Supplemental Digital Content is Available in the Text.
Key Words: perinatal HIV transmission, PMTCT, patient satisfaction, provider satisfaction, implementation science, task shifting
Abstract
Background:
High mother-to-child HIV transmission rates in Nigeria are coupled with a critical shortage of trained health personnel, dearth of infrastructure, and low levels of male involvement in HIV care. This study evaluated maternal and provider satisfaction with services for prevention of mother-to-child transmission within the context of an implementation science cluster-randomized trial that included task shifting to lower-cadre workers, male engagement, point-of-care CD4+ cell counts, and integrated mother–infant care.
Methods:
Patient and clinician satisfaction were measured at 6 control and 6 intervention sites using a 5-point Likert scale. Patient satisfaction was assessed at 6 weeks postpartum through a 22-item scale about the provider's ability to explain the health problem, time spent with the clinician, and motivation to follow prescribed treatment. Provider satisfaction was assessed through a 12-item scale about motivation, compensation, and training, with 4 additional questions about the impact of task shifting on job satisfaction to intervention arm providers.
Results:
We measured satisfaction among 340 mothers (intervention n = 160; control n = 180) and 60 providers (intervention n = 36; control n = 24). Total patient satisfaction (maximum 5) was higher in the intervention than control arm [median (interquartile range) = 4.61 (4.22–4.79) vs. 3.84 (3.22–4.22), respectively; P < 0.001]. Provider satisfaction was generally high, and was similar between the intervention and the control arms [median (interquartile range) = 3.60 (3.37–3.91) vs. 3.50 (3.08–4.25), respectively; P = 0.69]. Provider satisfaction dropped when questions on newly acquired provider roles were included [3.47 (3.25–3.72)]. Patient and provider satisfaction were not associated with uptake of antiretroviral therapy or mother–infant retention at 6 and 12 weeks postpartum.
Conclusions:
Satisfaction was higher among patients at intervention sites, and provider satisfaction decreased when newly assigned roles were factored in. Task shifting should include training and supportive oversight to ensure comfort with assigned tasks.
INTRODUCTION
Throughout Nigeria, health system challenges for scaling up of HIV services (eg, poor infrastructure, constraints on human resources, weak medical supply chains, competing priorities within the health system, HIV stigma and discrimination, and poor quality of service delivery) often lead to low levels of patient satisfaction.1–4 An implementation science strategy to addressing these barriers to high-quality HIV care would include a rigorous examination of approaches to applying evidence-based methods that impact patient satisfaction into routine practice. Health system responsiveness (HSR) measures quality of care based on patients' experiences within the health system.5–7 Patient satisfaction, a construct related to several HSR domains, is a subjective measure of a patient's perceived needs and expectations for health system interactions, and how these relate to their actual experiences and perceptions of care received.5,7,8 Significant associations have been found between adherence to antiretroviral therapy (ART) and follow-up among patients with HIV and the HSR domains of communication, dignity, promptness, and access to HIV care.8–10 Several other system-related factors associated with low patient satisfaction have also been associated with lower adherence to care, including, length and quality of the interaction with a physician and/or nurse, and shortages in health personnel and medical supplies.6,8,9,11–14
Task shifting, the system of delegating clinical responsibilities to less specialized health workers, is a well-established, feasible, and effective approach to building capacity and scaling up HIV services without compromising patient safety and quality of clinical care.15–18 The dearth of well-trained health care personnel to tackle the health challenges that abound in resource-limited settings like rural Nigeria makes the need for task shifting even more critical. Several studies have examined the impact of task shifting on multiple outcomes, including quality of clinical care, patient safety, health care costs, adherence to ART, and survival.17,19–21 Fewer studies have focused on the impact of task shifting on patient satisfaction with clinical services and the satisfaction of the lower-cadre workers with their new positions.13,22,23
Another bottleneck to scaling up prevention of mother-to-child transmission (PMTCT) services is the dearth of laboratory infrastructure. Simple, robust, and reliable point-of-care (PoC) devices can assure same-day results and impact ART uptake. Nurses in rural clinics can generate accurate CD4 and ART drug toxicity monitoring results using simple PoC devices, thereby strengthening the case for task shifting.24 Other factors that impact uptake of PMTCT services are integration of mother–infant services and the active involvement of male partners. The integration of postpartum mother–infant care through provision of same-venue services to mother–infant pairs permits HIV-infected women and their infants to be comanaged during postpartum clinic visits, thereby minimizing missed opportunities to delivering HIV care and infant services. Male participation in HIV care (eg, couple counseling and testing for HIV) increases PMTCT service uptake and adherence and is increasingly being promoted in HIV programs across Africa.25–27
This study is part of a broader study that examines the impact of a package of services for PMTCT (including task shifting, male partner engagement, PoC CD4 testing, and postpartum integration of mother–infant care) on select PMTCT outcomes. We surveyed a sample of patients and health care providers to assess whether our comprehensive site-level intervention package, and especially the task-shifting component, impacts patient and provider satisfaction. Findings from this study will help inform future strategies that include similar approaches within the context of implementation science interventions in analogous PMTCT program settings.
METHODS
Study Design and Setting
This study was part of a cluster-randomized trial conducted in 12 health facilities in rural Niger state, north central Nigeria. The protocol and primary results for the parent trial are described in other publications.28,29
Trial participants included HIV-infected women and their infants presenting for antenatal care (ANC) and/or delivery who met one of the following inclusion criteria: unknown HIV status at time of presentation; known HIV status with history of antiretroviral prophylaxis or treatment but not on treatment at the time of presentation; or known HIV status but treatment-naive. HIV-infected women who were on antiretroviral prophylaxis or treatment at the time of presentation to ANC were excluded from the study.
Twelve of 15 clinics in rural Niger state that were at the time supported by Friends in Global Health (FGH), Vanderbilt University's implementation partner for the U.S. President's Plan for AIDS Relief (PEPFAR), were matched with each other and then randomized within matched pairs to intervention (integrated PMTCT package, n = 6) or control (n = 6). Matching was based on monthly antenatal clinic volume, total patients with HIV seen during the past 6 months, urbanization of the area (rural or semiurban), and site accessibility (poor, fair, or easy).
Study Activities
For the control arm, HIV-infected women were referred to offsite comprehensive clinics for clinical and laboratory evaluation, and ART initiation. HIV clinical management was performed in accordance with current Nigeria PMTCT guidelines, as described earlier.28,30 The guidelines recommend initiating pregnant women with advanced WHO clinical stage disease (WHO stage 3 or 4) or advanced immunosuppression (CD4 count <350 cells/μL) on ART for their own health, or on ART as prophylaxis if they did not qualify for ART for their own health (option B). Breastfeeding mothers were continued on ART until 1 week after cessation of breastfeeding. HIV-exposed infants were started immediately after birth on daily nevirapine. Mothers were asked to bring their infants back to the clinic at 6 weeks for follow-up, continuation of nevirapine (if mother was not compliant with ART and was breastfeeding), immunizations, cotrimoxazole prophylaxis, and early infant diagnosis testing. The intervention sites received an integrated package of PMTCT services that included (1) devolved PMTCT tasks to trained midwives (task shifting); (2) PoC CD4+ cell and CD4% testing using the CyFlow miniPOC system (PartecGmbH, Görlitz, Germany); (3) integrated mother–infant care services (comanaging mother–infant pairs in the maternal and child health clinic and scheduling mother and infant visits to occur on the same visit date); (4) active influential family member (male partner), by inviting male partners to accompany their spouses to antenatal clinic visits using personalized letters; encouraging couple testing; and creating male-friendly clinic settings by engaging male counselors to see men who preferred to receive counseling from a man, amending standard operational procedures to include men in all appointments, and altering health facilities to permit privacy for couple counseling and testing; and (5) community involvement, which included recruitment and training of spouses of enrolled HIV-infected women as “community mobilization champions” to raise awareness of PMTCT among men in the community, and educating and encouraging men to accompany their spouses to PMTCT clinics and practice safe sex. “Community champions” also distributed condoms, linked men to couples counseling and testing services and solicited the support of community leaders and influential family members through one-on-one interactions and community forums.
The task-shifting component of the intervention was comprised of training, on-site mentoring, and continuous quality assurance. Three lower-cadre staff (2 nurses/midwives, 1 community health worker, and/or 1 pharmacist/pharmacy technician) at each intervention site underwent a 5-day HIV management training using curriculum adopted from the World Health Organization (WHO).31 A medical officer provided on-site mentoring through bimonthly chart reviews (quality assurance review) and consultation for complex cases. Information obtained from the chart reviews was shared with staff as feedback and for continuous quality assurance. The primary outcomes for the parent trial included maternal ART initiation and mother–infant retention at 6 and 12 weeks postpartum.
Patient Satisfaction Surveys
Patient satisfaction was assessed through a 22-item survey comprised of 5-point Likert-type scale items with response options including negative, somewhat negative, neither negative nor positive, somewhat positive, and positive. Items were adapted from a patient satisfaction questionnaire previously validated in Montreal, Canada.32 This scale measured patient perception of quality of care in regard to provider–patient relationship, technical aspects of care, and perceived outcomes of care. Items included assessments of perceived quality of examination, satisfaction with time spent by the provider to assess health concerns and provide sufficient feedback, level of comfort with the provider, and willingness to follow the providers' recommendations. The scale was tested at 2 randomly selected sites (1 site per study arm) in the local language (Hausa) among 15 patients before administration at all 12 sites to ensure content and face validity. Informed consent was collected by trained research assistants, who then administered the questionnaires on-site at the 6-week postpartum visit, or during home visits for clients who did not return at the 6-week time point (within 2 weeks of the missed appointment). All patients who participated in the parent study were eligible for the patient satisfaction survey.
Provider Satisfaction
Provider satisfaction was assessed in English, using 5-point (strongly agree, agree, neutral, disagree, strongly disagree) Likert-type items adapted from a previously published physician satisfaction survey.33 Items were assessed for face and content validity by a team of 6 Nigerian clinicians, translated into Hausa, and tested for understanding among 10 clinicians who were not part of the study. Nonphysician health care workers in the intervention group were asked 4 additional elements (not part of the original scale) which assessed adaptation to new task-shifted roles. These 4 items were (1) the physicians in the clinic are not supportive of my new role; (2) I am comfortable seeing patients in my new role; (3) my expanded responsibilities have made it difficult for me to attend to all of my job duties; and (4) I would have preferred additional training to complete my new job tasks. This confidential, written survey was administered on-site and included assessment of perceived quality of HIV program training, motivation to work in this role, support and mentorship from supervisors, and success in implementing PMTCT care at the site. Informed consent from all potential participants was obtained by research staff before surveys were given to clinical workers responsible for providing PMTCT services at intervention and control sites.
Data Collection
Study data in written form were collected and uploaded to a database managed using the Research Electronic Data Capture (REDCap) application hosted at Vanderbilt University.34 REDCap is a secure, web-based tool designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Statistical Analysis
We revalidated the patient satisfaction survey using maximum likelihood exploratory factor analysis with oblique rotation to examine the factor structure of the adapted instrument. Multiple factors emerged, and the internal reliability of each subscale was determined using Cronbach alpha. The satisfaction score was computed as a mean score of nonmissing items to yield a continuous score with possible range of 1–5. For the provider satisfaction survey, we did not perform factor analysis because the number of providers was too small (n = 60). The satisfaction score was computed as a combination of all nonmissing items to yield a continuous score with possible range of 1–5. Negatively worded items were reverse coded so that in all items, a higher score indicated higher satisfaction. Wilcoxon rank-sum test was used to compare satisfaction across study arms.
To determine the association between patient satisfaction and maternal ART uptake, we used a Poisson mixed-effects model, with study site and matched pairs as nested random effects and patient satisfaction as a fixed effect. The mixed-effects model also included as covariates the following maternal characteristics specified a priori as being important to ART uptake and retention: age, years of education, travel time to the facility, employment status, ethnicity, and time from HIV diagnosis (past/ANC versus delivery). Similar models were used to test for any association between patient satisfaction and postpartum retention of mother–infant pairs at 6 and 12 weeks. For the primary retention analyses, mother–infant pairs were excluded if the mother or infant died or was transferred/relocated within 14 weeks of delivery (5/369, 1.4%). An intent-to-treat approach was used for all analyses. R-software version 3.2.2 (www.r-project.org) was used for data analyses. Analysis scripts are available at http://biostat.mc.vanderbilt.edu/ArchivedAnalyses.
RESULTS
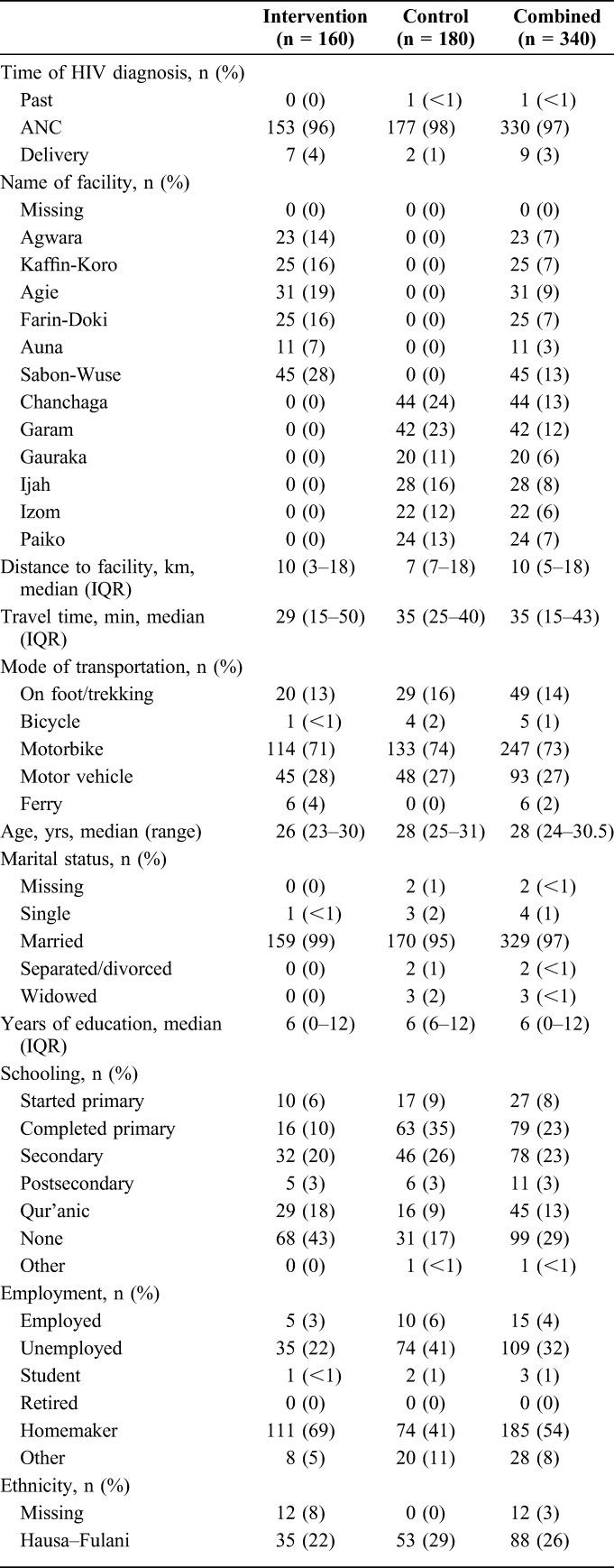
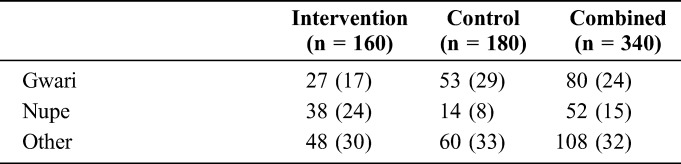
Of the 369 HIV-infected women who were eligible for the study, 340 participants (99.1%) completed patient satisfaction surveys (intervention n = 160; control n = 180; Table 1). The survey was administered during the 6-week postpartum visit for 214 (63%) participants (intervention n = 146; control n = 68). Most participants who completed the surveys reported being married (97%), homemakers (54%), and enrolled in the Sabon-Wuse health facility (intervention, 28%) or Chanchaga health facility (control, 24%). Almost all enrolled women (97%) reported being newly diagnosed as HIV infected while attending ANC. Patients in the intervention arm were slightly younger than those in the control {median age [interquartile range (IQR)] = 26 [23–30] years vs. 28 [25–31] years, respectively}. More than one-quarter of participants had no formal education (29%); this proportion was higher in the intervention arm than control (43% vs. 17%, respectively). Participants were comparable across arms for time of HIV diagnosis, distance to facility, and marital status.
TABLE 1.
Sociodemographic Characteristics of Study Participants Who Completed Satisfaction Survey by Trial Arm in 12 Rural Sites, Niger State, Nigeria
Patient Satisfaction
Factor analysis of patient satisfaction data resulted in 3 factor groupings: (1) interpersonal aspects of care (n = 7, Cronbach alpha = 0.92); (2) technical aspects of care (n = 8, Cronbach alpha = 0.90); and (3) outcomes of care (n = 3, Cronbach alpha = 0.93). Eighteen of the 22-item survey loaded onto the 3 factors and altogether were highly reliable with Cronbach alpha of 0.94. Total patient satisfaction was higher in the intervention arm than that in the control [median total satisfaction score (IQR) = 4.61 (4.22–4.79) vs. 3.84 (3.22–4.22), respectively; P < 0.001 from Wilcoxon rank-sum test]. When considering only the 214 participants surveyed on-site at the 6-week postpartum visit, patient satisfaction remained higher in intervention versus control sites [median total satisfaction score (IQR) = 4.61 (4.28–4.82) vs. 3.94 (3.50–4.22), respectively; P < 0.001]. Itemized results of patient satisfaction by study arm are depicted in Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A830.
We found little evidence (P < 0.10 for test of interaction) that patient satisfaction modified the effect of the intervention on the 3 primary outcomes in the trial. In addition, we found no statistically significant evidence of association between patient satisfaction and ART uptake [RR = 1.19 (95% CI: 0.89 to 1.59), P = 0.36], and with mother–infant retention at 6 weeks [RR = 1.18 (95% CI: 0.82 to 1.69), P = 0.36] or 12 weeks postpartum [RR = 1.30 (95% CI: 0.87 to 1.92), P = 0.20].
Provider Satisfaction
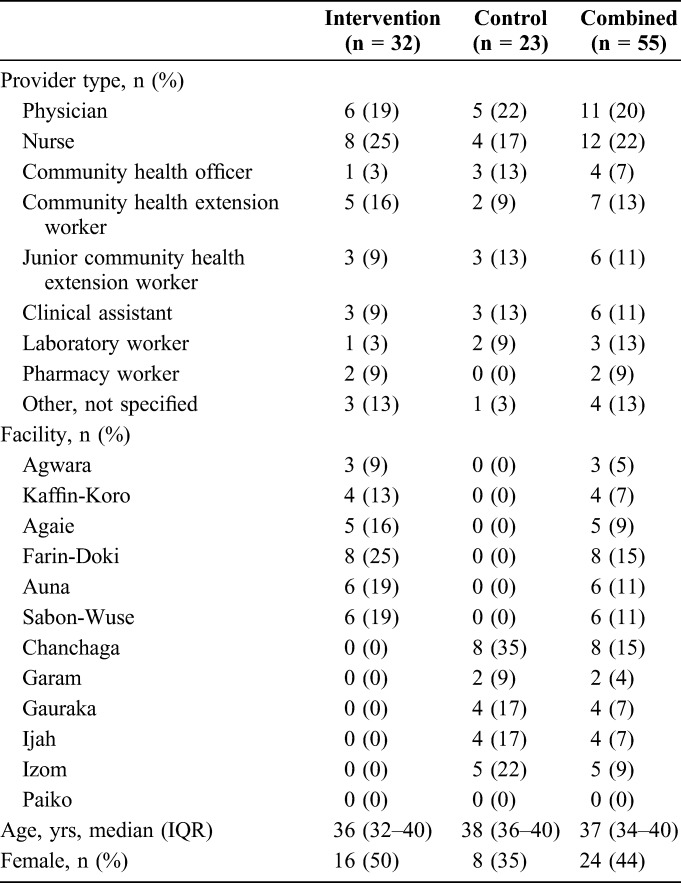
Of the 60 completed satisfaction surveys, only 55 (92%) surveys by providers were used for this analysis (intervention n = 32; control n = 23; Table 2); 5 surveys were completed by informatics personnel who do not see patients. The largest proportion of survey respondents included physicians (20%) or nurses (22%) and provided care at Farin-Doki health facility (intervention arm, 25%) or Chanchaga health facility (control arm, 35%). Providers in the intervention arm were slightly younger than those in the control arm [median age (IQR) = 36 (32–40) years vs. 38 (36–40) years, respectively]. The proportion of female providers was higher in the intervention arm than the control (50% vs. 35%, respectively).
TABLE 2.
Demographic Characteristics of Providers Who Completed Satisfaction Survey by Trial Arm in 12 Rural Sites, Niger State, North Central Nigeria
Total provider satisfaction was slightly higher in the intervention versus the control arm [median (IQR) = 3.6 (3.37–3.91) vs. 3.5 (3.08–4.25)], although we are unable to reject the null hypothesis that provider scores are equal across arms (Fig. 1; P = 0.69 from Wilcoxon rank-sum test). When the responses to the 4 additional task-shifting items were averaged into the intervention arm's provider satisfaction score, scores dropped [3.47 (3.25–3.72)]. Itemized results of provider satisfaction by study arm are provided in Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A830.
FIGURE 1.
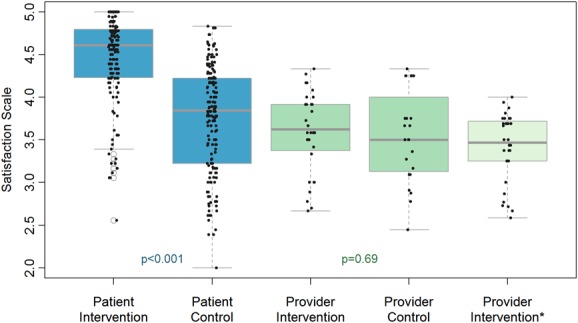
Box plot of satisfaction scores by patient or provider status and study arm, 12 sites, Niger state, north central Nigeria. “The edges of the box represent the first and third quartiles, with the median marked by the center line. Whiskers extend from the box 1.5×IQR (interquartile range; length of the box). Data outlying the whiskers are marked with points. Raw data is overlaid with a jitter to demonstrate the whole distribution. The values are from Wilcoxon rank sum tests comparing satisfaction across study arm and do not take into account the clustering that may occur within clinics. *This scale is only applicable to providers at Intervention sites, and represents the total of items applicable to both groups plus 4 items specific to the task-shifting intervention.”
DISCUSSION
We assessed patient and provider satisfaction after implementation of a PMTCT package of services in a PEPFAR-funded implementation science trial in rural Nigeria. Overall, we found patient satisfaction to be generally high, and significantly higher in the intervention arm than in the control, suggesting a positive impact of the multifaceted intervention protocol. Satisfaction among health care providers in the intervention arm was similar to the control arm, and any difference vanished when 4 items specific to newly acquired task-shifting roles were included in the analysis. Neither patient nor provider satisfaction was significantly associated with the primary outcomes of the parent trial (maternal ART uptake and maternal-infant retention in care at 6 and 12 weeks postpartum) following intervention arm and covariate adjustment.
There are few studies of provider or patient satisfaction with implementation of task-shifting activities in HIV settings. In Thailand, HIV-infected patients exposed to a nurse-led primary care service reported greater satisfaction with services than their counterparts who continued to be seen by physicians.22 Iwu and Holzemer23 reviewed studies of physician-to-nurse task shifting in HIV settings in Sub-Saharan Africa; they reported increased levels of job satisfaction, as indicated by feelings of stature, achievement, and morale among nurses in 3 studies. In Malawi, patients rated HIV prevention services more positively after the hospital workers (including physicians) received training in delivering peer-led HIV prevention interventions.35
The decrease in provider satisfaction for the intervention arm with the addition of the 4 items specific to acquisition of new, task-shifted roles is disconcerting and reveals their struggle with their new roles. These 4 items assessed perceptions on provider support, comfort in their new roles, their ability to attend their job duties, and satisfaction with the level of training they received to implement their new job tasks at the intervention sites. Inadequate provider training and support are documented barriers to sustainable task-shifting initiatives.36–43 Our findings suggest that task-shifting programs need to prioritize ensuring that providers are comfortable with assigned tasks, in addition to providing them with support and training.
Another commonly cited implementation barrier to sustainable task shifting is the increased workload on nurse providers. Reports from Lesotho highlight the increased workload, wherein nurse providers were providing an average of 45 consultations per day, exceeding the WHO recommended maximum of 30 consultations per day.44 In this example and others, the common solution is to further shift tasks to another cadre of lay counselors,44,45 shifting the pressure down the hierarchy of providers.
Although this study targeted patient and provider satisfaction and task-shifting roles, we cannot discount the contribution of male partner involvement, PoC CD4 testing, and integration of mother–infant services to the reported high overall patient and provider satisfaction. PoC CD4 testing eliminates the need for referral to hub clinics for laboratory testing, whereas the integration of mother–infant services through same-day same-venue mother–infant appointments negates the need for multiple clinic visits. In addition, PoC testing enables the acquisition of valuable skills by providers, which could positively impact their responses to the satisfaction survey items.
Our study is limited by small provider sample size, the cross-sectional single point-in-time nature of evaluating satisfaction instead of multiple time points after initial implementation, the possibility of social desirability bias associated with survey responses, the relatively low educational attainment of patient survey respondents, and the absence of analysis of specific intervention components impacting satisfaction scores. The strengths of our study include our use of validated instruments, a high response rate among patients, and the confidential nature of responses, thereby reducing the likelihood of social desirability bias.
In summary, we found that in an implementation science trial to optimize PMTCT services in rural Nigeria, overall patient satisfaction was generally high, and higher in the intervention arm compared with the control arm. A slight decrease in provider satisfaction was also observed with the addition of items specific to acquisition of new, task-shifted roles. This study addresses an important gap in the literature on patient and provider satisfaction with regard to strategies to improve PMTCT services in Nigeria, and can inform the successful implementation of future efforts to improve PMTCT services in similar resource-limited settings.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the following FGH Nigeria staff: Dr. Mukhtar Muhammad, Dr. Saidu Ishaq, Awwal Gambo, and Ibrahim Sodangi.
Footnotes
Supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, award number R01HD075075. This study was also supported by Friends in Global Health, Vanderbilt University's implementation partner for the U.S. President's Plan for AIDS Relief (PEPFAR). CMA received salary support from the National Institute of Mental Health (NIMH) Career Development Award K01MH107255-01.
The authors have no or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of the National Institutes of Health.
REFERENCES
- 1.Agu KA, Oqua D, Agada P, et al. Assessment of satisfaction with pharmaceutical services in patients receiving antiretroviral therapy in outpatient HIV treatment setting. Int J Clin Pharm. 2014;36:636–647. [DOI] [PubMed] [Google Scholar]
- 2.Aliyu MH, Varkey P, Salihu HM, Iliyasu Z, Abubakar IS. The HIV/AIDS epidemic in Nigeria: progress, problems and prospects. Afr J Med Med Sci. 2010;39:233–239. [PubMed] [Google Scholar]
- 3.Olowookere SA, Fatiregun AA, Ladipo MMA, et al. Reducing waiting time at a Nigerian HIV treatment clinic: opinions from and the satisfaction of people living with HIV/AIDS. J Int Assoc Physicians AIDS Care (Chic). 2012;11:188–191. [DOI] [PubMed] [Google Scholar]
- 4.Owolabi RS, Araoye MO, Osagbemi GK, et al. Assessment of stigma and discrimination experienced by people living with HIV and AIDS receiving care/treatment in University of Ilorin Teaching Hospital (UITH), Ilorin, Nigeria. J Int Assoc Physicians AIDS Care (Chic). 2012;11:121–127. [DOI] [PubMed] [Google Scholar]
- 5.De Silva A, Valentine N. A framework for measuring responsiveness. Geneva: World Health Organization; 2000. [Google Scholar]
- 6.Miller JS, Mhalu A, Chalamilla G, et al. Patient satisfaction with HIV/AIDS care at private clinics in Dar es Salaam, Tanzania. AIDS Care. 2014;26:1150–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peltzer K. Patient experiences and health system responsiveness in South Africa. BMC Health Serv Res. 2009;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poles G, Li M, Siril H, et al. Factors associated with different patterns of non-adherence to HIV care in Dar es Salaam, Tanzania. J Int Assoc Provid AIDS Care. 2014;13:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wouters E, Heunis C, van Rensburg D, Meulemans H. Patient satisfaction with antiretroviral services at primary health-care facilities in the Free State, South Africa—a two-year study using four waves of cross-sectional data. BMC Health Serv Res. 2008;8:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flickinger TE, Saha S, Moore RD, Beach MC. Higher quality communication and relationships are associated with improved patient engagement in HIV care. J Acquir Immune Defic Syndr. 2013;63:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RT, Camacho FT, Balkrishnan R. Willing to wait? The influence of patient wait time on satisfaction with primary care. BMC Health Serv Res. 2007;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wachira J, Naanyu V, Genberg B, et al. Health facility barriers to HIV linkage and retention in Western Kenya. BMC Health Serv Res. 2014;14:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asfaw E, Dominis S, Palen JGH, et al. Patient satisfaction with task shifting of antiretroviral services in Ethiopia: implications for universal health coverage. Health Policy Plan. 2014;29:ii50–ii58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzan-Monti M, Blanche J, Boyer S, et al. Benefits of task-shifting HIV care to nurses in terms of health-related quality of life in patients initiating antiretroviral therapy in rural district hospitals in Cameroon. HIV Med. 2015;16:307–318. [DOI] [PubMed] [Google Scholar]
- 15.Bemelmans M, Van Den Akker T, Ford N, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15:1413–1420. [DOI] [PubMed] [Google Scholar]
- 16.Gimbel-Sherr SO, Micek MA, Gimbel-Sherr KH, et al. Using nurses to identify HAART eligible patients in the Republic of Mozambique: results of a time series analysis. Hum Resour Health. 2007;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MB, Chapula BT, Chi BH, et al. Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kredo T, Adeniyi FB, Bateganya M, Pienaar ED. Task shifting from doctors to non-doctors for initiation and maintenance of antiretroviral therapy. Cochrane Database Syst Rev. 2014:CD007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bussmann C, Rotz P, Ndwapi N, et al. Strengthening healthcare capacity through a responsive, country-specific, training standard: the KITSO AIDS training program's sup-port of Botswana's National Antiretroviral Therapy Rollout. Open AIDS J. 2008;2:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaghan M, Ford N, Schneider H. A systematic review of task- shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torpey KE, Kabaso ME, Mutale LN, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PloS One. 2008;3:e2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aung MN, Moolphate S, Kitajima T, et al. Satisfaction of HIV patients with task-shifted primary care service versus routine hospital service in northern Thailand. J Infect Dev Ctries. 2015;9:1360–1366. [DOI] [PubMed] [Google Scholar]
- 23.Iwu EN, Holzemer WL. Task shifting of HIV management from doctors to nurses in Africa: clinical outcomes and evidence on nurse self-efficacy and job satisfaction. AIDS Care. 2014;26:42–52. [DOI] [PubMed] [Google Scholar]
- 24.Jani IV, Sitoe NE, Chongo PL, et al. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011;25:807–812. [DOI] [PubMed] [Google Scholar]
- 25.Msuya SE, Mbizvo EM, Hussain A, et al. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care. 2008;20:700–709. [DOI] [PubMed] [Google Scholar]
- 26.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manjate Cuco RM, M K, Bique Osman N, et al. Male partners' involvement in prevention of mother-to-child HIV transmission in sub-Saharan Africa: a systematic review. SAHARA J. 2015;12:87–105. [DOI] [PubMed] [Google Scholar]
- 28.Aliyu MH, Blevins M, Audet C, et al. Optimizing PMTCT service delivery in rural North-Central Nigeria: protocol and design for a cluster randomized study. Contemp Clin Trials. 2013;36:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliyu MH, Blevins M, Audet CM, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV. 2016;3:e202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Federal Ministry of Health (Nigeria). National Guidelines for Prevention of Mother-to-Child HIV Transmission (PMTCT). 4th ed 2010, Federal Ministry of Health Nigeria, Abuja, Nigeria. [Google Scholar]
- 31.WHO. Integrated Management of Pregnancy and Childbirth (IMPAC). Pregnancy, Childbirth, Postpartum and Newborn Care: A Guide for Essential Practice. Geneva: Emergency Plan for AIDS Relief (PEPFAR); 2006. [Google Scholar]
- 32.Haddad S, Potvin L, Roberge D, et al. Patient perception of quality following a visit to a doctor in a primary care unit. Fam Pract. 2000;17:21–29. [DOI] [PubMed] [Google Scholar]
- 33.Pathman DE, Konrad TR, Williams ES, et al. Physician job satisfaction, job dissatisfaction, and physician turnover. J Fam Pract. 2002;51:593. [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chimwaza AF, Chimango JL, Kaponda CP, et al. Changes in clients' care ratings after HIV prevention training of hospital workers in Malawi. Int J Qual Health Care. 2012;24:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asefa A, Mitike G. Prevention of Mother-to-Child Transmission (PMTCT) of HIV services in Adama town, Ethiopia: clients' satisfaction and challenges experienced by service providers. BMC Pregnancy Childbirth. 2014;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emdin CA, Chong NJ, Millson PE. Non-physician clinician provided HIV treatment results in equivalent outcomes as physician-provided care: a meta-analysis. J Int AIDS Soc. 2013;16:18445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivers LC, Jerome JG, Cullen KA, et al. Task-shifting in HIV care: a case study of nurse-centered community-based care in Rural Haiti. PLoS One. 2011;6:e19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mdege ND, Chindove S, Ali S. The effectiveness and cost implications of task-shifting in the delivery of antiretroviral therapy to HIV-infected patients: a systematic review. Health Policy Plan. 2013;28:223–236. [DOI] [PubMed] [Google Scholar]
- 41.O'Malley G, Asrat L, Sharma A, et al. Nurse task shifting for antiretroviral treatment services in Namibia: implementation research to move evidence into action. PLoS One. 2014;9:e92014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogbolu Y, Iwu EN, Zhu S, et al. Translating research into practice in low-resource countries: progress in prevention of maternal to child transmission of HIV in Nigeria. Nurs Res Pract. 2013;2013:848567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zachariah R, Ford N, Philips M, et al. Task shifting in HIV/AIDS: opportunities, challenges and proposed actions for sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2009;103:549–558. [DOI] [PubMed] [Google Scholar]
- 44.Cohen R, Lynch S, Bygrave H, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. J Int AIDS Soc. 2009;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emdin CA, Millson P. A systematic review evaluating the impact of task shifting on access to antiretroviral therapy in sub-Saharan Africa. Afr Health Sci. 2013;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


