Abstract
Background
Deleterious BRCA mutation carriers with breast cancer are at increased risk for additional breast cancer events. This study evaluated the impact that timing of identification of BRCA+ status has on surgical decision and outcome.
Methods
The authors reviewed all BRCA carriers at their institution whose breast cancer was diagnosed between January 1996 and June 2015. Patient surveys, medical records, and institutional databases were used to collect data. Differences in surgical choice were analyzed using the chi-square test, and rates of subsequent breast cancer events were estimated using the Kaplan–Meier method.
Results
The study investigated 173 BRCA carriers with breast cancer (100 BRCA1, 73 BRCA2). Of the women with known BRCA mutation before surgery and unilateral stages 0 to 3 breast cancer (n = 63), 12.7 % underwent lumpectomy, 4.8 % underwent unilateral mastectomy (UM), and 82.5 % underwent bilateral mastectomy (BM). These surgical choices differed significantly (p < 0.0001) from those of patients unaware of their mutation at the time of surgery (n = 93) (51.6 % had lumpectomy, 19.4 % had UM, 29 % had BM). Of the patients with BRCA mutation identified after surgery who underwent lumpectomy or UM, 36 (59 %) of 66 underwent delayed BM. The patients with BRCA+ known before diagnosis presented with significantly lower-stage disease (p = 0.02) at diagnosis (69 % stage 0 or 1) than those whose BRCA mutation was identified after cancer diagnosis (40 % stage 0 or 1).
Conclusions
The study findings showed that BRCA mutation status influences surgical decision. The rates of BM were higher for the patients with BRCA mutation known before surgery. Identification of BRCA mutation after surgery frequently leads to subsequent breast surgery. Genetic testing before surgery is important for patients at elevated risk for BRCA mutation.
In 1994 and 1995, BRCA1 and BRCA2 tumor-suppressor genes were respectively sequenced.1,2 Approximately 3 to 5 % of breast cancer cases are associated with germline mutations in BRCA1/BRCA2.3,4 Carriers of the BRCA1/BRCA2 mutations have an increased lifetime risk for the development of breast cancer,5,6 and mutation carriers with breast cancer are at increased risk for future breast cancer events.
Knowledge of a deleterious BRCA mutation can influence surgical treatment decisions for patients with a diagnosis of breast cancer.7 These women may opt for more aggressive surgical treatment such as bilateral mastectomy.8,9 For BRCA mutation carriers with a diagnosis of breast cancer who undergo breast-conserving surgery, some studies suggest that the risk of ipsilateral breast cancer is higher than for patients with sporadic breast cancer.10 Also, the risk of contralateral breast cancer development is significantly higher for BRCA mutation carriers than for noncarriers.10–12
The current study evaluated how the surgical decisions of BRCA mutation carriers with a diagnosis of breast cancer varied based on timing of identification of BRCA mutation status relative to surgery for their index breast cancer. Additionally, we sought to evaluate breast cancer outcomes by surgical procedure.
METHODS AND MATERIALS
Study Design
With Institutional Review Board approval, we reviewed all BRCA1/BRCA2 deleterious mutation carriers at our institution whose breast cancer was diagnosed between 1 January 1996 and 30 June 2015 using a systematic search of the electronic medical record. Individuals known to be BRCA mutation carriers through other research efforts were used to validate the search findings. A study coordinator reviewed the search results to verify testing results and abstract relevant genetic testing information.
From this cohort of BRCA carriers, female patients with a deleterious gene mutation who had a diagnosis of breast cancer were identified for this study. A chart review then extracted additional information from the medical record including age at breast cancer diagnosis, operation performed for the index breast cancer, subsequent breast surgery, timing of the BRCA test result, clinical and pathologic staging, histology, hormone receptor status, and information regarding systemic therapy and radiation therapy and patient outcome.
The patients were categorized into three groups: group 1 (patients whose BRCA+ status was known before their cancer diagnosis), group 2 (patients whose BRCA+ status was identified during their cancer workup, in the time between their breast cancer diagnosis and their index breast cancer surgery), group 3 (patients whose BRCA+ status was identified at any time after their index breast cancer surgery).
Statistical Analysis
Differences among the groups were assessed using the chi-square test for type of surgery, the Wilcoxon rank-sum test for age at diagnosis, and the Cochran-Armitage trend test for clinical stage. The Kaplan–Meier method with 95 % confidence intervals (CIs) calculated based on the logarithm of the survival function was used to estimate long-term outcomes including contralateral breast cancer, local-regional recurrence, and freedom from breast cancer, in which patients were counted as events if they experienced contralateral breast cancer, local-regional recurrence, or distant recurrence. Because many patients underwent second operations (e.g., delayed bilateral mastectomy) during the follow-up period, this time-varying covariate of surgery type was handled in two ways:
For simple Kaplan–Meier estimates, patients were censored at the time of the second surgery when estimation per procedure rates was performed.
For tests across surgery type, Cox proportional hazards regression with surgery type as a time-varying covariate was used to partition the follow-up time according different surgery types over time.
Analysis was performed using SAS (version 9.3) and the survival package in R (version 3.0.2).13 All p values lower than 0.05 were considered statistically significant.
RESULTS
A cohort of 191 female BRCA mutation carriers with a diagnosis of breast cancer was identified. Among these women, 11 (5.8 %) who had undergone their index surgery outside our institution without sufficient survey or medical record data to confirm the index surgical treatment were excluded from the study as well as another 4 patients (2.1 %) who had unknown timing of their BRCA mutation testing result. Two patients who had breast cancer identified as an occult malignancy at the time of bilateral prophylactic mastectomy and one patient who presented with breast cancer after bilateral prophylactic skin-sparing mastectomy were also excluded. Thus, the study cohort consisted of 173 women with sufficient data to classify the timing of BRCA mutation testing result relative to surgical decision making and to characterize the initial surgical management. Of these women, 100 (58 %) were BRCA1 deleterious mutation carriers, and 73 (42 %) were BRCA2 deleterious mutation carriers.
Table 1 shows the patient demographics, tumor characteristics, systemic and radiation treatment, and type of breast surgery for the 173 patients, of whom 160 (92 %) presented with unilateral breast cancer and 13 (8 %) presented with bilateral disease. Of the 173 patients, 15 (9 %) were known BRCA carriers who developed breast cancer (group 1), 56 (32 %) were found to be BRCA carriers at the time of cancer diagnosis but before surgery (group 2), and 102 (59 %) were found to be BRCA carriers after initial surgical treatment (group 3). Three of the 173 patients did not undergo primary surgery due to stage 4 disease at presentation, and one patient underwent axillary dissection alone due to axillary metastasis without breast primary surgery. The timing of BRCA mutation testing relative to cancer diagnosis did not differ significantly between the BRCA1 and BRCA2 carriers. Of the BRCA1 carriers, 12 % (12/100) had their mutation status identified before breast cancer diagnosis, whereas 4 % (3/73) of the BRCA2 mutation carriers were aware of their mutation before their breast cancer diagnosis (p = 0.073).
TABLE 1.
Characteristics of 173 female subjects
|
n = 173 n (%) |
|
|---|---|
| Median age at breast cancer diagnosis: years (range) | 45 (21–79) |
| Median age at genetic mutation testing: years (range) | 46 (21–79) |
| Timing of genetic testing relative to breast cancer diagnosis and surgery | |
| Genetic testing before breast cancer diagnosis | 15 (8.7) |
| Genetic testing after diagnosis but before surgery | 56 (32.4) |
| Genetic testing after surgery | 102 (59.0) |
| Laterality | |
| Bilateral breast cancer | 13 (7.5) |
| Unilateral breast cancer | 160 (92.5) |
| Clinical TNM stage | |
| 0 | 10 (7.3) |
| 1 | 49 (35.8) |
| 2 | 53 (38.7) |
| 3 | 22 (16.1) |
| 4 | 3 (2.2) |
| Unknown | 36 |
| Nodal status | |
| Negative | 98 (59.4) |
| Positive | 67 (40.6) |
| Unknown | 8 |
| Estrogen receptor status | |
| Positive | 86 (54.1) |
| Negative | 73 (45.9) |
| Unknown | 14 |
| HER2 status | |
| Positive | 10 (7.8) |
| Negative | 118 (92.2) |
| Unknown or not applicable | 45 |
| Initial breast surgery | |
| Lumpectomy | 60 (34.7) |
| Unilateral mastectomy | 21 (12.1) |
| Bilateral mastectomy | 88 (50.9) |
| Nonea | 4 (2.3) |
| Neoadjuvant systemic therapy | |
| None | 121 (72.9) |
| Neoadjuvant chemotherapy | 45 (27.1) |
| Neoadjuvant endocrine therapy | 0 (0.0) |
| Unknown | 7 |
| Adjuvant chemotherapy | |
| Yes | 90 (54.6) |
| No | 75 (45.5) |
| Unknown | 8 |
| Adjuvant radiation therapy | |
| Yes | 75 (45.5) |
| No | 90 (54.6) |
| Unknown | 8 |
TNM tumor-node metastasis, HER human epidermal growth factor receptor
This included three stage 4 patients who at presentation underwent no primary breast surgery and one patient with no breast primary tumor who underwent axillary lymph node dissection only as initial surgery
The median age at breast cancer diagnosis was 45 years (range 21–79 years), and the median age at identification of BRCA mutation status was 46 years (range 21–79 years). The median age at breast cancer diagnosis did not differ significantly by timing group and was 46 years (range 28–79 years) for group 1, 41 years (range 21–71 years) for group 2, and 45 years (range 25–72 years) for group 3 (p = 0.34).
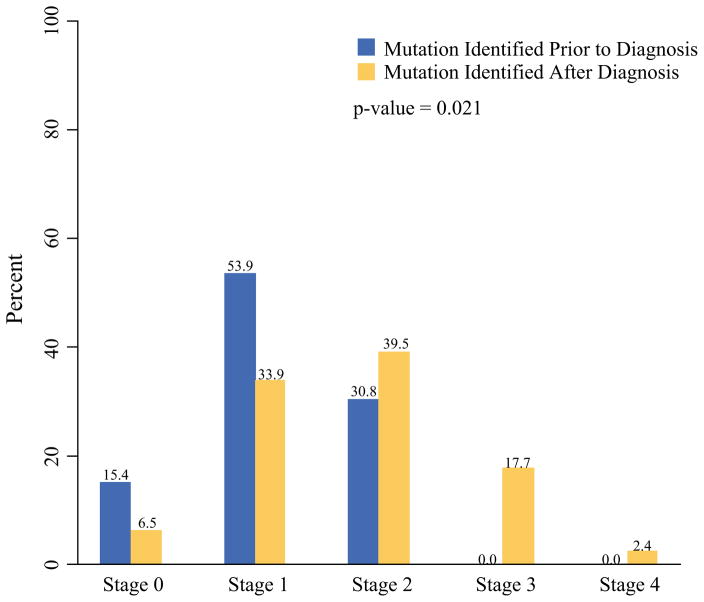
The women with BRCA mutation status known before diagnosis (group 1) demonstrated a significantly lower clinical stage at diagnosis (p = 0.02) than the women whose BRCA mutation was identified after their breast cancer diagnosis (groups 2 and 3) (Fig. 1). Of the patients with known BRCA mutation status prior to breast cancer development, 69 % presented with stage 0 or 1 disease, and none of these patients presented with either stage 3 or 4 disease. Of the known BRCA+ patients (Group 1) 73 % (11/15) were compliant with screening guidelines. Of the patients unaware of their BRCA mutation, 40 % presented with stage 0 or 1 disease, and 20 % presented with stage 3 or 4 disease.
FIG. 1.
Bar chart showing clinical stage distribution for female subjects whose BRCA mutation status was identified before breast cancer diagnosis versus after diagnosis
Clinical stage did not vary significantly between BRCA1 and BRCA2 carriers (p = 0.52), who had stage 0/1 in 44 % and 41 %, respectively. Estrogen receptor-negative (ER−) and human epidermal growth factor receptor 2-negative (HER2−) invasive cancer was significantly more likely in BRCA1 carriers (50 %) than in BRCA2 carriers (32 %) (p = 0.04). The findings showed no significant association between the BRCA gene and HER2 + disease (9 % BRCA1, 6 % BRCA2; p = 0.53). Receptor status did not vary significantly across groups 1, 2, and 3 (p = 0.25). The clinical stage was significantly higher for ER−/HER2− cancer (p = 0.03), with 50 % of cases classified as stage 2 and 27 % as stage 3 compared with 38 % classified as stage 2 and 16 % as stage 3 or 4 among other biologic subtypes, but was not significantly associated with the choice of surgery (p = 0.53).
Patients Presenting with Unilateral Cancer
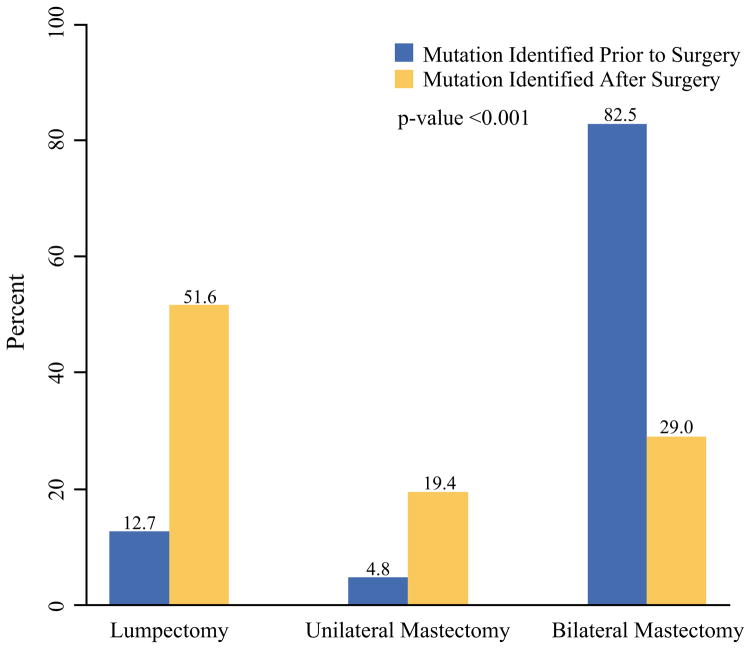
Among 156 women with unilateral breast cancer undergoing breast surgery for stages 0 to 3 disease, those with BRCA mutation known before surgery chose the following surgeries: lumpectomy (13 %), UM (5 %), and BM (83 %). These rates differed significantly (p < 0.0001) from the patients whose BRCA mutation was not identified until after surgery (lumpectomy, 52 %; UM, 19 %; and BM, 29 %) (Fig. 2). Among the patients with BRCA mutation identified after surgery who underwent lumpectomy or UM at their index surgery (n = 66), 26 (39 %) of 66 patients subsequently underwent BM for risk reduction after identification of mutation status, and another 2 (3 %) of the 66 patients underwent risk-reducing BM after initial surgery but before mutation testing. Additionally, 11 (17 %) of the 66 patients underwent subsequent BM as part of subsequent treatment of disease (6 for contralateral breast cancer, 4 for local-regional recurrence, and 1 for margin control). Thus, altogether, 39 (59 %) of the 66 patients underwent delayed bilateral mastectomy.
FIG. 2.
Bar chart showing distribution of initial surgery choice for 156 women with unilateral stages 0–3 breast cancer whose mutation status was identified before surgery versus after surgery
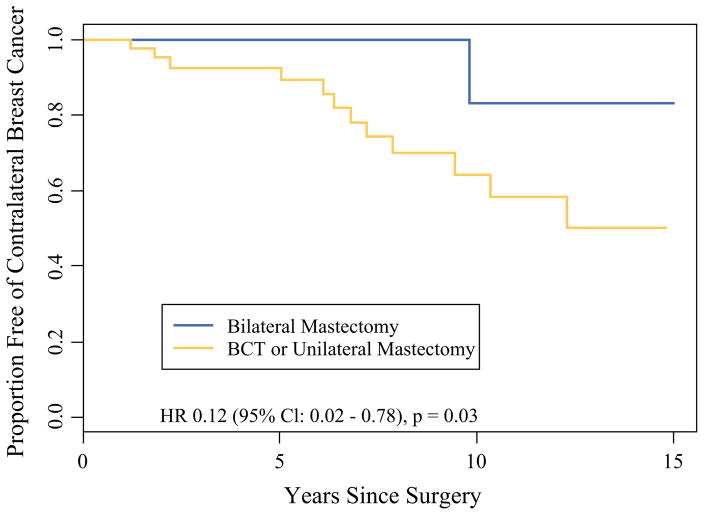
During a median follow-up period of 3.4 years (range 0–17 years), 33 of the patients with stages 0 to 3 unilateral breast cancer experienced one or more subsequent breast cancer events. The distribution of the first event type was 11 local-regional recurrences, 11 distant recurrences, and 11 contralateral breast cancers. The estimated 5-year freedom from breast cancer was 84 % (95 % CI 77–92 %) (Table 2). There was no significant difference across surgery types for this combined end point (p = 0.51) or for local-regional recurrence (p = 0.57). However, women undergoing BM saw a significantly reduced risk of contralateral breast cancer in unadjusted analysis (hazard ratio [HR], 0.12; 95 % CI 0.02–0.78; p = 0.03) (Fig. 3) and adjusted analysis (HR 0.14; 95 % CI 0.02–0.83; p = 0.03). One woman did experience contralateral breast cancer after skin-sparing contralateral prophylactic mastectomy. The contralateral breast cancer was in the mastectomy flap at the periphery of the breast.
TABLE 2.
Outcomes among 156 female subjects presenting with unilateral breast cancer
| 5-year % (95 % CI) |
10-year % (95 % CI) |
Unadjusted p value | p value adjusted for age and stage | |
|---|---|---|---|---|
| Local-regional recurrence | ||||
| Overall | 96.2 (92.5–100) | 83.7 (72.0–97.2) | ||
| Lumpectomy | 95.0 (88.3–100) | 80.0 (64.5–98.7) | 0.57 | 0.38 |
| Mastectomy | 97.0 (92.9–100) | 92.4 (83.2–100) | ||
| New contralateral primary breast cancer | ||||
| Overall | 97.2 (94.1–100) | 72.6 (58.3–90.5) | ||
| Lumpectomy or UM | 92.6 (84.9–100) | 64.3 (47.9–86.4) | 0.03 | 0.03 |
| BM | 100 | 83.3 (58.3–100) | ||
| Any subsequent breast cancer event | ||||
| Overall | 84.1 (77.0–91.8) | 56.4 (42.5–74.9) | ||
| Lumpectomy | 89.4 (80.0–100) | NAa | 0.51 | 0.36 |
| UM | 83.6 (64.9–100) | NAa | ||
| BM | 79.7 (68.6–92.5) | NAa | ||
UM unilateral mastectomy, BM bilateral mastectomy, NA not available
Sample size and number remaining at risk were too small to estimate
FIG. 3.
Kaplan–Meier estimates for the development of contralateral breast cancer among 156 female BRCA1/BRCA2 mutation carriers presenting with unilateral stages 0–3 breast cancer
Patients Presenting with Bilateral Cancer
Among 13 patients with bilateral breast cancer, 9 (69 %) underwent BM, and 4 (31 %) underwent bilateral lumpectomy. None of the four women electing bilateral lumpectomy knew of their BRCA mutation status at the time of surgical decision making, and three of the four women subsequently underwent BM (2 for risk reduction, 1 for margin control). Of the nine women electing BM, five knew of their mutation status before surgery, and four were unaware of their mutation. The estimated 5-year freedom from breast cancer in the bilateral cancer subset was 92 % (77–100 %).
DISCUSSION
Identification of BRCA mutation status influences the surgical decision making of patients with a diagnosis of breast cancer.14,15 Our study demonstrated that knowledge of BRCA mutation carrier status before surgical treatment has a significant impact on the type of surgical procedure patients choose. The findings showed that 83 % of the patients with known BRCA mutation status underwent BM compared with 29 % of the patients who were unaware of their BRCA mutation status at the time of their index surgery for breast cancer.
Comparison of outcomes between breast conservation and mastectomy for women with deleterious BRCA mutation is an area of ongoing debate and research. It is unlikely that a randomized clinical trial will ever be conducted. Therefore, results from retrospective studies must be used to answer this question.
Multiple studies have evaluated the rate of ipsilateral breast cancer recurrence in BRCA mutation carriers versus noncarriers, with conflicting results. In some studies, breast cancer patients with BRCA1/BRCA2 mutation who underwent lumpectomy for breast cancer had a greater risk of ipsilateral breast cancer than patients with sporadic breast cancer, and the risk increased with longer follow-up periods.16–18 The 10-year cumulative incidence of ipsilateral-breast cancer was 27 % for BRCA mutation carriers compared with 4 % for sporadic control subjects (HR 3.9; 95 % CI 1.1–13.8; p = 0.03).10 In comparison, other studies have shown no significant difference in local recurrence rates between BRCA mutation carriers and noncarriers among patients treated with lumpectomy and radiation.19–21
In terms of contralateral breast cancer risk, a meta-analysis showed that BRCA1/BRCA2 mutation carriers have a 3.5-fold increase in relative risk of contralateral breast cancer after a first breast cancer compared with noncarriers.10–12,22 A meta-analysis of 18 retrospective and 2 prospective cohorts reported on cumulative risk of secondary primary contralateral breast cancer in 1324 carriers of BRCA1/BRCA2 mutation. The cumulative 10-year risk of contralateral breast cancer was 27 % (95 % CI 21–33 %) for BRCA1 mutation carriers and 19 % (95 % CI 15–23 %) for BRCA2 mutation carriers compared with 5 % (95 % CI 3–7 %) for non-BRCA carriers.23
In our study, the women undergoing BM showed a significantly lower risk for contralateral breast cancer than those maintaining their contralateral breast.
Given recent evidence demonstrating higher rates of contralateral breast cancer development in this population, BM at the time of cancer diagnosis has been suggested as a reasonable surgical choice for BRCA mutation carriers.21,24,25 Furthermore, Metcalfe et al.26 reported a study with a 20-year follow-up period in which BRCA mutation carriers with breast cancer who underwent bilateral mastectomy had a 48 % reduction in death from breast cancer compared with women who underwent unilateral mastectomy (HR 0.52; 95 % CI 0.29–0.93; p = 0.03). Similarly, Heemskerk-Gerritsen et al.27 reported a lower mortality rate for BRCA mutation patients with unilateral breast cancer undergoing contralateral prophylactic mastectomy than for the surveillance group (9.6 and 21.6 per 1000 person-years of observation, respectively; adjusted HR 0.19; 95 % CI 0.29–0.82).
In our study of patients identified as BRCA mutation carriers after definitive surgery for their index breast cancer who underwent UM or lumpectomy, 59 % (39/66) ultimately underwent BM. Knowledge of BRCA mutation carrier status at the time of surgical decision making for their index cancer could have eliminated subsequent breast operations, which pose additional cost and morbidity, because many of these patients may have chosen to undergo BM as their initial operation. Genetic testing results can be difficult to obtain in a timely manner. In our practice, we currently prioritize the referral of patients meeting the criteria for genetic testing who will undergo primary surgery so that the information can be available before surgery.
The National Comprehensive Cancer Network guidelines recommend breast screening with magnetic resonance imaging (MRI) annually for BRCA mutation carriers 25–29 years of age, and for BRCA mutation carriers 30–75 years of age annual mammograms, and annual breast MRI.28 The combination of MRI and mammogram screening is based on the superior sensitivity shown through this approach.29,30 Patients with BRCA deleterious mutations at our institution are followed every 6 months with a breast exam, an annual MRI, and an annual mammography. Our study demonstrated that patients with known BRCA+ mutation status presented with a significantly earlier stage of breast cancer than patients unaware of their BRCA mutation carrier status. This is most likely due to high-risk screening and patient awareness in BRCA mutation carriers.
Although this study demonstrated clinically relevant information for BRCA mutation carriers, several imitations should be noted. This was a retrospective study from a single institution, and it had information gaps including patients’ preferences and physician recommendations, which also influence surgical decision making. Because not all the patients underwent their index surgical treatment at our institution, some clinical features such as clinical stage were missing for approximately 20 % of the patients. Yet, by including all the patients whose index breast surgery choice and timing of mutation testing could be discerned from sources including patient surveys, we were able to present a larger and more inclusive sample size for the primary aim. Finally, the moderate sample size and the relatively short median follow-up period of 41 months meant that analyses of outcomes such as recurrence were limited in precision, with wide confidence intervals for some estimates, and by low power for comparison across surgery groups.
In summary, this study provides strong evidence that knowledge of BRCA+ mutation status has an impact on definitive surgical decision making in index breast cancer treatment. The rates of bilateral mastectomy were significantly higher among the patients with known BRCA mutation status than among those whose BRCA mutation status was not known at time of surgical treatment. Identification of BRCA mutation after surgical treatment of the index breast cancer led to additional future surgeries for patients who underwent lumpectomy or UM as the surgical management of their initial unilateral breast cancer. Bilateral mastectomy decreased contralateral breast cancer risk significantly. This study further supports the importance of genetic testing before definitive surgical treatment of the index breast cancer of patients with elevated risk for genetic mutation.
Acknowledgments
This research was supported in part by a generous gift from the David F. and Margaret T. Grohne Family Foundation, an NCI Specialized Program of Research Excellence (SPORE) in Breast Cancer (CA116201), NIH Grants (CA116167 and CA192393), and a grant from the Breast Cancer Research Foundation
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–92. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 3.Risch HA, McLaughlin JR, Cole DEC, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 4.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–89. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasen HFA, Tesfay E, Boonstra H, et al. Early detection of breast and ovarian cancer in families with BRCA mutations. Eur J Cancer. 2005;41:549–54. doi: 10.1016/j.ejca.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Evans DG, Shenton A, Woodward E, Lalloo F, Howell A, Maher ER. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8:155. doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lokich E, Stuckey A, Raker C, Wilbur JS, Laprise J, Gass J. Preoperative genetic testing affects surgical decision making in breast cancer patients. Gynecol Oncol. 2014;134:326–30. doi: 10.1016/j.ygyno.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Mai PL, Lagos VI, Palomares MR, Weitzel JN. Contralateral risk-reducing mastectomy in young breast cancer patients with and without genetic cancer risk assessment. Ann Surg Oncol. 2008;15:3415–21. doi: 10.1245/s10434-008-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tercyak KP, Peshkin BN, Brogan BM, et al. Quality of life after contralateral prophylactic mastectomy in newly diagnosed high-risk breast cancer patients who underwent BRCA1/2 gene testing. J Clin Oncol. 2007;25:285–91. doi: 10.1200/JCO.2006.07.3890. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Etienne CA, Barile M, Gentilini OD, et al. Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol. 2009;16:3380–7. doi: 10.1245/s10434-009-0638-7. [DOI] [PubMed] [Google Scholar]
- 11.Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:443–55. doi: 10.1007/s10549-014-2890-1. [DOI] [PubMed] [Google Scholar]
- 12.Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:5887–92. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- 13.Therneau T, Grambsch P. Modeling survival data: extending the Cox model. Technometrics. 2002;44:85–6. doi: 10.1198/tech.2002.s656. [DOI] [Google Scholar]
- 14.Weitzel JN, McCaffrey SM, Nedelcu R, MacDonald DJ, Blazer KR, Cullinane CA. Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch Surg. 2003;138:1323–8. doi: 10.1001/archsurg.138.12.1323. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MD, Lerman C, Brogan B, et al. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22:1823–9. doi: 10.1200/JCO.2004.04.086. [DOI] [PubMed] [Google Scholar]
- 16.Pierce LJ, Phillips KA, Griffith KA, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010;121:389–98. doi: 10.1007/s10549-010-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105:812–22. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 18.Trainer AH, Lewis CR, Tucker K, Meiser B, Friedlander M, Ward RL. The role of BRCA mutation testing in determining breast cancer therapy. Nat Rev Clin Oncol. 2010;7:708–17. doi: 10.1038/nrclinonc.2010.175. doi:.10.1038/nrclinonc.2010.175. [DOI] [PubMed] [Google Scholar]
- 19.Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;24:2437–43. doi: 10.1200/JCO.2005.02.7888. [DOI] [PubMed] [Google Scholar]
- 20.Kirova YM, Savignoni A, Sigal-Zafrani B, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat. 2010;120:119–26. doi: 10.1007/s10549-009-0685-6. [DOI] [PubMed] [Google Scholar]
- 21.Robson M, Levin D, Federici M, et al. Breast conservation therapy for invasive breast cancer in Ashkenazi women with BRCA gene founder mutations. J Natl Cancer Inst. 1999;91:2112–7. doi: 10.1093/jnci/91.24.2112. [DOI] [PubMed] [Google Scholar]
- 22.Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:2328–35. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Molina-Montes E, Pérez-Nevot B, Pollán M, Sánchez-Cantalejo E, Espín J, Sánchez MJ. Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast. 2014;23:721–42. doi: 10.1016/j.breast.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002;359:1471–7. doi: 10.1016/S0140-6736(02)08434-9. [DOI] [PubMed] [Google Scholar]
- 25.Pierce LJ, Strawderman M, Narod SA, et al. Effect of radio-therapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol. 2000;18:3360–9. doi: 10.1200/JCO.2000.18.19.3360. [DOI] [PubMed] [Google Scholar]
- 26.Metcalfe K, Gershman S, Ghadirian P, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. 2014;348:g226. doi: 10.1136/bmj.g226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heemskerk-Gerritsen BA, Brekelmans CT, Menke-Pluymers MB, et al. Prophylactic mastectomy in BRCA1/2 mutation carriers and women at risk of hereditary breast cancer: long-term experiences at the Rotterdam Family Cancer Clinic. Ann Surg Oncol. 2007;14:3335–44. doi: 10.1245/s10434-007-9449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Center Guidelines. [Accessed 20 March 2016];Genetic/familial high risk assessment: breast and ovarian (verson 2.2016) http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf.
- 29.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148:671–9. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 30.Lord SJ, Lei W, Craft P, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43:1905–17. doi: 10.1016/j.ejca.2007.06.007. [DOI] [PubMed] [Google Scholar]