Abstract
Background:
Effective retention of HIV-infected mothers and their infants is fraught with multiple challenges, resulting in loss across the continuum of prevention of mother-to-child HIV transmission (PMTCT) care and missed opportunities to offer life-saving HIV prevention and treatment.
Methods:
The Mother Infant Retention for Health study is an individual-randomized study evaluating the effectiveness of active patient follow-up compared with standard of care on the combined outcome of attrition of HIV-infected women and their infants at 6 months postpartum. Lay counselors administered the active patient follow-up package of interventions, including individualized health education, use of flip charts during clinic visits, and at home, phone and short message service appointment reminders, active phone and physical tracking of patients immediately after missed clinic visits, and individualized retention and adherence support.
Results:
Use of study visits to indicate participant progression along the PMTCT cascade highlights the nature of loss among women and infants in PMTCT care because of issues such as pregnancy complications, infant deaths, and transfer out. Delay in implementation of Option B+, unanticipated slow enrollment, a health-care worker strike, rapid HIV test kit shortages, and changes in national PMTCT guidelines necessitated several modifications to the protocol design and implementation to ensure successful completion of the study.
Conclusions:
Flexibility when operationalizing an implementation science study is critical in the context of the shifting landscape in a noncontrolled “real-world” setting.
Trial Registration:
Clinicaltrials.gov NCT01962220.
Key Words: PMTCT, HIV, attrition, pregnant women, implementation research
INTRODUCTION
Prevention of mother-to-child HIV transmission (PMTCT) services in Sub-Saharan Africa (SSA) have been rapidly scaled up over the past decade with >73% of pregnant HIV-positive (HIV+) women reported to have received antiretroviral drugs (ARVs) for their benefit and to protect their infants.1 Although access to HIV testing and antiretroviral treatment (ART) is improving, retention in PMTCT care throughout pregnancy and the postpartum period remains a challenge in SSA. An estimated 220,000 children in SSA acquired HIV in 2014, primarily through perinatal exposure and in part because of incomplete and delayed translation of scientific findings to clinical and programmatic practice.2 Well-delineated individual, health system and structural barriers impact retention of mothers and infants throughout the PMTCT cascade, starting with HIV testing and continuing through pregnancy, delivery, and the postpartum period when breastfeeding.3–9
Kenya, with a national HIV seroprevalence of 5.6% and an estimated 75,000 HIV+ pregnant women in 2014, is committed to eliminating new pediatric HIV infections, signing on to the elimination agenda in 2012 to reduce mother-to-child HIV transmission to <5% and rolling out Option B+ in 2014.10–12 National rates of HIV testing during pregnancy (>90%) and provision of ARVs to HIV+ pregnant women (∼70%) are high.1 However, similar to many other countries in SSA, Kenya has experienced difficulties in retention and infant follow-up. Only 64% of HIV-exposed infants (HEI) received ARVs for PMTCT, and 72% received a virologic test within 2 months of birth. Interventions are needed to address the multiple barriers preventing HIV+ pregnant women and their infants from being effectively identified, linked to, and retained in care. These include structural barriers such as multiple visits to determine ART eligibility, clinic schedule constraints, long waiting times; behavioral factors such as little time to process HIV diagnosis, lack of disclosure support, lack of family or spousal support; and biomedical factors such as feeling too sick or healthy to initiate ART and drug side effects.13,14
In an effort to facilitate the translation of effective PMTCT interventions into practice, the US National Institutes of Health (NIH) and the President's Emergency Plan for AIDS Relief (PEPFAR) created the NIH/PEPFAR PMTCT Implementation Science (IS) Alliance. The Alliance created a platform for enhanced communication and collaboration among experts in IS research, PMTCT program implementation, and policy/advocacy with an aim to share best practices and coordinate efforts in providing effective, efficient, and sustained PMTCT services in real-world settings. The Alliance was anchored on a set of NIH-funded research teams conducting NIH/PEPFAR-funded IS studies, including the study described in this article, in 7 countries.15 Collectively, these IS studies hold great promise in addressing challenges in translating science to practice along the PMTCT cascade.
We designed this study to test a combination intervention to reduce loss to follow-up (LTFU) along the PMTCT cascade among women entering PMTCT services at 10 government health facilities in Nyanza, Kenya. The evidence-based components of this intervention address structural and behavioral factors, well described in the literature, that prevent pregnant and postpartum women from achieving the full benefit of PMTCT programs.3–9,16
This paper describes the study design and methods for the Mother Infant Retention for Health (MIR4Health) study and changes related to HIV and PMTCT services that occurred during study implementation. We also report on participant progression through the PMTCT cascade, highlighting threats, barriers, and adaptations to study implementation that are faced in real-world settings. The main results from the study will be reported in a subsequent publication.
STUDY DESIGN AND METHODS
The MIR4Health study was a prospective, individual-randomized trial comparing the existing standard of care (SOC) for PMTCT to the study intervention—active patient follow-up (APFU)—among pregnant HIV+ women and their infants receiving care at health facilities. The primary objective of the study was to evaluate the effectiveness of APFU as compared with SOC on the combined outcome of attrition among mothers, infants, and mother–infant pairs at 6 months postpartum. We chose 6-month follow-up instead of 18–24 months (when final infection status of the infant could be determined) because of the 2-year study time frame. Study staff used simple randomization using sequentially numbered opaque envelopes to assign participants to either APFU or SOC, with enrollment continuing until enrollment targets for each arm, across all health facilities, were met.
Study Setting and Participants
Study enrollment began in September 2013 at 5 government health facilities in western Kenya [Bondo District Hospital (DH), Ahero Sub-District Hospital (SDH), Ambira SDH, Masogo SDH, and Ukwala Health Center]. Three health facilities (Nyakach DH, Madiany DH, and Got Agulu SDH) were added in November 2013. Jaramogi Oginga Odinga Teaching and Referral Hospital and Siaya DH were added in January 2014. All 10 health facilities are supported by ICAP through funding from PEPFAR17 and have integrated HIV and PMTCT services into maternal and child health (MCH) services, allowing HIV+ pregnant women and their infants to receive antenatal care (ANC), PMTCT, and HIV services in the same clinic.
Women were eligible for enrollment if they were 16 years or older, diagnosed with HIV during the current pregnancy, confirmed pregnant, able to provide informed consent in English or Luo, and owned a cell phone or had access to one in their households. Eligibility was expanded in March 2014 to include women previously diagnosed with HIV infection at their first ANC visit because of slow study accrual. Women were excluded if they had a significant obstetric condition requiring urgent referral to another facility for specialized obstetric care, denied their HIV status, or stated an intention to move from the area before 6 months postpartum.
Study Visit Schedule and Care
All participants received routine ANC, delivery, and postpartum care along with PMTCT and HIV care at the MCH clinic per Kenyan national guidelines. Prenatal guidelines included a minimum of 4 ANC visits depending on gestational age at first ANC visit. PMTCT/HIV care in Kenya at study commencement was Option A [zidovudine starting at 14 weeks gestation through delivery with extended infant prophylaxis using nevirapine for the duration of breastfeeding for women with CD4 cell count >350 cells/mL or triple-drug ART for women with CD4 cell count <350 cells/mL]. Option B+ (ART for all pregnant women regardless of CD4 with tenofovir/lamivudine/efavirenz) was introduced in August 2014.18,19 Postdelivery, mothers and infants received follow-up care in the MCH until determination of final infection status of the infant when the mother was transferred to the ART clinic, along with her infant, if the infant was also HIV infected.
All participants attended up to 5 study visits that spanned the ANC period to 6 months postpartum and were scheduled to coincide with, but were conducted separately from, routine ANC and PMTCT/HIV visits (Fig. 1). All participants received a small stipend (Kenyan Shilling 400/5 USD) at each study visit. Study visit 1 occurred at the first ANC visit, and study visits 2 and 3 were scheduled to occur in the second and third trimester when information on demographics, HIV treatment knowledge and beliefs, social support, trauma/abuse, mental health, HIV disclosure/stigma, medication adherence, and side effects was collected. Study visits 4 and 5 were conducted at 6 weeks and 6 months postpartum, respectively, and focused on maternal and infant adherence, infant feeding practices, family planning, maternal mental health, and infant health. Clinic phlebotomists drew blood for maternal HIV RNA viral load at study visits 3 and 5, and clinic nurses collected dried blood samples from infants for DNA polymerase chain reaction testing at study visit 4 (routine early infant diagnosis) and study visit 5 (additional study-specific test). Participants who missed a study visit were contacted by study staff via telephone to reschedule the study visit. Those who could not reschedule were asked to complete a short questionnaire over the phone. All women who missed the final study visit were contacted by phone and visited at home to ascertain if they were still engaged in care and to document outcomes. Maternal follow-up in the study ended if there was a pregnancy loss or infant death.
FIGURE 1.
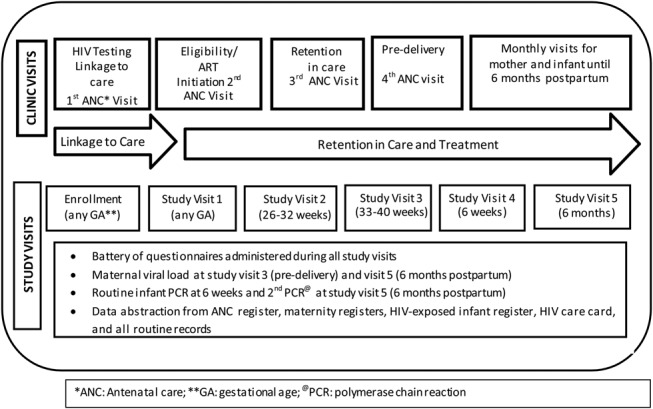
Study and clinic visit schedule.
MIR4Health Study Intervention
In addition to ANC and HIV services per the SOC, participants randomized to the intervention arm received a multicomponent combination intervention to address key structural and behavioral factors hindering retention for both mothers and infants. The study intervention (APFU) included 4 key components: (1) individualized PMTCT health education; (2) retention and adherence support; (3) phone and short message service (SMS) appointment reminders; (4) and follow-up and tracking for missed clinic visits. These 4 components were based on existing evidence of improving retention during pregnancy and the postpartum period and were considered feasible in resource-limited settings.20–26 The intervention was delivered by lay counselors called Mama Mshauri (mother mentor). These were community health-care workers who were hired and trained for this study. The Mama Mshauri underwent a 5-day practical training with study investigators, which included communication skills, PMTCT care, flip-chart use, and adherence and psychosocial support. They were introduced to participants during the randomization visit, met with them at each clinic visit, and visited assigned participants' homes on a monthly basis. Mama Mshauri assisted with expediting service provision, enhancing communication between participants and health providers, assisting participants to identify and problem-solve personal barriers to retention and adherence, providing psychosocial support and counseling, and reminding participants of upcoming clinic appointments via phone calls or SMS up to 1 week before scheduled appointments.
Study Outcomes
The primary study outcome is attrition at 6 months postpartum of mothers, infants, and mother–infant pairs. Attrition is defined as the proportion not retained in clinic care because of mother or infant death, LTFU, or pregnancy loss. Attrition specifically at 6 months is defined as no documented clinic attendance at 6 months (3–9 months window) for mother, infant, or mother–infant pair. Maternal clinic visits included ANC and HIV care visits (pre-ART or ART), and infants attendance at HEI care visits, as documented in routine collected medical records and registers. Secondary outcomes include measurements related to adherence, viral suppression, infant testing, disclosure, partner testing, and breastfeeding. These outcome findings will be the subject of future analyses.
Data Collection
Data were collected in 2 ways: (1) abstraction of routinely collected data from ANC, maternity, HIV care, and HEI care; and (2) completion of study questionnaires conducted as part of the 5 separate study visits. Routinely collected clinical data for each mother–infant pair was entered into a customized DHIS2 study database. To ensure accuracy and completeness, study staff undertook data quality assurance activities to validate data entered and to check for data entry errors. Data from the study questionnaires were entered into a separate Lime Survey database and were merged with DHIS2 clinical data for analysis.
Sample Size and Analysis Plan
MIR4Health was designed to enroll and randomize 340 HIV+ pregnant women, 170 in each arm. Assuming 90% of enrolled women would have a live birth,27 we planned for an effective sample size of 306 participants, 153 in each arm for the evaluation of outcomes that were conditional on live birth. With an estimated 40% attrition in the SOC arm for this sample size, we assumed 80% power in detecting attrition of ≤25% in the APFU/intervention arm at a significance level of 0.05.
In this paper, we describe study implementation and discuss the key implementation challenges and their impact on the study. We also report on proportions of participants who completed each study visit and losses that occur along the PMTCT cascade using study visits.
Ethical Considerations
Ethical approval for this study was provided by the Institutional Review Board of Columbia University Medical Center and the Ethical Review Committee of the Kenya Medical Research Institute. All participants provided written informed consent.
Study Adaptations and Challenges
Over the course of the study, modifications were made to accommodate various changes to standard service delivery (Table 1). Enrollment was slow at start-up because an increasing number of HIV+ women entering ANC had previously been diagnosed with HIV. To reach study accrual in the expected time frame, we increased the number of study facilities from 5 to 10 and revised study criteria to include known HIV+ women. Additionally, several programmatic changes impacted the study. Study materials and trainings were developed with the understanding that Option B+ would be endorsed as the new national PMTCT strategy. However, Option A remained in place until July 2014. Hence study materials including the flip chart had to be revised prior to study commencement. The adoption of Option B+ and implementation of routine viral load testing in August 2014 necessitated revision of study instruments and retraining of staff during study implementation. Additionally, study intervention components with the potential to affect study outcomes were introduced at study health facilities as part of routine care, including assignment of HIV+ mentor mothers to patients, use of SMS for adherence support, and community- or home-based HIV testing for family members.
TABLE 1.
Changes in the Operational Landscape That Impacted Study Implementation
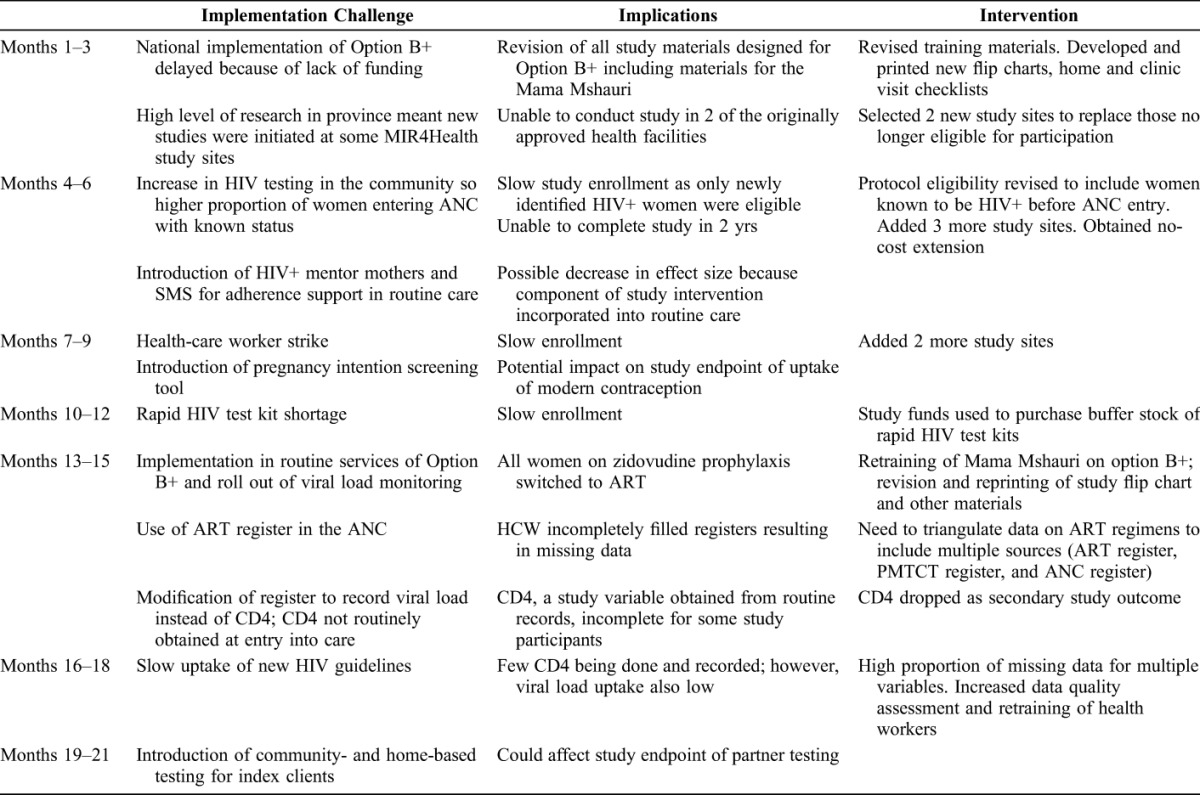
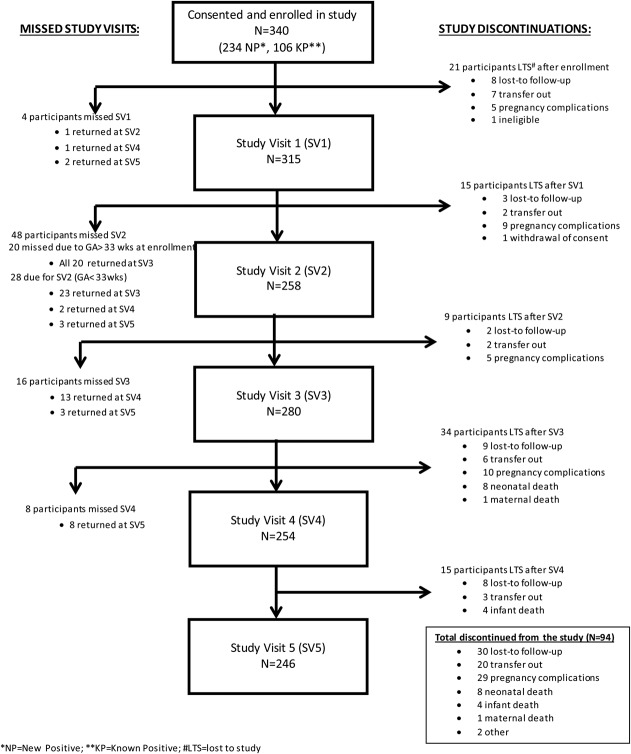
Figure 2 depicts participant progression through the PMTCT cascade using study visits. This cascade shows the number of women enrolled in the study who completed each study visit, those who missed a study visit but returned for a later study visit, and those who withdrew from the study. Some participants who “missed study visits” returned for a subsequent visit, whereas those who were “lost to study” never returned for another study visit. Among those “lost to study,” those lost to study follow-up (LTSF) never returned for a study visit. A total of 340 HIV+ pregnant women enrolled in the study at their first ANC visit; 315 (93%) completed study visit 1 and 246 (72%) completed study visit 5. Among the 94 women who discontinued the study, 29 (37%) experienced a pregnancy complication (miscarriage, ectopic pregnancy, false pregnancy, or still birth) and 12 (13%) experienced neonatal (8) or infant (4) death. Thirty women were lost to study follow-up and 20 transferred to another health facility; there was 1 maternal death and 2 study withdrawals (1 ineligible and 1 self-withdrawal). Study visit 2 had the highest number of missed visits (48), primarily because of advanced gestational age (>33 weeks) at enrollment (20). All 20 participants returned for study visit 3. Among the other 28 women who missed study visit 2, 23 returned at study visit 3 and 2 and 3 women at study visits 4 and 5, respectively.
FIGURE 2.
Study visit cascade—following participants through MIR4Health study visits, including missed study visits and reasons participants were lost to study.
DISCUSSION
MIR4Health is an individual-randomized IS study designed to evaluate the effectiveness of a combination intervention to improve retention of mother–infant pairs throughout the PMTCT cascade. It is one of several studies funded by the NIH/PEPFAR PMTCT IS Alliance to evaluate impact and effectiveness of evidence-based interventions to improve PMTCT and MCH outcomes.15 Our study design is innovative in several ways. First, it uses a combination of evidence-based interventions that address multiple barriers along the entire PMTCT cascade3–9,16,28; singular and isolated interventions often do not have the desired outcome of optimizing engagement across the entire cascade. Second, the use of lay health-care workers is consistent with the promotion of task sharing as an essential approach to scaling-up HIV services and implementing Option B+ and universal ART.25,29–32 Also, the proactive approach to patient follow-up expands on previous research and successful models to prevent LTFU, engage and empower HIV+ pregnant women to adhere to treatment, and anticipate potential barriers to retention.33–36 Lastly, the choice of the primary outcome, attrition of both mothers and infants recognizes that PMTCT services are expected to impact both MCH outcomes.
The study cascade, which closely resembles the PMTCT cascade, sheds light on important issues when reporting on PMTCT outcomes. The study cascade shows that women move in and out of care and have inconsistent attendance. Retention in PMTCT is complex and needs to reflect not only the continuity between the ANC and postnatal period but also include the variety of factors that impact mother and infant health outcomes.37 Pregnancy losses accounted for a third of study discontinuations and were substantially greater than predicted in our sample size calculations. Pregnancy losses seem to be common in this population of HIV+ women and may be overlooked as a key reason for high LTFU among women enrolled in PMTCT programs.27 Similarly, low retention in the postpartum period could result from pregnancy loss and poor birth outcomes, including infant death.38
IS research, by definition, situates itself within clinical services as a way to translate knowledge into practice; thus, it is subject to specific threats in the context of the rapidly changing landscape of HIV care, which presents unique challenges for study design and implementation. The MIR4Health study was conceived in 2011 and, at the time, the Kenya national program was planning to adopt and roll out Option B+ by July 2012. However, because of unanticipated delays, Option B+ was not implemented until August 2014. The delayed implementation of Option B+ required last-minute changes in study material and training of study staff. When Option B+ was finally implemented in August 2014, study accrual had been completed and women receiving prophylaxis under Option A were transitioned to ART under Option B+. Once again, study staff had to be retrained and materials such as counseling scripts and illustrative flip charts had to be revised. Furthermore, with the shift to Option B+, the importance of CD4 testing was de-emphasized and no longer obtained regularly as part of clinical care. Because laboratory results for the study were obtained through abstraction of routinely collected data, change in CD4 cell count, a secondary outcome, could not be measured. The change in policy is also likely to confound planned analyses of viral suppression by study arm. The switch from AZT prophylaxis to ART for women will likely have a more profound effect on viral suppression and could potentially affect the impact of the study intervention. The study also faced challenges with study accrual. The MIR4Health study was motivated by particularly low retention rates in Nyanza, Kenya, among pregnant women newly identified as HIV -infected on entry into PMTCT services. Quality assurance activities conducted within service delivery programs showed retention at 6 months postpartum at approximately 40% among newly diagnosed HIV+ pregnant women and 60%–75% among pregnant woman previously diagnosed (ICAP, program data). MIR4Health was designed specifically to address the low retention in the most vulnerable group, newly diagnosed women. However, with the rapidly changing and maturing HIV care and treatment landscape in Kenya, improved testing, ART, and PMTCT coverage meant more women with known HIV status entered ANC as compared with newly diagnosed.10 This necessitated a change in study eligibility criteria with adjustment of sample size based on revised retention estimates for the combined cohort.
Health facilities in Nyanza are responsive to the needs of clients and are constantly trying to improve care and treatment. As a result, some of the intervention components that were being trialed in the study were implemented by the facilities as part of routine service delivery during the study, including adherence support through SMS and the engagement of mother mentors. These interventions had the potential to improve outcomes in the control group and decrease the measurable impact of the MIR4Health combination intervention. These challenges highlight the limitation of IS studies situated in routine service programs; the lack of a controlled environment impacts study outcomes when clinics are eager to implement strategies to improve service delivery.
This study has both limitations and advantages to note. It relied on nonstudy staff for provision of routine services according to the SOC, including HIV tests, HIV clinical care, and service delivery documentation. This introduced a wide range of opportunities for nonstandard or nonoptimized provision of care and data collection and missing and incomplete data. Although the prospect of inaccurate data is a limitation, embedding the study within routine clinical care does offer the advantage of providing a more realistic picture of patient outcomes. The effectiveness of the intervention strategies has been established from previous research; however, IS efforts including our study can assess whether previous research findings are applicable to real-world settings. However, the uncontrolled clinic and policy factors were particular to the time and place of the study, and therefore may limit the generalizability of the findings.
CONCLUSIONS
The MIR4Health study evaluates a combination intervention to engage and retain HIV+ pregnant women and their infants in care. Review of the study visit cascade highlights the nature of retention among women and infants in PMTCT care. Pregnancy complications, infant deaths, and transfer to other facilities seem to explain a substantial proportion of loss along the care cascade. Despite the challenges and adaptations in implementing the study, if shown to be effective, the results are likely to be of interest to policymakers and implementers working to improve retention of HIV+ mother and their families.
Footnotes
Supported by the National Institutes of Health (NIH; award number: RO1 HD075163-01) and the President's Emergency Plan for AIDS Relief. NIH has approved this study, which complies with its policy on human subjects.
Presented in part at the Conference on Retroviruses and Opportunistic Infections, February 22–25, 2016, Boston, MA (Abstract No. 791).
The authors have no conflicts of interest to disclose.
E.J.A., R.F., W.R., and M.P.H. conceived the study design, developed and led the study implementation. D.C., C.B., M.S., and S.O.O. participated in study implementation. C.W. and Z.P. contributed to the data management and statistical analysis. All the authors contributed to writing the paper and approved the final manuscript.
REFERENCES
- 1.World Health Organization (WHO). Global Health Sector Response to HIV, 2000–2015: Focus on Innovations in Africa: Progress Report. Geneva, Switzerland: WHO; 2015. Available at: http://www.who.int/hiv/pub/progressreports/2015-progress-report/en. Accessed December 8, 2015. [Google Scholar]
- 2.Bhardwaj S, Carter B, Aarons GA, et al. Implementation research for the prevention of mother-to-child HIV transmission in sub-Saharan Africa: existing evidence, current gaps and new opportunities. Curr HIV/AIDS Rep. 2015;12:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bwirire LD, Fitzgerald M, Zachariah R, et al. Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Trans R Soc Trop Med Hyg. 2008;102:1195–2000. [DOI] [PubMed] [Google Scholar]
- 4.Gourlay A, Birdthistle I, Mburu G, et al. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588 Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3717402/. Accessed December 8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. [DOI] [PubMed] [Google Scholar]
- 6.Nassali M, Nakanjako D, Kyabayinze D, et al. Access to HIV/AIDS care for Mothers and Children in sub-Saharan Africa: adherence to the postnatal PMTCT program. AIDS Care. 2009;21:1124–1131. [DOI] [PubMed] [Google Scholar]
- 7.Clouse K, Pettifor A, Shearer K, et al. Loss to follow-up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Trop Med Int Health. 2013;18:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colvin CJ, Konopka S, Chalker JC, et al. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS One. 2014;9:e108150 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/. Accessed December 8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler MG, Lampe MA, Jamieson DJ, et al. Reducing the risk of mother-to-child human immunodeficiency virus transmission: past successes, current progress and challenges, and future directions. Am J Obstet Gynecol. 2007;197:S3–S9. [DOI] [PubMed] [Google Scholar]
- 10.Ng'ang'a A, Waruiru W, Ngare C, et al. The Status of HIV Testing and Counseling in Kenya: Results From a Nationally Representative Population-Based Survey. J Acquir Immune Defic Syndr. 2014;66(suppl 1):S27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joint IATT Technical Review Mission Report. Prevention of Mother-to-Child Transmission of HIV (PMTCT) and Pediatrics HIV/AIDS Care and Treatment. Republic of Kenya: UNICEF; 2010. [Google Scholar]
- 12.Kenya National AIDS Strategic Plan 2009/2010-2012/13: Delivering on Universal Access to Services. Nairobi, Kenya: National AIDS Control Council; 2009. [Google Scholar]
- 13.Duff P, Kipp W, Wild TC, et al. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambia J, Mandala J. A systematic review of interventions to improve prevention of mother- to-child HIV transmission service delivery and promote retention. J Int AIDS Soc. 2016;19:20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturke R, Harmston C, Simonds RJ, et al. A multi-disciplinary approach to implementation science: the NIH-PEPFAR PMTCT implementation science alliance. J Acquir Immune Defic Syndr. 2014;67:S163–S167. [DOI] [PubMed] [Google Scholar]
- 16.Lubega M, Musenze IA, Joshua G, et al. Sex inequality, high transport costs, and exposed clinic location: reasons for loss to follow-up of clients under prevention of mother-to-child HIV transmission in eastern Uganda—a qualitative study. Patient Prefer Adherence. 2013;7:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PEPFAR 3.0 Controlling the Epidemic: Delivering on the Promise of an AIDS-Free Generation. Available at: http://www.pepfar.gov/about/strategy/index.htm. Accessed December 8, 2015. [Google Scholar]
- 18.National AIDS and STI Control Program (NASCOP). Guidelines for Prevention to Mother to Child Transmission (PMTCT) HIV/AIDS in Kenya. 3rd ed NASCOP; Nairobi, Kenya, 2009. Available at: http://nascop.or.ke/library/pmtct/PMTCT%20Guideline-March%202010.pdf. Accessed December 8, 2015. [Google Scholar]
- 19.Ministry of Health; National AIDS and STI Control Program (NASCOP). Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: A Rapid Advice. NASCOP; Nairobi, Kenya, 2014. Available at: http://healthservices.uonbi.ac.ke/node/1963. Accessed December 8, 2015. [Google Scholar]
- 20.Torpey KE, Kabaso ME, Mutale LN, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PLoS One. 2008;3:e2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasvold PE, Wootton R. Use of Telephone and SMS reminders to improve attendance at Hospital appointments: a systematic review. J Telemed Telecare. 2011;17:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pop-Eleches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. [DOI] [PubMed] [Google Scholar]
- 24.Thomson KA, Cheti EO, Reid T. Implementation and outcomes of an active defaulter tracing system for HIV, prevention of mother to child transmission of HIV (PMTCT), and TB patients in Kibera, Nairobi, Kenya. Trans R Soc Trop Med Hyg. 2011;105:320–326. [DOI] [PubMed] [Google Scholar]
- 25.Naar-King S, Bradford J, Coleman S, et al. Retention in care of persons newly diagnosed with HIV: outcomes of the Outreach Initiative. AIDS Patient Care STDS. 2007;21:S40–S48. [DOI] [PubMed] [Google Scholar]
- 26.Zachariah R, Ford N, Philips M, et al. Task shifting in HIV/AIDS: opportunities, challenges and proposed actions for sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2009;103:549–558. [DOI] [PubMed] [Google Scholar]
- 27.Aluvaala J, Okello D, Murithi G, et al. Delivery outcomes and patterns of Morbidity and mortality for neonatal admissions in five Kenyan hospitals. J Trop Pediatr. 2015;61:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalembo FW, Zgambo M. Loss to follow-up: a major challenge to successful implementation of prevention of mother-to-child transmission of HIV-1 programs in sub-Saharan Africa. AIDS. 2012;31:589817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callaghan M, Ford N, Schneider H. A Systemic Review of task-shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MH, Ahmed S, Hosseinipour MC, et al. Implementation and operational research: the impact of option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2015;68:e77–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowley T, Mayers P. Trends in task shifting in HIV treatment in Africa: Effectiveness, challenges and acceptability to the health professions. African Journal of Primary Health Care & Family Medicine; 2015;7(1):807. 10.4102/phcfm.v7i1.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO). Policy Brief: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: What's New. Geneva, Switzerland: WHO; 2015. Available at: http://www.who.int/hiv/pub/arv/policy-brief-arv-2015/en/. Accessed December 8, 2015. [Google Scholar]
- 33.Braitstein P, Siika AM, Hogan J, et al. A clinician-nurse model to reduce early mortality and increase clinic retention among high-risk HIV-infected patients initiating combination antiretroviral treatment. J Int AIDS Soc. 2012;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otieno PA, Kohler PK, Bosire RK, et al. Determinants of failure to access care in mothers referred to HIV treatment programs in Nairobi, Kenya. AIDS Care. 2010;22:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradford JB. The promise of outreach for engaging and retaining out-of-care persons in HIV medical care. AIDS Patient Care STDS. 2007;21:S85–S91. [DOI] [PubMed] [Google Scholar]
- 36.Towne-Gold B, Ekouevi DK, Amani-Bose C, et al. Implementing family-focused HIV care and treatment: the first 2 years' experience of the mother-to-child transmission-plus program in Abidjan, Cote D'ivoire. Trop Med Int Health. 2009;14:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rollins NC, Becquet R, Orne-Gliemann J, et al. Defining and Analyzing Retention in care among pregnant and breastfeeding HIV-infected pregnant women: unpacking the data to interpret and improve PMTCT outcomes. J Acquir Immune Defic Syndr. 2014;67(suppl 2):S150–S156. [DOI] [PubMed] [Google Scholar]
- 38.Kim MH, Ahmed S, Hosseinipour MC, et al. The impact of option B+ on the infant PMTCT cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2015;70:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]