Abstract
Study Design.
Cross-sectional study.
Objective.
To explore the underlying anatomy of May-Thurner syndrome (MTS) using computed tomography (CT) and discuss its clinical significance for typing diagnosis.
Summary of Background Data.
Because the anatomical position of the corpse cannot fully illustrate the actual clinical situation in vivo, the diversity of MTS has not been fully elucidated yet.
Methods.
We retrospectively analyzed the data of 69 patients with MTS. By CT showing, patients were categorized to simple MTS (sMTS, 22 patients), lumbar degeneration-related MTS (dMTS, 33 patients) and other causes MTS (oMTS, 14 patients); meanwhile, a healthy control group were set. Evaluated indexes were onset age, course of disease, diameter of the iliac vein tunnel (IVTD), lumbar degeneration-related iliac vein compression (IVC), therapeutic effect, and diagnostic cutoff of risk IVTD prone to MTS.
Results.
The onset age of sMTS, dMTS, and oMTS were respectively 42.3 ± 6.5 years, 61.5 ± 10.6 years, and 53.1 ± 16.8 years (P < 0.001); courses were respectively 12.1 ± 9.2 days, 22.5 ± 7.6 days, and 6.8 ± 6.7 days (P = 0.002). IVTDs of sMTS, dMTS, oMTS, and the control were respectively 2.52 ± 0.50 mm, 2.29 ± 0.30 mm, 5.93 ± 2.21 mm, and 4.34 ± 1.61 mm (P < 0.001). Lumbar degeneration-related IVC in dMTS occurred at 41 places, including forward bulging or protruding intervertebral discs (51%,17/33), osteophytes (50%,16/33), and spondylolisthesis (19%, 8/33), but none happened in sMTS, oMTS, and the control. Eighty-six percent of sMTSs, 55% dMTSs, and none oMTSs needed intravenous stent-implanted operation to obtain effective treatment. MTS type (Waldχ2 = 6.092, P = 0.009), course (Waldχ2 = 4.618, P = 0.032), and treatment plan (Waldχ2 = 14.748, P < 0.001) markedly influence the therapeutic result. The cutoff of risk IVTD for sMTS and dMTS was 2.98 mm, which diagnostic sensitivity was 90% and specificity 100%.
Conclusion.
Owing to the distinct pathoanatomy and causes, diagnosis in classification of MTS by CT is helpful in accurate treatment program.
Level of Evidence: 3
Keywords: classification, computer, complications diagnostic use, iliac vein compression syndrome, lumbar degeneration, May-Thurner syndrome, therapy, tomography, x-ray
May-Thurner syndrome (MTS) was traditionally known as iliac vein compression syndrome (IVCS).1,2 Scholars have conducted many studies on the mechanism of MTS.3–7 Some thought the disease was a type of acquired lesion,8–11 and some believed it was more likely congenital abnormity;12,13 furthermore, some data from autopsy as aforesaid observations based on implied multifarious variances in MTS.14 But owing to the anatomical position of the corpse cannot fully illustrate the actual clinical situation in vivo, the diversity of the disease has not been fully elucidated yet. Therefore, this study further explored the etiology of MTS, but different from previous cadaver researches, this investigation was performed in vivo by computed tomography (CT).
MATERIALS AND METHODS
General Resources
We conducted a cross-sectional study from July, 2009 to June, 2014. The study utilized abdominal-pelvis part CT images of Southeast Hospital, Xiamen University as database.
Patient Selection
Consecutive patients with left low limb deep vein thrombosis (LDVT) were selected from the database of the time. Inclusion criteria include (i) definitive diagnosis of MTS by clinic and imageology, including ultrasound (US), CT, and digital subtraction angiography (DSA); (ii) clinical and imaging information are complete; (iii) treatment and follow-up results can be traced back. Exclusion criteria include LDVT caused by fracture of the lower limbs, pregnancy, venous disease, and other causes that did not pose a compression to the iliac vein.
Control Group
Control group includes (i) randomly selected from the database of the time, with sex and age, respectively matching with the patient group; (ii) abdominal-pelvic CT showed normal; and (iii) lumbar CT appeared clear.
Patient Classification
According to the causes of iliac vein compression showed by CT, patients were divided into three groups. (1) Simple MTS (sMTS): the left iliac vein is simply pressed by the right iliac artery from the front of it. (2) Lumbar degeneration-related MTS (dMTS): in addition to the right iliac artery compressing the opening of the left iliac vein, the lower lumbar degenerative changes, such as a bulging or protruding disc, osteophyte, sacral vertebrae spondylolisthesis, and anterior longitudinal ligament ossification, compress the iliac vein from the back. (3) Other causes MTS (oMTS): the LDVT resulted from diseases as abscess from lumbar tuberculosis or purulent inflammation and hematoma from compression fracture.
Patient Condition
The clinical manifestations included left lower limb swelling and pain over a period that ranged from 1 day to 6 months, with progressive worsening. Patients were admitted for further management after primarily being diagnosed as LDVT by emergency Doppler US. Iliolumbar plain CT and deep vein CT venography were subsequently performed for them to understand changes of the iliac veins and structures around it. Then angiography and interventional therapy were finished by DSA guidance.
Figure 1.
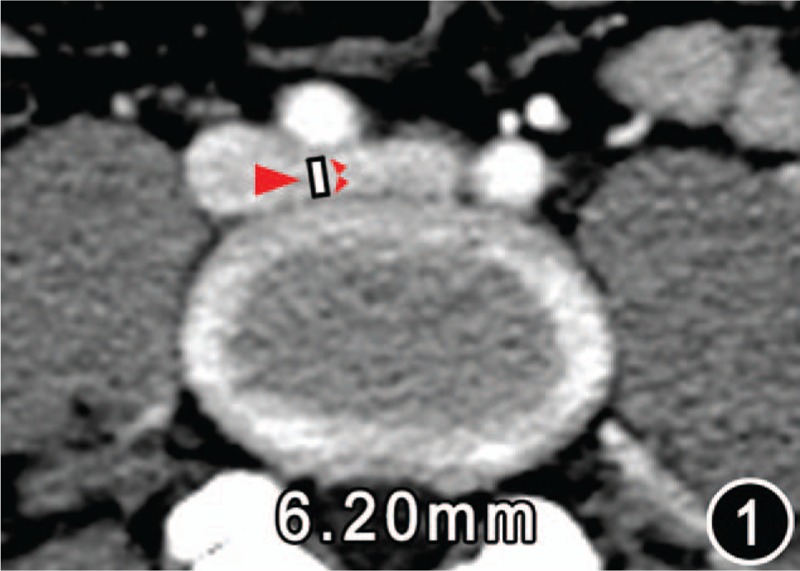
CT image of a female of age 40 years of control group. The map displayed the diameter (white line) of the left iliac vein tunnel (IVT, big red arrow) 6.20 mm, which was measured on the central cross-sectional CT image of the left iliac vein stretching across the anterospine from right to left. The anterior border of the IVT is the posterior wall of the right iliac artery (the front small red arrow); the posterior border is the anterior margin of the vertebral body (the rear small red arrow).
Figure 6.
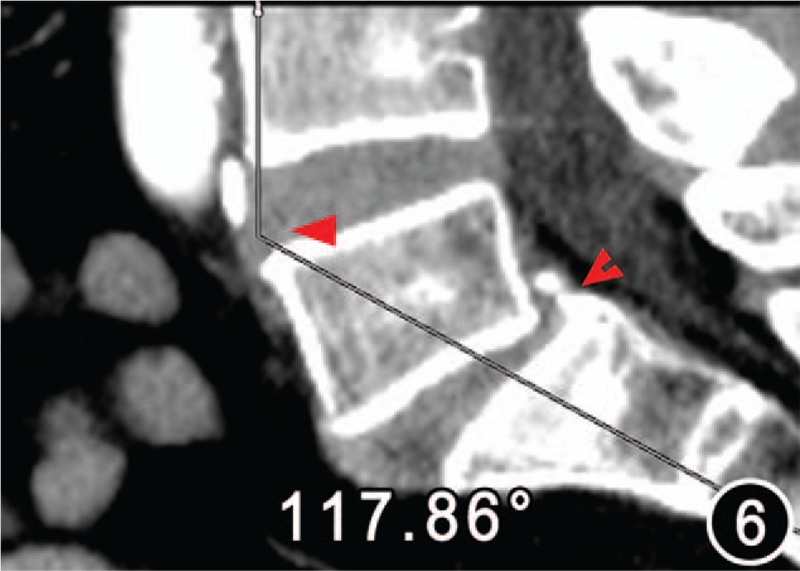
Low lumbar lordosis angle 117.86°of the same patient as Figure 5. The sagittal plane image demonstrated degenerative lower lumbar slippage (bifid arrow). The iliac vein tunnel (IVT) became narrow owing to the forward shift intervertebral disc of the fourth and fifth vertebral bodies, which caused the iliac vein compression (arrow). Thus, the IVT of the patient was considered secondum stenosis.
TABLE 1.
General Characteristics of Case and Control Groups
| Groups | Cases | Onset or Inclusion Age (yr.) | Course of Disease (day) | ||||
| Range | Range | ||||||
| sMTS | 22 | 31.0–54.0 | 42.3 ± 6.5 | 1.0–40.0 | 12.1 ± 9.2 | ||
| dMTS | 33 | 43.0–81.0 | 61.5 ± 10.6 | 2.0–120.0 | 22.5 ± 7.6 | ||
| oIVCS | 14 | 24.0–76.0 | 53.1 ± 16.8 | 3.0–30.0 | 6.8 ± 6.7 | ||
| Control | 380 | 15.0–92.0 | 47.4 ± 23.0 | – | --- | ||
| Statistical analysist*,† | 449 | F = 5.135,P = 0.002; (Ps-d = 0.008, Pd-c = 0.002; Ps-o, Ps-c, Pd-o and Po-c all p > 0.05) | F = 7.806,P = 0.002 ( Ps-d = 0.010, Pd-o = 0.010, Ps-o = 0.690) | ||||
*c indicates control group; d/dMTS, low lumbar degeneration-related May-Thurner syndrome; o/oIVCS, other cause iliac vein compression; s/sMTS simple May-Thurner syndrome.
Ps-c means comparison among sMTS and its control group, and so on.
†Bofferroni test was used in the statistical correction of multiple comparisons of means.
Figure 3.
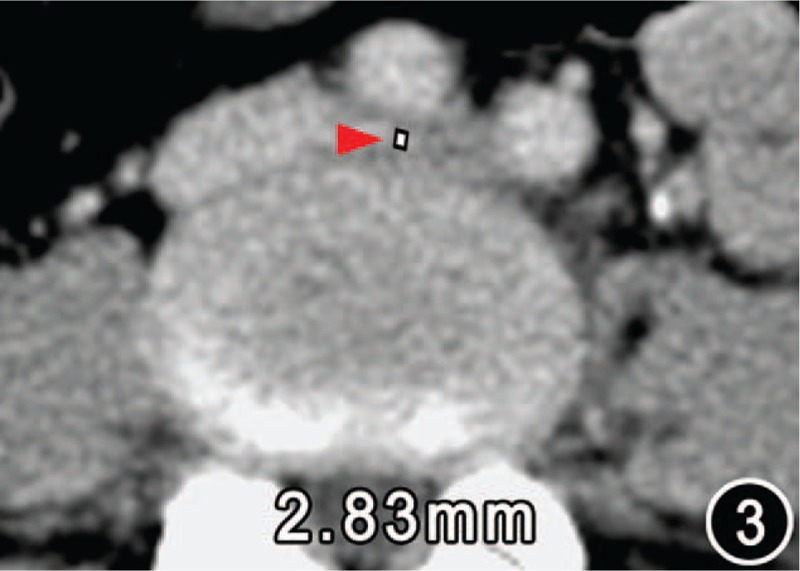
Diameter of the iliac vein tunnel (IVTD) 2.83 mm (arrow) of a 46-year-old female with simple MTS. No signs of degenerative changes oppressing the left iliac vein were found on the transection CT image. Therefore, the IVTD of the patient was deemed primary stenosis.
Figure 4.
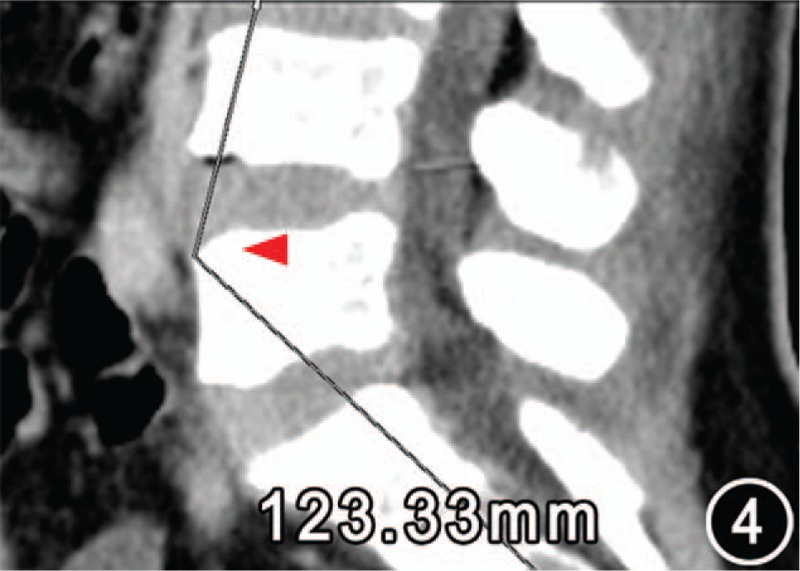
Low lumbar lordosis angle 123.33° (arrow) of the same patient as Figure 3. In addition, no signs of low lumbar degeneration-related iliac vein compression were found on the sagittal plane image.
Figure 5.

CT from a 68-year-old female with lumbar generation-related MTS presenting the diameter of the iliac vein tunnel 2.78 mm (arrow).
CT Imaging
Dual-source CT (Siemens Somatom Definition AS 4D CT; Siemens Company, Germany) was used. The scan range was from the diaphragmatic surface (the second hepatic door) to the popliteal vein. For direct lower extremity venous imaging, the left foot dorsal vein was punctured and injected with 370 mgI/mL of iopamidol iodine contrast (licence number H20053388, Bolaikexinyi Pharmaceutical Limited Liability Company, Shanghai), and scanning was performed from the foot to the head. The injection techniques included the use of a double cylinder high pressure syringe (Medrad Stellant D, Medrad Inc., Pennsylvania, U.S.A.), a 20G needle, 2 mL/s injection velocity, 80 mL total volume, with scanning beginning 35 s after the injection of contrast medium. The scanning parameters included 80 to 100 kV of tube voltage, 80 to 120 mAs of tube current (care dose4D and care kv were adopted to minimize the scan radiation dose), 1.5 mm pitch, 0.6 mm collimating, 1.25 mm reconstruction thickness, and B20f very smooth reconstruction algorithm. Patients received an average radiation dose of CTDI vol 27.06 to 40.40 mGy.
Observation Indices
Iliac vein tunnel (IVT) in front of the lumbar: The left iliac vein passes through the gap between the right iliac artery and the lumbar spine.
IVT diameter (IVTD): Measured on the central cross-sectional CT map of the left iliac vein stretching across the anterospine from right to left, the minimal sagittal diameter from the trailing edge of the right iliac artery to the leading edge of the vertebra is referred to as the IVTD.
Lower lumbar lordosis angle (LLLA): Measured on the CT middle sagittal plane of the lower spine obtained after multiple plane reconstruction by CT postprocessing, this angle is formed by two lines, one that is perpendicular to the superior border of the third lumbar and the other is the vertical of the superior margin of the first sacral vertebrae. The forward angle between the two lines is considered the LLLA.
Extent of thrombosis by US: (i) level 1, embolism limited to the iliac vein; (ii) level 2, embolism expanded to the femoral vein; (iii) level 3, embolism affected the popliteal vein.
Figure 2.
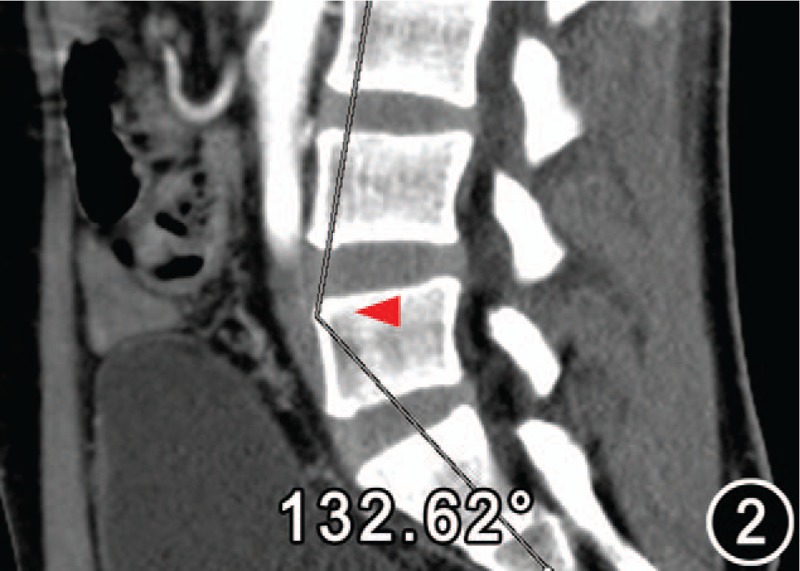
Low lumbar lordosis angle (red arrow) 132.62° measured on the reconstructed sagittal plane image from the same object as Figure 1. One side of the angle is perpendicular to the superior border of the third lumbar and the other is the vertical of the superior margin of the first sacral vertebrae.
Treatment Process and Effect Evaluation
According to documents15–18 and the author's practical experience, the therapeutic regimen was designed as programmed stage-treatment: the first phase, targeted intravenous catheter-directed thrombosis; the second phase, intravenous mechanically thromb-broken aspiration and balloon dilatation; the third phase, iliac venous stent implantation. Postoperative anticoagulation was maintained for 6 months.
Therapeutic effect were classified to effective and ineffective treatment.19–20 Follow-up phase from 6 to 36 months were reported.21 In this study, no recurrence within 12 months after therapy was deemed effective treatment, and relapse was considered invalid.
Evaluation Indexes
Sex incidence, onset age, course, IVTD, LLLA, lumbar degeneration-related iliac vein compression (LDRIVC) signs, compression sites, extent of venous thrombosis, therapeutic effect, and diagnostic cutoff of risk IVTD prone to MTS.
Statistical Analysis
Differences between case and control groups were statistically analyzed by t test for age, IVTD, and LLLA, andχ2 test for LDRIVC signs and compression sites. Differences in patient groups, including onset age, course, IVTD, LLLA, compression sites, extent of venous thrombosis, and therapeutic effect were evaluated with variance analysis. Diagnostic response of curve (ROC) analysis was applied to decide the cutoff of risk IVTD for MTS.
Logistic regression analysis was used to assess the influence factors for therapeutic effect. The independent variables were specified as the following: the course within 2 weeks was coded 1 and beyond 2 weeks was 2. sMTS was coded 1, dMTS was 2, and oMTS was 3. Level 1 of venous embolism was coded 1, level 2 was 2, and level 3 was 3. Treatment programs were respectively coded 1 for the first phase, 2 for the second phases, and 3 for the third phases. Dependent variable was therapeutic effect that invalid (relapse included) was 0 and valid was 1.
Images and therapeutic effects were assessed by two senior experts (with 20 and 14 years’ experience in muscular-skeletal radiological diagnosis and interventional therapy, respectively). Results were reached by both consulting consensus. All statistical analyses were performed using SPSS17.0 (SPSS Company, Chicago, IL). When unilateral P ≤ 0.05, the differences were considered to be significant.
RESULTS
General Characteristics of Case and Control Groups
Six-nine patients were finally enrolled, consisting of 22 sMTSs patients, 33 dMTSs patients, and 14 oMTSs patients. The control group had 380 health adults. In sMTS, six (27%, 6/22) were males and 16 (73%, 16/22) were females; in dMTS, males were 12 (36%, 12/33) and females were 21(64%, 21/33); in oMTS, six were lumbar spine tuberculosis, four inflammative abscess, and four lumbar compression fractures, with seven (50%, 7/14) males and seven (50%, 7/14) females. In control group, 179 were males and 201 were females. In case-group the LLLA were respectively 125.92 ± 4.85 (o) and 123.22 ± 6.11(o) for males and females (t = 2.012, P = 0.049), and in control-group the LLLA were respectively 136.64 ± 12.24 (o) and 134.05 ± 8.88 (o) for males and females (t = 2.280, P = 0.023). General information of all groups were presented in Table 1.
Indices of Image and Evaluations
The LDRIVC signs in dMTS occurred at 41 places, including forward bulging or protruding intervertebral discs (51% of incidence, 17/33), osteophytes (50%, 16/33), and lower lumbar spondylolisthesis (19%, 8/33). In oMTS, only one spondylolisthesis happened at the fifth lumbar vertebrae but that did not press into the iliac vein. IVTD and LLLA of case and control groups were measured as shown in Table 2. For sMTS and dMTS, the IVTD was positively correlation with LLLA (the former r = 0.801, P < 0.001; the latter r = 0.449, P = 0.009); but whether for oMTS or the control, the correlations between IVTD and LLLA did not show statistical significance (the former r = 0.094, P = 0.750; the latter r = 0.084, P = 0.103).
TABLE 2.
CT Measured Values of IVTD and LLLA in Case and Control Groups
| GroupS | IVTD (mm) (95% CI) | LLLA (o) (95% CI) |
| sMTS | 2.52 ± 0.50 (1.88–3.12) | 124.41 ± 3.93 (119.37–129.45) |
| dMTS | 2.29 ± 0.30 (1.77–2.82) | 121.82 ± 5.37 (117.71–125.93) |
| oIVCS | 5.93 ± 2.21 (5.13–6.74) | 129.50 ± 5.93 (123.19–135.82) |
| Control group | 4.34 ± 1.61 (4.18–4.50) | 135.54 ± 12.85 (134.33–136.76) |
| Statistical analysis* | F = 32.649, P < 0.001; (Ps-d = 1.000, Ps-o<0.001, Ps-c<0.001, Pd-o<0.001, Pd-c<0.001,Po-c = 0.001) | F = 18.654, P < 0.001; (Ps-d = 1.000, Ps-o = 1.000, Ps-c<0.001, Pd-o = 0.274, Pd-c<0.001,Po-c = 0.393) |
c indicates control group; d/dMTS, low lumbar degeneration-related May-Thurner syndrome; IVTD, diameter of the iliac vein tunnel; LLLA, lower lumbar lordosis angle; o/oIVCS, other cause iliac vein compression; s/sMTS, simple May-Thurner syndrome.
*Bofferroni test was used in the statistical correction of multiple comparisons of means.
In control group, 11 forward bulging or protruding disc (3%, 11/380) and 45 ventro-osteophytes (12%, 45/380) were detected for the segment of the fourth and fifth lumbar, but all these problem discs and osteophytes did not compress into the iliac vein.
The compression sites displayed by DSA and the extent of LDVT showed by US of various MTS consisted with that presented on CT images (See Table 3, Figures 7–8).
TABLE 3.
Sites of Iliac Vein Compression and Extent of thrombosis of Case Groups
| Groups | Cases | Sites of IVC by DSA | Extent of Thrombosis by US | |||||
| Right Ahead of the L4–5 Disc* | Left Front of the L5 | Right Ahead of the L5 | Right Front of the L5 | Limited to the Iliac Vein | Extended to the Femoral Vein | Extended to the Popliteal Vein | ||
| oMTS | 22 | 4 (18%) | 0 | 4 (18%) | 14 (64%) | 0 | 6 (27%) | 16 (73%) |
| dMTS | 33 | 9 (26%) | 3 (9%) | 18 (56%) | 3 (9%) | 6 (18%) | 10 (30%) | 17 (52%) |
| oIVCS | 14 | 0 | 0 | 0 | 0 | 4 (29%) | 6 (43%) | 4 (29%) |
| Test | 69 | χ2 = 19.305, P < 0.001 | — P = 0.033† | |||||
dMTS indicates low lumbar degeneration-related May-Thurner syndrome; DSA, digital subtraction angiography; IVC, iliac vein compression; oIVCS, other cause iliac vein compression; oMTS, May-Thurner syndrome; US, ultrasound sonography.
*the L4–5 disc, the disc between the fourth lumbar and the fifth lumbar.
†Fisher precise algorithm.
Figure 7.
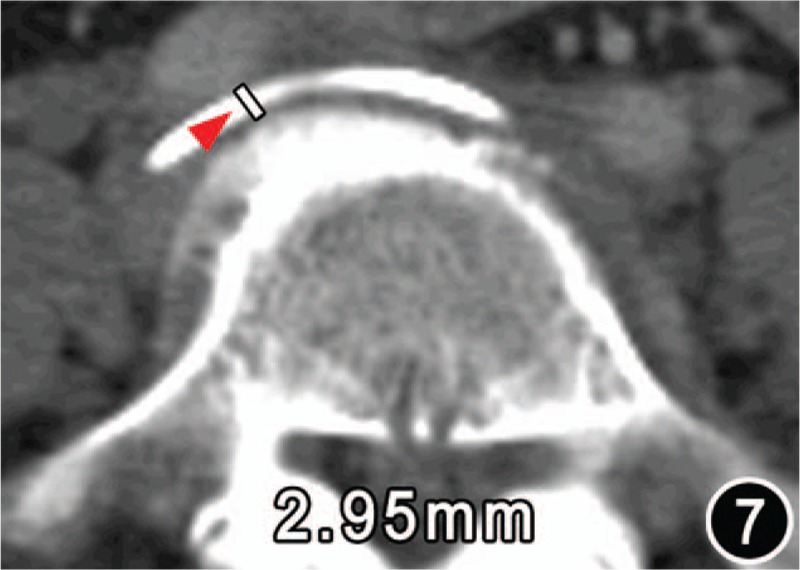
CT from a patient of age 67 years with lumbar degeneration-related MTS and presented the osteophyte (arrow) at the right front of the fifth lumbar protruding forward the iliac vein tunnel (IVT), which caused an acquired (or secondum) stenosis of the IVT.
Figure 8.
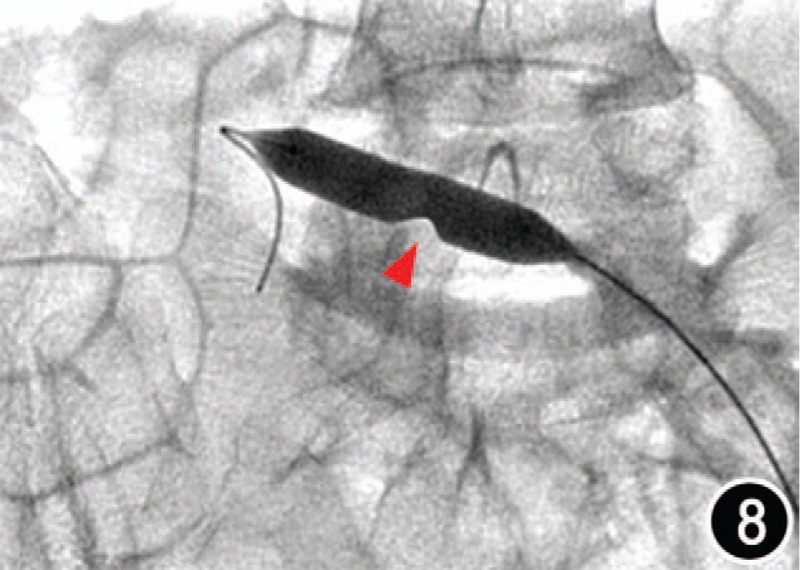
Map of intravenous balloon dilatation by digital subtraction angiography of the same patient as Figure 7, and showed a stenosis of the vein cavity corresponding to the osteophyte site (arrow).
Therapeutic Effect and Influencing Factors
In the first stage: 15 of 69 patients got valid treatment without recurrence during follow-up period, and 54 patients remained invalid were arranged to perform next therapeutic process. In the second stage: 26 of 54 patients gained primary effective treatment and 28 patients being still invalid; however, 9 of 26 valid patients relapsed during follow up, only 17 patients obtained final effective treatment. Invalid 28 patients and relapsed nine patients entered into the third stage treatment. After iliac vein stenting, remaining 37 patients achieved effective patency of the left iliac vein. See Table 4.
TABLE 4.
Interventional Treatment Results in Each Treatment Stage of Various IVCS
| Groups (Patients) | First Stage (69 Cases) | Second Stage (54 Cases) | Third Stage (37 Cases) | ||||||
| Valid | Invalid | Valid | Invalid | Valid | Invalid | ||||
| No Recur | Recur | No Recur | Recur | No Recur | Recur | ||||
| oMTS (22) | 0 | 0 | 22 | 3 | 3 | 16 | 19 | 0 | 0 |
| dMTS (33) | 5 | 0 | 28 | 10 | 6 | 12 | 18 | 0 | 0 |
| oIVCS (14) | 10 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
| X2 test | 27.270 | 12.186 | — | ||||||
| P | <0.001* | 0.002* | — | ||||||
dMTS indicates low lumbar degeneration-related May-Thurner syndrome; IVCS, iliac vein compression syndrome; oIVCS, other cause iliac vein compression; oMTS, May-Thurner syndrome.
*Valid cases were effective therapeutic patients without relapse; invalid cases included unresponsive patients and recurrent patients.
Logistic regression analysis demonstrated that IVCS type (β=1.567,Waldχ2 = 6.902, P = 0.009, odds ratio, OR, = 4.794), course(β= –1.908, Waldχ2 = 4.618, P = 0.032, OR = 0.148), and treatment plan(β=3.080, Waldχ2 = 14.748, P < 0.001, OR = 21.749) markedly influenced the therapeutic result, but extent of embolism not (β=-0.214, Waldχ2 = 0.149, P = 0.699, OR = 0.807).
Diagnostic Cutoff of Risk IVTD
Because of sMTS and dMTS had a common anatomical feature of narrow IVTD, both generally were named IVTD-narrow MTS. The IVTD of both was 2.41 ± 0.40 m) (95% CI: 1.83 –2.97 mm). Taken the IVTD of the control group as disease-free team, a ROC was obtained. The cutoff value of IVTD for risk of MTS was 2.98 mm and the area under curve (AUC) was 0.96, which diagnostic sensitivity was 90%, specificity 100% and Youden index 0.9.
DISCUSSION
Pathological Anatomy of MTS Based on CT
According to CT anatomical features, the authors classified MTS into three groups, sMTS, dMTS, and oMTS. Results showed that both sMTS and dMTS had common characteristics as narrow IVTD, small LLLA, and sex composition, but were markedly differences in onset age, LDRIVC signs, compressed sites of the iliac vein, extent of venous embolism, and therapeutic effect.
Forward bulging or protruding intervertebral discs, anterior vertebral osteophytes, and lumbar spondylolisthesis wedge-likely press into the iliac vein from the posterior direction, producing MTS presentation (named dMTS) as sMTS. This suggests that many patients, even those with congenitally narrow IVTD, do not necessarily develop to MTS without LDRIVC. Low lumbar degenerative changes induce or aggravate MTS. Although they both are caused by IVTD stenosis, sMTS is a congenital developmental variation, and may be referred as primary channel narrow; dMTS is complicated by lower lumbar degeneration and is considered secondary channel stenosis. As a physiological factor, LLLA was smaller in females than in males. Decreased LLLA would shorten the distance between the lumbar lordosis point and the iliac artery, leading to a decreased IVTD. For this reason, the disease is more common in females than in males. The oMTS did not exhibit abnormal small IVTD and LLLA, in contrast, which became slightly larger than that of the control owing to “mass effect” of hematoma or abscess. The oMTS had no regular patterns in sexual incidence, onset age, compression positions, and LDVT scopes.
Different Therapeutic Effects of Various MTSs
Once MTS occurs, vena cava filter and stent placement are generally the first line treatments.18,22,23 Simple catheter-directed intravenous thrombolysis usually could not recanalize the iliac vein or prevent the recanalized vein from relapsing. Complex treatments were recommented. In this study, 86% sMTSs and 52% dMTSs needed intravenous stent-implanted operation to obtain effective treatment, but none of oMTSs had stent-implanted operation before the iliac vein recanalization. Postoperative anticoagulation management for 6 months was inevitable to inhibit LDVT recurring.
Results indicated that MTS type, course, and treatment plan markedly influence the therapeutic result. Owing to the degeneration-related compressions develop slowly; the course of dMTS was longer than that of sMTS and oMTS. Compression sites of dMTS most commonly occurred at the right ahead of the fifth lumbar vertebra body or the fourth-fifth lumbar intervertebral discs, whereas that of sMTS often located the right front of the fifth lumbar vertebra body where is the origin of the left iliac vein, and which of oMTS were diffusely around the iliac vein and nonsolid compression. This may be the anatomical basis for extent of thrombosis caused by sMTS being serious than that of dMTS-caused and oMTS-originated. The differences of extent of venous embolism in three case groups were statistically significant, but wald χ2 test did not demonstrate extent of embolism had an effect on therapeutic result with significance, this might be because of less samples.
Cutoff of IVTD Value for Risk of sMTS and dMTS
The shape of the iliac vein and its peripheral interspaces and the lower lumbar spine curvature in cadavers are very different from that in vivo. IVTD in cadavers does not accurately reflect the true channel size in vivo. Confined by techniques and methods of study, in a long term no research reports on the relationship between IVTD and lower lumbar degenerative changes. CT has potential diagnostic superiority in subtle anatomy of vivo.24–26 Stenosis of the IVTD and compression of the iliac vein are the direct causes of MTS. For MTS, the key problem that imaging diagnosis needs to solve is the risk assessment and diagnosis in advance. Kibbe et al27 found, in a group of patients with LDVT diagnosed by CT-angiography, that the diameter of the iliac vein in 24% of the patients were reduced by more than 50%, and in 66% of patients, it was reduced by 25%. Carrs et al28 observed that the diameter of the left iliac vein decreased by 1 mm, the rate of incidence of LDVT increases by 1.68. In this study, the stenosis cutoff of risk IVTD was 2.98 mm for IVTD-narrow MTS, with AUC being 0.96 (mm)2, diagnostic sensitivity being 90%, specificity being 100% and Youden index being 0.9. This cutoff value was more direct, convenient, and practical in clinical diagnostic using than previous results. Although oMTS had a common characteristic of iliac vein compression as sMTS and dMTS, the IVTD became wider because of hematoma or abscess, and the soft compression factors decrease the difficulties of thrombolysis therapy.
Value and Limitation of this Study
Understanding IVTD is very important for early precaution of IVTD-narrow. The risk patient could change sleeping positions, such as the lateral position or the prone position, to enlarge the IVTD with forward lumbar curvature being reduced (because IVTD was positively correlation with LLLA), which prevents the iliac vein from being compressed by the pulsating right iliac artery and lumbar degenerative changes. Another important significance lies in introducing additional attention to the IVT and IVTD in CT diagnosis for lower back pain subjects, but traditional imaging diagnosis shows more solicitude for influences of the lumbar degeneration on the spinal canal or nerve root.
However, this study had some technical limitations. There was no imaging analysis for the structures inner the lumen of iliac vein, which might be important for accelerating thrombosis and affect the therapeutic effect. In addition, the sMTS and dMTS groups were all embolism patients based on the clinical presentation and lacked of prothrombosis and incomplete thrombosis patients. Therefore, the risk threshold of 2.98 mm is lower than the actual value. Future studies are aimed at fully leveraging the advantage of multimode imaging to reveal precise anatomy, pathology, and physiology changes in this disease to establish more effective preventive and curative measures.
Key Points
Different from previous cadaver researches, this investigation was performed to understand the underlying anatomy of May-Thurner syndrome (MTS) in vivo by CT.
MTS are categorized to simple MTS, lumbar degeneration-related MTS, and other causes MTS by the differences of the anatomy and causes of iliac vein compression showed by CT.
Three subtypes of MTS are distinct in onset age, course, lumbar degeneration-related iliac vein compression, compression site, extent of venous embolism, and therapeutic effect. Classified diagnosis of MTS by CT is helpful in accurate treatment program.
Acknowledgments
We would like to thank professor Zhen-qi Ding for his putting forward important intellectual concepts and help in clinical diagnosis of lumbar vertebral degenerative disease, and Yuan-miao Shou and Tong-ke Su for their help in image analysis.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
Zhangzhou municipal government funds were received in support of this work.
No relevant financial activities outside the submitted work.
References
- 1.Gil Martín AR, Carreras Aja M, Arrieta Ardieta I, et al. Cockett's syndrome, May-Thurner syndrome, or iliac vein compression syndrome. Radiologia 2014; 56:e5–e8. [DOI] [PubMed] [Google Scholar]
- 2.Mathur M, Cohen M, Bashir R. May-Thurner syndrome. Circulation 2014; 129:824–825. [DOI] [PubMed] [Google Scholar]
- 3.Virchow R. Uber die Erweiterung kleiner Gefasse. Arch Path Anat 1851; 3:427. [Google Scholar]
- 4.McMurrich JP. Th e occurrence of congenital adhesions in the common iliac veins, and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci 1908; 135:342–346. [Google Scholar]
- 5.Ehrich WE, Krumbhaar EB. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J 1943; 26:737–750. [Google Scholar]
- 6.Cockett FB, Th omas ML. Iliac compression syndrome. Brit J Surg 1965; 52:816. [DOI] [PubMed] [Google Scholar]
- 7.Narayan A, Eng J, Carmi L, et al. Iliac vein compression as risk factor for left- versus right-sided deep venous thrombosis: case-control study. Radiology 2012; 265:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrich WE, Krumbhaar EB. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J 1943; 26:18–31. [Google Scholar]
- 9.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957; 8:419–427. [DOI] [PubMed] [Google Scholar]
- 10.DiDio LJA. Normal and pathological structures on the inner surface of the left common iliac vein. Arq Cir Clin Experim 1949; 12:507–616. [Google Scholar]
- 11.Negus D, Fletcher EW, Cockett FB, et al. Compression and band formation at the mouth of the left common iliac vein. Br J Surg 1968; 55:369–374. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari E., Jr Su alcune interessanti malformazioni della vena iliaca commune di sinistra nell‘uomo. Briglie congenite tese attraverso illume. Cuore Circol 1940; 24:455–468. [Google Scholar]
- 13.Mitsuoka H, Ohta T, Hayashi S, et al. Histological study on the left common iliac vein spur. Ann Vasc Dis 2014; 7:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayalakshmi IB, Setty HS, Narasimhan C. Unusual cases of right-sided and left-sided May-Thurner syndrome. Cardiol Young 2014; 1–3.Jul 10. [DOI] [PubMed] [Google Scholar]
- 15.Brazeau NF, Harvey HB, Pinto EG, et al. May-Thurner syndrome: diagnosis and management (Review). Vasa [J] 2013; 42:96–105.doi: 10.1024/0301-1526/a000252. PMID: 23485836. [DOI] [PubMed] [Google Scholar]
- 16.Hu LY, Lou WS, Gu JP, et al. Early and long-term outcomes for postpartum deep vein thrombosis: the role of endovascular treatment. Chin J Radiol 2015; 49:386–390.doi: 10.3760/cma.j.issn:1005-1201.2015.05.015. [Google Scholar]
- 17.Song JH, He X, Lou WS, et al. Preliminary results of application of percutaneous AngioJet thrombectomy in patients with acute iliofemoral deep venous thrombosis. Chin J Radiol 2015; 49:758–762.doi:10.3760/cma.j.issn:1005-1201.2015.10.009. [Google Scholar]
- 18.Bækgaard N, Broholm R, Just S. Indications for stenting during thrombolysis. Phlebology 2013; 28:112–116.doi: 10.1177/0268355513476818. PMID: 23482545. [DOI] [PubMed] [Google Scholar]
- 19.Luo DY, Li HH, Long MY, et al. Comparison of therapeutic effect of surgical treatment and drug thrombolysis for acute deep venous thrombosis of the lower extremity. Chin J Gen Surg 2010; 25:876–879.doi: 10.3760/cma.j.issn.1007-631X.2010.11.006. [Google Scholar]
- 20.Yu WN, Zhang XQ, Wang YP, et al. Endovascular therapy for lower extremity deep venous thrombosis. Chin Arch Gen Surg 2013; 7:39–42. [Google Scholar]
- 21.Akimasa M, Norikazu Y, Yoshito O, et al. Early and long-term outcomes of venous stent implantation for iliac venous stenosis after catheter-directed thrombolysis for acute deep vein thrombosis [J]. Circ J 2014; 78:1234–1239.PMID:24583973. [DOI] [PubMed] [Google Scholar]
- 22.Xue GH, Huang XZ, Ye M, et al. Catheter-directed thrombolysis and stenting in the treatment of iliac vein compression syndrome with acute iliofemoral deep vein thrombosis: outcome and follow-up. Ann Vasc Surg 2014; 28:957–963. [DOI] [PubMed] [Google Scholar]
- 23.Budnur SC, Singh B, Mahadevappa NC, et al. Endovascular treatment of iliac vein compression syndrome (May-Thurner). Cardiovasc Interv Ther 2013; 28:101–105. [DOI] [PubMed] [Google Scholar]
- 24.Lamba R, Tanner DT, Sekhon S, et al. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics 2014; 34:93–115. [DOI] [PubMed] [Google Scholar]
- 25.McDermott S, Oliveira G, Ergül E, et al. May-Thurner syndrome: can it be diagnosed by a single MR venography study? Diagn Interv Radiol 2013; 19:44–48. [DOI] [PubMed] [Google Scholar]
- 26.Fong JK, Poh AC, Tan AG, et al. Imaging findings and clinical features of abdominal vascular compression syndromes. AJR Am J Roentgenol 2014; 203:29–36. [DOI] [PubMed] [Google Scholar]
- 27.Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg 2004; 39:937–943. [DOI] [PubMed] [Google Scholar]
- 28.Carr S, Chan K, Rosenberg J, et al. Correlation of the diameter of the left common iliac vein with the risk of lower-extremity deep venous thrombosis. J Vasc Interv Radiol 2012; 23:1467–1472. [DOI] [PubMed] [Google Scholar]


