Abstract
Background:
In 2014, Nigeria accounted for 33% of all new childhood HIV infections that occurred among the 22 Global Plan priority countries where 80% of HIV-infected women reside. Even with a vertical HIV transmission rate of 27%, only 6% of infants born to HIV-infected women in Nigeria receive early infant diagnosis (EID). This article reports rates of antiretroviral prophylaxis, EID, and mother-to-child transmission in a congregation-based Healthy Beginning Initiative (HBI) designed to increase HIV testing among pregnant women in southeast Nigeria.
Methods:
This is a nested cohort study of HIV-exposed infants (HEI) within the HBI trial originally designed as a 2-arm cluster randomized trial. HIV-infected mothers and infants were followed between January 2013 and August 2014.
Results:
Across both arms of the study, 72 HIV-infected women delivered 69 live infants (1 set of twins) and 4 had miscarriages. Of the 69 live-born HEI, HIV status was known for 71% (49/69), 16% (11/69) died before sample collection, and 13% (9/69) were lost to follow-up. Complete information was available for 84% of HEI (58/69), of which 64% (37/58) received antiretroviral prophylaxis. Among the 49 infants tested for HIV, 88% (43/49) received EID within 2 months and 12% (6/49) received antibody testing after 18 months. The mother-to-child transmission rate was 8.2% (4/49).
Conclusions:
EID was higher and HIV transmission rate was lower among the HBI participants compared to reported rates in 2014. However, further progress is needed to achieve goals of elimination of infant HIV infection.
Key Words: HIV, early infant diagnosis, linkage to care, prevention of mother-to-child transmission, PMTCT, Nigeria
INTRODUCTION
Mother-to-child transmission (MTCT) of HIV remains an important source of new HIV infections among the 22 priority countries identified in the Joint United Nations Program on HIV/AIDS Global Plan (referred to as Global Plan).1 In 2014, there were an estimated 1.2 million pregnant women living with HIV and 174,000 children (0–14 years old) newly infected in the priority countries.1 Considerable progress has been made to prevent MTCT. Between 2009 and 2014, there was a 48% decrease in new HIV infections among children in the Global Plan priority countries.1 However, some of these countries still face significant challenges in developing effective programs for PMTCT, such as poor access to screening and adherence to antiretroviral therapy (ART), and HIV stigma.2–4 To reduce the burden of HIV, it is important to improve HIV screening rates for pregnant women, increase the number of HIV-positive mothers who are linked to care and receive ART, increase the proportion of newborns who receive antiretroviral (ARV) prophylaxis, increase participation in early infant diagnosis (EID) through postnatal testing, and increase retention in long-term ART for HIV-positive mothers and children.
The setting for this study was Nigeria, and in 2014, an estimated 210,000 pregnant women in Nigeria were HIV positive.1 Nigeria is 1 of only 4 of the 22 priority countries with an HIV testing rate of less than 20% among pregnant women.5,6 Only 29% of HIV-infected pregnant women in Nigeria received ART compared to an average treatment rate of 77% in the other Global Plan priority countries.1,7 Nigeria accounted for 33% (n = 58,000) of all new childhood infections among the sub-Saharan Africa priority countries in 2014.1 About 90% of these infections are as a result of MTCT.8 Additionally, Nigeria has one of the lowest rates of EID for HIV at 6.3%, and only 12% of children living with HIV receive ART.1,5,9 Currently, there are nearly 400,000 HIV-infected children living in Nigeria.10
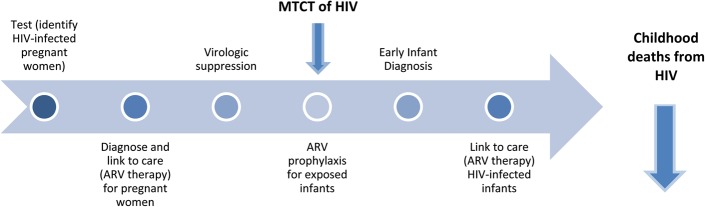
The implementation cascade for continuum of care for PMTCT and decreased childhood HIV deaths is shown in Figure 1.11 When HIV-infected pregnant women are promptly identified and placed on ART, and their exposed infants receive ARV prophylaxis, MTCT occurs in less than 1% of pregnancies.12–14 Without intervention, up to 45% of HIV-exposed infants (HEI) could be infected from their mothers.15 However, there are several barriers to implementing PMTCT interventions in Nigeria. An initial challenge to PMTCT is HIV testing among pregnant women. Barriers to testing in Nigeria include low perception of personal risk, lack of knowledge, poor access to screening sites, cost, confidentiality, HIV-related stigma, mistrust among couples, fear of knowing one's status and lack of male partner involvement for the pregnant woman.2–4,16–21
FIGURE 1.
Implementation cascade for continuum of care for PMTCT of HIV and decreased childhood HIV deaths.
Most pregnant women must access health clinics to receive HIV testing and to participate in available PMTCT interventions along the continuum of care (ie, ART, ARV prophylaxis for exposed infants, EID, and linkage to care/ART for HIV-infected infants).22,23 Such clinic-based approaches and interventions are challenging in Nigeria, as only 36% of births take place at a health facility.23 Although the number of health facilities that offer PMTCT programs has increased almost 10-fold from 2010 to 2014 (675–6546), the coverage of PMTCT remains low at 30%.7 Low EID rates may be because of low participation in antenatal and postnatal care by pregnant women, limited or no access to services in rural locations, and social and educational barriers.23–27 Because of these barriers, alternative and complementary interventions to PMTCT of HIV are needed to realize the Nigerian government's 2010–2015 National Strategic Framework goals of increasing access to quality HIV screening to 80% among pregnant women, at least 80% of all HEI having access to ARV prophylaxis, and at least 80% of all HEI having access to EID services by 2015.28
To develop an intervention to address barriers to PMTCT in Nigeria, we used the first 3 components of implementation science (IS) as characterized by the National Institutes of Health–the President's Emergency Plan for AIDS Relief (PEPFAR) PMTCT Implementation Science Alliance (The Alliance), which included the following: (1) understanding the implementation environment, (2) studying the actual process of implementation, and (3) testing innovative implementation approaches. These components were used to identify viable strategies for mitigating barriers to optimal HIV testing and PMTCT cascade completion by evaluating the implementation outcomes of the Healthy Beginning Initiative (HBI) through (1) studying the implementation environment of the initiative to assess factors, such as the behavior of pregnant women and their male partners with respect to HIV testing; (2) evaluating the use of innovative implementation processes to address major barriers impeding effective implementation; and (3) testing the new approach to PMTCT.
The purpose of this article was to report the impact of HBI on rates of ARV prophylaxis, EID, HIV status, and linkage to care among infants born to HIV-infected mothers. Additionally, we report on how the involvement of implementers throughout the process impacted the intervention.
METHODS
Research Planning Meetings
Because of the high rates of HIV infection among infants in Nigeria, a working group was initiated in Enugu, Nigeria in 2010 to identify IS approaches that would increase the components of the continuum of care for PMTCT of HIV by reducing barriers. Members of the working group were drawn from local organizations involved with HIV education, advocacy groups, faith-based organizations, members of support groups for people living with HIV, representatives of women's groups, hospitals, delivery centers, and representatives from government agencies. The working group made the decision to evaluate a culturally adapted, family-centered congregation-based approach to HIV testing and linkage to care titled HBI. Members of the working group became partners and collaborators in this study. Each component of HBI was focused on reducing barriers to PMTCT that had been identified by the working group.
Study Setting and Design
A total of 40 churches in Enugu State of Nigeria participated in the HBI.29 The population in Enugu State is predominately Christian, with church attendance approaching 90% and an extensive network of faith-based institutions.30–32 Religious leaders are well-informed about HIV/AIDS and leverage their position for HIV prevention and interventions.33 Each church had a health team (HT) comprised of the priest, women's and men's group leaders, and 2 volunteer health advisors, 1 of whom is trained to perform venipuncture.29 Prevention, education, Treatment, Training and Research-Global Solutions (PeTR-GS) conducted training workshops for all study staff and church-based volunteer health advisors.
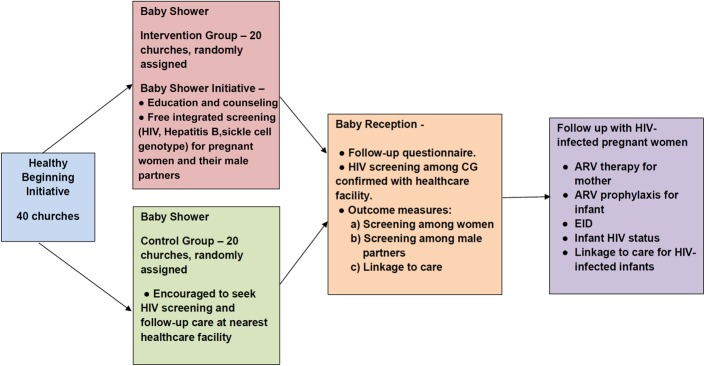
The initial HBI study used a 2-arm cluster randomized trial to evaluate the feasibility and acceptability of a congregation-based baby shower intervention delivered by lay health advisors at local churches (intervention) versus a clinic-based approach (control) on HIV testing. The 40 churches were randomly assigned (1:1) to either the intervention arm or the control arm (Fig. 2). We present a brief synopsis of HBI here; however, details of the study protocol, sample size, and power analysis are published elsewhere.29,34 Enrollment began in January 2013 and was completed by September 2013. Follow-up of enrolled participants was completed by August 2014.
FIGURE 2.
Healthy Beginning Initiative.
Participant Recruitment
Participant recruitment occurred at the level of the churches.29 Monthly prayer sessions for pregnant women were used to identify pregnant women and their male partners and for recruitment. During this routine prayer session, the priest asked pregnant women and their male partners to step up to the altar for prayers. For the study, after praying for a healthy pregnancy and delivery, the priest introduced HBI and the HT and described the program's objectives. Pregnant women and their male partners were invited to voluntarily participate in the study. Pregnant women could participate even if their male partner was unavailable or chose not to participate.
Baby Shower
The baby shower took place one Sunday a month, which was designated as the “Healthy Beginning Sunday,” and pregnant women and their families were invited to attend. The baby shower was similar for both study arms with the exception of the intervention. Participants in both groups were provided a gift and refreshments at the baby shower. Participants in the control arm were referred to the closest health care facility and encouraged to seek prenatal screening, HIV testing, and follow-up care there, which was considered standard of care. Participants in the intervention arm received an educational program that included healthy habits and information about important laboratory screenings during pregnancy. Additionally, participants in the intervention arm were offered free integrated laboratory screenings during the baby shower, including tests for HIV, hepatitis B, and sickle cell genotype. This integrated testing was designed to reduce the stigma associated with HIV-only screening.
Baby Reception
Participants in both groups were invited to a baby reception immediately following infant baptisms in church 6–8 weeks after delivery. The baby reception was used for follow-up and post-delivery linkage to care. Self-reported prenatal screenings in the control arm were confirmed with the health care facilities. Women in the control arm were offered integrated HIV testing at the baby reception if they did not receive testing during pregnancy. HIV-infected women and infants were linked to facility-based care.
Follow-up
No HIV treatment was provided at the HBI church sites; however, treatment was provided at health care facility hubs to which HBI sites were linked for care of all positive participants, including infant and mother pairs, and male partners in both the control and the intervention arms. Standard of care for HBI participants included the following: (1) all pregnant mothers received Option B+ at the treatment sites. Although this was not yet the national guideline in Nigeria, it was part of PEPFAR Partners' program guideline; (2) maternal ARV: first line for all PMTCT was TDF/3TC/EFV except where contraindicated; (3) infant prophylaxis was Nevirapine for 6 weeks; (4) all infants were recommended to receive EID at 6–8 weeks; and (5) breastfeeding was recommended to all mothers. AFASS (affordable, feasible, accessible, sustainable, and safe) was taught, but all mothers were supported to breastfeed. Infants born to HIV-infected women were followed until final HIV status was determined. Results presented are for the cohort of infants from HBI born to HIV-infected women from both the intervention and the control arms and include the infants' receipt of ARV prophylaxis, EID, their HIV status, and linkage to care for HIV-infected infants.
Ethical Approval
The study was approved by the Institutional Review Board of the University of Nevada, Reno, and the Nigerian National Health Research Ethics Committee.
RESULTS
Infant Outcomes
A total of 3002 pregnant women participated in the HBI [intervention group (IG) = 1647, control group (CG) = 1355]. Previously published results showed that women in churches randomized to the intervention arm were more likely to be screened for HIV, linked to care, and on ARV therapy during pregnancy.35 Of the 3002 women who participated in HBI, 2254 received HIV testing [IG = 1514 (92%), CG = 740 (55%)]. A total of 72 of the 2254 women tested (3.19%) were identified to be HIV infected and gave birth to 69 live infants (including 1 set of twins). Four pregnancies ended in a miscarriage. Overall, 71% (49/69) of infants received an HIV test, 16% (11/69) died before their blood samples were collected for HIV testing, and 13% (9/69) were lost to follow-up. Among the 69 live births, complete information was available for 84% (58/69).
As shown in Figure 3, 64% (37/58) of the infants for whom information was available received antiretroviral prophylaxis (Nevirapine for 6 weeks). Among the 49 who were tested for HIV, 88% (43/49) received EID by polymerase chain reaction within 2 months and 12% (6/49) received an HIV antibody test after 18 months. Among the 49 with an HIV test result, 8.2% (4/49) were HIV positive. The 4 positive infants had a positive EID within 2 months of birth and a positive antibody test after 18 months. Of the 39 negative EID tests, 25 (64%) had an antibody test after 18 months and all were negative. Although 43 infants received EID within 2 months of birth, the average time to receive results was 5.6 months for infants tested within 2 months. Of the 4 HIV-infected infants, 3 initiated ART (AZT/3TC/Nevirapine), and treatment support is provided on an ongoing basis to the caregiver.
FIGURE 3.
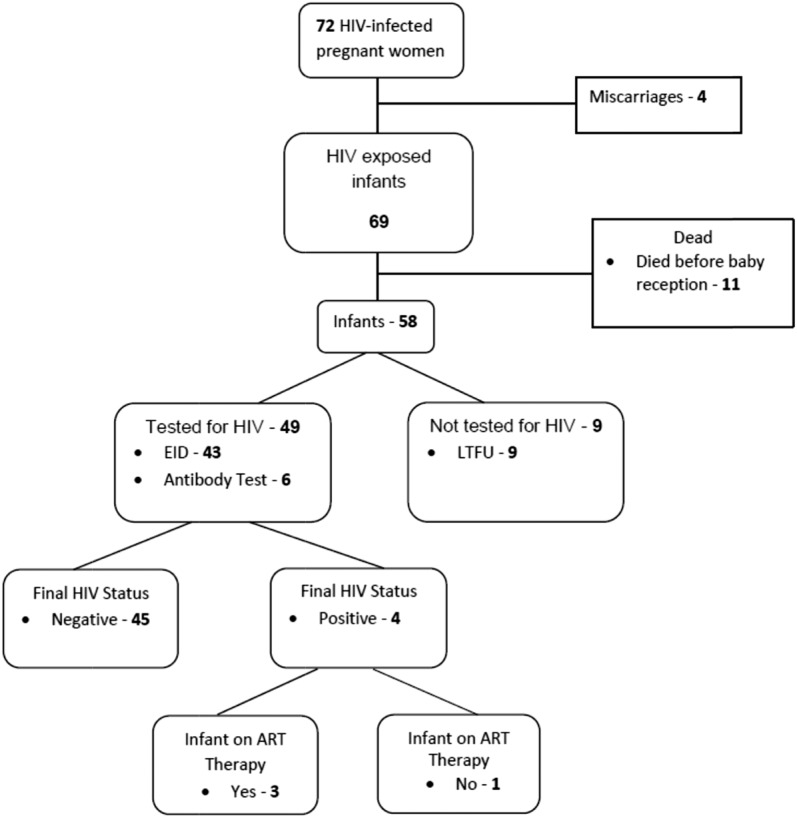
HIV testing, status, and care for HIV-exposed infants.
Implementation Environment and Process Involvement
HBI was developed as a culturally adapted, family-centered approach that relied on the wide distribution of churches and church-based community networks to address barriers to HIV testing for pregnant women in the IG by providing education and counseling (knowledge), on-site, free, and integrated testing (HIV, sickle cell genotype, and hepatitis B) rather than HIV-only testing (aimed at reducing access barriers, reducing cost, and addressing stigma), and culturally appropriate baby showers (aimed at reducing losses to follow-up and increase linkage to care postdelivery).
We believe that involving the church community in the implementation process was critical to the success of HBI. We spent time engaging the clergy leadership, explaining the objectives of the intervention and getting buy-in. Once the priests and members of the HT were trained, we had very little turnover among the teams. Priests and the volunteer HT members are trusted in the community and able to interact with the study participants more so than any external, non–community-based research team would have. Additionally, because the HTs are composed of volunteers, HBI has been sustained in the community. Even though recruitment into the trial ended in 2013, communities elected to continue the program because of its popularity. Each of the participating sites is provided baby shower gifts and testing for sickle cell by the HealthySunrise Foundation, a nonprofit organization. HIV testing is provided free through the local PEPFAR supported partner PeTR-GS.
DISCUSSION
HBI was successful in increasing participation along the PMTCT cascade. Compared to published national rates in Nigeria, we saw an increase in rates of exposed infants receiving ART prophylaxis, EID, and ARV therapy for HIV-infected infants.5,9 Additionally, the transmission rate among this group was 8.2%. This rate is lower than transmission rates found in other studies in Nigeria, which approach 30%.36 However, in this study, the number of HIV-infected infants identified was small (n = 4), and comparison made to national data need to consider this small number.
Although we approached the Nigerian government's National Strategic Framework goals of 80% of pregnant women receiving HIV testing among the IG, we did not meet the goal of at least 80% of all HEI receiving ARV prophylaxis and at least 80% of all HEI receiving EID.28,37 We were not able to obtain the HIV status of 29% of the infants either because they died before HIV testing was completed or they were lost to follow-up. Additionally, although 43 infants had EID within 2 months of birth, on average, it took 5.6 months to receive a result. This was because of processing delays that occurred at the laboratory facility during this period (eg, lack of reagents, breakdown of laboratory equipment). This was identified as an area for improvement to be able to start HIV-infected infants on ART closer to the timing of EID.
HIV testing for the intervention arm of HBI was offered in a location where people, including pregnant women, congregate. The church locations were high-yielding, as nearly 90% of people in the Enugu State and much of southeast Nigeria attend church at least once a week, and churches are prevalent in every village in Nigeria.30 However, once HIV-infected pregnant women were identified in either group (intervention or control arm), they were linked to care and provided follow-up care at the nearest health care facility. Because not every village has a health care facility, some pregnant women were required to travel long distances to receive follow-up care prenatally (ART for pregnant women), at birth (ARV prophylaxis for exposed newborns), and after birth (ART of HIV-infected infants). In sub-Saharan Africa countries, referral of HIV-infected pregnant women to facilities has not produced significant increases in PMTCT uptake mainly because of issues with patient transportation to health care facilities and poor provider tracking of referrals.37,38 Although HBI eliminated a barrier to PMTCT by providing accessible HIV testing sites, it did not eliminate barriers to PMTCT that exist at the health care facility level where ART was provided, as observed in similar studies.
Building on the success of HBI for HIV testing and linkage to care for pregnant women, our next IS activity will be to identify the role that HBI can play in PMTCT activities further along the continuum of care. We could engage churches as sites for ART distribution, a form of community ART, for pregnant women and engage the HTs in a support role for retention in care of HIV-infected mothers and children. It is feasible for interventions, such as motivational interviewing to be used to train the HTs that implement HBI, to act as promontoras and provide support and counseling to increase retention in care.
HBI could offer EID by providing free, integrated HIV testing for newborns at the baby receptions and in follow-up and providing ART for HIV-infected infants. Because relatively few births occur within the health care system in Nigeria, HBI could be used to distribute infant ARV prophylaxis by working with traditional birth attendants in the communities.39 Studies have found that with training, traditional birth attendants are able to administer ART peripartum, enhancing PMTCT.40 Few intervention studies have targeted critical steps for PMTCT further down the continuum of care, and IS research is needed to improve EID uptake, ARV prophylaxis for exposed newborns, and linkage to care for HIV-infected infants.41
A lesson learned for future implementation of HBI in other countries and settings is to engage leaders, advocates, and the community throughout the process. By doing so, the initiative is more likely to be adopted by the community and can be useful for future scale-up activities. In the 40 churches, we are currently scaling up services to include screening for other diseases (eg, hypertension, breast cancer) by training the HT to take vital signs (eg, blood pressure) or teaching women to do self-breast examinations for cancer screening. The scalability of the HBI approach will serve as a valuable health resource in resource-limited communities.
By using the components of IS as characterized by The Alliance, HBI was able to increase participation along the continuum of care for PMTCT by increasing rates of HIV screening and linkage to care among pregnant women, ARV prophylaxis and EID for exposed infants, and ARV therapy for HIV-infected infants. The success of HBI is attributed to engagement of key partners, such as local HIV program implementers, advocacy groups, faith-based organizations, and HIV support groups, as well as representatives from women's groups, hospitals, delivery centers, and government agencies throughout the initiative. The success of HBI can be built on to deliver additional important health screenings and linkage to care for the community.
ACKNOWLEDGMENTS
The authors are grateful to HealthySunrise Foundation, Sunrise Foundation, Bishop John Okoye (Catholic Bishop of Awgu Diocese), Arch. Bishop Emmanuel Chukwuma (Anglican Bishop of Enugu), Bishop Callistus Onaga (Catholic Bishop of Enugu), and Arch. Bishop Amos Madu (Anglican Bishop of Oji-River). Their support was instrumental to the successful implementation of HBI. HBI implementation would not have been possible without the support and tireless effort of the priests in the participating churches. The church-based Volunteer Health Advisors took ownership of the program and made the process of recruitment and implementation smooth for our study team and participants. This study would have been impossible to conduct without the support of PeTR-GS (our PEPFAR-supported partner), staff, and volunteers.
Footnotes
Supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Mental Health, the President's Emergency Plan for AIDS Relief under award number R01HD075050 (E.E.E.). The funding agencies played no role in the study conception, design, data collection, data analysis, data interpretation, or writing of the report.
Presented at the Consortium of Universities for Global Health Conference, April 9–11, 2016, San Francisco, CA.
The authors have no funding or conflicts of interest to disclose.
The corresponding author, Dr. E. E. Ezeanolue, had full access to all the data in the study and had final responsibility for the decision to submit for publication. The trial was registered with ClinicalTrials.gov, identifier number NCT 01795261. Full study operating procedure manual is available on the website.
REFERENCES
- 1.UNAIDS. 2015 Progress Report on the Global Plan. Available at: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf. Update 2015. Accessed April 12, 2016. [Google Scholar]
- 2.Hardon A, Vernooij E, Bongololo-Mbera G, et al. Women's views on consent, counseling and confidentiality in PMTCT: a mixed-methods study in four African countries. BMC Public Health. 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monjok E, Smesny A, Essien EJ. HIV/AIDS-related stigma and discrimination in Nigeria: review of research studies and future directions for prevention strategies. Afr J Reprod Health. 2009;13. [PMC free article] [PubMed] [Google Scholar]
- 4.Turan JM, Bukusi EA, Onono M, et al. HIV/AIDS stigma and refusal of HIV testing among pregnant women in rural Kenya: results from the MAMAS study. AIDS Behav. 2011;15:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAID. Joint United Nations Programmes on HIV/AIDS, Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Update 2014. Accessed July 3, 2015. [Google Scholar]
- 6.UNAID. Joint United Nations Programmes on HIV/AIDS, 2013 Progress Report on the Global Plan towards Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/unaidspublication/2013/20130625_progress_global_plan_en.pdf. Update 2014. Accessed July 3, 2015. [Google Scholar]
- 7.National Agency for the Control of AIDS (NACA). Global AIDS Response Country Progress Report: Nigeria GARPR 2015. Available at: http://www.unaids.org/sites/default/files/country/documents/NGA_narrative_report_2014.pdf. Update 2015. Accessed April 12, 2016. [Google Scholar]
- 8.World Health Organization. Sexual and Reproductive Health: Prevention of Mother-to-child Transmission of HIV (PMTCT) and Family Planning (FP). Available at: http://www.who.int/reproductivehealth/topics/linkages/pmtct/en/. Update 2016. Accessed April 12, 2016. [Google Scholar]
- 9.UNAID. Joint United Nations Programmes on HIV/AIDS. The Gap Report. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- 10.UNAIDS. Nigeria: HIV and AIDS Estimates. Available at: http://www.unaids.org/sites/default/files/epidocuments/NGA.pdf. Update 2013. Accessed July 3, 2015. [Google Scholar]
- 11.Kim MH, Ahmed S, Buck WC, et al. The tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. 2012;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tubiana R, Le Chenadec J, Rouzioux C, et al. Factors associated with mother-to-child transmission of HIV-1 despite a maternal viral load <500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1). Clin Infect Dis. 2010;50:585–596. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Achievements in public health. reduction in perinatal transmission of HIV infection–united states, 1985-2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–597. [PubMed] [Google Scholar]
- 14.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(1 February):458–465. [DOI] [PubMed] [Google Scholar]
- 15.Coutsoudis A, Pillay K, Kuhn L, et al. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, south Africa. AIDS. 2001;15:379–387. [DOI] [PubMed] [Google Scholar]
- 16.Nunn A, Zaller N, Cornwall A, et al. Low perceived risk and high HIV prevalence among a predominantly African American population participating in Philadelphia's rapid HIV testing program. AIDS Patient Care STDS. 2011;25:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz DA, Kiarie JN, John-Stewart GC, et al. Male perspectives on incorporating men into antenatal HIV counseling and testing. PLoS One. 2009;4:e7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morfaw F, Mbuagbaw L, Thabane L, et al. Male involvement in prevention programs of mother to child transmission of HIV: a systematic review to identify barriers and facilitators. Syst Rev. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auvinen J, Kylma J, Suominen T. Male involvement and prevention of mother-to-child transmission of HIV in Sub-Saharan Africa: an integrative review. Curr HIV Res. 2013;11:169–177. [DOI] [PubMed] [Google Scholar]
- 20.Auvinen J, Kylmä J, Välimäki M, et al. Barriers and resources to PMTCT of HIV: Luba-Kasai men's perspective in Lusaka, Zambia. J Assoc Nurses AIDS Care. 2013;24:554–568. [DOI] [PubMed] [Google Scholar]
- 21.Aluisio A, Richardson BA, Bosire R, et al. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federal Ministry of Health, Nigeria. National HIV Sero-prevalence Sentinel Survey 2010. Technical Report, Department of Public Health. Available at: http://www.nigeriaaids.org/documents/2010_National%20HIV%20Sero%20Prevalence%20Sentinel%20Survey.pdf. Update 2010. Accessed July 3, 2015. [Google Scholar]
- 23.National Population Commission (NPC) [Nigeria] and ICF International. Nigeria demographic and Health Survey 2013. Abuja, Nigeria and MD: NPC and ICF International; 2014. [Google Scholar]
- 24.Umeobika J, Ezebialu I, Ezenyeaku C, et al. Knowledge and perception of mother to child transmission of human immunodeficiency virus among south eastern Nigerian pregnant women. J HIV Hum Reprod. 2013;1:15. [Google Scholar]
- 25.Olugbenga-Bello A, Adebimpe W, Osundina F, et al. Perception on prevention of mother-to-child-transmission (PMTCT) of HIV among women of reproductive age group in Osogbo, southwestern Nigeria. Int J Womens Health. 2013;5:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asekun-Olarinmoye E, Asekun-Olarinmoye I, Adebimpe W, et al. Community attitude towards the reproductive rights and sexual life of people living with HIV/AIDS in Olorunda local government area, Osogbo, Nigeria. HIV AIDS (Auckl). 2013;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNICEF. Cotrimoxazole Prophylaxis for HIV-exposed and HIV-infected Infants and Children; Practical Approaches to Implementation and Scale up 2010. 2010. Available at: http://www.unicef.org/aids/files/CotrimoxazoleGuide_2009.pdf. Accessed July 3, 2015. [Google Scholar]
- 28.National Agency for the Control of AIDS. The 2010-2015 HIV/AIDS Strategic Plan. Available at: http://nigeria.unfpa.org/pdf/nationalframweworkfullversion.pdf. Update 2010. Accessed July 3, 2015. [Google Scholar]
- 29.Ezeanolue EE, Obiefune MC, Yang W, et al. Comparative effectiveness of congregation- versus clinic-based approach to prevention of mother-to-child HIV transmission: study protocol for a cluster randomized controlled trial. Implement Sci. 2013;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Values Survey. World Survey Wave 6: 2010–2014. Available at: http://www.worldvaluessurvey.org/WVSOnline.jsp. Accessed January 20, 2015. [Google Scholar]
- 31.Pew Research Center. Tolerance and Tension: Islam and Christianity in Sub-saharan Africa. Available at: http://www.pewforum.org/files/2010/04/sub-saharan-africa-full-report.pdf. Updated 2010. Accessed February 10, 2015. [Google Scholar]
- 32.Pew Research Center. Christianity in Sub-saharan Africa. 2012. Available at: http://www.pewforum.org/newassets/images/reports/sub-saharan-africa/sub-saharan-africa-fullreport. Accessed February 10, 2015. [Google Scholar]
- 33.Ucheaga DN, Hartwig KA. Religious leaders' response to AIDS in Nigeria. Glob Public Health. 2010;5:611–625. [DOI] [PubMed] [Google Scholar]
- 34.Ezeanolue EE, Obiefune MC, Ezeanolue CO, et al. Effect of a congregation-based intervention on uptake of HIV testing and linkage to care in pregnant women in nigeria (baby shower): a cluster randomised trial. Lancet Glob Health. 2015;3:e692–e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezeanolue E, Obiefune M, Ezeanolue C, et al. Impact of a congregation-based intervention on uptake of HIV testing among pregnant women in Nigeria: the baby shower cluster randomized trial. Lancet Glob Health. 2015;3(11):e692–e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Audu RA, Salu OB, Musa AZ, et al. Estimation of the rate of mother to child transmission of HIV in Nigeria. Afr J Med Med Sci. 2006;35:121–124. [PubMed] [Google Scholar]
- 37.Hamela G, Kabondo C, Tembo T, et al. Evaluating the benefits of incorporating traditional birth attendants in HIV prevention of mother to child transmission service delivery in Lilongwe, Malawi. Afr J Reprod Health. 2014;18(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- 38.Audet CM, Salato J, Blevins M, et al. Educational intervention increased referrals to allopathic care by traditional healers in three high HIV-prevalence rural districts in Mozambique. PloS One. 2013;8:e70326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassey E, Elemuwa C, Anukam K. Knowledge of, and attitudes to, acquired immune deficiency syndrome (AIDS) among traditional birth attendants (TBAs) in rural communities in cross river state, Nigeria. Int Nurs Rev. 2007;54:354–358. [DOI] [PubMed] [Google Scholar]
- 40.Brennan AT, Thea DM, Semrau K, et al. In-Home HIV testing and nevirapine dosing by traditional birth attendants in rural Zambia: a feasibility study. J Midwifery Womens Health. 2014;59:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhardwaj S, Carter B, Aarons GA, et al. Implementation research for the prevention of mother-to-child HIV transmission in Sub-Saharan Africa: existing evidence, current gaps, and new opportunities. Curr HIV/AIDS Rep. 2015;12:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]