Abstract
Background
Since systemic lupus erythematosus (SLE) affects women of reproductive age, pregnancy is a major concern.
Objective
To identify predictors of adverse pregnancy outcome (APO) in inactive or stable active SLE patients
Design
Prospective Cohort
Setting
Multicenter
Patients
385 patients (49% non-Hispanic White; 31% prior nephritis) with SLE in PROMISSE. Exclusion criteria were: proteinuria >1000 mg/24 hour, creatinine >1.2 mg/dL, prednisone >20 mg/day, or multi-fetal pregnancy.
Measurements
APO included: fetal/neonatal death; birth <36 weeks due to placental insufficiency, hypertension, or preeclampsia; and small for gestational age (SGA) <5%. Disease activity was assessed by SLEPDAI and physician's global assessment (PGA).
Results
APO occurred in 19.0% (95% CI: 15.2% - 23.2%) of pregnancies, fetal death (4%), neonatal death (1%), preterm delivery (9%), and SGA (10%). Severe flares in the second and third trimester occurred in 2.5% and 3.0%, respectively. Baseline predictors of APO included lupus anticoagulant positive (OR = 8.32, 95% CI: 3.59-19.26), antihypertensive use (OR = 7.05, 95% CI: 3.05 - 16.31), PGA>1 (OR = 4.02, 95% CI: 1.84 - 8.82) and platelets (OR = 1.33 per 50K decrease, 95% CI:1.09-1.63); non-Hispanic White was protective (OR = 0.45, 95% CI: 0.24-0.84). Maternal flares, higher disease activity, and smaller increase in C3 later in pregnancy also predicted APO. Among women without baseline risk factors, the APO rate was 7.8%. For those either LAC positive, or LAC negative but non-White or Hispanic and taking antihypertensives, APO rate was 58%; fetal/neonatal mortality 22%.
Limitations
Excluded patients with high disease activity.
Conclusions
In pregnant SLE patients with inactive or stable mild/moderate disease, severe flares are infrequent, and absent specific risk factors, outcomes are favorable.
Primary Funding Source
National Institutes of Health
Introduction
Systemic lupus erythematosus (SLE) primarily affects women of childbearing age. Absent treatment with cytotoxic agents, SLE does not adversely impact fertility (1, 2), but fetal and maternal health during pregnancy are a concern. Advice regarding safety and timing of pregnancy requires identification of clinical and laboratory parameters that predict outcomes.
It has been suggested that SLE pregnancies result in high rates of preterm birth, preeclampsia, and fetal loss compared to pregnancies in healthy women (3-10). Previous studies have identified active disease, hypocomplementemia, anti-ds DNA antibodies, prior nephritis, and antiphospholipid antibodies (aPL) (6-8, 10-13) as risk factors for adverse pregnancy outcomes (APO). Effects of pregnancy on SLE activity and contribution of disease activity to APO remain unclear (10, 14-18). Currently, SLE patients are advised to consider pregnancy during periods of minimal and stable disease (19). However, data supporting this advice are based on retrospective or prospective single-center studies involving few patients, have limited generalizability to multi-ethnic populations, and are controversial (3-10).
To develop more robust data to inform patients and their physicians regarding pregnancy in SLE, we leveraged the PROMISSE Study (Predictors of pRegnancy Outcome: bioMarkerIn antiphospholipid antibody Syndrome and Systemic lupus Erythematosus). PROMISSE is the largest multi-center, multi-ethnic and multi-racial study to prospectively assess the frequency of APO, clinical and laboratory variables that predict APO, and pregnancy-associated flare rates in SLE women with inactive or mild/moderate activity at conception.
Methods
Study Design
PROMISSE is a multicenter, prospective observational study of pregnancies in women with SLE (≥4 revised ACR criteria) (20), SLE and aPL, aPL alone, and healthy women at low risk of APO (≥1 successful pregnancy, no prior fetal death, and <2 miscarriages <10 weeks' gestation). Criteria for the healthy controls were designed to minimize factors unrelated to SLE that might impact outcome. This paper focuses on the SLE patients with or without aPL (Appendix Figure 1). Patients with aPL were previously reported (21).
Patient Population
Pregnant patients were enrolled between September 2003 and December 2012 at 8 U.S. and 1 Canadian site. Institutional review boards approved the protocol and consent forms; written informed consent was obtained from patients. Consecutive pregnant women meeting inclusion criteria were recruited up to 12 weeks' gestation precluding ascertainment of first trimester losses. Only one pregnancy for each patient was included.
Enrollment inclusion criteria were: singleton intrauterine pregnancy; age 18-45 years; hematocrit >26%. Since the overall goal of PROMISSE was to identify risk factors for and mechanisms of APO specifically attributable to lupus and/or aPL, other potential causes of APO were excluded: prednisone >20 mg/day; urine protein (mg)/creatinine (gram) ratio ≥1000; erythrocyte casts on urinalysis; serum creatinine >1.2 mg/dL; diabetes mellitus; blood pressure ≥140/90 mmHg at screening.
Definition of SLE Disease Activity and Flares during Pregnancy
Investigators used the Systemic Lupus Erythematosus Pregnancy Disease Activity Index (SLEPDAI), an instrument incorporating history, physical exam, and laboratory measures to gauge lupus activity. The SLEPDAI was modified to discount physiologic changes of pregnancy that mimic disease activity to assure attribution to lupus (19, 22, 23).
A flare composite was used to define mild/moderate or severe flares, similar to that used in the SELENA (Safety of Estrogens in Lupus Erythematosus, National Assessment) trial, except SLEPDAI was substituted for SELENA SLEDAI (24) instrument. The composite includes: a) SLEPDAI score on the instrument; b) assessment of new or worsening disease activity, medication changes, and hospitalizations not captured on the SLEPDAI score; and c) physician's global-assessment (PGA) (range 0 to 3, with 0 indicating inactive disease and 3 severe disease). Study investigators were trained with “paper” pregnant SLE patients and case-report forms prepared by JPB (gold standard). The average correlation between investigator responses with the gold standard was 0.89 (95% CI: 0.83-0.95) and mean scores were all within ±15% of the gold standard. Inter-rater reliability estimated by the intraclass correlation coefficient was also high: 0.78 (95% CI: 0.61-0.89). In some situations, SLEPDAIs and PGAs were not completed because required serologic data and/or complete blood count (CBC) were unavailable. Flare status was then based on review of medical records and evidence of a clinical change and/or addition of new medications.
Adverse Pregnancy Outcomes
APO included one or more of the following: 1) fetal death after 12 weeks' gestation unexplained by chromosomal abnormalities, anatomic malformation, or congenital infection; 2) neonatal death prior to hospital discharge due to complications of prematurity and/or placental insufficiency (e.g. abnormal fetal surveillance test(s), abnormal Doppler flow velocimetry waveform analysis suggestive of fetal hypoxemia, oligohydramnios (25); 3) preterm delivery or termination of pregnancy <36 weeks due to gestational hypertension, preeclampsia, or placental insufficiency; 4) small for gestational age (SGA) neonate, defined as birth weight <5th percentile absent anatomical or chromosomal abnormalities.
Screening and Follow-Up Visits
Screening evaluation included history, ACR criteria, physical examination, CBC, comprehensive metabolic panel, urinalysis, and random or 24-hour urine collection for protein/creatinine ratio (if dipstick >1+). Serological profiles (anti-dsDNA, anti-SSA/Ro, SSB/La, and C3 and C4) were determined at local laboratories. Tests for aPL included anticardiolipin antibodies (IgG, IgM, and IgA), anti-β2 glycoprotein I (IgG, IgM), and lupus anticoagulant (LAC) and were performed at core laboratories (21).Tests were repeated each trimester. SLEPDAI and PGA were scored at screening, second trimester (20-23 weeks) and third trimester (32-35 weeks) visits.
Statistical Analysis
Bivariate associations of APO status and each predictor variable were evaluated with chi-square and Fisher's exact tests for categorical variables and T-test for continuous variables. Multivariable analyses were conducted using logistic regression models. Three separate models were fit to allow for greater flexibility in modeling the potentially time-varying relationship between predictors that were measured repeatedly during pregnancy and APO. Model 1 included variables measured at screening to identify baseline characteristics that are predictive of an APO occurring at any time. Model 2 considered these baseline variables plus variables measured at 20-23 weeks to predict APO after 23 weeks, and Model 3 considered additional variables measured at 32-35 weeks to predict late APO. Decisions regarding variable selection at each step of model development were based on both clinical factors and statistical significance. For example, change in C3 is routinely monitored in SLE patients and was therefore prioritized for inclusion in models 2 and 3. When a continuous variable such as PGA yielded similar results whether dichotomized using a clinically justified cut point or in the original scale, the more clinically interpretable binary form was chosen. Ethnicity/race was dichotomized to non-Hispanic White versus all other groups because of sample size and clinical considerations. Potential confounding by enrollment site was also evaluated. The c-statistic was computed to assess the model's ability to discriminate between patients with and without APO. Leave-one-out cross-validation was conducted to evaluate the degree of over-fitting the model to the data (26).
Four patients who were lost to follow-up were excluded. Missing data in the predictor variables was addressed using the Markov chain Monte Carlo multiple imputation approach in the SAS procedure, PROC MI. The rates of missing data were 0% – 7% for baseline variables, 2% - 24% for 20-23 week variables and 5% - 27% for 32-35 week variables. The imputation model included outcome, all predictors in each logistic regression model, variables with missing values at the relevant visit for that model, and the following auxiliary variables deemed to influence the missing data value: past history of renal disease, thrombosis, thrombocytopenia, fetal death and heparin use for model 1, and platelet count, PGA, SLEPDAI, and C3 at the prior visits for models 2 and 3. Forty imputed data sets were generated for each model and results were combined with PROC MIANALYZE. To evaluate the robustness of our results, sensitivity analyses were conducted using the complete case approach for handling missing data, simpler imputation models that included final predictors and outcome variable only, and more complex imputation models. All analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC). Statistical significance was defined as a two-sided P-value <0.05.
Role of Funding Source
The funding source had no role in design, conduct, analysis, and decision to submit this manuscript for publication.
Results
Study Population and Pregnancy Outcomes
Of 741 pregnant women screened for PROMISSE, 385 SLE patients with documented outcomes were included (Appendix Figure 1). Patients were recruited from 9 sites. Forty-eight percent of patients were Non-Hispanic White, and 35% were African American or Hispanic White. Patients were inactive or had stable mild/moderate activity at entry, with a mean SLEPDAI of 2.8 (SD=3.0) and mean PGA of 0.39 (SD=0.54). In 91%, entry urine protein excretion was <500mg/day. Among 120 patients with a history of renal disease, available biopsy results included 19 Class III, 29 Class IV, 21 Class V, 8 Class III and V, and 2 Class IV/V.
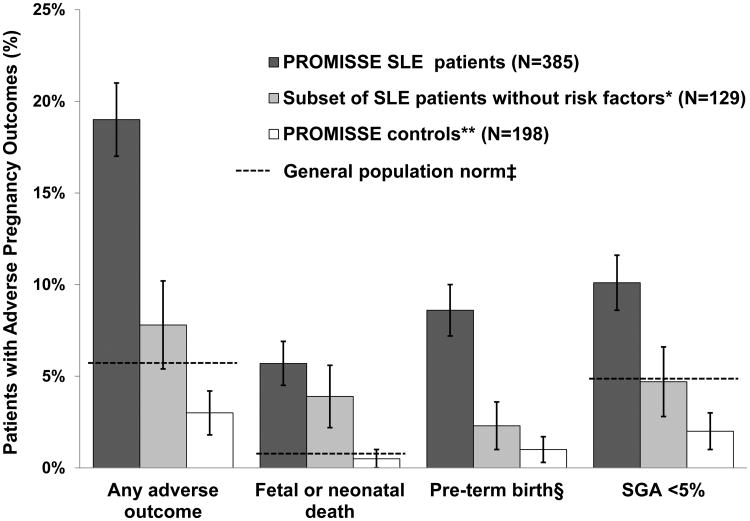
One or more APO occurred in 19.0% (95% CI: 15.2% - 23.2%) of SLE patients. Fetal death occurred in 4%, neonatal death in 1%, indicated preterm delivery in 9%, and SGA neonate in 10% (Figure 1,Table 1). Seventeen patients (4.4%) had more than one outcome. Preeclampsia after 36 weeks (not included in the PROMISSE APO definition) occurred in 2%.APO rates were 15.4% (95% CI: 11.7% - 19.7%) in SLE patients without aPL and 43.8% (95% CI: 29.5% - 58.8%) in those with aPL. In contrast, 3% (95% CI: 1.1% - 6.4%) of PROMISSE controls had one or more APO. Congenital heart block (CHB) occurred in 1/154 anti-Ro exposed fetuses. Neonatal outcomes are presented in Appendix Table 1.
Figure 1. Frequency of Adverse Pregnancy Outcomes in Patients and Healthy Controls.
Error bars represent 95% confidence bounds.
*SLE patients with no risk factors are non-Hispanic White, LAC negative, PGA ≤1, platelet count >100,000 and not treated with antihypertensive medications at baseline. They constitute a subset of “All SLE”.
† Because women enrolled in the PROMISSE control group had at least one successful pregnancy, no history of fetal death, no more than 1 miscarriage <10 weeks' gestation, and no underlying medical problems requiring treatment, it was anticipated that their pregnancy outcomes would be better than those in unselected healthy patients, particularly nulliparous women. In addition to the APOs in the bar graph, the healthy control patients had 10 (5.1%) adverse outcomes that did not meet the study primary outcome definition: ≤ 23 weeks: 2 genetic terminations; >23 to ≤ 35 weeks: 3 premature preterm rupture of membranes and/or spontaneous preterm births, 2 placental abruptions, 1 delivery for fetal indications (supraventricular tachycardia and hydrops); >35 weeks: 3 premature preterm rupture of membranes and/or spontaneous preterm births (event numbers may add to more than total because some women had more than one adverse outcome).
‡ When available, pregnancy outcomes in the general population are represented by the broken line (27, 28). No general population norm line is available for the study definition used for pre-term birth (see below).
§ Pre-term birth is defined as delivery at <36 weeks and indicated by gestational hypertension, preeclampsia, or placental insufficiency.
SGA = small for gestational age
Table 1. Pregnancy outcomes in SLE patients*.
| Gestational Age of Pregnancy Outcome in Completed Weeks | |||
|---|---|---|---|
| ≤23 weeks | >23 to ≤35 weeks | >35 weeks | |
| Pregnancies with PROMISSE Study-defined Adverse Pregnancy Outcomes** | 11 | 32 | 30 |
| Average gestational age at end of pregnancy (weeks) | 18.2 | 29.6 | 37.7 |
| Study APOs | |||
| Fetal death | 11 | 5 | 2 |
| Neonatal death | 0 | 5 | 0 |
| Birth < 36 wks due to placental insufficiency, gestational hypertension, or preeclampsia | 4 | 27 | 2 |
| Small-for-gestational age (birthweight <5th percentile). Patients with SGA had the following other pregnancy complications: | 3 | 9 | 27 |
| a) Premature preterm rupture of membranes and/or premature labor | 0 | 0 | 4 |
| b) Delivery > 35 weeks with GHTN/PE, oligohydramnios | 0 | 0 | 3e |
| Pregnancies with outcomes that were not in the PROMISSE Study definitions** | 4 | 20 | 52 |
| Average gestational age at end of pregnancy (weeks) | 19.6 | 32.1 | 38.7 |
| Termination of pregnancy | 1a | 2b | 0 |
| Incompetent cervix | 2 | 0 | 0 |
| Premature preterm rupture of membranes and/or premature labor | 1 | 14 | 14 |
| Delivery > 35 weeks with GHTN/PE, oligohydramnios | 0 | 0 | 28f |
| Delivery for other obstetric indications | 0 | 2c | 5g |
| Delivery for maternal indications | 0 | 2d | 6h |
| Uncomplicated pregnancies | 0 | 0 | 236 |
See Figure 1 for pregnancy outcomes in healthy control population
May add up to more than the total because patients may have multiple outcomes.
Elective
1 CHB, 1 Trisomy 18
1 placental abruption, 1 poor obstetric Hx
2 SLE flares
2 PE/GHTN>36W, 1 oligohydramnios
12 PE/GHTN>36W, 16 oligohydramnios
3 chorioamnionitis, 2 poorobstetricHx
5 thrombocytopenia, 1 congestive heart failure
Bivariate Analyses of Risk Factors of APO
Demographic and Past Medical History
Mothers with APO were more likely to be African-American, to have BMI >30, prior fetal demise >10 weeks of gestation, past renal disease, and prior thrombosis, compared to mothers without APO (Table 2). APO rates also differed according to site of enrollment, reflecting inherent differences in the clinical and demographic characteristics of patients treated at those centers.
Table 2. Characteristics of Pregnant SLE Patients at Baseline and Their Association with Adverse Pregnancy Outcome.
| Patient Characteristic | Total N=385 (%) | No AP0 N=312 (%) | APO N=73 (%) | P-value* | |
|---|---|---|---|---|---|
|
| |||||
| Demographic | Site, N (%) | 0.033 | |||
| NYU | 95 (24.7) | 82 (26.3) | 13 (17.8) | ||
| Toronto | 95 (24.7) | 77 (24.7) | 18 (24.7) | ||
| Johns Hopkins | 86 (22.3) | 75 (24.0) | 11 (15.1) | ||
| Hospital for Special | 47 (12.2) | 33 (10.6) | 14 (19.2) | ||
| Surgery | 29 (7.5) | 23 (7.4) | 6 (8.2) | ||
| University of Utah | 22 (5.7) | 14 (4.5) | 8 (11.0) | ||
| Oklahoma | 8 (2.1) | 6 (1.9) | 2 (2.7) | ||
| University of Chicago | 2 (0.5) | 1 (0.3) | 1 (1.4) | ||
| Columbia | 1 (0.3) | 1 (0.3) | 0 (0.0) | ||
| Northwestern | |||||
|
| |||||
| Ethnicity/Race, N (%) | 0.053 | ||||
| Non-Hispanic White | 184 (47.8) | 157 (50.3) | 27 (37.0) | ||
| Hispanic White | 58 (15.1) | 43 (13.8) | 15 (20.6) | ||
| African American | 78 (20.3) | 58 (18.6) | 20 (27.4) | ||
| Asian | 42 (10.9) | 36 (11.5) | 6 (8.2) | ||
| Other | 10 (2.6) | 10 (3.2) | 0 (0.0) | ||
| Don't know/Refused to answer | 13 (3.4) | 8 (2.6) | 5 (6.9) | ||
|
| |||||
| BMI, N (%) | 0.048 | ||||
| <25 | 217 (60.3) | 183 (62.9) | 34 (49.3) | ||
| 25-30 | 82 (22.8) | 65 (22.3) | 17 (24.6) | ||
| >30 | 61 (16.9) | 43 (14.8) | 18 (26.1) | ||
| Missing | 25 | 21 | 4 | ||
|
| |||||
| Mean Age (SD), N=385 | 30.93(4.9) | 31.04 (4.8) | 30.49 (5.3) | 0.39 | |
|
| |||||
| Clinical History | Pregnancy Number, N (%) | 0.36 | |||
| 1 | 159 (41.3) | 135 (43.3) | 24 (32.9) | ||
| 2 | 102 (26.5) | 82 (26.3) | 20 (27.4) | ||
| 3 | 66 (17.1) | 51 (16.4) | 15 (20.6) | ||
| 4+ | 58 (15.1) | 44 (14.1) | 14 (19.2) | ||
|
| |||||
| Renal Disease, N (%) | 0.042 | ||||
| No | 265 (68.8) | 222 (71.2) | 43 (58.9) | ||
| Yes | 120 (31.2) | 90 (28.9) | 30 (41.1) | ||
|
| |||||
| Platelets <100,000, N (%) | 0.058 | ||||
| No | 319 (82.9) | 264 (84.6) | 55 (75.3) | ||
| Yes | 66 (17.1) | 48 (15.4) | 18 (24.7) | ||
|
| |||||
| Prior Thrombosis, N (%) | 0.014 | ||||
| No | 354 (92.0) | 292 (93.6) | 62 (84.9) | ||
| Yes | 31 (8.1) | 20 (6.4) | 11 (15.1) | ||
|
| |||||
| Prior Fetal Death > 10 | 0.002 | ||||
| weeks, N (%) | 344 (89.4) | 286 (91.7) | 58 (79.5) | ||
| No | 41 (10.7) | 26 (8.3) | 15 (20.6) | ||
| Yes | |||||
|
| |||||
| Prior Premature Birth <34 | 0.104 | ||||
| weeks, N (%) | 378 (98.2) | 308 (98.7) | 70 (95.9) | ||
| No | 7 (1.8) | 4 (1.3) | 3 (4.1) | ||
| Yes | |||||
|
| |||||
| Laboratory Values | Anti-dsDNA, N (%) | 0.51 | |||
| Negative | 219 (58.4) | 180 (59.2) | 39 (54.9) | ||
| Positive | 156 (41.6) | 124 (40.8) | 32 (45.1) | ||
| Missing | 10 | 8 | 2 | ||
|
| |||||
| aPL***, N (%) | <0.001 | ||||
| Negative | 337 (87.5) | 285 (91.4) | 52 (71.2) | ||
| Positive | 48 (12.5) | 27 (8.7) | 21 (28.8) | ||
|
| |||||
| LAC, N (%) | <0.001 | ||||
| Negative | 351 (91.2) | 297 (95.2) | 54 (74.0) | ||
| Positive | 34 (8.8) | 15 (4.8) | 19 (26.0) | ||
|
| |||||
| Anti-Ro, N (%) | 0.21 | ||||
| Negative | 221 (59.1) | 175 (57.6) | 46 (65.7) | ||
| Positive | 153 (40.9) | 129 (42.4) | 24 (34.3) | ||
| Missing | 11 | 8 | 3 | ||
|
| |||||
| Anti-La, N (%) | 0.57 | ||||
| Negative | 311 (84.3) | 253 (83.8) | 58 (86.6) | ||
| Positive | 58 (15.7) | 49 (16.2) | 9 (13.4) | ||
| Missing | 16 | 10 | 6 | ||
|
| |||||
| Protein > 500mg/day, N (%) | 0.40 | ||||
| No | 339 (91.1) | 277 (91.7) | 62 (88.6) | ||
| Yes | 33 (8.9) | 25 (8.3) | 8 (11.4) | ||
| Missing | 13 | 10 | 3 | ||
|
| |||||
| Platelets | |||||
| Mean (SD), N=379 | 253.04 (84.3) | 259.13 (85.8) | 226.17(71.6) | 0.003 | |
| < 100,000, N (%) | |||||
| No | 370 (97.6) | 304 (98.4) | 66 (94.3) | 0.065 | |
| Yes | 9 (2.4) | 5 (1.6) | 4 (5.7) | ||
| Missing | 6 | 3 | 3 | ||
|
| |||||
| Low Complement**, N (%) | 0.019 | ||||
| No | 252 (66.0) | 213 (68.7) | 39 (54.2) | ||
| Yes | 130 (34.0) | 97 (31.3) | 33 (45.8) | ||
| Missing | 3 | 2 | 1 | ||
|
| |||||
| Mean C3 in g/L (SD), N=364 | 1.05 (0.3) | 1.05 (0.3) | 1.02 (0.3) | 0.31 | |
|
| |||||
| Mean C4 in g/L (SD), N=358 | 0.20 (0.1) | 0.20 (0.1) | 0.19 (0.1) | 0.60 | |
|
| |||||
| Disease Activity | SLEPDAI | ||||
| Mean (SD), N=381 | 2.79 (3.0) | 2.58 (2.9) | 3.68 (3.2) | 0.005 | |
| > 4 | 0.009 | ||||
| No | 311 (81.6) | 260 (84.1) | 51 (70.8) | ||
| Yes | 70 (18.4) | 49 (15.9) | 21 (29.2) | ||
| Missing | 4 | 3 | 1 | ||
|
| |||||
| PGA | |||||
| Mean (SD), N=367 | 0.39 (0.5) | 0.35 (0.5) | 0.59 (0.6) | 0.005 | |
| > 1 | |||||
| No | 328 (89.4) | 275 (91.7) | 53 (79.1) | 0.003 | |
| Yes | 39 (10.6) | 25 (8.3) | 14 (20.9) | ||
| Missing | 18 | 12 | 6 | ||
|
| |||||
| Current Medications | Glucocorticoids, N (%) | 0.29 | |||
| No | 232 (60.3) | 192 (61.5) | 40 (54.8) | ||
| Yes | 153 (39.7) | 120 (38.5) | 33 (45.2) | ||
|
| |||||
| Hydroxychloroquine, N (%) | 0.38 | ||||
| No | 136 (34.3) | 107 (34.3) | 29 (39.7) | ||
| Yes | 249 (64.7) | 205 (65.7) | 44 (60.3) | ||
|
| |||||
| Azathioprine, N (%) | 0.184 | ||||
| No | 316 (82.1) | 260 (83.3) | 56 (76.7) | ||
| Yes | 69 (17.9) | 52 (16.7) | 17 (23.3) | ||
|
| |||||
| Heparin, N (%) | 0.009 | ||||
| No | 302 (78.4) | 253 (81.1) | 49 (67.1) | ||
| Yes | 83 (21.6) | 59 (18.9) | 24 (32.9) | ||
|
| |||||
| Aspirin, N (%) | 0.48 | ||||
| No | 250 (64.9) | 200 (64.1) | 50 (68.5) | ||
| Yes | 135 (35.1) | 112 (35.9) | 23 (31.5) | ||
|
| |||||
| Antihypertensives, N (%) | < 0.001 | ||||
| No | 352 (91.4) | 295 (94.6) | 57 (78.1) | ||
| Yes | 33 (8.6) | 17 (5.5) | 16 (21.9) | ||
APO = adverse pregnancy outcome; anti-dsDNA = anti-double stranded DNA; LAC = lupus anticoagulant; SLEPDAI = Systemic Lupus Erythematosus Pregnancy Disease Activity Index; PGA = physician's global assessment
P-values based on available data; proportion of missing data < 10% for all variables
Low is defined as C3 and/or C4 below normal value in the local laboratory
APL is defined as aCL IgG ≥40 GPL units; IgM ≥40 MPL units and/or positive LAC: RVVT, Kaolin, dilute TTI or PTT LA and/or anti-β2GPI IgG ≥40 GPL units; IgM ≥40 MPL units at least twice between 6 weeks and 5 years apart of which one must be during the PROMISSE pregnancy at a core lab, as previously described (21).
Laboratory Values at Screening Visit
The proportion of mothers classified as aPL-positive was higher in patients with APO compared to those without APO. Mothers with APO were more likely, at screening to have positive LAC, low platelet count, and low complement level (C3, C4, or CH50 below laboratory normal), although overall mean levels of C3 and C4 were in the normal range and not significantly different between those with or without APO. The proportions of patients with anti-ds DNA antibodies, anti-Ro, anti-La and urinary protein >500 mg/day were also similar across groups.
Medications and Disease Activity at Screening Visit
Baseline SLEPDAI and PGA were significantly higher in those with APO. Use of antihypertensive medications and heparin was also more common among patients with APO. Of 33 patients receiving antihypertensive medications, 13 had no evidence of prior nephritis and 28 had no proteinuria; of 83 patients receiving any type of heparin, 29 met study criteria for aPL positivity and 26 had prior thrombosis. Glucocorticoids were not associated with APO.
Maternal Disease Activity during Follow-Up
Table 3 summarizes disease activity measures and laboratory parameters obtained at 20-23 weeks on 370 patients still pregnant at 23 weeks, and variables measured at 32-35 weeks for 318 patients at 35 weeks. Among patients with known flare status, 12.7% (95% CI: 9.4% - 16.5%) had a mild/moderate flare and 2.5% (95% CI: 1.1% - 4.7%) had a severe flare at 20-23 weeks; 9.6% (95% CI: 6.5% - 13.5%) had a mild/moderate flare and 3.0% (95% CI: 1.4% - 5.6%) had a severe flare at 32-35 weeks. Severe flares included nephritis (9), pleuritis (6), arthritis (5), thrombocytopenia (3), and cerebritis, myositis, and pericarditis (1 each) with some patients having more than one organ system involved.
Table 3. Association of Laboratory Parameters and Maternal Lupus Disease Activity During Pregnancy with Adverse Outcomes.
| 20 – 23 Week Measures* | Total N=370 (%) | No APO N=308 (%) | APO N=62 (%) | P-value** |
|---|---|---|---|---|
|
| ||||
| Anti-dsDNA | ||||
| Negative | 188 (60.5) | 162 (61.8) | 26 (53.1) | 0.25 (0.45) |
| Positive | 123 (39.6) | 100 (38.2) | 23 (46.9) | |
| Missing | 59 | 46 | 13 | |
|
| ||||
| Change in protein > 500mg/day from baseline | 0.22 (0.27) | |||
| No | 346 (97.2) | 290 (97.6) | 56 (94.9) | |
| Yes | 10 (2.8) | 7 (2.4) | 3 (5.1) | |
| Missing | 14 | 11 | 3 | |
|
| ||||
| Mean Platelets (SD), N=318 | 241.90 (76.9) | 246.87 (75.4) | 218.64 (80.6) | 0.013(0.004) |
| <100,000, N (%) | ||||
| No | 308 (96.9) | 0.079 (0.063) | ||
| Yes | 10 (3.1) | 256 (97.7) | 52 (92.9) | |
| Missing | 52 | 6 (2.3) | 4 (7.1) | |
| 46 | 6 | |||
|
| ||||
| Low complement | ||||
| No | 226 (72.2) | 195 (74.1) | 31 (62.0) | 0.079 (0.167) |
| Yes | 87 (27.8) | 68 (25.9) | 19 (38.0) | |
| Missing | 57 | 45 | 12 | |
|
| ||||
| C3 | ||||
| Mean in g/L (SD), N=300 | 1.14 (0.3) | 1.17 (0.3) | 1.03 (0.3) | 0.002(0.007) |
| Mean increase from baseline (SD),N=289 | 0.10 (0.2) | 0.11 (0.2) | 0.04 (0.2) | 0.025(0.009) |
|
| ||||
| C4 | ||||
| Mean in g/L (SD), N=295 | 0.20 (0.1) | 0.20 (0.1) | 0.20 (0.1) | 0.96(0.79) |
| Mean increasefrom baseline (SD),N=281 | 0.00 (0.1) | 0.00 (0.0) | 0.01 (0.1) | 0.71(0.73) |
|
| ||||
| Mean SLEPDAI (SD), N=319 | 2.22 (2.5) | 1.93 (2.1) | 3.67 (3.4) | <0.001(<0.001) |
| >4 | ||||
| No | 280(87.8) | 241 (90.9) | 39 (72.2) | <0.001(<0.001) |
| Yes | 39 (12.2) | 24 (9.1) | 15 (27.8) | |
| Missing | 51 | 43 | 8 | |
|
| ||||
| Mean PGA (SD), N=301 | 0.38 (0.5) | 0.34 (0.5) | 0.57 (0.6) | 0.015(0.009) |
| > 1 | ||||
| No | 277 (92.0) | 234 (94.0) | 43 (82.7) | 0.011(0.019) |
| Yes | 24 (8.0) | 15 (6.0) | 9 (17.3) | |
| Missing | 69 | 59 | 10 | |
|
| ||||
| Flare | ||||
| No | 308 (84.9) | 269 (88.5) | 39 (66.1) | <0.001 (<0.001) |
| Mild/moderate | 46 (12.7) | 30 (9.9) | 16 (27.1) | |
| Severe | 9 (2.5) | 5 (1.6) | 4 (6.8) | |
| Unknown | 7 | 4 | 3 | |
|
| ||||
| 32 – 35 Week Measures* | TotalN=318 (%) | No APON=288 (%) | APON=30 (%) | P-value** |
|
| ||||
| Anti-dsDNA | ||||
| Negative | 160 (65.3) | 146 (65.5) | 14 (63.6) | 0.86 (0.93) |
| Positive | 85 (34.7) | 77 (34.5) | 8 (36.4) | |
| Missing | 73 | 65 | 8 | |
|
| ||||
| Change in protein > 500mg/day from second trimester | ||||
| No | 285 (97.3) | 261 (97.8) | 24 (92.3) | 0.152(0.104) |
| Yes | 8 (2.7) | 6 (2.3) | 2 (7.7) | |
| Missing | 25 | 21 | 4 | |
|
| ||||
| Mean Platelets (SD), N=258 | 233.98 (75.6) | 234.65 (75.4) | 227.46 (79.2) | 0.66(0.61) |
| <100,000, N (%) | ||||
| No | 254 (98.5) | 0.32(0.46) | ||
| Yes | 4 (1.6) | 231 (98.7) | 23 (95.8) | |
| Missing | 60 | 3 (1.3) | 1 (4.2) | |
| 54 | 6 | |||
|
| ||||
| Low complement | ||||
| No | 191 (77.0) | 177 (78.3) | 14 (63.6) | 0.118(0.120) |
| Yes | 57 (23.0) | 49 (21.7) | 8 (36.4) | |
| Missing | 70 | 62 | 8 | |
|
| ||||
| C3 | ||||
| Mean in g/L (SD), N=241 | 1.25 (0.3) | 1.26 (0.3) | 1.13 (0.4) | 0.147(0.027) |
| Mean increase from baseline (SD), N=234 | 0.19 (0.2) | 0.20 (0.2) | 0.14 (0.2) | 0.18(0.019) |
|
| ||||
| C4 | ||||
| Mean in g/L (SD), N=239 | 0.21 (0.1) | 0.22 (0.1) | 0.21 (0.1) | 0.90(0.80) |
| Mean increase from baseline (SD), N=231 | 0.01 (0.1) | 0.01 (0.1) | 0.01 (0.1) | 0.96(0.59) |
|
| ||||
| Mean SLEPDAI (SD), N=265 | 1.99 (2.7) | 1.83 (2.5) | 3.74 (4.4) | 0.051(<0.001) |
| >4, N (%) | ||||
| No | 239 (90.2) | 221 (91.3) | 18 (78.3) | 0.060(0.006) |
| Yes | 26(9.8) | 21 (8.7) | 5 (21.7) | |
| Missing | 53 | 46 | 7 | |
|
| ||||
| Mean PGA (SD), N=262 | 0.31 (0.5) | 0.30 (0.4) | 0.44 (0.6) | 0.31 (0.038) |
| >1, N (%) | ||||
| No | 247 (94.3) | 228 (95.0) | 19 (86.4) | 0.120 (0.047) |
| Yes | 15 (5.7) | 12 (5.0) | 3 (13.6) | |
| Missing | 56 | 48 | 8 | |
|
| ||||
| Flare | ||||
| No | 264 (87.4) | 249 (89.3) | 15 (65.2) | <0.001 (<0.001) |
| Mild/moderate | 29 (9.6) | 25 (9.0) | 4 (17.4) | |
| Severe | 9 (3.0) | 5 (1.8) | 4 (17.4) | |
| Unknown | 16 | 9 | 7 | |
APO = adverse pregnancy outcome; anti-dsDNA = anti-double stranded DNA; SLEPDAI = Systemic Lupus Erythematosus Pregnancy Disease Activity Index; PGA = physician's global assessment
20-23 week data are based on patients who had not delivered by 23 weeks and 32-35 week data are based on patients who had not delivered by 35 weeks.
Top p-value based on available data, bottom p-value in () based on multiply imputed data (see Statistical Analysis).
At both 20-23 weeks and 32-35 weeks, smaller increases in C3 from baseline, higher SLEPDAI, and higher PGA scores were observed in patients with APO. Platelet counts were also significantly lower at 20-23 weeks in those with APO. Patients with APO were more likely to have flared in second or third trimesters. Albeit rare, an increase in proteinuria of >500 mg/day from the prior visit during second or third trimesters was not associated with subsequent APO. Anti-dsDNA antibodies and C4 levels in the second or third trimester did not differ between patients with or without APO.
Multivariable Analyses for Risk Factors of APO
Baseline variables which were independently predictive of APO at any time included LAC, antihypertensive use, PGA score>1, and lower platelet count. Non-Hispanic White ethnicity/race was associated with a lower risk of APO, compared to the other race/ethnic groups combined. LAC status and antihypertensive use on risk of APO were strongly related to risk of APO. Similar baseline predictors of APO were identified in analyses restricted to primigravid patients (results not shown).
Variables at screening that predicted APO after 23 weeks were identical to predictors of APO at any time: antihypertensive use, LAC, PGA score >1, and lower platelet count, with non-Hispanic White ethnicity/race associated with lower risk of APO (Table 4 Model 2). In addition, maternal flares in second trimester, increase in SLEPDAI score, and less of an increase in C3 level from baseline, predicted APO after 23 weeks. Adjusted odds of an APO was nearly 6-fold higher among women who had a severe flare compared to those who did not.
Table 4. Predictors of Adverse Pregnancy Outcomes from Logistic Regression models.
| Predictor Variable | Model 1: APO at any time during pregnancy (N=385, Events = 73) | Model 2: APO after 23 weeks (N=370, Events = 62) | Model 3: APO after 35 weeks (N = 318, Events = 30) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|
| ||||||
| Non-Hispanic White : Yes vs No | 0.45 (0.24-0.84) | 0.013 | 0.44 (0.21-0.93) | 0.032 | 0.40 (0.15-1.08) | 0.071 |
|
| ||||||
| Current Antihypertensive Use: Yes vs No | 7.05 (3.05-16.31) | <0.001 | 13.14 (4.79-36.04) | <0.001 | 7.69 (2.37-24.96) | <0.001 |
|
| ||||||
| LAC status: Positive vs Negative | 8.32 (3.59-19.26) | <0.001 | 7.80 (2.83-21.45) | <0.001 | 4.04 (1.05-15.61) | 0.043 |
|
| ||||||
| PGA: > 1 vs ≤ 1 | 4.02 (1.84-8.82) | <0.001 | 3.78 (1.47-9.73) | 0.006 | 3.20 (0.93-10.96) | 0.064 |
|
| ||||||
| Platelet Count (per 50,000 decrease) | 1.33 (1.09-1.63) | 0.006 | 1.41 (1.12-1.78) | 0.003 | 1.35 (1.01-1.82) | 0.046 |
|
| ||||||
| 20-23 weeks | ||||||
|
| ||||||
| Flare: | ||||||
| Mild/Moderate vs None | 3.14 (1.25-7.90) | 0.0150 | ||||
| Severe vs None | 5.87 (1.15-29.96) | .033 | ||||
|
| ||||||
| Change in C3 from baseline (per 0.10 g/L decrease) | 1.24 (1.03-1.50) | 0.025 | ||||
|
| ||||||
| SLEPDAI (per 2 point increase) | 1.43 (1.06-1.94) | 0.020 | ||||
|
| ||||||
| 32-35 weeks | ||||||
|
| ||||||
| Flare: | ||||||
| Mild/Moderate vs None | 1.95 (0.55-7.00) | 0.30 | ||||
| Severe vs None | 9.60 (1.95-47.35) | 0.006 | ||||
|
| ||||||
| Change in C3 from baseline (per 0.10 g/L decrease) | 1.21 (0.97-1.50) | 0.087 | ||||
|
| ||||||
| C-statistic | 0.78 (95% CI: 0.71-0.84) | 0.84 (95% CI: 0.77-0.90) | 0.79 (95% CI: 0.70 – 0.89) | |||
|
| ||||||
| Cross-validated C-statistic | 0.76 (95% CI: 0.69 – 0.83) | 0.81 (95% CI: 0.74-0.88) | 0.75 (95% CI:0.64-0.85) | |||
LAC = lupus anticoagulant; PGA = physician's global assessment; SLEPDAI = Systemic Lupus Erythematosus Pregnancy Disease Activity Index; Missing data for predictor variables was addressed using multiple imputation (see Statistical Analysis)
Many baseline and second trimester variables associated with risk of APO were also predictive of APO after 35 weeks, but reduced sample size and number of events diminished power (Table 4 Model 3). Statistically significant predictors were antihypertensive use at screening, LAC status, lower platelet count, and occurrence of severe flare between 32-35 weeks. The magnitudes of the effects of Non-Hispanic White ethnicity/race, PGA>1 and change in C3 on risk of APO were similar to those in models 1 and 2, but not did reach statistical significance. The limited sample size and number of events may not support a model with this number of covariates; thus, these results should be interpreted cautiously.
In sensitivity analyses using alternative approaches for handling missing data, the main results and inferences remained the same for models 1 and 2 (Appendix Table 2). Model 3 was more sensitive to how missing data were handled, but baseline antihypertensive and low platelet count, and severe flare in third trimester were consistently predictive of APO after 35 weeks across the different approaches considered.
Among 129 women known to be non-Hispanic White, not on antihypertensive therapy, LAC negative, PGA≤1 at screening, and platelet count >100,000, only 10 (7.8%, 95% CI: 3.8% - 13.8%) had an APO at any time and fetal or neonatal death rate was 3.9% (95% CI: 1.3% - 8.8%). In comparison, APO rate was 58.0% (95% CI: 43.2% - 71.8%) and fetal/neonatal death rate 22.0% (95% CI: 11.5% - 36.0%) in the combined group of 50 women known to be either LAC positive, or LAC negative but non-White or Hispanic and treated with antihypertensive medications. Seven of 8 LAC positive patients with either PGA >1, on antihypertensive medication or with platelet counts <100,000 at screening experienced an APO.
Discussion
In our large cohort of prospectively followed SLE patients with inactive or stable mild/moderate activity at conception, 81% of pregnancies were uncomplicated, 5% ended in fetal or neonatal death, and severe maternal flares occurred in <3%. Importantly, the rate of APOs was <8%, with fetal or neonatal deaths accounting for fewer than half of these events in non-Hispanic White women with a PGA score ≤1, negative LAC, no antihypertensives, and platelet count >100,000. The frequency of CHB was half the reported rate of 2% (29, 30) perhaps due to a protective effect of hydroxychloroquine (31, 32).
Physicians often use anti-dsDNA antibodies and complement to anticipate clinical outcome. In this study, anti-dsDNA positivity was not associated with APO. However, patients with APO had baseline complement levels below normal ranges more often, although first trimester mean C3 and C4 values were in the normal range and not predictive of APO. Despite mean levels of C3 and C4 remaining in the normal range as pregnancy progressed, less of an increase in C3 levels from baseline to second trimester was predictive of an APO after 23 weeks. Interpretation of complement is confounded in pregnancy because circulating complement reflects both synthesis (enhanced by estrogen), and consumption (33). The absence of an increase in complement during pregnancy suggests increased complement activation with generation of anaphylatoxins which drive poor placental vascularization and trophoblast injury (34).
Other studies of pregnant women with SLE have assessed serological variables. In a single-center study of 40 pregnancies in women with mild/moderate lupus activity, clinical and laboratory variables evaluated between 20-28 weeks revealed 9 of 38 live births were preterm with low C4, the only marker associated with this outcome (19). A retrospective study of 267 pregnancies in 203 non-Hispanic SLE patients, one third of whom had APO, showed clinical and serologic activity (positive anti-dsDNA or low complement)in the second trimester was associated with fetal loss and preterm birth, even in those with low clinical activity (11). Our observations suggest that low complement levels in second and third trimesters were not helpful, but those with an APO had less of an increase in C3 later in pregnancy compared to baseline.
Findings from PROMISSE suggest that there is an increased risk of APO in patients with history of nephritis, although the association was not significant in multivariable analyses. A retrospective study of 107 pregnancies in 83 SLE patients found higher frequencies of preterm delivery (30%) and preeclampsia (28%) in women with past nephritis compared to 11% and 16%, respectively, in women without prior nephritis (11). Other retrospective studies did not find a relationship between previous nephritis and APO, and inclusion of more than one pregnancy in the same patient detracts from their reliability (7, 35). While a recent prospective study limited to patients with past lupus nephritis concluded that patients with quiescent disease at conception had favorable pregnancy outcomes (36), a meta-analysis of 37 studies with 1,842 patients showed that prior nephritis was associated with higher rates of preeclampsia (37).
Limitations of this study are acknowledged. The predictive models have not been externally validated and the number of adverse events in model 3 was limited. Given the timing of recruitment, PROMISSE did not address first trimester losses. Additional biomarkers should be evaluated to identify high risk patients and define mechanisms of APO in SLE patients.
Our study is the largest prospective study to date investigating pregnancy in SLE. In patients with inactive or stable mild/moderate activity, pregnancy is safer for mother and child than was considered in the past, with good outcomes in 81% of patients. Because women with high activity (e.g. active nephritis or prednisone >20 mg/day) were excluded, our findings may not apply to these individuals. Importantly, in the absence of baseline features indicative of risk (LAC positive, antihypertensive medications, PGA >1, Hispanic or non-White ethnicity/race, low platelet count), pregnancy outcomes are highly favorable. Patients with risk factors identified for APO should be monitored more closely and considered for future interventional trials to prevent APO.
Supplementary Material
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Acknowledgments
Supported by: NIH/NIAMS RO1 AR49772 (PROMISSE Study, MDL, MK, DWB, JB, MG, CL, JM, MP, FP, LS, MDS, JES)
Mary Kirkland Center for Lupus Research (JES, MDL)
NIH AR43727 (MP)
Appendix.
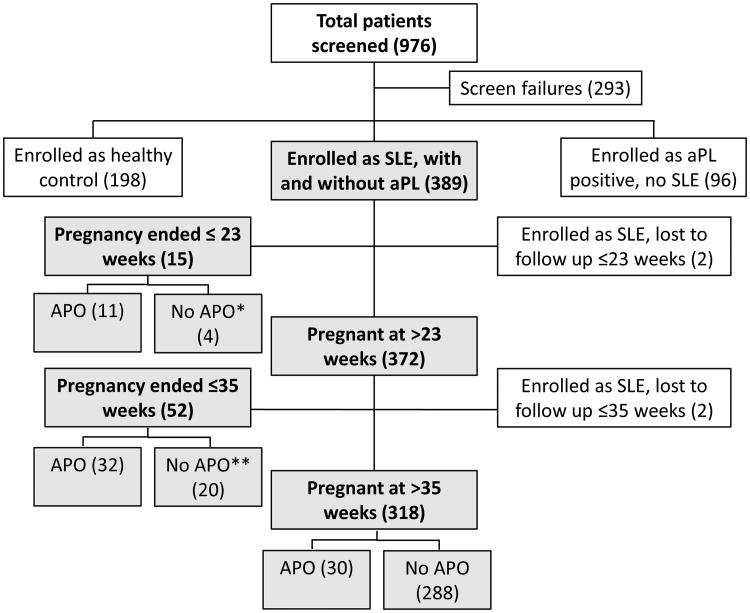
Appendix Figure 1. Patient enrollment and classification for the PROMISSE Study.
Pregnant women at <12 weeks' gestation with antiphospholipid antibody (aPL) positivity and systemic lupus erythematosus (SLE) and healthy controls were screened. Exclusion criteria included: multifetal pregnancy, prednisone >20 mg/day, blood pressure ≥140/90 mm Hgurine protein (mg)/creatinine (gram) ratio ≥1,000 mg protein/gm creatinine on 24-hour urine or spot urine collection, serum creatinine >1.2 mg/dl, screened too late in pregnancy, and previously enrolled in PROMISSE. Healthy pregnant women were enrolled if they had ≥ one successful pregnancy, no history of fetal death, and no more than 1 miscarriage <10 weeks' gestation and no antiphospholipid antibodies. The current study included all PROMISSE patients who met American College of Rheumatology criteria for systemic lupus erythematosus (SLE). Number of subjects is shown in parenthesis.
*Four patients whose pregnancy ended at <23 weeks did not meet study criteria for primary study outcome: elective termination (1), incompetent cervix (2), PPROM (1).See Table 1.
**Twenty patients whose pregnancy ended at <35 weeks did not meet study criteria for primary study outcome: indicated delivery for poor obstetric history (1), for SLE flare (1), for bleeding placental abruption (1); termination for complete heart block (1); fetal death due to trisomy 18 (1); PPROM and/or premature labor (15).See Table 1.
APO = adverse pregnancy outcome; aPL = antiphospholipid antibodies; PPROM = premature preterm rupture of membranes
Appendix Table 1. Neonatal Outcomes (includes only live-born neonates).
| Gestational Age of Pregnancy Outcome in Completed Weeks | ||
|---|---|---|
| >23 to ≤35 weeks (N) | >35 weeks (N) | |
| Live births from pregnancies with PROMISSE Study-defined APOs | 27 | 28 |
| 5 minute Apgar <7 | 40.7% (27) * | 3.8% (26) |
| 5 minute Apgar <5 | 40.7% (27) | 3.8% (26) |
| Need for ventilator support (CPAP or intubation) | 84.2% (19) | 10.5% (19) |
| Neonatal hospitalization >5 days (in neonates living to hospital discharge) | 88.2% (17) | 21.7% (23) |
| Neonatal death prior to discharge due to complications of prematurity | 5a | 0 |
| Other neonatal complications | 1b | 1d |
| Live births from pregnancies from pregnancies without PROMISSE Study-defined APOs | 20 | 288 |
| 5 minute Apgar <7 | 5.6% (18) | 0.4% (229) |
| 5 minute Apgar <5 | 0% (18) | 0.4% (229) |
| Need for ventilator support (CPAP or intubation) | 41.7% (12) | 2.6% (229) |
| Neonatal hospitalization >5 days | 71.4% (14) | 2.6% (229) |
| Other neonatal complications | 1c | 4e |
Includes 5 cases of extreme prematurity, including 2 cases with sepsis and 1 case with respiratory distress syndrome
1 case of sepsis
1 case of blindness
1 case of sepsis and respiratory distress syndrome
Includes 1 case of tachycardia, 1 case of hydronephrosis, 1 case of pneumothorax and 1 case of Tetralogy of Fallot with severe pulmonary atresia
% subjects who are positive for the parameter; N=total number of patients with available data for the specific parameter
Footnotes
ClinicalTrials.gov identifier: NCT00198068
Contributor Information
Jill P. Buyon, New York University School of Medicine, New York.
Mimi Y. Kim, Albert Einstein College of Medicine, New York.
Marta M. Guerra, Hospital for Special Surgery, New York.
Carl A. Laskin, University of Toronto, Toronto.
Michelle Petri, Johns Hopkins University School of Medicine, Baltimore.
Michael D. Lockshin, Hospital for Special Surgery and Weill Cornell Medical College, New York.
Lisa Sammaritano, Hospital for Special Surgery and Weill Cornell Medical College, New York.
D. Ware Branch, University of Utah Health Sciences Center and Intermountain Healthcare, Salt Lake City.
T. Flint Porter, University of Utah Health Sciences Center and Intermountain Healthcare, Salt Lake City.
Allen Sawitzke, University of Utah Health Sciences Center, Salt Lake City.
Joan T. Merrill, Oklahoma Medical Research Foundation and the University of Oklahoma Health Sciences Center, Oklahoma City.
Mary D. Stephenson, University of Illinois at Chicago, Chicago.
Elisabeth Cohn, Hospital for Special Surgery, New York.
Lamya Garabet, Hospital for Special Surgery, New York, Oest fold Hospital Trust, Fredrikstad, Norway.
Jane E. Salmon, Hospital for Special Surgery and Weill Cornell Medical College, New York.
References
- 1.Silva CA, Leal MM, Leone C, Simone VP, Takiuti AD, Saito MI, et al. Gonadal function in adolescents and young women with juvenile systemic lupus erythematosus. Lupus. 2002;11(7):419–25. doi: 10.1191/0961203302lu219oa. [DOI] [PubMed] [Google Scholar]
- 2.Ostensen M. New insights into sexual functioning and fertility in rheumatic diseases. Best Pract Res Clin Rheumatol. 2004;18(2):219–32. doi: 10.1016/j.berh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Allbritton J. Fetal outcome of lupus pregnancy: a retrospective case-control study of the Hopkins Lupus Cohort. J Rheumatol. 1993;20(4):650–6. [PubMed] [Google Scholar]
- 4.Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA. Outcome of lupus pregnancy: a controlled study. Rheumatology (Oxford) 2000;39(9):1014–9. doi: 10.1093/rheumatology/39.9.1014. [DOI] [PubMed] [Google Scholar]
- 5.Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008;199(2):127 e1–6. doi: 10.1016/j.ajog.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Arfaj AS, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus. 2010;19(14):1665–73. doi: 10.1177/0961203310378669. [DOI] [PubMed] [Google Scholar]
- 7.Ko HS, Ahn HY, Jang DG, Choi SK, Park YG, Park IY, et al. Pregnancy outcomes and appropriate timing of pregnancy in 183 pregnancies in Korean patients with SLE. Int J Med Sci. 2011;8(7):577–83. doi: 10.7150/ijms.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Zhao Y, Song Y, Zhang W, Bian X, Yang J, et al. Pregnancy in women with systemic lupus erythematosus: a retrospective study of 111 pregnancies in Chinese women. J Matern Fetal Neonatal Med. 2012;25(3):261–6. doi: 10.3109/14767058.2011.572310. [DOI] [PubMed] [Google Scholar]
- 9.Shand AW, Algert CS, March L, Roberts CL. Second pregnancy outcomes for women with systemic lupus erythematosus. Ann Rheum Dis. 2013;72(4):547–51. doi: 10.1136/annrheumdis-2011-201210. [DOI] [PubMed] [Google Scholar]
- 10.Smyth A, Oliveira GH, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5(11):2060–8. doi: 10.2215/CJN.00240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clowse ME, Magder LS, Petri M. The clinical utility of measuring complement and anti-dsDNA antibodies during pregnancy in patients with systemic lupus erythematosus. J Rheumatol. 2011;38(6):1012–6. doi: 10.3899/jrheum.100746. [DOI] [PubMed] [Google Scholar]
- 12.Bramham K, Hunt BJ, Bewley S, Germain S, Calatayud I, Khamashta MA, et al. Pregnancy outcomes in systemic lupus erythematosus with and without previous nephritis. J Rheumatol. 2011;38(9):1906–13. doi: 10.3899/jrheum.100997. [DOI] [PubMed] [Google Scholar]
- 13.Lynch A, Marlar R, Murphy J, Davila G, Santos M, Rutledge J, et al. Antiphospholipid antibodies in predicting adverse pregnancy outcome. A prospective study. Ann Intern Med. 1994;120(6):470–5. doi: 10.7326/0003-4819-120-6-199403150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 1991;34(12):1538–45. doi: 10.1002/art.1780341210. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Irastorza G, Lima F, Alves J, Khamashta MA, Simpson J, Hughes GR, et al. Increased rate of lupus flare during pregnancy and the puerperium: a prospective study of 78 pregnancies. Br J Rheumatol. 1996;35(2):133–8. doi: 10.1093/rheumatology/35.2.133. [DOI] [PubMed] [Google Scholar]
- 16.Wong KL, Chan FY, Lee CP. Outcome of pregnancy in patients with systemic lupus erythematosus. A prospective study. Arch Intern Med. 1991;151(2):269–73. [PubMed] [Google Scholar]
- 17.Lockshin MD. Pregnancy does not cause systemic lupus erythematosus to worsen. Arthritis Rheum. 1989;32(6):665–70. doi: 10.1002/anr.1780320602. [DOI] [PubMed] [Google Scholar]
- 18.Urowitz MB, Gladman DD, Farewell VT, Stewart J, McDonald J. Lupus and pregnancy studies. Arthritis Rheum. 1993;36(10):1392–7. doi: 10.1002/art.1780361011. [DOI] [PubMed] [Google Scholar]
- 19.Clowse ME, Wallace DJ, Weisman M, James A, Criscione-Schreiber LG, Pisetsky DS. Predictors of preterm birth in patients with mild systemic lupus erythematosus. Ann Rheum Dis. 2013;72(9):1536–9. doi: 10.1136/annrheumdis-2012-202449. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Lockshin MD, Kim M, Laskin CA, Guerra M, Branch DW, Merrill J, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheum. 2012;64(7):2311–8. doi: 10.1002/art.34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buyon JP, Kalunian KC, Ramsey-Goldman R, Petri MA, Lockshin MD, Ruiz-Irastorza G, et al. Assessing disease activity in SLE patients during pregnancy. Lupus. 1999;8(8):677–84. doi: 10.1191/096120399680411272. [DOI] [PubMed] [Google Scholar]
- 23.Levy RA, Vilela VS, Cataldo MJ, Ramos RC, Duarte JL, Tura BR, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401–4. doi: 10.1191/096120301678646137. [DOI] [PubMed] [Google Scholar]
- 24.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–8. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 25.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 26.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning: with Applications in R. New York: Springer; 2013. [Google Scholar]
- 27.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282–91. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep. 2012;60(8):1–22. [PubMed] [Google Scholar]
- 29.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44(8):1832–5. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117(4):485–93. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 31.Izmirly PM, Kim MY, Llanos C, Le PU, Guerra MM, Askanase AD, et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis. 2010;69(10):1827–30. doi: 10.1136/ard.2009.119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126(1):76–82. doi: 10.1161/CIRCULATIONAHA.111.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abramson SB, Buyon JP. Activation of the complement pathway: comparison of normal pregnancy, preeclampsia, and systemic lupus erythematosus during pregnancy. Am J Reprod Immunol. 1992;28(3-4):183–7. doi: 10.1111/j.1600-0897.1992.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 34.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203(9):2165–75. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saavedra MA, Cruz-Reyes C, Vera-Lastra O, Romero GT, Cruz-Cruz P, Arias-Flores R, et al. Impact of previous lupus nephritis on maternal and fetal outcomes during pregnancy. Clin Rheumatol. 2012;31(5):813–9. doi: 10.1007/s10067-012-1941-4. [DOI] [PubMed] [Google Scholar]
- 36.Fischer-Betz R, Specker C, Brinks R, Aringer M, Schneider M. Low risk of renal flares and negative outcomes in women with lupus nephritis conceiving after switching from mycophenolate mofetil to azathioprine. Rheumatology (Oxford) 2013;52(6):1070–6. doi: 10.1093/rheumatology/kes425. [DOI] [PubMed] [Google Scholar]
- 37.Smyth A, Radovic M, Garovic VD. Women, kidney disease, and pregnancy. Adv Chronic Kidney Dis. 2013;20(5):402–10. doi: 10.1053/j.ackd.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.