Supplemental Digital Content is Available in the Text.
A patient's experience of pain for the first 48 hours of an admission can be visualized with graphical pain score trajectories, providing clinically useful information.
Keywords: Pain score, Trajectory, Quality improvement, Hospital
Abstract
Pain care for hospitalized patients is often suboptimal. Representing pain scores as a graphical trajectory may provide insights into the understanding and treatment of pain. We describe a 1-year, retrospective, observational study to characterize pain trajectories of hospitalized adults during the first 48 hours after admission at an urban academic medical center. Using a subgroup of patients who presented with significant pain (pain score >4; n = 7762 encounters), we characterized pain trajectories and measured area under the curve, slope of the trajectory for the first 2 hours after admission, and pain intensity at plateau. We used mixed-effects regression to assess the association between pain score and sociodemographics (age, race, and gender), pain medication orders (opioids, nonopioids, and no medications), and medical service (obstetrics, psychiatry, surgery, sickle cell, intensive care unit, and medicine). K-means clustering was used to identify patient subgroups with similar trajectories. Trajectories showed differences based on race, gender, service, and initial pain score. Patients presumed to have dissimilar pain experiences (eg, sickle vs obstetrical) had markedly different pain trajectories. Patients with higher initial pain had a more rapid reduction during their first 2 hours of treatment. Pain reduction achieved in the 48 hours after admission was approximately 50% of the initial pain, regardless of the initial pain. Most patients' pain failed to fully resolve, plateauing at a pain score of 4 or greater. Visualizing pain scores as graphical trajectories illustrates the dynamic variability in pain, highlighting pain responses over a period of observation, and may yield new insights for quality improvement and research.
1. Introduction
Hospitalized patients often experience pain. Barriers to optimal pain care include patient factors (eg, reluctance to report pain, fear of addiction), physician factors (eg, fear of overdose, addiction, distrust of subjective data, lawsuits), health system issues (eg, low priority, opiate policies), and inadequate pain assessment.16,18–20 Clinical guidelines have not improved pain care.16,19,20 This is primarily because translation of guidelines into practice has been affected by poor implementation leading to unintended consequences including oversedation.24 In a 22-site collaborative, the accuracy of pain assessment, presence of a care plan, and pain care education were associated with only modest declines in the percentage of patients reporting moderate or severe pain (from 24% to 17%).6 Additionally, the Center for Medicare and Medicaid Services reports that only 71% of hospitalized patients reported that their pain was “always well controlled.”1
The experience of pain during hospitalization is dynamic, affected by a host of factors including the nature of the illness or injury, genetics, sociodemographic characteristics, mood, fatigue, and previous and current drug therapy. Although pain scores are often thought to be an important clinical outcome, research has shown that they are highly individualized4,9 and are often confounded by emotional rather than sensory responses to pain.5 As a result, there is variability in pain measurement and understanding, and little direct connection between increased measurement of pain and improved pain outcomes.2
One potential technique for contextualizing a patient's pain during hospitalization is to represent it as a trajectory. A pain trajectory is a graphical representation of a patient's pain scores over an observation period.2–4,23 Compared with a single pain score, trajectories represent a patient's pain experience over an episode of care and draw attention to speed of onset of initial pain relief, stability of pain relief, and overall amount of pain relief achieved.
Much of the prior research on pain trajectories has focused on specific subpopulations (eg, postoperative patients). Studies used self-reported pain to describe the directionality and trends in pain intensity (ie, increase, decrease, and no change),2,3 long-term pain trajectories among adolescents,10 and effects of exercise interventions on pain.23 Here, we characterize pain trajectories among a cohort of adult inpatients—a group large and diverse enough to illustrate significant variation in the experience of pain.
As part of a larger study on the safe and effective use of opioids for medical inpatients, we sought to examine the usefulness of pain trajectories as a method for characterizing the experience of pain among hospitalized adults. We sought to answer the following questions:
-
1. What is the functional form of the pain score trajectories?
a. How rapidly does pain relief occur?
b. Does the pain score stabilize? When and at what level does this stabilization occur?
2. Are there discernible types of trajectories corresponding to clusters of patients with similar pain experiences during hospitalization?
3. How do trajectories vary as a function of age, gender, race/ethnicity, initial pain score, and medical service?
2. Methods
2.1. Setting and participants
This study was conducted at the University of Illinois Hospital (UIH). University of Illinois Hospital and Health Science System is a 495-bed tertiary, urban academic medical center that includes an emergency department (ED) and 23 primary and specialty care clinics. In fiscal year 2015, UIH had approximately 47,000 ED visits, 20,000 hospitalizations, and 7000 inpatient surgeries. The adjusted mean length of stay was 5.7 days.
At the hospital, the nursing staff recorded the pain of hospitalized patients using the numerical rating scale (a 11-point scale ranging from 0 to 10). This assessment was performed at discrete but irregular time intervals. Pain scores were saved in the patient's medical record with a timestamp. All adult patients (aged 18-89 years) presenting with an initial pain score >4, admitted and discharged from UIH between January 1, 2014 and December 31, 2014 were included. Using the hospital's enterprise data warehouse, we obtained age, race, gender, medical service, subsequent pain scores and times, and all medication orders. The institutional review board of the University of Illinois at Chicago approved the study.
2.2. Data analysis
2.2.1. Data preparation
Patients presenting with significant pain (pain score >4) were included. Significant pain was defined as pain severe enough to limit functioning, determined to be between 5 and 10.6,21 A recent literature review also suggests that a score between 5 and 10 generally represents moderate to severe pain.25
Before data analysis, we performed several transformations. Medication orders placed for each patient were grouped into 1 of the 3 categories: (1) not having received an order for an analgesic (“no medications”), (2) only nonopioid analgesics (“nonopioids”), or (3) any opioid analgesic (“opioid”). Patients in category (3) may have received additional nonopioid analgesia. Based on a list of unique medications that were ordered for patients in our cohort, 1 reviewer (WLG) classified the analgesics into opioids or nonopioids (n = 328 unique medications). This classification was based on evaluating whether an analgesic contained an opioid or not. Mixed analgesics (eg, Vicodin) were classified as containing opioid, hence grouped under the “opioid” category.
Next, we organized data from each patient encounter, sorting it chronologically by time of pain score entry, with the time of the initial pain score recording denoted as time zero. The time of each additional pain score was recorded as the time elapsed since time zero, until the predetermined cut-off value of 48 hours was reached. We had 2 reasons for using a 48-hour observation window. First, we were interested in the acute period that followed immediately after admission. As such, the 48-hour window provided opportunities to investigate a period of rapid (and significant) changes in the pain intensity due to immediate and focused treatment. Second, we specifically focused on the pain-related event that may have brought the patient to the hospital. Although using extended time periods (eg, until discharge) may be useful, it also may introduce new events (eg, surgical procedures) that can create variations in the pain trajectory.
The decision to use time of first pain measurement as time zero, instead of time of admission, was based on the need for a logical and unambiguous starting time for the pain trajectory. Other choices for time zero were administrative, based on when orders are placed, beds are found, or the ED makes a triage decision. Patients unable to give a pain assessment (eg, sedation, paralysis, or intubation) were excluded, as it would be unclear whether they were admitted with pain. Additionally, if a patient was admitted to the hospital from the ED and met our inclusion criteria, then all preceding pain scores were included for analysis. Finally, we used the discharge service to denote the patient's location of service. For example, if a patient was admitted through the ED and transferred to a medical intensive care unit then discharged from the general medicine floor unit, the location for that patient encounter was categorized as general medicine.
We removed all spurious values before the main analysis (pain scores >10, negative values; comprising <0.5% of the data set). We performed preliminary data processing using R (www.r-project.org). We used a smoothed, rolling mean fitting function provided by the ggplot2 package in R to represent the observed pain trajectories graphically. This smoothing function relies on a locally weighted scatter plot smoothing approach.8
2.2.2. Statistical analysis plan
We computed descriptive statistics and then estimated mixed-effects regression models of the pain trajectories.2,4,23 The dependent variable in these models was the pain score, and the independent variables were time, patient characteristics, medical service, and medication orders.
Mixed-effects regression provides an effective mechanism for measuring time-varying longitudinal phenomena, as these models include “random effects” that account for the correlation among repeated assessments of the same participants.15 The individual random effects capture how an individual patient's trajectory deviates from the mean trajectory.
There are several advantages of using mixed-effects regression modeling for analysis of pain trajectories: first, since these are regression models, time is considered to be a continuous function, evaluated at discrete points, and therefore the number of longitudinal measurements for each patient does not have to be the same, nor do they have to be evaluated at the similar intervals.13,15 In our case, the number of pain score measurements (M = 13.45, SD = 6.85), and the intervals of measurements over the 48-hour period varied across patients. These variations were likely based on the medical service or specific conditions of the patient. Second, mixed-effects models allow for investigating the effects of both time-invariant (eg, gender, race) and time-varying covariates on changes in pain intensity. Third, and most importantly for our study, as opposed to traditional methods, mixed-effects models estimate and quantify each individual's variation from the mean. In our case, this was based on an empirical Bayes (EB) estimation.15 These individual variations can then be analyzed to identify subgroups of patients who vary from the mean trajectory in similar ways. Other reported studies on pain trajectories have used similar models.2,4,23
We also investigated the effects of several covariates, including patient demographic characteristics, service locations, and analgesia orders on the shape of pain trajectories. We applied an analytic approach developed by Gibbons et al.12 for modeling time-varying longitudinal phenomena. This included 3 steps: (1) fit a mixed-effects polynomial regression model to the pain score data, (2) generate EB estimates for each polynomial coefficient for each patient, and (3) cluster the EB estimates corresponding to each patient to identify sub sets of patients with similar pain trajectories.
The first step involved fitting a mixed-effects regression model to the pain scores. Similar to Gibbons et al.,12 we used a third-degree polynomial function of time to model the shape of the overall pain trajectory. The 4 coefficients (constant, linear, quadratic, and cubic) of the third-degree polynomial were treated as random effects. The population parameters were estimated using maximum marginal likelihood estimation. These estimates were then used to generate the EB estimates for the pain trajectory for each patient in our data set.12 Additionally, to understand the factors that affected the slope of the trajectory, we also included interactions of time with gender, age, race, and medical service.
In the second step, we used the EB estimates for the model coefficients for each patient. The EB estimates can be used to derive each patient's variation from the mean pain score trajectory, and adding the estimated mean coefficients (ie, fixed-effects) provides an individual's personal pain trajectory. For each patient, EB estimates were represented as a vector, and k-means clustering method was used for cluster analysis.14 We used a scree plot to determine the optimal number of clusters to analyze.11 Once the clusters were identified, for each group, we plotted mean pain trajectories, computed area under the curve (AUC), and characterized the subgroup's demographics.
Mixed-effects regression and the generation of EB estimates were performed using SuperMix (version 1.2; Scientific Software International). For each of the clusters, the AUC was computed using the R statistical package. This AUC function matches the approximation for the integration function using the trapezoidal rule.
To quantify the relationship between initial pain score and the slope of the pain score trajectory, we used the correlation between the intercept term and the linear time term (provided in the SuperMix regression output). To further analyze and visualize this relationship, we divided the data in to 3 subgroups based on the initial pain score (initial pain = 5 or 6; 7 or 8; 9 or 10). For each subgroup, a pain trajectory was developed and the following variables were determined: Vo, the rate of decrease of pain score in the first 2 hours after the initial pain score (ie, slope for the first 2 hours); and Pplateau, the mean pain score for the last 24 hours recording (ie, from 24 to 48 hours).
3. Results
3.1. General characteristics of pain scores
We extracted 104,414 pain scores from 7762 admissions involving 5418 unique patients (Table 1). Patients were predominantly female (64.5%) and African American (60.3%), with an average age of 43.3 years. The observed mean pain score trajectory for the entire sample, along with the fitted regression line, is shown in Figure 1. For 27% of the patient encounters, there were no pain measurements after the 48-hour period, indicating that these patients were likely discharged before the end of the 48-hour window.
Table 1.
Demographic characteristics of all encounters.
Figure 1.

Observed data and fitted regression line for N = 7762 encounters with adult inpatients admitted with pain score >4. The solid line represents observed data with geometric smoothing; the dotted line shows the fitted curve from the polynomial regression models. X-axis (“Days”) denotes the time elapsed, in days, since the initial pain measurement. Y-axis (“Pain”) denotes the pain score.
The main effects of age, gender, race, and the use of opioids on pain scores are included Appendix A Table 1 (available online as Supplemental Digital Content at http://links.lww.com/PAIN/A331). Figure 2 shows pain score trajectories by gender, race, and type of medication order. For every 1-year increase in age, pain scores decreased on average by 0.02 points (P < 0.001). Men had lower average pain scores (β = −0.61, P < 0.001) than women. Patients on the sickle cell service (β = 2.07, P < 0.001) and intensive care units (β = 0.46, P < 0.001) had higher average pain scores than patients on medicine units. Compared with patients who did not have orders for any pain medications, the average pain scores of those who had orders for medications were higher (for those who had opioid orders: β = 2.07, P < 0.001; for those who had non-opioid orders: β = 0.82, P < 0.001).
Figure 2.
Observed 48-hour pain trajectories with rolling mean scatter plot smoothing, clockwise from upper left: (A) by gender; (B) by race (African American, White, Hispanic, and Other); and (C) by analgesic medication exposure (no meds, nonopioids only, any opioids). X-axis (“Days”) denotes the time elapsed, in days, since the initial pain measurement. Y-axis (“Pain”) denotes the pain score.
There were significant interactions of gender, race, and medications with the linear, quadratic, and cubic time terms. Compared with men, pain scores of women declined more rapidly (P < 0.001). With respect to race, compared with African Americans, Hispanic patients' pain scores declined more rapidly, but pain scores of patients reporting their race as “other” had a slower decline. Compared with patients having no medication orders, pain scores for patients who had an opioid order had a slower decline in pain scores.
In our regression model, there was a significant correlation between time and the intercept term (r = 0.38) showing that the slope of the pain trajectory was correlated with the initial pain score. Figure 3 shows the trajectories for patients with pain scores categorized into 3 levels of initial pain: 5 or 6, 7 or 8, and 9 or 10. For each trajectory, an initial rate of pain reduction, Vo and Pplateau, the mean pain score for the last 24 hours of observation, were computed (Table 2).
Figure 3.
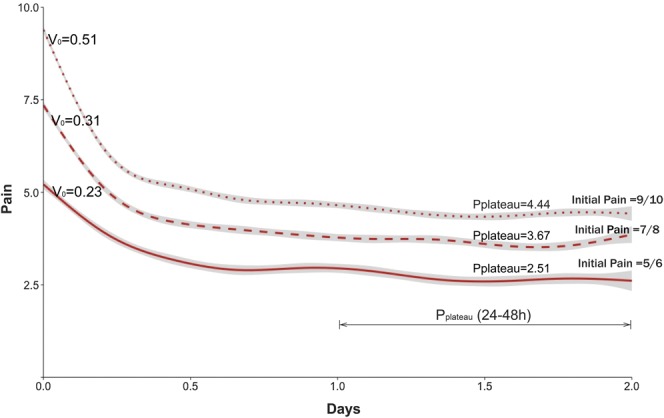
Observed average pain trajectories for patients with 3 levels of initial pain (5 or 6, 7 or 8, and 9 or 10): Vo is the rate of decrease of pain score during the first 2 hours after initial pain recording and Pplateau is the average pain during the 24 hours (24-48 hours) of pain recording. X-axis (“Days”) denotes the time elapsed, in days, since the initial pain measurement. Y-axis (“Pain”) denotes the pain score.
Table 2.
Percentage of decrease of pain for patients with different levels of initial presenting pain: 5/6, 7/8 and 9/10.
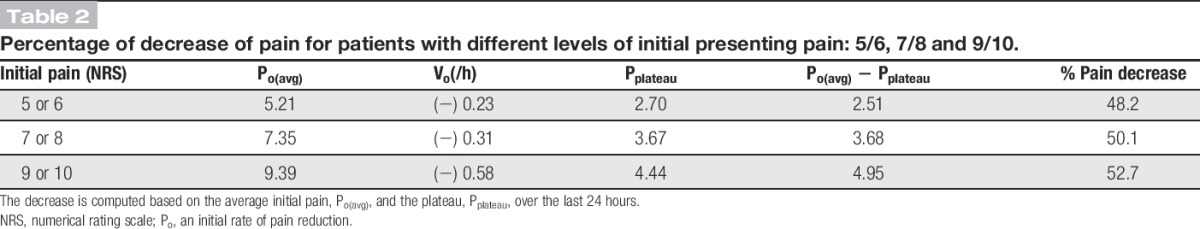
We found that the rate of decrease of pain score during the first 2 hours (Vo) decreased with decreasing initial pain. Similarly, Pplateau also decreased as the initial pain score decreased (Fig. 3). The difference between the initial pain score and Pplateau was not constant: for pain scores 9 or 10, 4.95 points: for pain scores 7 or 8, 3.68 points; and for pain scores 5 or 6, 2.51 points. The percentage reduction, however, was fairly constant across the 3 groups, ranging from 48.2% to 52.7%.
3.2. Pain trajectory clusters
We selected a 4-cluster solution based on the scree plot (See Appendix A Figure 1, available online as Supplemental Digital Content at http://links.lww.com/PAIN/A331), which illustrated that after 4 clusters, additional clusters produced only minimal change in the within-group sum of squares. Table 3 summarizes the properties of each of the clusters, and Figure 4 shows the average observed trajectories and the fitted regression lines.
Table 3.
Pain score, demographics, service location, and medication order type for each cluster.

Figure 4.
Observed and fitted 48-hour pain score trajectories for patients in 4 clusters. The solid lines are the observed data with geometric smoothing. The dotted lines are the fitted curves from the polynomial regression models, using mean empirical Bayes estimates from each cluster. The corresponding area under the curve (AUC) values are also shown. X-axis (“Days”) denotes the time elapsed, in days, since the initial pain measurement. Y-axis (“Pain”) denotes the pain score.
Cluster 1 included relatively younger patients, with the highest proportion of African Americans with the highest initial and average pain. Opioids had been ordered for most of these patients (97.5%), and this group contained the highest proportion of patients being treated in the sickle cell disease unit (36.5%). Cluster 2 included patients with a lower initial and average pain than cluster 1. The pain was persistent and without rapid resolution. Almost all patients received orders for opioids (94.8%) and were predominantly discharged from a medicine service. Cluster 3 included the highest percentage of women (68.8%), with discharges from medical (47.7%), obstetrical (23.3%), and surgical (17.7%) services. Eighty-seven percent had received an order for an opioid analgesic. Exclusive use of nonopioids was more common in cluster 3 than in clusters 1 and 2. Although the initial pain was similar to cluster 2, the resolution was more rapid, with a slight rebound. Cluster 4 included relatively older patients and was characterized by a very rapid resolution and the largest rebound increase during the last 24 hours. These patients were predominantly nonsurgical. Compared with the other subgroups, only about two-thirds of patients had an opioid order and >10% received no analgesia orders at all.
The normalized AUC for the mean pain score trajectory for each cluster was as follows: cluster 1: 0.69, cluster 2: 0.52, cluster 3: 0.34, and cluster 4: 0.15.
4. Discussion
For hospitalized patients, sustainable improvements in pain control have been difficult to achieve. We explored the possibility that new understandings and opportunities for improvement might be opened up by representing pain scores as continuous, graphical trajectories. Our objective was to examine the usefulness of pain score trajectories as a way of representing the experience of pain among a large cohort of hospitalized patients who were admitted with significant pain.
By representing pain scores as graphical trajectories, we were able to highlight several characteristics regarding the experience of pain for hospitalized patients. Pain trajectories varied based on race, gender, service, and initial pain, in clinically plausible ways. For the overwhelming majority of patients, pain scores declined substantially during the first 12 hours but minimally thereafter. But across different types of patients, the speed of the onset of relief, the absolute reduction in pain, and the final level of pain relief achieved differed substantially. We also found that the rate of decline in pain score was correlated with the initial pain score such that the higher the level of initial pain, the more rapid the initial decline. It is likely that this effect might be slightly exaggerated by the effect of “regression to the mean” on patients with an initial pain score of 10. However, the relationship with initial pain score likely still holds based on our finding regarding the variation in pain scores for patients with initial scores <10.
During the first 48 hours, pain scores did not stabilize at the same level for all patients but depended on the initial pain score. Regardless of the initial level of pain, the total amount of pain relief amounted to roughly half the initial pain. Although our analysis does not make it clear why there was a plateau at such high levels of pain for many patients, this finding is noteworthy. We can speculate that it may be due to patient and/or clinician expectations, or the culture of patient–nurse or nurse–physician communication regarding pain.
Our approach was exploratory, descriptive, and formative intended to raise questions and generate hypotheses rather than to provide definitive answers to the central dilemmas of pain care. Using this approach, we uncovered a number of novel patterns and generated several hypotheses. In the rest of this section, we describe the implications of our methodological approach for generating pain trajectories and the use of these trajectories for research and quality improvement efforts.
As described previously, we used a mixed-effects regression approach to characterize pain trajectories and used clustering techniques to classify the trajectories. The trajectories we generated showed some discriminant validity in the sense that they were able to separate out patients who are known to have quite different experiences of pain. In other words, each cluster represented groups of patients who were similar to one another but distinguishable from patients in other clusters based on clinical and/or sociodemographic characteristics. For example, cluster 1 had the highest proportion of African American men and the highest proportion of patients in the sickle cell unit; patients in this cluster also had the highest pain and limited pain resolution. By contrast, cluster 4 included older patients with rapid pain resolution. Although this analysis was based on a single institution, our approach is generalizable and can be used to generate and evaluate clusters of pain trajectories from similar data sets.
Additional methodological approaches that we used also could be considered for investigating the quality of pain care. For example, is it possible to use the AUC of the trajectory as a measure of the quality of pain relief over an entire episode of care? It is well known that pain scores are related to patient satisfaction scores.13,22 We recognize that patient satisfaction with pain care is caught up in a complex dynamic wherein patients may demand more pain relief and staff may resist.26 Still, this work suggests a variety of new hypotheses about the relationship between properties of a pain score trajectory and patient satisfaction (eg, Is AUC of the pain score trajectory a better predictor of patient satisfaction than some discrete measure of pain?). With proper case-mix adjustment, it may be possible to compare different areas of a hospital, and even different hospitals, to identify factors that explain variability in the quality of pain care.
The analysis of clusters of pain trajectories suggests a number of questions for further research and provides opportunities for quality improvement. The 4 identified clusters that emerged raise several questions that warrant further exploration. For example, for patients in clusters 2, 3, and 4, why is there only limited pain relief after the first 12 hours? Similarly, why does the pain score for patients in cluster 1 not decrease <5? What accounts for the difference in the speed of onset of relief and the absolute magnitude of relief across all 4 clusters? Why do patients in clusters 3 and 4 have a rebound in their pain intensity after 24 hours? Why do patients in clusters 1 and 2 still have a high level of pain at 48 hours? With more careful analysis and control of clinical variables such as diagnosis, procedures, and medication administration, it should be possible to explain some of these phenomena.
One of the novel contributions of using graphical pain score trajectory is to bring such new questions to our attention. By representing pain as a time series, the dynamic properties of pain are rendered visible, and these dynamic properties can then be the targets of quality improvement. The trajectories can encourage us to think about pain more holistically, at the level of the entire episode of hospitalization, rather than the way clinicians typically view them as discrete measurements in time. Furthermore, enhancing the pain trajectories to include clinical events such as painful procedures and medication administrations can help in potentially translating the use of pain trajectories to routine clinical use. It must be acknowledged that in our current analysis, pain trajectories lack details regarding medication administration and procedures.
Enhancing the visual detail in pain trajectories and translating their use to routine clinical practice will require new visualization strategies. For example, to make trajectories more useful clinically, it will be helpful to have visual representations that can facilitate the comparison of multiple patients' pain trajectories and their actual variations over time in relation to key clinical events. Adding these and other features, as part of a user-centered design process led by clinicians, is a topic for future work.
4.1. Limitations
Several factors limit the conclusions that can be drawn from this initial study. This was a single-site study, carried out at an urban, minority-serving, academic, tertiary care hospital. As such, the findings may not generalize to other hospital settings. We did not control for a wide variety of factors that are known to affect the experience of pain, most notably admitting diagnosis, the presence or absence or timing of painful procedures, or the time of day. This was primarily because of the wide range of the diagnoses in our cohort (unique primary diagnoses, n = 1330; unique procedures, n = 1050; unique diagnoses-related group codes, n = 570). Given the exploratory nature of this study and potential lack of statistical power for subgroup analysis based on diagnosis, we focused on generating an overall perspective on the pain trajectories. However, clinical diagnosis is an important consideration for interpreting pain trajectories. For example, the observed racial differences in pain trajectories are provocative, but due to the lack of control for diagnoses, it is not clear whether this reflects true racial differences in pain experience or just a result of an underlying (but, unmeasured) correlation between race and diagnosis, such as sickle cell disease.
We did not control for the specific type of medication used, the time of administration, dose, or route of administration. There was significant variation in pain at the level of an individual patient that is not apparent in our graphs of mean pain score trajectories. The individual variation was captured in our mixed-effects regression models but not in the graphs. Finally, the numerical pain score was presumed to be a valid proxy for subjective pain intensity, despite potential measurement error related to patient reporting and nurse documentation.
5. Conclusion
Representing pain with continuous, graphical pain score trajectories reveals several dynamic aspects of the experience of pain among hospitalized patients. Although these properties have not previously received much attention, they appear to capture important information regarding the overall experience of pain that is relevant to both providers and patients. This way of representing pain score data may provide new targets for quality improvement and new information to guide clinical decision making.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article to disclose.
This project was supported by grant U19HS021093 from the Agency for Healthcare Research and Quality (AHRQ). The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A331.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Center for Medicare and Medicaid Services data.medicare.gov. 2015. Available from: https://data.medicare.gov/Hospital-Compare/Patient-survey-HCAHPS-National/99ue-w85f. Accessed June 6, 2016.
- [2].Chapman CR, Donaldson GW, Davis JJ, Bradshaw DH. Improving individual measurement of postoperative pain: the pain trajectory. J Pain 2011;12:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chapman CR, Fosnocht D, Donaldson GW. Resolution of acute pain following discharge from the emergency department: the acute pain trajectory. J Pain 2012;13:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chapman CR, Zaslansky R, Donaldson GW, Shinfeld A. Postoperative pain trajectories in cardiac surgery patients. Pain Res Treat 2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clark WC, Yang JC, Tsui SL, Ng KF, Clark SB. Unidimensional pain rating scales: a multidimensional affect and pain survey (MAPS) analysis of what they really measure. PAIN 2002;98:241–7. [DOI] [PubMed] [Google Scholar]
- [6].Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. PAIN 1996;67:267–73. [DOI] [PubMed] [Google Scholar]
- [7].Cleeland CS, Reyes-Gibby CC, Schall M. Rapid improvement in pain management: the Veterans Health Administration and the Institute for Health care Improvement Collaborative. Clin J Pain 2003;19:298–305. [DOI] [PubMed] [Google Scholar]
- [8].Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–36. [Google Scholar]
- [9].de C Williams AC, Davies HT, Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. PAIN 2000;85:457–63. [DOI] [PubMed] [Google Scholar]
- [10].Dunn KM, Jordan KP, Mancl L, Drangsholt MT, Le Resche L. Trajectories of pain in adolescents: a prospective cohort study. PAIN 2011;152:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Everitt BS, Landau S, Leese M. Cluster analysis. London: Edward Arnold, 1993. [Google Scholar]
- [12].Gibbons RD, Lazar NA, Bhaumik DK, Sclove SL, Chen HY, Thulborn KR, Sweeney JA, Hur K, Patterson D. Estimation and classification of fMRI hemodynamic response patterns. NeuroImage 2004;22:804–14 [DOI] [PubMed] [Google Scholar]
- [13].Hanna MN, Gonzalez-Fernandez M, Barrett AD, Williams KA, Pronovost P. Does patient perception of pain control affect patient satisfaction across surgical units in a tertiary teaching hospital? Am J Med Qual 2012;27:411–16. [DOI] [PubMed] [Google Scholar]
- [14].Hartigan JA, Wong MA. A k-means clustering algorithm. Appl Stat 1979:100–8. [Google Scholar]
- [15].Hedeker D, Gibbons RD, Longitudinal data analysis. Hoboken, NJ; John Wiley & Sons, 2006. [Google Scholar]
- [16].Institute for Clinical Systems Improvement. Health care guideline: Assessment and management of acute pain. Bloomington: Institute for Clinical Systems Improvement, 2007. [Google Scholar]
- [17].Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982:963–74. [PubMed] [Google Scholar]
- [18].Lin RJ, Reid MC, Liu LL, Chused AE, Evans AT. The barriers to high-quality inpatient pain management a qualitative study. Am J Hosp Palliat Med 2015;32:594–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lippe PM, Brock C, David J, Crossno R, Gitlow S. The first national pain medicine summit—final summary report. Pain Med 2010;11:1447–68. [DOI] [PubMed] [Google Scholar]
- [20].Macintyre PE, Scott DA, Schug SA, Visser EJ, Walker SM. Acute pain management: scientific evidence. Melbourne: Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine, 2010. [Google Scholar]
- [21].Serlin RC, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. PAIN 1995;61:277–84. [DOI] [PubMed] [Google Scholar]
- [22].Tedore T, Weinberg R, Witkin L, Giambrone GP, Faggiani SL, Fleischut PM. Acute pain management/regional anesthesia. Anesthesiol Clin 2015;33:739–51. [DOI] [PubMed] [Google Scholar]
- [23].Treat-Jacobson D, Henly SJ, Bronas UG, Leon AS, Henly GA. The pain trajectory during treadmill testing in peripheral artery disease. Nurs Res 2011;60:S38–49. [DOI] [PubMed] [Google Scholar]
- [24].Vila H, Jr, Smith RA, Augustyniak MJ, Nagi PA, Soto RG, Ross TW, Cantor AB, Strickland JM, Miguel RV, The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg 2005;101:474–80, table of contents. [DOI] [PubMed] [Google Scholar]
- [25].Woo A, Lechner B, Fu T, Wong CS, Chiu N, Lam H, Pulenzas N, Soliman H, DeAngelis C, Chow E. Cut points for mild, moderate, and severe pain among cancer and non-cancer patients: a literature review. Ann Palliat Med 2015;4:176–83. [DOI] [PubMed] [Google Scholar]
- [26].Wright AP, Becker WC, Schiff GD. Strategies for flipping the script on opioid overprescribing. JAMA Intern Med 2016;176:7–8. [DOI] [PubMed] [Google Scholar]




