EARLY INTERVENTIONS IMPROVE OUTCOME
Sepsis in the year 2016 remains the most expensive disease treated in hospitals and is the most common cause of in-hospital deaths in the United States (1). However, over the last 15 years, since the introduction of early goal-directed therapy (EGDT) and the Surviving Sepsis Campaign (SSC), there has been a consistent and historic reduction in mortality (2). The reduction from a historic mortality of 46.5% to less than 30% was validated when a trio of multinational trials named Protocolized Care for Early Septic Shock (ProCESS), Australasian Resuscitation in Sepsis Evaluation (ARISE), and Protocolized Management in Sepsis (ProMISe) “compared” various forms of resuscitation strategies (2, 3). This independently obtained historic mortality of 46.5% from an international task force of experts is identical to that of the original EGDT trial (2). Thus, it is absolutely clear that a protocolized approach consisting of early detection (lactate and fluid challenge), antibiotic therapy, source control, prevention of sudden cardiopulmonary events, and early hemodynamic optimization improves outcomes.
Even with unprecedented and replicated mortality benefit, many have proposed to dissemble the original EGDT trial and its components (4). ProCESS, ARISE, and ProMISe attempted to replicate and examine the efficacy of EGDT and have shown all time low mortalities, equal mortality reduction in all arms with no harm of EGDT. For some, these trials have made EGDT synonymous with an early liberal fluid strategy and its negative consequences (5–8). In rebuttal to our distinguished colleagues Genga and Russell (9); we advocate that treating early sepsis is not a time to be hydrophobic. Early fluid therapy in the context of a physiologically based protocol such as EGDT improves mortality for severe sepsis and septic shock.
THE EBB AND FLOW PHASE OF FLUID MANAGEMENT
In 1942, Cuthbertson (10) described the metabolic response to inflammation, injury, and shock using the concept of the “ebb and flow” phase of critical illness.
“During the ebb-phase or resuscitation phase, there is low cardiac output, poor tissue perfusion and a cold and clammy patient. During the flow phase which is a staccato affair, the patient struggles to break from the grip of the ebb-phase which last about 3 days. Upon entering the flow-phase, the swollen patient has an increased cardiac output, normal tissue perfusion where diuresis occurs and body weight falls steady.”
This eloquent clinical description serves as the framework for the clinical principles of fluid management in early sepsis, Figures 1 and 2.
Figure 1.
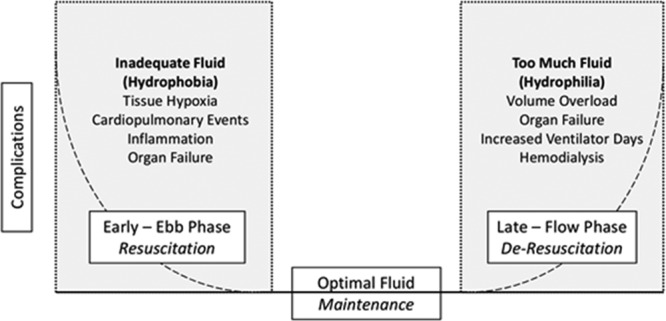
Optimal fluid therapy.
Figure 2.
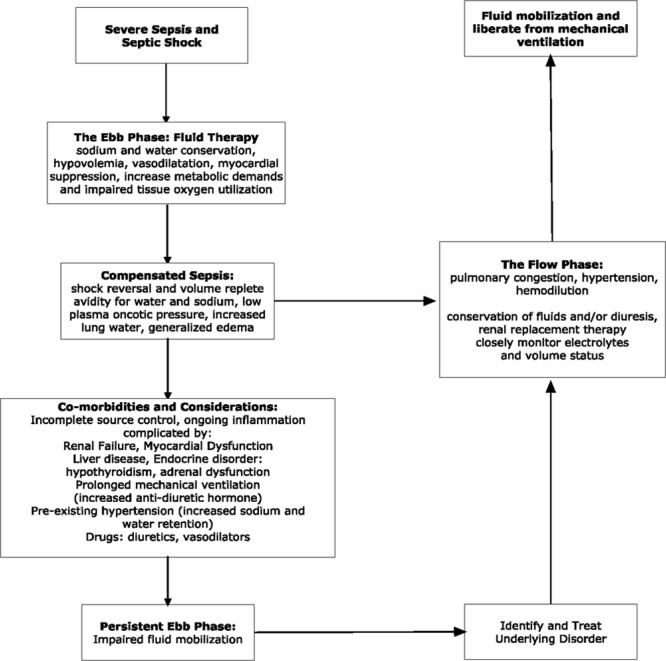
The dynamic landscape of fluid management. Reproduced from Schuller et al (11) and Rivers et al (12).
MECHANISMS FOR THE DEVELOPMENT OF HYPOVOLEMIA IN SEPSIS
Sepsis-induced hypovolemia can be a result of vomiting (poor intake), diarrhea, sweating, edema, peritonitis, or other exogenous losses. Further contributions to hypovolemia may result from vasodilatation, peripheral blood pooling, extravasation of fluid into the interstitial space, and increased capillary endothelial permeability. Hypovolemia and vasodilatation can be exaggerated in patients with comorbidities such as cardiovascular and renal disease where chronic therapy includes diuretics and afterload reduction. All of these mechanisms result in a decrease in intravascular volume which gives rise to a critical reduction in ventricular preload (central venous pressure [CVP]), ventricular diastolic pressure, stroke volume, cardiac output, systemic oxygen delivery, and an increase in systemic oxygen demands (decreased central venous oxygen saturation [Scvo2]) (13).
Compensatory responses as a reaction to decreased circulating blood volume are mediated by the activation of the neuroendocrine system. A redistribution of blood flow away from skeletal muscle beds and the splanchnic viscera supports vital organ blood flow to the heart and brain (14–16), with the augmentation of myocardial contractility leading to increased stroke volume (14). The constriction of arterial and venous capacitance vessels, particularly in the splanchnic bed, augments the venous return (14, 15). The activation of the renin-angiotensin axis releases aldosterone from the adrenal cortex. Changes in serum osmolarity lead to arginine-vasopressin release from the posterior pituitary. Both enhance fluid retention (15, 17–19). Comorbidities, such as congestive heart failure, renal failure, liver disease, and adrenal dysfunction, may modify this salt and water homeostasis and the dynamic “ebb and flow” landscape.
Microcirculatory changes observed in early sepsis such as acidosis, pyrexia, and increased RBC 2,3-diphosphoglycerate occur, creating a local tissue environment to enhance the unloading of oxygen to tissues. Multiple factors may contribute to microvascular alterations, including driving pressure, alterations in RBC rheology, viscosity (local hematocrit), and leukocyte adhesion to endothelial cells, endothelial dysfunction, and interstitial edema. This is further complicated by the use of vasopressors. The use of techniques evaluating the microcirculation to optimize volume therapy remains to be validated (20, 21).
EARLY FLUID THERAPY ATTENUATES THE EARLY PATHOGENESIS
The hemodynamic picture of early sepsis is hypotension, decreased CVP, decreased cardiac index, and decreased Scvo2 (13, 22). When the efficacy of antibiotics, cardiovascular support (fluids and dopamine titrated by intravascular monitoring to hemodynamic endpoints), and a combination of these two therapies in dogs with septic shock was compared, survival rates were 0%, 13%, 13%, and 43% in groups receiving no therapy (controls), antibiotics alone, cardiovascular support alone, or combined therapy, respectively (23). Although survivors and nonsurvivors in the combined therapy group required similar quantities of fluid therapy, nonsurvivors gained significantly more weight, suggesting abnormal vascular permeability with extravascular retention of fluids in the nonsurvivors indicating a more pronounced ebb phase. Thus, fluid overload is a result of the method of resuscitation, disease pathogenesis, and underlying comorbidities rather than the clinician’s over prescription of fluid administration.
Early fluid therapy modulates initial inflammation. In human models of endotoxemia, isotonic prehydration significantly attenuates concentrations of proinflammatory cytokines (tumor necrosis factor-α, interleukin [IL]-8, and IL-1β), while enhancing concentration of anti-inflammatory cytokines such as IL-10. This effect is associated with a reduction of endotoxin-induced symptoms and fever, while the endotoxin-induced changes in hemodynamic variables remain unchanged. More importantly, the peak activity of the inflammatory response is between 1 and 6 hours after introduction of the insult, which gives rise to the concept of early and late resuscitation as distinct therapeutic entities (24).
DIAGNOSTIC, THERAPEUTIC, AND OUTCOME IMPLICATIONS OF FLUID THERAPY
Optimizing fluid therapy not only modulates inflammation and increases microvascular perfusion but also decreases the need for vasopressor therapy, steroid use, and more invasive monitoring (20, 24–26). When a clinician is confronted with a profoundly hypotensive patient with an infection, fluid therapy is indicated. However, the clinical assessment of volume status is insensitive, nonspecific, and one of the most challenging clinical assessments. In a post hoc analysis of the Fluids and Catheters Treatment Trial (FACTT) in treatment of acute lung injury (ALI), this hypothesis was examined, which compared physical examination findings of ineffective circulation (capillary refill time > 2 s, skin mottling, and cool extremities) to variables obtained from pulmonary artery catheters. It was found that these physical examination findings are not useful predictors of a low cardiac index or low mixed venous oxygen saturation (27, 28).
TITRATION OF FLUIDS—A WORK IN PROGRESS
Titration of fluid therapy is performed with numerous methodologies. Frequently, more than one method is required to make an accurate assessment. History, physical examination, dynamic, static and volumetric devices, and ultrasound and metabolic variables can be used, each with certain limitations. Inherent in measuring intravascular pressures is the inference that pressure equals volume. There are many instances for which the pressure within an intracardiac chamber may be elevated and the intravascular volume status may be diminished. The goal is to infuse “adequate” volume to restore perfusion before the onset of irreversible tissue damage without raising cardiac filling pressure to a level that produces hydrostatic pulmonary edema (29, 30). Hemodynamic monitoring used to accomplish these goals can vary and have not been shown to have outcomes superiority (31–33). Although much maligned as a predictor of fluid responsiveness, the general use of CVP in the treatment of severe sepsis and septic shock has been associated with improved outcomes (34–36).
For example, a patient with a CVP of 30 mm Hg, mean arterial pressure (MAP) of 70 mm Hg, heart rate of 80 beats/min, lactate of 5 mM/L, and normal hemoglobin and arterial oxygen saturation and Scvo2 of 44% would get an inotrope. Interestingly, this scenario is associated with increased fluid administration because the CVP is lowered with improved ventricular compliance (37). A patient with a CVP of 4 mm Hg, MAP of 70 mm Hg, heart rate of 80 beats/min, lactate of 5 mM/L, and normal hemoglobin and arterial oxygen saturation and Scvo2 of 44% would get fluid therapy. If CVP was taken in isolation in the first scenario, one would not assume fluid therapy is indicated and some may mistakenly use a diuretic. An elevated CVP has been associated with increased mortality as an interpretation of volume overload when in reality it may be myocardial suppression (38). Thus, using all endpoints in an algorithmic approach is the foundation of a comprehensive resuscitation (39). The discussion regarding CVP is best summarized by Sondergaard et al (35) who stated:
“Knowledge of the CVP is essential for the measurement of the volume state, the performance of the heart and the SVR. It enters considerations of heart, volume and power efficiency. The CVP provides a floating ground for the differential measurement of intravascular pressures. It does not inherently measure preload or the volume state but its measurement is essential to their calculation. Once the above principles are understood, precise control of the circulation becomes a straightforward mathematically predictable process.”
FLUID THERAPY—EARLY
A hypotensive episode is associated with an increased risk of death, and the response to an adequate fluid challenge improves upon this discriminatory value for risk stratification (40–43). Early fluid therapy targeted to endpoints is associated with a decrease in systemic inflammation, vasopressor use, and mortality and must be distinguished from late aggressive fluid therapy (24, 40, 44–48). Multiple studies have shown that the fluid challenge of 30 mL/kg fluid volume within 3 hours of presentation is associated with increased MAP, normalization of Scvo2, and decreased vasopressor use at 6 hours. There is also a 19% relative reduction in in-hospital 30-day mortality and hospital length of stay (40, 42, 43). This benefit is realized even in patients with a history of renal and heart failure (43). The benefits of fluid administration are maximal when initiated within 30 minutes, making the fluid challenge an important aspect of early sepsis care. Lee et al (40, 49) concluded that “earlier fluid resuscitation may account for the lack of outcome differences between the original EGDT publication and the more recent three trials (ProCESS, ARISE, and ProMISe) and may have contributed to the overall low 60-day in-hospital mortality rate of 19%.”
From hospital arrival (prerandomization period) to the end of the 6-hour study period, the total fluid volume was similar and ranged from 3.5 to 5.5 L for the original EGDT publication and ProCESS, ARISE, and ProMISe study groups. Because of the greater lead time prior to enrollment into these trials, between 2 and 2.6 L of fluid was given prior to randomization. There is striking similarity in the amount of fluid given in all trials that approximates the intravascular volume of 5 L. However, the comparative differences in fluid therapy was 1,482 mL (42.4%), 697 mL (16%), 175 mL (4.1%), and 229 mL (4.4%) between the EGDT and usual or control care treatment groups in the EGDT, ProCESS, ARISE, and ProMISe trials, respectively (Table 1).
TABLE 1.
Comparison of Treatments in Sepsis Trials
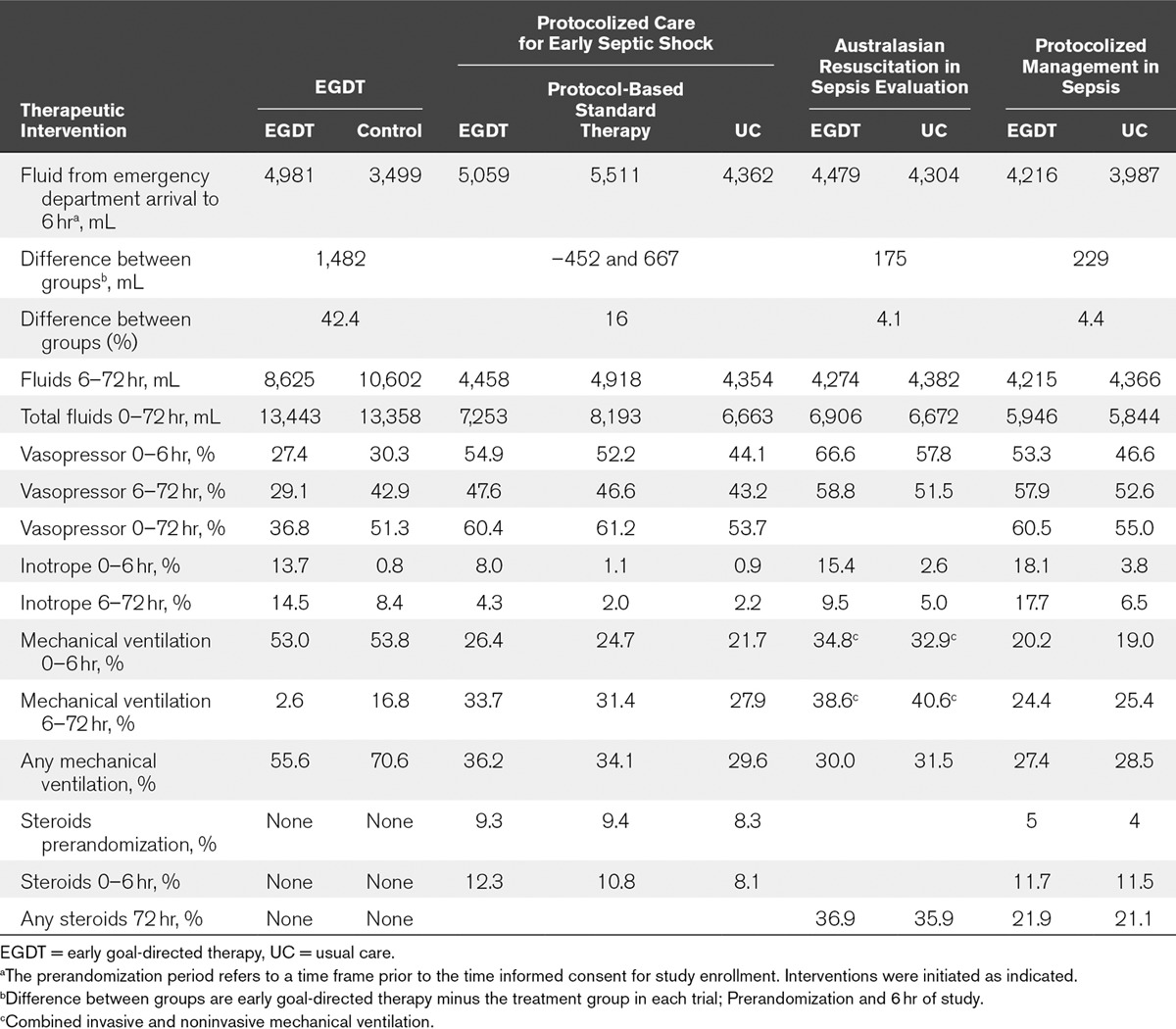
In the EGDT study, this significantly greater volume therapy or treatment effect during the resuscitation (prerandomization and first 6 hr) was associated with a greater reduction (13.8%) in vasopressor therapy, less volume therapy (2 L or 23%), and lower mechanical ventilation rates (14.2%) between the EGDT and control group over the subsequent 6–72-hour time period. These results were observed in the absence of aggressive glucose control, steroid use, protective lung strategies, and conservative fluid management strategies. These were present during the conduction of the ProCESS, ARISE, and ProMISe trials.
VASOPRESSOR THERAPY
ProCESS, ARISE, and ProMISe trials used vasopressor therapy twice as much compared to the EGDT trial. The more frequent administration of vasopressors in ProCESS, ARISE, and ProMISe may result in a hemodynamic phenotype of “vasodilatory septic shock” which is associated with a lower mortality risk as described by Hernandez et al (50). These findings may further indicate that early vasopressor administration instead of continued fluid therapy may be beneficial once the resuscitation is complete (51). Thus, it seems that ProCESS, ARISE, and ProMISe showed us that 4–6 L of fluid is generally required for early hemodynamic optimization.
The introduction of early vasopressor use is intriguing but challenging (51). Vasodilatory shock after adequate volume resuscitation is the requisite for the physiologic use of corticosteroids. This definition will be altered when vasopressors are introduced at an earlier stage before adequate fluid resuscitation. Waechter et al (52) reports that vasopressor use in the first hour may be associated with increased mortality in patients with greater illness severity. Vasopressor use also increases CVP which may impact volume therapy when this endpoint is artificially elevated (53).
Vasopressor therapy elevates the intensity of patient care because vasopressors require monitoring (i.e., intraarterial pressure monitoring). By placing a patient on vasopressors when adequate volume therapy may have reversed the hypotension, one is placing the patient in a higher level of care and potentially increasing healthcare resource consumption. With EGDT, vasopressor use diminishes by 13.8% over the subsequent 72 hours.
FLUID THERAPY—LATER
When comparing the original EGDT to ProCESS, ARISE, and ProMISe between 6 and 72 hours, significantly more volume therapy (almost two-fold) was administered in the EGDT study in both treatment groups compared to the three follow-up trials. There was an approximate two-fold increase in vasopressor therapy in ProCESS, ARISE, and ProMISe compared to the EGDT trial during this time period.
ProCESS, ARISE, and ProMISe had mechanical ventilation rates approximately half that of the EGDT and the reference literature (54–56). For example, the SSC database from 2005 to 2008 reports a mechanical ventilation rate of 52.4% (7,877/15,022 of patients), which is almost identical to the EGDT study. Mortality rates in this report were 48.3%, 45.7%, and 33.0% in mechanically ventilated patients with ALI, without ALI, and without mechanical ventilation, respectively. The use of protective lung strategies and conservative fluid management strategies for ALI was not used in the EGDT study as it preceded these trials.
The FACTT trial isolated the manipulation of volume therapy as a controlled intervention which began an average of 43 hours after ICU admission and 24 hours after the establishment of ALI (11). Although there was no difference in 60-day mortality, patients in the conservative strategy group had significantly improved lung and CNS function (decreased need for sedation) along with mechanical ventilation and thus ICU care. There was a statistically significant 0.3-day increase in cardiovascular failure free days in the liberal compared to the conservative fluid group. The increased early volume in the EGDT led to a decreased need for mechanical ventilation over the first 72 hours of hospitalization. This may be due to the modulating effects on IL-8, which has been identified as an ALI culprit within the first 72 hours of presentation (57, 58).
The findings of the FACTT trial brought attention to the negative consequences of the overuse of fluid administration. In order to generalize these results and avoid mitigating the salutary findings, multiple variables must be considered when applying a conservative fluid management approach. The exclusion of patients on hemodialysis, overt renal insufficiency, heart failure, and the relatively young age of the patients studied (age, 50 yr) make the FACTT trial a departure from the reality that many clinicians will face in the treatment of severe sepsis and septic shock.
FLUID REMOVAL-OPTIMAL TIMING
Although pathogenically well described, the clinical landmark that separates the ebb from flow phase is frequently indistinct and complex. It requires meticulous attention to past medical history (i.e., renal failure and congestive heart failure), cumulative fluid balance, relevant hemodynamic variables, laboratory findings (hemodilution and renal function), and physical examination findings of fluid overload, Figure 2. In the absence of early recognition of the flow phase, the complications of pulmonary edema, myocardial complications, respiratory insufficiency, and the continued need for ventilatory support results. Fluid conservation, diuretic therapy, and the institution of renal replacement therapy are clinical decisions made in the flow phase especially in patients with renal insufficiency and cardiac disease (59). When renal replacement therapy is required in the treatment of septic shock, mortality approaches 50%. The optimal timing of initiating renal replacement therapy in the presence of acute kidney injury is not clearly established (60–62).
The body of observational evidence over the last 10 years points towards the deleterious effects of persistently positive fluid balances in septic shock. Nevertheless, only one large clinical trial has demonstrated the efficacy and safety of fluid removal in ICU patients without cardiovascular dysfunction. The best intervention for active fluid management may be the avoidance of unnecessary fluid loading with the best evidence-based approaches, whereas active fluid withdrawal may have a later role. More evidence from randomized clinical trials is needed to guide physicians in the art and science of late fluid management in septic shock (63).
HYDROPHOBIA IS UNWARRANTED IN EARLY SEPSIS CARE
Early titrated fluid administration modulates inflammation, improves microvascular perfusion, organ function, and outcomes.
Fluid therapy is determined by the method of resuscitation along with various clinically measured variables, disease pathogenesis, and underlying comorbidities rather than the clinician’s over prescription of fluid administration.
Contrary to popular belief, recent EGDT septic shock trials used similar amounts resuscitation fluids in the acute phase when compared to the original EGDT trial. This was associated all time lows in mortality.
Patients who suffer the negative consequences of fluid overload are already at high risk because of preexisting and acquired comorbidities.
A comprehensive understanding of “ebb and flow” combined with closely monitored early fluid therapy, accumulation and timely removal is of most importance.
Fluid therapy should be treated as any drug which involves consideration of the type, dose, duration of treatment, and toxicity (64).
Hydrophobia may be synonymous with rabies but should not be with early sepsis management.
ACKNOWLEDGMENT
We thank Stephanie Stebens, MLIS, AHIP (Librarian, Sladen Library, K-17, Henry Ford Hospital, 2799 West Grand Blvd, Detroit, MI, 48202) for her help with the article.
Footnotes
Dr. Jaehne received funding from Henry Ford Hospital (part-time employee) and ASPIRUS Hospital Iron River, MI (part-time employee). Dr. Rivers disclosed other support. He currently conducts research for Abbott Laboratories, Alere, Spectral Diagnostics, and the National Institutes of Health. The early goal-directed therapy study was performed without extra mural (academic or industry) funding. All catheters used and equipment in the study were paid by Henry Ford Hospital to Edwards Lifesciences.
REFERENCES
- 1.Torio CM, Moore BJ. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; National inpatient hospital costs: The most expensive conditions by payer, 2013. HCUP statistical brief #204. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp. Accessed August 17, 2016. [PubMed] [Google Scholar]
- 2.Nguyen HB, Jaehne AK, Jayaprakash N, et al. Early goal-directed therapy in severe sepsis and septic shock: Insights and comparisons to ProCESS, ProMISe, and ARISE. Crit Care. 2016;20:160. doi: 10.1186/s13054-016-1288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar-Hari M, Phillips GS, Levy ML, et al. Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis RJ. Disassembling goal-directed therapy for sepsis: A first step. JAMA. 2010;303:777–779. doi: 10.1001/jama.2010.203. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: The ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015;41:1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 6.Cronhjort M, Hjortrup PB, Holst LB, et al. Association between fluid balance and mortality in patients with septic shock: A post hoc analysis of the TRISS trial. Acta Anaesthesiol Scand. 2016;60:925–933. doi: 10.1111/aas.12723. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Kollef MH. Conservative fluid therapy in septic shock: An example of targeted therapeutic minimization. Crit Care. 2014;18:481. doi: 10.1186/s13054-014-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neyra JA, Li X, Canepa-Escaro F, et al. Cumulative fluid balance and mortality in septic patients with or without acute kidney injury and chronic kidney disease. Crit Care Med. 2016;44:1891–1900. doi: 10.1097/CCM.0000000000001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genga K, Russell JA. Early liberal fluids for sepsis patients are harmful. Crit Care Med. 2016 Apr 7 doi: 10.1097/CCM.0000000000001829. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Cuthbertson DP. Post-shock metabolic response. The Lancet. 1942;239:433–436. [Google Scholar]
- 11.Wiedemann HP, Wheeler AP, Bernard GR, et al. National Heart Lung, Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 12.Rivers EP, Jaehne AK, Eichhorn-Wharry L, et al. Fluid therapy in septic shock. Curr Opin Crit Care. 2010;16:297–308. doi: 10.1097/MCC.0b013e32833be8b3. [DOI] [PubMed] [Google Scholar]
- 13.Rosário AL, Park M, Brunialti MK, et al. SvO(2)-guided resuscitation for experimental septic shock: Effects of fluid infusion and dobutamine on hemodynamics, inflammatory response, and cardiovascular oxidative stress. Shock. 2011;36:604–612. doi: 10.1097/SHK.0b013e3182336aa4. [DOI] [PubMed] [Google Scholar]
- 14.Chien S. Role of the sympathetic nervous system in hemorrhage. Physiol Rev. 1967;47:214–288. doi: 10.1152/physrev.1967.47.2.214. [DOI] [PubMed] [Google Scholar]
- 15.Reilly PM, Wilkins KB, Fuh KC, et al. The mesenteric hemodynamic response to circulatory shock: An overview. Shock. 2001;15:329–343. doi: 10.1097/00024382-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 16.Gann DS, Carlson DE, Byrnes GJ, et al. Role of solute in the early restitution of blood volume after hemorrhage. Surgery. 1983;94:439–446. [PubMed] [Google Scholar]
- 17.Givertz MM. Manipulation of the renin-angiotensin system. Circulation. 2001;104:E14–E18. doi: 10.1161/hc3001.094733. [DOI] [PubMed] [Google Scholar]
- 18.Cumming AD, Driedger AA, McDonald JW, et al. Vasoactive hormones in the renal response to systemic sepsis. Am J Kidney Dis. 1988;11:23–32. doi: 10.1016/s0272-6386(88)80170-7. [DOI] [PubMed] [Google Scholar]
- 19.Danser AH. Local renin-angiotensin systems: The unanswered questions. Int J Biochem Cell Biol. 2003;35:759–768. doi: 10.1016/s1357-2725(02)00178-4. [DOI] [PubMed] [Google Scholar]
- 20.Boldt J, Ince C. The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: A review. Intensive Care Med. 2010;36:1299–1308. doi: 10.1007/s00134-010-1912-7. [DOI] [PubMed] [Google Scholar]
- 21.Ospina-Tascón GA, Madriñán-Navia H. Should microcirculation monitoring be used to guide fluid resuscitation in severe sepsis and septic shock? Rev Bras Ter Intensiva. 2015;27:92–95. doi: 10.5935/0103-507X.20150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astiz ME, Rackow EC, Kaufman B, et al. Relationship of oxygen delivery and mixed venous oxygenation to lactic acidosis in patients with sepsis and acute myocardial infarction. Crit Care Med. 1988;16:655–658. doi: 10.1097/00003246-198807000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Natanson C, Danner RL, Reilly JM, et al. Antibiotics versus cardiovascular support in a canine model of human septic shock. Am J Physiol. 1990;259:H1440–H1447. doi: 10.1152/ajpheart.1990.259.5.H1440. [DOI] [PubMed] [Google Scholar]
- 24.Dorresteijn MJ, van Eijk LT, Netea MG, et al. Iso-osmolar prehydration shifts the cytokine response towards a more anti-inflammatory balance in human endotoxemia. J Endotoxin Res. 2005;11:287–293. doi: 10.1179/096805105X58715. [DOI] [PubMed] [Google Scholar]
- 25.Packman MI, Rackow EC. Optimum left heart filling pressure during fluid resuscitation of patients with hypovolemic and septic shock. Crit Care Med. 1983;11:165–169. doi: 10.1097/00003246-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–1106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 27.Strehlow MC. Early identification of shock in critically ill patients. Emerg Med Clin North Am. 2010;28:57–66, vii. doi: 10.1016/j.emc.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Grissom CK, Morris AH, Lanken PN, et al. National Institutes of Health/National Heart, Lung and Blood Institute Acute Respiratory Distress. Association of physical examination with pulmonary artery catheter parameters in acute lung injury. Crit Care Med. 2009;37:2720–2726. doi: 10.1097/ccm.0b013e3181a59532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weil MH, Shubin H, Rosoff L. Fluid repletion in circulatory shock: Central venous pressure and other practical guides. JAMA. 1965;192:668–674. doi: 10.1001/jama.1965.03080210012003. [DOI] [PubMed] [Google Scholar]
- 30.Aya HD, Ster IC, Fletcher N, et al. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44:880–891. doi: 10.1097/CCM.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 31.Sevransky JE. Dynamic measures to determine volume responsiveness: Logical, biologically plausible, and unproven. Crit Care Med. 2016;44:1923–1926. doi: 10.1097/CCM.0000000000001997. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Lu B, Sheng X, et al. Accuracy of stroke volume variation in predicting fluid responsiveness: A systematic review and meta-analysis. J Anesth. 2011;25:904–916. doi: 10.1007/s00540-011-1217-1. [DOI] [PubMed] [Google Scholar]
- 33.Richard JC, Bayle F, Bourdin G, et al. Preload dependence indices to titrate volume expansion during septic shock: A randomized controlled trial. Crit Care. 2015;19:5. doi: 10.1186/s13054-014-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walkey AJ, Wiener RS, Lindenauer PK. Utilization patterns and outcomes associated with central venous catheter in septic shock: A population-based study. Crit Care Med. 2013;41:1450–1457. doi: 10.1097/CCM.0b013e31827caa89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sondergaard S, Parkin G, Aneman A. Central venous pressure: Soon an outcome-associated matter. Curr Opin Anaesthesiol. 2016;29:179–185. doi: 10.1097/ACO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 36.Eskesen TG, Wetterslev M, Perner A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2016;42:324–332. doi: 10.1007/s00134-015-4168-4. [DOI] [PubMed] [Google Scholar]
- 37.Mark DG, Morehouse JW, Hung YY, et al. In-hospital mortality following treatment with red blood cell transfusion or inotropic therapy during early goal-directed therapy for septic shock: A retrospective propensity-adjusted analysis. Crit Care. 2014;18:496. doi: 10.1186/s13054-014-0496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 39.Rivers EP, Yataco AC, Jaehne AK, et al. Oxygen extraction and perfusion markers in severe sepsis and septic shock: Diagnostic, therapeutic and outcome implications. Curr Opin Crit Care. 2015;21:381–387. doi: 10.1097/MCC.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Ramar K, Park JG, et al. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: A retrospective cohort study. Chest. 2014;146:908–915. doi: 10.1378/chest.13-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones AE, Yiannibas V, Johnson C, et al. Emergency department hypotension predicts sudden unexpected in-hospital mortality: A prospective cohort study. Chest. 2006;130:941–946. doi: 10.1378/chest.130.4.941. [DOI] [PubMed] [Google Scholar]
- 42.Leisman D, Wie B, Doerfler M, et al. Association of fluid resuscitation initiation within 30 minutes of severe sepsis and septic shock recognition with reduced mortality and length of stay. Ann Emerg Med. 2016;68:298–311. doi: 10.1016/j.annemergmed.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 43.Liu VX, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193:1264–1270. doi: 10.1164/rccm.201507-1489OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. 2005;33:2194–2201. doi: 10.1097/01.ccm.0000182798.39709.84. [DOI] [PubMed] [Google Scholar]
- 45.Cohen R. Use of corticosteroids in septic shock. Minerva Anestesiol. 2011;77:190–195. [PubMed] [Google Scholar]
- 46.Practice parameters for hemodynamic support of sepsis in adult patients in sepsis. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27:639–660. doi: 10.1097/00003246-199903000-00049. [DOI] [PubMed] [Google Scholar]
- 47.Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 48.Yacopetti N, Alexandrou E, Spencer TR, et al. Central venous catheter insertion by a clinical nurse consultant or anaesthetic medical staff: A single-centre observational study. Crit Care Resusc. 2010;12:90–95. [PubMed] [Google Scholar]
- 49.Pettilä V, Hjortrup PB, Jakob SM, et al. Control groups in recent septic shock trials: A systematic review. Intensive Care Med. 2016 Jul 23 doi: 10.1007/s00134-016-4444-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Hernandez G, Castro R, Romero C, et al. Persistent sepsis-induced hypotension without hyperlactatemia: Is it really septic shock? J Crit Care. 2011;26:435.e9–435.e14. doi: 10.1016/j.jcrc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Bai X, Yu W, Ji W, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care. 2014;18:532. doi: 10.1186/s13054-014-0532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waechter J, Kumar A, Lapinsky SE, et al. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group. Interaction between fluids and vasoactive agents on mortality in septic shock: A multicenter, observational study. Crit Care Med. 2014;42:2158–2168. doi: 10.1097/CCM.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 53.Frank ED. A shock team in a general hospital. Anesth Analg. 1967;46:740–745. [PubMed] [Google Scholar]
- 54.Bouchard J, Soroko SB, Chertow GM, et al. Program to Improve Care in Acute Renal Disease (PICARD) Study Group. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 55.Payen D, de Pont AC, Sakr Y, et al. Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuller D, Mitchell JP, Calandrino FS, et al. Fluid balance during pulmonary edema. Is fluid gain a marker or a cause of poor outcome? Chest. 1991;100:1068–1075. doi: 10.1378/chest.100.4.1068. [DOI] [PubMed] [Google Scholar]
- 57.Rivers EP, Kruse JA, Jacobsen G, et al. The influence of early hemodynamic optimization on biomarker patterns of severe sepsis and septic shock. Crit Care Med. 2007;35:2016–2024. doi: 10.1097/01.ccm.0000281637.08984.6e. [DOI] [PubMed] [Google Scholar]
- 58.Miller EJ, Cohen AB, Matthay MA. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med. 1996;24:1448–1454. doi: 10.1097/00003246-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Ren HS, Li M, Zhang YJ, et al. High-volume hemofiltration combined with early goal-directed therapy improves alveolar-arterial oxygen exchange in patients with refractory septic shock. Eur Rev Med Pharmacol Sci. 2016;20:355–362. [PubMed] [Google Scholar]
- 60.Wierstra BT, Kadri S, Alomar S, et al. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: A systematic review and evidence synthesis. Crit Care. 2016;20:122. doi: 10.1186/s13054-016-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaudry S, Hajage D, Schortgen F, et al. AKIKI Study Group. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 62.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN Randomized Clinical Trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 63.Besen BA, Taniguchi LU. Negative fluid balance in sepsis: When and how? Shock. 2016 Jul 21 doi: 10.1097/SHK.0000000000000701. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.McDermid RC, Raghunathan K, Romanovsky A, et al. Controversies in fluid therapy: Type, dose and toxicity. World J Crit Care Med. 2014;3:24–33. doi: 10.5492/wjccm.v3.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]


