drug-specific T-lymphocyte responses in multiallergic patients with cystic fibrosis. Chem Res Toxicol. 2013;26:963–975. doi: 10.1021/tx400124m. [DOI] [PubMed] [Google Scholar]
- 192.El-Ghaiesh S, Monshi MM, Whitaker P, Jenkins R, Meng X, Farrell J, Elsheikh A, Peckham D, French N, Pirmohamed M, et al. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. J Pharmacol Exp Ther. 2012;341:597–610. doi: 10.1124/jpet.111.190900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tanvarasethee B, Buranapraditkun S, Klaewsongkram J. The potential of using enzyme-linked immunospot to diagnose cephalosporin-induced maculopapular exanthems. Acta Derm Venereol. 2013;93:66–69. doi: 10.2340/00015555-1386. [DOI] [PubMed] [Google Scholar]
- 194.Bensaid B, Rozieres A, Nosbaum A, Nicolas JF, Berard F. Amikacin-induced drug reaction with eosinophilia and systemic symptoms syndrome: delayed skin test and ELISPOT assay results allow the identification of the culprit drug. J Allergy Clin Immunol. 2012;130:1413–1414. doi: 10.1016/j.jaci.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 195.Phatharacharukul P, Klaewsongkram J. A case of sulfasalazine-induced hypersensitivity syndrome confirmed by enzyme-linked immunospot assay. Allergy Asthma Immunol Res. 2013;5:415–417. doi: 10.4168/aair.2013.5.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Phillips E, Bartlett JA, Sanne I, Lederman MM, Hinkle J, Rousseau F, Dunn D, Pavlos R, James I, Mallal SA, et al. Associations between HLA-DRB1*0102, HLA-B*5801, and hepatotoxicity during initiation of nevirapine-containing regimens in South Africa. J Acquir Immune Defic Syndr. 2013;62:e55–57. doi: 10.1097/QAI.0b013e31827ca50f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99–105. doi: 10.1007/s00403-008-0895-5. [DOI] [PubMed] [Google Scholar]
- 198.Diez RA. HLA-B27 and agranulocytosis by levamisole. Immunol Today. 1990;11:270. doi: 10.1016/0167-5699(90)90109-m. [DOI] [PubMed] [Google Scholar]
- 199.Ozkaya-Bayazit E, Akar U. Fixed drug eruption induced by trimethoprim-sulfamethoxazole: evidence for a link to HLA-A30 B13 Cw6 haplotype. J Am Acad Dermatol. 2001;45:712–717. doi: 10.1067/mjd.2001.117854. [DOI] [PubMed] [Google Scholar]
- 200.Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 201.O'Donohue J, Oien KA, Donaldson P, Underhill J, Clare M, MacSween RN, Mills PR. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717–720. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, Day CP, Ruiz-Cabello F, Donaldson PT, Stephens C, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hautekeete ML, Horsmans Y, Van Waeyenberge C, Demanet C, Henrion J, Verbist L, Brenard R, Sempoux C, Michielsen PP, Yap PS, et al. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology. 1999;117:1181–1186. doi: 10.1016/s0016-5085(99)70404-x. [DOI] [PubMed] [Google Scholar]
- 204.Romano A, De Santis A, Romito A, Di Fonso M, Venuti A, Gasbarrini GB, Manna R. Delayed hypersensitivity to aminopenicillins is related to major histocompatibility complex genes. Ann Allergy Asthma Immunol. 1998;80:433–437. doi: 10.1016/s1081-1206(10)62997-3. [DOI] [PubMed] [Google Scholar]
- 205.Roujeau JC, Huynh TN, Bracq C, Guillaume JC, Revuz J, Touraine R. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 1987;123:1171–1173. [PubMed] [Google Scholar]
- 206.Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, Naldi L, Bouwes-Bavinck JN, Sidoroff A, de Toma C, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 207.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Drug Metab Rev. 2012;44:116–126. doi: 10.3109/03602532.2011.605790. [DOI] [PubMed] [Google Scholar]
- 208.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Semin Liver Dis. 2009;29:400–411. doi: 10.1055/s-0029-1240009. [DOI] [PubMed] [Google Scholar]
- 209.Likanonsakul S, Rattanatham T, Feangvad S, Uttayamakul S, Prasithsirikul W, Tunthanathip P, Nakayama EE, Shioda T. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai patients. AIDS Res Ther. 2009;6:22. doi: 10.1186/1742-6405-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, Tantisiriwat W, Charoenyingwattana A, Sura T, Takahashi A, et al. Genome-wide association study identifies variations in 6p21. 3 associated with nevirapine-induced rash. Clin Infect Dis. 2011;53:341–348. doi: 10.1093/cid/cir403. [DOI] [PubMed] [Google Scholar]
- 211.Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, Tantisiriwat W, Charoenyingwattana A, Sura T, Chantratita W, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19:139–146. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- 212.Gao S, Gui XE, Liang K, Liu Z, Hu J, Dong B. HLA-dependent hypersensitivity reaction to nevirapine in Chinese Han HIV-infected patients. AIDS Res Hum Retroviruses. 2012;28:540–543. doi: 10.1089/AID.2011.0107. [DOI] [PubMed] [Google Scholar]
- 213.Gatanaga H, Yazaki H, Tanuma J, Honda M, Genka I, Teruya K, Tachikawa N, Kikuchi Y, Oka S. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS. 2007;21:264–265. doi: 10.1097/QAD.0b013e32801199d9. [DOI] [PubMed] [Google Scholar]
- 214.Littera R, Carcassi C, Masala A, Piano P, Serra P, Ortu F, Corso N, Casula B, La Nasa G, Contu L, et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006;20:1621–1626. doi: 10.1097/01.aids.0000238408.82947.09. [DOI] [PubMed] [Google Scholar]
- 215.Yuan J, Guo S, Hall D, Cammett AM, Jayadev S, Distel M, Storfer S, Huang Z, Mootsikapun P, Ruxrungtham K, et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011;25:1271–1280. doi: 10.1097/QAD.0b013e32834779df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vitezica ZG, Milpied B, Lonjou C, Borot N, Ledger TN, Lefebvre A, Hovnanian A. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–541. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]
- 217.Martin AM, Nolan D, James I, Cameron P, Keller J, Moore C, Phillips E, Christiansen FT, Mallal S. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–99. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 218.Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 219.Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, Lai E, Davies K, Handley A, Dow DJ, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 220.Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13:1285–1306. doi: 10.2217/pgs.12.108. [DOI] [PubMed] [Google Scholar]
- 221.Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, Yu YX, Chen MF, Low HQ, Li JH, et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med. 2013;369:1620–1628. doi: 10.1056/NEJMoa1213096. [DOI] [PubMed] [Google Scholar]
- 222.Petz LD. Immunologic cross-reactivity between penicillins and cephalosporins: a review. J Infect Dis. 1978;137(Suppl):S74–S79. doi: 10.1093/infdis/137.supplement.s74. [DOI] [PubMed] [Google Scholar]
- 223.Callero A, Berroa F, Infante S, Fuentes-Aparicio V, Alonso-Lebrero E, Zapatero L. Tolerance to cephalosporins in nonimmediate hypersensitivity to penicillins in pediatric patients. J Investig Allergol Clin Immunol. 2014;24:134–136. [PubMed] [Google Scholar]
- 224.Phillips E, Knowles SR, Weber EA, Blackburn D. Cephalexin tolerated despite delayed aminopenicillin reactions. Allergy. 2001;56:790. doi: 10.1034/j.1398-9995.2001.056008790.x. [DOI] [PubMed] [Google Scholar]
- 225.Novalbos A, Sastre J, Cuesta J, De Las Heras M, Lluch-Bernal M, Bombin C, Quirce S. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001;31:438–443. doi: 10.1046/j.1365-2222.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- 226.Herbert ME, Brewster GS, Lanctot-Herbert M. Medical myth: Ten percent of patients who are allergic to penicillin will have serious reactions if exposed to cephalosporins. West J Med. 2000;172:341. doi: 10.1136/ewjm.172.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Noguerado-Mellado B, Pinto Fernandez C, Pineda-Pineda R, Martinez Lezcano P, Alvarez-Perea A, De Barrio Fernandez M. Cross-reactivity between carbapenems: two case reports. J Allergy Clin Immunol Pract. 2014;2:816–817. doi: 10.1016/j.jaip.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 228.Kruppa A, Scharffetter-Kochanek K, Krieg T, Hunzelmann N. Immediate reaction to roxithromycin and prick test cross-sensitization to erythromycin and clarithromycin. Dermatology. 1998;196:335–336. doi: 10.1159/000017907. [DOI] [PubMed] [Google Scholar]
- 229.Tham SN, Kwok YK, Chan HL. Cross-reactivity in fixed drug eruptions to tetracyclines. Arch Dermatol. 1996;132:1134–1135. [PubMed] [Google Scholar]
- 230.Rudzki E, Rebandel P. Cross-reactions with 4 aminoglycoside antibiotics at various concentrations. Contact Dermatitis. 1996;35:62. doi: 10.1111/j.1600-0536.1996.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 231.Garcia-Rubio I, Martinez-Cocera C, Robledo Echarren T, Vazquez Cortes S. Fixed exanthema from systemic tobramycin. J Investig Allergol Clin Immunol. 2006;16:264–265. [PubMed] [Google Scholar]
- 232.Yung MW, Rajendra T. Delayed hypersensitivity reaction to topical aminoglycosides in patients undergoing middle ear surgery. Clin Otolaryngol Allied Sci. 2002;27:365–368. doi: 10.1046/j.1365-2273.2002.00597.x. [DOI] [PubMed] [Google Scholar]
- 233.Gastaminza G, Anda M, Audicana MT, Fernandez E, Munoz D. Fixed-drug eruption due to metronidazole with positive topical provocation. Contact Dermatitis. 2001;44:36. [PubMed] [Google Scholar]
- 234.Blanca-Lopez N, Andreu I, Torres Jaen MJ. Hypersensitivity reactions to quinolones. Curr Opin Allergy Clin Immunol. 2011;11:285–291. doi: 10.1097/ACI.0b013e3283489bc3. [DOI] [PubMed] [Google Scholar]
- 235.Chang B, Knowles SR, Weber E. Immediate hypersensitivity to moxifloxacin with tolerance to ciprofloxacin: report of three cases and review of the literature. Ann Pharmacother. 2010;44:740–745. doi: 10.1345/aph.1M579. [DOI] [PubMed] [Google Scholar]
- 236.Aranda A, Mayorga C, Ariza A, Dona I, Rosado A, Blanca-Lopez N, Andreu I, Torres MJ. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011;66:247–254. doi: 10.1111/j.1398-9995.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- 237.Neuman MG, Cohen LB, Nanau RM. Quinolones-induced hypersensitivity reactions. Clin Biochem. 2015;48:716–739. doi: 10.1016/j.clinbiochem.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 238.Macias E, Moreno E, Davila I, Laffond E, Ruiz A, Batista JC, Lorente F. Reaction to teicoplanin with tolerance to vancomycin. J Investig Allergol Clin Immunol. 2008;18:71–72. [PubMed] [Google Scholar]
- 239.Kwon HS, Chang YS, Jeong YY, Lee SM, Song WJ, Kim HB, Kim YK, Cho SH, Kim YY, Min KU. A case of hypersensitivity syndrome to both vancomycin and teicoplanin. J Korean Med Sci. 2006;21:1108–1110. doi: 10.3346/jkms.2006.21.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McElrath MJ, Goldberg D, Neu HC. Allergic cross-reactivity of teicoplanin and vancomycin. Lancet. 1986;1:47. doi: 10.1016/s0140-6736(86)91933-1. [DOI] [PubMed] [Google Scholar]
- 241.Perrin-Lamarre A, Petitpain N, Trechot P, Cuny JF, Schmutz JL, Barbaud A. Glycopeptide-induced cutaneous adverse reaction: results of an immunoallergic investigation in eight patients. Ann Dermatol Venereol. 2010;137:101–105. doi: 10.1016/j.annder.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 242.Hsiao SH, Chou CH, Lin WL, Lee EJ, Liao LH, Chang HJ, Yeh PY, Lin CY, Wu TJ. High risk of cross-reactivity between vancomycin and sequential teicoplanin therapy. J Clin Pharm Ther. 2012;37:296–300. doi: 10.1111/j.1365-2710.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- 243.de Vries E, van Weel-Sipman MH, Vossen JM. A four-year-old child with teicoplanin allergy but no evidence of cross-reaction with vancomycin. Pediatr Infect Dis J. 1994;13:167. doi: 10.1097/00006454-199402000-00026. [DOI] [PubMed] [Google Scholar]
- 244.Strom BL, Schinnar R, Apter AJ, Margolis DJ, Lautenbach E, Hennessy S, Bilker WB, Pettitt D. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628–1635. doi: 10.1056/NEJMoa022963. [DOI] [PubMed] [Google Scholar]
- 245.Wall GC, Dewitt JE, Haack S, Fornoff A, Eastman DK, Koenigsfeld CF. Knowledge and attitudes of American pharmacists concerning sulfonamide allergy cross-reactivity. Pharm World Sci. 2010;32:343–346. doi: 10.1007/s11096-010-9389-6. [DOI] [PubMed] [Google Scholar]
- 246.Wulf NR, Matuszewski KA. Sulfonamide cross-reactivity: is there evidence to support broad cross-allergenicity? Am J Health Syst Pharm. 2013;70:1483–1494. doi: 10.2146/ajhp120291. [DOI] [PubMed] [Google Scholar]
- 247.Lehmann DF. The metabolic rationale for a lack of cross-reactivity between sulfonamide antimicrobials and other sulfonamide-containing drugs. Drug Metab Lett. 2012;6:129–133. doi: 10.2174/187231212804096664. [DOI] [PubMed] [Google Scholar]
- 248.Johnson KK, Green DL, Rife JP, Limon L. Sulfonamide cross-reactivity: fact or fiction? Ann Pharmacother. 2005;39:290–301. doi: 10.1345/aph.1E350. [DOI] [PubMed] [Google Scholar]
- 249.Nishijima T, Gatanaga H, Teruya K, Mizushima D, Aoki T, Watanabe K, Kinai E, Honda H, Yazaki H, Tanuma J, et al. Skin rash induced by ritonavir-boosted darunavir is common, but generally tolerable in an observational setting. J Infect Chemother. 2014;20:285–287. doi: 10.1016/j.jiac.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 250.Zawodniak A, Lochmatter P, Beeler A, Pichler WJ. Cross-reactivity in drug hypersensitivity reactions to sulfasalazine and sulfamethoxazole. Int Arch Allergy Immunol. 2010;153:152–156. doi: 10.1159/000312632. [DOI] [PubMed] [Google Scholar]
- 251.Lin KY, Cheng CY, Yang CJ, Tsai MS, Hsieh SM, Sun HY, Sheng WH, Chen MY, Chang SY, Cheng SH, et al. Skin rash related to once-daily boosted darunavir-containing antiretroviral therapy in HIV-infected Taiwanese: incidence and associated factor. J Infect Chemother. 2014;20:465–470. doi: 10.1016/j.jiac.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 252.Borras-Blasco J, Navarro-Ruiz A, Borras C, Castera E. Adverse cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. J Antimicrob Chemother. 2008;62:879–888. doi: 10.1093/jac/dkn292. [DOI] [PubMed] [Google Scholar]
- 253.Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, Durham SR, Jacobsen L, Malling HJ, Mosges R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 254.Buijs BS, van den Berk GE, Boateng CP, Hoepelman AI, van Maarseveen EM, Arends JE. Cross-reactivity between darunavir and trimethoprim-sulfamethoxazole in HIV-infected patients. AIDS. 2015;29:785–791. doi: 10.1097/QAD.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 255.Lin KY, Hung CC. Clinical relevance of cross-reactivity between darunavir and trimethoprim-sulfamethoxazole in HIV-infected patients. AIDS. 2015;29:2213–2214. doi: 10.1097/QAD.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 256.Carr A, Tindall B, Penny R, Cooper DA. Patterns of multiple-drug hypersensitivities in HIV-infected patients. AIDS. 1993;7:1532–1533. doi: 10.1097/00002030-199311000-00023. [DOI] [PubMed] [Google Scholar]
- 257.Beumont MG, Graziani A, Ubel PA, MacGregor RR. Safety of dapsone as Pneumocystis carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with allergy to trimethoprim/sulfamethoxazole. Am J Med. 1996;100:611–616. doi: 10.1016/s0002-9343(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 258.Lin D, Li WK, Rieder MJ. Cotrimoxazole for prophylaxis or treatment of opportunistic infections of HIV/AIDS in patients with previous history of hypersensitivity to cotrimoxazole. Cochrane Database Syst Rev. 2007:CD005646. doi: 10.1002/14651858.CD005646.pub2. [DOI] [PubMed] [Google Scholar]
- 259.Leoung GS, Stanford JF, Giordano MF, Stein A, Torres RA, Giffen CA, Wesley M, Sarracco T, Cooper EC, Dratter V, et al. Trimethoprim-sulfamethoxazole (TMP-SMZ) dose escalation versus direct rechallenge for Pneumocystis Carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with previous adverse reaction to TMP-SMZ. J Infect Dis. 2001;184:992–997. doi: 10.1086/323353. [DOI] [PubMed] [Google Scholar]
- 260.Mehta U, Maartens G. Is it safe to switch between efavirenz and nevirapine in the event of toxicity? The Lancet Infectious Diseases. 2007;7:733–738. doi: 10.1016/S1473-3099(07)70262-1. [DOI] [PubMed] [Google Scholar]
- 261.Gangar M, Arias G, O'Brien JG, Kemper CA. Frequency of cutaneous reactions on rechallenge with nevirapine and delavirdine. Ann Pharmacother. 2000;34:839–842. doi: 10.1345/aph.19258. [DOI] [PubMed] [Google Scholar]


