Abstract
Thirty samples from thirteen Sicilian monofloral honeys by the local black honeybee, and two honeydew honeys, were studied to assess phenol content, reducing power and antioxidant capacity as well as correlations among these parameters. Honeys from Apiaceae showed the highest phenol amount and capacity to reduce ferric ion and stable chemical radicals, whereas honeys from Leguminosae the lowest. All honeys were active against myoglobin-derived radicals usually formed in red meat after storage and/or heating and significant correlation (p = 0.023) was found between flavonoid content and deactivation rate of this radical. Dill > almond > tangerine > thistle > sulla honeys inhibited formation of lipoperoxides in either iron/ascorbate or azoinitiator -induced membrane lipid oxidation, whereas eucalyptus honey was mostly effective in the metal-dependent model. Honeys by black honeybee possess remarkable reducing power and antioxidant potential against radicals of interest in dietary foodstuffs.
Keyword: Food science
1. Introduction
Honey is the result of multifarious relationships between bees and their environment. Organoleptic characteristics and properties of honeybee honey may widely vary according to the species of flowers and plants, the environment where plants grow, and the insect itself. The Sicilian black honeybee (Apis mellifera ssp. sicula) is an African subspecies of Apis mellifera. Because of the African origin the Sicilian black bee had the benefit of adapting in hot and hard lands including the regions of the Mediterranean area like Sicily (Italy). The black honeybee differs from the more common Apis mellifera ssp. ligustica for both morphologic and physiologic characteristics, such as color and wing dimensions, better resistance to high temperature, higher ability of impollination, and higher physical and immunological resistance. Established in Sicily since the last ice age of the Quaternary, the Sicilian black bee risked extinction in the 70s of last century when beekeepers began to import the subspecies ligustica, erroneously considered more docile and productive, from northern Italy. Things had a turning point when a group of entomologists and beekeepers managed to keep the sicula subspecies genetically pure by transferring some old hives on the island of Ustica, about 60 km off the coast of Sicily, where the selected bees were raised without the risk of contamination. A Slow Food Presidium founded in 2008 (www.slowfood.org) then started a reintegration plan in western Sicily, including fertilization stations to reproduce the Sicilian black honeybee in purity and periodically check the purity of the subspecies by genetic screening. Nowadays eight beekeepers in western Sicily preserve and breed the Apis mellifera ssp. sicula queens and their swarms, thus producing the only honey entirely made by the black honeybee.
In view of the characteristics of the Sicilian black honeybee, scientific interest has grown for components and biological properties of its honey. Chemical characteristics and antimicrobial properties of some monofloral Sicilian honeys produced by Apis mellifera ssp. sicula have recently been reported (Mannina et al., 2015; Tenore et al., 2012). This study extends knowledge on functional properties of honeys produced by the Sicilian black honeybee assessing their antiradical and antioxidant capacity in several both chemical and biological in vitro models. Thirty samples of monofloral honeys from thirteen botanical species and two honeydew honeys were assayed in comparison with the manuka honey from New Zealand, known as innovative and with specific biomedical properties all over the world (Molan, 1999; Stephens et al., 2010; Carter et al., 2016). With respect to manuka honey, honeys by black honeybee possess remarkable reducing power and antioxidant potential against radicals relevant in dietary foodstuffs.
2. Material and methods
2.1. Honey samples
Thirty samples of A. mellifera ssp. sicula honeys from the floral sources reported below were obtained from eight beekeepers in the area of western Sicily (Italy) in 2015: acacia (Robinia pseudoacacia L.), almond (Prunus dulcis L.), astragalus (Astragalus membranaceus F.), carob (Ceratonia siliqua, L.), dill (Anethum graveolens L.), eucalyptus (Eucalyptus globules L.), ferula (Ferula communis L.), lemon (Citrus limon, L.), medlar (Mespilus germanica L.), orange (Citrus arantium L.), sulla (Hedysarum coronarium L.), tangerine (Citrus reticulate L.), thistle (Silybum marianum L.). In addition, A. mellifera ssp. sicula honeydew honeys from eucalyptus and citrus were also studied. The botanical origin of honey samples was assessed by palynological analysis. The honeys were kept at room temperature in the dark in airtight containers for less than 3 months until the analysis. One commercial sample of Manuka honey (20+ active Royal Bee, New Zealand), was obtained from a local store (Palermo, Italy). The floral source of the honeydew honeys in the study was assumed on the basis of the species growing in the region where the honeys were produced and on statements of the beekeepers.
Artificial honey (100 g), prepared by dissolving 1.5 g sucrose, 7.5 g maltose, 40.5 g fructose and 33.5 g glucose in 17 ml of distilled water and mixing for 1 h.
2.2. Total phenol and flavonoid content
The Folin–Ciocalteu reaction, based on the reduction of phosphotungstic–phosphomolybdic acid (Folin–Ciocalteu's reagent) to blue reaction products in alkaline solution (Singleton et al., 1999), was used to determine total phenol content (TPC). Honey solutions (10% w/v) were prepared in distilled water and aliquots (100–500 μL), in a final volume of 3.5 mL of water, were mixed with 0.5 mL Folin–Ciocalteu reagent for 4 min. Then 2 ml of 10% sodium carbonate and 4 mL of water were added. Incubation at room temperature for 90 min was carried out, then absorbance of the reaction mixture was measured spectrophotometrically in a DU640 Beckman spectrophotometer (Beckman, Milan, Italy), at 700 nm against a blank without honey. Quantitation was by reference to gallic acid (5–100 μg/mL), and results expressed as gallic acid equivalents (GAE, milligrams/100 g honey).
Total flavonoid content (TF) was determined according to the colorimetric assay developed by Zhishen et al. (1999). Aliquots of honey solutions (20–200 μL) were mixed with distilled water (final volume 4 mL). At baseline, 0.3 mL of NaNO2 (5%, w/v) were added, followed by 0.3 mL AlCl3 (10% w/v) after 5 min, and by the addition of 2 mL of NaOH (1 M) 6 min later. The volume was then immediately increased to 10 mL by the addition of 2.4 mL distilled water. The mixture was vigorously shaken to ensure adequate mixing, and the absorbance measured at 510 nm. A calibration curve was prepared using a solution of quercetin (10–25 μg/mL) and the results expressed as milligram quercetin equivalents (QE) per 100 g honey.
2.3. Reducing capacity test
Ferric ion reducing antioxidant power (FRAP) assay was carried out according to Saxena et al. (2010) with some modifications. One milliliter of honey solution in water (10% w/v) was mixed with 1 mL of 0.2 M phosphate buffer (pH 6.6). The reaction mixture was mixed with 1 mL of potassium ferricyanide (1%) and incubated for 20 min at 50 °C. Trichloroacetic acid (10%, 1 mL) was added followed by thorough mixing via vortex mixer. The reaction mixture thus obtained was centrifuged at 1000 g, for 10 min. A 2.5 mL aliquot of supernatant was mixed with 5 mL of double distilled water and 0.5 mL of FeCl3 solution (0.1%). Absorbance was measured at 700 nm. Ascorbic acid (0–100 μg/mL) was used as a reference compound and the results were expressed as milligrams of ascorbic acid equivalents (AAE) per 100 g honey.
2.4. Radical scavenging activity assays
2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)•+ radical cation was prepared following the method of Pellegrini et al. (1999) by reacting ABTS with potassium persulfate. The stock solutions included 7.0 mM ABTS solution and 140 mM potassium persulfate solution. The working solution was prepared by mixing 1 mL ABTS solution with 18 μL potassium persulfate solution and allowing them to react for 18 h at room temperature in the dark. The solution was then diluted by mixing 1 mL ABTS•+ solution with 5 mM phosphate saline buffer to obtain an absorbance of 0.700 ± 0.020 units at 734 nm. Aliquots (10 μL) of properly diluted honey solution, were allowed to react with 1 mL of the ABTS•+ solution for 15 min in the dark and then the absorbance at 734 nm was measured again.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity was measured according Brand-Williams et al. (1995). Aliquots (10 μL) of properly diluted honey solution, were added to 1 mL of DPPH ethanol solution (1 × 10−4 mol/L) and the absorbance was determined at 515 nm after a 30 min incubation at room temperature, in the dark.
Perferryl-myoglobin radical (•Mb[FeIV=O]) was formed by oxidation of horse met-Mb, at 37 °C, with H2O2. Briefly, in a reaction mixture containing 100 μM met-Mb in 0.1 M sodium acetate, pH 5.0, 100 μМ H2O2 was added either in the absence or in the presence of 10 μL of properly diluted honey solution. Spectrophotometric scans were monitored at 400–800 nm (Patel et al., 1996) before and 5 min after the H2O2 addition. The hypervalent iron-concentration was calculated from the absorbance at 556 nm, the point at which the two forms differ most (ε = 3.6 mMcm−1) (Grinberg et al., 1994). Myoglobin spectral changes in the visible region were measured on a Beckman DU 640 spectrophotometer (Beckman, Milan, Italy), equipped with a temperature controller.
The ABTS•+, DPPH and •Mb[FeIV=O] radical scavenging activities of honeys were evaluated in comparison with Trolox, the water-soluble analog of vitamin E, and the results expressed as micromol of Trolox equivalents (TE) per 100 g of honey.
2.5. Metal -dependent and -independent lipid oxidation of bovine brain microsomes
Bovine brain microsomes were prepared from a tissue homogenate in 10 mM phosphate buffer saline, pH 7.4 (PBS), by differential centrifugation. Microsomes in PBS (2 mg protein/mL) were incubated at 37 °C either in the absence (control) or in the presence of honey samples (0.5 mg whole honey/mL). Lipid oxidation was induced by either azo-initiator [2,2′-azobis (2-amidino-propane) dihydrochloride] (AAPH) or FeCl3/ascorbic acid; formed lipid hydroperoxides were monitored after reaction with thiobarbituric acid (TBA), as TBA-reactive substances (TBA-RS, Gentile et al., 2007). The results were presented as nmol of MDA equiv/mg of protein, using the molar extinction coefficient of 156,000.
Proteins in microsomal preparations were determined by the Bio Rad colorimetric method (Bradford, 1976).
2.6. Statistical analyses
Graph Pad INSTAT software (GraphPad software, San Diego, CA, USA) was used to calculate the means and standard deviations of three independent experiments involving duplicate analyses for each sample/condition. A one-way ANOVA was performed on the means to determine whether they differed significantly. P values < 0.05 were regarded as significant. The degree of linear relationship between two variables was measured using the Pearson product moment correlation coefficient (r).
3. Results and discussion
This work evaluates and compares total phenols and flavonoid content, as well as antiradical and antioxidant capacities, of thirty samples of monofloral honeys produced by A. mellifera ssp. Sicula from thirteen botanical species and of two honeydew honeys, obtained from different beekeepers in western Sicily. Water solutions of whole honey have been used throughout the study. Artificial honey was used as a reference throughout to disclose the eventual contribution from characteristic reducing sugar components to the antioxidant capacity of honeys. The artificial honey did not show any activity in all assays.
3.1. Total phenol and flavonoid content
The TPC and TF of honeys ranged from 16.5 ± 0.8 to 133.3 ± 4.8 mg GAE/100 g and 4.0 ± 0.01 to 82.1 ± 0.23 QE/100 g, respectively (Table 1). Phenolic and flavonoid content of honey is related with the floral origin (Amiot et al., 1989; Ferreres et al., 1991; Gil et al., 1995; Martos et al., 2000a, Martos et al., 2000b; Tomás-Barberán et al., 2001; Vit et al., 1997). In accordance, TPC and TF in the honeys by A. mellifera ssp. sicula varied with the plant family, with the lowest value in honeys from Leguminosae (acacia, astragalus, sulla and carob) and the highest in those from Apiaceae (ferula and dill) (Table 1). The amount of TPC and TF of honey from Rosaceae (almond, medlar) in our study were lower than those reported by Tenore et al. (2012) in honeys of the same monofloral origin produced by the Sicilian black honeybee in 2011. Climate variability and seasonal factors can influence the content of phenol components in honeys, as previously reported by other authors. (Al-Mamary et al., 2002; Yao et al., 2005; Cowan, 1999). Commercial honey of manuka (L.scoparium, L.), used as a “gold standard” showed values of TPC and TF two times lower than those observed in ferula and dill honeys, and comparable to those found in honey from eucalyptus, a species belonging to the same Myrtaceae family as manuka (Table 1).
Table 1.
A compilation of dataa from 30 honey and 2 honeydew honey samples produced by the Sicilian black honeybees (Apis mellifera ssp. sicula).
| Floral origin | N° of sample | TPC | TF | FRAP | DPPH• |
ABTS•+ |
•Mb[FeIV=O] deactivation rate |
|---|---|---|---|---|---|---|---|
| mg GAE/100g | mg QE/100g | mg AAE/100g | μmolTE/100g | ||||
| Leguminosae | |||||||
| Sulla | 1 | 16.5 ± 0.8 | 4.8 ± 0.02 | 17.3 ± 0.6 | 23.9 ± 1.1 | 30.4 ± 1.1 | 7.8 ± 0.3 |
| 2 | 17.8 ± 0.5 | 5.6 ± 0.03 | 19.3 ± 0.8 | 25.7 ± 0.9 | 33.1 ± 0.9 | 8.5 ± 0.5 | |
| 3 | 20.1 ± 0.9 | 4.4 ± 0.02 | 16.1 ± 0.7 | 26.9 ± 1.2 | 32.7 ± 0.8 | 7.7 ± 0.6 | |
| 4 | 19.2 ± 0.9 | 4.0 ± 0.01 | 18.3 ± 0.8 | 22.3 ± 1.0 | 31.5 ± 1.0 | 6.9 ± 0.4 | |
| Acacia | 5 | 18.2 ± 0.7 | 7.6 ± 0.04 | 21.1 ± 1.1 | 24.1 ± 2.3 | 25.5 ± 1.2 | 4.6 ± 0.3 |
| Carob tree | 6 | 25.3 ± 1.8 | 8.3 ± 0.30 | 60.9 ± 2.5 | 40.2 ± 2.2 | 49.3 ± 1.9 | 4.8 ± 0.3 |
| Astragalus | 7 | 30.3 ± 1.2 | 10.9 ± 0.05 | 69.4 ± 2.8 | 17.5 ± 0.8 | 46.2 ± 2.3 | 4.2 ± 0.3 |
| 8 | 37.8 ± 2.0 | 12.1 ± 0.06 | 71.3 ± 3.0 | 22.9 ± 1.2 | 54.8 ± 3.1 | 3.9 ± 0.3 | |
| Rutaceae | |||||||
| Tangerine | 9 | 29.4 ± 1.1 | 8.5 ± 0.03 | 22.9 ± 0.8 | 8.5 ± 0.3 | 19.2 ± 0.7 | 4.4 ± 0.3 |
| 10 | 32.4 ± 1.7 | 9.0 ± 0.04 | 24.7 ± 1.2 | 18.4 ± 0.6 | 27.5 ± 0.6 | 4.1 ± 0.2 | |
| Lemon | 11 | 30.8 ± 1.2 | 6.5 ± 0.06 | 30.4 ± 1.2 | 22.0 ± 0.8 | 38.9 ± 0.9 | 3.2 ± 0.2 |
| Orange | 12 | 36.5 ± 1.1 | 8.1 ± 0.04 | 24.3 ± 0.8 | 26.5 ± 0.6 | 29.3 ± 7.4 | 3.4 ± 0.2 |
| 13 | 25.4 ± 0.7 | 9.5 ± 0.04 | 23.9 ± 0.9 | 29.1 ± 0.8 | 37.5 ± 6.9 | 3.9 ± 0.2 | |
| Asteraceae | |||||||
| Thistle | 14 | 28.4 ± 1.1 | 17.1 ± 0.05 | 47.7 ± 1.9 | 37.3 ± 1.1 | 49.9 ± 1.9 | 2.4 ± 0.3 |
| 15 | 35.1 ± 0.9 | 18.2 ± 0.07 | 49.2 ± 2.5 | 40.1 ± 1.2 | 55.5 ± 1.6 | 2.7 ± 0.1 | |
| 16 | 49.9 ± 0.8 | 16.9 ± 0.08 | 44.1 ± 2.2 | 32.9 ± 0.8 | 51.7 ± 1.8 | 2.8 ± 0.1 | |
| 17 | 34.0 ± 1.3 | 17.4 ± 0.04 | 33.1 ± 2.0 | 41.2 ± 1.4 | 53.6 ± 1.1 | 2.9 ± 0.1 | |
| Rosaceae | |||||||
| Medlar | 18 | 38.2 ± 0.9 | 12.9 ± 0.11 | 32.2 ± 2.0 | 38.1 ± 2.4 | 45.1 ± 1.9 | 7.8 ± 0.2 |
| 19 | 65.1 ± 1.2 | 13.9 ± 0.10 | 44.5 ± 1.7 | 32.9 ± 3.3 | 62.3 ± 2.3 | 7.3 ± 0.2 | |
| Almond | 20 | 47.7 ± 2.8 | 9.3 ± 0.12 | 47.2 ± 1.5 | 122.3 ± 7.3 | 200.5 ± 8.1 | 3.7 ± 0.2 |
| 21 | 43.1 ± 1.9 | 9.2 ± 0.13 | 43.8 ± 2.1 | 115.1 ± 4.2 | 160.5 ± 6.3 | 3.2 ± 0.3 | |
| Myrtaceae | |||||||
| Eucaliptus | 22 | 55.2 ± 3.1 | 27.4 ± 0.09 | 62.8 ± 0.9 | 180.6 ± 8.7 | 220.6 ± 8.7 | 4.2 ± 0.2 |
| 23 | 54.8 ± 2.2 | 31.1 ± 0.10 | 67.3 ± 1.0 | 194.3 ± 9.3 | 225.3 ± 7.4 | 5.3 ± 0.3 | |
| 24 | 56.7 ± 1.9 | 29.8 ± 0.08 | 74.2 ± 0.8 | 185.9 ± 8.4 | 222.0 ± 8.8 | 4.4 ± 0.2 | |
| 25 | 57.0 ± 2.3 | 32.1 ± 0.12 | 75.2 ± 1.3 | 190.3 ± 7.2 | 221.7 ± 8.3 | 5.1 ± 0.3 | |
| Apiaceae | |||||||
| Ferula | 26 | 108.2 ± 3.1 | 55.8 ± 0.14 | 93.7 ± 3.6 | 150.4 ± 6.3 | 269.6 ± 9.3 | 6.6 ± 0.4 |
| 27 | 132.0 ± 2.7 | 51.5 ± 0.14 | 110.2 ± 4.9 | 114.2 ± 5.5 | 228.2 ± 8.5 | 6.0 ± 0.5 | |
| Dill | 28 | 95.1 ± 3.9 | 75.1 ± 0.22 | 159.3 ± 6.3 | 238.4 ± 6.8 | 240.1 ± 9.8 | 10.3 ± 0.8 |
| 29 | 120.6 ± 5.5 | 80.1 ± 0.34 | 173.6 ± 6.9 | 235.3 ± 9.4 | 270.3 ± 9.9 | 10.1 ± 0.5 | |
| 30 | 133.3 ± 4.8 | 82.1 ± 0.23 | 165.3 ± 7.3 | 226.2 ± 10.1 | 242.3 ± 9.7 | 11.8 ± 0.6 | |
| Honeydew | |||||||
| Citrus | 31 | 100.8 ± 4.5 | 68.8 ± 0.15 | 120.2 ± 6.7 | 196.4 ± 6.3 | 255.3 ± 7.4 | 3.0 ± 0.2 |
| Eucaliptus | 32 | 110.3 ± 4.8 | 75.3 ± 0.21 | 179.3 ± 5.5 | 180.3 ± 7.5 | 260.2 ± 8.2 | 3.0 ± 0.2 |
| Manuka | 50.3 ± 1.9 | 37.1 ± 0.32 | 111.9 ± 6.3 | 119.1 ± 4.5 | 165.2 ± 7.4 | 3.7 ± 0.3 | |
aTPC, total phenol content; TF, total flavonoids; FRAP, Ferric reducing antioxidant power; GAE, gallic acid equivalent; QE, quercetin equivalent; AAE, ascorbic acid equivalent; TE, trolox equivalent. Each value is the mean ± SD of three separate experiments. Manuka honey was used as golden reference.
We also assayed two different honeydew honeys, a type of honey produced by the honeybee by processing the sweet secretions of some insects (Aphids and Hemiptera) as they feed on plant sap. TPC and TF in the honeydew honeys from eucalyptus and citrus were respectively two-fold and three-fold higher than those measured in the nectar honeys from the same species/families (Table 1), and higher than almost all other monofloral honeys. This was not unexpected. Honeydew honeys have been reported to possess higher total phenol and flavonoid content, and higher antioxidant power than many nectar honeys (Escriche et al., 2014; Escuredo et al., 2013; Rodríguez Flores et al., 2015; Tuberoso et al., 2011). The TPC and TF reducing components of the two honeydews, however, were substantially comparable with those in the ferula and dill monofloral honeys (Table 1). On the whole, the total amount of reducing components (as TPC) in the Sicilian honeys by black honeybee was among the highest evaluated in several either multifloral (8.6 to 34 mg GAE/100 g honey) (Di Marco et al., 2012), or monofloral (6.0 to 27.6 GAE/100 g honey) (Pichichero et al., 2009) honey varieties from other Italy's regions and from many countries abroad, including South Africa, India, Czech Republic, Portugal, Romania, Venezuela, Slovenia and Pakistan, reported in the range of 32.59–114.75; 47–98; 8.25–24.25; 22.61–72.77; 2– 125.00; 38.15–182.10; 2.77–28.57 and 1.33–155.16 GAE mg/100 g, respectively (Al et al., 2009; Bertoncelj et al., 2007; Ferreira et al., 2009; Lachman et al., 2010; Meda et al., 2005; Saxena et al., 2010; Vit et al., 2009; Noor et al., 2014).
3.2. Reducing capacity and radical scavenging activity
FRAP assay provides a direct estimation of reductants in a sample, and is based on the ability of the analyte to reduce the Fe3+/Fe2+ couple. The honey from the Apiaceae nectar showed the highest ferric ion reducing capacity, with a mean value for dill of 165.66 ± 6.8 AAE/100 g, whereas sulla honey the lowest one, with a mean value of 17.75 ± 0.72 AAE/100 g (Table 1). Manuka honey showed a TRAP higher than the majority of Sicilian honeys, however it was 21% lower than that of honeys from dill and ferula Apiaceae nectar (mean value 140.4 ± 5.8 AAE/100 g) (Table 1). One study on forty Czech Republic honeys reported FRAP values ranging from 8.72 to 22.29 AAE/100 g (Lachman et al., 2010; Lellau and Liebezeit, 2003).
Radical-scavenging ability of honeys and honeydew honeys by Apis mellifera ssp. sicula was evaluated by DPPH and ABTS•+ decoloration assays in comparison with Trolox. Both tests measure the free radical scavenging activity of samples in solution, by donating of one electron (Huang et al., 2005). The radical scavenging ability of the thirty honey samples resulted slightly higher in the ABTS test (from 19.2 ± 0.7 to 270.3 ± 9.9 μmol TE/100 g) than in the DPPH test (from 8.5 ± 0.3 to 238.4 ± 6.8 μmol TE/100 g) (Table 1). Dill honey showed the highest antiradical capacity, while tangerine honey was the least active. Honeydew honeys and honeys produced by Sicilian black honeybee from dill, ferula and eucalyptus nectars, appeared 1.5- to 2-fold more effective than manuka honey to reduce chemical radicals, whereas almond honey showed a comparable DPPH and ABTS•+ radical scavenging activity (Table 1).
The perferryl-myoglobin radical is an oxidant species of interest in the food chemistry. Metmyoglobin and hydrogen peroxide can be formed in the red meat by spontaneous autoxidation of oxyMb and react to form perferryl-Mb, which comprises a globin-based radical and an oxoferryl moiety. The reducing activity of honeys by Apis mellifera ssp. Sicula toward perferryl-myoglobin radical (•Mb[FeIV=O]) was investigated by monitoring the spectral changes of the H2O2-dependent conversion of MetMb in •Mb[FeIV=O], either in the absence or in the presence of honey. When 100 μM met-Mb and 100 μM H2O2 were incubated at 37 °C, the amount of perferryl-Mb after a 5-min incubation (61.3 ± 2.1 μM, n = 9) was calculated from the molar extinction coefficient of the difference in the absorption of the two protein forms at 556 nm. The decrement of the perferryl form in the presence of suitable amounts of honey was converted in deactivation rate and compared with the reducing capacity of Trolox used as a reference (Table 1). Our data indicated that dill honey, followed by sulla and medlar with comparable activities, had the highest reduction rate whereas thistle honey the lowest. In addition, the honeydew honeys appeared poorly effective in reducing •Mb[FeIV=O]. Finally, the deactivation rate of manuka honey was among the lowest (Table 1).
The correlations between the honey total phenol and flavonoid contents, and the reducing and radical scavenging capacity, measured as FRAP, DPPH, ABTS•+ and •Mb[FeIV=O], evaluated by Pearson correlation coefficient (r) confirmed that the phenolic components were involved in the overall reducing capacity of all honeys. Table 2 shows the correlation matrix obtained for each pair of variables. Good correlations (0.774 ≤ r ≤ 0.959, p < 0.0001) were observed between either TPC or TF and parameters related to both ferric ions reducing power (FRAP) and scavenging activity against chemical radicals (DPPH, ABTS•+). Tenore et al. (2012) came to similar conclusions by examining a smaller number of Sicilian black bee honey samples.
Table 2.
Correlation matrix (Pearson correlation coefficient) between all the parameters measured in honey samples produced by Apis mellifera ssp. Sicula.
| TPC | TF | FRAP | DPPH | ABTS•+ | |
|---|---|---|---|---|---|
| TF | 0.919 (<0.0001) |
||||
| FRAP | 0.883 (<0.0001) |
0.956 (<0.0001) |
|||
| DPPH | 0.774 (<0.0001) |
0.851 (<0.0001) |
0.819 (<0.0001) |
||
| ABTS•+ | 0.854 (<0.0001) |
0.843 (<0.0001) |
0.815 (<0.0001) |
0.959 (<0.0001) |
|
| •MbFeIV=O | 0.316 | 0.399 (0.023) |
0.356 | 0.334 | 0.237 |
Numbers in brackets are p value at the 95.0%.
No correlation existed between the •Mb[FeIV=O] deactivation rate of the black bee honeys and their TPC, whereas a slight but significant correlation (p = 0.023) was observed with the flavonoid amount (Table 2). This suggests that specific phenolic components, rather than their amount, can account for the perferryl-Mb reducing capacity of honeys. Radical trapping capacity of reducing compounds is not correlated to the redox properties alone but depends also on the ability of distribution of the antioxidant in the phase of the oxidizing probe. In other terms, the reducing capacity of radical scavengers measured through the DPPH and ABTS•+ cannot be simply translated to their activity against •Mb[FeIV=O], since interaction with the transient radical site of the protein, or access to the heme hydrophobic pocket demands suitable size, three-dimensional structure and physicochemical feature of the compound (Jongberg et al., 2014). As reported (Mannina et al., 2015; Tenore et al., 2012), phenolic acids represent approximately 83% of TPC, whereas quercetin and kaempferol are the most representative flavonoids in lemon, orange, medlar, almond, sulla and dill honey produced by Apis mellifera ssp. sicula. Interestingly, the highest amount of rutin, a flavonol shown to be effective in reducing the perferryl form of heme-proteins (Grinberg et al., 1994), has been reported in sulla (Mannina et al., 2015) and medlar (Tenore et al., 2012) honeys, that indeed were highly effective in deactivating the perferryl-Mb radical species, despite a low or modest TPC (Table 1).
Met-Mb and Mb radicals have been associated with oxidative alteration of proteins and lipids occurring in stored meat, (Kroger-Ohlsen et al., 2002), or even during meat digestion in the stomach. (Baron and Andersen, 2002; Lapidot et al., 2005; Gorelik et al., 2005; Tesoriere et al., 2007). Indeed lipid hydroperoxides formed under oxidative conditions, or from heated meat, may stimulate the pseudo-peroxidase activity of met-Mb (Lapidot et al., 2005), resulting in the generation of hypervalent iron ferryl species, in turn inducing lipid and protein oxidation (Kanner and Harel, 1985). Phenol-rich extracts from plants and herbs have been shown to protect meat and meat products efficiently against rancidity, as a result of their capacity to scavenge reactive radicals, including those derived from heme-proteins (Shah et al., 2014). Our observations now suggest such a potential also in honeys.
3.3. Antioxidant capacity of honeys against peroxyl radicals
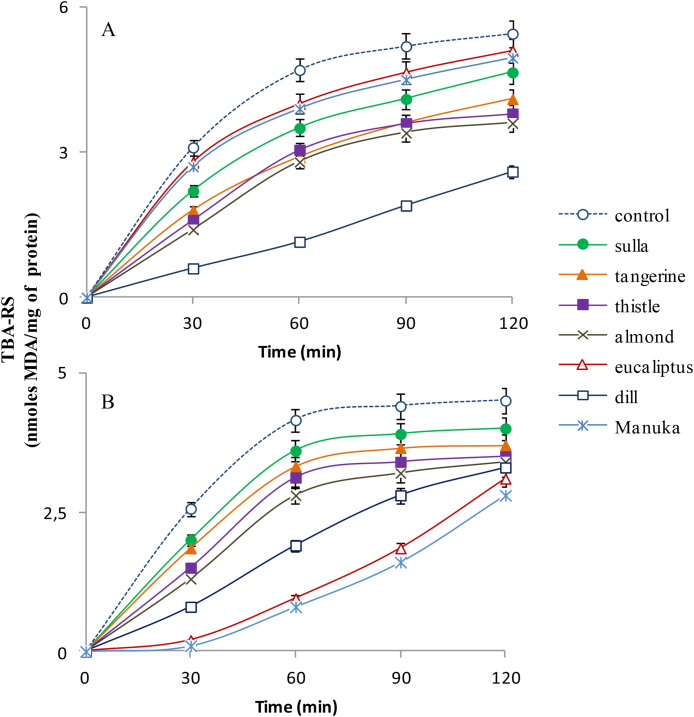
Reactive oxygen species (ROS) such as peroxyl radicals play a key role in the unwanted chain reactions of lipid autoxidation in biological systems and food. Dietary antioxidants often broadly include ROS scavengers inhibiting radical chain reactions and metal chelators (Huang et al., 2005). The effect of Sicilian honeys in protecting polyunsaturated fatty acid from oxidation was assessed utilizing an in vitro model of either azoinitiator or iron-dependent lipid oxidation of bovine brain microsomes. We investigated sulla (samples n°3, 4 in Table 1), tangerine (samples n°9, 10), almond (samples n°20, 21), eucalyptus (samples n°24, 25), dill (samples n°29, 30) and thistle (samples n°15, 16) as representative honeys from plants of the different families, i.e. Leguminosae, Rutaceae, Rosaceae, Myrtaceae, Apiaceae and Asteraceae, respectively. Manuka honey was also assayed for comparison. Lipid oxidation, induced in microsomes (2 mg protein/mL) by either the water-soluble azoinitiator AAPH or Fe+3/ascorbate, started immediately and a maximum of TBA-RS was measured at 60 min (Fig. 1 A, B, control). Peroxyl-radical scavenging capacity of honeys was calculated from the percent inhibition of MDA formed with respect to control after a 60 min incubation. Dill, almond, tangerine, thistle, and sulla honeys, in this order, inhibited formation of lipoperoxides in both models, with a slightly higher effectiveness in the azoinitiator (76%, 41%, 35%, 38%, 26% inhibition, respectively), than Fe+3/ascorbate -dependent oxidation (50%, 31%, 25%, 20%, 14% inhibition, respectively) oxidation. Eucalyptus and manuka honeys, instead, showed a remarkably higher antioxidant effect in the metal (77% and 80% inhibition, respectively) than AAPH-dependent oxidation (15% and 17% inhibition, respectively, suggesting combined peroxyl radical-scavenging ability and metal-chelating activity of their components (Fig. 1 A, B).
Fig. 1.
Time-course of TBA-RS formation during the AAPH-induced (A), and Fe3+/ascorbate-induced (B) microsomal oxidation, either in the absence (control) or in the presence of 10 mg honey per ml reaction mixture. Microsomes at 2 mg protein per mL reaction mixture, were submitted to peroxidation by AAPH and Fe3+/ascorbate, respectively, as reported in Methods. Each value is the mean ± SD of n determinations performed in duplicate on different honey samples of the same nectar (sulla, n = 4; tangerine, n = 2; almond, n = 2; thistle, n = 4; eucalyptus, n = 4; dill, n = 3, Manuka, n = 1).
Honey flavonoids, and in particular quercetin, have been considered as lipid soluble compounds able to interact with cell membrane and protect against AAPH-induced lipid oxidation (Alvarez-Suarez et al., 2012). Indeed, the highest amount of TF was measured in dill honey, while according to other studies (Tenore et al., 2012) almond honey by the Sicilian black bee contains high amounts of quercetin (2.35 mg/100 g honey). On the other hand, the eucalyptus and manuka honeys show around one half TF than dill honey (Table 1), whereas their small amounts of quercetin (0.3–0.5 mg/100 g honey), luteolin (0.3–0.4 mg/100 g honey) and kaempferol (0.13–0.17 mg/100 g manuka honey; 0.05–0.07 mg/100 g eucalyptus honey) (Marshall et al., 2014; Martos et al., 2000a; Yao et al., 2003) are possibly responsible for iron-chelating properties (Ren et al., 2008).
4. Conclusion
The honey market is currently very attractive, especially for local and regional products endowed with special properties. While assessing the reducing capacities of whole honeys produced by the A. mellifera ssp sicula in western Sicily, we showed that a number of monofloral honeys are to be ranked among the richest in phenol components and radical scavenging activities on a global scale. Antioxidants in their natural context do not act individually. It should be noted that with assaying whole honey solutions we considered the activity of the entire pool of phytochemicals, their eventual interactions and correlation with other honey constituents, as it can occur with a dietary foodstuff. In this context, for the first time we showed that honey can neutralize oxoferryl radicals from oxidation of myoglobin, an activity that does not correlate with the total phenol content, but appears to depend on certain flavonoids occurring in some of the honeys examined. Myoglobin-derived radicals strongly contribute to development of lipid oxidation in red meat, either stored or during digestion. In addition to the activity against lipoperoxyl radicals, scavenging of perferryl myoglobin may concur to explain the protective activity of honey against rancidity of stored meat (Antony et al., 2006; Johnston et al., 2005) and encourages honey as a novel ingredient in meat cooking or industrial preparations.
A growing body of scientific research, especially on the manuka honey, has recently revealed many molecular mechanisms that may rationalize well known medicinal properties of honey (Carter et al., 2016), and may contribute to expand preventive and even therapeutic uses. Our findings encourage further studies on the Sicilian black honeybee honey. The activity of monofloral honey in cell environment where specific reducing components may modulate redox-regulated pathways relevant to gene expression and cell function is now under investigation in our laboratory.
Declarations
Author contribution statement
Alessandro Attanzio: Conceived and designed the experiments; Performed the experiments.
Luisa Tesoriere: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mario Allegra: Conceived and designed the experiments; Analyzed and interpreted the data.
Maria A. Livrea: Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Al M.L., Daniel D., Moise A., Bobis O., Laslo L., Bogdanov S. Physicochemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009;112:863–867. [Google Scholar]
- Al-Mamary M., Al-Meeri A., Al-Habori M. Antioxidant activities and total phenolic contents of different types of honey. Nutr. Res. 2002;22:1041–1047. [Google Scholar]
- Alvarez-Suarez J.M., Giampieri F., Damiani E., Astolfi P., Fattorini D., Regoli F., Quiles J.L., Battino M. Radical-scavenging activity, protective effect against lipid peroxidation and mineral contents of monofloral Cuban honeys. Plant Foods Hum. Nutr. 2012;67:31–38. doi: 10.1007/s11130-011-0268-7. [DOI] [PubMed] [Google Scholar]
- Amiot M.J., Aubert S., Gonnet M., Tacchini M. Les composés phénoliques des miels: étude préliminaire sur l’identification et la quantification par familles. Apidologie. 1989;20:115–125. [Google Scholar]
- Antony S., Rieck J.R., Acton J.C., Han I.Y., Halpin E.L., Dawson P.L. Effect of dry honey on the shelf life of packaged turkey slices. Poult. Sci. 2006;85:1811–1820. doi: 10.1093/ps/85.10.1811. [DOI] [PubMed] [Google Scholar]
- Baron C.P., Andersen H.J. Myoglobin-induced lipid oxidation. A review. J. Agric. Food Chem. 2002;50:3887–3897. doi: 10.1021/jf011394w. [DOI] [PubMed] [Google Scholar]
- Bertoncelj J., Dobersek U., Jamnik M., Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105:822–828. [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter D.A., Blair S.E., Cokcetin N.N., Bouzo D., Brooks P., Schothauer R., Harry E.J. Therapeutic manuka honey: no longer so alternative. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco G., Canuti L., Imperi S., Leonardi D., Canini A. Nutraceutical properties of honey and pollen produced in a natural park. J. Agr. Sci. 2012;3:187–200. [Google Scholar]
- Escriche I., Kadar M., Juan-Borrás M., Domenech E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey - Impact of industrial thermal treatment. Food Chem. 2014;142:135–143. doi: 10.1016/j.foodchem.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Escuredo O., Míguez M., Fernández-González M., Seijo M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013;138:851–856. doi: 10.1016/j.foodchem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Ferreira I.C.F.R., Aires E., Barreira J.C.M., Estevinho L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. [Google Scholar]
- Ferreres F., Tomás-Barberán F.A., Gil M.I., Tomás-Lorente F. An HPLC technique for flavonoid analysis in honey. J. Sci. Food Agr. 1991;56:49–56. [Google Scholar]
- Gentile C., Tesoriere L., Butera B., Fazzari M., Monastero M., Allegra M., Livrea M.A. Antioxidant activity of Sicilian Pistachio (Pistacia vera L. Var. Bronte) nut extract and its bioactive components. J. Agric. Food Chem. 2007;55:643–648. doi: 10.1021/jf062533i. [DOI] [PubMed] [Google Scholar]
- Gil M.I., Ferreres F., Ortiz A., Subra E., Tomas-Barberan F.A. Plant phenolic metabolites and floral origin of rosemary honey. J. Agric. Food Chem. 1995;43:2833–2838. [Google Scholar]
- Gorelik S., Lapidot T., Shaham I., Granit R., Ligumsky M., Kohen R., Kanner J. Lipid peroxidation and coupled vitamin oxidation in simulated gastric fluid inhibited by dietary polyphenols: health implications. J. Agric. Food Chem. 2005;53:3397–3402. doi: 10.1021/jf040401o. [DOI] [PubMed] [Google Scholar]
- Grinberg L.N., Rachmilewitz E.A., Newmark H. Protective effects of rutine against hemoglobin oxidation. Biochem. Pharmacol. 1994;48:643–649. doi: 10.1016/0006-2952(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Jongberg S., Lund M.N., Skibsted L.H., Davies M.J. Competitive reduction of perferrylmyoglobin radicals by protein thiols and plant phenols. J. Sci. Food Agr. 2014;62:11279–11288. doi: 10.1021/jf5041433. [DOI] [PubMed] [Google Scholar]
- Johnston J.E., Sepe H.A., Miano C.L., Brannan R.G., Alderton A.L. Honey inhibits lipid oxidation in ready-to-eat ground beef patties. Meat Sci. 2005;70:627–631. doi: 10.1016/j.meatsci.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Kanner J., Harel S. Initiation of membranal lipid peroxidation by activated metmyoglobin. Arch. Biochem. Biophys. 1985;237:314–321. doi: 10.1016/0003-9861(85)90282-6. [DOI] [PubMed] [Google Scholar]
- Kroger-Ohlsen M., Carlsen C.U., Andersen M.L., Skibsted L.H. Pseudoperoxidase activity of myoglobin: Pigment catalyzed formation of radicals in meat systems. In: Morelle M., Shahidi F., Ho C., editors. Free Radicals in Food. American Chemical Society; Washington DC: 2002. pp. 138–150. [Google Scholar]
- Lachman J., Orsak M., Hejtmankova L., Kovarova E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT–Food Sci. Techno. 2010;43:52–58. [Google Scholar]
- Lapidot T., Granit R., Kanner J. Lipid peroxidation by free iron ions and myoglobin as affected by dietary antioxidants in simulated gastric fluids. J. Agric. Food Chem. 2005;53:3383–3390. doi: 10.1021/jf040402g. [DOI] [PubMed] [Google Scholar]
- Lellau T.F., Liebezeit G. Cytotoxic and antitumor activities of ethanolic extracts of salt marsh plants from the lower Saxonian Wadden Sea, Southern North Sea. Pharm. Biol. 2003;4:293–300. [Google Scholar]
- Mannina L., Sobolev A.P., Di Lorenzo A., Vista S., Tenore G.C., Daglia M. Chemical composition of different botanical origin honeys produced by Sicilian black honeybees (Apis mellifera ssp. sicula) J. Agric. Food Chem. 2015;63:5864–5874. doi: 10.1021/jf506192s. [DOI] [PubMed] [Google Scholar]
- Marshall S.M., Schneider K.R., Cisneros K.V., Gu L. Determination of antioxidant capacities, α-dicarbonyls, and phenolic phytochemicals in Florida varietal honeys using HPLC-ESI-MS (n.) J. Agric. Food Chem. 2014;62:8623–8631. doi: 10.1021/jf501329y. [DOI] [PubMed] [Google Scholar]
- Martos I., Ferreres F., Yao L., D’Arcy B., Caffin N., Tomás-Barberán F.A. Flavonoids in monospecific Eucalyptus honeys from Australia. J. Agric. Food Chem. 2000;48:4744–4748. doi: 10.1021/jf000277i. [DOI] [PubMed] [Google Scholar]
- Martos I., Ferreres F., Tomás-Barberán F.A. Identification of flavonoid markers for the botanical origin of eucalyptus honey. J. Agric. Food Chem. 2000;48:1498–1502. doi: 10.1021/jf991166q. [DOI] [PubMed] [Google Scholar]
- Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- Molan P.C. The role of honey in the management of wounds. J. Wound Care. 1999;8:415–418. doi: 10.12968/jowc.1999.8.8.25904. [DOI] [PubMed] [Google Scholar]
- Noor N., Sarfraz R.A., Ali S., Shahid M. Antitumour and antioxidant potential of some selected Pakistani honeys. Food Chem. 2014;143:362–366. doi: 10.1016/j.foodchem.2013.07.084. [DOI] [PubMed] [Google Scholar]
- Patel R.P., Svistunenko D.A., Darley-Usmar V.M., Symons M.C., Wilson M.T. Redox cycling of human methaemoglobin by H2O2 yields persistent ferryl iron and protein based radicals. Free Radical Res. 1996;25:117–123. doi: 10.3109/10715769609149916. [DOI] [PubMed] [Google Scholar]
- Pellegrini N., Re R., Yang M., Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2'-azinobis(3-ethylenebenzothiazoline- 6-sulfonic acid) radical cation decolorization assay. Methods Enzymol. 1999;299:379–389. [Google Scholar]
- Pichichero E., Lorena C., Canini A. Characterisation of the phenolic and flavonoid fractions and antioxidant power of Italian honeys of different botanical origin. J. Sci. Food Agr. 2009;89:609–616. [Google Scholar]
- Ren J., Meng S., Lekka C.H., Kaxiras E. Complexation of flavonoids with iron: structure and optical signatures. J. Phys. Chem. 2008;112:1845–1850. doi: 10.1021/jp076881e. [DOI] [PubMed] [Google Scholar]
- Rodríguez Flores M.S., Escuredo O., Seijo M.C. Assessment of physicochemical and antioxidant characteristics of Quercus pyrenaica honeydew honeys. Food Chem. 2015;166:101–106. doi: 10.1016/j.foodchem.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Shah M.A., Bosco S.J.D., Mir S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014;98:21–33. doi: 10.1016/j.meatsci.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Saxena S., Gautam S., Sharma A. Physical: biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010;118:391–397. [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Stephens J.M., Schlothauer R.C., Morris B.D., Yang D., Fearnley L., Greenwood D.R., Loomes K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and manuka honeys. Food Chem. 2010;120:78–86. [Google Scholar]
- Tenore G.C., Ritieni A., Campiglia P., Novellino E. Nutraceutical potential of monofloral honeys produced by the Sicilian black honeybees (Apis mellifera ssp. sicula) Food Chem. Toxicol. 2012;50:1955–1961. doi: 10.1016/j.fct.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Tesoriere L., Butera D., Gentile C., Livrea M.A. Bioactive components of Sicilian caper (Capparis spinosa L.) and antioxidant effects in a simulated red meat gastric digestion. Agric. Food Chem. 2007;55:8465–8471. doi: 10.1021/jf0714113. [DOI] [PubMed] [Google Scholar]
- Tomás-Barberán F.A., Martos I., Ferreres F., Radovic B.S., Anklam E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agr. 2001;81:485–496. [Google Scholar]
- Tuberoso C.I., Jerković I., Bifulco E., Marijanović Z. Biodiversity of Salix spp: honeydew and nectar honeys determined by RP-HPLC and evaluation of their antioxidant capacity. Chem. Biodivers. 2011;8:872–879. doi: 10.1002/cbdv.201000359. [DOI] [PubMed] [Google Scholar]
- Vit P., Soler C., Tomás-Barberán F.A. Profiles of phenolic compounds of Apis mellifera and Melipona spp: Honeys from Venezuela. Zeitschrift fur Lebensmittel-Untersuchung und-Forschung A. 1997;204:43–47. [Google Scholar]
- Vit P., Rodriguez-Malaver A., Roubik D.W., Moreno E., Souza B.A., Sancho M.T. Expanded parameters to assess the quality of honey from Venezuelan bees (Apis mellifera) JAAS. 2009;3:72–81. [Google Scholar]
- Yao L., Datta N., Tomás-Barberán T.A., Ferreres F., Martos I., Singanuong R. Flavonoids: phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003;81:159–168. [Google Scholar]
- Yao L., Jiang Y., Singanusong R., Datta N., Raymont K. Phenolic acids in Australian Melaleuca, Guioa, Lophostemon: Banksia and Helianthus honeys and their potential for floral authentication. Food Res. Int. 2005;38:651–658. [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]