Supplemental Digital Content is available in the text.
Keywords: mesenchymal stromal cells, neutrophils, phagocytosis, sepsis, specialized proresolving lipid mediators
Abstract
Objectives:
Mesenchymal stromal cells are being investigated as a cell-based therapy for a number of disease processes, with promising results in animal models of systemic inflammation and sepsis. Studies are ongoing to determine ways to further improve the therapeutic potential of mesenchymal stromal cells. A gas molecule that improves outcome in experimental sepsis is carbon monoxide. We hypothesized that preconditioning of mesenchymal stromal cells with carbon monoxide ex vivo would promote further therapeutic benefit when cells are administered in vivo after the onset of polymicrobial sepsis in mice.
Design:
Animal study and primary cell culture.
Setting:
Laboratory investigation.
Subjects:
BALB/c mice.
Interventions:
Polymicrobial sepsis was induced by cecal ligation and puncture. Mesenchymal stromal cells, mesenchymal stromal cells-conditioned with carbon monoxide, fibroblasts, or fibroblasts-conditioned with carbon monoxide were delivered by tail vein injections to septic mice. The mice were assessed for survival, bacterial clearance, and the inflammatory response during sepsis in each of the groups. Mesenchymal stromal cells were also assessed for their ability to promote bacterial phagocytosis by neutrophils, the production of specialized proresolving lipid mediators, and their importance for mesenchymal stromal cells function using gene silencing.
Measurements and Main Results:
Ex vivo preconditioning with carbon monoxide allowed mesenchymal stromal cells to be administered later after the onset of sepsis (6 hr), and yet maintain their therapeutic effect with increased survival. Carbon monoxide preconditioned mesenchymal stromal cells were also able to alleviate organ injury, improve bacterial clearance, and promote the resolution of inflammation. Mesenchymal stromal cells exposed to carbon monoxide, with docosahexaenoic acid substrate, produced specialized proresolving lipid mediators, particularly D-series resolvins, which promoted survival. Silencing of lipoxygenase pathways (5-lipoxygenase and 12/15-lipoxygenase), which are important enzymes for specialized proresolving lipid mediator biosynthesis, resulted in a loss of therapeutic benefit bestowed on mesenchymal stromal cells by carbon monoxide.
Conclusions:
Taken together, these data suggest that production of specialized proresolving lipid mediators contribute to improved mesenchymal stromal cell efficacy when exposed to carbon monoxide, resulting in an improved therapeutic response during sepsis.
Sepsis is a disease process initiated by an underlying severe infection that leads to a systemic inflammatory response (1–4). The desired outcome in sepsis is for the invading microorganism(s) to be cleared, followed by resolution of the inflammatory process (5), allowing homeostasis to be achieved and permanent organ dysfunction avoided. Although in the past the resolution of inflammation was felt to be a passive process, it has been demonstrated that specialized proresolving lipid mediators (SPMs) control this process in an orchestrated manner, stopping neutrophil influx and activating resolution pathways (6). These bioactive mediators are synthesized from omega-3 fatty acids (docosahexaenoic acid [DHA] and eicosapentaenoic acid). Omega-6 fatty acids also contribute to the production of SPMs; however, the arachidonic acid (AA)–derived mediators also include the proinflammatory eicosanoids (6).
Even with recent advances in standard of care, severe sepsis and septic shock remain associated with high rates of morbidity and mortality (7, 8). Investigators have recently begun to explore cell-based therapies for sepsis, including the use of mesenchymal stromal cells (MSCs) (9, 10). MSCs are considered to be a promising platform for cell-based therapy, and in preclinical animal models, the administration of MSCs has shown improved outcome in polymicrobial sepsis induced by cecal ligation and puncture (CLP) in mice (11–15). The benefit of treating with MSCs during CLP, or single organism bacterial peritonitis, appears to be better when given early (1–4 hr) after the onset of sepsis (11–13, 15), whereas later administration (6 hr) may require the addition of antibiotics for improved survival (14). Thus, timing of MSC administration in regard to the onset of sepsis is a very important factor determining therapeutic benefit.
Heme oxygenase (HO)-1 is an important cytoprotective molecule during experimental sepsis in mice (16–19). We previously demonstrated the benefits of MSCs when administered after the onset of polymicrobial sepsis in mice, although these effects were not dependent on endogenous HO-1 (12). A product of HO-1 degradation of heme, carbon monoxide (CO) has also been shown to be beneficial therapeutically in animal models of sepsis (16, 20, 21), and inhaled CO gas accelerates the resolution of inflammation in zymosan-induced peritonitis (22). Furthermore, ex vivo conditioning of cells (23, 24) or even tissues (25) with CO has shown protection from cell and organ injury. Thus, in the present study, we wanted to determine whether CO preconditioning would improve MSC function and therapeutic efficacy in experimental polymicrobial sepsis, and to determine potential mechanisms contributing to this effect.
MATERIAL AND METHODS
Isolation of MSCs and Lung Fibroblasts
Mouse MSCs were harvested from the compact bone of BALB/c mice (12 mice/harvest) as described (12), and lung fibroblasts were used as control mesenchymal cells. MSCs of passage 3–6 were used for experiments. Human MSCs were obtained from either Texas A&M Health Science Center, Institute for Regenerative Medicine, or Lonza. The cells were characterized using a BD fluorescent-activated cell sorting (FACS) Canto II (Becton Dickinson Biosciences, Bedford, MA) and analyzed using FlowJo software (Becton Dickinson Biosciences). Antibodies used for immunophenotyping of cells are listed in Supplemental Table 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/C8).
CO Exposure
Cells were exposed to ambient air or CO gas at 250 parts per million (ppm) for 4 hours in a chamber that was humidified and maintained at 37°C as described (26).
Gene Silencing
For the 5-lipoxygenase (LOX) and 12/15-LOX silencing experiments, lentiviral transfection was used. For detailed information, see Supplemental Methods (Supplemental Digital Content 2, http://links.lww.com/CCM/C9).
CLP
To induce polymicrobial sepsis, we used an established murine model of CLP (two-thirds cecum ligated, two 21-gauge needle punctures) as described (12, 16).
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Staining
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was performed in the spleen, and the area of positive staining was assessed as described (27).
Aspartate Aminotransferase, Alanine Aminotransferase, and Creatinine Assays
Aspartate aminotransferase (AST, alanine aminotransferase, and creatinine plasma levels were measured by commercially available colorimetric assay kits (BioVision, Milpitas, CA).
Assessment of Blood/Peritoneal Colony-Forming Units
Serial dilutions of whole blood were performed, and aliquots were cultured on Luria-Bertani agar plates as described (12). Colony-forming units (CFUs) were counted following overnight incubation at 37°C. In addition, serial dilutions of peritoneal lavages were also performed, and CFUs were counted.
Neutrophil Isolation
Isolation of mouse and human neutrophils is described in the Supplemental Methods (Supplemental Digital Content 2, http://links.lww.com/CCM/C9).
Phagocytosis Assay
Neutrophil phagocytosis of green fluorescent protein–labeled bacteria, ± MSCs, is described in the Supplemental Methods (Supplemental Digital Content 2, http://links.lww.com/CCM/C9). Bacterial phagocytosis was measured by flow cytometry as described (12), and Supplemental Figure 1 (Supplemental Digital Content 3, http://links.lww.com/CCM/C10; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21) shows our gating strategy and a representative flow cytometry pseudocolor density plot for neutrophil phagocytosis.
Efferocytosis Assay
The measurement of macrophages phagocytizing dead neutrophils from peritoneal fluid after CLP injury, ± MSCs, is described in the Supplemental Methods (Supplemental Digital Content 2, http://links.lww.com/CCM/C9). Supplemental Figure 2 (Supplemental Digital Content 4, http://links.lww.com/CCM/C11; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21) demonstrates representative flow cytometry pseudocolor density plots of efferocytosis assays in permeabilized (A) and nonpermeabilized (B) cells.
Lipid Mediator Profiling Using Liquid Chromatography-Tandem Mass Spectrometry–Based Metabololipidomics
Measurement of lipid mediator profiles was performed as described (28). For detailed information, see the Supplemental Methods (Supplemental Digital Content 2, http://links.lww.com/CCM/C9).
Quantitative Real-Time Polymerase Chain Reaction, Western Blotting, and 3-(4,5-Dimethylthiazol-2-yl)2,5-Diphenyl-Tetrazolium Bromide Viability Assay
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed as described (29). Primer sequences are shown in Supplemental Table 2 (Supplemental Digital Content 5, http://links.lww.com/CCM/C12). For further details regarding qRT-PCR, Western blotting, or 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide viability assays, see the Supplemental Methods (Supplemental Digital Content 2, http://links.lww.com/CCM/C9).
Statistical Analysis
Data are expressed as mean ± sem. Comparisons of mortality were made by analyzing Kaplan-Meier survival curves, and then the log-rank test was performed to assess the differences in survival. For comparisons between two groups, we used Student unpaired t test. One-way analysis of variance was used for analysis of more than two groups. When data were not normally distributed, nonparametric analyses were performed using Mann-Whitney U or Kruskal-Wallis testing, respectively. The numbers of samples per group (n), or the numbers of experiments, are specified in the figure legends. Statistical significance is accepted at p value less than 0.05.
Study Approval
The care and use of animals for all experiments were approved by the Harvard Medical Area Standing Committee on Animals, Harvard Medical School. Experiments involving human blood donation by consented volunteers, for isolation of neutrophils, were approved by the Partners Healthcare Institutional Review Board.
RESULTS
Preconditioning of MSCs With CO Sustains the Efficacy of the Cells for Survival Benefit in CLP-Induced Sepsis
MSCs phenotypically adhered to plastic and expressed markers of mesenchymal origin (CD105, CD90.2, CD73, CD140b, CD29, CD44) and Sca1, but not markers of hematopoietic origin (such as CD45, CD11b, and bone marrow lineage markers) or major histocompatibility complex II (Supplemental Fig. 3A, Supplemental Digital Content 6, http://links.lww.com/CCM/C13; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21). In addition, the cells showed multipotency in vitro by differentiating into osteoblasts, adipocytes, and chondrocytes (Supplemental Fig. 3B, Supplemental Digital Content 6, http://links.lww.com/CCM/C13; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21). To understand the effect of CO preconditioning on MSCs, we administered the cells in vivo to mice after the onset of CLP-induced sepsis. MSCs or control fibroblasts—exposed to ambient air or CO at 250 ppm × 4 hours—were initially IV administered 2 hours (5.0 × 105 cells), 24 hours (2.5 × 105 cells), and 48 hours (2.5 × 105 cells) after CLP surgery. The administration of MSCs increased survival (65%) compared with fibroblasts (22%) after the onset of sepsis (Fig. 1A). Preconditioning of MSCs with CO tended to further increase survival (88%) compared with nonconditioned MSCs, although this was not a statistically significant increase. However, mice receiving MSCs preconditioned with CO had a marked increase in survival compared with mice receiving fibroblasts or fibroblasts preconditioned with CO (37%). To determine whether the effects of CO were dependent on the expression of HO-1, we preconditioned either wild-type or HO-1–deficient MSCs and administered the cells 2, 24, and 48 hours after CLP surgery. Supplemental Figure 4 (Supplemental Digital Content 7, http://links.lww.com/CCM/C14; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21) demonstrates that the mice had a nearly identical survival after the onset of sepsis, when receiving either wild-type or HO-1–deficient MSCs preconditioned with CO. Finally, we administered MSCs later after the onset of sepsis (6 hr), and in only one dose (5.0 × 105), to further investigate the effects of CO preconditioning on MSCs (Fig. 1B). In this setting, mice receiving MSCs preconditioned with CO had a significantly improved survival (76%) compared with mice treated with MSCs that were not exposed to CO (38%). These data demonstrate that CO preconditioning of MSCs sustained their beneficial effects when administered later after the onset of sepsis, and in a single dose of fewer total cells. In contrast, the efficacy of MSCs not preconditioned with CO was lost when given later and in a single dose (Fig. 1B). Exposure of MSCs to CO did not alter baseline viability of the cells, and in the presence of oxidative stress (H2O2), CO preconditioning increased MSC survival (Supplemental Fig. 5, Supplemental Digital Content 8, http://links.lww.com/CCM/C15; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21).
Figure 1.
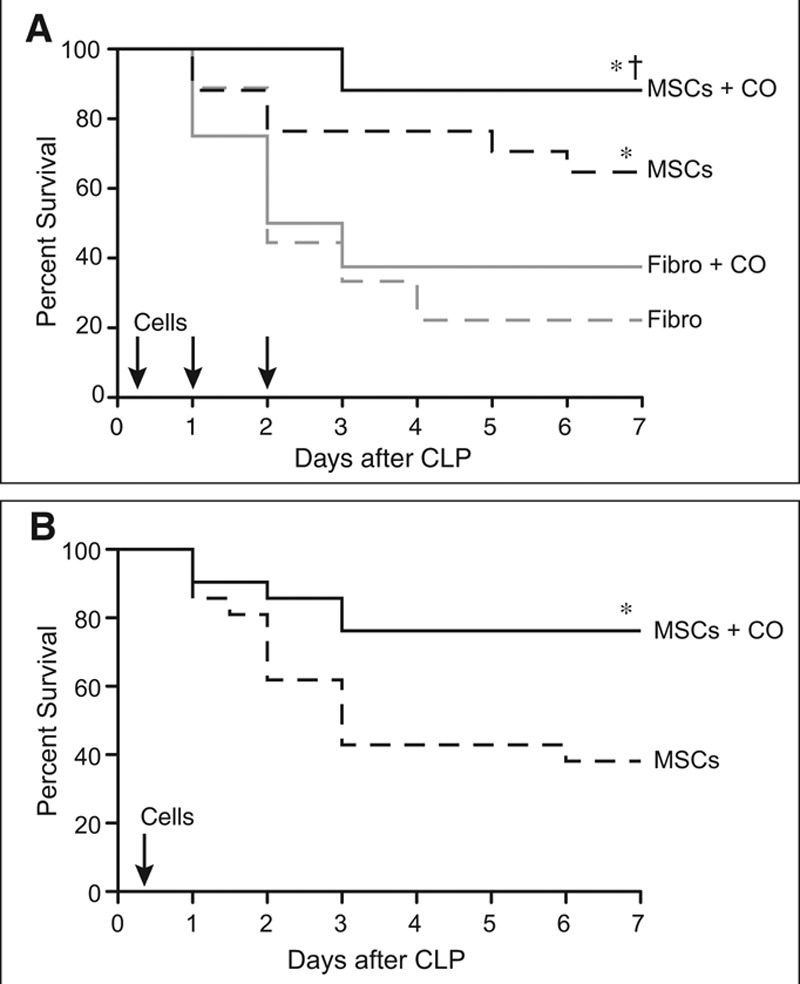
Administration of mesenchymal stromal cells (MSCs) preconditioned with carbon monoxide (CO) improves efficacy of the cells and survival in cecal ligation and puncture (CLP)-induced sepsis. A, BALB/c mice were randomly separated into four groups: fibroblasts (Fibro), dashed gray line, n = 9; Fibro+CO, solid gray line, n = 8; MSCs, dashed black line, n = 17; and MSCs+CO, solid black line, n = 17. All mice were subjected to CLP, and 2 hr later the mice were treated with 5 × 105 cells by tail vein injection. Treatments were repeated at 24 and 48 hr after surgery with 2.5 × 105 cells. Survival of mice was monitored for 7 d, and data are presented as a Kaplan-Meier survival curve, and analyzed by log-rank test. * MSCs versus Fibro, p = 0.034; * MSCs+CO versus Fibro, p = 0.0002; and † MSCs+CO versus Fibro+CO, p = 0.003. B, BALB/c mice were randomly separated into two groups: MSCs, dashed black line, n = 21; and MSCs+CO, solid black line, n = 21. All mice were subjected to CLP, and after 6 hr the mice were treated with 5 × 105 cells by tail vein injection. Survival of mice and data analysis as described in (A). *MSCs+CO versus MSCs, p = 0.016.
Preconditioning of MSCs With CO Further Decreases Organ Injury Associated With Polymicrobial Sepsis
One of the most sensitive organs to the injurious effects of sepsis is the spleen (30). Postmortem spleens from patients dying of sepsis have shown that apoptosis of lymphocytes in the white pulp is a prominent feature of injury (31, 32). In the present study, representative images of splenic tissue 24 hours after CLP surgery showed a marked increase in TUNEL staining in mice receiving fibroblast control cells compared with sham mice (Fig. 2A). The administration of MSCs 6 hours after the onset of sepsis resulted in reduced cell death within the spleens of mice receiving MSCs preconditioned with CO (Fig. 2A). The reduction in liver and kidney injury was also evident in mice receiving MSCs preconditioned with CO. Plasma levels of AST, alanine transaminase, and plasma creatinine were increased in septic mice receiving fibroblasts compared with sham-operated mice (Fig. 2, B and C respectively). However, plasma levels of liver enzymes were decreased in mice receiving MSCs compared with control fibroblasts, and further decreased in mice treated with MSCs preconditioned with CO (Fig. 2B). In regard to creatinine, only administration of MSCs preconditioned with CO was able to reduce the level during sepsis (Fig. 2C).
Figure 2.
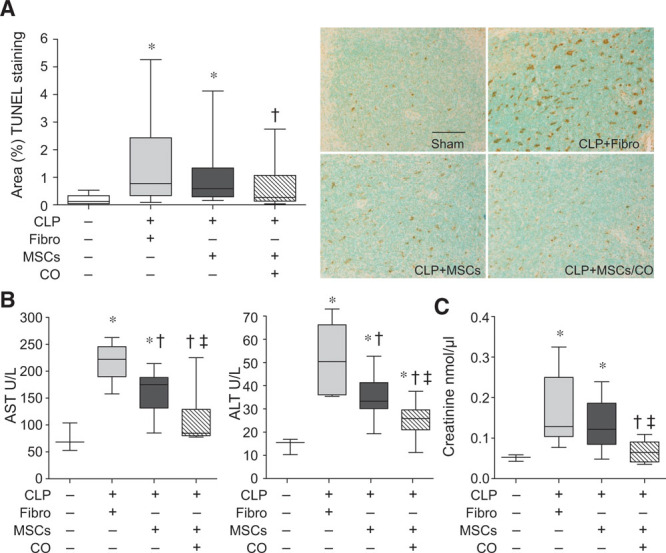
Administration of mesenchymal stromal cells (MSCs) preconditioned with carbon monoxide (CO) decreases tissue injury in cecal ligation and puncture (CLP)-induced sepsis. Mice were subjected to sham or CLP surgery (CLP+fibroblasts [Fibro], CLP+MSCs, CLP+MSCs/CO). A total of 5 × 105 cells were administered by tail vein injection 6 hr after CLP. At 24 hr, mice were anesthetized, and blood and spleens were harvested. A, Spleen injury was evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (brown staining, right), and quantitated (left). The bar in the sham represents a length of 50 μm. Data are presented as box plots, which show median values and interquartile ranges. Analysis was done by Kruskal-Wallis testing (also used in B and C). Sham, white bar, n = 3; CLP+Fibro, light gray bar, n = 7; CLP+MSCs, dark gray bar, n = 8; CLP+MSCs/CO, striped bar, n = 8. p = 0.0001, with significant comparisons * versus sham and † versus CLP+Fibro. B, Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) data are shown as box plots. Sham, n = 3; CLP+Fibro, light gray bar, n = 6; CLP+MSCs, dark gray bar, n = 8; CLP+MSCs/CO, striped bar, n = 9. p = 0.002 and p = 0.0008, respectively, with significant comparisons * versus sham, † versus CLP+Fibro, and ‡ versus CLP+MSCs. C, Plasma creatinine levels are shown as box plots. Sham, n = 3; CLP+Fibro, light gray bar, n = 6; CLP+MSCs, dark gray bar, n = 6; CLP+MSCs/CO, striped bar, n = 5. p = 0.018, and significant comparisons of specific groups * versus sham, † versus CLP+Fibro, and ‡ versus CLP+MSCs.
Preconditioning of MSCs With CO Improves Bacterial Clearance and Increases Neutrophil Phagocytosis
Bacterial clearance was assessed from the peritoneum and blood 24 hours after the onset of polymicrobial sepsis. Mice undergoing CLP-induced sepsis, and receiving control fibroblasts, revealed a marked increase in CFUs for bacteria in the peritoneum (Fig 3A, left) and blood (Fig. 3A, right) compared with sham mice. Mice receiving MSCs, compared with fibroblasts, 6 hours after CLP surgery showed a trend for decreased bacterial CFUs. However, bacterial CFUs in both the peritoneum and blood (Fig. 3, A and B) were significantly decreased in the mice receiving MSCs preconditioned with CO.
Figure 3.
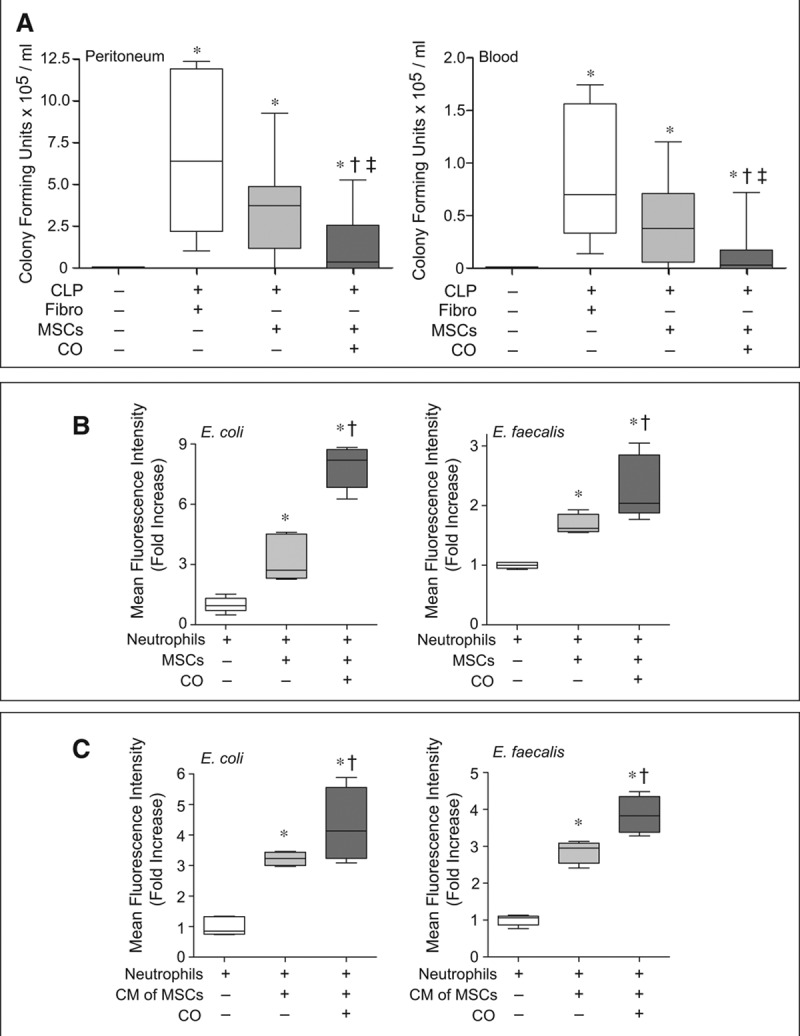
Administration of mesenchymal stromal cells (MSCs) preconditioned with carbon monoxide (CO) improves bacterial clearance in cecal ligation and puncture (CLP)-induced sepsis and increases bacterial phagocytosis by neutrophils. A, Mice were subjected to sham or CLP surgery (CLP+fibroblasts [Fibro], CLP+MSCs, CLP+MSCs/CO). A total of 5 × 105 cells were administered by tail vein injection 6 hr after CLP. At 24 hr, peritoneal fluid (left) and blood samples (right) were collected and analyzed. Data are presented as box plots, which show median values and interquartile ranges. Analysis was done by Kruskal-Wallis testing (also used in B and C). Left, sham, n = 11; CLP+Fibro, white box, n = 6; CLP+MSCs, light gray box, n = 9; CLP+MSCs/CO, dark gray box, n = 12. Right, sham, n = 7; CLP+Fibro, white box, n = 6; CLP+MSCs, light gray box, n = 10; CLP+MSCs/CO, dark gray box, n = 12. p < 0.0001 left and p = 0.001 right, with significant comparisons * versus sham, † versus CLP+Fibro, and ‡ versus CLP+MSCs. B, Isolated neutrophils were incubated with green fluorescent protein (GFP)-labeled E. coli or E. faecalis in the presence of MSCs (light gray bars), MSCs+CO (dark gray bars) or in the absence of MSCs (white bars). Data are presented as box plots, n = 5–7 per groups from three independent experiments. p < 0.0008 for E. coli and p = 0.0022 for E. faecalis, with significant comparisons * versus no MSCs, and † versus MSCs. C, Isolated neutrophils were incubated with GFP-labeled E. coli or E. faecalis with addition of conditioned medium (CM) from MSCs (light gray bars), MSCs+CO (dark gray bars), or basal medium (white bars). Data are presented as box plots, n = 4–5 per group from three independent experiments. p < 0.0064 for E. coli and p < 0.0047 for E. faecalis, with significant comparisons * versus basal medium, and † versus CM of MSCs.
We previously demonstrated that the interaction of MSCs with neutrophils is critical for bacterial clearance after polymicrobial sepsis (12). In the present study, MSCs promoted an increase in phagocytosis of Gram-negative Escherichia coli and Gram-positive Enterococcus faecalis by neutrophils, and exposure of MSCs to CO led to a further increase in neutrophil phagocytosis (Fig. 3B). Similarly, conditioned medium from MSCs was able to increase neutrophil phagocytosis, which was further increased by the conditioned medium from MSCs exposed to CO (Fig. 3C).
Preconditioning of MSCs With CO Promotes the Resolution of Inflammation During Polymicrobial Sepsis
Next we investigated whether MSCs would increase the clearance of apoptotic neutrophils by macrophages in the peritoneum (efferocytosis), which is an important process during the resolution of inflammation (33). Treatment with MSCs 6 hours after the onset of CLP-induced sepsis was able to increase peritoneal efferocytosis compared with fibroblasts; however, MSCs preconditioned with CO had a more marked increase in efferocytosis (Fig 4A).
Figure 4.
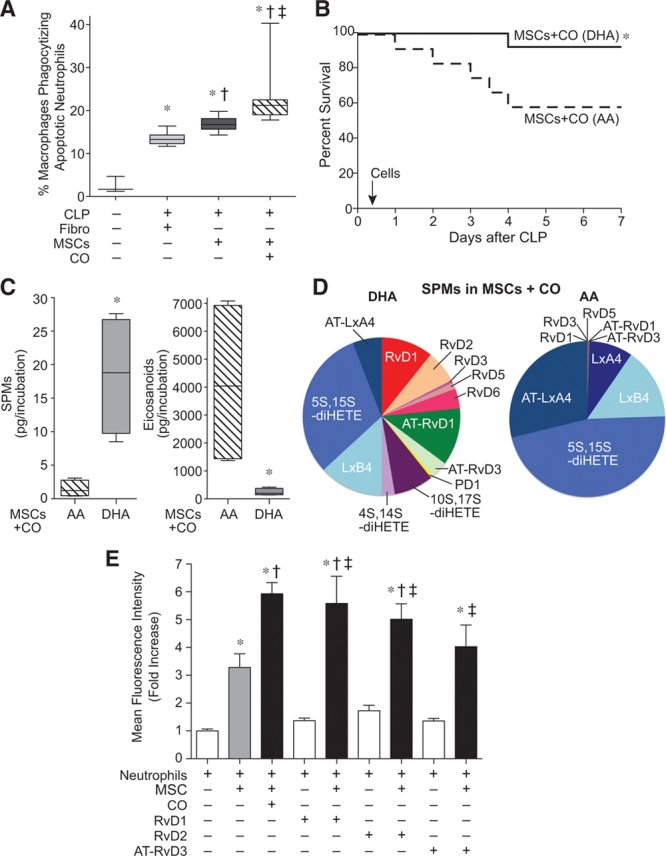
Carbon monoxide (CO) preconditioning induces production of specialized proresolving lipid mediators (SPMs) in mesenchymal stromal cells (MSCs), which mimic the CO effect by enhancing neutrophil phagocytosis of bacteria. A, Mice were subjected to sham or cecal ligation and puncture (CLP) surgery (CLP+fibroblasts [Fibro], CLP+MSCs, CLP+MSCs/CO). A total of 5 × 105 cells were administered by tail vein injection 6 hr after CLP. At 24 hr, peritoneal fluid was collected and efferocytosis assays were performed. Data are presented as box plots, which show median values and interquartile ranges. Sham, n = 3; CLP+Fibro, light gray bar, n = 6; CLP+MSCs, dark gray bar, n = 6; CLP+MSCs/CO, striped bar, n = 7. Analysis was done by Kruskal-Wallis testing (p = 0.0004), with significant comparisons * versus sham, † versus CLP+Fibro, and ‡ versus CLP+MSCs. B, Mice were subjected to CLP, and 6 hr later, the mice received 5 × 105 MSCs treated with CO in the presence of docosahexaenoic acid (DHA) (10 μM), solid black line, n = 12 or MSCs-treated with CO in the presence of arachidonic acid (AA) (10 μM), dashed black line, n = 12. Survival of mice was monitored for 7 d and data are presented as a Kaplan-Meier survival curve, and analyzed by log-rank test. *versus MSCs+CO (AA), p = 0.040. C, Human MSCs were exposed to CO in the presence of DHA or AA, followed by lipid mediator profiling. Total SPMs are shown left, and total eicosanoids right. Data are presented as box plots, n = 4 samples per group from two independent experiments. Analysis was done by Mann-Whitney U testing. * versus MSCs+CO in the presence of AA (p = 0.03 for SPMs and p = 0.03 for eicosanoids). D, Representative pie charts of SPM levels in MSCs conditioned with CO, in the presence of DHA or AA. E, Human neutrophils were incubated with green fluorescent protein-labeled E. coli alone (white bars, n = 16), in the presence of MSCs (gray bar, n = 16), or in the presence of CO-conditioned MSCs (black bar, n = 16) or MSCs conditioned with resolvin D1 (RvD1, 10 nM), Rv D2 (10 nM), or aspirin-triggered Rv D3 (AT-RvD3, 10 nM) (black bars, n = 8). Data are presented as fold increase in mean fluorescence intensity, mean ± sem, from three independent experiments. Analysis was done by one-way analysis of variance (p < 0.0001), with significant comparisons * versus no MSCs, † versus MSCs, and ‡ versus its RvD control. HETE = hydroxyeicosatetraenoic acid, Lx = lipoxin, PD1 = Protectin D1.
Coinciding with the process of efferocytosis, SPMs are produced to help orchestrate the resolution of inflammation (6, 33). Because culture conditions alone, in contrast to the in vivo setting, do not provide the necessary substrates for production of SPMs, we incubated CO preconditioned MSCs with either DHA or AA to help elucidate the mediators contributing to the beneficial MSC response. Figure. 4B demonstrates that administration of CO preconditioned MSCs to mice 6 hours after the onset of CLP-induced sepsis led to a significantly improved survival when the cells were exposed to DHA (92% survival), compared with cells exposed to AA (58% survival). The cells exposed to DHA predominantly expressed SPMs, whereas the cells exposed to AA expressed eicosanoids (Fig. 4C).
To determine SPMs produced by MSCs preconditioned with CO, we performed liquid chromatography-tandem mass spectrometry. Supplemental Figure 6 (Supplemental Digital Content 9, http://links.lww.com/CCM/C16; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21) shows representative multiple reaction monitoring traces (A) for the measured lipid mediators and accompanying tandem mass spectrometry spectra (B) used for identification. As seen in Figure. 4D, SPMs were greater in DHA exposed cells, compared with AA exposed cells, particularly D-series resolvins (Rv). An entire profile of SPMs is shown in Supplemental Table 3 (Supplemental Digital Content 10, http://links.lww.com/CCM/C17), and a complete list of abbreviations is provided in Supplemental Table 4 (Supplemental Digital Content 11, http://links.lww.com/CCM/C18). We next used representative D-series Rv (RvD1, RvD2, and aspirin triggered [AT]-RvD3) to assess their role in the interaction of MSCs with neutrophils to promote bacterial phagocytosis. To confirm this response was applicable in human cells, we used human MSCs and neutrophils. MSCs increased the phagocytosis of E. coli by neutrophils, and MSCs preconditioned with CO produced an enhanced phagocytic response (Fig. 4E). Incubation of neutrophils with RvD1, RvD2, or AT-RvD3 alone showed evidence of neutrophil phagocytosis of E. coli. However, when MSCs were exposed to either RvD1 or RvD2, and then incubated with neutrophils, phagocytosis was robustly increased compared with neutrophils exposed to RvDs alone or MSCs alone, to a level analogous to neutrophils incubated with MSCs preconditioned with CO (Fig. 4E). Exposure of MSCs to AT-RvD3 did not promote neutrophil phagocytosis to a level significantly greater than neutrophils exposed to MSCs alone.
5-LOX and 12/15-LOX Are Critical Enzymes for CO-Induced MSC Function and Benefit in Sepsis
LOXs are the enzymes responsible for the biosynthesis of SPMs, with both 5-LOX and 12/15-LOX pathways contributing to the production of Rv from DHA (34, 35). To further assess the role of SPMs in promoting neutrophil phagocytosis by MSCs, we pretreated MSCs with the LOX inhibitor baicalein, and then incubated ambient air- or CO-exposed MSCs with neutrophils. Baicalein blunted the ability of ambient air-exposed MSCs to promote neutrophil phagocytosis, and the LOX inhibitor abolished the enhanced phagocytic response of neutrophils exposed to CO preconditioned MSCs (Fig. 5A). This lack of a CO response was evident in both human (Fig. 5A, left) and mouse (Fig. 5A, right) MSCs. To delineate the response of LOX enzymes to CO, we exposed either MSCs or control fibroblasts to ambient air or CO (250 ppm) and then assessed the level of 5-LOX or 12/15-LOX messenger RNA (mRNA) by qRT-PCR. The basal levels of both 5-LOX and 12/15-LOX were low in fibroblasts and not inducible by CO. Interestingly, the basal level of 5-LOX was significantly greater in MSCs compared with fibroblasts, and CO caused a further increase in 5-LOX expression (Fig. 5B, top). In contrast, the basal level of 12/15-LOX was not significantly different between fibroblasts and MSCs; however, the exposure of CO caused a marked increase in the level of 12/15-LOX (Fig. 5B, bottom). Thus, in MSCs, both the 5-LOX and 12/15-LOX enzymes are induced by exposure to CO.
Figure 5.
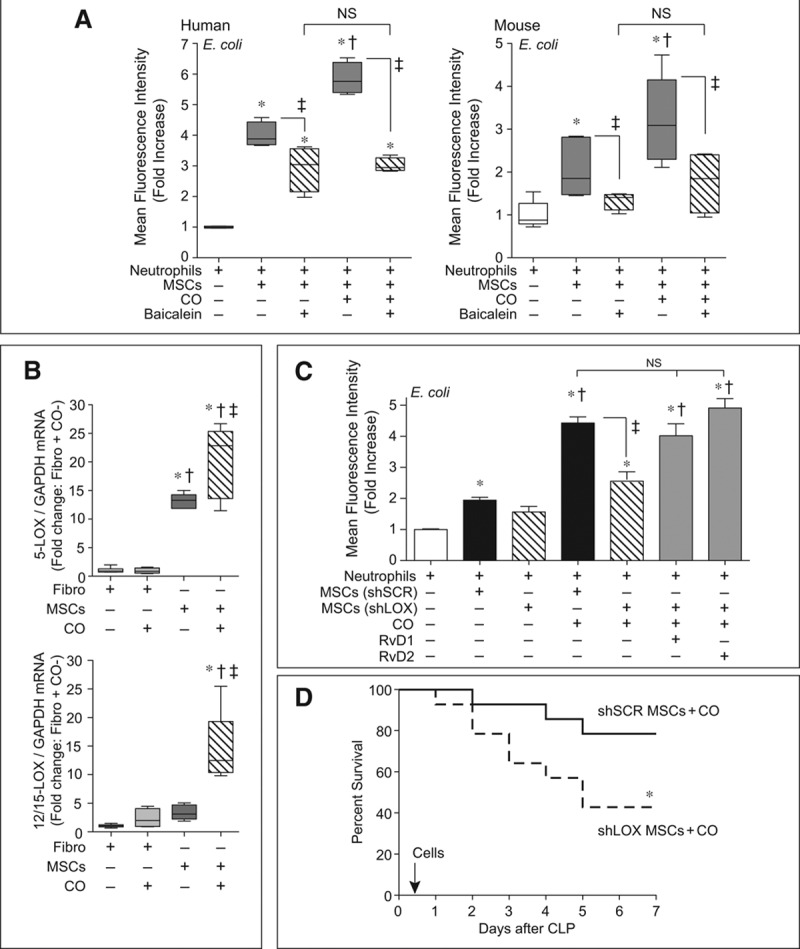
Lipoxygenase pathways contribute to the carbon monoxide (CO)-mediated effects of mesenchymal stromal cells (MSCs) on neutrophil phagocytosis, and silencing of 5-lipoxygenase (LOX) and 12/15-LOX results in a loss of improved MSC efficacy by CO conditioning. A, MSCs were pretreated with baicalein (10 μM) or vehicle, and then cocultured with human (left) or mouse (right) neutrophils in the presence or absence of green fluorescent protein (GFP)-labeled E. coli. Data are presented as box plots, which show median values and interquartile ranges. Analysis was done by Kruskal-Wallis testing (also used in B). n = 4–5 per group from three independent experiments. p = 0.0016 and p = 0.0062, human and mouse, respectively, and significant comparisons of specific groups * versus no MSCs, † versus MSCs, and ‡ between designated groups. B, Fibroblasts (Fibro) and MSCs were incubated with CO or ambient air for 4 hr, and then RNA was extracted and quantitative real-time polymerase chain reaction performed for 5-LOX (upper) and 12/15-LOX (lower). Data are presented as box plots, n = 4–6 per group from three independent experiments. p = 0.0008 for 5-LOX and 12/15-LOX, with significant comparisons * versus Fibro, † versus Fibro+CO, and ‡ versus MSCs. C, MSCs were silenced with scrambled (SCR), or both 5-LOX and 12/15-LOX = small hairpin RNAs (shRNAs) (method used to silence 5-LOX and 12/15 LOX (shLOX [defined in the narrative of the article]). The MSCs were exposed to CO or ambient air, and then cocultured with neutrophils in the presence of GFP-labeled E. coli. shLOX MSCs exposed to CO were also exposed to resolvin D1 (RvD1, 10 nM) or Rv D2 (10 nM). Data are presented as fold increase in mean fluorescence intensity, mean ± sem, n = 6–12 per group from three independent experiments. Analysis was done by one-way analysis of variance (p < 0.0001) with significant comparisons * versus no MSCs, † versus MSCs (SCR shRNA sequence [shSCR]), and ‡ between designated groups. D, BALB/c mice were randomly separated into two groups, shSCR MSCs+CO (solid black line, n = 14) or shLOX MSCs+CO (dashed black line, n = 14). Six hours after cecal ligation and puncture (CLP), the mice were treated with 5 × 105 cells by tail vein injection. Animal survival was monitored for 7 d, and data are presented as a Kaplan-Meier survival curve, and analyzed by log-rank test. * versus shSCR MSCs+CO, p = 0.049. GAPDH = glyceraldehyde-3-phosphate dehydrogenase, mRNA = messenger RNA, NS = not significant.
Finally, to determine whether the 5-LOX and 12/15-LOX enzymes are critical for MSC function, we silenced these enzymes in mouse MSCs (silencing of 5-LOX and 12/15 LOX [shLOX] [as described in the narrative]) or introduced a scrambled shRNA sequence (shSCR). Supplemental Figure 7 (Supplemental Digital Content 12, http://links.lww.com/CCM/C19; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21) demonstrates a reduction in 5-LOX and 12/15-LOX mRNA, protein, and a reduction in RvD1 and RvD2 production in shLOX cells. Although shSCR MSCs produced an increase in neutrophil phagocytosis, there was no significant increase in neutrophil phagocytosis by shLOX MSCs (targeting both 5-LOX and 12/15-LOX enzymes). Even more impressive, shLOX MSCs preconditioned with CO had a markedly blunted effect on neutrophil phagocytosis compared with SCR shRNA MSCs preconditioned with CO (Fig. 5C). This blunted neutrophil phagocytosis response was rescued by administration of exogenous RvD1 or RvD2. Silencing of 5-LOX or 12/15-LOX enzymes individually did not significantly blunt the MSC response (Supplemental Fig. 8, Supplemental Digital Content 13, http://links.lww.com/CCM/C20; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21), suggesting that both enzymes contribute to the ability of MSCs to promote neutrophil phagocytosis, particularly after preconditioning with CO. This effect of silencing both 5-LOX and 12/15-LOX in MSCs was also translated to their in vivo response. shLOX MSCs preconditioned with CO and administered to septic mice 6 hours after CLP had a decreased survival (43%) compared with shSCR MSCs exposed to CO (79%) (Fig. 5D). These data suggest that silencing of 5-LOX and 12/15-LOX enzymes led to a loss of improved efficacy afforded to MSCs by preconditioning with CO. Interestingly, the survival rate of mice receiving shLOX MSCs preconditioned with CO (43%) is comparable with the survival rate of mice receiving MSCs not preconditioned with CO (38%), when injected 6 hours after CLP.
DISCUSSION
Gas molecules have been proposed as therapeutic agents for a variety of disease processes, including lung and even systemic illnesses (36, 37). One such gas is CO, which has been shown to be beneficial therapeutically in animal models of sepsis (16, 20, 21). Although preclinical studies and clinical trials are ongoing to more fully evaluate the safety and effectiveness of administering CO in vivo for the treatment of human diseases (38), the goal of the present study was to promote the beneficial effects of CO on MSCs ex vivo and then administer the cells after the onset of polymicrobial sepsis in mice.
Studies have investigated various molecular and genetic modifications of MSCs to improve cell viability and function, with particular interest in cardiovascular diseases (9, 39). MSCs are known to repair injured tissues without evidence for significant engraftment or differentiation, through paracrine actions (40). Conditioning of MSCs with cytokines, growth factors, and toll-like receptor ligands has also been performed in an effort to increase their therapeutic potential, with an interest in modulation of inflammatory responses (41–43). To our knowledge, the present study is the first use of preconditioning MSCs with CO ex vivo to treat experimental sepsis.
HO-1 and its product CO have known cytoprotective properties (44), and it has been shown in MSCs that overexpression of HO-1 or incubation with compounds that release CO can increase cell viability during myocardial ischemia and neural differentiation, respectively (45, 46). We also show that in the setting of increased oxidative stress, as occurs during sepsis, preconditioning of MSCs with CO is able to increase cell viability (Supplemental Fig. 5, Supplemental Digital Content 8, http://links.lww.com/CCM/C15; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21). Furthermore, we now demonstrate that MSCs preconditioned with CO have improved efficacy, allowing therapy to be administered at later time points after the onset of sepsis, and with fewer total cells, whereas nonconditioned MSCs lose their therapeutic efficacy. Thus, CO preconditioned MSCs lead to increased survival (Fig. 1), less organ injury (Fig. 2), and improved bacterial clearance (Fig. 3) during experimental sepsis.
Once the invading bacteria are cleared, the resolution of inflammation is necessary to regain homeostasis and prevent organ injury during sepsis. SPMs are produced to coordinate the resolution response (6, 33, 35). In the presence of DHA substrate, MSCs exposed to CO predominantly produced SPMs and improved survival when administered after the onset of sepsis, whereas MSCs exposed to AA substrate resulted in eicosanoid production and reduced survival (Fig. 4). When assessing the differences in SPMs, the production of Rv family members was greater in cells exposed to DHA versus AA (Fig. 4). Interestingly, it was previously shown by Spite et al (47) that RvD2 improved survival in CLP-induced polymicrobial sepsis by regulating the inflammatory response, phagocytosis, and bacterial clearance. Taken together, these data suggest that production of SPMs (particularly D-series Rv) may contribute to the improved therapeutic response of MSCs exposed to CO ex vivo.
To confirm the importance of SPM production in MSCs preconditioned with CO, we exposed MSCs to a LOX inhibitor (baicalein) or silenced both 5-LOX and 12/15-LOX enzymes in MSCs. Either maneuver resulted in a loss of enhanced neutrophil phagocytosis induced by CO preconditioned MSCs (Fig. 5, A and C). In addition, silencing of both 5-LOX and 12/15-LOX resulted in a loss of survival benefit for MSCs preconditioned with CO, and a loss of MSCs efficacy when administered 6 hours after CLP-induced sepsis (Fig. 5D).
It has been suggested that exposure to CO promotes a beneficial response, in part, through a positive feedback loop increasing the production of its parent enzyme HO-1 (22, 48). In addition, the proresolving lipid mediator lipoxin A4 (LXA4) is known to increase HO-1 expression (49). However, we found that endogenous HO-1 was not required for the beneficial CO preconditioning response of MSCs during sepsis because HO-1–deficient cells were as effective as wild-type MSCs in protecting mice from CLP-induced mortality (Supplemental Fig. 4, Supplemental Digital Content 7, http://links.lww.com/CCM/C14; legend, Supplemental Digital Content 14, http://links.lww.com/CCM/C21).
Recently it was shown that human MSCs cocultured with alveolar epithelial type II cells have increased production of LXA4 in the presence of proinflammatory cytokines (50). MSCs were also able to promote the resolution of acute lung injury induced by E. coli lipopolysaccharide, and this occurred in part by production of LXA4 (50). Conversely, in our study, LxA4 was expressed by CO preconditioned MSCs only in the presence of AA (Fig. 4D; and Supplemental Table 3, Supplemental Digital Content 10, http://links.lww.com/CCM/C17), which resulted in the production of predominantly eicosanoids (Fig. 4C) and led to decreased survival in CLP-induced sepsis (Fig. 4B). The imbalance of increased production of AA-derived proinflammatory eicosanoids, and less production of SPMs (i.e., LxA4), likely contributed to a worse outcome. Taken together, these data suggest that LXA4 is not playing as significant of a role in the MSC response to CO exposure, and its effect on outcome during polymicrobial sepsis.
Although the present study advanced our understanding of the therapeutic effects of MSCs in sepsis, we are presently limited to using a mouse model of disease. Furthermore, MSCs were not used in conjunction with antibiotics and hemodynamic resuscitation, standards of care for human patients, and they will need to be tested in the future. We believe that conditioning of MSCs with CO has the potential for future application; however, in this early stage of investigation, CO-conditioned MSCs are not immediately translatable to clinical application.
CONCLUSION
In the present study, we demonstrate that preconditioning of MSCs with CO gas improved MSC function and therapeutic efficacy during sepsis. Data including inhibition or silencing of LOX enzymes (5-LOX and 12/15-LOX) support the concept that the increased beneficial response of MSCs exposed to CO occurred in part through the production of SPMs, which contributed to the interaction of MSCs with neutrophils to promote bacterial phagocytosis, resolution of inflammation, and increased survival. We propose that MSCs preconditioned with CO may have future implications for the treatment of sepsis, allowing a beneficial response even with a delayed initiation of therapy.
ACKNOWLEDGMENTS
We thank Paul C. Norris and Iliyan K. Vlasakov for their assistance with the analyses of specialized proresolving lipid mediators.
Supplementary Material
Footnotes
*See also p. 2296.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by National Institutes of Health grants HL108801 (to Drs. Fredenburgh, Baron, Choi, Serhan, and Perrella), HL102897 (to Dr. Perrella) and American Heart Association grant 11SDG722018 (to Dr. Liu).
Dr. Perrella received support for article research from the National Institutes of Health (NIH). His institution received funding from the NIH (National Heart, Lung, and Blood Institute [NHLBI]). Dr. Tsoyi received support for article research from the NIH. His institution received funding from the NIH. Drs. Dalli and Colas received support for article research from the NIH. Drs. Ghanta and Ith received support for article research from the NIH. Their institutions received funding from the NIH. Dr. Fredenburgh received support for article research from the NIH. Her institution received funding from the NHLBI. Drs. Baron and Choi received support for article research from the NIH. Dr. Serhan received support for article research from the NIH and disclosed other support (SAB member Corbus, SAB member Inflammation Foundation, SAB Solutex. He is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. He is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company [no current relationship but still hold founder stock]. His interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies). Dr. Liu received support for article research from the NIH and the AHA. Her institution received funding from the NIH and the AHA. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE. Surviving sepsis: Going beyond the guidelines. Ann Intensive Care. 2011;1:17. doi: 10.1186/2110-5820-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opal SM, Dellinger RP, Vincent JL, et al. The next generation of sepsis clinical trial designs: What is next after the demise of recombinant human activated protein C?. Crit Care Med. 2014;42:1714–1721. doi: 10.1097/CCM.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 9.Wannemuehler TJ, Manukyan MC, Brewster BD, et al. Advances in mesenchymal stem cell research in sepsis. J Surg Res. 2012;173:113–126. doi: 10.1016/j.jss.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 10.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Rey E, Anderson P, González MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 12.Hall SR, Tsoyi K, Ith B, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: The importance of neutrophils. Stem Cells. 2013;31:397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 15.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung SW, Liu X, Macias AA, et al. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maines MD, Gibbs PE. 30 some years of heme oxygenase: From a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun. 2005;338:568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 18.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ryter SW, Morse D, Choi AM. Carbon monoxide and bilirubin: Potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol. 2007;36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoetzel A, Dolinay T, Schmidt R, et al. Carbon monoxide in sepsis. Antioxid Redox Signal. 2007;9:2013–2026. doi: 10.1089/ars.2007.1762. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Lee SJ, Coronata AA, et al. Carbon monoxide confers protection in sepsis by enhancing beclin 1-dependent autophagy and phagocytosis. Antioxid Redox Signal. 2014;20:432–442. doi: 10.1089/ars.2013.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang N, Shinohara M, Dalli J, et al. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013;190:6378–6388. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardini C, Zannoni A, Bacci ML, et al. Protective effect of carbon monoxide pre-conditioning on LPS-induced endothelial cell stress. Cell Stress Chaperones. 2010;15:219–224. doi: 10.1007/s12192-009-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira HL, Queiroga CS, Alves PM. Pre-conditioning induced by carbon monoxide provides neuronal protection against apoptosis. J Neurochem. 2008;107:375–384. doi: 10.1111/j.1471-4159.2008.05610.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakao A, Faleo G, Shimizu H, et al. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008;74:1009–1016. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- 26.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 27.Fredenburgh LE, Velandia MM, Ma J, et al. Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J Immunol. 2011;187:5255–5267. doi: 10.4049/jimmunol.1101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colas RA, Shinohara M, Dalli J, et al. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsoyi K, Geldart AM, Christou H, et al. Elk-3 is a KLF4-regulated gene that modulates the phagocytosis of bacteria by macrophages. J Leukoc Biol. 2015;97:171–180. doi: 10.1189/jlb.4A0214-087R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tinsley KW, Grayson MH, Swanson PE, et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 33.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 34.Kohli P, Levy BD. Resolvins and protectins: Mediating solutions to inflammation. Br J Pharmacol. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryter SW, Choi AM. Gaseous therapeutics in acute lung injury. Compr Physiol. 2011;1:105–121. doi: 10.1002/cphy.c090003. [DOI] [PubMed] [Google Scholar]
- 37.Baumgart K, Radermacher P, Wagner F. Applying gases for microcirculatory and cellular oxygenation in sepsis: Effects of nitric oxide, carbon monoxide, and hydrogen sulfide. Curr Opin Anaesthesiol. 2009;22:168–176. doi: 10.1097/ACO.0b013e328328d22f. [DOI] [PubMed] [Google Scholar]
- 38.Ryter SW, Choi AM. Carbon monoxide: Present and future indications for a medical gas. Korean J Intern Med. 2013;28:123–140. doi: 10.3904/kjim.2013.28.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trivedi P, Tray N, Nguyen T, et al. Mesenchymal stem cell therapy for treatment of cardiovascular disease: Helping people sooner or later. Stem Cells Dev. 2010;19:1109–1120. doi: 10.1089/scd.2009.0465. [DOI] [PubMed] [Google Scholar]
- 40.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: Implications on therapeutic potential. Mediators Inflamm. 2010;2010:865601. doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perdoni C, McGrath JA, Tolar J. Preconditioning of mesenchymal stem cells for improved transplantation efficacy in recessive dystrophic epidermolysis bullosa. Stem Cell Res Ther. 2014;5:121. doi: 10.1186/scrt511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Akker F, de Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: Immunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm. 2013;2013:181020. doi: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morse D, Lin L, Choi AM, et al. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbagallo I, Tibullo D, Di Rosa M, et al. A cytoprotective role for the heme oxygenase-1/CO pathway during neural differentiation of human mesenchymal stem cells. J Neurosci Res. 2008;86:1927–1935. doi: 10.1002/jnr.21660. [DOI] [PubMed] [Google Scholar]
- 46.Tsubokawa T, Yagi K, Nakanishi C, et al. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298:H1320–H1329. doi: 10.1152/ajpheart.01330.2008. [DOI] [PubMed] [Google Scholar]
- 47.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vummaleti SV, Branduardi D, Masetti M, et al. Theoretical insights into the mechanism of carbon monoxide (CO) release from CO-releasing molecules. Chemistry. 2012;18:9267–9275. doi: 10.1002/chem.201103617. [DOI] [PubMed] [Google Scholar]
- 49.Biteman B, Hassan IR, Walker E, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 2007;21:2257–2266. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- 50.Fang X, Abbott J, Cheng L, et al. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J Immunol. 2015;195:875–881. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]


