Supplemental Digital Content is Available in the Text.
This descriptive case series among adults documents that pain can return temporarily at healed, previously pain-free injury sites during acute opioid withdrawal.
Keywords: Pain, Substance withdrawal syndrome, Opioid, Opioid dependence, Hyperalgesia, Opioid-induced hyperalgesia, Self-report, Mixed methods
Abstract
Withdrawal pain can be a barrier to opioid cessation. Yet, little is known about old injury site pain in this context. We conducted an exploratory mixed-methods descriptive case series using a web-based survey and in-person interviews with adults recruited from pain and addiction treatment and research settings. We included individuals who self-reported a past significant injury that was healed and pain-free before the initiation of opioids, which then became temporarily painful upon opioid cessation—a phenomenon we have named withdrawal-associated injury site pain (WISP). Screening identified WISP in 47 people, of whom 34 (72%) completed the descriptive survey, including 21 who completed qualitative interviews. Recalled pain severity scores for WISP were typically high (median: 8/10; interquartile range [IQR]: 2), emotionally and physically aversive, and took approximately 2 weeks to resolve (median: 14; IQR: 24 days). Withdrawal-associated injury site pain intensity was typically slightly less than participants' original injury pain (median: 10/10; IQR: 3), and more painful than other generalized withdrawal symptoms which also lasted approximately 2 weeks (median: 13; IQR: 25 days). Fifteen surveyed participants (44%) reported returning to opioid use because of WISP in the past. Participants developed theories about the etiology of WISP, including that the pain is the brain's way of communicating a desire for opioids. This research represents the first known documentation that previously healed, and pain-free injury sites can temporarily become painful again during opioid withdrawal, an experience which may be a barrier to opioid cessation, and a contributor to opioid reinitiation.
1. Introduction
A growing appreciation of the deleterious effects of short- and long-term opioid use8,27,38,42,56,57,90,97,99,103,114,122,130 has spurred the need to address barriers to opioid cessation. Among these barriers, pain during or right after opioid withdrawal may be key.82,107 Generalized myalgias and arthralgias are well known to occur during opioid withdrawal,53 and were described as “internal rheumatism” almost 2 centuries ago.110
It is known that opioid use itself can cause adaptations in the central nervous system that lead to increased pain sensitivity, termed opioid-induced hyperalgesia (OIH),3,6,10,21,32,41,65,68,84,91,92 clinically first described over a century ago.2 Opioid-induced hyperalgesia is at times confused with tolerance, which may be distinct in both mechanism and treatment.6,29,51 Once opioids are stopped, the pain sensitivity can continue or seem to heighten temporarily because any pain-relieving effect opioids may have provided is eliminated, and the drug-opposite effect can take time to subside.139 Also, in preclinical models, opioid withdrawal induces central changes in neurotransmitters, along with neuroimmune and neuroinflammatory mediators involved in nociception,43,44 thus potentially intensifying pain beyond OIH. A general increase in pain sensitivity after opioid cessation can occur after acute4,55 or chronic66,109,132,144 opioid exposure, referred to by a variety of names including withdrawal-induced hyperalgesia (WIH). For individuals with chronic noncancer pain (CNCP) or an opioid use disorder (OUD), severity of pain during and immediately after opioid withdrawal can be a risk factor for restarting opioids.17,63,82,107,124 As a result, dose reduction or elimination can be difficult for those who take opioids, and can create enormous challenges in the doctor–patient relationship.52
Along with the above pain syndromes, we have observed patients who report that pain can reoccur at their old, previously healed, and previously pain-free injury sites during rapid opioid cessation, and that this reoccurring pain can resolve once the opioid withdrawal syndrome is over. To our knowledge, no previous studies have described such a pain experience.
Qualitative research can supplement quantitative results and help record new experiences, point to possible etiologies, and assist with future directions in research and treatment.36 Therefore, we undertook an exploratory mixed-methods study to document the existence and characteristics of this pain phenomenon that we have named withdrawal-associated injury site pain (WISP).
2. Methods
This nonconsecutive exploratory case series was derived from data gathered through an online survey and semistructured qualitative interviews using a convergent mixed-methods design.36,105
We were unable to identify any previously published validated instruments to assess injury site pain during opioid withdrawal. Therefore, we created and iteratively tested a pilot survey, administering it to target populations followed by individual-focused interviews, until no further changes were required for external consistency. We developed 5 screening questions and a descriptive survey containing 35 questions, with some options for narrative responses. Survey content validity was achieved through face validation and context validation. The survey literacy level was assessed to be grade 7.4 on the Flesch–Kincaid scale.54,79 In the survey given to participants, WISP was referred to as “recurrent pain” which was updated to WISP in Supplement 1 for congruency with this report (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A341). Instead of taking the online survey, participants living close to the research team could choose an in-person semistructured interview using online survey questions plus semistructured qualitative questions to further explore their perceptions and beliefs. The lead author (L.M. Rieb) conducted all interviews. New questions evolved as themes and nuances of the WISP experience emerged.
We enrolled a diverse cohort of patients reporting daily opioid consumption, regardless of the reason they had taken opioids (CNCP or OUD) because the neurophysiologic changes induced by opioids that affect withdrawal are likely the same in both populations.9,62 We recruited a convenience sample using posters and bookmarks, as well as snowball sampling methods. Posters purposefully sought participants who had pain at old injury sites during opioid withdrawal and were placed at 20 facilities across Canada, including inpatient and outpatient detoxification treatment facilities, out-patient pain management clinics, primary care methadone clinics, and a local inner-city research facility, between November 2013 and June 2015. Interview participants gave written consent to participate. The University of British Columbia's Behavioral Ethics Review Board and the Vancouver Coastal Health Research Institute approved the study protocol.
Eligible participants were 18 years or older and acknowledged being able to read English at a grade 8 level or above. Individuals were excluded from the interview if they appeared impaired from substance or medication use. The 5 initial screening questions probed for the presence of WISP:
(1) Use of opioids daily for 3 months or more, and
(2) A significant painful injury that was healed and pain-free for at least 3 months before starting opioids, and
(3) During that pain-free time was off all other pain medications, and
(4) Two weeks or more off opioids since starting daily use, and
(5) Temporary return of pain at the old healed injury site when stopping opioids.
If the participant answered “yes” to all 5 screening questions, they met criteria for having WISP. These participants could choose to continue to the additional descriptive survey. Participants self-administering the online survey were given options to link to resources for emotional support, to enter a draw for a gift, and to return to the website later for a copy of the results. In addition, participants who agreed to be interviewed received a $20 honorarium. Interview recruitment continued until predominant theme saturation occurred.
Demographic characteristics of participants included in this study are summarized using descriptive statistics, reporting percentages and total counts for dichotomous or categorical values as well as medians and interquartile range (IQR) for continuous values. Quantitative frequency analysis was performed on the survey questions using SPSS V.23.
The qualitative interviews were recorded and transcribed. These qualitative data were thematically coded using NVivo version 11.1.1 (1707) by the lead author (L.M. Rieb). In analyzing these qualitative data, both deductive and inductive approaches were used,19 beginning with themes from the survey and expanding with themes that emerged from the interviews.
3. Results
During the study period, 58 people completed screening, including 31 by interview. Among these participants, there were 47 who met criteria for WISP. The average age was 46 years (IQR: 20). Thirty-four (72%) of these participants went on to complete the full descriptive survey (23 by interview). Among those interviewed, 21 answered questions beyond the survey and are included in the qualitative analysis (2 were excluded for fatigue and confusion with open-ended questions).
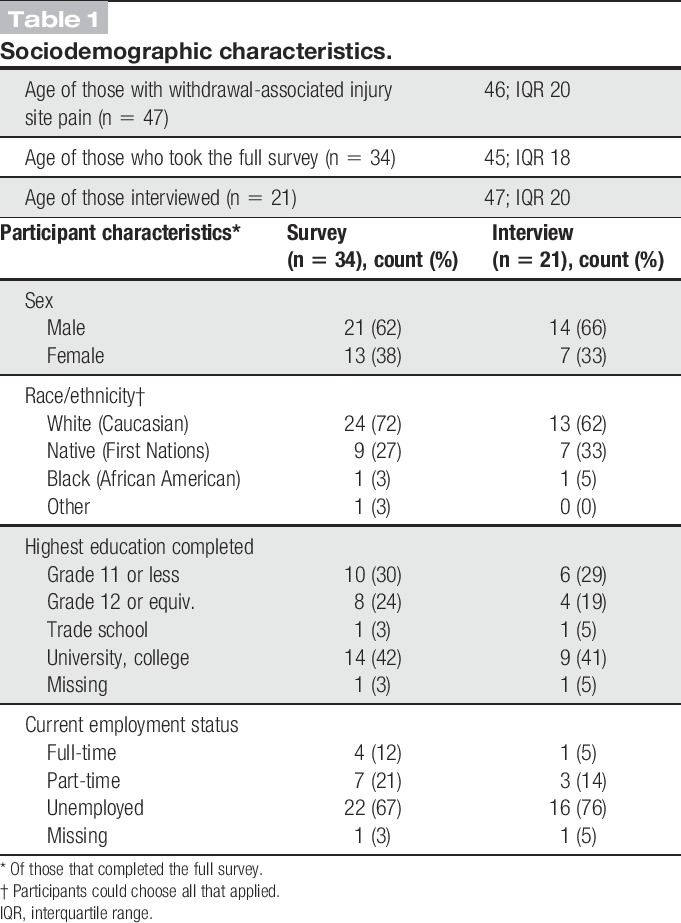
The sociodemographic data of those who completed the full descriptive survey (along with the subset interviewed) are outlined in Table 1. For the survey, the median participant age was 45 years (IQR: 18). Most participants were male, white, unemployed, with a grade 12 education or higher. Most were recruited from an inner-city research office, followed by primary care clinics that offered methadone programs, and several from residential treatment facilities and pain clinics in British Columbia, Canada. All of the interviews took place in Vancouver. Those interviewed had similar age and sociodemographic characteristics as the overall sample.
Table 1.
Sociodemographic characteristics.
Interspersed below with the quantitative data are participant quotes from the themes that emerged during the qualitative interviews.
3.1. Bodily experiences of withdrawal-associated injury site pain
Although there was a range of experiences reported, most participants found WISP to be intense and aversive both physically and emotionally. As one participant noted, “Oh God, I was in hell.”
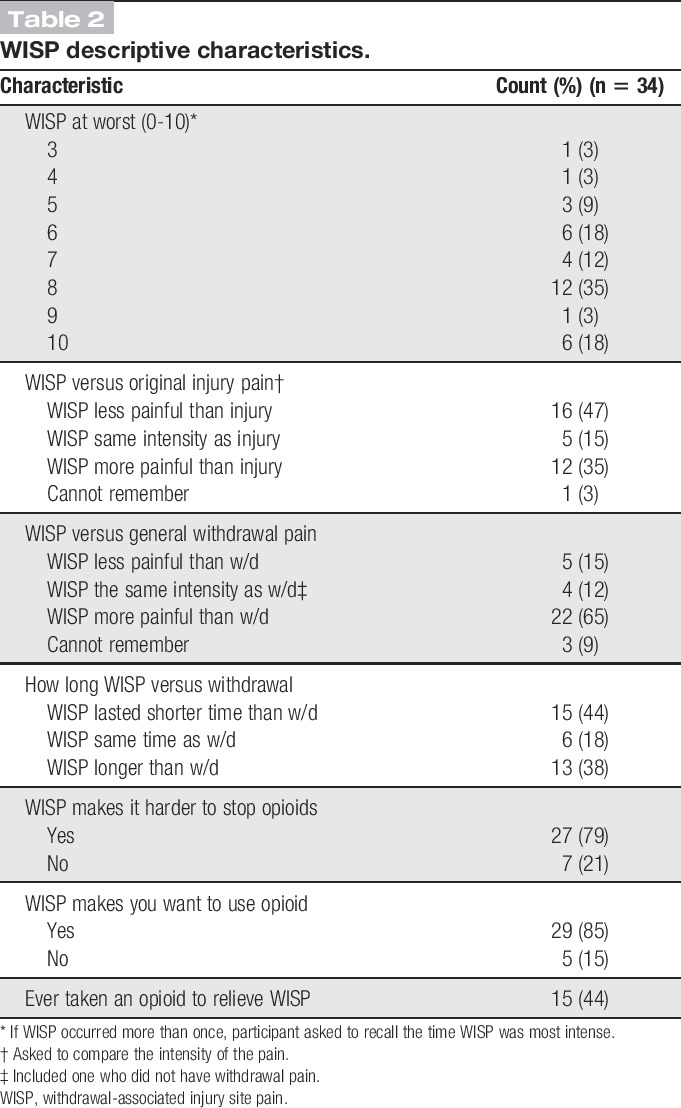
The surveyed descriptive characteristics of the experience of WISP are outlined in Table 2. Most participants recalled WISP as being severely painful: The most commonly recalled pain score for WISP on an 11-point Likert scale (0-10) was 8 (IQR: 2). When asked to compare intensity of pain, WISP was recalled by most participants (22; 65%) as being more painful than their generalized withdrawal pain, and as the same or less painful than the original injury, although 13 (38%) felt WISP was even more painful than their original injury and was often compared with their original injury in quality. For example, one participant remarked on WISP being like a “flashback of the original injury”:
“God, it felt just like it did when it was healing when it was broken, yeah. I don't know how—any other way to describe it.” Participant #2, 53 year-old white male, original injury—fractured arm at age 12
Table 2.
WISP descriptive characteristics.
There was often a distinction made between WISP and generalized withdrawal pain:
“I was pounding my legs…old injury sites are horrendous. So, like it's more severe in those spots. The other part you can like go, get through with a hot cloth, or whatever, with Gravol and stuff, but old injury sites come back with like, severe severity.” Participant #17, 58 year old Indigenous female, original injury—foot fractures requiring plating and lower leg injuries requiring fasciotomies after a home invasion, capture, and repeated assault with a hammer.
In the interviews, some participants described potential inflammatory and neuropathic symptoms of WISP. For example:
“It's just almost like a shooting up the back of my leg, combined with pressure in that area, as well as, you know, I could feel my skin stretching and the sensitivity to touch was increased.” Participant # 5, 35 year old white male, original injury—right ankle tendon tear requiring casting.
The above engineer also reported swelling of his right (but not left) ankle during opioid withdrawal. In a variation on this theme, another participant endorsed always feeling stiffness along with pain at his old healed injury site:
“Yeah, it was restricted motion… I think the texture, the back of my wrist, I think it became a bit woody and … I deliberately went through wrist stretching exercises…[to get my] wrist flexibility back.”—Participant #1, 62 year-old white male physician, original injury—soft tissue inflammation and infection in the dorsum of his left wrist from injecting fentanyl.
This participant felt WISP and the associated stiffness were postacute withdrawal phenomena, which occurred temporarily as withdrawal faded. However, all others interviewed reported that WISP began during the time of other withdrawal features, but could extend longer. There was one participant who had no other opioid withdrawal symptom but pain at his old injury site.
Typically, participants reported that the contralateral area to the injury site did not hurt in withdrawal or did so in a manner that was “much, much less, not even notable.” In this regard, the person acted as their own control, indicating WISP as somehow distinct from generalized withdrawal pain.
On average, participants noted it took about 2 weeks (median: 14; IQR: 24; range 1-70, with outliers at 120 and 365 days) for WISP to resolve after stopping opioids. By 30 days, WISP was finished in 28 (82%) of participants, although for 6 (18%) it lasted longer than a month.
3.2. Emotional aspects of withdrawal-associated injury site pain
Participants spoke of the “emotional pain” of opioid withdrawal, in general, and of WISP, in particular, during which the trauma or emotional distress associated with the original injury could be re-experienced. For example:
“There's also not just physical pain… I was run over by a semi so I suffered some physical injuries that come up in withdrawal, but also there's anxiety from it too…It's like PTSD from that big time”—Participant #8, 38 year old white male with previous multiple bilateral lower leg and foot fractures after being struck and pulled underneath a semi-trailer.
3.3. Withdrawal-associated injury site pain affect on opioid use behavior
Twenty-seven survey participants (79%) felt that having WISP made it harder to come off opioids, and 29 (86%) reported that having WISP made them want to take an opioid to relieve the pain. From the interviews, it was clear that many of our participants had stopped opioids multiple times, and 15 (44%) of those surveyed reported having taken an opioid to relieve WISP during one of their attempts at detoxification. However, for a few interviewees, having WISP “…made me glad that I stopped taking opiates.”
3.4. Mitigators of withdrawal-associated injury site pain
There were 19/34 (56%) participants who could recall taking one or more nonopioid medications or substances that helped relieve WISP. Most of them (17 [89%]) listed nonsteroidal anti-inflammatory drugs (NSAIDs), most frequent to least: ibuprofen, naproxen, and ketorolac. Six (32%) mentioned acetaminophen, and 3 (16%) listed either gabapentin or pregabalin. One person each named ketamine, phenobarbital, cyclobenzaprine, alcohol, cannabis, and a topical herbal remedy containing menthol and camphor. Several commented that opioid rotation to buprenorphine before tapering lessened WISP. One felt rotation to methadone before detoxification was helpful and another that methadone maintenance suppressed WISP; however, several participants remarked that methadone maintenance was the hardest opioid use to come off of in terms of WISP and general withdrawal symptoms. A few participants found that calming techniques assisted in lowering WISP.
Half of those surveyed believed that if they had been told by a health care provider about WISP they would have “absolutely” had more courage to get through the opioid cessation process.
3.5. Original injury characteristics
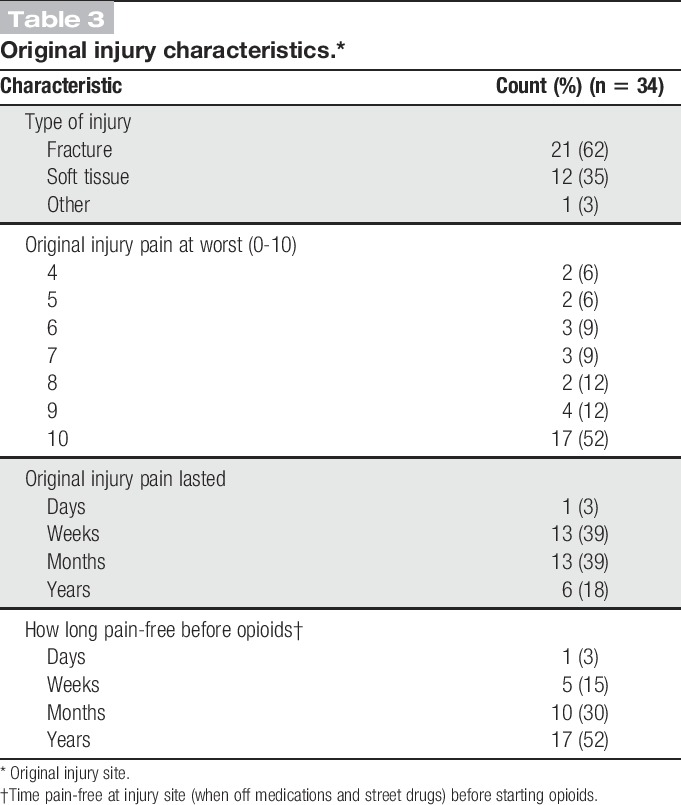
Characteristics of the original injury reported by participants are listed in Table 3. Participants reported their original injury usually as having occurred many years before the survey (median: 17; IQR: 14.5). Fracture was the most commonly reported type of injury (21 [62%] of cases) at times involving instrumentation or subsequent infection, followed by soft tissue injuries (abscesses, strain/sprains, blunt trauma, incision site pain), and one case of dislocation. Most participants rated the original injury pain as severe: On an 11-point Likert scale (0-10), the median pain was 10 (IQR: 3). The original injury pain typically lasted for weeks or months. Participants reported that they had a long pain-free span lasting months or, more commonly, years between their injury and their initiation of opioids (for a separate issue). The median time between the original injury and having WISP was 7 years (IQR: 14).
Table 3.
Original injury characteristics.*
3.6. Opioid withdrawal characteristics
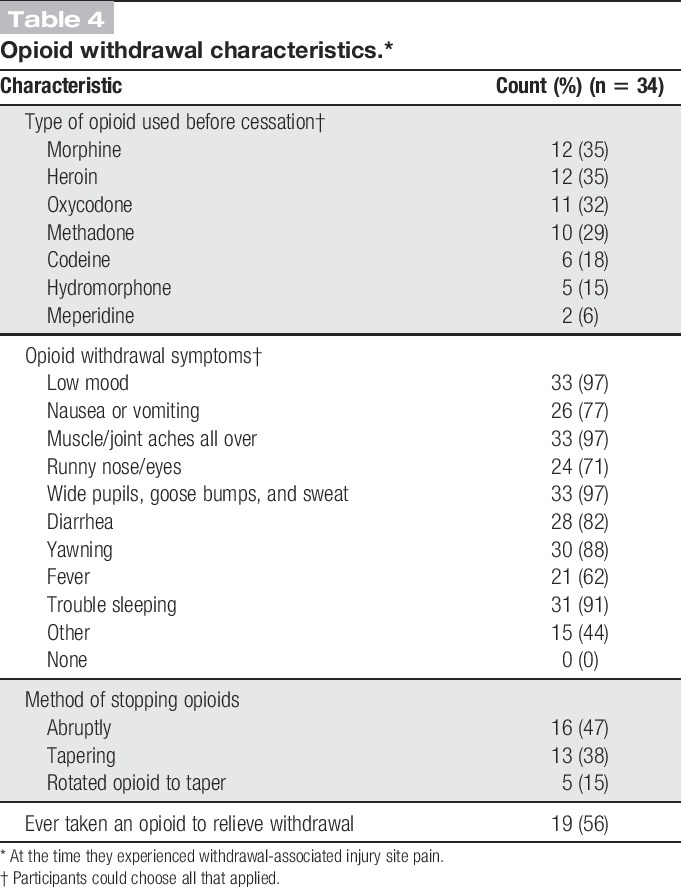
Characteristics of the withdrawal syndrome are listed in Table 4. The most commonly used opioids before cessation and experiencing WISP were morphine, heroin, oxycodone, and methadone. Most participants surveyed had stopped their opioid use abruptly. Most participants recalled significant opioid withdrawal symptoms which lasted on average 2 weeks (median: 13 days; IQR: 25; range 2-80 with an outlier at 143). By 30 days, 27 (79%) of the participants were over withdrawal. Nineteen (56%) survey participants reported restarting opioids to make generalized withdrawal symptoms go away during one of the often many attempts at detoxification.
Table 4.
Opioid withdrawal characteristics.*
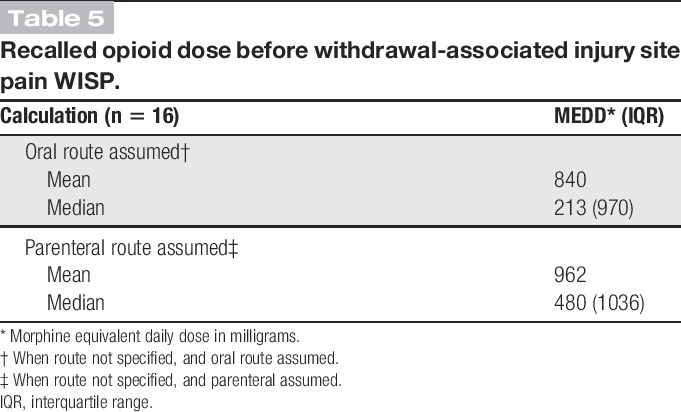
Sixteen of those interviewed recalled the doses of the opioids used just before stopping and getting WISP. These were converted to morphine equivalent daily dose (MEDD) and averaged (Table 5). These high doses are conservative calculations because we did not include additional opioids used concurrently if the amount consumed was not recalled.
Table 5.
Recalled opioid dose before withdrawal-associated injury site pain WISP.
3.7. Theories about the origin of withdrawal-associated injury site pain
Participants tried to conceptualize WISP within the context of their lived experience. Withdrawal-associated injury site pain was characterized as a “mystery” by some participants. Furthermore, although there were those who expressed that it was “all part of the drug withdrawal,” there were others who reported that they thought they had developed a new disorder, like “arthritis.” Still other participants believed that they had improper healing of the original injury, with one participant noting, “I don't think it healed right.”
One participant combined the view of a possible underlying injury with lack of endogenous opioids during withdrawal being the cause of WISP:
“I think [WISP occurs] because lack of my own body producing a pain killer. That it's just sensitive due to the injury… And when I'm in withdrawal my body's way too sensitive and there's pain there that's not being handled, right… [Then] my body kicks in its own morphine to cover up because it helps with the, I don't know, the tolerance and the damage that's done there.” Participant # 8, as above.
A number of interviewees felt that WISP “might be psychological.” Another took this concept further and spoke of it as a “ghost pain.” A more elaborate version of this theme was the concept of the brain trying to play a trick on the participant as part of drug craving:
“I thought, okay, it's such a strong pull to do the drugs that my brain figured out that because I started taking opiates when I sprained my ankle, it's going to start kicking the pain out at the ankle to get more opiates…because my brain was subconsciously craving it… the primal part of my brain, it still wants to communicate. And it communicates in basic level, right. So it's going to be pain, pleasure, pain, pleasure, right… I honestly do believe it's a form of communication between the primal part of your brain and your pre-frontal cortex.” Participant # 5, as above.
Among those interviewed, a few participants experienced with multiple withdrawal episodes suggested that it was opioid use itself that not only produced WISP but after a while could cause their old healed injury sites to hurt with use:
“In hindsight… I never correlated oxy use to actually producing pain. And in my experience, that's pretty much what happened. Addiction promoted pain.” Participant #10, 34 year-old white female, original injury—minor right knee twist playing basketball in junior high school.
There was one participant who later had WISP, who identified that opioid use at the time of his original injury had also seemed to increase his pain:
“When I got beaten up, when I broke my tailbone I started using Tylenol 3s and stuff like that and then it increased it instead. That was weird.” Participant #18, 39 year-old Indigenous female, original injury—coccyx fracture from being kicked.
3.8. Current opioid use
At the time of the survey, 21 (61%) participants were no longer taking opioids, and the rest reported one or more of the following: Eleven (32%) were in a methadone or buprenorphine/naloxone maintenance program, 9 (27%) were using opioids because of addiction, 7 (21%) were using opioids for pain at a different site, and notably 6 (18%) were taking opioids for pain at the old healed injury site that they knew could be pain-free after opioid cessation.
4. Discussion
From the descriptions provided by our participants, the first clinical picture of WISP has been drawn. For those who have had a previously healed injury that was pain-free when daily opioids began, injury site pain can return with a vengeance upon opioid cessation but vanish like a wisp sometime after withdrawal is over, an experience that can influence opioid use behavior.
Participants on average rated the intensity of WISP as severe (8/10), almost as painful as their original injury (10/10), and more painful than their generalized withdrawal pain. Also some participants interviewed described neuropathic and inflammatory symptoms accompanying WISP. The severity of WISP and of the original injury pain is interesting because there is evidence that an intense barrage of pain signaling from the periphery, especially if it involves tissue inflammation, infection, or damage (as with our subjects), can alter areas of the central nervous system to become more pain sensitive, a contributor to central sensitization.12,64,117,140–142
Opioid use can also cause or worsen neuroimmune and neuroinflammatory responses and add to central sensitization through the release of prostaglandins, chemokines, and cytokines (eg, tumor necrosis factor, substance P, interleukin 2), including those from microglial cells after toll-like receptor 4 stimulation.6,39,60,68,73,80,112,133,134 Along with opioid, neurokinin 1, and toll-like receptor 4 antagonists, NSAIDS and gabapentinoids have been found to reduce neuroinflammation and mitigate OIH and/or WIH in some1,5,14,18,34,45,46,61,70,83,85,94,100,121,127,135,137 but not all50 circumstances. In our study, a subset of participants named NSAIDs most frequently and gabapentinoids third for relieving WISP, supporting a possible neuroinflammatory component.
Additional aspects of central sensitization that have been implicated in OIH and WIH involve changes in ascending and descending central pathways (including the dorsal horn, rostral ventromedial medulla, raphe nucleus, and somatosensory cortex) and involve gamma-Aminobutyric acid, dynorphin, cyclic adenosine monophosphate, protein kinase, glutamate, N-methyl-D-aspartate receptors, and other components.13,15,16,22,25,26,39,43,58,86,92,93,98,128,129,145 Among our participants, just one person each named ketamine, phenobarbital, alcohol, and cannabis as relieving WISP. Although these may be coincidental, there is evidence that these or related substances may mitigate OIH, WIH, or other forms of pain through modulation of one of the above mechanisms, some of which are also involved with gabapentinoids.6,74,94,111,113,116
An opioid overuse pain syndrome has been previously proposed that incorporates physical and emotional components driving opioid use.89 In the narratives reported here, physical pain and emotional suffering were intertwined during opioid withdrawal and contributed to the aversive nature of the experience of WISP. Future research may determine if WISP and potential injury site swelling may in part be caused by adrenoceptor stimulation from catecholamine release during opioid withdrawal. This mechanism has been implicated in WIH.15 Sympathetic outflow, including anxiety states can modulate neuroinflammation and pain including joint swelling.106,118 Norepinephrine can also induce glial cells to release proinflammatory cytokines.60,68 Clinically, both propranolol and clonidine have been shown to decrease OIH.28,81
Thus, it is speculated that OIH and WIH may play key roles in WISP: As pain sensitivity increases due to opioid use, and then further during withdrawal, residual underlying sensitivity from the injury (either peripheral or central) may be uncovered, exacerbated by sympathetic outflow, including anxiety from pain.
Withdrawal-associated injury site pain was typically recalled to last about 2 weeks, similar to the timing of withdrawal. By 1 month, WISP was resolved in 82% of participants. Previous documentation has shown that the nervous system takes time to reset after prolonged opioid exposure.95 Previous heroin users, those withdrawn from methadone, and patients with chronic low back pain withdrawn from prescription opioids have all been shown to have abnormal heat or cold pain perception for somewhere between one and 5 months after opioid cessation.108,109,126,132 Opioid rotation before taper was reported by a few of our participants to be helpful. Unfortunately, opioid substitution therapy has not always been shown to improve OIH in heroin users and may instead worsen it,33 although low dose methadone may assist in OIH prevention35 and has been shown to allow pain control in cancer patients unresponsive to rapid escalation of other opioids.96
Our group reported extensive generalized withdrawal symptoms with opioid cessation. Two other studies in CNCP patients did not find “significant” withdrawal symptoms after opioid cessation, measuring on average 4 on the Clinical Opiate Withdrawal Scale (COWS), yet their participants had WIH to cold or heat pain.66,144 One explanation for the discrepancy may be that a low COWS score still may be recalled as withdrawal. Other explanations may include study population differences in initial opioid dose, duration of opioid use, rate of opioid cessation, presence of opioid rotation, other medications used during tapering, and number of times detoxified—all of which have been shown to influence OIH or WIH.14,24,30,44,47,66,74,104,124,131
Comparatively low opioid doses, along with taper instead of abrupt cessation may in part explain why a number of studies have reported a lack of temporary CNCP pain escalation (or even improvement in pain) immediately after opioid cessation,11,82,102,125,131 although their dropouts may have had a different experience. Also, it is unknown in the other studies if the original source of CNCP was injury.
This study further outlined participant theories regarding the etiology of WISP. Attribution of WISP to normal withdrawal, or speculation about re-injury was predicted. However, the idea that the opioid-dependent brain is sending a pain signal to try to trick the person to take more opioids was an unexpected finding. Certainly, craving is a risk factor for relapse.138 Also evidence exists that pain modulates dopamine release from the mesolimbic system.123 However, the concept of drug craving triggering pain has not been previously well documented. This brings up the possibility that beyond physiologic dependence, addiction may be a driver for WISP. Also, opioid-induced glial cell activation can activate systems responsible for drug reward and dependence.31,68,69,71,101
Some participants had insight that taking opioids may cause or contribute to WISP, and involve endogenous pain systems. Perhaps if we had not recruited specifically for those who only had injury site pain during withdrawal, we may have had more narratives demonstrating a potential link between OIH and WISP.
The vast majority of our participants felt that having WISP made it harder to come off opioids, and made them want to use an opioid again, which 44% did during one of multiple detoxification episodes. Although at the time of the survey most participants were off opioids, it is poignant that almost one-fifth of the participants had restarted opioids to relieve pain at their old healed injury site — the one they knew could be pain-free once they came off. Thus, by implication, they were taking opioids chronically to relieve WISP (although new pathology is possible). In general, for those with OUDs pain may be a risk factor for continued substance use,40,63,107,119 yet the source of the pain is rarely differentiated,40,78,115 so it is unknown if injury site pain was playing a role in previous study populations.
There are limitations to our study. First, it was not designed to measure prevalence. Also, pain intensity and duration may be subject to recall bias, although reliable and valid in some circumstances.20,37,120 Technical barriers to online survey self-completion became apparent so the interview process was expanded. The principal investigator did all of the interviews and coding so there is a possibility of investigator and interview bias.143 Information on cigarette smoking status and alcohol cessation were not elicited yet may influence the success of opioid elimination67 and add to pain during withdrawal.7,72 We did not question psychological status, which can affect WIH23 and alter recalled acute pain intensity59 and opioid utilization48,49 as well as affect relapse and dropout rates when stopping opioids.63,77,88 We did not look at genetic, sex, and hormonal differences, all of which can impact OIH.75,76,87,136 Finally, we did not recruit controls with no previous significant injury, although many of our participants acted as their own control (injury-free on the contralateral side).
This research represents the first known documentation that previously healed, and pain-free injury sites can temporarily become painful again when stopping opioids. This experience seems to be a barrier to opioid cessation and raises an important question. Are there patients labeled with CNCP who in fact have WISP? Given the findings in this study, a prospective observational cohort study could document WISP incidence, mitigating factors, influence on opioid detoxification, as well as correlation with OIH and WIH. Ultimately, a randomized controlled trial of treatments for WISP could be undertaken to reduce suffering in those individuals attempting to discontinue opioids.
Conflict of interest Statement
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank the study participants for their contributions to the research, as well as current and past researchers and staff. We would specifically like to thank Dr. Michael Klein for conceptual support of the original study design; Dr. Brittany Dennis for her review of the manuscript; and Dr. Kathy Hornby, Jane Liu, and Victor Trot for their research assistance. Dr. L. M. Rieb received funding through the Clinical Scholars Program, Department of Family Practice, University of British Columbia, the College of Family Physicians of BC, and the US National Institutes of Health via a National Institute of Drug Abuse (NIDA) sponsored Canadian Addiction Medicine Research Fellowship through St. Paul's Hospital (R25 DA037756-02).
Dr. W. V. Norman is supported with a Chair in Applied Public Health Research from the Canadian Institutes for Health Research and the Public Health Agency of Canada, and as a Scholar of the Michael Smith Foundation for Health Research. Dr. E. Wood is supported in part by a Tier 1 Canada Research Chair in Inner-City Medicine award. Dr. M.-J. Milloy is supported in part by the United States National Institutes of Health (R01-DA051525). Drs. M.-J. Milloy and R. McNeil are supported by Scholar Awards from the Michael Smith Foundation for Health Research and New Investigator Awards from the Canadian Institutes of Health Research. The University of British Columbia has received an unstructured gift from NG Biomed to support Dr. M.-J. Milloy's research. These funders had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A341.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Akbari E. The role of cyclo-oxygenase inhibitors in attenuating opioid-induced tolerance, hyperalgesia, and dependence. Med Hypotheses 2012;78:102–6. [DOI] [PubMed] [Google Scholar]
- [2].Albutt C. On the abuse of hypodermic injections of morphia. Practitioner 1870;3:327–30. [Google Scholar]
- [3].Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006;104:570–87. [DOI] [PubMed] [Google Scholar]
- [4].Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. PAIN 2003;106:49–57. [DOI] [PubMed] [Google Scholar]
- [5].Arout CA, Caldwell M, McCloskey DP, Kest B. C-Fos activation in the periaqueductal gray following acute morphine-3beta-D-glucuronide or morphine administration. Physiol Behav 2014;130:28–33. [DOI] [PubMed] [Google Scholar]
- [6].Arout CA, Edens E, Petrakis IL, Sofuoglu M. Targeting opioid-induced hyperalgesia in clinical treatment: neurobiological considerations. CNS Drugs 2015;29:465–86. [DOI] [PubMed] [Google Scholar]
- [7].Baiamonte BA, Valenza M, Roltsch EA, Whitaker AM, Baynes BB, Sabino V, Gilpin NW. Nicotine dependence produces hyperalgesia: role of corticotropin-releasing factor-1 receptors (CRF1Rs) in the central amygdala (CeA). Neuropharmacology 2014;77:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ballantyne JC. Opioid therapy in chronic pain. Phys Med Rehabil Clin N Am 2015;26:201–18. [DOI] [PubMed] [Google Scholar]
- [9].Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med 2012;172:1342–3. [DOI] [PubMed] [Google Scholar]
- [10].Bannister K. Opioid-induced hyperalgesia: where are we now? Curr Opin Support Palliat Care 2015;9:116–21. [DOI] [PubMed] [Google Scholar]
- [11].Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag 2006;2:277–82. [DOI] [PubMed] [Google Scholar]
- [12].Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol 2013;74:630–6. [DOI] [PubMed] [Google Scholar]
- [13].Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res 1990;7:185–203. [DOI] [PubMed] [Google Scholar]
- [14].Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clinic Proc 2015;90:828–42. [DOI] [PubMed] [Google Scholar]
- [15].Bie B, Fields HL, Williams JT, Pan ZZ. Roles of alpha1- and alpha2-adrenoceptors in the nucleus raphe magnus in opioid analgesia and opioid abstinence-induced hyperalgesia. J Neurosci 2003;23:7950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bie B, Peng Y, Zhang Y, Pan ZZ. cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci 2005;25:3824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blondell RD, Ashrafioun L, Dambra CM, Foschio EM, Zielinski AL, Salcedo DM. A clinical trial comparing tapering doses of buprenorphine with steady doses for chronic pain and coexistent opioid addiction. J Addict Med 2010;4:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bornemann-Cimenti H, Lederer AJ, Wejbora M, Michaeli K, Kern-Pirsch C, Archan S, Rumpold-Seitlinger G, Zigeuner R, Sandner-Kiesling A. Preoperative pregabalin administration significantly reduces postoperative opioid consumption and mechanical hyperalgesia after transperitoneal nephrectomy. Br J Anaesth 2012;108:845–9. [DOI] [PubMed] [Google Scholar]
- [19].Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. PAIN 2008;139:146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brush DE. Complications of long-term opioid therapy for management of chronic pain: the paradox of opioid-induced hyperalgesia. J Med Toxicol 2012;8:387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. PAIN 1988;32:89–94. [DOI] [PubMed] [Google Scholar]
- [23].Carcoba LM, Contreras AE, Cepeda-Benito A, Meagher MW. Negative affect heightens opiate withdrawal-induced hyperalgesia in heroin dependent individuals. J Addict Dis 2011;30:258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci 2001;21:4074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Celerier E, Laulin JP, Larcher A, Le Moal M, Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res 1999;847:18–25. [DOI] [PubMed] [Google Scholar]
- [26].Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci 2010;30:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med 2015;162:276–86. [DOI] [PubMed] [Google Scholar]
- [28].Chu LF, Cun T, Ngai LK, Kim JE, Zamora AK, Young CA, Angst MS, Clark DJ. Modulation of remifentanil-induced postinfusion hyperalgesia by the beta-blocker propranolol in humans. PAIN 2012;153:974–81. [DOI] [PubMed] [Google Scholar]
- [29].Chu LF, D'Arcy N, Brady C, Zamora AK, Young CA, Kim JE, Clemenson AM, Angst MS, Clark JD. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. PAIN 2012;153:1583–92. [DOI] [PubMed] [Google Scholar]
- [30].Cohen SP, Christo PJ, Wang S, Chen L, Stojanovic MP, Shields CH, Brummett C, Mao J. The effect of opioid dose and treatment duration on the perception of a painful standardized clinical stimulus. Reg Anesth Pain Med 2008;33:199–206. [DOI] [PubMed] [Google Scholar]
- [31].Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 2012;134:219–45. [DOI] [PubMed] [Google Scholar]
- [32].Compton MA. Cold-presser pain tolerance in opiate and cocaine abusers — correlates of drug type and use status. J Pain Symptom Manage 1994;9:462–73. [DOI] [PubMed] [Google Scholar]
- [33].Compton P, Canamar CP, Hillhouse M, Ling W. Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. J Pain 2012;13:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Compton P, Kehoe P, Sinha K, Torrington MA, Ling W. Gabapentin improves cold-pressor pain responses in methadone-maintained patients. Drug Alcohol Depend 2010;109:213–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Craig DS. Very-low-dose methadone for the prevention of opioid hyperalgesia. J Palliat Med 2013;16:1172–3. [DOI] [PubMed] [Google Scholar]
- [36].Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. Thousand Oaks: SAGE Publications, 2011. [Google Scholar]
- [37].Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend 1998;51:253–63; discussion 267-258. [DOI] [PubMed] [Google Scholar]
- [38].Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012;379:55–70. [DOI] [PubMed] [Google Scholar]
- [39].DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist 2004;10:40–52. [DOI] [PubMed] [Google Scholar]
- [40].Dennis BB, Bawor M, Naji L, Chan CK, Varenbut J, Paul J, Varenbut M, Daiter J, Plater C, Pare G, Marsh DC, Worster A, Desai D, Thabane L, Samaan Z. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse 2015;9:59–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. PAIN 2001;90:91–6. [DOI] [PubMed] [Google Scholar]
- [42].Dowell D, Haegerich TM, Chou R. Cdc guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- [43].Dunbar SA, Karamian I, Roberts L, Zhang J. Increased prostaglandin E2 release and activated Akt/beta-catenin signaling pathway occur after opioid withdrawal in rat spinal cord. Anesthesiology 2006;105:154–9. [DOI] [PubMed] [Google Scholar]
- [44].Dunbar SA, Karamian I, Yeatman A, Zhang J. Effects of recurrent withdrawal on spinal GABA release during chronic morphine infusion in the rat. Eur J Pharmacol 2006;535:152–6. [DOI] [PubMed] [Google Scholar]
- [45].Dunbar SA, Karamian I, Zhang HH. Ketorolac prevents recurrent withdrawal induced hyperalgesia but does not inhibit tolerance to spinal morphine in the rat. Eur J Pain 2007;11:1–6. [DOI] [PubMed] [Google Scholar]
- [46].Dunbar SA, Karamov IG, Buerkle H. The effect of spinal ibuprofen on opioid withdrawal in the rat. Anesth Analg 2000;91:417–22. [DOI] [PubMed] [Google Scholar]
- [47].Dunbar SA, Pulai IJ. Repetitive opioid abstinence causes progressive hyperalgesia sensitive to N-methyl-D-aspartate receptor blockade in the rat. J Pharmacol Exp Ther 1998;284:678–86. [PubMed] [Google Scholar]
- [48].Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain 2010;26:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Edlund MJ, Martin BC, Fan MY, Braden JB, Devries A, Sullivan MD. An analysis of heavy utilizers of opioids for chronic noncancer pain in the TROUP study. J Pain Symptom Manage 2010;40:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Eisenach JC, Tong CY, Curry RS. Failure of intrathecal ketorolac to reduce remifentanil-induced postinfusion hyperalgesia in humans. PAIN 2015;156:81–7. [DOI] [PubMed] [Google Scholar]
- [51].Eisenberg E, Suzan E, Pud D. Opioid-induced hyperalgesia (OIH): a real clinical problem or just an experimental phenomenon? J Pain Symptom Manage 2015;49:632–6. [DOI] [PubMed] [Google Scholar]
- [52].Esquibel AY, Borkan J. Doctors and patients in pain: conflict and collaboration in opioid prescription in primary care. PAIN 2014;155:2575–82. [DOI] [PubMed] [Google Scholar]
- [53].Farrell M. Opiate withdrawal. Addiction 1994;89:1471–5. [DOI] [PubMed] [Google Scholar]
- [54].Flesch R. A new readability yardstick. J Appl Psychol 1948;32:221–33. [DOI] [PubMed] [Google Scholar]
- [55].Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth 2014;112:991–1004. [DOI] [PubMed] [Google Scholar]
- [56].Franklin GM. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2014;83:1277–84. [DOI] [PubMed] [Google Scholar]
- [57].Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 2006;174:1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci 2002;22:6747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gedney JJ, Logan H, Baron RS. Predictors of short-term and long-term memory of sensory and affective dimensions of pain. J Pain 2003;4:47–55. [DOI] [PubMed] [Google Scholar]
- [60].Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gustorff B, Kozek-Langenecker S, Kress HG. Gabapentin: the first preemptive anti-hyperalgesic for opioid withdrawal hyperalgesia? Anesthesiology 2003;98:1520–1; author reply 1521–1522. [DOI] [PubMed] [Google Scholar]
- [62].Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ, Rounsefell B. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain 2009;10:316–22. [DOI] [PubMed] [Google Scholar]
- [63].Heiwe S, Lonnquist I, Kallmen H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain 2011;15:966–70. [DOI] [PubMed] [Google Scholar]
- [64].Hoheisel U, Mense S. Inflammation of the thoracolumbar fascia excites and sensitizes rat dorsal horn neurons. Eur J Pain 2015;19:419–28. [DOI] [PubMed] [Google Scholar]
- [65].Hooten WM, Lamer TJ, Twyner C. Opioid-induced hyperalgesia in community-dwelling adults with chronic pain. PAIN 2015;156:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hooten WM, Mantilla CB, Sandroni P, Townsend CO. Associations between heat pain perception and opioid dose among patients with chronic pain undergoing opioid tapering. Pain Med 2010;11:1587–98. [DOI] [PubMed] [Google Scholar]
- [67].Hooten WM, Townsend CO, Bruce BK, Warner DO. The effects of smoking status on opioid tapering among patients with chronic pain. Anesth Analg 2009;108:308–15. [DOI] [PubMed] [Google Scholar]
- [68].Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 2007;7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 2012;32:11187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci 2008;28:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jacobsen JH, Watkins LR, Hutchinson MR. Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. Int Rev Neurobiol 2014;118:129–63. [DOI] [PubMed] [Google Scholar]
- [72].Jochum T, Boettger MK, Burkhardt C, Juckel G, Bar KJ. Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain 2010;14:713–18. [DOI] [PubMed] [Google Scholar]
- [73].Johnson JL, Hutchinson MR, Williams DB, Rolan P. Medication-overuse headache and opioid-induced hyperalgesia: a review of mechanisms, a neuroimmune hypothesis and a novel approach to treatment. Cephalalgia 2013;33:52–64. [DOI] [PubMed] [Google Scholar]
- [74].Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology 2005;103:147–55. [DOI] [PubMed] [Google Scholar]
- [75].Juni A, Cai M, Stankova M, Waxman AR, Arout C, Klein G, Dahan A, Hruby VJ, Mogil JS, Kest B. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology 2010;112:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Juni A, Klein G, Kowalczyk B, Ragnauth A, Kest B. Sex differences in hyperalgesia during morphine infusion: effect of gonadectomy and estrogen treatment. Neuropharmacology 2008;54:1264–70. [DOI] [PubMed] [Google Scholar]
- [77].Kanof PD, Aronson MJ, Ness R. Organic mood syndrome associated with detoxification from methadone-maintenance. Am J Psychiatry 1993;150:423–8. [DOI] [PubMed] [Google Scholar]
- [78].Karasz A, Zallman L, Berg K, Gourevitch M, Selwyn P, Arnsten JH. The experience of chronic severe pain in patients undergoing methadone maintenance treatment. J Pain Symptom Manage 2004;28:517–25. (Erratum appears in J Pain Symptom Manage. 2004 Dec;28:626 Note: Arnstein, Julia [corrected to Arnsten, Julia H]). [DOI] [PubMed] [Google Scholar]
- [79].Kincaid JP, Fishburne JRP, Rogers RL, Chissom BS, Naval technical training command Millington Tn research B. Derivation of new readability formulas (automated readability index, fog count and flesch reading ease formula) for navy enlisted personnel. 1975. Available at: http://www.dtic.mil/dtic/tr/fulltext/u2/a006655.pdf. Accessed November 1, 2015.
- [80].King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. PAIN 2005;116:276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schuttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology 2003;99:152–9. [DOI] [PubMed] [Google Scholar]
- [82].Krumova EK, Bennemann P, Kindler D, Schwarzer A, Zenz M, Maier C. Low pain intensity after opioid withdrawal as a first step of a comprehensive pain rehabilitation program predicts long-term nonuse of opioids in chronic noncancer pain. Clin J Pain 2013;29:760–9. [DOI] [PubMed] [Google Scholar]
- [83].Lee C, Lee HW, Kim JN. Effect of oral pregabalin on opioid-induced hyperalgesia in patients undergoing laparo-endoscopic single-site urologic surgery. Korean J Anesthesiol 2013;64:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lee M, Silverman S, Hansen H, Patel V, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011;14:145–61. [PubMed] [Google Scholar]
- [85].Li Q. Antagonists of toll like receptor 4 maybe a new strategy to counteract opioid-induced hyperalgesia and opioid tolerance. Med Hypotheses 2012;79:754–6. [DOI] [PubMed] [Google Scholar]
- [86].Li X, Clark JD. Hyperalgesia during opioid abstinence: mediation by glutamate and substance p. Anesth Analg 2002;95:979–84. [DOI] [PubMed] [Google Scholar]
- [87].Liang DY, Li X, Clark JD. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain 2013;14:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Liebmann PM, Lehofer M, Moser M, Legl T, Pernhaupt G, Schauenstein K. Nervousness and pain sensitivity: II. Changed relation in ex-addicts as a predictor for early relapse. Psychiatry Res 1998;79:55–8. [DOI] [PubMed] [Google Scholar]
- [89].Manchikanti L, Atluri S, Boswell MV, Hansen HC. Opioid overuse pain syndrome. J Opioid Manag 2014;10:81. [DOI] [PubMed] [Google Scholar]
- [90].Manchikanti L, Atluri S, Hansen H, Benyamin RM, Falco FJ, Helm Ii S, Kaye AD, Hirsch JA. Opioids in chronic noncancer pain: have we reached a boiling point yet? Pain Physician 2014;17:E1–10. [PubMed] [Google Scholar]
- [91].Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. PAIN 2002;100:213–17. [DOI] [PubMed] [Google Scholar]
- [92].Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep 2006;10:67–70. [DOI] [PubMed] [Google Scholar]
- [93].Mao JR, Sung BK, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 2002;22:8312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Martinez V, Cymerman A, Ben Ammar S, Fiaud JF, Rapon C, Poindessous F, Judet T, Chauvin M, Bouhassira D, Sessler D, Mazoit X, Fletcher D. The analgesic efficiency of combined pregabalin and ketamine for total hip arthroplasty: a randomised, double-blind, controlled study. Anaesthesia 2014;69:46–52. [DOI] [PubMed] [Google Scholar]
- [95].Mattick RP, Hall W. Are detoxification programmes effective? Lancet 1996;347:97–100. [DOI] [PubMed] [Google Scholar]
- [96].Mercadante S, Ferrera P, Arcuri E, Casuccio A. Opioid-induced hyperalgesia after rapid titration with intravenous morphine: switching and re-titration to intravenous methadone. Ann Palliat Med 2012;1:10–13. [DOI] [PubMed] [Google Scholar]
- [97].Modesto-Lowe V, Brooks D, Petry N. Methadone deaths: risk factors in pain and addicted populations. J Gen Intern Med 2010;25:305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Morgan MM, Heinricher MM, Fields HL. Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal-cord with rostral ventromedial medulla. Neuroscience 1992;47:863–71. [DOI] [PubMed] [Google Scholar]
- [99].Murphy Y, Goldner EM, Fischer B. Prescription opioid use, harms and interventions in Canada: a review update of new developments and findings since 2010. Pain Physician 2015;18:E605–614. [PubMed] [Google Scholar]
- [100].Narai Y, Imamachi N, Saito Y. Gabapentin augments the antihyperalgesic effects of diclofenac sodium through spinal action in a rat postoperative pain model. Anesth Analg 2012;115:189–93. [DOI] [PubMed] [Google Scholar]
- [101].Narita M, Suzuki M, Kuzumaki N, Miyatake M, Suzuki T. Implication of activated Astrocytes in the development of drug dependence differences between methamphetamine and morphine. Ann N Y Acad Sci 2008;2008:96–104. [DOI] [PubMed] [Google Scholar]
- [102].Nilsen HK, Stiles TC, Landro NI, Fors EA, Kaasa S, Borchgrevink PC. Patients with problematic opioid use can be weaned from codeine without pain escalation. Acta Anaesthesiol Scand 2010;54:571–9. [DOI] [PubMed] [Google Scholar]
- [103].Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010;CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nosyk B, Sun H, Evans E, Marsh DC, Anglin MD, Hser YI, Anis AH. Defining dosing pattern characteristics of successful tapers following methadone maintenance treatment: results from a population-based retrospective cohort study. Addiction 2012;107:1621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Pluye P, Hong QN. Combining the power of stories and the power of numbers: mixed methods research and mixed studies reviews. Annu Rev Public Health 2014;35:29–45. [DOI] [PubMed] [Google Scholar]
- [106].Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther 2014;16:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Potter JS, Chakrabarti A, Domier CP, Hillhouse MP, Weiss RD, Ling W. Pain and continued opioid use in individuals receiving buprenorphine-naloxone for opioid detoxification: secondary analyses from the clinical trials network. J Subst Abuse Treat 2010;38:S80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Prosser JM, Steinfeld M, Cohen LJ, Derbyshire S, Eisenberg DP, Cruciani RA, Galynker Abnormal heat and pain perception in remitted heroin dependence months after detoxification from methadone-maintenance. Drug Alcohol Depend 2008;95:237–44. [DOI] [PubMed] [Google Scholar]
- [109].Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: new evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend 2006;82:218–23. [DOI] [PubMed] [Google Scholar]
- [110].Quincey Td. Confessions of an English opium-eater and other writings. Oxford: Oxford University Press, 2013. [Google Scholar]
- [111].Quinlan J. The use of a subanesthetic infusion of intravenous ketamine to allow withdrawal of medically prescribed opioids in people with chronic pain, opioid tolerance and hyperalgesia: outcome at 6 months. Pain Med 2012;13:1524–5. [DOI] [PubMed] [Google Scholar]
- [112].Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci 2002;22:9980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ramasubbu C, Gupta A. Pharmacological treatment of opioid-induced hyperalgesia: a review of the evidence. J Pain Palliat Care Pharmacother 2011;25:219–30. [DOI] [PubMed] [Google Scholar]
- [114].Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA 2016;315:2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 2003;289:2370–8. [DOI] [PubMed] [Google Scholar]
- [116].Russo R, D'Agostino G, Mattace Raso G, Avagliano C, Cristiano C, Meli R, Calignano A. Central administration of oxytocin reduces hyperalgesia in mice: implication for cannabinoid and opioid systems. Peptides 2012;38:81–8. [DOI] [PubMed] [Google Scholar]
- [117].Rygh LJ, Svendsen F, Hole K, Tjolsen A. Natural noxious stimulation can induce long-term increase of spinal nociceptive responses. PAIN 1999;82:305–10. [DOI] [PubMed] [Google Scholar]
- [118].Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol 2012;88:1–25. [DOI] [PubMed] [Google Scholar]
- [119].Savage SR, Kirsh KL, Passik SD. Challenges in using opioids to treat pain in persons with substance use disorders. Addict Sci Clin Pract 2008;4:4–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sisk AL, Grover B, Steflik DE. Long-term memory of acute postsurgical pain. J Oral Maxillofac Surg 1991;49:353–8; discussion 358-359. [DOI] [PubMed] [Google Scholar]
- [121].Stoicea N, Russell D, Weidner G, Durda M, Joseph NC, Yu J, Bergese SD. Opioid-induced hyperalgesia in chronic pain patients and the mitigating effects of gabapentin. Front Pharmacol 2015;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. PAIN 2013;154(suppl 1):S94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Taylor AM, Becker S, Schweinhardt P, Cahill C. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. PAIN 2016;157:1194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tennant FS, Jr, Rawson RA. Outpatient treatment of prescription opioid dependence: comparison of two methods. Arch Intern Med 1982;142:1845–7. [PubMed] [Google Scholar]
- [125].Townsend CO, Kerkvliet JL, Bruce BK, Rome JD, Hooten WM, Luedtke CA, Hodgson JE. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. PAIN 2008;140:177–89. [DOI] [PubMed] [Google Scholar]
- [126].Treister R, Eisenberg E, Lawental E, Pud D. Is opioid-induced hyperalgesia reversible? A study on active and former opioid addicts and drug naive controls. J Opioid Manag 2012;8:343–9. [DOI] [PubMed] [Google Scholar]
- [127].Troster A, Sittl R, Singler B, Schmelz M, Schuttler J, Koppert W. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology 2006;105:1016–23. [DOI] [PubMed] [Google Scholar]
- [128].Vanderah TW, Ossipov MH, Lai J, Malan TP, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. PAIN 2001;92:5–9. [DOI] [PubMed] [Google Scholar]
- [129].Vera-Portocarrero LP, Ossipov MH, Lai J, King T, Porreca F. Descending facilitatory pathways from the rostroventromedial medulla mediate naloxone-precipitated withdrawal in morphine-dependent rats. J Pain 2011;12:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Volkow ND, McLellan AT. Opioid abuse in chronic pain – Misconceptions and mitigation strategies. New Engl J Med 2016;374:1253–63. [DOI] [PubMed] [Google Scholar]
- [131].Vorobeychik Y, Chen L, Bush MC, Mao J. Improved opioid analgesic effect following opioid dose reduction. Pain Med 2008;9:724–7. [Erratum appears in Pain Med. 2008 Nov;9:1228]. [DOI] [PubMed] [Google Scholar]
- [132].Wang H, Akbar M, Weinsheimer N, Gantz S, Schiltenwolf M. Longitudinal observation of changes in pain sensitivity during opioid tapering in patients with chronic low-back pain. Pain Med 2011;12:1720–6. [DOI] [PubMed] [Google Scholar]
- [133].Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A 2012;109:6325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun 2007;21:131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 2009;30:581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Waxman AR, Juni A, Kowalczyk W, Arout C, Sternberg WF, Kest B. Progesterone rapidly recruits female-typical opioid-induced hyperalgesic mechanisms. Physiol Behav 2010;101:759–63. [DOI] [PubMed] [Google Scholar]
- [137].Wei X, Wei W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J Anesth 2012;26:236–41. [DOI] [PubMed] [Google Scholar]
- [138].Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 2005;5:9–19. [DOI] [PubMed] [Google Scholar]
- [139].White JM. Pleasure into pain: the consequences of long-term opioid use. Addict Behav 2004;29:1311–24. [DOI] [PubMed] [Google Scholar]
- [140].Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–8. [DOI] [PubMed] [Google Scholar]
- [141].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152:S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–8. [DOI] [PubMed] [Google Scholar]
- [143].Wynder EL. Investigator bias and interviewer bias: the problem of reporting systematic error in epidemiology. J Clin Epidemiol 1994;47:825–7. [DOI] [PubMed] [Google Scholar]
- [144].Younger J, Barelka P, Carroll I, Kaplan K, Chu L, Prasad R, Gaeta R, Mackey S. Reduced cold pain tolerance in chronic pain patients following opioid detoxification. Pain Med 2008;9:1158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zhao YL, Chen SR, Chen H, Pan HL. Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-D-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J Biol Chem 2012;287:25073–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


